Valorization of Aronia melanocarpa Pomace: A Sustainable Source of Bioactive Compounds for Developing Colored Healthcare Textiles, Biomedical Hydrogels, and Green Corrosion Inhibitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of Aronia Pomace Extract
2.3. Utilization of Aronia Pomace Extract for One-Step Dyeing and Functionalization of Textiles
2.4. Synthesis of Starch/Gelatin (SG) Hydrogels, Functionalization, and Characterization
2.5. Preparation of the Working Electrode and Solutions and Electrochemical Testing
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Aronia Pomace Ethanol Extract
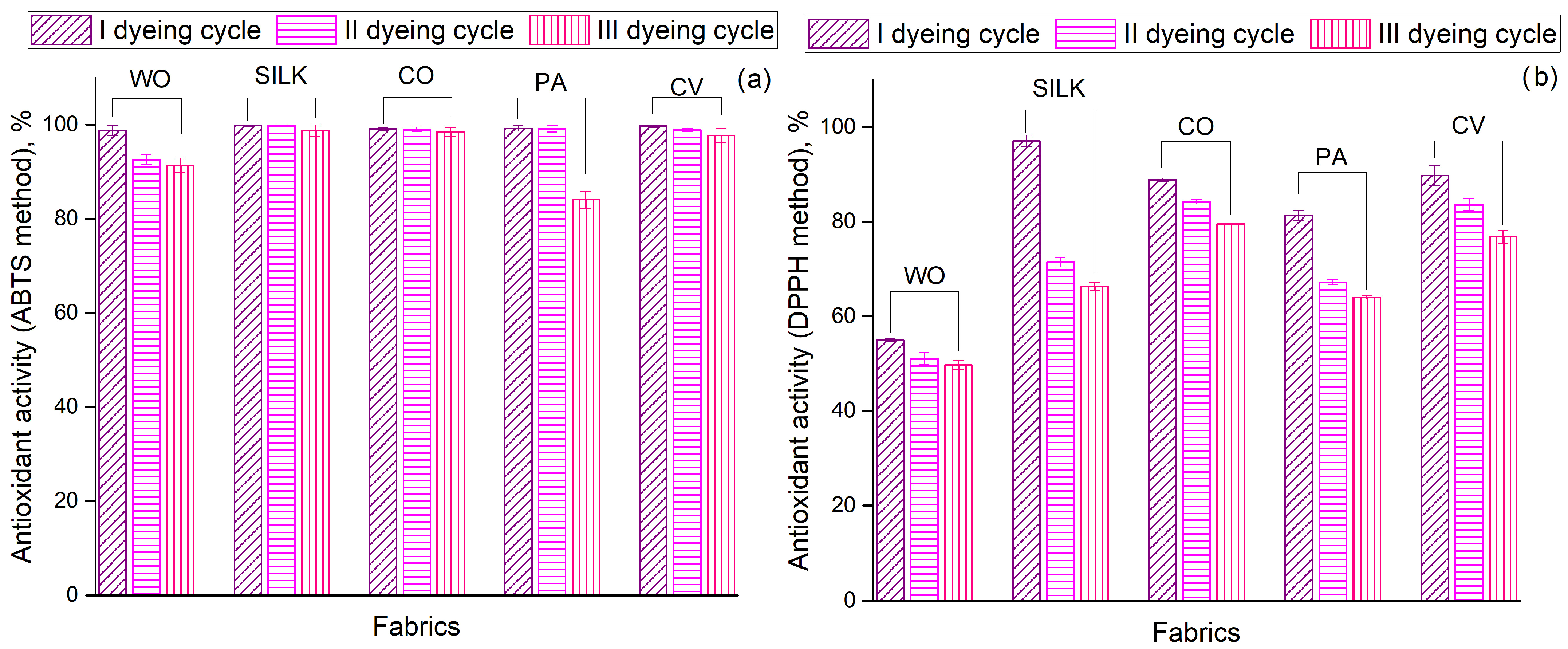
3.2. Obtaining Colored Bioactive Fabrics
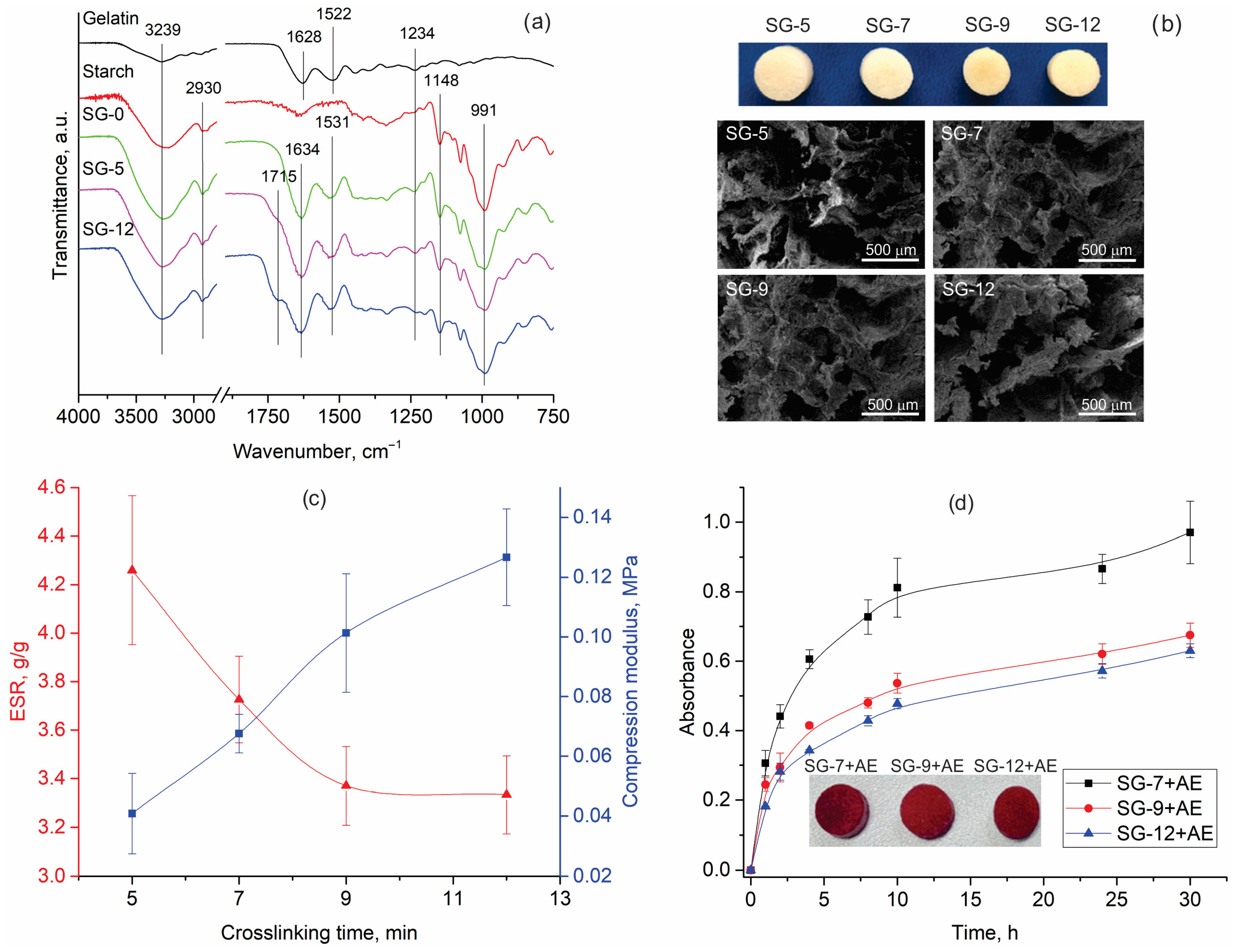
3.3. Bioactive Hydrogels
3.4. Green Corrosion Inhibitor Based on Aronia Pomace Extract
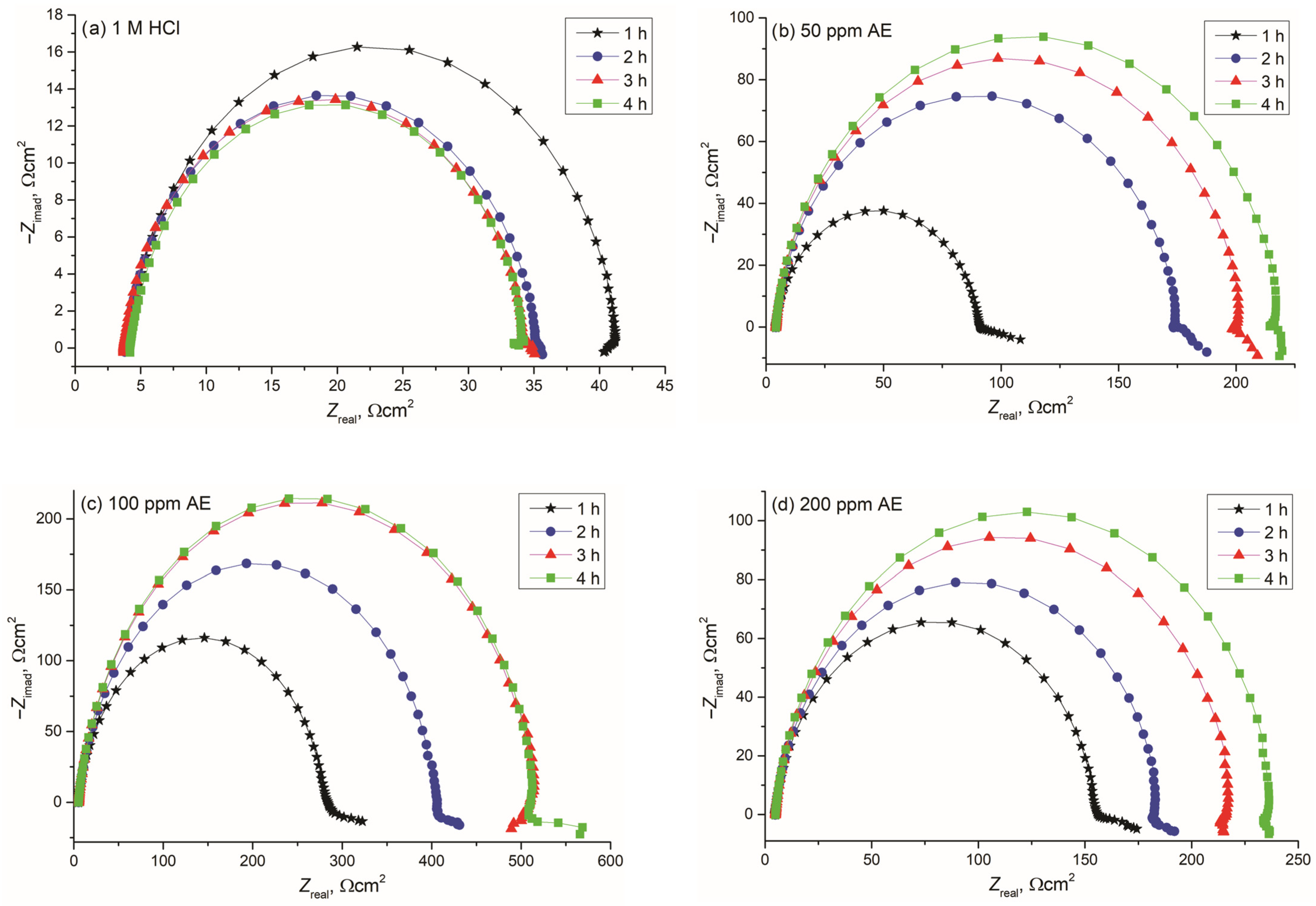
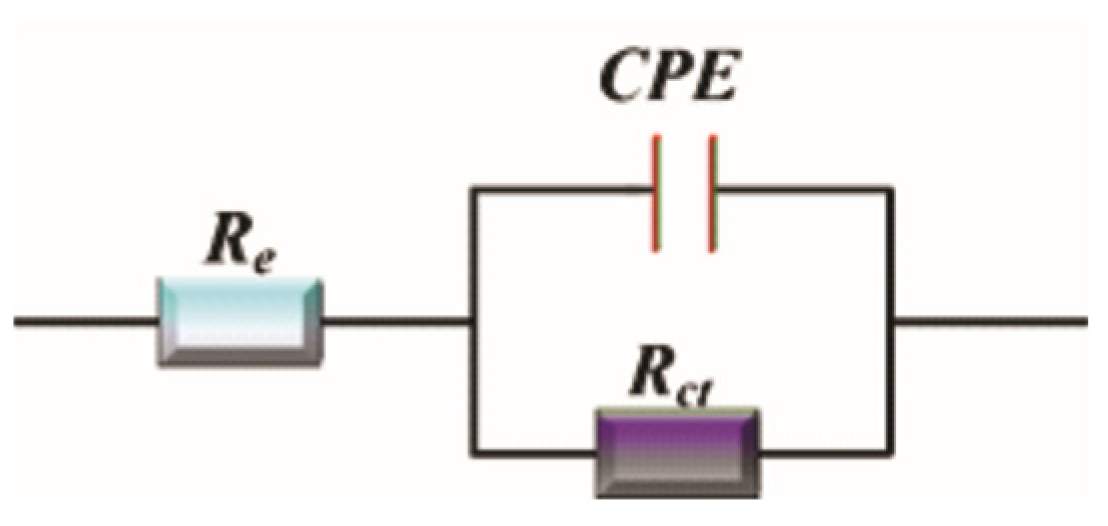
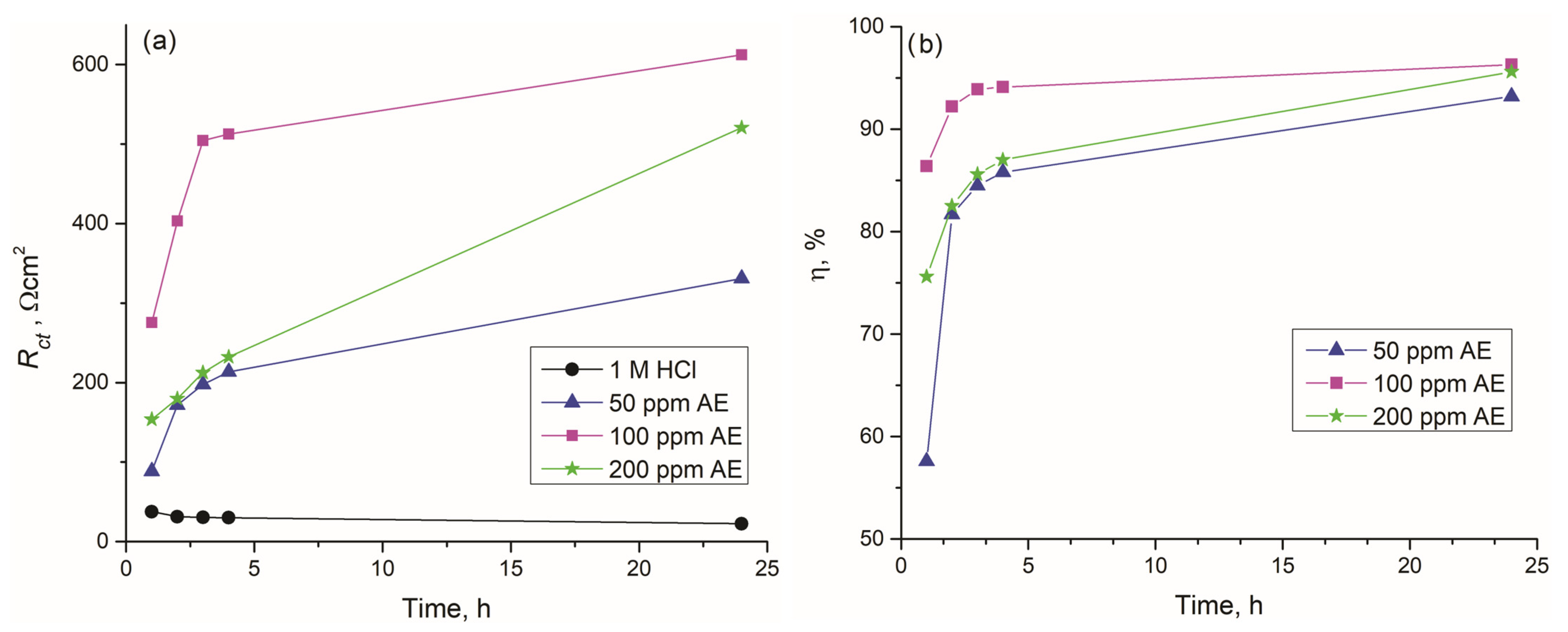
3.4.1. Electrochemical Impedance Spectroscopy (EIS) Measurements
3.4.2. Polarization Measurements
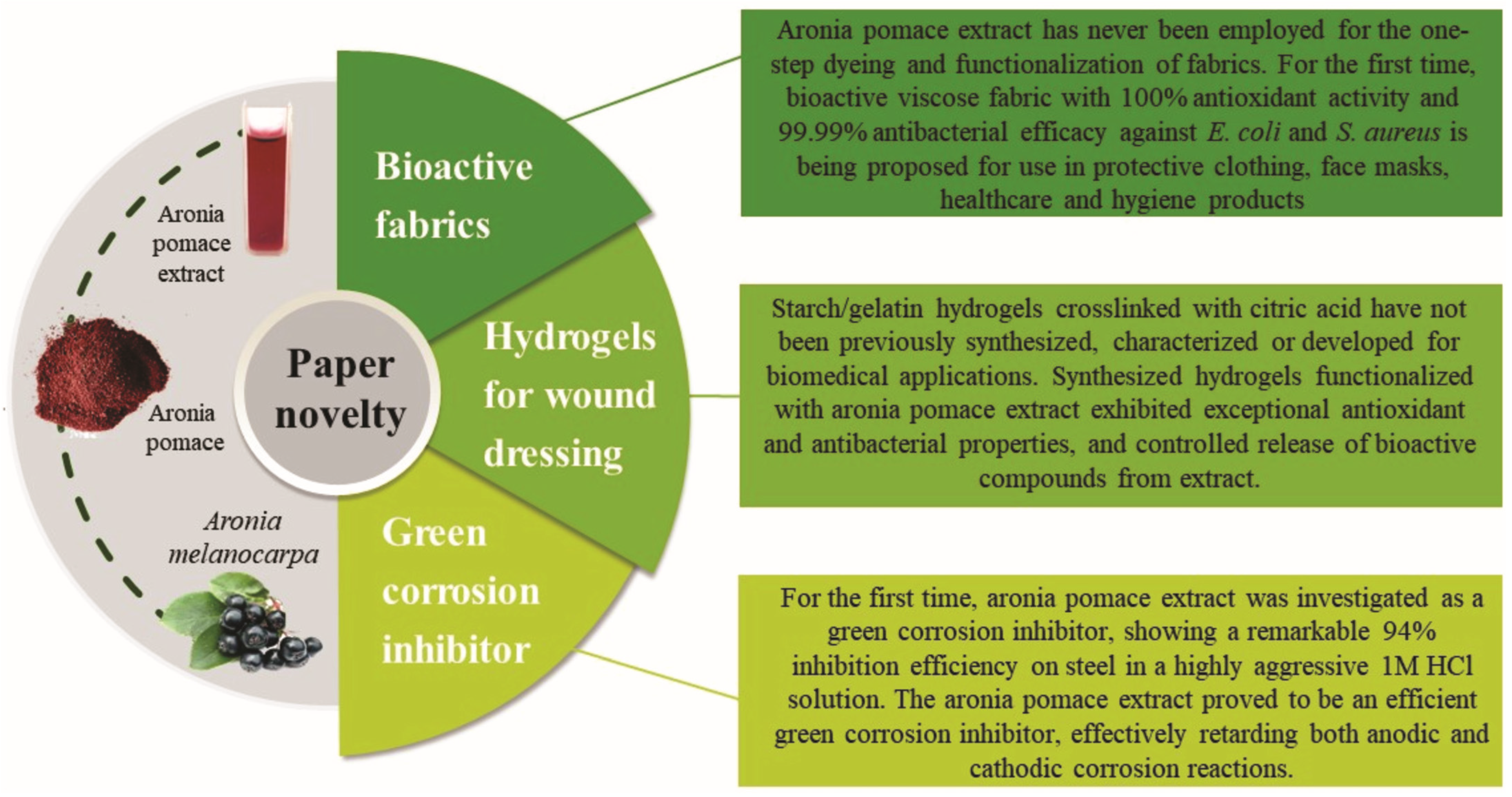
3.5. Advances Beyond Existing Methods and Connection of the Findings with Industrial Feasibility or Scalability
- From extraction to novel products
- Choice and validation of a scalable and mild extraction method
- One-step dyeing and functionalization of textiles with a focus on dyebath reusability
- Development and characterization of starch/gelatin hydrogels functionalized with aronia pomace extract with tunable properties and controlled release
- Electrochemical demonstration of a green corrosion inhibitor
3.6. Study Limitations
4. Conclusions
- Sustainable textile dyeing and functionalization, wherein cotton and viscose fabrics were found suitable for colored disposable bioactive textiles, including protective clothing, face masks, and healthcare and hygiene products, due to their strong antioxidant activity (>97% ABTS, >76% DPPH) and antibacterial efficacy (>75% against E. coli, >80% against S. aureus).
- Development of novel starch/gelatin hydrogels for wound dressing, with compression modulus of 0.068–0.127 MPa and equilibrium swelling ratios of 3.33–3.73 g/g. Hydrogels functionalized with aronia pomace extract exhibited over 99% ABTS antioxidant activity and antibacterial efficacy exceeding 70% against E. coli and 97% against S. aureus, along with controlled release of bioactive compounds, demonstrating suitability for wound care applications.
- Green corrosion inhibition, where the extract demonstrated inhibition efficiency above 96% for carbon steel at 100 ppm, predominantly retarding the cathodic reaction, confirming its effectiveness as a sustainable corrosion inhibitor.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, S.; Lee, J.; Son, H.; Lee, J.U.; Park, C.; Yoo, H.Y. Improved recovery of antioxidants from aronia juice processing residue via optimization of extraction variables based on multi-prediction models. Sustain. Chem. Pharm. 2024, 39, 10156. [Google Scholar] [CrossRef]
- Melo, P.S.; Massarioli, A.P.; Denny, C.; dos Santos, L.F.; Franchin, M.; Pereira, G.E.; Vieira, T.M.; Rosalen, P.L.; de Alencar, S.M. Winery by-products: Extraction optimization, phenolic composition and cytotoxic evaluation to act as a new source of scavenging of reactive oxygen species. Food Chem. 2015, 181, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Ju, Y.; Bao, N.; Luo, Y.L.; Huang, L.L.; Cao, N.X.; Liu, M.Z.; Bo, J.N.; Zhang, S.; Yan, Y. Effects of polyphenol-rich Aronia melanocarpa pomace feeding on growth performance, biochemical profile, and meat quality in pigs at weaned and finishing stages. Livest. Sci. 2021, 252, 104674. [Google Scholar] [CrossRef]
- Petreska Stanoeva, J.; Damjanovski, V.; Cichna-Markl, M.; Stefova, M. Anthocyanin fingerprinting as an authentication testing tool for blueberry, aronia, and pomegranate juices. Eur. Food Res. Technol. 2024, 250, 751–762. [Google Scholar] [CrossRef]
- Petrov Ivanković, A.; Ćorović, M.; Milivojević, A.; Simović, M.; Banjanac, K.; Veljković, M.; Bezbradica, D. Berries pomace valorization: From waste to potent antioxidants and emerging skin prebiotics. Int. J. Fruit Sci. 2024, 2, 85–101. [Google Scholar] [CrossRef]
- Gao, N.; Sun, X.; Li, D.; Gong, E.; Tian, J.; Si, X.; Jiao, X.; Xing, J.; Wang, Y.; Meng, X.; et al. Optimization of anthocyanidins conversion using chokeberry pomace rich in polymeric proanthocyanidins and cellular antioxidant activity analysis. LWT 2020, 133, 109889. [Google Scholar] [CrossRef]
- Witczak, T.; Stępień, A.; Gumul, D.; Witczak, M.; Fiutak, G.; Zięba, T. The influence of the extrusion process on the nutritional composition, physical properties and storage stability of black chokeberry pomaces. Food Chem. 2021, 334, 127548. [Google Scholar] [CrossRef]
- Zhang, M.-Q.; Zhang, J.; Zhang, Y.-T.; Sun, J.-Y.; Prieto, M.A.; Simal-Gandara, J.; Putnik, P.; Li, N.-Y.; Liu, C. The link between the phenolic composition and the antioxidant activity in different small berries: A metabolomic approach. LWT 2023, 182, 114853. [Google Scholar] [CrossRef]
- Kitryte, V.; Kraujaliene, V.; Sulniute, V.; Pukalskas, A.; Venskutonis, P.R. Chokeberry pomace valorization into food ingredients by enzyme-assisted extraction: Process optimization and product characterization. Food Bioprod. Process. 2017, 105, 36–50. [Google Scholar] [CrossRef]
- Simić, V.M.; Rajković, K.M.; Stojičević, S.S.; Veličković, D.T.; Nikolić, N.C.; Lasić, M.L.; Karabegović, I.T. Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep. Purif. Technol. 2016, 160, 89–97. [Google Scholar] [CrossRef]
- Andrade, T.A.; Hamerski, F.; Fetzer, D.E.L.; Roda-Serrat, M.C.; Corazza, M.L.; Norddahl, B.; Errico, M. Ultrasound-assisted pressurized liquid extraction of anthocyanins from Aronia melanocarpa pomace. Sep. Purif. Technol. 2021, 276, 119290. [Google Scholar] [CrossRef]
- Ivanovska, A.; Savić Gajić, I.; Mravik, Ž.; Reljić, M.; Ilić-Tomić, T.; Savić, I.; Luxbacher, T.; Lađarević, J. Transforming discarded walnut green husk into a resource of valuable compounds for colored bioactive textiles with a focus on circular economy concept. Dyes Pigments 2024, 231, 112406. [Google Scholar] [CrossRef]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa products and by-products for health and nutrition: A review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Aprodu, I.; Chitescu, C.L.; Grigore-Gurgu, L.; Dumitrașcu, L. Investigation of the antioxidant and antimicrobial properties of ultrasound-assisted extracted phenolics from Aronia melanocarpa pomace. Appl. Sci. 2025, 15, 7070. [Google Scholar] [CrossRef]
- Winkler, D.A.; Breedon, M.; White, P.; Hughes, A.E.; Sapper, E.D.; Cole, I. Using high throughput experimental data and in silico models to discover alternatives to toxic chromate corrosion inhibitors. Corros. Sci. 2016, 106, 229–235. [Google Scholar] [CrossRef]
- Petrov Ivanković, A.; Milivojević, A.; Ćorović, M.; Simović, M.; Banjanac, K.; Jansen, P.; Vukoičić, A.; van den Bogaard, E.; Bezbradica, D. In Vitro evaluation of enzymatically derived blackcurrant extract as prebiotic cosmetic ingredient: Extraction conditions optimization and effect on cutaneous microbiota representatives. Chem. Biol. Technol. Agric. 2023, 10, 125. [Google Scholar] [CrossRef]
- ASTM E2149-20; Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents Under Dynamic Contact Conditions. ASTM International: West Conshohocken, PA, USA, 2020.
- AATCC 100-2019; Antibacterial Finishes on Textile Materials. AATCC: Research Triangle Park, NC, USA, 2019.
- Glaser, T.K.; Plohl, O.; Vesel, A.; Ajdnik, U.; Ulrih, N.P.; Hrnčič, M.K.; Bren, U.; Fras Zemljič, L. Functionalization of polyethylene (PE) and polypropylene (PP) material using chitosan nanoparticles with incorporated resveratrol as potential active packaging. Materials 2019, 12, 2118. [Google Scholar] [CrossRef]
- Hong, K.H. Effect of biomordanting with Aronia melanocarpa leaf extract on coloring and functionalizing of wool and cotton fabrics dyed with A. melanocarpa fruit extract. Polym. Bull. 2024, 81, 9235–9251. [Google Scholar] [CrossRef]
- Ugrinović, V.; Marković, M.; Božić, B.; Panić, V.; Veljović, Đ. Poly(methacrylic acid) hydrogels crosslinked by poly(ethylene glycol) diacrylate as pH-responsive systems for drug delivery applications. Hem. Ind. 2023, 77, 235–249. [Google Scholar] [CrossRef]
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa fruits as a rich dietary source of chlorogenic acids and anthocyanins: 1H-NMR, HPLC-DAD, and chemometric studies. Molecules 2020, 25, 3234. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhu, J.; Tong, Y.; Kong, Y.; Tan, C.; Wang, M.; Wan, M.; Meng, X. Antibacterial characteristics and mechanisms of action of Aronia melanocarpa anthocyanins against Escherichia coli. LWT 2021, 150, 112018. [Google Scholar] [CrossRef]
- Pavun, L.; Spasojević, D.; Ivanovska, A.; Lađarević, J.; Milenković, M.; Uskoković-Marković, S. Characterization of tea water extracts and their utilization for dyeing and functionalization of fabrics of different chemical compositions. Maced. J. Chem. Chem. Eng. 2023, 42, 263–273. [Google Scholar] [CrossRef]
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Renuka Devi, P.; Veera Ravi, A. Fungal pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi. 2020, 6, 68. [Google Scholar] [CrossRef]
- Srivastava, P.; Ramesh, M.; Kaushik, P.; Kumari, A.; Aggarwal, S. Pyocyanin pigment from Pseudomonas species: Source of a dye and antimicrobial textile finish—A review. Proc. Indian Natl. Sci. Acad. 2022, 88, 542–550. [Google Scholar] [CrossRef]
- Hamieau, M.; Loulergue, P.; Szydłowska-Czerniak, A. A green solvent extraction of antioxidants from herbs and agro-food wastes: Optimization and capacity determination. Appl. Sci. 2024, 14, 2936. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical scavenging mechanisms of phenolic compounds: A quantitative structure-property relationship (QSPR) study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An overview on starch-based sustainable hydrogels: Potential applications and aspects. J. Polym. Environ. 2022, 30, 19–50. [Google Scholar] [CrossRef]
- Sisso, A.M.; Boit, M.O.; De Forest, C.A. Self-healing injectable gelatin hydrogels for localized therapeutic cell delivery. J. Biomed. Mater. Res. A 2020, 108, 1112–1121. [Google Scholar] [CrossRef]
- Lipatova, I.M.; Yusova, A.A. Effect of mechanical activation on starch crosslinking with citric acid. Int. J. Biol. Macromol. 2021, 185, 688–695. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Mallick, S.P.; Sagiri, S.S.; Singh, V.K.; Pal, K.; Pradhan, D.K.; Bhattacharya, M.K. Effect of processed starches on the properties of gelatin-based physical hydrogels: Characterization, in vitro drug release and antimicrobial studies. Polym. Plast. Technol. Eng. 2014, 53, 700–715. [Google Scholar] [CrossRef]
- Chhabra, R.; Peshattiwar, V.; Pant, T.; Deshpande, A.; Modi, D.; Sathaye, S.; Tibrewala, A.; Dyawanapelly, S.; Jain, R.; Dandekar, P. In Vivo studies of 3D starch-gelatin scaffolds for full-thickness wound healing. ACS Appl. Bio Mater. 2020, 18, 2920–2929. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Disper. Sci. Technol. 2019, 41, 690–698. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sun, T.L.; Zhang, A.K.; Ikura, Y.; Nakajima, T.; Nonoyama, T.; Kurokawa, T.; Ito, O.; Ishitobi, H.; Gong, J.P. Tough physical double-network hydrogels based on amphiphilic triblock copolymers. Adv. Mater. 2016, 28, 4884–4890. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-based hydrogels applied in drug delivery: An overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Liu, J.; Chinga-Carrasco, G.; Cheng, F. Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 2016, 23, 3129–3143. [Google Scholar] [CrossRef]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef]
- Petkovska, J.; Geskovski, N.; Marković, D.; Dimova, V.; Mirakovski, D.; Radetić, M.; Jordanov, I. Chitosan-pectin multilayer coating with anthocyanin grape dye as pH indicating wound dressing: Synthesis and characterization. Carbohydr. Polym. Technol. Appl. 2024, 7, 100438. [Google Scholar] [CrossRef]
- Ivanovska, A.; Milenković, J.; Lađarević, J.; Mihajlovski, K.; Dojčinović, B.; Ugrinović, V.; Škaro Bogojević, S.; Kostić, M. Harnessing the power of green and rooibos tea aqueous extracts for obtaining colored bioactive cotton and cotton/flax fabrics intended for disposable and reusable medical textiles. Cellulose 2024, 31, 9523–9542. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Sun, X.; Zhang, Q.; Wang, R.; Zhao, J.; Aslam, R.; Sun, Y.; Yan, Y.; Li, X. Seaweed extract as green corrosion inhibitor for carbon steel in hydrochloric acid solution. Colloid. Surf. A 2020, 700, 134751. [Google Scholar] [CrossRef]
- Guo, L.; Tan, B.; Li, W.; Li, Q.; Zheng, X.; Obot, I.B. Banana leaves water extracts as inhibitor for X70 steel corrosion in HCl medium. J. Mol. Liq. 2021, 327, 114828. [Google Scholar] [CrossRef]
- Shahini, M.H.; Ramezanzadeh, M.; Ramezanzadeh, B.; Bahlakeh, G. The role of ethanolic extract of Stachys byzantina’s leaves for effective decreasing the mild-steel (MS) degradation in the acidic solution; coupled theoretical/experimental assessments. J. Mol. Liq. 2021, 329, 115571. [Google Scholar] [CrossRef]
- El Azzouzi, M.; Azzaoui, K.; Warad, I.; Hammouti, B.; Shityakov, S.; Sabbahi, R.; Saoiabi, S.; Youssoufi, M.H.; Akartasse, N.; Jodeh, S.; et al. Moroccan, Mauritania, and senegalese gum Arabic variants as green corrosion inhibitors for mild steel in HCl: Weight loss, electrochemical, AFM and XPS studies. J. Mol. Liq. 2022, 347, 118354. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Yang, X.B.; Zhang, Y.; Xu, N. Artemisia verlotiorum extract as green corrosion inhibitor for enhanced corrosion resistance of mild steel in acidic solution. J. Mol. Liq. 2025, 419, 126811. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Ferreira Fagundes, T.d.S.; dos Santos, N.E.; Rocha, T.S.d.M.; Garrett, R.; Borges, R.M.; Muricy, G.; Valverde, A.L.; Ponzio, E.A. Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. Electrochim. Acta 2019, 312, 137–148. [Google Scholar] [CrossRef]
- Cherrad, S.; Alrashdi, A.A.; Lee, H.S.; El Aoufir, Y.; Lgaz, H.; Satrani, B.; Ghanmi, M.; Aouane, E.M.; Chaouch, A. Cupressus arizonica fruit essential oil: A novel green inhibitor for acid corrosion of carbon steel. Arab. J. Chem. 2022, 15, 103849. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ramezanzadeh, M.; Haddadi, S.A.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M. Persian liquorice extract as a highly efficient sustainable corrosion inhibitor for mild steel in sodium chloride solution. J. Clean. Prod. 2019, 210, 660–672. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wang, C.; Li, Y.; Wu, Y. Exploring the potential of plant extracts as corrosion inhibitors: A comprehensive review. Prog. Org. Coat. 2025, 198, 108915. [Google Scholar] [CrossRef]




| Compound | λ, nm | Rt, min | Concentration, µmol/L |
|---|---|---|---|
| neochlorogenic acid | 310 | 11.07 | 21.21 ± 0.34 |
| chlorogenic acid | 310 | 17.34 | 27.00 ± 1.28 |
| cyanidin-3-galactoside | 520 | 25.64 | 547.72 ± 4.26 |
| cyanidin-3-glucoside | 520 | 26.78 | 54.55 ± 1.07 |
| cyanidin-3-arabinoside | 520 | 28.18 | 210.19 ± 2.23 |
| cyanidin-3-xyloside | 520 | 32.45 | 27.30 ± 0.15 |
| Fabric | I Dyeing Cycle | II Dyeing Cycle | III Dyeing Cycle | ΔE (I, II) | ΔE (II, III) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |||
| WO | 58.42 | 6.63 | 5.20 | 58.12 | 3.74 | 2.61 | 59.37 | 1.40 | 2.75 | 3.89 | 2.66 |
| SILK | 62.97 | 12.98 | −5.53 | 67.25 | 10.79 | −7.51 | 66.36 | 9.11 | −9.17 | 5.20 | 8.96 |
| CO | 73.33 | 10.16 | 0.39 | 74.26 | 11.33 | −1.13 | 73.65 | 10.79 | −1.24 | 2.13 | 0.82 |
| PA | 70.51 | 3.92 | 3.67 | 76.81 | 4.71 | 1.17 | 75.38 | 4.87 | 0.94 | 6.82 | 1.46 |
| CV | 72.97 | 14.68 | −3.56 | 75.86 | 13.55 | −4.22 | 75.29 | 12.99 | −6.42 | 3.17 | 2.34 |
| Fabric | I Dyeing Cycle | II Dyeing Cycle | III Dyeing Cycle | |||
|---|---|---|---|---|---|---|
| E. coli | S. aureus | E. coli | S. aureus | E. coli | S. aureus | |
| WO | / | / | / | / | / | / |
| SILK | / | / | / | / | / | / |
| CO | 99.99% | 99.99% | 86.36 ± 2.21% | 99.90 ± 0.07% | / | 99.86 ± 0.11% |
| PA | / | / | / | / | / | / |
| CV | 99.99% | 99.99% | 80.91 ± 3.11% | 99.79 ± 0.18% | 75.45 ± 1.45% | 97.73 ± 1.84% |
| Sample | ABTS Antioxidant Activity | Antibacterial Activity | |
|---|---|---|---|
| E. coli | S. aureus | ||
| SG-7+AE | 99.99% | 70.91 ± 1.12% | 97.42 ± 1.63% |
| SG-9+AE | 99.99% | 78.18 ± 2.34% | 98.07 ± 0.29% |
| SG-12+AE | 99.91% | 74.54 ± 1.09% | 99.99% |
| Solution | Ecorr, mV vs. SCE | η, % | |
|---|---|---|---|
| 1 M HCl | −454 | 335 | / |
| 50 ppm AE | −475 | 26.5 | 92.1 |
| 100 ppm AE | −477 | 10.9 | 96.7 |
| 200 ppm AE | −490 | 19.0 | 94.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugrinović, V.; Simović, A.; Ćorović, M.; Mihajlovski, K.; Lađarević, J.; Bajat, J.; Ivanovska, A. Valorization of Aronia melanocarpa Pomace: A Sustainable Source of Bioactive Compounds for Developing Colored Healthcare Textiles, Biomedical Hydrogels, and Green Corrosion Inhibitor. Sustain. Chem. 2025, 6, 46. https://doi.org/10.3390/suschem6040046
Ugrinović V, Simović A, Ćorović M, Mihajlovski K, Lađarević J, Bajat J, Ivanovska A. Valorization of Aronia melanocarpa Pomace: A Sustainable Source of Bioactive Compounds for Developing Colored Healthcare Textiles, Biomedical Hydrogels, and Green Corrosion Inhibitor. Sustainable Chemistry. 2025; 6(4):46. https://doi.org/10.3390/suschem6040046
Chicago/Turabian StyleUgrinović, Vukašin, Anđela Simović, Marija Ćorović, Katarina Mihajlovski, Jelena Lađarević, Jelena Bajat, and Aleksandra Ivanovska. 2025. "Valorization of Aronia melanocarpa Pomace: A Sustainable Source of Bioactive Compounds for Developing Colored Healthcare Textiles, Biomedical Hydrogels, and Green Corrosion Inhibitor" Sustainable Chemistry 6, no. 4: 46. https://doi.org/10.3390/suschem6040046
APA StyleUgrinović, V., Simović, A., Ćorović, M., Mihajlovski, K., Lađarević, J., Bajat, J., & Ivanovska, A. (2025). Valorization of Aronia melanocarpa Pomace: A Sustainable Source of Bioactive Compounds for Developing Colored Healthcare Textiles, Biomedical Hydrogels, and Green Corrosion Inhibitor. Sustainable Chemistry, 6(4), 46. https://doi.org/10.3390/suschem6040046









