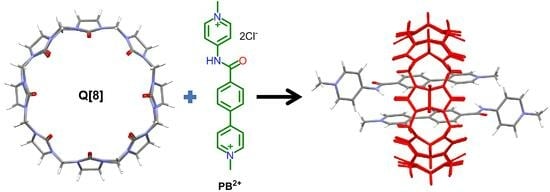
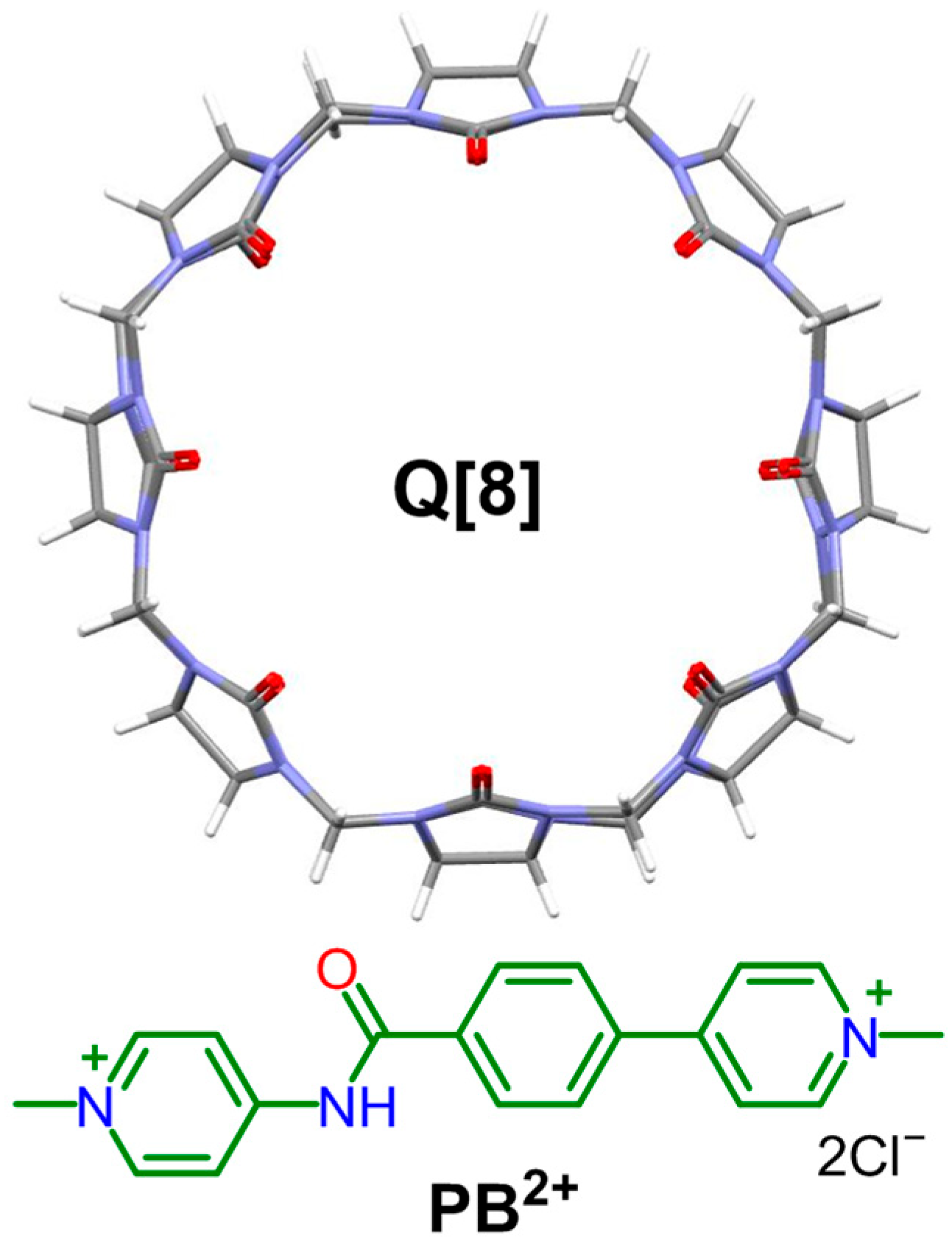
A Study of the Inclusion Complex Formed Between Cucurbit[8]uril and N,4-Di(pyridinyl)benzamide Derivative
Abstract
1. Introduction
2. Materials and Methods
- Synthesis of compound PB2+
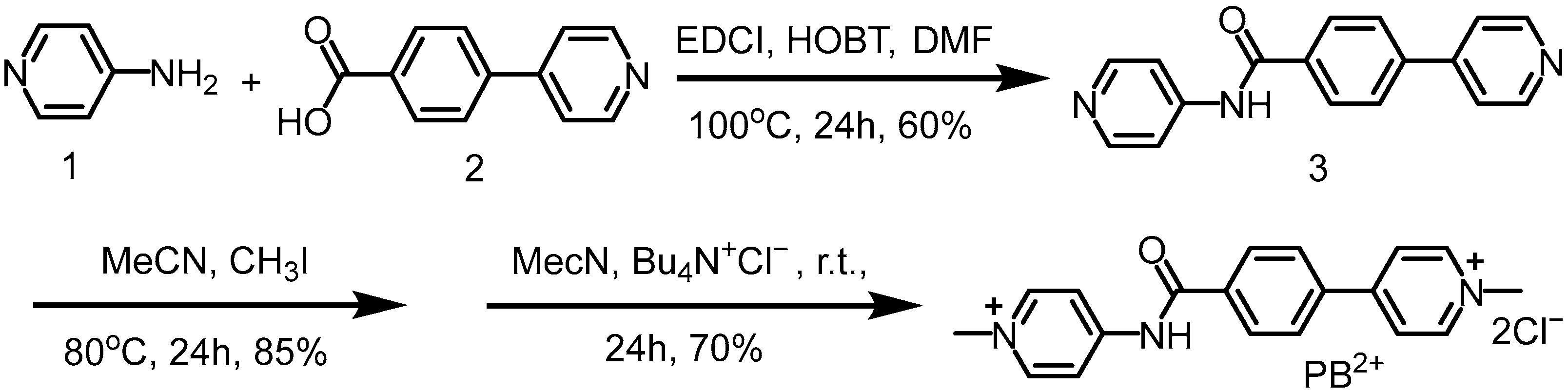 Compound PB2+. Compound 1 (4-Aminopyridine, 0.52 g, 5.1 mmol), 2 (1.0 g, 5.0 mmol), EDCI (N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride) (0.96 g, 5.0 mmol), and HOBT (1-Hydroxybenzotriazole) (0.68 g, 5.0 mmol) were dissolved in DMF (10 mL) and stirred at 60 °C for 24 h. After the reaction was completed, it was allowed to cool to room temperature. The resulting precipitate was filtered, washed with ethanol three times, and dried in vacuum to obtain compound 3 as a white solid.
Compound PB2+. Compound 1 (4-Aminopyridine, 0.52 g, 5.1 mmol), 2 (1.0 g, 5.0 mmol), EDCI (N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride) (0.96 g, 5.0 mmol), and HOBT (1-Hydroxybenzotriazole) (0.68 g, 5.0 mmol) were dissolved in DMF (10 mL) and stirred at 60 °C for 24 h. After the reaction was completed, it was allowed to cool to room temperature. The resulting precipitate was filtered, washed with ethanol three times, and dried in vacuum to obtain compound 3 as a white solid.- Nuclear magnetic resonance measurements.
- UV–vis absorption.
- Isothermal titration calorimetry (ITC) experiments.
- High-resolution electrospray mass spectrometer (HR ESI–MS).
- Quantum chemistry calculations.
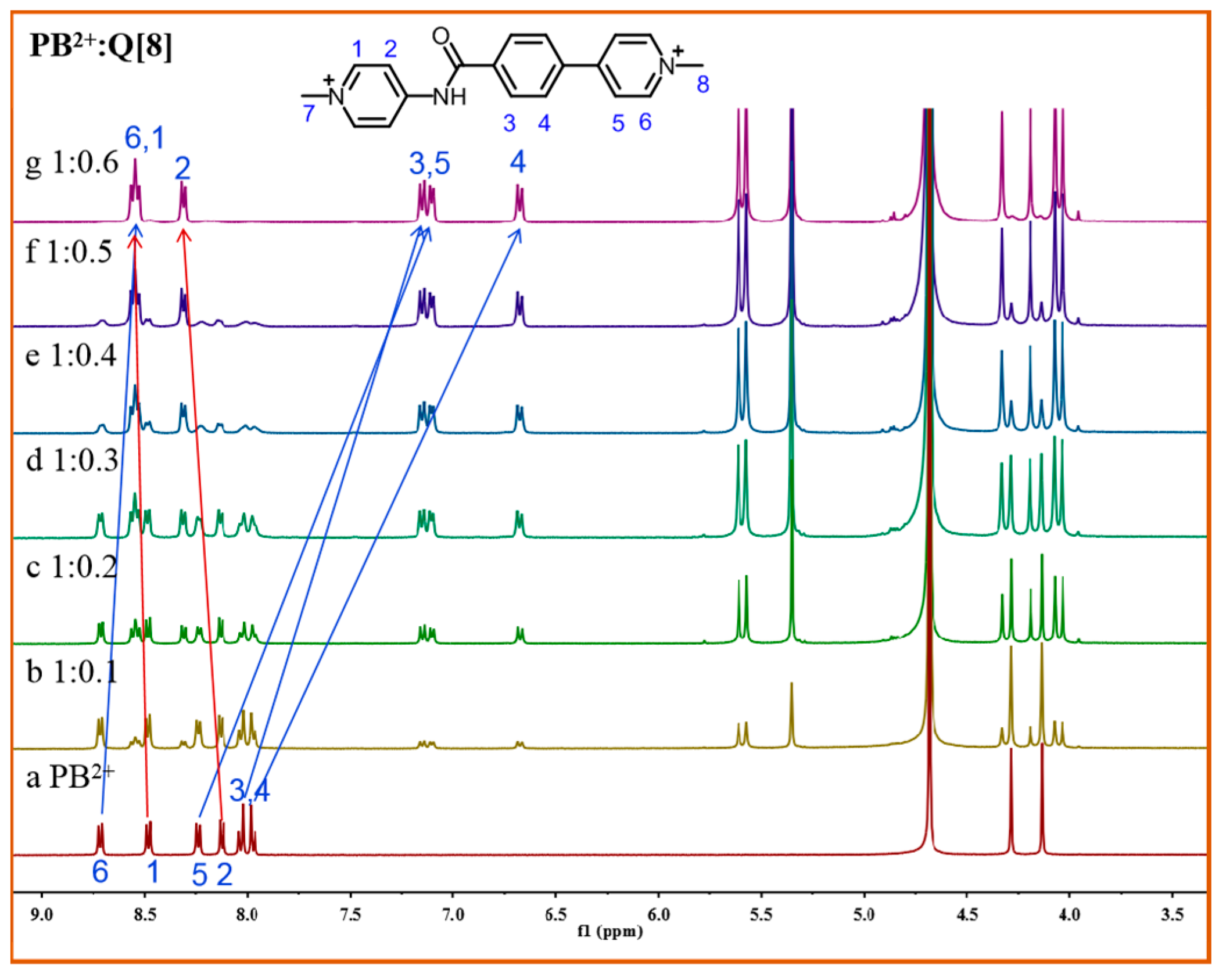
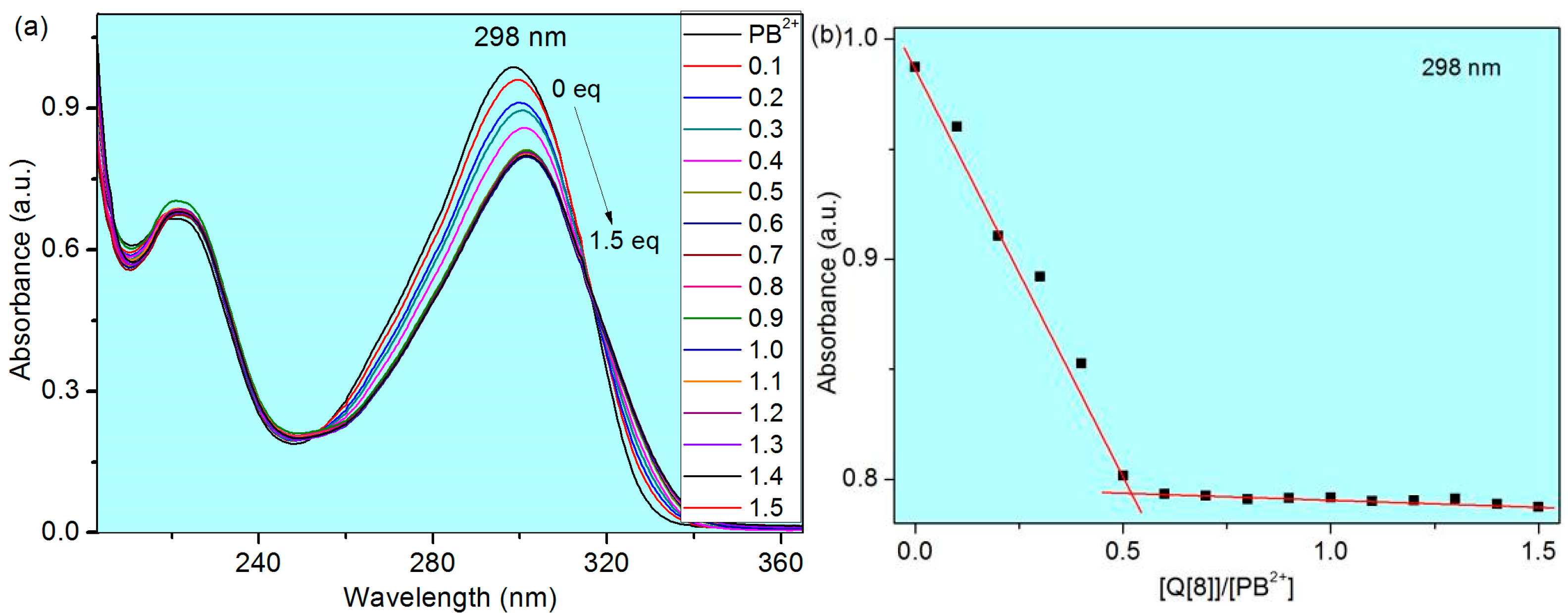
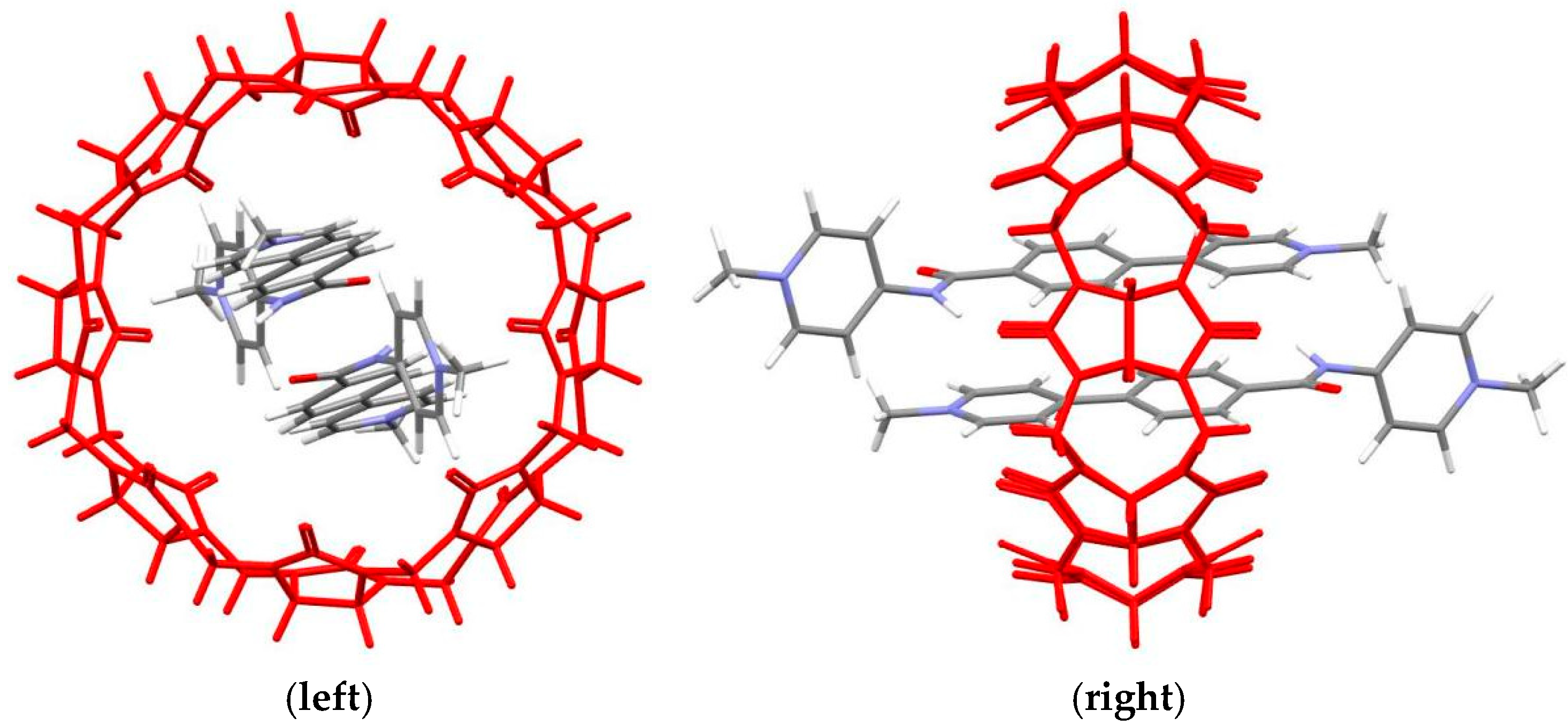
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Yuan, B.; Zhang, X.; Scherman, O.A. Supramolecular Chemistry at Interfaces: Host-Guest Interactions for Fabricating Multifunctional Biointerfaces. Acc. Chem. Res. 2014, 47, 2106–2115. [Google Scholar] [CrossRef]
- Ghale, G.; Nau, W.M. Dynamically Analyte-Responsive Macrocyclic Host-Fluorophore Systems. Acc. Chem. Res. 2014, 47, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Chen, K.; Redshaw, C. Stimuli-responsive mechanically interlocked molecules constructed from cucurbit[n]uril homologues and derivatives. Chem. Soc. Rev. 2023, 52, 1428–1455. [Google Scholar] [CrossRef] [PubMed]
- Shetty, D.; Khedkar, J.K.; Park, K.M.; Kim, K. Can we beat the biotin-avidin pair: Cucurbit[7]uril-based ultrahigh affinity host-guest complexes and their applications. Chem. Soc. Rev. 2015, 44, 8747–8761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, C.; Yang, K.; Chen, X.; Wang, R. Cucurbituril-Based Supramolecular Polymers for Biomedical Applications. Angew. Chem. Int. Edit. 2022, 61, e202206763. [Google Scholar] [CrossRef]
- Nie, H.; Wei, Z.; Ni, X.-L.; Liu, Y. Assembly and Applications of Macrocyclic-Confinement-Derived Supramolecular Organic Luminescent Emissions from Cucurbiturils. Chem. Rev. 2022, 122, 9032–9077. [Google Scholar] [CrossRef]
- Guan, W.-L.; Chen, J.-F.; Liu, J.; Shi, B.; Yao, H.; Zhang, Y.M.; Wei, T.-B.; Lin, Q. Macrocycles-assembled AIE supramolecular polymer networks. Coordin. Chem. Rev. 2024, 507, 215717. [Google Scholar] [CrossRef]
- Ziganshina, A.Y.; Ko, Y.H.; Jeon, W.S.; Kim, K. Stable π-dimer of a tetrathiafulvalene cation radical encapsulated in the cavity of cucurbit[8]uril. Chem. Commun. 2004, 7, 806–807. [Google Scholar] [CrossRef]
- Ni, X.-L.; Chen, S.; Yang, Y.; Tao, Z. Facile Cucurbit[8]uril-Based Supramolecular Approach To Fabricate Tunable Luminescent Materials in Aqueous Solution. J. Am. Chem. Soc. 2016, 138, 6177–6183. [Google Scholar] [CrossRef]
- Rabbani, R.; Masson, E. Probing Interactions between Hydrocarbons and Auxiliary Guests inside Cucurbit[8]uril. Org. Lett. 2017, 19, 4303–4306. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Ma, X.; Tian, H. Visible-Light-Excited Room-Temperature Phosphorescence in Water by Cucurbit[8]uril-Mediated Supramolecular Assembly. Angew. Chem. Int. Edit. 2020, 59, 9928–9933. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.; Chang, S.L.; Clements, N.; Hirani, Z.; Kimberly, L.B.; Odoi-Adams, K.; Suating, P.; Taylor, H.F.; Trauth, S.A.; Urbach, A.R. Molecular recognition of peptides and proteins by cucurbit[n]urils: Systems and applications. Chem. Soc. Rev. 2024, 53, 11519–11556. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-K.; Zhang, W.; Liu, Z.; Zhang, H.; Zhang, B.; Liu, Y. Supramolecular Pins with Ultralong Efficient Phosphorescence. Adv. Mater. 2021, 33, 2007476. [Google Scholar] [CrossRef]
- Yang, B.; Yu, S.-B.; Zhang, P.-Q.; Wang, Z.-K.; Qi, Q.-Y.; Wang, X.-Q.; Xu, X.-H.; Yang, H.-B.; Wu, Z.-Q.; Liu, Y.; et al. Self-Assembly of a Bilayer 2D Supramolecular Organic Framework in Water. Angew. Chem. Int. Edit. 2021, 60, 26268–26275. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xu, Y.; Wang, S.; Pang, Q.; Liu, S. Metal-organic rotaxane frameworks constructed from a cucurbit[8]uril-based ternary complex for the selective detection of antibiotics. Chem. Commun. 2023, 59, 5890–5893. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.-Z.; Han, N.; Liu, H.; Xing, L.-B. Supramolecular organic framework nanosheets for efficient singlet oxygen generation: A multilevel energy transfer approach for photocatalytic Minisci reactions. J. Mater. Chem. A. 2025, 13, 13789–13796. [Google Scholar] [CrossRef]
- Li, Y.; Yan, C.; Li, Q.; Cao, L. Successive construction of cucurbit[8]uril-based covalent organic frameworks from a supramolecular organic framework through photochemical reactions in water. Sci. China Chem. 2022, 65, 1279–1285. [Google Scholar] [CrossRef]
- Yan, C.; Li, Q.; Miao, X.; Zhao, Y.; Li, Y.; Wang, P.; Wang, K.; Duan, H.; Zhang, L.; Cao, L. Chiral Adaptive Induction of an Achiral Cucurbit[8]uril-Based Supramolecular Organic Framework by Dipeptides in Water. Angew. Chem. Int. Edit. 2023, 62, e202308029. [Google Scholar] [CrossRef]
- Chen, D.-T.; Zhang, X.-W.; Xia, Q.-X.; Yang, X.-N.; Wang, C.-H.; Shan, P.-H.; Xiao, X. A novel styrylpyridine derivatives: Supramolecular assembly with cucurbit[8]uril for detection of difenzoquat, white light material and beyond. J. Mol. Liq. 2023, 385, 122414. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Shao, A.; Wu, Z.; He, Z.; Tian, H.; Ma, X. Aqueous up-conversion organic phosphorescence and tunable dual emission in a single-molecular emitter. Chem. Sci. 2025, 16, 6290–6297. [Google Scholar] [CrossRef]
- Geng, Q.-X.; Wang, F.; Cong, H.; Tao, Z.; Wei, G. Recognition of silver cations by a cucurbit[8]uril-induced supramolecular crown ether. Org. Biomol. Chem. 2016, 14, 2556–2562. [Google Scholar] [CrossRef]
- Tabet, A.; Forster, R.A.; Parkins, C.C.; Wu, G.; Scherman, O.A. Modulating stiffness with photo-switchable supramolecular hydrogels. Polym. Chem. 2019, 10, 467–472. [Google Scholar] [CrossRef]
- Gao, Z.-Z.; Lin, R.L.; Bai, D.; Tao, Z.; Liu, J.X.; Xiao, X. Host-guest complexation of cucurbit[8]uril with two enantiomers. Sci. Rep. 2017, 7, 44717. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, X.; Wen, X.; Yu, H.; Li, B.; Wang, M.; Liu, M.; Wu, G. Chiral ring-in-ring complexes with torsion-induced circularly polarized luminescence. Chem. Sci. 2025, 16, 7858–7863. [Google Scholar] [CrossRef]
- Tang, B.; Xu, W.; Xu, J.-F.; Zhang, X. Transforming a Fluorochrome to an Efficient Photocatalyst for Oxidative Hydroxylation: A Supramolecular Dimerization Strategy Based on Host-Enhanced Charge Transfer. Angew. Chem. Int. Edit. 2021, 60, 9384–9388. [Google Scholar] [CrossRef]
- Xu, W.; Du, Y.; Ma, H.; Tang, X.; Xu, J.-F.; Zhang, X. A cucurbit[8]uril-mediated host-guest complex for red-light photocatalysis. Org. Chem. Front. 2024, 11, 6327–6332. [Google Scholar] [CrossRef]
- Zhou, X.; Bai, X.; Shang, F.; Zhang, H.-Y.; Wang, L.-H.; Xu, X.; Liu, Y. Supramolecular assembly activated single-molecule phosphorescence resonance energy transfer for near-infrared targeted cell imaging. Nat. Commun. 2024, 15, 4787. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-F.; Huo, M.; Kong, J.; Liu, Y. Cucurbituril-Confined Tetracation Supramolecular 2D Organic Framework for Dual-Emission TS-FRET. Adv. Opt. Mater. 2024, 12, 2400453. [Google Scholar] [CrossRef]
- Niu, Q.; Ye, Y.; Su, L.; He, X.; Li, Z.; Liu, Y. Ultrastrong room-temperature phosphorescence in cucurbit[8]uril-mediated crystalline supramolecules for ratiometric detection of phenethylamine. Sci. China Chem. 2025, 68, 2671–2679. [Google Scholar] [CrossRef]
- Lin, F.; Yu, S.-B.; Liu, Y.-Y.; Liu, C.-Z.; Lu, S.; Cao, J.; Qi, Q.-Y.; Zhou, W.; Li, X.; Liu, Y.; et al. Porous Polymers as Universal Reversal Agents for Heparin Anticoagulants through an Inclusion-Sequestration Mechanism. Adv. Mater. 2022, 34, 2200549. [Google Scholar] [CrossRef]
- Yu, S.-B.; Lin, F.; Tian, J.; Yu, J.; Zhang, D.-W.; Li, Z.-T. Water-soluble and dispersible porous organic polymers: Preparation, functions and applications. Chem. Soc. Rev. 2022, 51, 434–449. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Pu, Z.-H.; Dai, X.; Bai, Q.-H.; Tao, Z.; Xiao, X. Cucurbit[8]uril-modulated discrete pyrene dimers fluorescent sensor for nitroaromatic compounds and latent fingerprints visualization. Sens. Actuator B-Chem. 2024, 418, 136312. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Q.; Pan, D.; Xu, X.; Bai, Q.-H.; Wang, C.-H.; Zeng, X.; Xiao, X. Supramolecular phosphorescent assemblies based on cucurbit[8]uril and bromophenylpyridine derivatives for dazomet recognition. Spectrochim. Acta A. 2025, 330, 125695. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Guan, M.; Li, Q.; Xu, J.; Cai, M.; Xu, J.; Liu, Q. Study of supramolecular organic frameworks for purification of hydrogen through molecular dynamics simulations. Sep. Purif. Technol. 2024, 335, 126106. [Google Scholar] [CrossRef]
- Zhang, D.-S.; Zhang, W.-Z.; Ding, X.-Y.; Xiao, J.; Wang, W.; Zhang, Y.-K.; Zhang, R.-H.; Hu, H.; Zhang, Y.-Z.; Geng, L.; et al. A novel flake-shaped supramolecular building block based metal-organic framework: Structure analysis and selective dye adsorption properties. Polyhedron 2023, 244, 116595. [Google Scholar] [CrossRef]
- Gao, Z.-Z.; Xu, Y.-Y.; Wang, Z.-K.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Porous [Ru(bpy)3]2+-Cored Metallosupramolecular Polymers: Preparation and Recyclable Photocatalysis for the Formation of Amides and 2 Diazo-2-phenylacetates. ACS Appl. Polym. Mater. 2020, 2, 4885–4892. [Google Scholar] [CrossRef]
- Gao, Z.-Z.; Shen, L.; Hu, Y.-L.; Sun, J.-F.; Wei, G.; Zhao, H. Supramolecular Crystal Networks Constructed from Cucurbit[8]uril with Two Naphthyl Groups. Molecules 2023, 28, 63. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, F.-F.; Sun, J.-F.; Gao, Z.-Z. Cucurbit[8]uril-controlled[2+2]photodimerization of styrylpyridinium molecule. Inorg. Chem. Commun. 2022, 141, 109536. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.-K.; Gao, Z.-Z.; Zong, Y.; Sun, J.-D.; Zhou, W.; Wang, H.; Ma, D.; Li, Z.-T.; Zhang, D.W. Porous organic polymer overcomes the post-treatment phototoxicity of photodynamic agents and maintains their antitumor efficiency. Acta Biomater. 2022, 150, 254–264. [Google Scholar] [CrossRef]
- Gao, Z.-Z.; Wang, Z.-K.; Wei, L.; Yin, G.; Tian, J.; Liu, C.-Z.; Wang, H.; Zhang, D.-W.; Zhang, Y.-B.; Li, X.; et al. Water-Soluble 3D Covalent Organic Framework that Displays an Enhanced Enrichment Effect of Photosensitizers and Catalysts for the Reduction of Protons to H2. ACS Appl. Mater. Interfaces 2020, 12, 1404–1411. [Google Scholar] [CrossRef]
- Day, A.I.; Arnold, A.P.; Blanch, R.J.; Snushall, B.J. Controlling Factors in the Synthesis of Cucurbituril and Its Homologues. J. Org. Chem. 2001, 66, 8094–8100. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, I.-S.; Kim, S.-Y.; Lee, E.; Kang, J.-K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New Cucurbituril Homologues: Syntheses, Isolation, Characterization, and X-ray Crystal Structures of Cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Tian, J.; Xu, Z.-Y.; Zhang, D.-W.; Wang, H.; Xie, S.-H.; Xu, D.-W.; Ren, Y.-H.; Wang, H.; Liu, Y.; Li, Z.-T. Supramolecular metalorganic frameworks that display high homogeneous and heterogeneous photocatalytic activity for H2 production. Nat. Commun. 2016, 7, 11580. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Wu, Z.-Y.; Wang, Z.-K.; Wang, H.; Zhang, D.-W.; Liu, Y.; Li, Z.-T. A woven supramolecular metal-organic framework comprising a ruthenium bis(terpyridine) complex and cucurbit[8]uril: Enhanced catalytic activity toward alcohol oxidation. ChemPlusChem 2020, 85, 1498–1503. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yang, M.; Yang, W.; Gao, Z.; Zhao, H.; Wei, G.; Sun, J. A Study of the Inclusion Complex Formed Between Cucurbit[8]uril and N,4-Di(pyridinyl)benzamide Derivative. Organics 2025, 6, 26. https://doi.org/10.3390/org6020026
Wang Z, Yang M, Yang W, Gao Z, Zhao H, Wei G, Sun J. A Study of the Inclusion Complex Formed Between Cucurbit[8]uril and N,4-Di(pyridinyl)benzamide Derivative. Organics. 2025; 6(2):26. https://doi.org/10.3390/org6020026
Chicago/Turabian StyleWang, Zhikang, Mingjie Yang, Weibo Yang, Zhongzheng Gao, Hui Zhao, Gang Wei, and Jifu Sun. 2025. "A Study of the Inclusion Complex Formed Between Cucurbit[8]uril and N,4-Di(pyridinyl)benzamide Derivative" Organics 6, no. 2: 26. https://doi.org/10.3390/org6020026
APA StyleWang, Z., Yang, M., Yang, W., Gao, Z., Zhao, H., Wei, G., & Sun, J. (2025). A Study of the Inclusion Complex Formed Between Cucurbit[8]uril and N,4-Di(pyridinyl)benzamide Derivative. Organics, 6(2), 26. https://doi.org/10.3390/org6020026