1. Introduction
Fractures of the distal upper limb—those involving the phalanges, metacarpals, and carpal bones—continue to impose a heavy workload on emergency departments: an analysis of the Nationwide Emergency Department Sample identified 2.12 million upper-extremity fractures in 2016, representing 1.5% of total Emergency Department (ED) attendances [
1,
2]; phalangeal injuries were the single most common subtype, and treatment costs for open fractures were almost double those for closed injuries [
3].
Although the great majority of hand and wrist fractures are closed, roughly one in twenty present with a breach in the soft-tissue envelope—an incidence of open injury first highlighted in a 2001 database study that estimated 5% of hand fractures to be open [
2,
4]. These figures underline both the persistent frequency and the distinctive resource implications of distal upper-limb fractures, especially when compound wounds are involved [
5]. Although any open fracture carries an elevated infection risk [
6], contemporary series show that open hand fractures are infected far less often than open long-bone injuries [
7]: early reports cited rates up to 11% [
8], but modern cohorts controlled for confounders document figures closer to 0–6%, supporting the long-held view that the hand’s rich vascularity and ease of access confer relative resilience [
9].
Several pragmatic features set open hand fractures apart from their tibial or femoral counterparts. First, the anatomy lends itself to regional anesthesia: a simple digital nerve injection or a Bier intravenous block provides painless operating conditions at the bedside, allowing thorough irrigation, meticulous debridement, and even provisional K-wire fixation to be completed in the ED [
10]. In a 145-patient series managed chiefly under local or digital blocks, definitive treatment carried an infection rate of only 1.4% [
8], and national database work has since documented a steady shift in irrigation-and-debridement for finger and hand fractures from the operating theatre to the ED without an accompanying rise in re-operations or readmissions [
11,
12].
Second, the long-debated “six-hour rule” that still guides many protocols for open tibial fractures has little empirical support in the hand [
8,
13]. A prospective multicentre cohort of 983 adults found no increase in surgical-site infection when debridement was delayed up to four days (median 20 h) provided intravenous antibiotics were started promptly [
13], while a tertiary-hospital audit of 232 open hand injuries—92% treated under local anesthesia—reported a deep-infection rate of just 1.3%, again unrelated to time-to-surgery [
14]. Reviews of the broader open-fracture literature likewise conclude that, beyond grossly contaminated or ischaemic wounds, there is “little to no literature that has been able to support” an absolute six-hour cut-off; what matters more is rapid antibiotic administration and a well-executed debridement when appropriate staff and facilities are available [
15,
16,
17].
The Gustilo–Anderson classification [
18,
19], initially developed for use in long bones, is not ideal in classifying hand fractures. The parameters that define each Gustilo–Anderson grade and the hand’s distinctive patterns of soft-tissue coverage and vascular compromise make the scale a poor fit for open fractures of the fingers and palm. Cut-offs of 1 cm and 10 cm, meaningful for long bones, lose practical relevance when applied to such a small anatomical unit. Likewise, the indications and reconstructive options for covering exposed tibial or femoral shafts (for example, in type IIIB injuries) differ markedly from those available in the hand [
20,
21]. Open hand fractures also arise from several distinctive mechanisms, the most common being a direct cut or penetrating insult in which a sharp implement—such as a power saw—slices through the tissues and bone [
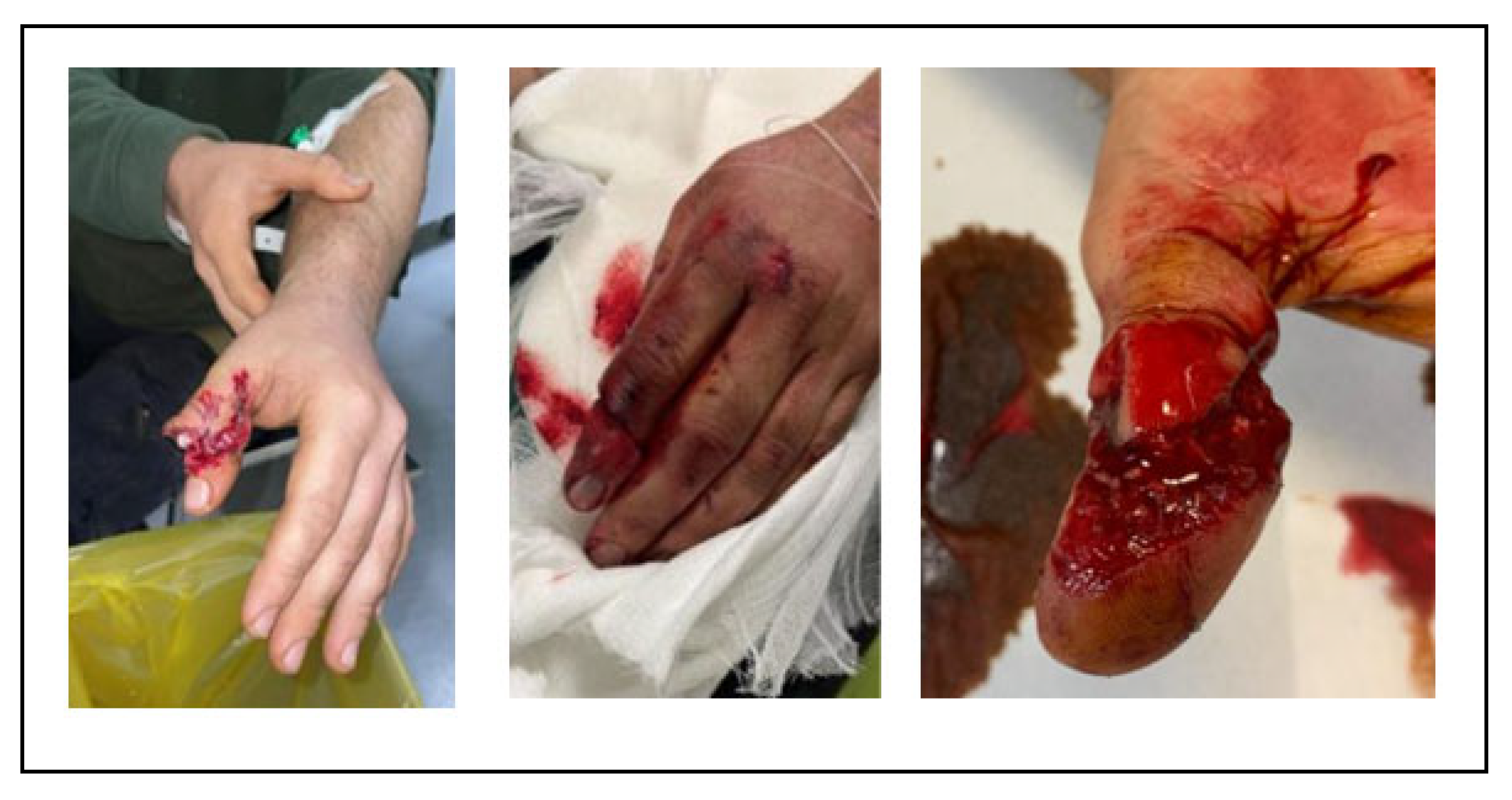
12] (
Figure 1).
Because current protocols still extrapolate from long-bone data, a hand-specific pathway is warranted—one that foregrounds immediate IV antibiotics, allows a flexible but prompt surgical window after adequate bedside debridement, and recognizes the lower baseline infection risk when counselling patients and allocating resources [
4,
16].
For this reason, a new tailored classification was created in 2016 by Tulipan et al. [
7], proposing to give less emphasis on wound size and instead taking into account fracture location, the extent of contamination, the integrity of soft-tissue coverage, and the viability of the vascularity [
1].
The present study evaluates whether time-to-surgery in open phalangeal fractures is associated with infection, radiographic union, and time to return to work. We did not undertake inter-limb comparisons, and our conclusions are limited to this cohort.
2. Materials and Methods
This was a single-centre retrospective cohort study reported in accordance with STROBE principles. Because of the observational design, the study cannot establish causality and is intended to be hypothesis-generating.
From October 2023 to January 2025, we retrospectively reviewed all patients treated for open phalanx fractures at our hospital’s Hand Surgery Unit. An open fracture was defined as any volar or dorsal finger wound with direct bone exposure. This strict definition was selected to ensure that cases reflected a true communication between the external environment and the fracture site, rather than superficial lacerations without osseous exposure; it also harmonizes with pragmatic ED decision-making where the presence of bone exposure drives the need for operative management and targeted antimicrobial prophylaxis [
15,
16,
17]. To reduce misclassification bias, wounds with doubtful exposure were discussed by two attending hand surgeons based on operative notes and peri-operative images.
2.1. Inclusion and Exclusion Criteria
Inclusion criteria were (1) any wound of the finger determining bone exposure; (2) any patient older than 18 years old; (3) patients with complete follow-up (until bone consolidation).
Exclusion criteria were (1) patients needing replant and/or revascularization; (2) patients with neurological impairment and any condition making difficult to evaluate post-op function; (3) patients with pregress trauma on the same digit. We excluded metacarpal and carpal injuries to avoid heterogeneity related to differing biomechanics, soft-tissue envelopes, and reconstructive options [
20,
21].
2.2. Data Collection
Data was collected using internal database, screening surgical records with the following diagnosis, according to ICD-9 codes: “816.1: Open fracture of one or more phalanges of hand”, “834.1: Open dislocation of finger”, or “883: Open wound of finger(s)”. Operative notes were used to confirm every fracture. For each case we recorded sex and age; injury mechanism (power-saw laceration, sharp cut, crush, animal bite, or other); injured digit and phalanx (proximal, middle, or distal); time from emergency-department arrival to surgery (≤24 h, 24–72 h, or >72 h); associated soft-tissue damage (tendon, nerve, or artery); and the procedure performed (osteosynthesis, tendon repair, nerve repair, or arterial repair). Out-patient charts were then reviewed to establish fracture-healing time—defined, unless otherwise noted, as the date of K-wire removal—and to document infections or other complications such as amputation or postoperative dislocation. Finally, patients were contacted by telephone to determine the interval until return to work and to collect additional demographic details, including job type (manual versus office work). Patients were divided into three groups according to time from emergency-department arrival to surgery: Group A, ≤24 h; Group B, 24–72 h; and Group C, ≥72 h.
To improve internal validity, data extraction was conducted by two independent reviewers with adjudication by a senior author in the case of disagreement. Missing data were handled by pairwise deletion for the variable under analysis; no imputation was performed given the modest dataset size and the retrospective nature of the study. The decision to use timing from ED arrival, rather than time from injury, reflects the reality that many patients arrive after unknown delays and that hospital-based processes (triage, antibiotics, imaging, and anesthesia) are the points amenable to quality improvement [
15,
16,
17].
2.3. Surgical Treatment
Prior to surgical treatment, patients were treated with one-shot ev 2.2 g of amoxicillin/clavulanic acid if not contraindicated and subsequently switched to oral administration (1 g, 3 times per day). All wounds were washed with 1 L saline solution and 500 mL iodine solution and temporarily immobilized with gauze, bandages, and eventual splinting.
The decision regarding the timing relied on various elements such as the availability of the emergency operating room and surgeon advice, as well as tissue conditions after initial washout (e.g., ability to close primarily vs. need for staged coverage) [
15,
16,
17].
All surgeries were performed by experienced hand surgeons at our institution. Surgical treatment was variable among patients, reflecting real-world practice and the spectrum of injury. According to the type and location of injury, regional block or local anesthesia was administered. Fractures were treated with percutaneous K-wires in all cases, and wires were always left out of the skin rather than buried. Flexor and extensor tendon injuries were not repaired only in the case of critical terminal vascularization; in all other cases, a 4-strand core suture with running epitendinous suture was used for flexor tendons and a 2-strand core suture for extensor tendons injuries. Nerves were repaired if a gap smaller than 1 cm was present. When possible, the wound was closed primarily; otherwise, local flaps or dermal substitutes were used, in line with the principle that reliable coverage reduces dead space and bacterial load, and facilitates early motion [
20,
21].
Across the cohort, osseous stabilization was performed with percutaneous K-wires in all fractures, with wires left externalized. All patients received the same antibiotic protocol (amoxicillin/clavulanate unless contraindicated) and standardized bedside irrigation before surgery. Variations in soft-tissue procedures (tendon/nerve/skin coverage) reflected injury patterns rather than surgeon preference.
2.4. Post-Op Management
All patients were treated with antibiotics (amoxicillin/clavulanic acid 1 g, 3 times per day as standard care) for at least 6 days after surgery. Clinical follow-up focused on the early detection of superficial surgical-site infection (SSI) versus deep infection/osteomyelitis and on the timely initiation of hand therapy once stability and tendon protection allowed. Analgesic regimens and edema control were standardized across groups to reduce confounding on pain-limited mobilization.
2.5. Statistical Analysis
Because postoperative infection was recorded as a dichotomous variable (present/absent), the three groups were compared with a 3 × 2 chi-square test of independence—an approach appropriate for categorical data and proportions rather than continuous variables. Degrees of freedom were set to 2 (number of groups), and expected cell counts were checked to verify that the chi-square assumptions were met.
We performed a one-way fixed-effects ANOVA based on summary statistics (group means, standard deviations, and sample sizes) for non-dichotomous variables. In each ANOVA, we partitioned total variation into “between-groups” and “within-groups” components, calculated the corresponding mean squares, and derived the F statistic with its associated degrees of freedom. Because some groups were very small or variance-free, we explicitly noted the potential violation of normality and homoscedasticity assumptions and used Welch ANOVA or non-parametric alternatives where raw data were available. Statistical analysis was performed using SPSS 29.0.
Given the retrospective design and fixed sample, we did not conduct a formal a priori power analysis; post hoc sensitivity indicates that only medium-to-large effects would be detectable at α = 0.05 with the present group sizes, which cautions against over-interpreting small between-group differences.
3. Results
A total of 90 patients were finally included. The study population had a mean age of 49.8 ± 17.6 years and was predominantly male, with women accounting for only 8.9% of cases. Manual labourers made up 42.2% of the cohort, reflecting a sizable occupational exposure to high-risk tools. Mechanistically, injuries were largely attributable to power-saw accidents, which produced 44% of the open hand fractures; sharp blade lacerations contributed for 13%, crush injuries 31%, and animal bites 12%.
Anatomically, fractures were distributed almost evenly across the phalanges: 32% involved the proximal segment (F1), 32% the middle segment (F2), and 36% the distal segment (F3). Regarding the affected digits, 17% of fractures occurred in the thumb, 25% in the index finger, 25% in the middle finger, 12% in the ring finger, and 21% in the little finger, highlighting a slight predominance of injuries to the radial digits.
Concomitant soft-tissue injuries were frequent: flexor tendons were lacerated in 13.3% of fractures, extensor tendons in 35.6%, digital nerves in 24.4%, and digital arteries in 22.2%.
In total, 22.22% of patients (20) were included in Group A and were treated within 24 h. The same number of patients were included in Group B and were treated between 24 and 72 h. A total of 60 patients (55.6%) were treated after 72 h and included in Group C. Baseline demographics and injury characteristics were comparable among the three timing groups; no statistically significant differences were observed in age, sex, or mechanism of injury (
Table 1).
All injuries were classified according to Tulipan [
7]. As we included phalanges only, all injuries were classified as type 1. We recorded equal distribution of type 1a and type 1b in the three groups and no injury classified as type 1c, as revascularization was an exclusion criterion.
The three groups were compared according to three different outcomes: infection rate, time of bone consolidation, and time to return to work.
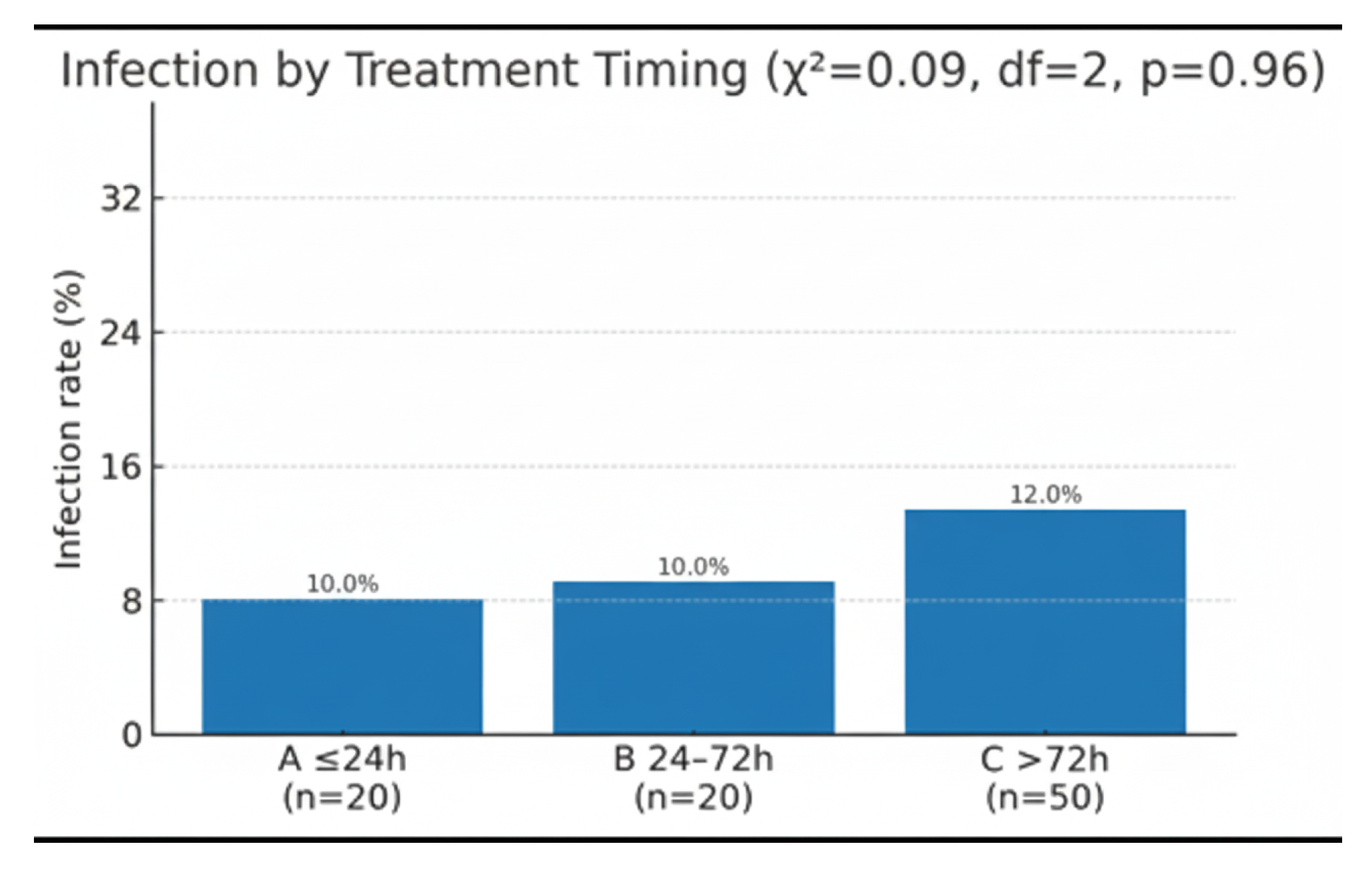
Group A recorded 2 infections among 20 patients (10%), Group B likewise 2/20 (10%), and Group C 6/50 (12%). The overall incidence was therefore 10/90 (11%). A chi-square test of independence produced χ
2 = 0.09 with 2 degrees of freedom, giving a two-tailed
p-value of 0.96. Infection rates were thus statistically indistinguishable across the three cohorts (
Figure 2)
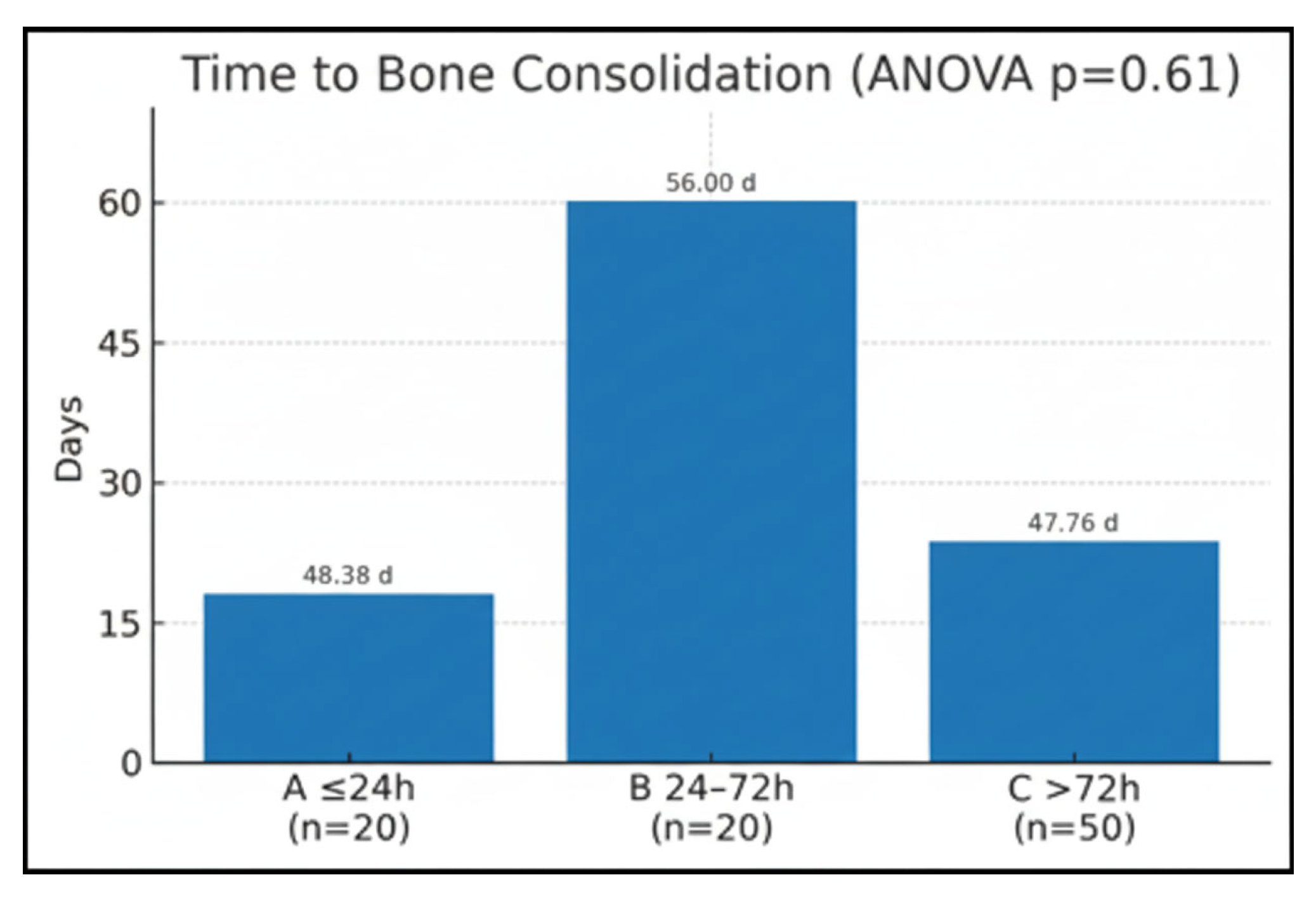
Time of bone consolidation was compared between the groups (Group A = 48.38 ± 16.23; Group B = 56 ± 2.93; Group C = 47.76 ± 14.37). A one-way analysis of variance was applied to compare the variable across the three study groups. The test returned a two-tailed
p = 0.61, indicating that the differences among the group means were not statistically significant (
Figure 3).
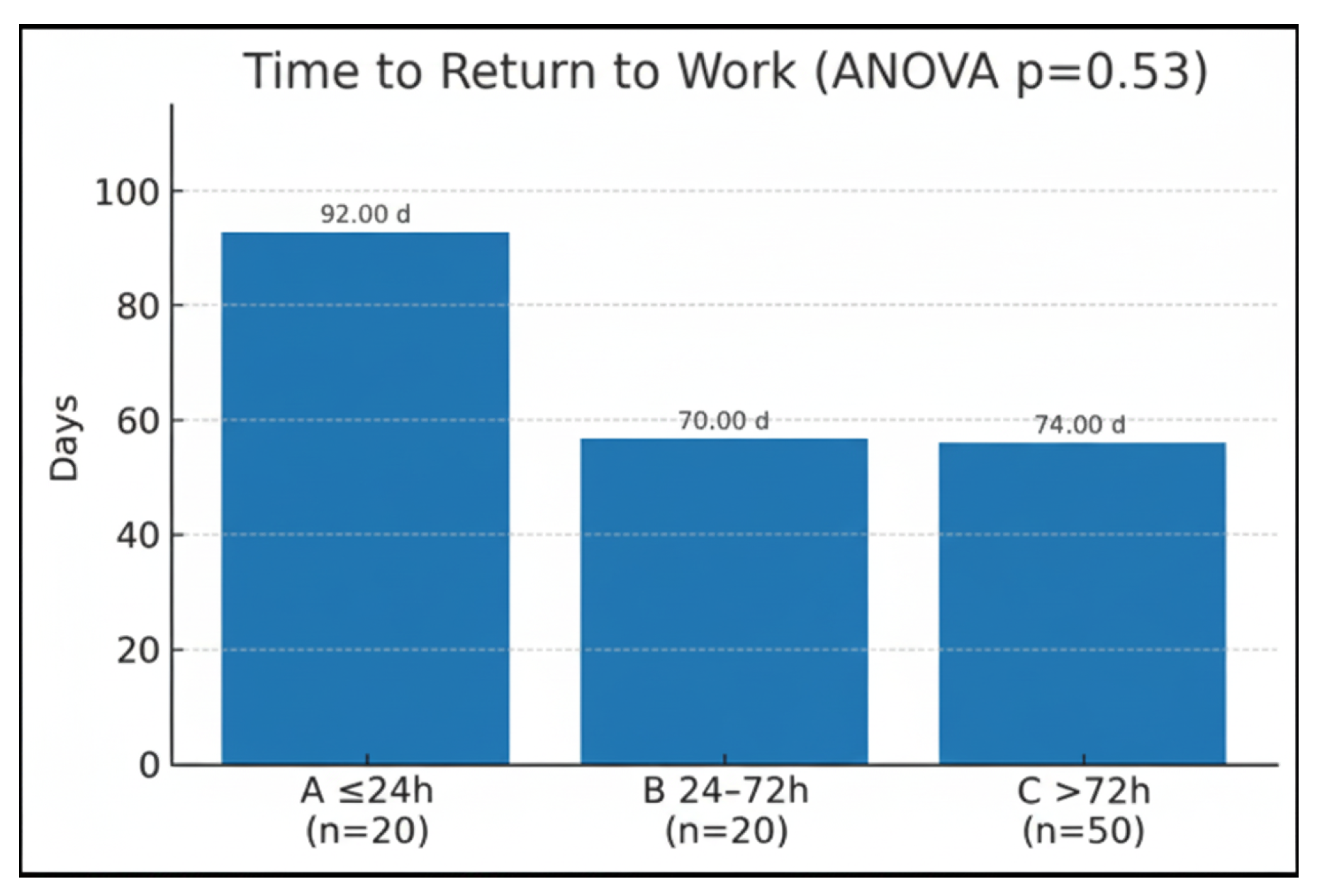
Return to work was compared using a one-way fixed-effects ANOVA. The analysis produced a two tailed
p =0.53, indicating that the differences were not statistically significant (
Figure 4).
4. Discussion
One of the most relevant observations emerging from this work is that, unlike what is consistently emphasized for fractures of the lower extremities, timing alone does not appear to be the primary determinant of infection or functional outcome in open hand fractures. Although we did not compare upper and lower limbs, the prior hand-specific literature notes practical differences from long-bone injuries (e.g., anesthesia, soft-tissue handling, and service setting). Our data are limited to open phalangeal fractures and do not address inter-limb differences. This finding aligns with the systematic review by Ketonis et al. [
4], which showed that debridement performed within six hours was associated with an infection rate of 4.2%, compared with 3.6% when surgery occurred within twelve hours—differences that were not statistically significant. Subsequent studies likewise failed to demonstrate a clear relationship between surgical delay and infection risk in open hand fractures [
8,
20,
21]. In this context, the so-called “six-hour rule,” still applied to long-bone trauma, seems less applicable to the hand, provided that prompt antibiotic therapy and adequate irrigation are achieved [
15,
16,
17,
22]. Nevertheless, in our cohort, infections—though rare—were more frequent beyond the 72 h threshold, suggesting that extreme delays may still confer some incremental risk in selected patients.
Our results indicate that the critical determinants of postoperative infection lie more in the quality of soft-tissue coverage and the extent of osseous damage than in time-to-surgery. Open hand fractures are frequently associated with soft-tissue loss, bone comminution, and tendon or neurovascular injuries—all of which compromise perfusion and local immunity [
23,
24]. In a large retrospective series, Jia et al. [
25] identified inadequate soft-tissue coverage, bone loss/comminution, serum albumin level, neutrophil count, and BMI as independent risk factors for postoperative SSI in open hand fractures. In a cohort of 846 patients, Atthakomol et al. [
9] likewise reported that comminution/bone loss, neurovascular injuries, and inadequate soft-tissue coverage are independent predictors of infection.
These data highlight that biological and anatomical variables outweigh purely temporal ones in predicting outcomes.
In practical terms, achieving a stable skeletal construct is essential to facilitate immediate soft-tissue reconstruction. Stable fixation provides a scaffold for vascularized flap coverage, reducing dead space and contamination. Improved outcomes with primary osseous and soft-tissue reconstruction have been reported across the literature [
26]. Conversely, a staged approach—initial debridement followed by delayed fixation and closure—remains preferable when heavy contamination, questionable tissue viability, or systemic host factors (e.g., diabetes or immunosuppression) elevate the risk of infection [
27,
28]. This underscores the need for individualized surgical decision-making rather than rigid adherence to predefined time thresholds.
Another relevant consideration involves classification systems. The Gustilo–Anderson scheme, although historically widespread, remains suboptimal for the hand. Its wound-length thresholds and emphasis on energy transfer do not translate well to small anatomical units. The Tulipan classification [
7], by contrast, de-emphasizes wound size and integrates fracture location, contamination, and soft-tissue coverage (
Table 2). According to Tulipan et al. [
7], while immediate antibiotics are mandatory in all cases, debridement and fracture management (excluding injuries requiring urgent revascularization) may be scheduled on a semi-elective or delayed basis. In the emergency department, irrigation (washout) is the only required procedure. Our data support the same conclusion.
While this classification rightly emphasizes the role of soft tissues, it does not account for bone loss, which is a key consideration when deciding surgical timing. Establishing a stable osseous construct provides the platform for immediate soft-tissue reconstruction [
26]. Improved outcomes with primary osseous and soft-tissue reconstruction have been reported across the literature. A staged approach is preferable when heavy contamination obscures tissue viability or when infection risk is high due to injury- or host-related factors [
27,
28].
Beyond infection, our study explored two additional patient-centred outcomes—time to bone union and time to return to work—both relevant for shared decision-making and resource planning. Neither outcome differed significantly across timing groups. This suggests that in the hand, provided antibiotics are administered promptly and debridement is executed with due diligence, functional recovery is driven more by the biology of the wound (coverage and tissue viability), skeletal stability, and rehabilitation protocols than by narrow timing thresholds. In this sense, open phalangeal fractures behave differently from high-energy long-bone injuries where time-to-debridement often acts as a stronger surrogate for contamination control [
15,
16,
17,
20,
21].
A further clinical implication is the suitability of regional anesthesia and ED-based washout for many cases [
10,
11,
12]. These strategies reduce theatre occupancy and can shorten time-to-antibiotics and time-to-irrigation—factors repeatedly linked to better outcomes in the open-fracture literature [
15,
16,
17]—without compromising safety when patient selection is appropriate. Power-saw lacerations and sharp cuts, for example, often allow for swift, thorough irrigation and primary closure under block, whereas crush injuries and animal bites may require a more cautious staged course in view of soft-tissue viability and microbiological load [
5,
6,
12].
Finally, our findings support the rationale for hand-specific pathways that formalize (1) immediate IV antibiotics on ED arrival; (2) prompt, copious irrigation and careful debridement at bedside when feasible; (3) early decision on primary closure versus local flap/dermal substitute; (4) stable K-wire fixation to support soft-tissue reconstruction; (5) a flexible surgical window for definitive procedures in the absence of dysvascularity or gross contamination [
4,
7,
16]. Such pathways may be particularly impactful in centres with high volumes of occupational hand injuries, where predictable logistics and standard operating procedures help align staff, materials, and patient counselling.
This study has important limitations. First, its retrospective design introduces risks of selection bias, information bias, and confounding by indication (e.g., operating room availability and perceived wound complexity) that cannot be fully eliminated. Second, although inclusion was limited to open phalangeal fractures and osseous fixation was uniformly by percutaneous K-wires, the cohort remains heterogeneous in mechanism, contamination, and associated soft-tissue injury; this reflects real-world practice but limits generalizability and may dilute small timing effects. Third, the limited number of infection events (n = 10) constrains statistical power and precludes robust multivariable adjustment without overfitting. To mitigate these issues, we verified baseline comparability among timing groups and conducted stratified sensitivity analyses, which did not change the direction of the findings. Finally, this is a single-centre study with unit-specific pathways, and external validity may be limited. Overall, the results should be viewed as hypothesis-generating and supportive of individualized decision-making rather than prescriptive timing thresholds.
Future work should prospectively validate risk-stratified timing, incorporating variables highlighted by recent hand-specific series—soft-tissue coverage feasibility, bone loss/comminution, and neurovascular compromise—alongside host factors such as nutritional status and systemic comorbidities [
7,
8,
9,
25,
26,
27,
28]. Pragmatic trials or registries comparing ED-based andOR-based debridement under standardized protocols would also be valuable to delineate indications, resource utilization, and patient-reported outcomes. Finally, incorporating return-to-work and hand function metrics into core outcome sets would better align research endpoints with what matters to patients and health-system planners.