Abstract
Background: Odontogenic myxoma (OM) is a rare, benign, but locally aggressive tumor of odontogenic mesenchymal origin. This study aims to expand current knowledge by integrating a concise literature review with a detailed case report of a surgically complex pediatric OM, treated using a biologically advantageous reconstructive technique. Methods: In this study, we report the case of an eight-year-old girl presenting with a large OM that caused complete disruption of the architecture of the left hemimandible. Due to the tumor’s size and bone involvement, radical resection was necessary. A modified extraoral facelift approach was employed to ensure adequate surgical access while avoiding intraoral incisions and minimizing visible scarring. Immediate mandibular reconstruction was performed using an autologous costochondral graft. Discussion: Although infrequently used in modern surgical practice, the costochondral graft offers unique advantages in pediatric patients due to its inherent growth potential and capacity for long-term biological integration. In this case, the graft allowed restoration of mandibular continuity and form with minimal donor site morbidity, demonstrating its viability even today. Conclusions: This case underlines the importance of tailored reconstructive strategies in pediatric OM. The costochondral graft provided excellent functional and esthetic results, with four-year follow-up confirming stable anatomical remodeling and bone regeneration.
1. Introduction
Odontogenic myxoma (OM) is a benign, non-encapsulated tumor of odontogenic origin. Among odontogenic tumors, OM ranks third in frequency—accounting for approximately 1–17% of solid odontogenic neoplasms—following ameloblastomas and odontomas [1,2]. OM exhibits a slight female predilection, with a female-to-male ratio of approximately 1.5:1, and it most commonly occurs between the second and fourth decades of life. The mandible is the most frequently affected site, particularly the posterior and angular regions [3,4,5]. However, cases involving the maxilla and zygomatic bone have also been documented [6,7].
Clinically, OM typically presents as a slow-growing, gelatinous, non-encapsulated mass with a gray- white to reddish appearance. Over time, the lesion may cause progressive swelling, leading to symptoms such as pathological tooth mobility, malocclusion, and altered sensation, including dysesthesia or hypoesthesia [3,8]. Radiologically, OM characteristically appears as a multilocular radiolucent lesion with a “honeycomb” or “soap bubble” pattern on CT imaging, often accompanied by expansion and thinning of the surrounding bone cortices [9]. Currently, no universally accepted treatment algorithm exists for OM; given the reported recurrence rate of 10–45%, many authors advocate for radical surgical excision with at least a 1 cm margin of healthy tissue. Nonetheless, more conservative approaches, such as curettage and enucleation, are also proposed in selected cases [10].
In pediatric patients, the rarity of the disease and the dynamic nature of craniofacial growth necessitate individualized therapeutic planning. Treatment decisions are often influenced by tumor extent, anatomical involvement, and reconstructive considerations. In this context, even single-case reports can provide valuable guidance for clinical decision-making and contribute to the limited literature available on pediatric OM. The case presented herein highlights the surgical and reconstructive challenges associated with this rare tumor in children and supports the need for ongoing reporting and discussion.
2. Materials and Methods
We collected comprehensive radiographic, photographic, clinical, and anamnestic documentation of an 8-year-old female patient diagnosed with mandibular odontogenic myxoma and treated at the Maxillofacial Surgery Unit of Salus Italo-Albanian Hospital of Tirana in March 2021. Informed consent was obtained from the patient’s parents both for the surgical procedure and for the publication of clinical and photographic data. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
In addition, we performed a review of the available literature indexed in PubMed, focusing on documented cases of odontogenic myxoma with mandibular involvement in the pediatric population.
2.1. Literature Review
Odontogenic myxoma of the mandible in pediatric patients is an uncommon entity, with limited cases described in the literature. In this review, we analyzed 10 published articles reporting on pediatric cases of mandibular odontogenic myxoma, encompassing a total of 16 patients aged between 1 and 10 years (Table 1). The reported cases include a range of surgical treatments, from conservative approaches such as enucleation and curettage to more extensive procedures like segmental mandibulectomy. Follow-up durations varied from 6 months to over 25 years.

Table 1.
Literature review of cases of odontogenic myxoma with mandibular localization.
Conservative surgery has been applied in younger patients and in cases with smaller lesions or more favorable locations. These techniques typically result in shorter recovery times and preserve mandibular growth potential. However, long-term follow-up data suggest a potential for late 1 recurrence, especially when radical margins are not achieved. On the other hand, segmental mandibulectomy is often favored in larger, expansive, or structurally disruptive lesions, offering a higher level of oncological safety. This approach consistently demonstrates low recurrence rates, even over follow-up periods exceeding a decade, although it introduces challenges in immediate reconstruction and long-term craniofacial development.
Reconstruction strategies in pediatric patients remain individualized and are influenced by age, growth potential, and defect size. In most cases, follow-up durations range from several months to over 20 years, reinforcing the importance of long-term surveillance, especially after conservative management.
2.2. Case Presentation
An 8-year-old female patient was referred to the Department of Oral and Maxillofacial Surgery of the Salus Italo-Albanian Hospital of Tirana for evaluation of a progressively enlarging, firm swelling involving the middle and lower thirds of the left hemiface (Figure 1). The lesion had been first noted approximately one year prior and had shown continuous enlargement over time. Intraoral examination revealed a mass involving the left mandibular alveolar ridge, with increased mobility of the posterior mandibular teeth upon palpation.
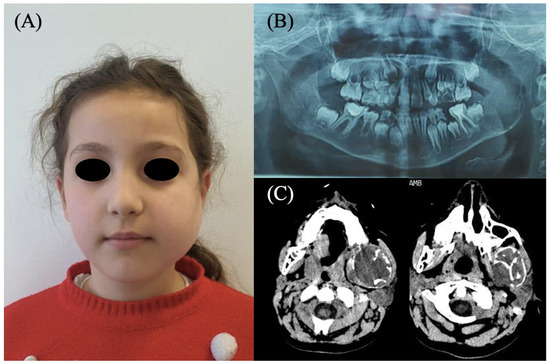
Figure 1.
(A) An 8-year-old patient with evident swelling of the middle and lower third of the left hemiface. (B) Panoramic radiograph and (C) CT scan revealing a large mass involving the body, angle, and ramus of the left mandible.
A panoramic radiograph demonstrated a multilocular radiolucent lesion with a “soap bubble” appearance, involving the entire left hemimandible up to the mental foramen, with extension to both the coronoid and condylar processes. Preoperative computed tomography (CT) confirmed the expansive lesion, extending from the mid-body of the mandible to the ascending ramus, including the coronoid and condylar apophyses (Figure 1).
The patient was otherwise in good general health, with no systemic symptoms such as fever, weight loss, or anorexia. Vital signs and routine laboratory investigations were within normal limits. No cervical lymphadenopathy was noted, and apart from the facial swelling, the clinical examination was unremarkable.
Given the tumor’s size and the degree of mandibular destruction, a radical surgical resection was necessary, requiring equally demanding reconstructive planning. A modified extraoral facelift approach was employed to achieve wide surgical exposure while minimizing visible scarring and avoiding intraoral incisions—thereby reducing the risk of contamination of the biological reconstructive site. The choice of this access was based on several prerequisites: the lesion was a benign neoplasm, not confined to the bone tissue, and amenable to removal in fragments. Considering the young age of the patient, an extraoral approach offering the most favorable esthetic outcome was preferred. Furthermore, since a biological reconstruction with an osteochondral graft was planned and no adjuvant radiotherapy was required, the external approach was selected to further minimize the risk of graft contamination that could occur with a transmucosal endoral access. Although technically more demanding than a transcervical approach, the facelift access ensures superior cosmetic results.
After creating a wide subcutaneous flap, the platysma muscle was exposed from the sternocleidomastoid to the mandibular margin. The superficial cervical fascia was dissected, identifying the marginal branch of the facial nerve. Using the FIABB nerve stimulator, both the marginal and cervical branches of the facial nerve were traced and preserved. The anterior border of the sternocleidomastoid and, deeper, the posterior belly of the digastric muscle were exposed, allowing access to the mandibular border and the tumor. Once the key anatomical structures were isolated and protected, the tumor was removed in fragments together with the residual mandibular cortical, from the region of the mandibular foramen up to the condyle. The surgical field provided adequate visibility to complete the resection while preserving the oral mucosa.
Immediate reconstruction was performed using an autologous costochondral graft, a technique rarely used in current practice but particularly suited for pediatric mandibular reconstruction due to its potential for growth and biological integration (Figure 2).
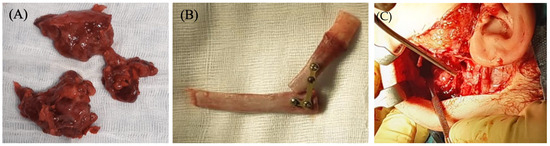
Figure 2.
(A) Left mandibular neoplasm excised. (B) Costochondral graft shaped to reconstruct the defect resulting from the left hemimandibulectomy. (C) Facelift approach and extended surgical exposure of the interested hemimandibular region.
3. Results
No intraoperative complications or postoperative adverse events were observed; evaluation of facial nerve function revealed no signs of impairment. The patient underwent an uneventful hospital stay of 7 days, during which she received broad-spectrum antibiotic therapy, analgesics, and daily local wound care.
Histopathological examination confirmed a benign mesenchymal tumor composed of monomorphic spindle cells arranged in short, interwoven bundles within a diffusely myxoid stroma (Figure 3). The mitotic index was low (1 mitosis per 10 HPF), with a Ki67/MIB1 proliferation rate of 7%. Immunohistochemistry revealed weak diffuse positivity for CD99, while all other markers tested (p63, CD34, CD68/PGM1, S-100, EMA, MUC-4) were negative. No necrosis was present, and fragments of bone tissue were observed interspersed with tumor cells.
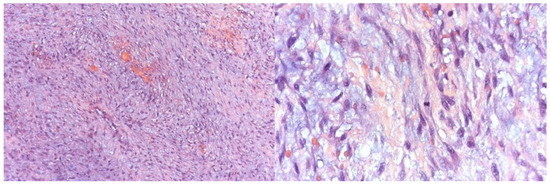
Figure 3.
Histological examination of tumor sections under light microscopy demonstrating features suggestive of an odontogenic myxoma.
Postoperative follow-up focused on monitoring mandibular growth and potential compensatory changes in the maxilla. The patient began orthodontic therapy with functional appliances, along with scheduled radiological assessments. At 6 months, CT imaging demonstrated remodeling of the costochondral graft’s cartilaginous component, which formed a neocondyle morphologically comparable to the contralateral side. Residual native mandibular bone appeared integrated with the bony portion of the graft (Figure 4).
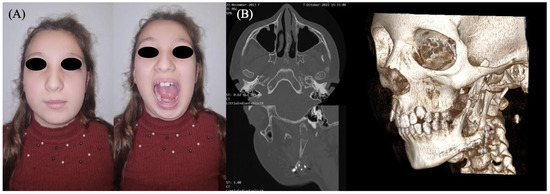
Figure 4.
(A) Patient at two years post-surgery. (B) Follow-up CT images showing the formation of a neocondyle and osseous growth of the costochondral graft, harmoniously integrated with the native mandible.
At four years postoperatively, development of the maxillomandibular complex was deemed satisfactory, with preserved growth symmetry on the left hemiface aside from a mild volume deficiency attributable to the intrinsic difference between the graft and native mandibular tissue (Figure 5). A slight limitation in right lateral mandibular deviation was observed but deemed clinically acceptable. Maximal mouth opening was within normal limits, and the patient reported no difficulties with mastication or speech. The reconstructed mandibular segment provided adequate contour and functional stability, although its volume was not sufficient to support prosthetic rehabilitation at this stage. These findings were assessed through serial morpho-functional clinical evaluations and imaging studies, including CT scans performed at 6 months, 2 years, and 4 years after surgery. Orthodontic therapy continues to support proper dental eruption and occlusion. The need for secondary bone grafting for pre-prosthetic purposes, followed by implant-prosthetic rehabilitation, will be reassessed upon completion of skeletal growth.
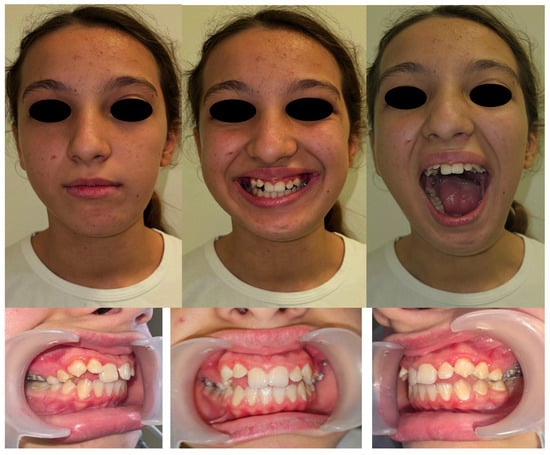
Figure 5.
Clinical evaluation and dental occlusion of the patient at four years post-surgery.
4. Discussion
Odontogenic myxoma (OM) is a benign but locally aggressive odontogenic tumor, characterized by its infiltrative behavior and significant potential for recurrence. Although it predominantly arises in the second to fourth decades of life, its occurrence in pediatric patients is exceptionally rare, with fewer than twenty documented cases in the literature. The rarity of the tumor in childhood complicates the establishment of standardized treatment guidelines, and consequently, each case must be approached with a highly individualized surgical strategy.
Maxillofacial surgery as a discipline often requires demolitive procedures in the context of malignant or benign neoplasms, severe craniofacial dysmorphisms, or major traumatic injuries [19,20,21]. These resections are typically followed by complex reconstructive procedures, which may involve vascularized free flaps, regional pedicled flaps, or bone grafts [22,23]. Within this broader reconstructive paradigm, pediatric mandibular OM poses unique challenges; the need for oncological safety through radical excision must be balanced with the preservation of growth potential and the minimization of long-term functional and esthetic sequelae.
In the case described, the tumor’s extensive involvement of the left hemimandible precluded any conservative approach such as curettage or enucleation. Radical resection was therefore unavoidable, but the reconstructive strategy required careful consideration. Harvesting tissue from growth-active donor sites such as the fibula, while effective for large defects in adults, may carry long-term morbidity risks in children, including altered limb development. Although vascularized fibular flaps represent a reliable reconstructive option, they lack growth potential and could result in facial asymmetry over time. Preserving the fibula was also considered advisable, given the possibility of secondary reconstruction after skeletal maturity and the risk of tumor recurrence, which would make a less invasive and more easily revisable reconstruction preferable. Alloplastic materials were excluded due to their inability to accommodate mandibular growth. Consequently, a biologically adaptive solution was favored over purely technical alternatives, prioritizing growth potential and long-term facial symmetry in this pediatric patient. For this reason, an autologous costochondral graft was selected.
Costochondral grafts have been historically employed in pediatric mandibular reconstruction because of the intrinsic growth potential of their cartilaginous component [22,24,25]. This biological property allows a more harmonious development of the maxillomandibular complex, reducing the risk of asymmetry and occlusal discrepancies that are frequently observed with rigid grafting techniques or non-growing reconstructive materials. In the present case, this strategy enabled satisfactory restoration of mandibular continuity and symmetry while minimizing donor site morbidity. Although the costochondral graft offers several advantages in the pediatric population, it does not provide a predictable basis for long-term implant-prosthetic rehabilitation, which can instead be more reliably achieved through definitive reconstruction with a free flap, such as the fibula or the iliac crest [26,27,28,29,30,31,32].
The surgical approach was also a critical determinant of the favorable outcome. A modified extraoral facelift incision was utilized, chosen to simultaneously maximize surgical exposure and minimize visible scarring. Furthermore, avoiding intraoral incisions reduced the risk of graft contamination and subsequent infection. However, this technique requires meticulous dissection to safeguard the branches of the facial nerve, underscoring the technical demands of such a procedure.
Histopathological analysis confirmed the diagnosis of odontogenic myxoma, and close postoperative monitoring was essential to detect recurrence and to assess graft integration. Follow-up imaging demonstrated progressive remodeling of the costochondral graft, with neocondyle formation and osseous integration, supporting its long-term biological viability.
5. Conclusions
In conclusion, the management of pediatric mandibular odontogenic myxoma exemplifies the complexity of maxillofacial oncologic and reconstructive surgery. Radical resection remains the cornerstone of oncologic safety, but in children, reconstructive strategies must account for ongoing craniofacial growth and psychosocial well-being. The use of a costochondral graft in this context represents a biologically favorable and functionally effective option, achieving stable long-term outcomes. This case highlights the importance of personalized, growth-conscious reconstructive planning in pediatric maxillofacial surgery.
Author Contributions
Conceptualization, F.L. and M.S.; methodology, F.L. and A.M.M.; software, V.C.; validation, F.L., E.B.P. and M.S.; formal analysis, A.M.M.; investigation, A.M.M. and V.C.; resources, F.L.; data curation, V.C.; writing—original draft preparation, F.L.; writing—review and editing, A.M.M.; visualization, E.B.P.; supervision, F.L.; project administration, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the approval waived by the Institutional Review Board of Salus Hospital, Tirana on 6 May 2024, as the procedure presented in the case report is routinely performed in clinical practice and does not involve investigational or experimental interventions.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
All data generated in this study are included in the published article.
Conflicts of Interest
The authors declare no conflicts of interest. None of the authors have any financial relationships or interests, including membership, employment, consultancies, stock/share ownership, honoraria, grants or other funding, paid expert testimonies, or patent-licensing arrangements, that could inappropriately influence or bias this work. Additionally, the authors declare no non-financial conflicts of interest, including personal or professional relationships, affiliations, or personal beliefs, that could affect the objectivity of this study. The authors declare no conflicts of interest between any company or private practice and this manuscript. No funders or companies had any role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Li, T.J.; Sun, L.S.; Luo, H.Y. Odontogenic myxoma: A clinicopathologic study of 25 cases. Arch. Pathol. Lab. Med. 2006, 130, 1799–1806. [Google Scholar] [CrossRef]
- Regezi, J.A.; Kerr, D.A.; Courtney, R.M. Odontogenic tumors: Analysis of 706 cases. J. Oral. Surg. 1978, 36, 771–778. [Google Scholar]
- Martínez-Mata, G.; Mosqueda-Taylor, A.; Carlos-Bregni, R.; de Almeida, O.P.; Contreras-Vidaurre, E.; Vargas, P.A.; Cano-Valdéz, A.M.; Domínguez-Malagón, H. Odontogenic myxoma: Clinico-pathological, immunohistochemical and ultrastructural findings of a multicentric series. Oral. Oncol. 2008, 44, 601–607. [Google Scholar] [CrossRef]
- Odukoya, O. Odontogenic tumors: Analysis of 289 Nigerian cases. J. Oral. Pathol. Med. 1995, 24, 454–457. [Google Scholar] [CrossRef]
- Kangur, T.T.; Dahlin, D.C. Myxomatous tumors of the jaws. J. Oral. Surg. 1975, 33, 523–528. [Google Scholar]
- Rotenberg, B.W.; Daniel, S.J.; Nish, I.A.; Ngan, B.Y.; Forte, V. Myxomatous lesions of the maxilla in children: A case series and review of management. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Nikunj, A.M.; Mishra, B.; Elangovan, B.; Rajkhokar, D. Peripheral Odontogenic Myxoma of Zygoma and Orbital Region—A Unique Case Report with Review of Literature. Ann. Maxillofac. Surg. 2024, 14, 112–115. [Google Scholar] [CrossRef]
- Dotta, J.H.; Miotto, L.N.; Spin-Neto, R.; Ferrisse, T.M. Odontogenic Myxoma: Systematic review and bias analysis. Eur. J. Clin. Invest. 2020, 50, e13214. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, A.B.; Arayasantiparb, R.; Juengsomjit, R.; Lam-Ubol, A. Central Odontogenic Myxoma: A Radiographic Analysis. Int. J. Dent. 2021, 2021, 1093412. [Google Scholar] [CrossRef]
- Ghosh, B.C.; Huvos, A.G.; Gerold, F.P.; Miller, T.R. Myxoma of the jaw bones. Cancer 1973, 31, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Ataman, M.; Sarioglu, T.; Ayas, K. Myxoma of the mandible. Int. J. Pediatr. Otorhinolaryngol. 1993, 27, 183–186. [Google Scholar] [CrossRef]
- Shahoon, H.; Esmaeili, M.; Nikhalat, M.; Farokhi, E. Odontogenic Fibromyxoma and Odontogenic Cyst in an Eight year old Boy: Three-year Follow-up. J. Dent. Res. Dent. Clin. Dent. Prospect. 2009, 3, 103–105. [Google Scholar]
- Mauro, A.; Lipari, L.; Tortorici, S.; Leone, A.; Gerbino, A.; Buscemi, M. Expression of MMP-2 and MMP-9 in odontogenic myxoma in a child: Report of a clinical case. Odontology 2013, 101, 233–238. [Google Scholar] [CrossRef]
- Kleiber, G.M.; Skapek, S.X.; Lingen, M.; Reid, R.R. Odontogenic myxoma of the face: Mimicry of cherubism. J. Oral. Maxillofac. Surg. 2014, 72, 2186–2191. [Google Scholar] [CrossRef]
- Haser, G.C.; Su, H.K.; Hernandez–Prera, J.C.; Khorsandi, A.S.; Wang, B.Y.; Urken, M.L. Pediatric odontogenic fibromyxoma of the mandible: Case report and review of the literature. Head Neck 2016, 38, E25–E28. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tanaka, K.; Hirai, H.; Marukawa, E.; Izumo, T.; Harada, H. Appropriate surgical margin for odontogenic myxoma: A review of 12 cases. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2018, 126, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.V.; Rocha, A.C.; Ceccheti, M.M.; Gallo, C.d.B.; Alves, F.A. Odontogenic myxoma in a child treated with enucleation and curettage. Autops. Case Rep. 2018, 8, e2018042. [Google Scholar] [CrossRef] [PubMed]
- Shupak, R.; Cho, J.J. Mandibular odontogenic myxoma in a paediatric patient. BMJ Case Rep. 2020, 13, e236926. [Google Scholar] [CrossRef] [PubMed]
- Spinzia, A.; Renzetti, P.; Bongiorno, A.; Laganà, F. Orbital Floor Fractures Comparing Different Kinds of Reconstruction: A Proposal for Restoration of Physiological Anatomy. J. Craniofac. Surg. 2021, 32, e128–e134. [Google Scholar] [CrossRef]
- Prior, A.; Allegretti, L.; Melloni, I.; Bovio, M.; Laganà, F.; Ceraudo, M.; Zona, G. Traumatic subarachnoid hemorrhage related to ophthalmic artery avulsion: A case report. Acta Neurochir. 2018, 160, 913–917. [Google Scholar] [CrossRef]
- Laganà, F.; Arcuri, F.; Spinzia, A.; Bianchi, B. Maxillomandibular Advancement for Obstructive Sleep Apnea Syndrome: Long-Term Results of Respiratory Function and Reverse Face-Lift. J. Craniofac. Surg. 2023, 34, 1760–1765. [Google Scholar] [CrossRef]
- Khavanin, N.; White, M.J.; Walsh, J.M.; Steinberg, J.P. Mandibular Reconstruction Following Central Giant Cell Granuloma Resection in Primary Dentition: A Case for the Use of a Costochondral Graft. Cleft Palate Craniofac. J. 2021, 58, 260–268. [Google Scholar] [CrossRef]
- Arcuri, F.; Laganà, F.; Bianchi, B.; Ferrari, S.; Ferri, A. Double Arterialized Scapular Tip Free Flap for Mandibular Reconstruction. J. Craniofac. Surg. 2023, 34, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, A.; Yadav, P.; Bhutia, O.; Kaur, K.; Dekyi, T.; Pandey, R. Growth Outcome and Jaw Functions Are Better After Gap Arthroplasty Plus Costochondral Graft Reconstruction Than Gap Arthroplasty Alone in Pediatric Temporomandibular Joint Ankylosis Patients: A Cluster Randomized Controlled Trial. J. Oral. Maxillofac. Surg. 2021, 79, 2548–2561. [Google Scholar] [CrossRef]
- Castellon, L.; Jerez, D.; Mayorga, J.; Fuenzalida, C. Remodeling of Costochondral Graft After Mandibular Reconstruction. J. Oral. Maxillofac. Surg. 2017, 75, e1–e226. [Google Scholar] [CrossRef]
- Zhao, X.; Ding, M.; Diarra, D.; Ouyang, S.; Lin, H.; Zhou, R.; Zeng, B.; Ma, L.; Liu, B.; Wu, T. Iliac crest free flap versus fibula free flap for mandibular reconstruction: Cost-effectiveness analysis in a Chinese population. J. Craniomaxillofac. Surg. 2025, 53, 1399–1406. [Google Scholar] [CrossRef]
- Zavattero, E.; Ramieri, G.M.; Agrò, G.D.; Fasolis, M.; Garzino-Demo, P.; Borbon, C. Implant Dental Rehabilitation of Fibula-Free Flap Reconstructed Jaws. J. Craniofac. Surg. 2021, 32, e134–e136. [Google Scholar] [CrossRef]
- Tarsitano, A.; Ciocca, L.; Scotti, R.; Marchetti, C. Morphological results of customized microvascular mandibular reconstruction: A comparative study. J. Craniomaxillofac. Surg. 2016, 44, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.Y.; Kim, D.D.; Ghali, G.E. Maxillofacial Reconstruction Using Vascularized Fibula Free Flaps and Endosseous Implants. Oral. Maxillofac. Surg. Clin. N. Am. 2019, 31, 259–284. [Google Scholar] [CrossRef]
- Beltramini, G.; Beltramini, G.A.; Rossi, D.; Bolzoni, A.; Piva, A.; Laganà, F. Implants outcome inserted in different sites. J. Biol. Regul. Homeost Agents 2020, 34 (Suppl. S2), 13–17. [Google Scholar] [PubMed]
- Attia, S.; Wiltfang, J.; Streckbein, P.; Wilbrand, J.-F.; El Khassawna, T.; Mausbach, K.; Howaldt, H.-P.; Schaaf, H. Functional and aesthetic treatment outcomes after immediate jaw reconstruction using a fibula flap and dental implants. J. Craniomaxillofac. Surg. 2019, 47, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Lonie, S.; Herle, P.; Paddle, A.; Pradhan, N.; Birch, T.; Shayan, R. Mandibular reconstruction: Meta-analysis of iliac- versus fibula-free flaps. ANZ J. Surg. 2016, 86, 337–342. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).