Unlock the Potentials to Further Improve CO2 Storage and Utilization with Supercritical CO2 Emulsions When Applying CO2-Philic Surfactants
Abstract
1. Introduction
2. Experiment Description
2.1. Materials
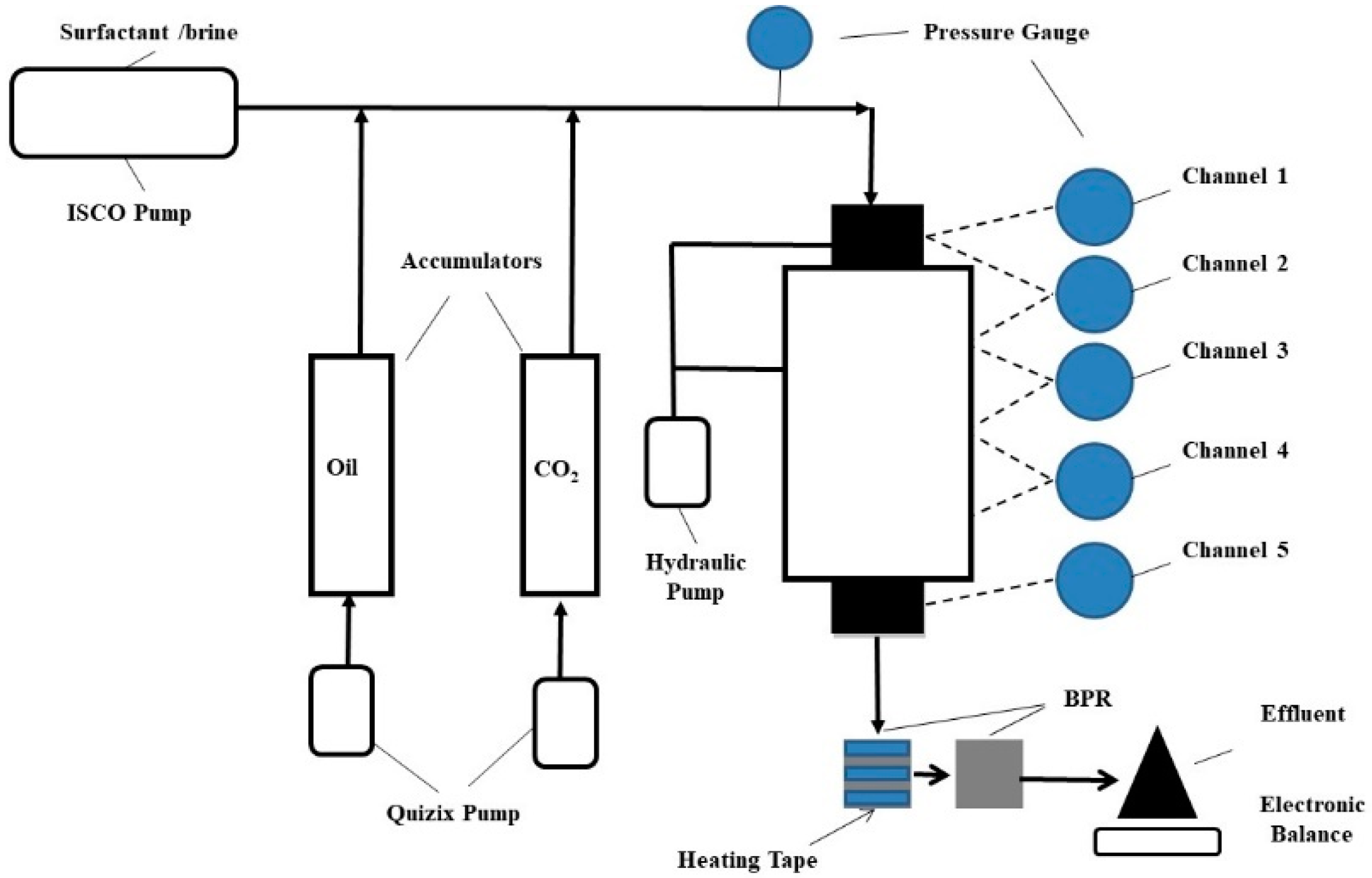
2.2. Experimental Apparatus and Procedures
2.2.1. Static Adsorption
2.2.2. Phase Behavior Test
2.2.3. Two-Phase Coreflooding without Artificial Fracture
2.2.4. Three-Phase Coreflooding with Artificial Fracture
3. Simulation Description
3.1. Simulation Deck
3.2. Foam Model
3.3. Injection Strategy
4. Results and Discussion
4.1. Experiments
4.1.1. Static Adsorption
4.1.2. Phase Behavior Test
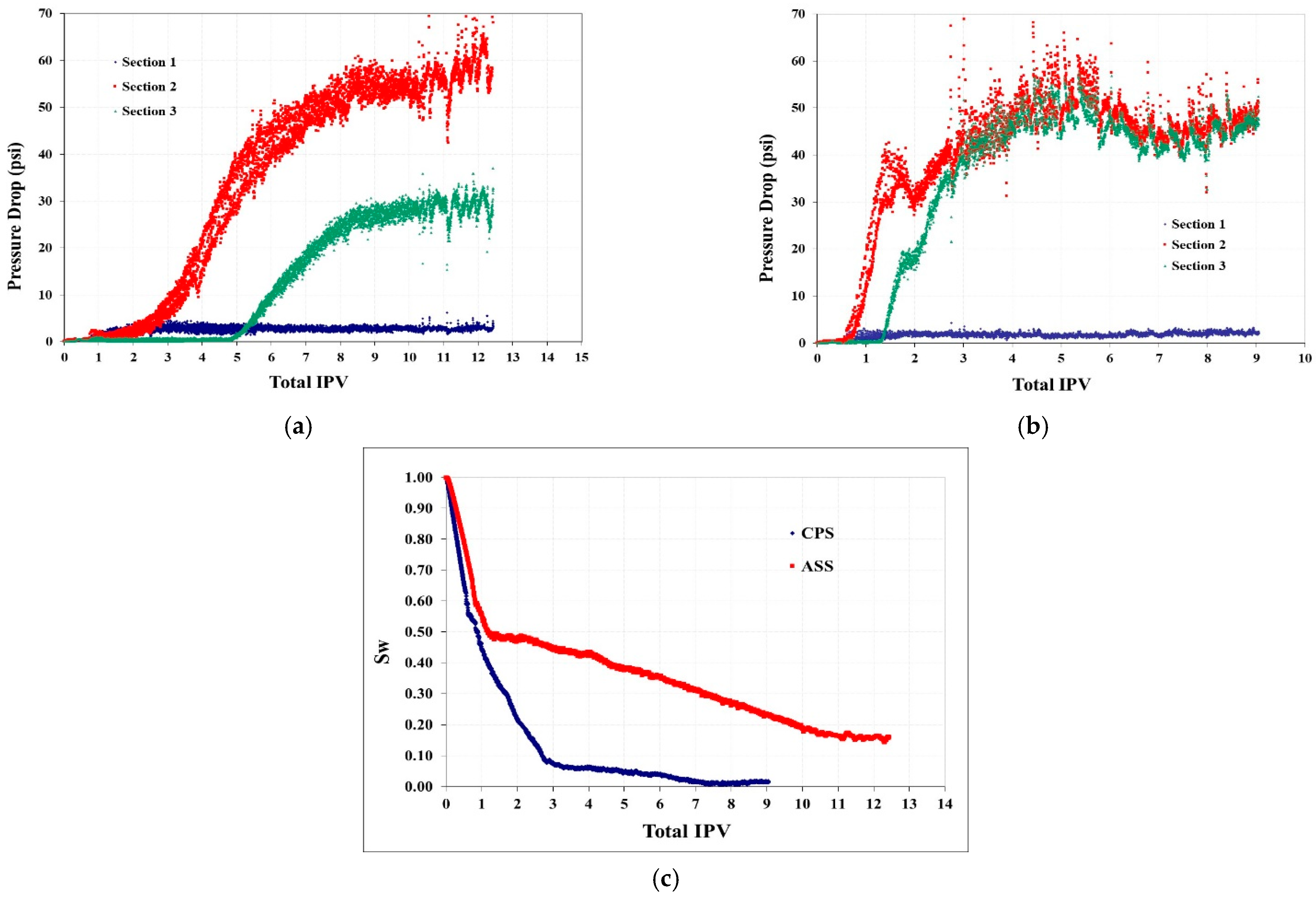
4.1.3. Two-Phase Flooding with Unfractured Berea Sandstone
4.1.4. Two-Phase Flooding with Unfractured Silurian Dolomite
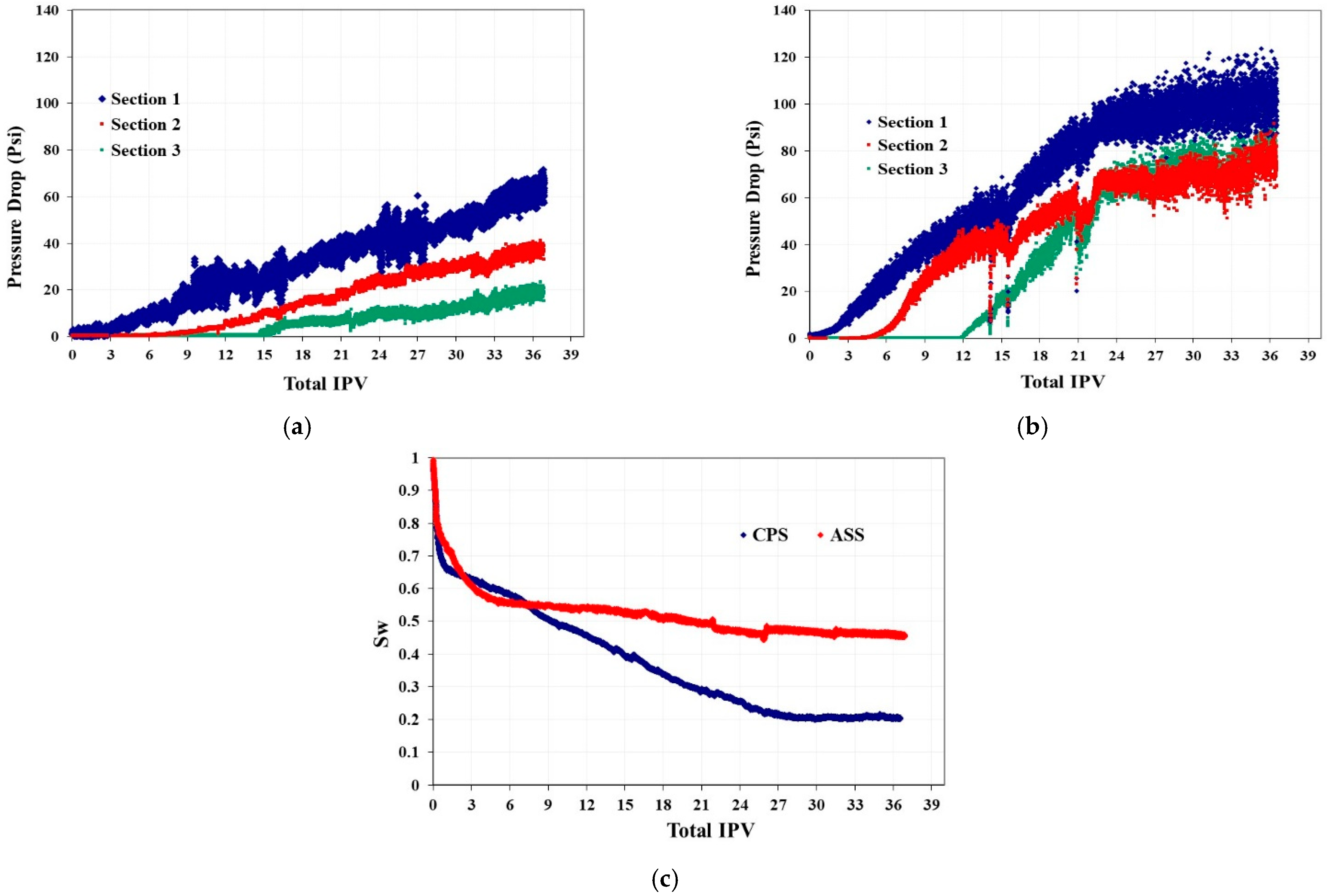
4.1.5. Three-Phase CO2 & W/G Floodings with Fractured Silurian Dolomite
4.1.6. Three-Phase ScCO2 Emulsion Floodings with Fractured Silurian Dolomite
4.2. Simulations
5. Conclusions
5.1. Experiments
- The employed anionic aqueous soluble surfactant displays more than four times higher adsorption on Silurian dolomite than selected CO2-philic one, which is ascribed to stronger electrostatic attraction owing to opposite charges. The behaviors of nonionic CPS are dominated by hydrogen bonding, which is weaker than the electrostatic force.
- Both ASS (CD-1045) and CPS (2EH-PO5-EO15) show poor affinity to Wasson oil, particularly the latter. In brief, the observations here eliminate the concern of surfactant loss to W/O interface, and both surfactants are mainly used for foaming purposes.
- In the two-phase unfractured Berea sandstone system, CPS emulsion propagates much faster, which is attributed to its dual phase partition capacity before strong foam generation upstream, but to its superior film stabilization ability when it further propagates downstream. The better emulsion stability is also supported by the much faster pressure buildup and higher plateau pressure drops, which in turn facilitate CPS emulsion to achieve higher fluid displacement efficiency and CO2 storage.
- Similar relative behaviors are observed in the two-phase unfractured Silurian dolomite system, with even sharper contrast in the displacement rate and CO2 storage. CPS emulsion could reduce brine saturation to less than 0.1 in 2.8 TIPV, and CO2 saturation could be as high as 97% eventually.
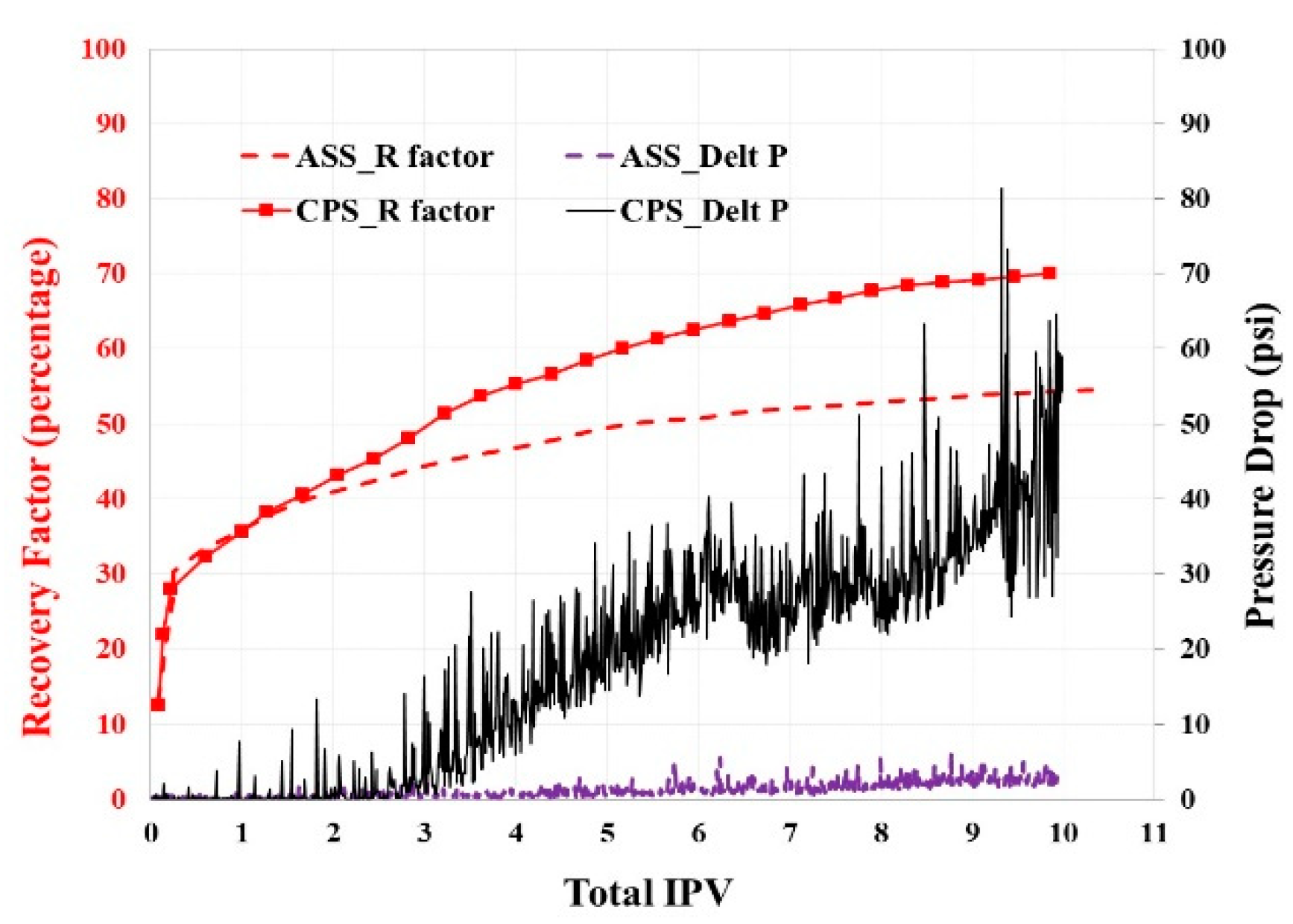
- In the three-phase system with fractured Silurian dolomite, either continuous ScCO2 injection or co-injection of brine and ScCO2 provides relatively poor performances with negligible pressure drops, and oil recoveries are 24% and 35% respectively, even though MMP is achieved. It seems like here it is dominated by the lack of sufficient mobility control, and only the regions near the fracture are swept.
- The selected ASS (CD-1045) indeed improves oil recovery to 54%, with more than a forty times increase of the pressure drop. When replacing the stabilizer agent with CPS, it is further enhanced to 70%, with another over 10 time pressure drop increase, which also indicates the far better CO2 storage.
5.2. Simulations
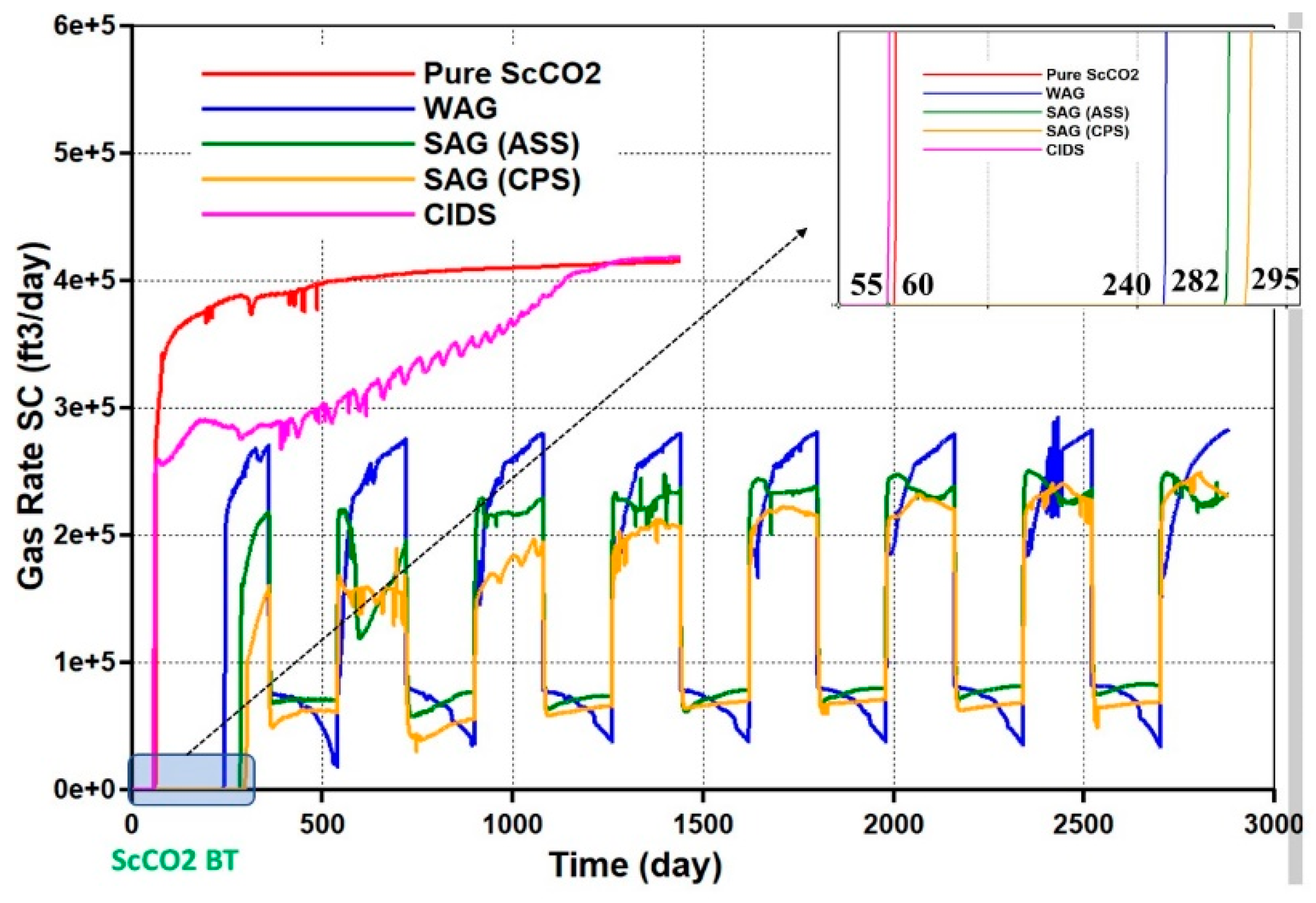
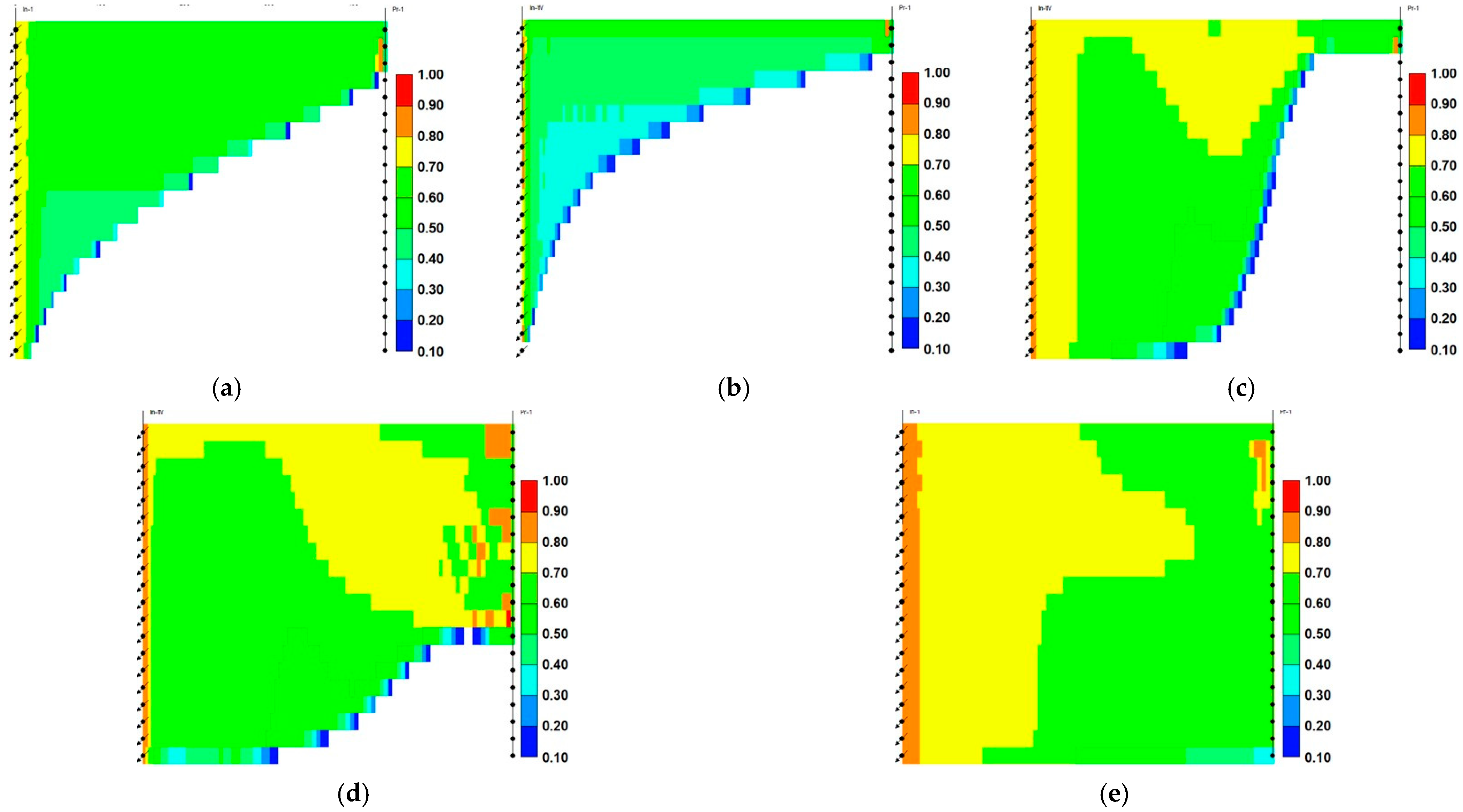
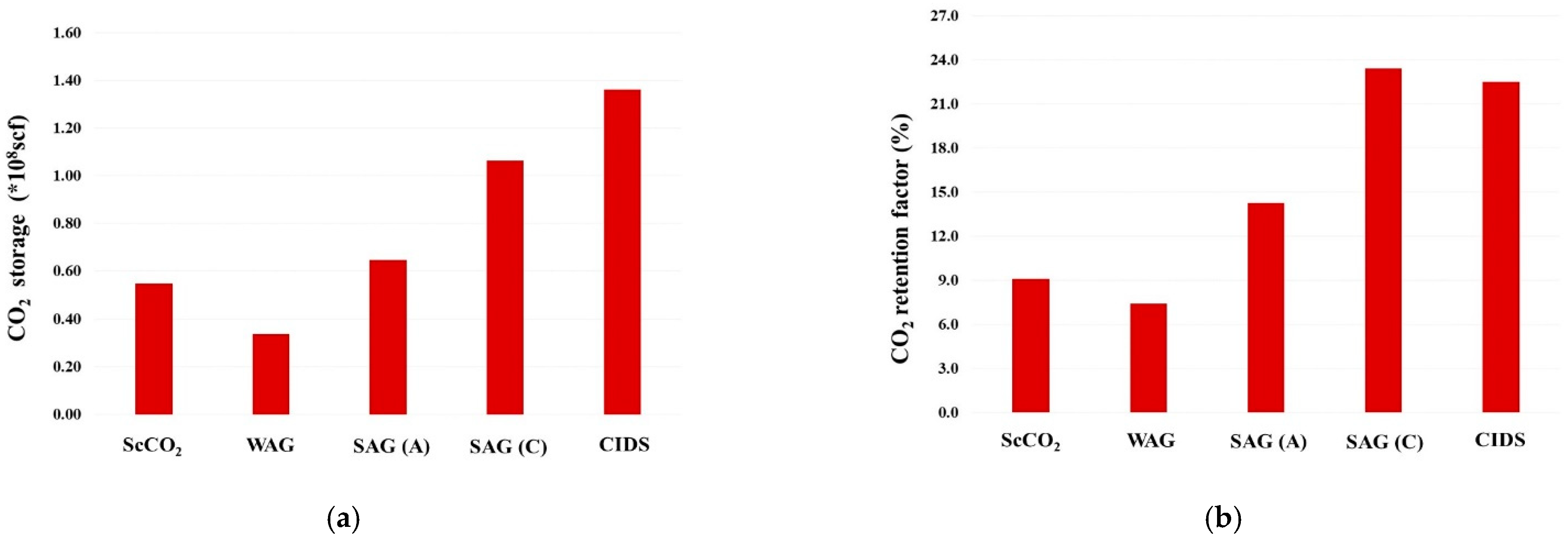
- Relative to ScoCO2 and WAG injections, it is necessary to employ a surfactant for proper mobility control. CPS emulsion could further slow down the gas BT and reduce the CO2 production rate, relative to ASS emulsion. In particular, CPS emulsion not only extends the mixing zone pronouncedly deeper into the reservoir, but also pulls the override zone downwards to provide better conformance control there. CIDS injection could even sweep the entire reservoir area. Accordingly, CPS emulsion stores more than 63% CO2 than ASS emulsion, while another 28% improvement is achieved by novel CIDS injection. Meanwhile, CPS emulsion is also 65% more efficient on CO2 retention than a conventional one.
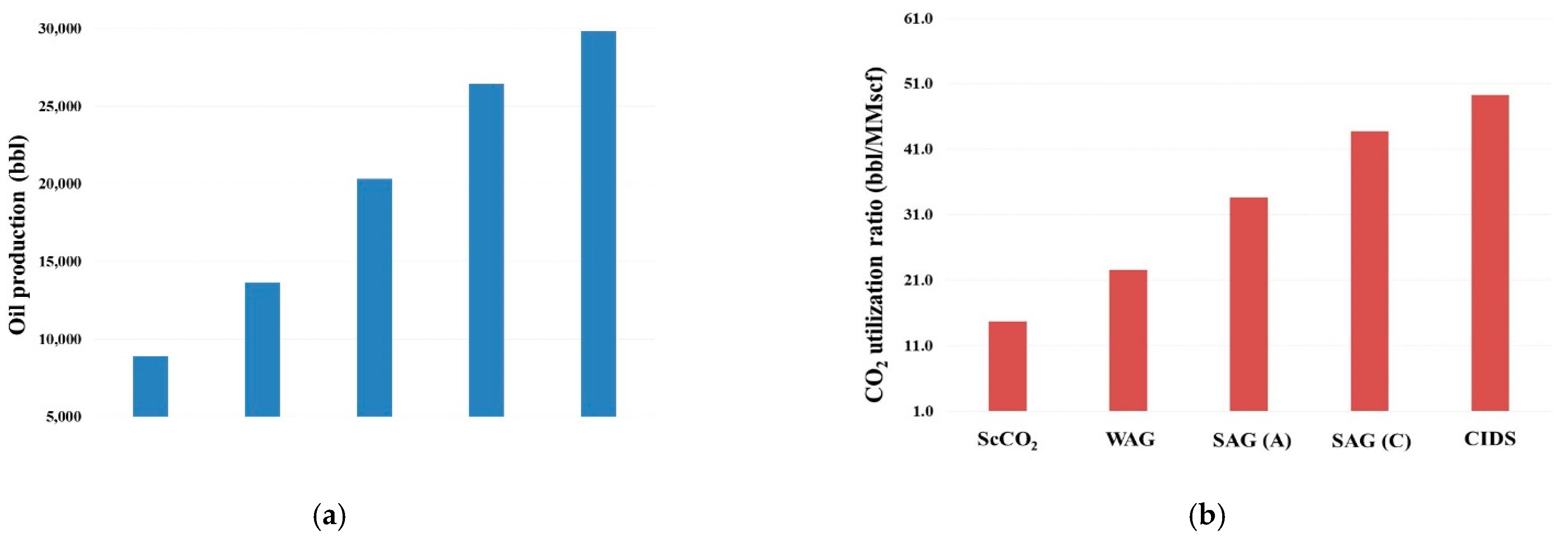
- Conventional ASS emulsion would leave an unswept surfactant vacuum zone on the topmost layer, owing to water slumping, while the dual phase partition capacity of CPS endues its possibility to present in those layers to realize the mobility control and facilitate the CO2 diversion downwards. In turn, the alternating injection of CPS emulsion doubles the oil production than WAG, and produces 30% more than ASS emulsion, while CIDS injection could provide another 13% more oil recovery. The variation of the CO2 utilization ratio is consistent with oil production among tested cases.
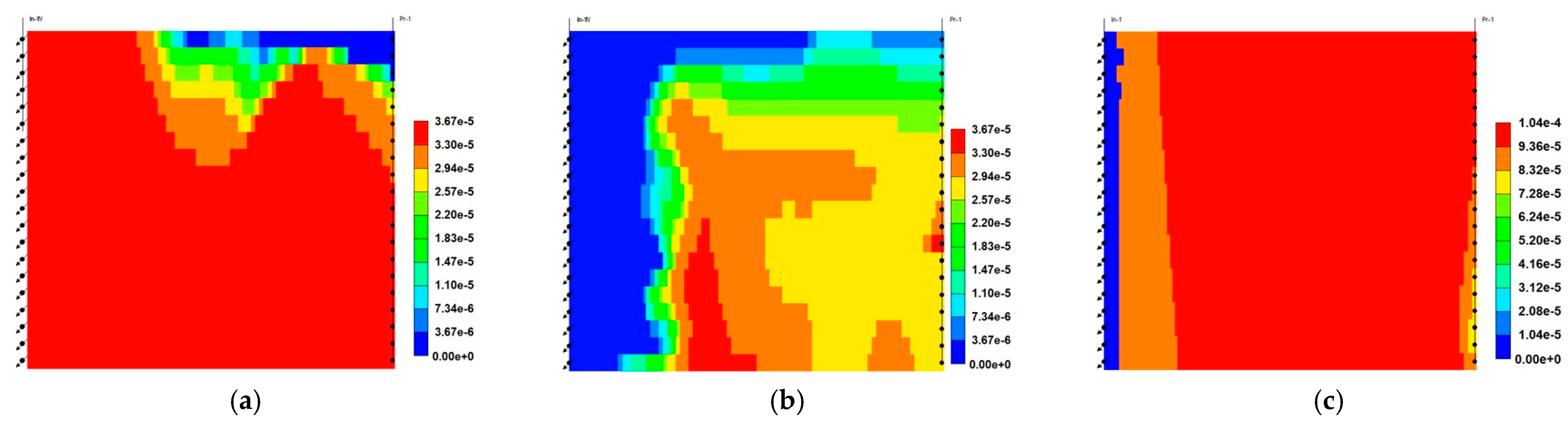
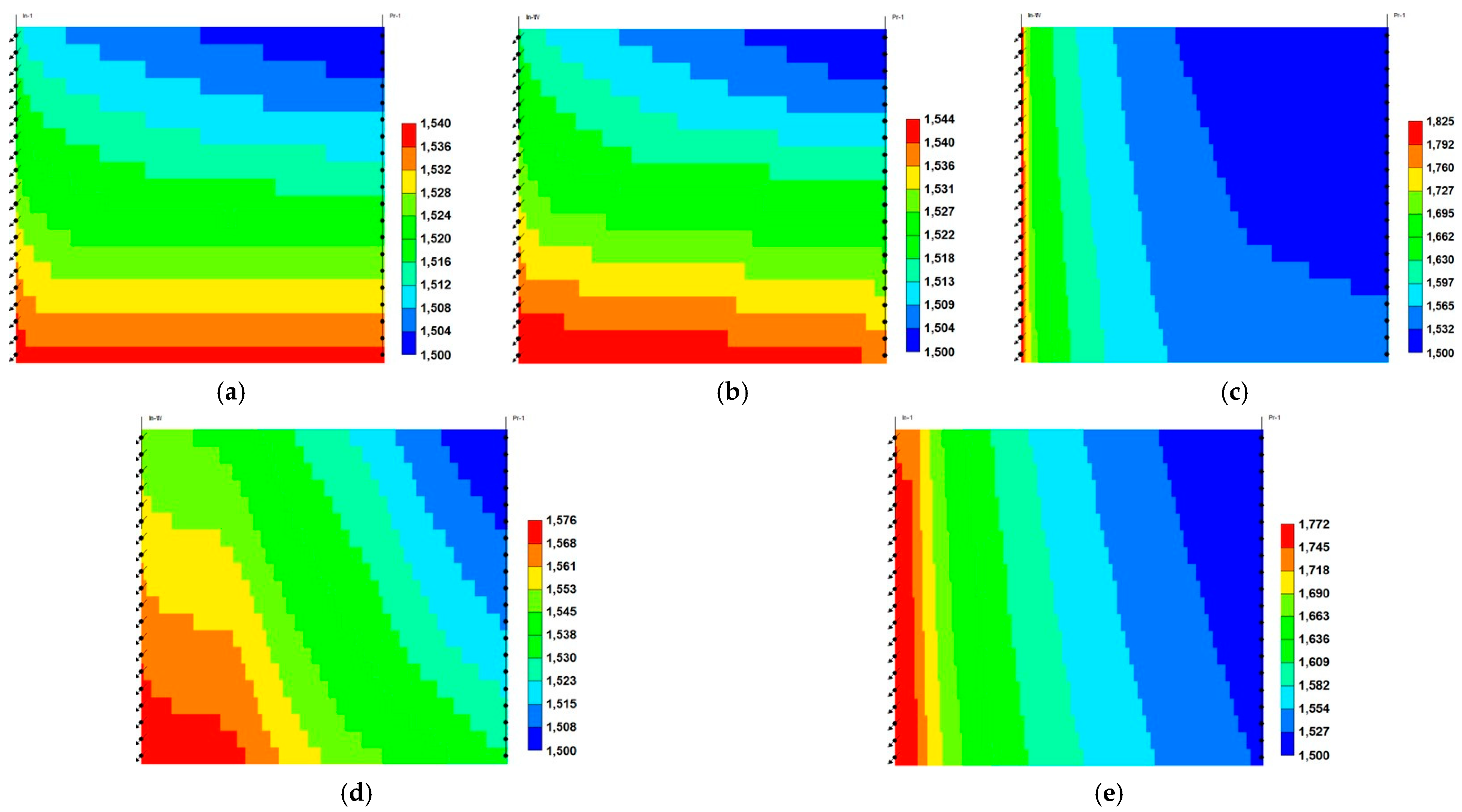
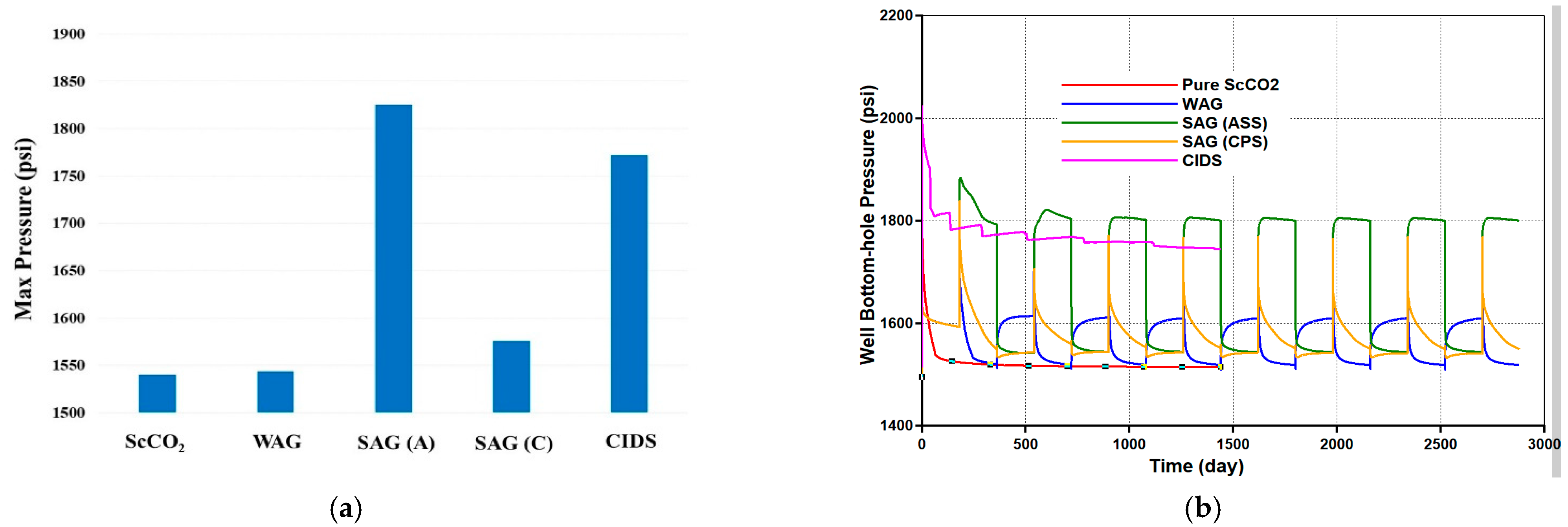
- The pressure distribution of ASS emulsion is uneven and the pressure gradient is sharp near the wellbore, which implies low energy utilization efficiency. On the contrary, CPS emulsion provides a much more even pressure gradient. In addition, there exists a CPS vacuum zone near the wellbore, which is caused by the combination of ScCO2 extraction to water, the dynamic partition of CPS into injected ScCO2 stream and the foam dry-out effect. Thereby, CPS emulsion demonstrates significantly lower BHP and much higher injectivity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| epdry | Exponent in the equation for foam dry out effect |
| epsurf | Exponent in the equation for surfactant concentration effect |
| fmdry | Critical water saturation for foam collapsing |
| fmmob | Reference mobility reduction factor |
| fmsurf | Critical surfactant concentration for “strong” foam (mole fraction) |
| F1 | Effect of surfactant concentration for foam |
| F7 | Effect of foam dry out by waster saturation |
| FM | Foam mobility reduction factor |
| K | G/L partition coefficient |
Appendix A. Selected CPS Thermodynamic Properties at 1500 psi and 35 °C (Ren et al. 2014)
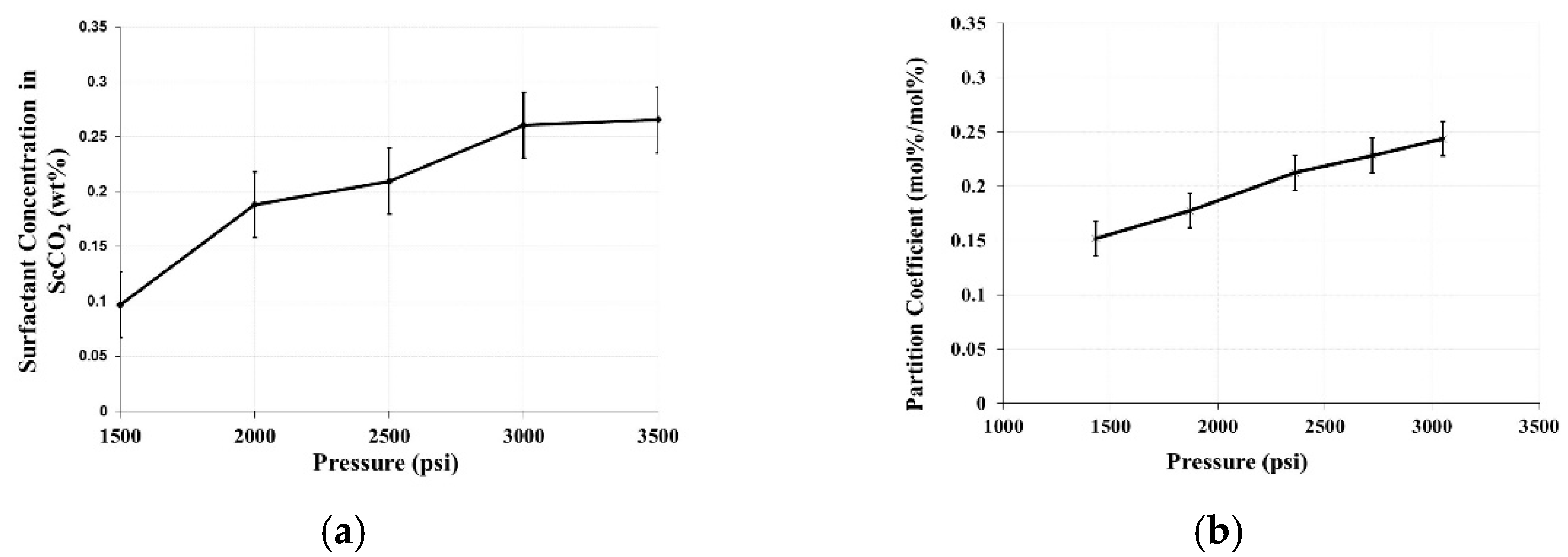
References
- Bejestani, D.Z.; Rostami, B.; Khosravi, M.; Kazemi, K. Effect of petrophysical matrix properties on bypassed oil recovery from a matrix-fracture system during CO2 near-miscible injection: Experimental investigation. Int. J. Multiph. Flow 2016, 85, 123–131. [Google Scholar] [CrossRef]
- Bond, D.C.; Holbrook, C.C. Gas Drive Oil Recovery Process. U.S. Patent No. 2866507, December 1958. [Google Scholar]
- Hiraski, G.J. The Steam-Foam Process. J. Pet. Technol. 1989, 41, 449–456. [Google Scholar] [CrossRef]
- M’Barki, O.; Ma, K.; Ren, G.; Mateen, K.; Bourdarot, G.; Morel, D.C.; Nguyen, Q.P. Repeatable Steady-State Foam Experimental Data and Investigations of Foam Hysteresis in a Sand Pack. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 9–11 October 2017. [Google Scholar]
- Hirasaki, G.; Lawson, J. Mechanisms of Foam Flow in Porous Media: Apparent Viscosity in Smooth Capillaries. Soc. Pet. Eng. J. 1985, 25, 176–190. [Google Scholar] [CrossRef]
- Falls, A.; Musters, J.; Ratulowski, J. The Apparent Viscosity of Foams in Homogeneous Bead Packs. SPE Reserv. Eng. 1989, 4, 155–164. [Google Scholar] [CrossRef]
- Dhanuka, V.V.; Dickson, J.L.; Ryoo, W.; Johnston, K.P. High internal phase CO2-in-water emulsions stabilized with a branched nonionic hydrocarbon surfactant. J. Colloid Interface Sci. 2006, 298, 406–418. [Google Scholar] [CrossRef]
- Alkan, H.; Goktekin, A.; Satman, A. A Laboratory Study of CO2-Foam Process for Bati Raman Field, Turkey. In Proceedings of the Middle East Oil Show, Bahrain, 16–19 November 1991; Available online: https://onepetro.org/SPEMEOS/proceedings-abstract/91MEOS/All-91MEOS/SPE-21409-MS/53677 (accessed on 24 December 2020).
- Lee, H.O.; Heller, J.P. Laboratory Measurements of CO2-Foam Mobility. SPE Reserv. Eng. 1990, 5, 193–197. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Jiao, T.; Niu, J. Performance Comparison Between Internal Olefin Sulfonates and Alpha Olefin Sulfonates. J. Surfactants Deterg. 2016, 20, 183–191. [Google Scholar] [CrossRef]
- Tsau, J.-S.; Grigg, R.B. Assessment of Foam Properties and Effectiveness in Mobility Reduction for CO2-Foam Floods. In Proceedings of the 1997 SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 18–21 February 1997. [Google Scholar]
- Yin, G.; Grigg, R.B.; Svec, Y. Oil Recovery and Surfactant Adsorption during CO2-Foam Flooding. In Proceedings of the 2009 Offshore Technology Conference, Houston, TX, USA, 4–7 May 2009. [Google Scholar]
- Patil, P.D.; Knight, T.; Katiyar, A.; Vanderwal, P.; Scherlin, J.; Rozowski, P.; Ibrahim, M.; Sridhar, G.B.; Nguyen, Q.P. CO2 Foam Field Pilot Test in Sandstone Reservoir: Complete Analysis of Foam Pilot Response. J. Pet. Technol. 2018, 70, 70–71. [Google Scholar]
- Garrett, P.R. The Science of Defoaming—Theory, Experiment and Applications; Surfacing Science Series; Taylor & Francis Group: Abingdon, UK, 2020; Volume 155. [Google Scholar]
- Lobo, L.A.; Nikolov, A.D.; Wasan, D.T. Foam stability in the presence of oil: On the importance of the second virial coefficient. J. Dispers. Sci. Technol. 1989, 10, 143–161. [Google Scholar] [CrossRef]
- Denkov, N.D.; Marinova, K.G.; Tcholakova, S.S. Mechanistic understanding of the modes of action of foam control agents. Adv. Coll. Interface Sci. 2014, 206, 57–67. [Google Scholar] [CrossRef]
- Bergeron, V.; Fagan, M.E.; Radke, C.J. Generalized entering coefficients: A criterion for foam stability against oil in porous media. Langmuir 1993, 9, 1704–1713. [Google Scholar] [CrossRef]
- Koczo, K.; Lobo, L.; Wasan, D. Effect of oil on foam stability: Aqueous foams stabilized by emulsions. J. Colloid Interface Sci. 1992, 150, 492–506. [Google Scholar] [CrossRef]
- Henry, R.L.; Fisher, D.R.; Pennell, S.P.; Honnert, M.A. Field Test of Foam to Reduce CO2 Cycling. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 21–24 April 1996. [Google Scholar]
- Chou, S.; Vasicek, S.; Pisio, D.; Jasek, D.; Goodgame, J. CO2 Foam Field Trial at North Ward-Estes. In Proceedings of the SPE Annual Technical Conference and Exhibition, Washington, DC, USA, 4–7 October 1992. [Google Scholar]
- Tsau, J.-S.; Heller, J. CO2 Foam Field Verification Pilot Test at EVGSAU: Phase IIIA—Surfactant Performance Characterization and Quality Assurance. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 17–20 April 1994. [Google Scholar]
- Martin, F.D.; Stevens, J.E.; Harpole, K.J. CO2-Foam Field Test at the East Vacuum Grayburg/San Andres Unit. SPE Reserv. Eng. 1995, 10, 266–272. [Google Scholar] [CrossRef]
- Hoefner, M.L.; Evans, E.M. CO2 Foam: Results from Four Developmental Field Trials. SPE Reserv. Eng. 1995, 10, 273–281. [Google Scholar] [CrossRef]
- Mukherjee, J.; Nguyen, Q.P.; Scherlin, J.; Vanderwal, P.; Rozowski, P. CO2 Foam Pilot in Salt Creek Field, Natrona County, WY: Phase III: Analysis of Pilot Performance. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14–18 April 2018. [Google Scholar]
- Sanders, A.W.; Jones, R.M.; Linroth, M.A.; Nguyen, Q.P. Implementation of a CO2 Foam Pilot Study in the SACROC Field: Performance Evaluation. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. [Google Scholar]
- Svorstol, I.; Vassenden, F.; Mannhardt, K. Laboratory Studies for Design of a Foam Pilot in the Snorre Field. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 21–24 April 1996. [Google Scholar]
- Xu, Q.; Rossen, W. Experimental Study of Gas Injection in a Surfactant-Alternating-Gas. SPE Reserv. Eval. Eng. 2004, 7, 438–448. [Google Scholar] [CrossRef]
- Xing, D.; Wei, B.; McLendon, W.J.; Enick, R.M.; McNulty, S.; Trickett, K.; Mohamed, A.; Cummings, S.; Eastoe, J.; E Rogers, S.; et al. CO2-Soluble, Nonionic, Water-Soluble Surfactants That Stabilize CO2-in-Brine Foams. SPE J. 2012, 17, 1172–1185. [Google Scholar] [CrossRef]
- McLendon, W.; Koronaios, P.; Enick, R.M.; Biesmans, G.; Salazar, L.C.; E Miller, A.; Soong, Y.; McLendon, T.; Romanov, V.; Crandall, D. Assessment of CO2-soluble non-ionic surfactants for mobility reduction using mobility measurements and CT imaging. J. Pet. Sci. Eng. 2014, 119, 196–209. [Google Scholar] [CrossRef]
- Le, V.Q.; Nguyen, Q.P.; Sanders, A. A Novel Foam Concept with CO2 Dissolved Surfactants. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar]
- Tiffin, D.L.; Sebastian, H.M.; Bergman, D.F. Displacement Mechanism and Water Shielding Phenomena for a Rich-Gas/Crude-Oil System. SPE Reserv. Eng. 1991, 6, 193–199. [Google Scholar] [CrossRef]
- Akbar, M.; Vissapragada, B.; Alghamdi, A.H.; Allem, D. A Snapshot of Carbonate Reservoir Evaluation. Oilfoeld Rev. 2000, 12, 20–21. [Google Scholar]
- Sloan, R. Quantification of Uncertainty in Recovery Efficiency Predictions: Lessons Learned from 250 Mature Carbonate Fields. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5–8 October 2003. [Google Scholar]
- Chen, P.; Mohanty, K.K. Wettability Alteration in High Temperature Carbonate Reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Alshehri, A.J.; Kovscek, A.R. Experimental and Numerical Study of Gravity Effects on Oil Recovery in Fractured Carbonates. In Proceedings of the 7th International Petroleum Technology Conference, Doha, Qatar, 19–22 January 2014. [Google Scholar]
- Chahardowli, M.; Zholdybayeva, A.; Farajzadeh, R.; Bruining, H. Solvent-enhanced Spontaneous Imbibition in Fractured Reservoirs. In Proceedings of the 75th EAGE Conference & Exhibition incorporating SPE EUROPEC 2013, London, UK, 10–13 June 2013. [Google Scholar]
- Eide, O.; Ersland, G.; Brattekas, B.; Haugen, A.; Graue, A.; Ferno, M.A. CO2 EOR by Diffusive Mixing in Fractured Reservoirs. Petrophysics 2015, 56, 23–31. [Google Scholar]
- Farajzadeh, R.; Wassing, B.; Boerrigter, P. Foam assisted gas–oil gravity drainage in naturally-fractured reservoirs. J. Pet. Sci. Eng. 2012, 94–95, 112–122. [Google Scholar] [CrossRef]
- Alavian, S.A.; Whitson, C.H. Scale Dependence of Diffusion in Naturally Fractured Reservoirs for CO2 Injection. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Morel, D.; Bourbiaux, B.; Latil, M.J.; Thiebot, B. Diffusion Effects in Gasflooded Light-Oil Fractured Reservoirs. SPE Adv. Technol. Ser. 1993, 1, 100–109. [Google Scholar] [CrossRef]
- Denney, D. History Matching Thermally Assisted Gas/Oil Gravity Drainage in Fractured Reservoirs. J. Pet. Technol. 2008, 60, 51–55. [Google Scholar] [CrossRef]
- Karimaie, H.; Torsæter, O. Low IFT gas–oil gravity drainage in fractured carbonate porous media. J. Pet. Sci. Eng. 2010, 70, 67–73. [Google Scholar] [CrossRef]
- Yan, W.; Miller, C.A.; Hirasaki, G.J. Foam sweep in fractures for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282–283, 348–359. [Google Scholar] [CrossRef]
- Ferno, M.A.; Gauteplass, J.; Pancharoen, M.; Haugen, A.; Graue, A.; Kovscek, A.R.; Hirasaki, G.J. Experimental Study of Foam Generation, Sweep Efficiency and Flow in a Fracture Network. SPE J. 2016, 21, 1140–1150. [Google Scholar] [CrossRef]
- Abdalla, M.S. Appraisal of Techniques Applied to Stimulate the Deep Khuff Gas Wells. In Proceedings of the Middle East Oil Show, Bahrain, 11–14 March 1989. [Google Scholar]
- Fjelde, I.; Zuta, J.; Duyilemi, O.V. Oil Recovery from Matrix during CO2-Foam Flooding of Fractured Carbonate Oil Reservoirs. In Proceedings of the Europec/EAGE Conference and Exhibition, Rome, Italy, 9–12 June 2008. [Google Scholar]
- Zuta, J.; Fjelde, I. Transport of CO2-Foaming Agents during CO2-Foam Processes in Fractured Chalk Rock. SPE Reserv. Eval. Eng. 2010, 13, 710–719. [Google Scholar] [CrossRef]
- Castro, S.H.L.; Restrepo, A.; Ocampo, A. Use of Divergent Fluids as an Alternative for Enhanced Recovery in Naturally Fractured Cores. In Proceedings of the Latin American and Caribbean Petroleum Engineering Conference, Cartagena de Indias, Colombia, 31 May–3 June 2009. [Google Scholar]
- Zuta, J.; Fjelde, I.; Berenblyum, R. Oil Recovery during CO2-Foam in Fractured Chalk Rock at Reservoir Conditions. In Proceedings of the International Symposium of the Society of Core Analysts, Noordwijk, The Netherlands, 27–30 September 2009. [Google Scholar]
- Panahi, H. Improving the Recovery Factor of Heavy Crude Reservoirs by Co-injecting CO2 and Other Conventional Gaseous Injecting Materials at Immiscibility Condition with Foam. In Proceedings of the SPE International Petroleum Conference in Mexico, Puebla Pue, Mexico, 7–9 November 2004. [Google Scholar]
- Haugen, Å.; Fernø, M.A.; Graue, A.; Bertin, H.J. Experimental Study of Foam Flow in Fractured Oil-Wet Limestone for Enhanced Oil Recovery. SPE Reserv. Eval. Eng. 2012, 15, 218–228. [Google Scholar] [CrossRef]
- Consani, K.A.; Smith, R.D. Observations on the solubility of surfactants and related molecules in carbon dioxide at 50 °C. J. Supercrit. Fluids 1990, 3, 51–56. [Google Scholar] [CrossRef]
- Eastoe, J.; Paul, A.; Nave, S.; Steytler, D.C.; Robinson, B.H.; Rumsey, E.; Thorpe, M.; Heenan, R.K. Micellization of Hydeocarbon Surfactants in Supercritical Carbon Dioxide. J. Am. Chem. Soc. 2001, 123, 988–989. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.; Holm, L. Method for recovering oil from subterranean formations. U.S. Patent 3,342,256, 19 September 1967. [Google Scholar]
- Fink, R.; Hancu, D.; Valentine, R.; Beckman, J.E. Toward the Development of “CO2-philic” Hydrocarbons. 1. Use of Side-Chain Functionalization to Lower the Miscibility Pressure of Polydimethylsiloxanes in CO2. J. Phys. Chem. B 1999, 103, 6441–6444. [Google Scholar] [CrossRef]
- Hoefling, T.A.; Newman, D.A.; Enick, R.M.; Beckman, E.J. Effect of Strcuture on the Cloud-Point Curves of Silicone-Based Amphiphiles in Supercritical Carbon Dioxide. J. Supercrit. Fluids 1993, 6, 165–171. [Google Scholar] [CrossRef]
- Ryoo, W.; Webber, S.E.; Johnston, K.P. Water-in-Carbon Dioxide Microemulsions with Methylated Branched Hydrocarbon Surfactants. Ind. Eng. Chem. Res. 2003, 42, 6348–6358. [Google Scholar] [CrossRef]
- Sagisaka, M.; Ono, S.; James, C.; Yoshizawa, A.; Mohamed, A.; Guittard, F.; Enick, R.M.; Rogers, S.E.; Czajka, A.; Hill, C.; et al. Anisotropic reversed micelles with fluorocarbon-hydrocarbon hybrid surfactants in supercritical CO2. Colloids Surf. B Biointerfaces 2018, 168, 201–210. [Google Scholar] [CrossRef]
- Elhag, A.S.; Chen, Y.; Chen, H.; Reddy, P.P.; Cui, L.; Worthen, A.J.; Ma, K.; Hirasaki, G.J.; Nguyen, Q.P.; Biswal, S.L.; et al. Switchable Amine Surfactants for Stable CO2/Brine Foams in High Temperature, High Salinity Reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Sagir, M.; Tan, I.M.; Mushtaq, M.; Talebian, S.H. FAWAG Using CO2 Philic Surfactants for CO2 Mobility Control for Enhanced Oil Recovery Applications. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Ren, G.; Sanders, A.W.; Nguyen, Q.P. New method for the determination of surfactant solubility and partitioning between CO2 and brine. J. Supercrit. Fluids 2014, 91, 77–83. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B.; Cheremisin, A. CO2 Capture by Injection of Flue Gas or CO2–N2 Mixtures into Hydrate Reservoirs: Dependence of CO2 Capture Efficiency on Gas Hydrate Reservoir Conditions. Environ. Sci. Technol. 2018, 52, 4324–4330. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Farahani, M.V.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Chen, Y.H.; Fan, L.S.; Kim, H.; Gao, X.; Ling, T.C.; Chiang, T.C.; Pei, S.L.; Gu, G. CO2 mineralization and utilization by alkaline solid wastes for potential carbon reduction. Nat. Sustain. 2020, 3, 399–405. [Google Scholar] [CrossRef]
- Trogus, F.J.; Sophany, T.; Schechter, R.S.; Wade, W.H. Static and Dynamic Adsorption of Anionic and Nonionic Surfactants. Soc. Pet. Eng. J. 1977, 17, 337–344. [Google Scholar] [CrossRef]
- Princen, H.; Goddard, E. The effect of mineral oil on the surface properties of binary surfactant systems. J. Colloid Interface Sci. 1972, 38, 523–534. [Google Scholar] [CrossRef]
- Bourrel, M.; Schechter, R.S. Microemulsions and Related Systems; Marcel Dekker: New York, NY, USA, 1988. [Google Scholar]
- Ren, G.; Mateen, K.; Ma, K.; Luo, H.; Bourdarot, G.; Morel, D.; Nguyen, N.; Nguyen, Q. In-depth Experimental Studies of Low-Tension Gas (LTG) in High Salinity and High Temperature Sandstone Reservoir. In Proceedings of the IOR 2019—20th European Symposium on Improved Oil Recovery; European Association of Geoscientists & Engineers, Pau, France, 8–10 April 2019. [Google Scholar]
- McAuliffe, C.D. Crude-Oil-Water Emulsions to Improve Fluid Flow in an Oil Reservoir. J. Pet. Technol. 1973, 25, 721–726. [Google Scholar] [CrossRef]
- Zanganeh, M.N.; Rossen, W.R. Optimization of Foam Enhanced Oil Recovery: Balancing Sweep and Injectivity. SPE Reserv. Eval. Eng. 2013, 16, 51–59. [Google Scholar] [CrossRef]
- Vassenden, F.; Holt, T. Experimental Foundation for Relative Permeability Modeling of Foam. SPE Reserv. Eval. Eng. 2000, 3, 179–185. [Google Scholar] [CrossRef]
- Chen, M.; Yortsos, Y.; Rossen, W. A Pore-Network Study of the Mechanisms of Foam Generation. In Proceedings of the SPE International Petroleum Conference in Mexico, Puebla Pue, Mexico, 7–9 November 2004. [Google Scholar]
- Ashoori, E.; Van Der Heijden, T.L.; Rossen, W.R. Fractional-Flow Theory of Foam Displacements with Oil. SPE J. 2010, 15, 260–273. [Google Scholar] [CrossRef]
- Patzek, T.W. Description of Foam Flow in Porous Media by the Population Balance Method; American Chemical Society: Washington, DC, USA, 1988; pp. 326–341. [Google Scholar]
- Li, B.; Hirasaki, G.J.; Miller, C.A. Upscaling of Foam Mobility Control to Three Dimensions. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 26–29 April 2006. [Google Scholar]
- Mahani, H.; Keya, A.L.; Berg, S.; Nasralla, R. Electrokinetics of Carbonate/Brine Interface in Low-Salinity Waterflooding: Effect of Brine Salinity, Composition, Rock Type, and pH on?-Potential and a Surface-Complexation Model. SPE J. 2017, 22, 53–68. [Google Scholar] [CrossRef]
- Laurier, L.; Schramm, K.M.; Novosad, J.J. Electrokinetic properties of reservoir rock particles. Colloids Surf. 1991, 55, 309–333. [Google Scholar]
- Jian, G.; Puerto, M.C.; Wehowsky, A.; Dong, P.; Johnston, K.P.; Hirasaki, G.J.; Biswal, S.L. Static Adsorption of an Ethoxylated Nonionic Surfactant on Carbonate Minerals. Langmuir 2016, 32, 10244–10252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Elhag, A.S.; Poon, B.M.; Cui, L.; Ma, K.; Liao, S.Y.; Omar, A.; Worthen, A.J.; Hirasaki, G.J.; Nguyen, Q.P.; et al. Ethoxylated Cationic Surfactants for CO2 EOR in High Temperature, High Salinity Reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. [Google Scholar]
- Mukherjee, J.; Norris, S.O.; Nguyen, Q.P.; Scherlin, J.M.; Vanderwal, P.G.; Abbas, S. CO2 Foam Pilot in Salt Creek Field, Natrona County, WY: Phase I: Laboratory Work, Reservoir Simulation, and Initial Design. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Liu, Y.; Grigg, R.; Bai, B. Salinity, pH, and Surfactant Concentration Effects on CO2-Foam. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 2–4 February 2005. [Google Scholar]
- Chen, Y.; Elhag, A.S.; Cui, L.; Worthen, A.J.; Reddy, P.P.; Noguera, J.A.; Ou, A.M.; Ma, K.; Puerto, M.; Hirasaki, G.J.; et al. CO2-in-Water Foam at Elevated Temperature and Salinity Stabilized with a Nonionic Surfactant with a High Degree of Ethoxylation. Ind. Eng. Chem. Res. 2015, 54, 4252–4263. [Google Scholar] [CrossRef]
- Rossen, W.R. Minimum pressure gradient for foam flow in porous media: Effect of interactions with stationary lamellae. J. Colloid Interface Sci. 1990, 139, 457–468. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, K.; Farajzadeh, R.; Puerto, M.; Biswal, S.L.; Hirasaki, G.J. Effect of Surfactant Partitioning Between Gaseous Phase and Aqueous Phase on CO2 Foam Transport for Enhanced Oil Recovery. Transp. Porous Media 2016, 114, 777–793. [Google Scholar] [CrossRef]
- Bernard, G.G.; Jacobs, W. Effect of Foam on Trapped Gas Saturation and on Permeability of Porous Media to Water. Soc. Pet. Eng. J. 1965, 5, 295–300. [Google Scholar] [CrossRef]
- Holm, L. The Mechanism of Gas and Liquid Flow Through Porous Media in the Presence of Foam. Soc. Pet. Eng. J. 1968, 8, 359–369. [Google Scholar] [CrossRef]
- Adkins, S.S.; Chen, X.; Nguyen, Q.P.; Sanders, A.W.; Johnston, K.P. Effect of branching on the interfacial properties of nonionic hy-drocarbon surfactants at the air-water and carbon dioxide-water interfaces. J. Colloid Interface Sci. 2010, 346, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Grigg, R.B. Improving CO2 Efficiency for Recovering Oil in Heterogeneous Reservoirs; DOE Contract No. DE-FG26-01BC15364; National Petroleum Technology Program (U.S.): Tulsa, OK, USA, 2004.
- Chambers, K.T.; Radke, C.J. Capillary Phenomena in Foam Flow through Porous Media. In Interfacial Phenomena in Petroleum Recovery; Morrow, N.R., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1991. [Google Scholar]
- Kovscek, A.; Radke, C. Fundamentals of foam transport in porous media. In Foams: Fundamentals and Applications in the Petroleum Industry; American Chemical Society: Washington, DC, USA, 1994; pp. 115–163. [Google Scholar]
- Uleberg, K.; Høier, L. Miscible Gas Injection in Fractured Reservoirs. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 13–17 April 2002. [Google Scholar]
- Sanchez, N.L. Management of Water Alternating Gas (WAG) Injection Projects. In Proceedings of the Latin American and Caribbean Petroleum Engineering Conference, Caracas, Venezuela, 21–23 April 1999. [Google Scholar]
- Falls, A.; Hirasaki, G.; Patzek, T.; Gauglitz, D.; Miller, D.; Ratulowski, T. Development of a Mechanistic Foam Simulator: The Population Balance and Generation by Snap-Off. SPE Reserv. Eng. 1988, 3, 884–892. [Google Scholar] [CrossRef]
- Stone, H.L. Vertical, Conformance in An Alternating Water-Miscible Gas Flood. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 26–29 September 1982. [Google Scholar]
- Li, D.; Ren, B.; Zhang, L.; Ezekiel, J.; Ren, S.; Feng, Y. CO2-sensitive foams for mobility control and channeling blocking in enhanced WAG process. Chem. Eng. Res. Des. 2015, 102, 234–243. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xue, F.; Wang, Y.; Ren, B.; Zhang, L.; Ren, S. CO2 foam flooding for im-proved oil recovery: Reservoir simulation models and influencing factors. J. Pet. Sci. Eng. 2015, 133, 838–850. [Google Scholar] [CrossRef]



| EXP | Surfactant | Injection Gas Fraction | Rock Type | Measured Matrix Permeability (md) | Measured Composite Core Permeability (md) | Porosity (Fraction) | Initial So (Fraction) | |
|---|---|---|---|---|---|---|---|---|
| Two-phase without fracture | CO2 Emulsion | ASS | 0.75 | Sandstone | 296 | N/A | 0.212 | N/A |
| CO2 Emulsion | CPS | 0.75 | Sandstone | 312 | N/A | 0.202 | N/A | |
| CO2 Emulsion | ASS | 0.75 | Dolomite | 146 | N/A | 0.172 | N/A | |
| CO2 Emulsion | CPS | 0.75 | Dolomite | 157 | N/A | 0.169 | N/A | |
| Three-phase with fracture | Pure CO2 | N/A | 1 | Dolomite | 132 | 899 | 0.17 | 0.51 |
| W&G | N/A | 0.75 | Dolomite | 167 | 1098 | 0.17 | 0.52 | |
| CO2 Emulsion | ASS | 0.75 | Dolomite | 143 | 967 | 0.165 | 0.46 | |
| CO2 Emulsion | CPS | 0.75 | Dolomite | 160 | 1023 | 0.165 | 0.49 | |
| Fmmob | Fmsurf | Epsurf | Fmdry | Epdry |
|---|---|---|---|---|
| 100 | 0.000033399 | 1 | 0.15 | 1 |
| Injection Strategy | Aqueous/CO2 Cycle Ratio | Used Surfactants | Unit Slug Size (Day) | Aqueous Rate (bbl/d) | CO2 Rate (scf/d) | Surfactant Concentration (Mole Fraction) | Injection Time (Days) |
|---|---|---|---|---|---|---|---|
| Pure CO2 | - | - | - | - | 420,000 | - | 1440 |
| WAG | 1:1 | - | 180 | 45 | 315,000 | - | 2880 |
| SAG | 1:1 | A or C | 180 | 45 | 315,000 | 3.34 × 10−5 | 2880 |
| Novel CIDS | - | C | - | - | 420,000 | 2.63 × 10−5 | 1440 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, G.; Ren, B.; Li, S.; Zhang, C. Unlock the Potentials to Further Improve CO2 Storage and Utilization with Supercritical CO2 Emulsions When Applying CO2-Philic Surfactants. Sustain. Chem. 2021, 2, 127-148. https://doi.org/10.3390/suschem2010009
Ren G, Ren B, Li S, Zhang C. Unlock the Potentials to Further Improve CO2 Storage and Utilization with Supercritical CO2 Emulsions When Applying CO2-Philic Surfactants. Sustainable Chemistry. 2021; 2(1):127-148. https://doi.org/10.3390/suschem2010009
Chicago/Turabian StyleRen, Guangwei, Bo Ren, Songyan Li, and Chao Zhang. 2021. "Unlock the Potentials to Further Improve CO2 Storage and Utilization with Supercritical CO2 Emulsions When Applying CO2-Philic Surfactants" Sustainable Chemistry 2, no. 1: 127-148. https://doi.org/10.3390/suschem2010009
APA StyleRen, G., Ren, B., Li, S., & Zhang, C. (2021). Unlock the Potentials to Further Improve CO2 Storage and Utilization with Supercritical CO2 Emulsions When Applying CO2-Philic Surfactants. Sustainable Chemistry, 2(1), 127-148. https://doi.org/10.3390/suschem2010009






