Abstract
Background: Total knee arthroplasty (TKA) is the gold standard for advanced hemophilic arthropathy. However, surgical management in hemophilic patients is complex due to joint deformities, bleeding risk, and systemic comorbidities. This study aimed to compare the surgical outcomes and cost-effectiveness of TKA in hemophilic versus non-hemophilic patients. Methods: This prospective study included 50 patients treated between 2010 and 2024 at Elias University Hospital, Romania. Group 1 included 20 male patients with severe hemophilia (2 with inhibitors); Group 2 included 30 non-hemophilic male patients. Data collection was standardized and conducted preoperatively, at 6 and 12 months postoperatively, and annually thereafter for up to 14 years following surgery. The mean follow-up duration across the cohort was 7.3 ± 3.9 years (range: 0.5–14 years), allowing for consistent long-term evaluation of clinical and functional outcomes. Study included operative time, transfusion requirements, hospitalization length, perioperative complications, functional outcomes (Knee Society Score—KSS), quality of life (EQ-5D), and cost per quality-adjusted life year (QALY). Results: Hemophilic patients had significantly longer operative times (154.5 vs. 88.7 min; p < 0.001), higher transfusion rates (45% vs. 20%, p < 0.047), and longer hospital stays (mean 12.3 vs. 6.6 days). Perioperative complications occurred in 90% of hemophilic patients (anemia requiring transfusion: 45%; compressive hematomas: 10%; wound dehiscence: 15%) compared to 10% in controls. Non-hemophilic patients achieved superior postoperative functional scores. Mean preoperative KSS was 32.25 ± 11.24 and postoperatively, the mean score increased to 98 ± 1.34. The mean preoperative KSS in the hemophilic group was 31 ± 13.93 and postoperative KSS was 74.5 ± 19.92. The cost per QALY was €2506 in the hemophilic group versus €1258 in controls. The economic assessment was conducted from the hospital perspective, focusing on direct medical costs incurred during hospitalization and the perioperative period. Cost components included factor replacement therapy, surgical and anesthesia costs, hospital stay, laboratory investigations, blood transfusions, and management of postoperative complications. Conclusion: Although TKA improves quality of life and function in hemophilic patients, it is associated with higher complication rates and costs. These findings highlight the need for careful patient selection and informed consent when considering TKA in hemophilic patients.
1. Introduction
Hemophilia is an inherited X-linked disorder caused by a deficiency of coagulation factor VIII (hemophilia A) or factor IX (hemophilia B) [1,2,3,4,5].
The hallmark clinical sign in patients with hemophilia is represented by intra-articular bleeding, which in its early stages is reversible, but subsequently progresses to joint ankylosis and, over time, leads to joint deformities [6,7,8,9].
Caregivers must initiate early treatment, starting in childhood, with the goal of preventing the development of chronic arthropathy [10,11,12,13].
The standard treatment for advanced hemophilic arthropathy is total knee arthroplasty (TKA) [14].
Surgeons performing TKA in this population must be proficient in revision TKA techniques and familiar with the use of specialized implants [15,16].
Given the rarity of hemophilia (1:5000–10,000 males) and limited published surgical series, multi-institutional outcome data are critically needed to establish best practices. While TKA outcomes in hemophilic patients have been reported [14,15,16], comparative cost-effectiveness data versus non-hemophilic patients remain scarce, particularly in Eastern Europe. This article presents our experience with TKA in patients with hemophilia treated at the Orthopedic Department of Elias University Hospital. The primary objective was to compare postoperative functional outcomes (KSS) and complication rates between hemophilic and non-hemophilic TKA patients. This comparison quantifies the additional surgical complexity, complication burden, and healthcare costs associated with hemophilic arthropathy, informing preoperative counseling and resource allocation. We hypothesized that hemophilic patients would demonstrate increased perioperative complications and healthcare costs compared to controls, yet still achieve clinically meaningful functional improvement.
2. Materials and Methods
2.1. Design
This study represents a prospective cohort design, with longitudinal follow-up and standardized data collection at predefined intervals. Although data were later extracted from an institutional registry for analysis, patient enrollment and follow-up were conducted prospectively according to a predefined protocol from our institutional Orthopedic Department of Elias University Hospital, Bucharest, Romania. The database include all hemophilic patients who underwent TKA between 2010, and 2024, with a minimum follow-up duration of six months. Data collection was standardized and conducted preoperatively, at 6 and 12 months postoperatively, and annually thereafter for up to 14 years following surgery. The mean follow-up duration across the cohort was 7.3 ± 3.9 years (range: 0.5–14 years), allowing for consistent long-term evaluation of clinical and functional outcomes. All patients completed minimum 6-month follow-up with no loss to follow-up. The study was approved by the local Ethics Committee. Although the time interval for the study is long, the surgical techniques and implant technology did not suffer major changes. The surgical team was mainly the same. Non-hemophilic controls were consecutively selected (1–2 per hemophilic patient) during the same hospitalization period to minimize temporal bias.
2.2. Inclusion Criteria
Group 1: 20 male patients with hemophilia who underwent TKA.
Group 2: 30 non-hemophilic male patients who underwent TKA. Inclusion criteria for Group 2 was: male sex with knee arthritis. Exclusion criteria included female sex.
2.3. Procedure
Viral status (HBV, HCV, HIV) was documented. Liver function was assessed, as chronic viral infections can lead to hepatitis, cirrhosis, thrombocytopenia, and increased bleeding risk. In such cases, fresh frozen plasma or platelet transfusions may be required. HIV-positive patients were at increased risk for postoperative infections due to immunosuppression. The indication for postoperative blood transfusion was established for patients presenting with a hemoglobin level below 8 g/dL or symptomatic anemia (manifested by tachycardia, hypotension, dizziness, or decreased oxygen saturation). Severe hemophilia was defined as a baseline clotting factor VIII or IX activity level below 1%, consistent with the World Federation of Hemophilia (WFH) classification criteria. Hemoglobin was measured on postoperative days 1 and 2 or when drain output exceeded 500 mL.
In Group 1, two patients had pre-existing inhibitors against recombinant clotting factors, and one developed inhibitors postoperatively.
Diagnosis and severity of hemophilia (mild, moderate, or severe) were confirmed. Baseline clotting factor levels, inhibitor status, and replacement regimen were recorded. A comprehensive written treatment and monitoring plan was provided by the Hemophilia Comprehensive Care Team (CCT), including dosing schedules and recovery protocols (pre-, intra-, and postoperative). Consistency in using the same replacement product was recommended to reduce pharmacokinetic variability and inhibitor formation.
Mandatory laboratory tests included: platelet count, APTT, fibrinogen, prothrombin time, FVIII/IX levels, inhibitor titers, and blood type.
Factor VIII/IX levels were monitored immediately postoperatively and then daily during hospitalization, with therapy dosages adjusted accordingly.
Patients were admitted two days prior to elective surgery for optimal preparation and perioperative planning. Blood products were cross-matched in advance. The coagulation laboratory was notified to prepare for intensive monitoring.
A comprehensive preoperative evaluation of the entire musculoskeletal system was performed, including both knees, hips, and ankles, given the frequent presence of multi-joint involvement that may influence surgical planning. Standard imaging included anteroposterior (AP), lateral, sunrise, and notch views of both knees. In addition, standing long-leg alignment radiographs (three-joint views) and a CT scan were required to thoroughly assess limb alignment, anatomical variations, and the extent of osseous defects in some cases.
2.4. Surgical Management
The standard surgical approach consisted of a midline incision and medial parapatellar arthrotomy. In cases of joint stiffness or patellar maltracking, extended exposures including quadricepsplasty was performed.
Synovectomy is an essential step of total knee arthroplasty (TKA) and was performed routinely in all cases. In the advanced cases, joint stiffness or even ankylosis was observed. Therefore, thorough soft tissue release was performed during surgery. When residual flexion contracture was still present despite adequate soft tissue release, additional bone resection was done from the distal femur alone or both the distal femur and proximal tibia, to achieve full extension and proper implant positioning.
Patella resurfacing was performed for all cases in both groups. In hemophilic patients, the bone resected from the patella was minimal to preserve bone stock for potential future revision.
Due to prolonged axial deformities—such as varus or valgus malalignment—and significant muscle atrophy, the risk of intraoperative joint instability in hemophilic patients is substantially higher than in primary total knee arthroplasty (TKA) for osteoarthritis. To effectively address this instability, we have used rotating-hinge prostheses (RT-PLUS from Smith Nephew, Watford, Hertfordshire, England) for the majority of hemophilic patients. All non-hemophilic patients were treated with PS implants (Nexgen by Zimmer, Warsaw, IN, USA). The study is significantly confounded by the difference in implant types used between the two groups. That will impact the validity of directly comparing the primary outcomes, particularly the functional Knee Society Scores (KSS). Constrained implants are biomechanically different and are typically reserved for more complex or revision scenarios.
All patients received 1 g of intravenous tranexamic acid administered 30 min prior to incision [14]. Cemented implants were used in all cases, with the least amount of constraint necessary to achieve a stable knee joint.
We did not use a tourniquet during total knee arthroplasty (TKA) in any of the patient groups. Meticulous hemostasis was performed at the end of the procedure prior to wound closure to prevent postoperative hematoma. An active suction drain was placed intra-articularly and maintained for 3 days in hemophilic patients and for 2 days in non-hemophilic patients, due to the higher risk of bleeding in hemophilia patients.
Pharmacological thromboprophylaxis was administered to all non-hemophilic patients. In contrast, only mechanical thromboprophylaxis—consisting of early mobilization and compressive dressings—was used in the hemophilic group.
Patients followed standardized rehabilitation protocol with early mobilization on postoperative day 1, progressive weight-bearing, and range-of-motion exercises.
Operative time, intraoperative blood loss, and coagulation factor levels were systematically recorded. Hemoglobin levels were assessed at multiple time points: preoperatively upon the patient’s arrival on the day of surgery, in the recovery room for selected cases, and daily during the postoperative hospital stay.
Postoperative complications were categorized as early (occurring within ≤90 days after surgery) and late (manifesting beyond 90 days postoperatively). The severity of each adverse event was graded according to the Clavien–Dindo classification, distinguishing between minor complications requiring pharmacologic or bedside interventions (Grades I–II) and major complications necessitating surgical procedures (Grades III–V) [17]. Implant survival was assessed using implant revision as the primary endpoint, and all reoperations—regardless of cause—were recorded.
All patients underwent both preoperative and postoperative clinical evaluations, during which data were collected regarding pain, range of motion, and knee joint stability. Functional outcomes assessed using the original Knee Society Score by the surgical team at each follow-up.
2.5. Statistical Analisys
To assess the overall quality of life in patients from both study groups, the EuroQol-5 Dimension (EQ-5D) questionnaire was administered both preoperatively and postoperatively. This instrument provided data on general mobility, self-care ability, performance of usual activities, pain levels, and the degree of anxiety or depression.
The results obtained from the EQ-5D questionnaire were converted based on reference data reflecting the general health status of the general population. Following this conversion, a score was assigned to each patient, indicating their quality of life. The minimum score is 0, representing death, while the maximum score is 1, indicating perfect health. Using this score, the Quality-Adjusted Life Years (QALY) metric was calculated, reflecting the number of life years adjusted for quality of life. To assess the cost-effectiveness of total knee arthroplasty (TKA) in the two study groups, the cost/QALY ratio was used. The used formula was
U is utility (extracted from the EQ-5D) and t time (in years) that the patient is in that utility.
QALYs = U × t
The economic assessment was conducted from the hospital perspective, focusing on direct medical costs incurred during hospitalization and the perioperative period. Cost components included factor replacement therapy, surgical and anesthesia costs, hospital stay, laboratory investigations, blood transfusions, and management of postoperative complications. The major cost drivers were the factor replacement therapy (representing the largest expenditure due to high unit costs and individualized dosing schedules) and length of hospitalization, particularly in hemophilic patients who required prolonged inpatient monitoring. Additional contributors included implant costs, transfusion requirements, and treatment of postoperative infections or wound complications. Indirect costs such as loss of productivity, caregiver burden, or social support were not included, as the analysis was confined to the institutional hospital perspective.
Data Analysis Strategy
- Study OverviewA retrospective comparative analysis between two cohorts:
- -
- Group 1 (Hemophilic patients)—n1 = 20
- -
- Group 2 (Non-hemophilic controls)—n2 = 30
- Data Entry and Preparation
Data were entered into a secure database (Excel → SPSS/R/Python Pandas IBM SPSS Statistics (Version 26.0, IBM Corp., Armonk, NY, USA), R software (Version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria), and Python (Version 3.10) with the Pandas library (Version 1.5.3)). Each variable was coded as follows:
| Variable | Type | Coding/Description |
| Group | Categorical | 0 = Non-hemophilic, 1 = Hemophilic |
| Operative_time | Continuous (min) | Numeric value |
| Transfusion | Binary categorical | 0 = No, 1 = Yes |
| Hospital_stay | Continuous (days) | Numeric value |
| KSS_pre | Continuous (points) | Knee Society Score before TKA |
| KSS_post | Continuous (points) | Knee Society Score at last follow-up |
Missing data were handled using listwise deletion. Outliers were assessed via z-scores (>3 SD) or visual inspection of boxplots.
- 3.
- Data Distribution Assessment
Normality was tested using the Shapiro–Wilk test (α = 0.05) and visualized through Q–Q plots. Homogeneity of variance was verified using Levene’s test. Depending on the results, either parametric (Student’s t-test) or non-parametric (Mann–Whitney U test) methods were applied.
- 4.
- Statistical Tests
| Operative time | Continuous | Independent-samples t-test | Welch correction if variances unequal |
| Transfusion rate | Categorical (binary) | Fisher’s Exact test | Used instead of χ2 due to small, expected counts |
| Hospital stay | Continuous | Independent-samples t-test | Two-tailed |
| KSS pre-op | Continuous | Independent-samples t-test | Tests baseline comparability |
| KSS post-op | Continuous | Independent-samples t-test | Evaluates outcome improvement |
| ΔKSS (post—pre) | Continuous (derived) | Paired t-test within groups | Measures improvement magnitude |
- 5.
- Significance and Effect Sizes
Statistical significance was set at p < 0.05 (two-tailed). 95% Confidence Intervals (CI) were reported for all mean differences. Effect sizes were calculated as Cohen’s d for continuous variables (0.2 small, 0.5 medium, 0.8 large) and odds ratios (OR ± 95% CI) for categorical outcomes.
- 6.
- Interpretation Framework
Results were interpreted in light of clinical significance, not only statistical difference. p-values were supplemented with effect sizes and confidence intervals to reflect the magnitude of effect. Adjusted interpretation was applied where small sample sizes might induce Type II error. Cost variability was discussed separately to reflect temporal trends (2010–2024).
- 7.
- Optional Extension: Cost–Effectiveness Subanalysis
For economic evaluation, cost per QALY gained was calculated between groups. Costs were adjusted by year using inflation indexing (WHO PPP or Eurostat GDP deflator). Sensitivity analysis was performed across cost percentiles.
Acknowledge: Sample size was determined by convenience sampling due to rarity of hemophilia (no formal power calculation performed).
3. Results
The primary endpoint of the study was the functional outcome assessed by the Knee Society Score (KSS) at the latest follow-up. Secondary endpoints included the rate and severity of postoperative complications (classified according to Clavien–Dindo), perioperative blood transfusion rate, and the cost per quality-adjusted life year (QALY) gained.
3.1. Demographic and Clinical Characteristics
The demographic and clinical characteristics of the study groups are summarized in Table 1.

Table 1.
Demographic and clinical characteristics of the study groups.
3.2. Operative Metrics and Hospitalization
The mean operative time in the non-hemophilic group was 88.71 ± 10.17 min (range: 69–110; mode: 85, 95), whereas in the hemophilic group, it was significantly longer at 154.54 ± 18.36 min (range: 120–180).
The rate of postoperative blood transfusions was 45% in the hemophilic group—2.25 times higher than the 20% observed in the non-hemophilic cohort. The difference in transfusion rates between hemophilic (45%) and non-hemophilic (20%) groups was statistically significant (p = 0.047), indicating a higher transfusion requirement among hemophilic patients. The calculated odds ratio was 4.2, indicating a significantly elevated transfusion risk in hemophilic patients.
The mean length of hospital stay for non-hemophilic patients was 6.57 ± 1.63 days (range: 4–12; mode: 6), while hemophilic patients required significantly longer hospitalization with a mean of 12.3 ± 2.38 days (range: 7–15), representing a 1.87-fold increase (Table 2).

Table 2.
Comparison of operative time, transfusion rate, and hospital stay between hemophilic and non-hemophilic groups. Statistical significance for inter-group differences is reported in Section 3.2.
3.3. Functional Outcomes (Knee Society Score—KSS)
Functional outcomes were assessed using the original Knee Society Score by the surgical team at each follow-up. In the non-hemophilic group, the mean preoperative KSS was 32.25 ± 11.24, with a confidence interval between 26.5 and 38. Postoperatively, the mean score increased to 98 ± 1.34, with a confidence interval between 97 and 98.72—indicating a nearly threefold improvement.
In the hemophilic group, the mean preoperative KSS was 31, with a median of 34.5, mode of 35, and standard deviation of ±13.93. The confidence interval ranged from 19.85 to 42.15, with scores varying from 7.5 (min) to 49.5 (max), (p < 0.001). Preoperative KSS showed marked dispersion (mean 31.0 ± 23.8; 95% CI 19.85–42.15; n = 20), reflecting substantial baseline heterogeneity (severity stage, inhibitor status, deformity).
Postoperatively, the mean KSS rose to 74.5, with a median of 80, a mode of 90, and a standard deviation of ±19.92. The confidence interval was between 58.86 and 90.44, with values ranging from 50 to 94.5.
3.4. Implant Types and Survivorship
In the non-hemophilic group, 100% of patients received primary prosthetic implants.
In the hemophilic group, 80% of patients (n = 16) required highly constrained prostheses (only rotating hinge), due to severely altered joint anatomy. Only 20% (n = 4) received primary implants, representing a rate five times lower than in the control group.
Implant survivorship was 100% for all patients. At the most recent follow-up, no radiographic evidence of osteolysis, radiolucent lines, or component migration was observed.
3.5. Perioperative Complications
Complications occurred in 90% of hemophilic and 20% of non-hemophilic patients. According to the Clavien–Dindo classification, most events were Grades I–II, including anemia managed conservatively or with transfusion. Grade III complications included five compressive hematomas requiring surgical evacuation, three cases of wound dehiscence necessitating revision, and one intraoperative femoral condyle fracture (Grade IIIb) stabilized with plate and screws. No Grade IV (life-threatening) or Grade V (death) events were observed. (Figure 1).
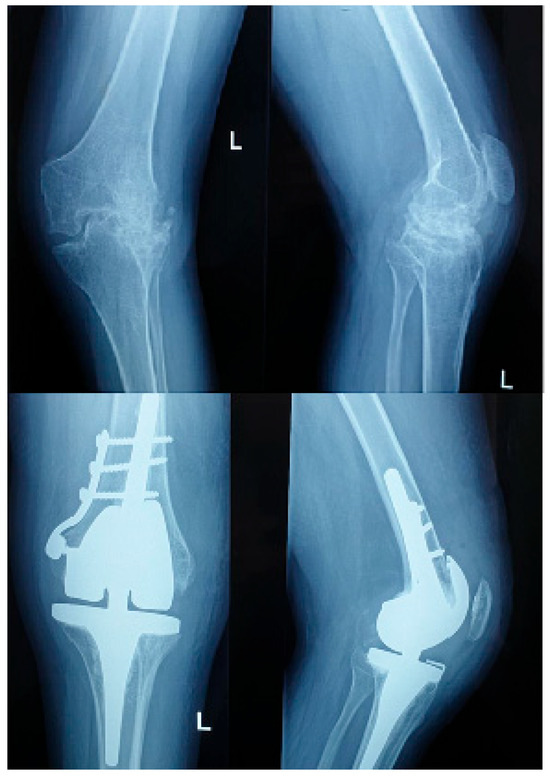
Figure 1.
Pre and postoperative X ray of a hemophilic patient with inhibitors.
Among the 20 hemophilic patients, three (15%) had factor inhibitors—two pre-existing and one developing postoperatively. Compared with inhibitor-negative patients, those with inhibitors exhibited markedly more complex perioperative courses. The mean operative time was prolonged (172.0 ± 6.5 min vs. 151.2 ± 17.9 min, p = 0.048), and the transfusion requirement was higher (100% vs. 37.5%, p = 0.021). The mean hospital stay was significantly longer (15.7 ± 2.1 days vs. 11.8 ± 2.2 days, p = 0.012). All inhibitor-positive patients developed postoperative complications: two experienced compressive hematomas requiring surgical evacuation (Clavien–Dindo Grade IIIb), and one sustained an intraoperative medial femoral condyle fracture, stabilized with plate and screws (Grade IIIb).
The mean intraoperative blood loss was significantly higher in the hemophilic cohort compared to non-hemophilic controls. Hemophilic patients experienced an average blood loss of 612 ± 178 mL, whereas the non-hemophilic group had a mean of 348 ± 92 mL (p < 0.001). Blood loss correlated positively with operative time (r = 0.63, p < 0.01) and transfusion requirement, and was notably higher in patients with inhibitors (mean: 790 ± 120 mL, p = 0.021 vs. inhibitor-negative).
3.6. Cost-Effectiveness (QALY Analysis)
Health-related quality of life, assessed using the EQ-5D index, improved significantly in both groups postoperatively. The mean preoperative EQ-5D score was 0.42 ± 0.11 in the hemophilic group and 0.61 ± 0.09 in the non-hemophilic group (p < 0.001). Postoperatively, the mean EQ-5D score increased to 0.78 ± 0.10 in hemophilic patients and 0.86 ± 0.07 in controls (p = 0.032). The overall gain in EQ-5D was greater in the hemophilic cohort (+0.36 vs. +0.25), reflecting a substantial postoperative improvement in quality of life despite more complex perioperative management.
Quality-Adjusted Life Years (QALY) were calculated using EQ-5D–derived utility scores:
- Control group (non-hemophilic): Mean QALY = 2.4
- Study group (hemophilic): Mean QALY = 2.5
The cost-effectiveness ratio (cost/QALY) was also analyzed (Table 3):

Table 3.
Quality-Adjusted Life Years (QALY) and Cost-Effectiveness.
- Control group: €1258 per QALY
- Hemophilic group: €2506 per QALY (approximately 2× higher)
This substantial difference reflects the higher complexity, increased complication rates, need for replacement therapy, and extended hospitalization in hemophilic patients.
4. Discussion
This comparative study demonstrated that hemophilic TKA patients experienced 4.5-fold higher complication rates (90% vs. 20%), doubled hospitalization costs (€2506 vs. €1258/QALY), yet achieved meaningful functional improvement (KSS + 43.5 points).
Studies emphasizes that, with appropriate multidisciplinary management and individualized perioperative protocols, satisfactory functional and clinical outcomes can be achieved in this high-risk population. Continuous multidisciplinary management, including close postoperative monitoring and adjunctive antifibrinolytic therapy when appropriate, is essential to reduce bleeding complications and optimize surgical outcomes in this high-risk population. Inhibitor presence complicates perioperative management significantly [18,19,20,21].
According to current clinical protocols, the concentration of deficient coagulation factors—factor VIII in Hemophilia A or factor IX in Hemophilia B—should reach up to 120% at the time of anesthetic induction. An additional 20–30% should be maintained during the first 3–4 h of surgery [22].
A frequently observed deformity in these patients is the absence of the physiological posterior tibial slope, thought to be caused by ambulation in a genu flexum (flexed-knee) posture during growth. Typical radiographic features include enlarged epiphyses, widened intercondylar notches, and massive osteophytes, which may obscure true joint lines and result in prosthesis malalignment. The mediolateral-to-anteroposterior dimension ratio is often increased, and the patella is usually larger than that seen in non-hemophilic osteoarthritis patients. These abnormalities must be carefully accounted for during preoperative planning [23].
4.1. Technical Considerations
TKA in hemophilic patients often requires:
- Extensive bone resections to correct deformities.
- Use of highly constrained implants and smaller-sized components.
- Asymmetric resections, which may cause ligament imbalance.
- Soft tissue releases, especially of the lateral collateral ligament and popliteus tendon, in cases of genu valgum.
- Osteotomies and posterior capsulotomy for improved extension.
Surgical exposure in hemophilic patients can be particularly challenging due to extensive intra-articular adhesions, arthrofibrosis, and limited range of motion [24,25,26,27,28].
Perioperative antibiotic prophylaxis does not differ from that used in standard TKA. First-generation cephalosporins are the agents of choice. In patients with allergies, clindamycin or vancomycin is recommended [29]. The use of a tourniquet remains controversial: some surgeons prefer it for intraoperative blood control, while others avoid it to allow better monitoring of active bleeding [30].
One notable limitation of this study is related to the comparability of the two groups. In hemophilic patients, osteoarthritis is typically diagnosed at a much more advanced stage, often when the native ligamentous structures of the knee are no longer functionally competent. As a result of extensive bone destruction and marked ligamentous instability commonly observed in this population, constrained or semi-constrained implants with a higher degree of intrinsic stability were often selected for primary total knee arthroplasty.
In contrast, patients in the control group—who presented with primary gonarthrosis—underwent TKA using standard, posterior-stabilized primary implants. In our routine clinical practice, constrained prostheses are generally reserved for revision procedures following the failure of primary implants. However, due to the severe joint deterioration associated with hemophilic arthropathy, these implants were used as the primary option in the hemophilic group.
This discrepancy in implant selection introduces a confounding variable that limits the direct comparability of surgical outcomes. Although all patients underwent what was technically classified as primary TKA, the functional and biomechanical differences between implant types—driven by the underlying pathology—make it challenging to interpret the results as a pure comparison between two homogeneous primary arthroplasty groups. Similarly, the comparison does not reflect a conventional primary-versus-revision scenario, further complicating the interpretation of outcome differences between the cohorts.
4.2. Thromboprophylaxis and Thromboembolic Risk
Pharmacologic thromboprophylaxis should be considered only in patients with additional risk factors [31].
Current guidelines from both the American Academy of Orthopaedic Surgeons (AAOS) advise against the use of chemoprophylaxis in patients with bleeding diathesis. The rationale is that the inherent coagulation deficits in hemophilic patients may eliminate the need for anticoagulant therapy following total knee arthroplasty (TKA) [32,33].
In our study we did not administer chemoprophylaxis in hemophilic patients undergoing TKA. Instead, we prioritize early ambulation and mechanical thromboprophylaxis. Notably, we have not observed any cases of symptomatic venous thromboembolism (VTE) in our patient groups.
4.3. Outcomes
The functional outcomes and survival rates of total knee arthroplasty (TKA) in patients with hemophilia are generally inferior to those observed in patients with osteoarthritis [34].
However, these outcomes have been improving due to advances in implant design and more effective perioperative coagulation factor replacement strategies [9]. These findings are consistent with the results of Moore et al., whose meta-analysis demonstrated significant postoperative improvements in knee range of motion (ROM) among hemophilic patients undergoing total knee arthroplasty, despite higher complication rates and perioperative challenges [35].
One of the key factors influencing postoperative ROM is preoperative mobility, which is often more limited in patients with hemophilia due to chronic arthropathy [36].
In our study, non-hemophilic patients achieved excellent outcomes with a mean postoperative KSS of 98, very close to the maximum score. In contrast, hemophilic patients had a mean postoperative KSS of 74.5, approximately 1.31 times lower than the control group. This is consistent with the literature, which reports that long-standing joint destruction and polyarticular involvement in hemophilia result in lower functional outcomes after TKA [37]. The 24-point KSS deficit likely reflects preoperative disease severity, polyarticular involvement, and constrained implant biomechanics rather than surgical technique alone.
The mean age at the time of surgery in the hemophilic group was 40 years, compared to 68.4 years in the non-hemophilic group—a difference of 1.71-fold. This confirms earlier findings that early-onset hemarthroses accelerate the need for surgical intervention in hemophilia.
4.4. Complications
Early perioperative complications were markedly higher in the hemophilic cohort (90%) versus the control group (20%). Anemia was the most common complication, followed by hematomas and wound dehiscence. One patient with inhibitors suffered an intraoperative fracture, requiring additional internal fixation. These findings align with previous research highlighting the elevated perioperative risk in hemophilic patients [38]. There are some risks of transfusion especially at hemophilic patients. Risk of alloimmunization and inhibitor formation from exposure to plasma-derived products. Potential transmission of viral pathogens (although extremely rare with modern screening). Volume overload and iron accumulation in patients with multiple transfusions. Interaction between transfused plasma and infused clotting factors. Therefore, transfusions should be administered only when clinically indicated and always under hematologic supervision in a Comprehensive Hemophilia Care Center.
The bleeding diathesis inherent to hemophilic patients, combined with local factors such as poor bone quality, bone loss, soft tissue fibrosis, muscle atrophy, axial deformities, and knee flexion contractures, contributes to a higher risk of complications following total knee arthroplasty (TKA). Reported complications in this patient population include infection, periprosthetic fracture, persistent bleeding, neurovascular injury, inhibitor development, prosthetic loosening, patellar clunk syndrome, patellar subluxation, anterior knee pain, and, in some cases, the need for component removal [39].
Infection remains the most devastating complication following total knee arthroplasty (TKA). While the risk of periprosthetic joint infection (PJI) in the general population is typically less than 1%, patients with bleeding disorders experience a markedly higher incidence, with an average reported infection rate around 7% or higher [40].
We did not observe any infections in our study groups, which may be attributed to the short follow-up duration and the relatively small sample size.
Blood loss is a well-recognized complication of total knee arthroplasty (TKA) in patients with bleeding diathesis. Reported rates of blood transfusion following TKA in individuals with hemophilia range from 29.1% to 58%, exposing patients to potential complications such as alloantibody formation, infection, allergic reactions, prolonged hospitalization, and delayed rehabilitation [41].
The most frequent complication observed in our study was anemia, affecting 16 hemophilic patients, of whom 8 required blood transfusions. Additionally, 3 patients developed compressive hematomas at the surgical site, which required surgical evacuation.
The transfusion requirement in hemophilic patients was notably higher—45%, compared to 20% in controls—a 2.25-fold increase. The odds ratio for needing a transfusion in the hemophilic group was 4.2, indicating significantly elevated transfusion risk.
Although blood transfusion itself represents a therapeutic intervention, not a complication, it was reported in this study to reflect the clinical impact of postoperative anemia, which constitutes the underlying complication. Anemia was among the most frequent adverse events in hemophilic patients, often necessitating transfusion to restore hemodynamic stability and ensure adequate oxygen delivery. Therefore, transfusion rates are presented as an indirect indicator of postoperative bleeding severity and hemostatic challenge, rather than as complications per se.
4.5. Cost per QALY and Cost-Effectiveness for TKA
Total knee arthroplasty (TKA) relieves pain and improves quality of life for persons with advanced knee osteoarthritis.
According to studies one of the main determinants of cost-effectiveness for TKA is the degree of pre-operative dysfunction. Those patients with poorer pre-operative function derived greater benefit [42].
QALY calculations represent postoperative utility maintained over the mean follow-up period (7.3 ± 3.9 years), rather than incremental gains from baseline.
While both groups achieved improvements in quality-adjusted life years (QALYs), the cost per QALY was significantly higher in hemophilic patients:
- Control group: €1258/QALY
- Hemophilic group: €2506/QALY
When a high-volume hospital is available, TKAs performed in a high-volume hospital confer even greater value per dollar spent than TKAs performed in low-volume centers [43].
To better contextualize the value of the intervention—in this case, total knee arthroplasty—it is advisable to benchmark the incremental cost-effectiveness ratio (ICER) against internationally accepted willingness-to-pay thresholds for one quality-adjusted life year (QALY) gained. A commonly referenced standard is that proposed by the World Health Organization (WHO), which considers an intervention to be cost-effective if the cost per QALY gained is less than 1 to 3 times the gross domestic product (GDP) per capita.
Applying this framework to the Romanian context, where the GDP per capita is approximately €17,000 (based on the most recent data), a medical intervention such as knee replacement surgery can be considered cost-effective if its ICER falls below €17,000–€51,000 per QALY. This approach allows for a more objective interpretation of the procedure’s economic value and supports informed decision-making in the allocation of healthcare resources within the public health system.
One of the primary limitations of this study is the relatively small sample size, which may impact the generalizability of the findings. Due to the limited number of patients included, statistical power is reduced, potentially affecting the robustness of the observed associations and outcomes. This limitation is further compounded by the inherent challenges in recruiting patients with hemophilia, a rare inherited bleeding disorder. The low prevalence of the condition, combined with the strict inclusion criteria required for surgical interventions, significantly restricted the eligible patient population. As a result, patient accrual was slow and required extended timeframes, limiting the feasibility of a larger-scale study. Future research involving multicenter collaboration and larger cohorts is necessary to confirm these findings and enhance their external validity. The substantial age disparity and different implant requirements between groups limit direct outcome comparisons. Additionally, short minimum follow-up (6 months) precludes assessment of long-term implant survival and late infectious complications.
Additionally, although no postoperative infections were observed in the hemophilic cohort throughout the study period—spanning approximately 15 years—this finding must be interpreted with caution. The absence of infections may reflect effective perioperative protocols but requires cautious interpretation given the small sample and the fact that late-onset infections (>1 year) are common in hemophilia [44]. Longer follow-up is needed. Consequently, no definitive conclusions can be drawn regarding the potential influence of comorbid conditions—such as viral status (e.g., hepatitis B, hepatitis C, or HIV)—on postoperative outcomes in hemophilic patients undergoing total knee arthroplasty. Larger studies with stratified analyses are required to elucidate the role of these factors.
This investigation presents several notable strengths. First, all procedures were performed using a standardized surgical technique by an experienced and consistent orthopedic team over the entire study period, minimizing variability in operative approach and postoperative management. Second, the study includes a comprehensive cost analysis incorporating factor replacement therapy, hospitalization, and complication management costs—elements rarely examined together in hemophilia arthroplasty research. Third, it represents a rare comparative design, directly contrasting hemophilic and non-hemophilic cohorts within the same institution and under uniform perioperative protocols, thereby enhancing internal validity and clinical relevance.
Future studies should aim to establish multicenter registries with extended follow-up periods, allowing for more robust assessment of long-term implant survivorship, complication patterns, and cost-effectiveness in hemophilic arthroplasty.
5. Conclusions
This is the first Romanian series and among few comparative studies quantifying the 2-fold cost differential and 90% complication burden in hemophilic TKA, providing evidence-based expectations for preoperative counseling. This study also provides rare comparative cost-effectiveness data, filling knowledge gap in Eastern European hemophilic TKA outcomes.
Total knee arthroplasty (TKA) remains the standard treatment for managing severe hemophilic arthropathy, particularly in patients with advanced joint destruction and poor response to conservative therapy. Although considered controversial in younger individuals, TKA is now recognized as both effective and indicated for hemophilic patients, especially given the early onset and progressive nature of joint damage in this population.
Successful outcomes depend heavily on meticulous preoperative planning.
Compared to controls, hemophilic patients require 74% longer operative time, 4.5-fold higher complication rates, and twice the cost per QALY. €2506/QALY remains well below Romanian thresholds (€17,000–51,000/QALY), supporting continued access.
Despite 90% complication rates and doubled costs, TKA provides clinically important improvement (KSS + 43.5 points).
Findings from single-center series (n = 20) require multicenter validation.
Surgeons should counsel patients to expect substantial pain relief but realistic KSS scores (mean 74 vs. 98), prolonged hospitalization, and 90% likelihood of complications requiring management.
Outcomes support continued use of multidisciplinary teams, though impact warrants prospective evaluation. This ensures optimal surgical timing, minimizes complications and achieves excellent short-term implant survival, but longer follow-up is needed.
Author Contributions
Conceptualization, G.S. and H.O.; methodology, G.S. and N.G.; software, R.D.; validation, H.O., N.G., M.R.; formal analysis, R.D. and G.S.; resources, M.R.; writing—original draft preparation, G.S.; writing—review and editing, G.S.; supervision, H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Elias University Hospital, Bucharest, Romania, approval number: 104062025, approved on 4 June 2025.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Srivastava, A.; Brewer, A.K.; Mauser-Bunschoten, E.P.; Key, N.S.; Kitchen, S.; Llinas, A.; Ludlam, C.A.; Mahlangu, J.N.; Mulder, K.; Poon, M.C.; et al. Guidelines for the Management of Hemophilia. Haemophilia 2013, 19, e1–e47. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Tuddenham, E.G. The Hemophilias—From Royal Genes to Gene Therapy. N. Engl. J. Med. 2001, 344, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, V.S.; Key, N.S.; Ljung, L.R.; Manco-Johnson, M.J.; van den Berg, H.M.; Srivastava, A. Definitions in Hemophilia: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2014, 12, 1935–1939. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. How Hemophilia Is Inherited. Available online: https://www.cdc.gov/hemophilia/testing/how-hemophilia-is-inherited.html (accessed on 18 August 2025).
- Mehta, P.; Reddivari, A.K.R. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551607/ (accessed on 18 August 2025).
- Braden, B.; Wenke, A.; Karich, H.J.; Dietrich, C.F.; Scharrer, I.; Caspary, W.F.; Lembcke, B. Risk of Gastrointestinal Bleeding Associated with Helicobacter Pylori Infection in Patients with Haemophilia or von Willebrand Syndrome. Helicobacter 1998, 3, 184–187. [Google Scholar] [CrossRef]
- Mena, A.K.; Jayalakjshmi, S.; Prasa, V.S.S.; Murthy, J.M.K. Spinal epidural haematoma in a patient with haemophilia-B. Spinal Cord 1998, 36, 658–660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aledort, L.M.; Kroner, B.; Mariani, G. Hemophilia treatment: Imune tolerance induction. Treatment duration analysis and economical consideration. Haematologica 2000, 85, 83–85. [Google Scholar]
- Roosendaal, G.; van Rinsum, A.C.; Vianen, M.E.; van den Berg, H.M.; Lafeber, F.P.; Bijlsma, J.W. Haemophilic arthropathy resembles degenerative rather than inflammatory joint disease. Histopathology 1999, 34, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Berntorp, E. Methods of haemophilia care delivery: Regular prophylaxis versus episodic treatment. Haemophilia 1995, 1, 3–7. [Google Scholar] [CrossRef]
- Ljung, R.C.R. Prophylactic infusion regimens in the management of haemophilia. Thromb. Haemost. 1999, 82, 525–530. [Google Scholar]
- Schramm, W.; Berger, K. Economics of prophylactic treatment. Haemophilia 2003, 9 (Suppl. S1), 111–116. [Google Scholar] [CrossRef] [PubMed]
- Botistella, L.R. Maintenance of musculoskeletal function in patients with haemophilia. Haemophilia 1998, 4, 26–32. [Google Scholar] [CrossRef]
- Lachiewicz, P.F.; Inglis, A.E.; Insall, J.N.; Sculco, T.P.; Hilgartner, M.W.; Bussel, J.B. Total knee arthroplasty in hemophilia. J. Bone Jt. Surg. Am. 1985, 67, 1361–1366. [Google Scholar] [CrossRef]
- Goddard, N.J.; Rodiguez-Merchan, E.C.; Wiedel, J.D. Total knee replacement in haemophilia. Haemophilia 2002, 8, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Atilla, B.; Caglar, O.; Pekmezci, M.; Buyukasik, Y.; Tokgozoglu, A.; Alpaslan, M. Pre-operative flexion contracture determines the functional outcome of haemophilic arthropathy treated with total knee arthroplasty. Haemophilia 2012, 18, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien–Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Strauss, A.; Schmolders, J.; Friedrich, M.J.; Pflugmacher, R.; Müller, M.; Goldmann, G.; Oldenburg, J.; Pennekamp, P.H. Outcome after total knee arthroplasty in haemophilic patients with stiff knees. Haemophilia 2015, 21, e300–e305. [Google Scholar] [CrossRef]
- Mortazavi, S.J.; Bagheri, N.; Farhoud, A.; Hadi Kalantar, S.; Ghadimi, E. Total Knee Arthroplasty in Patients with Hemophilia: What Do We Know? Arch. Bone Jt. Surg. 2020, 8, 470–478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Federation of Hemophilia. WFH Guidelines for the Management of Hemophilia, 3rd ed.; WFH: Montreal, QC, Canada, 2020; Available online: https://www1.wfh.org/publications/files/pdf-1873.pdf (accessed on 18 April 2020).
- Danielson, H.; Ekman, M.; Näsman, M.; Ljung, R.; Berntorp, E. Total joint replacement in inhibitor-positive haemophilia: Long-term outcome analysis in fifteen patients. World J. Orthop. 2017, 8, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Wiedel, J.; Geraghty, S.; Funk, S. Joint replacement surgery in hemophilia. World Fed. Hemoph. 2010, 50, 1–10. [Google Scholar]
- Mortazavi, S.M.; Haghpanah, B. Anthropometric study of the hemophilic knee joints undergoing total knee arthroplasty. In Proceedings of the WFH 15th Int Musculoskeletal Congress, Seoul, Republic of Korea, 5–7 May 2017; p. 14. [Google Scholar]
- Frauchigera, L.H.; Harstalla, R.; Kajahna, J.; Andersonb, S.; Egglia, S. Bilateral total knee arthroplasty in a patient with haemophilia A, high inhibitor titre and aneurysma spurium of the popliteal artery. Swiss Med. Wkly. 2010, 140, w13094. [Google Scholar] [CrossRef]
- Innocenti, M.; Civinini, R.; Carulli, C.; Villano, M.; Linari, S.; Morfini, M. A modular total knee arthroplasty in haemophilic arthropathy. Knee 2007, 14, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Heeg, M.; Meyer, K.; Smid, W.; Horn, J.V.; Meer, J.V.D. Total knee and hip arthroplasty in haemophilic patients. Haemophilia 1998, 4, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Park, C.H.; Bae, D.K.; Song, S.J. How much preoperative flexion contracture is a predictor for residual flexion contracture after total knee arthroplasty in hemophilic arthropathy and rheumatoid arthritis? Knee Surg Relat Res 2002, 34, 20. [Google Scholar] [CrossRef]
- Lapierre, P.B.; Guyen, O.; Chavane, H.; Lienhart, A.; Carret, J.P.; Bejui-Hugues, J. Early- to mid-term results of total knee arthroplasty in hemophilic knees: A review of 34 cases. In Proceedings of the World Federation of Hemophilia 10th Musculoskeletal Congress, Montréal, QC, Canada, 1–4 May 2007; p. 15. [Google Scholar]
- Mortazavi, S.M.; Firoozabadi, M.A.; Najafi, A.; Mansouri, P. Evaluation of outcomes of suction drainage in patients with haemophilic arthropathy undergoing total knee arthroplasty. Haemophilia 2017, 23, e310–e315. [Google Scholar] [CrossRef] [PubMed]
- Kubes, R.; Salaj, P.; Hromadka, R.; Vcelak, J.; Kubena, A.A.; Frydrychova, M.; Magerský, Š.; Burian, M.; Ošťádal, M.; Vaculik, J. Range of motion after total knee arthroplasty in hemophilic arthropathy. BMC Musculoskelet Disord. 2018, 19, 162. [Google Scholar]
- Hermans, C. Perioperative thromboprophylaxis in patients with hemophilia and von Willebrand disease undergoing major orthopedic surgery. In Proceedings of the 20th Congress of the European Hematology Association, Vienna, Austria, 11–14 June 2015. [Google Scholar]
- AAOS. AAOS Guideline on Preventing Venous Thromboembolic Disease in Patients Undergoing Elective Hip and Knee Arthroplasty. 2018. Available online: https://www.aaos.org/research/guidelines/vte/vte_full_guideline.pdf (accessed on 18 April 2020).
- Pradhan, S.M.; Key, N.S.; Boggio, L.; Pruthi, R. Venous thrombosis prophylaxis in haemophilics undergoing major orthopaedic surgery: A survey of haemophilia treatment centres. Haemophilia 2009, 15, 1337–1338. [Google Scholar] [CrossRef]
- Schick, M.; Stucki, G.; Rodriguez, M.; Meili, E.; Huber, E.; Michel, B.; Brühlmann, P. Haemophilic; arthropathy: Assessment of quality of life after total knee arthroplasty. Clin. Rheumatol. 1999, 18, 468–472. [Google Scholar] [CrossRef]
- Moore, M.F.; Tobase, P.; Allen, D.D. Meta-analysis: Outcomes of total knee arthroplasty in the haemophilia population. Haemophilia 2016, 22, e275–e285. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.; Gu, Y.; Zhang, J.; Wang, P.; Tong, P.; Lv, S. Total knee arthroplasty in patients with haemophilic arthropathy is effective and safe according to the outcomes at a mid-term follow-up. J. Orthop. Traumatol. 2022, 23, 31. [Google Scholar] [CrossRef] [PubMed]
- Fenelon, C.; Murphy, E.P.; Fahey, E.J.; Murphy, R.P.; O’Connell, N.M.; Queally, J.M. Total knee arthroplasty in hemophilia: Survivorship and outcomes—A systematic review and meta-analysis. J. Arthroplast. 2022, 37, 581–592.e1. [Google Scholar] [CrossRef]
- Shen, S.N.; Wu, D.X.; Lv, S.J.; Tong, P.J. Hidden blood loss of total knee arthroplasty in hemophilia arthritis: An analysis of influencing factors. BMC Musculoskelet Disord. 2022, 23, 587. [Google Scholar] [CrossRef] [PubMed]
- Cancienne, J.M.; Werner, B.C.; Browne, J.A. Complications After TKA in Patients With Hemophilia or Von Willebrand’s Disease. J. Arthroplast. 2015, 30, 2285–2289. [Google Scholar] [CrossRef]
- Jamsen, E.; Varonen, M.; Huhtala, H.; Lehto, M.U.; Lumio, J.; Konttinen, Y.T.; Moilanen, T. Incidence of prosthetic joint infections after primary knee arthroplasty. J. Arthroplast. 2010, 25, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Solimeno, L.P.; Mancuso, M.E.; Pasta, G.; Santagostino, E.; Perfetto, S.; Mannucci, P.M. Factors influencing the long-term outcome of primary total knee replacement in haemophiliacs: A review of 116 procedures at a single institution. Br. J. Haematol. 2009, 145, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Stan, G.; Orban, H.; Orban, C. Cost Effectiveness Analysis of Knee Osteoarthritis Treatment. Chirurgia 2015, 110, 368–374. [Google Scholar] [PubMed]
- Losina, E.; Walensky, R.P.; Kessler, C.L.; Emrani, P.S.; Reichmann, W.M.; Wright, E.A.; Holt, H.L.; Solomon, D.H.; Yelin, E.; Paltiel, A.D.; et al. Cost-effectiveness of total knee arthroplasty in the United States: Patient risk and hospital volume. Arch Intern. Med. 2009, 169, 1113–1121, discussion 1121–1122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez-Merchan, E.C.; De la Corte-Rodriguez, H.; Alvarez-Roman, T.; Gomez-Cardero, P.; Encinas-Ullan, C.A.; Jimenez-Yuste, V. Complications and Implant Survival of Total Knee Arthroplasty in People with Hemophilia. J. Clin. Med. 2022, 11, 6244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).