Tannins from Acacia mearnsii De Wild as a Sustainable Alternative for the Development of Latent Fingerprints
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Acquisition
2.2. Ash Production
2.3. Composite Preparation
2.4. Characterization
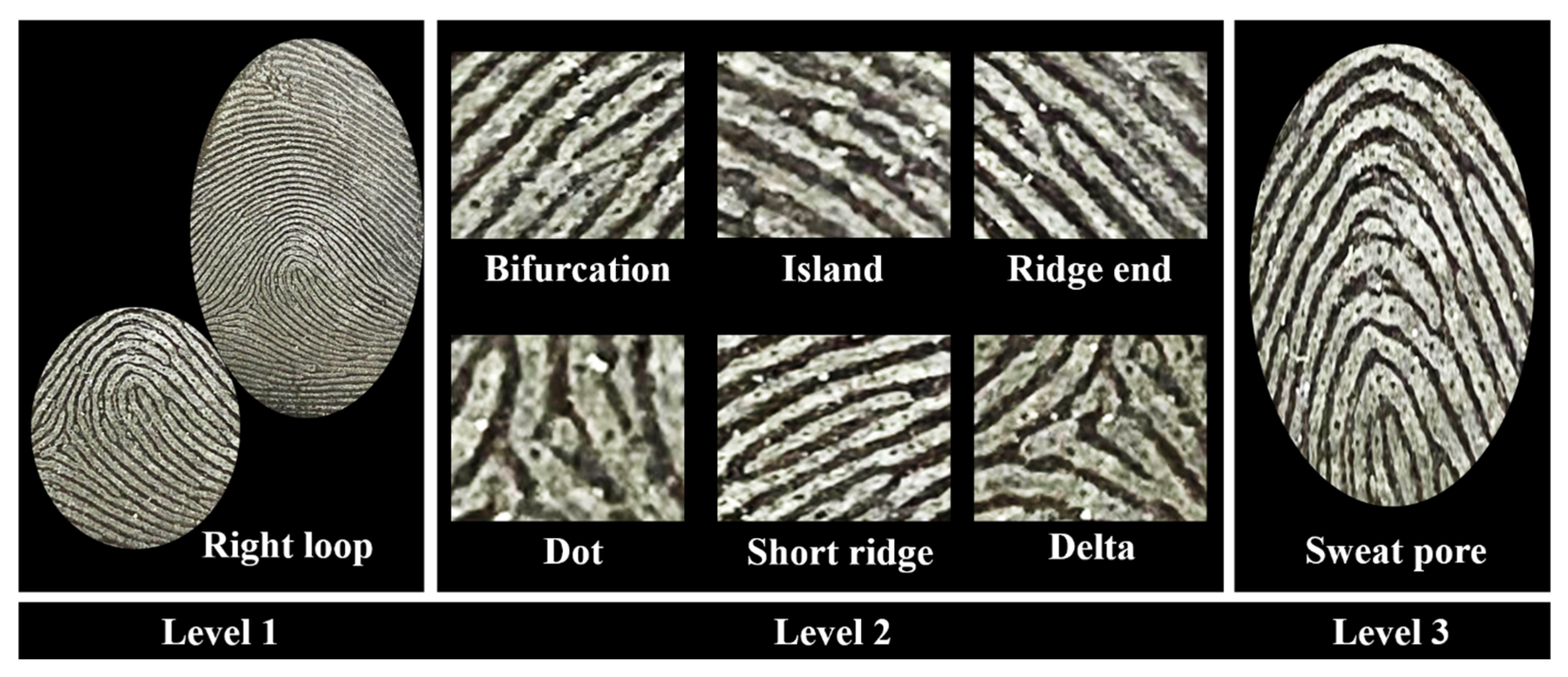
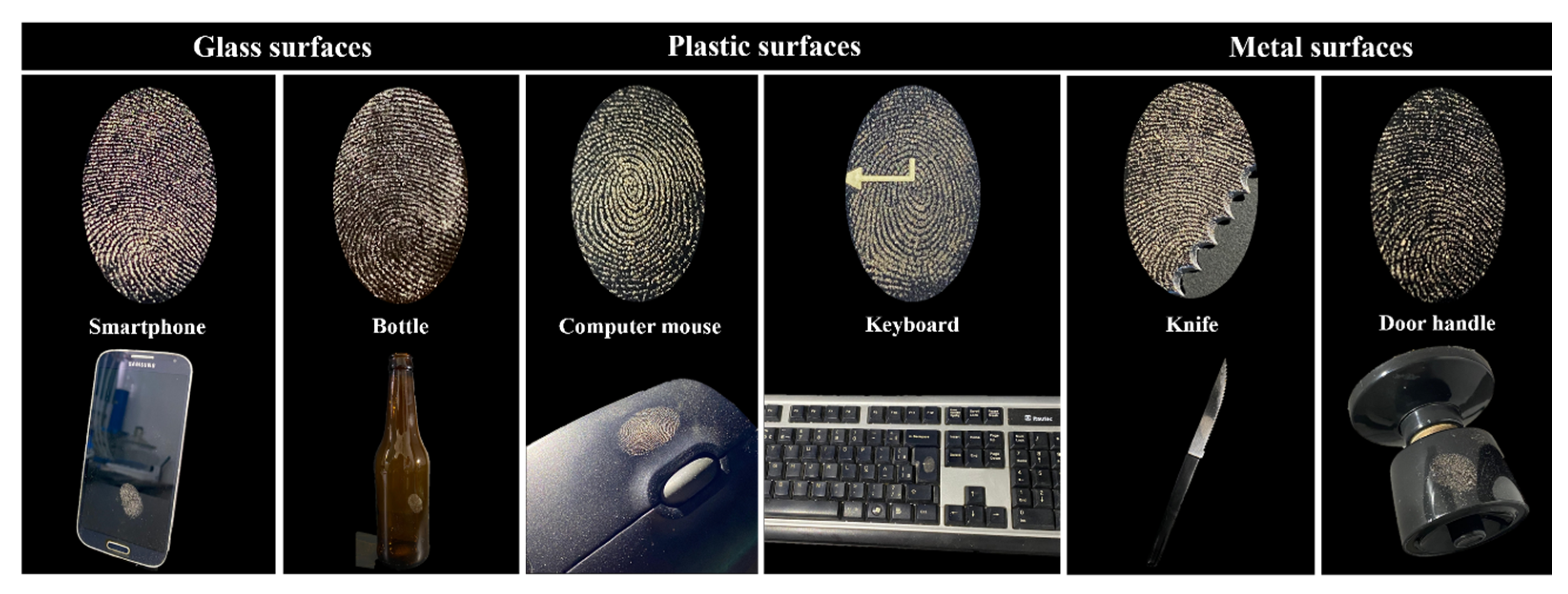
2.5. Papilloscopy
3. Results and Discussion
3.1. Chemical Characterization
3.2. Papilloscopic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LFPs | Latent Fingerprints |
| IBGE | Brazilian Institute of Geography and Statistics |
| UV–Vis | Ultraviolet–Visible Spectroscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| FAAS | Flame Atomic Absorption Spectrometer |
| FAES | Flame Atomic Emission Spectrometer |
References
- Silveira, C.I.; Pereira, S.A.; de Leon, V.B.; Camacho, J.T.; Leitzke, A.F.; Freitag, R.A.; Pereira, C.M.P.; Rosa, B.N.D. Reutilização Do Ácido Acetilsalicílico Preparado Nas Aulas De Química Orgânica Experimental Como Revelador De Impressões Digitais: Uma Aplicação No Ensino De Química. Química Nova 2024, 48, e-20250062. [Google Scholar] [CrossRef]
- Bueno, D.T.; Leitzke, A.F.; Crizel, R.L.; Jansen-Alves, C.; Bertizzolo, E.G.; da Silva, J.P.; Sejanes, G.Q.; Mariotti, K.C.; de Pereira, C.M.P. Characterization of Bixin by UV-Visible Spectroscopy and HPLC, and Its Application as Latent Fingermark Developer. Analytica 2024, 5, 107–118. [Google Scholar] [CrossRef]
- Christofdis, G.; Morrissey, J.; Birkett, J.W. Detection of fngermarks—Applicability to metallic surfaces: A literature review. J. Forensic Sci. 2018, 63, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Leitzke, A.F.; Bueno, D.T.; Poletti, T.; Maron, G.K.; Lopes, B.V.; Morais, E.V.; Inacio, A.P.O.L.; Silveira, C.I.; Silva, J.P.; Dias, D.; et al. The effectiveness of natural indigo/kaolinite composite powder in the development of latent fingermarks. Egypt. J. Forensic Sci. 2024, 14, 19. [Google Scholar] [CrossRef]
- da Rosa, B.N.; da Rosa, M.P.; Poletti, T.; de Lima, N.P.K.; Maron, G.K.; Lopes, B.V.; Mariotti, K.C.; Beck, P.H.; Carreño, N.L.V.; Pereira, C.M.P. Green composites from thiophene chalcones and rice husk lignin: An alternative of powder for latent fingermark. Surfaces 2022, 5, 481–488. [Google Scholar] [CrossRef]
- Leitzke, A.F.; Berneira, L.M.; Rosa, B.N.D.; Moreira, B.C.; Mariotti, K.D.C.; Venzke, D.; Pereira, C.M. A Química de Produtos Naturais aplicados a reveladores de impressões digitais latentes. Química Nova 2022, 45, 424–434. [Google Scholar] [CrossRef]
- Vadivel, R.; Nirmala, M.; Anbukumaran, K. Commonly available, everyday materials as non-conventional powders for the visualization of latent fingerprints. Forensic Chem. 2021, 24, 100339. [Google Scholar] [CrossRef]
- da Rosa, B.N.; Maron, G.K.; Lopes, B.V.; Rocha, A.C.S.; de Moura Gatti, F.; Machado, J.O.A.; Barichello, J.M.; Mariotti, K.C.; Trossini, G.H.G.; Carreño, N.L.V.; et al. Dimethylaminochalcones with silicon dioxide and zinc oxide as latent fingermark developer powder. Mater. Chem. Phys. 2023, 295, 127033. [Google Scholar] [CrossRef]
- Nicolodi, C.; Da Rosa, B.N.; Da Silva, C.C.; Berneira, L.M.; Pacheco, B.S.; Poletti, T.; Venzke, D.; Mariotti, K.C.; Pereira, C.M.P. Aplicação de condimentos na revelação de impressões digitais latentes: Um experimento no ensino de química. Quim. Nova 2019, 42, 962–970. [Google Scholar] [CrossRef]
- da Rosa, B.N.; Venzke, D.; Poletti, T.; de Lima, N.P.K.; Camacho, J.T.; Mariotti, K.C.; dos Santos, M.A.Z.; Pizzuti, L.; Carreño, N.L.V.; Pereira, C.M.P. Microwave assisted synthesis of thiocarbamoylpyrazoles and application as an alternative latent fingermark developer. J. Braz. Chem. Soc. 2020, 31, 1327–1331. [Google Scholar] [CrossRef]
- da Rosa, B.N.; Mariotti, K.C.; Pacheco, B.S.; Silva, C.C.; Carreño, N.L.V.; Nicolodi, C.; Poletti, T.; Silva, A.C.A.; Silva, A.F.; Giongo, J.L.; et al. Fluorescent phenylthiazoles: Application as latent fingermark and their cytotoxicity against NOK-SI cell line. Chem. Data Collect. 2021, 33, 100700. [Google Scholar] [CrossRef]
- Venzke, D.; Poletti, T.; Rosa, B.N.; Berneira, L.M.; Lima, N.P.K.; Oliveira, T.F.; Carreño, N.L.V.; Mariotti, K.C.; Duarte, L.S.; Nobre, S.M.; et al. Preparation of fluorescent bisamides: A new class of fingermarks developers. Chem. Data Collect. 2021, 33, 100680. [Google Scholar] [CrossRef]
- Pacheco, B.S.; Da Silva, C.C.; Da Rosa, B.N.; Mariotti, K.C.; Nicolodi, C.; Poletti, T.; Segatto, N.V.; Collares, T.; Seixas, F.K.; Paniz, O.; et al. Monofunctional curcumin analogues: Evaluation of green and safe developers of latent fingerprints. Chem. Pap. 2021, 75, 3119–3129. [Google Scholar] [CrossRef]
- Passos, L.F.; Berneira, L.M.; Poletti, T.; Mariotti, K.C.; Carreño, N.L.V.; Hartwig, C.A.; Pereira, C.M.P. Evaluation and characterization of algal biomass applied to the development of fingermarks on glass surfaces. Aust. J. Forensic Sci. 2021, 53, 337–346. [Google Scholar] [CrossRef]
- Lima, N.P.; Rosa, B.N.; Poletti, T.; Moreira, B.C.; Leitzke, A.F.; Mariotti, K.C.; Carreño, N.L.V.; Pereira, C.M. As clássicas hidrazonas como reveladores de impressões digitais: Uma proposta de química orgânica experimental. Química Nova 2023, 46, 215–221. [Google Scholar] [CrossRef]
- Poletti, T.; Berneira, L.M.; Bueno, D.T.; da Silva, C.C.; da Silva, R.; Pereira, C.M. Chemical evaluation and application of cinnamaldehyde-derived curcumins as potential fingerprint development agents. Talanta Open 2022, 6, 100133. [Google Scholar] [CrossRef]
- Bueno, D.T.; Leitzke, A.F.; da Silva, J.P.; Bonemann, D.H.; Sejanes, G.Q.; da Rosa, B.N.; Poletti, T.; Maron, G.K.; Lopes, B.V.; Goularte, M.P.; et al. Application of Κ-Carrageenan for One-Pot Synthesis of Hybrids of Natural Curcumin with Iron and Copper: Stability Analysis and Application in Papiloscopy. Colorants 2025, 4, 3. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant polyphenols: Structure, occurrence and bioactivity. Stud. Nat. Prod. Chem. 2003, 28, 257–312. [Google Scholar]
- Sun, X.; Zhang, J.; You, Y. Enhancement of Cu (II) removal by carbon disulfide modified black wattle tannin gel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125594. [Google Scholar] [CrossRef]
- Hameed, Y.T.; Idris, A.; Hussain, S.A.; Abdullah, N. A tannin-based agent for coagulation and flocculation of municipal wastewater: Chemical composition, performance assessment compared to Polyaluminum chloride, and application in a pilot plant. J. Environ. Manag. 2016, 184, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Oliveira, A.L.; de Freitas, A.D.S.; Rodrigues, J.S.; do Carmo, K.P.; Ferreira, M.; Botaro, V.R. Enhancement of UV-C irradiation protection of polystyrene matrix by tannin (Acacia mearnsii De Wild) additivation: Accelerated aging test. J. Mater. Res. 2023, 38, 1593–1608. [Google Scholar] [CrossRef]
- Prietsch, A.V.G.; Weimer, P.; Spies, L.M.; Haubert, R.; Nunes, A.J.S.; Kreutz, O.C.; Morisso, F.D.P.; Maluf, R.W.; Moura, A.B.D.; Ziulkoski, A.L.; et al. Isolation of Valuable Bioactive Compounds from Aerial Parts of Black Wattle, a Forest Solid Waste. Waste Biomass Valorization 2021, 12, 4953–4964. [Google Scholar] [CrossRef]
- Venter, P.B.; Senekal, N.D.; Kemp, G.; Amra-Jordaan, M.; Khan, P.; Bonnet, S.L.; Van Der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 3: The chemical composition of wattle (Acacia mearnsii) bark extract. Phytochemistry 2012, 83, 153–167. [Google Scholar] [CrossRef]
- Cesprini, E.; De Iseppi, A.; Giovando, S.; Tarabra, E.; Zanetti, M.; Šket, P.; Marangon, M.; Tondi, G. Chemical characterization of cherry (Prunus avium) extract in comparison with commercial mimosa and chestnut tannins. Wood Sci. Technol. 2022, 56, 1455–1473. [Google Scholar] [CrossRef]
- Rodrigues, T.L.; Pedroso, P.D.C.; de Freitas, J.H.C.; Carvalho, A.C.P.; Flores, W.H.; Morais, M.M.; da Rosa, G.S.; de Almeida, A.R.F. Obtaining of a rich-cellulose material from black wattle (Acacia mearnsii De Wild.) bark residues. Environ. Sci. Pollut. Res. 2023, 30, 113055–113067. [Google Scholar] [CrossRef] [PubMed]
- IAL—Instituto Adolfo Lutz. Métodos Físico-Químicos Para Análise de Alimentos, 4th ed.; IAL: São Paulo, Brazil, 2008; 1020p. [Google Scholar]
- Sears, V.G.; Bleay, S.M.; Bandey, H.L.; Bowman, V.J. A methodology for finger mark research. Sci. Justice 2012, 52, 145–160. [Google Scholar] [CrossRef]
- da Cruz, J.A.; da Silva, A.B.; Ramin, B.B.S.; Souza, P.R.; Popat, K.C.; Zola, R.S.; Kipper, M.J.; Martins, A.F. Poly (vinyl alcohol)/cationic tannin blend films with antioxidant and antimicrobial activities. Mater. Sci. Eng. C 2020, 107, 110357. [Google Scholar] [CrossRef]
- Facchi, D.P.; Lima, A.C.; de Oliveira, J.H.; Lazarin-Bidóia, D.; Nakamura, C.V.; Canesin, E.A.; Bonafé, E.G.; Monteiro, J.P.; Visentainer, J.V.; Muniz, E.C.; et al. Polyelectrolyte complexes based on alginate/tanfloc: Optimization, characterization and medical application. Int. J. Biol. Macromol. 2017, 103, 129–138. [Google Scholar] [CrossRef]
- Lütke, S.F.; Igansi, A.V.; Pegoraro, L.; Dotto, G.L.; Pinto, L.A.; Cadaval Jr, T.R. Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. J. Environ. Chem. Eng. 2019, 7, 103396. [Google Scholar] [CrossRef]
- Cataldo, F.; Ursini, O.; Angelini, G. A Green Synthesis of Colloidal Silver Nanoparticles and Their Reaction with Ozone. Chem. Bull. 2013, 2, 700–705. [Google Scholar]
- Li, G.W.; Hong, L.; Tong, M.S.; Deng, H.H.; Xia, X.H.; Chen, W. Determination of tannic acid based on luminol chemiluminescence catalyzed by cupric oxide nanoparticles. Anal. Methods 2015, 7, 1924–1928. [Google Scholar] [CrossRef]
- Costadinnova, L.; Hristova, M.; Kolusheva, T.; Stoilova, N. Conductometric Study of The Acidity Properties of Tannic Acid (Chinese Tannin). J. Univ. Chem. Technol. Metall. 2012, 47, 289–296. [Google Scholar]
- Rodrigues, L.A.; Parmentier, J.; Parra, J.B.; Thim, G.P. Preparation of nodular carbon cryogel from simple and inexpensive polycondensation reaction of commercial modified black wattle tannin. J. Sol-Gel Sci. Technol. 2013, 6, 519–526. [Google Scholar] [CrossRef]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Masson, E.; Celzard, A. The use of tannin to prepare carbon gels. Part I: Carbon aerogels. Carbon 2011, 49, 2773–2784. [Google Scholar] [CrossRef]
- Caldeira, M.V.W.; Neto, R.M.R.; Schumacher, M.V. Content and exportation of micronutrients in black wattle (Acacia mearnsii de wild.) of Australian batemans bay provenance. Rev. Árvore 2003, 27, 9–14. [Google Scholar] [CrossRef]
- Krug, F.J.; Rocha, F.R.P. Métodos de Preparo de Amostras para Análise Elementar; EditSBQ: São Paulo, Brazil, 2016; 572p. [Google Scholar]
- De Alcaraz-Fossoul, J.; Mestres Patris, C.; Balaciart Muntaner, A.; Barrot Feixat, C.; Gené Badia, M. Determination of latent fingerprint degradation patterns—A real fieldwork study. Int. J. Leg. Med. 2013, 127, 857–870. [Google Scholar] [CrossRef]
- Girod, A.; Ramotowski, R.; Weyermann, C. Composition of fingermark residue: A qualitative and quantitative review. Forensic Sci. Int. 2012, 223, 10–24. [Google Scholar] [CrossRef]
- Gomes, F.M.; de Pereira, C.M.P.; Mariotti, K.C.; Pereira, T.M.; dos Santos, N.A.; Romão, W. Study of latent fingerprints. Forensic Chem. 2023, 35, 100525. [Google Scholar] [CrossRef]
- Poletti, T.; Berneira, L.M.; Passos, L.F.; da Rosa, B.N.; de Pereira, C.M.; Mariotti, K.D.C. Preliminary efficiency evaluation of development methods applied to aged sebaceous latent fingermarks. Sci. Justice 2021, 61, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Low, W.Z.; Khoo, B.E.; Aziz, Z.B.A.; Low, L.W.; Teng, T.T.; Bin Abdullah, A.F.L. Application of acid-modified Imperata cylindrica powder for latent fingerprint development. Sci. Justice 2015, 55, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Cadd, S.; Islam, M.; Manson, P.; Bleay, S. Fingerprint composition and aging: A literature review. Sci. Justice 2015, 55, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Sebastiany, A.P.; Pizzato, M.C.; Del Pino, J.C.; Salgado, T.D.M. A utilização da ciência forense e da investigação criminal como estratégia didática na compreensão de conceitos científicos. Educ. Química 2013, 24, 49–56. [Google Scholar] [CrossRef]
- Sodhi, G.S.; Kaur, J. Powder method for detecting latent fingerprints: A review. Forensic Sci. Int. 2001, 120, 172–176. [Google Scholar] [CrossRef]
- Singh, H.; Kour, S.; Selvaraj, M. Magnetically separable template assisted iron nanoparticle for the enhancement of latent fingerprints. J. Indian. Chem. Soc. 2022, 99, 100661. [Google Scholar] [CrossRef]
- Atri, S.; Bumbrah, G.S.; Verma, G.; Joshi, B. Nanostructured assessment of copper oxide for latent fingerprint recognition. J. Radiat. Res. Appl. Sci. 2024, 17, 101018. [Google Scholar] [CrossRef]
- Fieldhouse, S. Consistency and reproducibility in fingermark deposition. Forensic Sci. Int. 2011, 207, 96–100. [Google Scholar] [CrossRef]
- Croxton, R.S.; Baron, M.G.; Butler, D.; Kent, T.; Sears, V.G. Variation in amino acid and lipid composition of latent fingerprints. Forensic Sci. Int. 2010, 199, 93–102. [Google Scholar] [CrossRef]




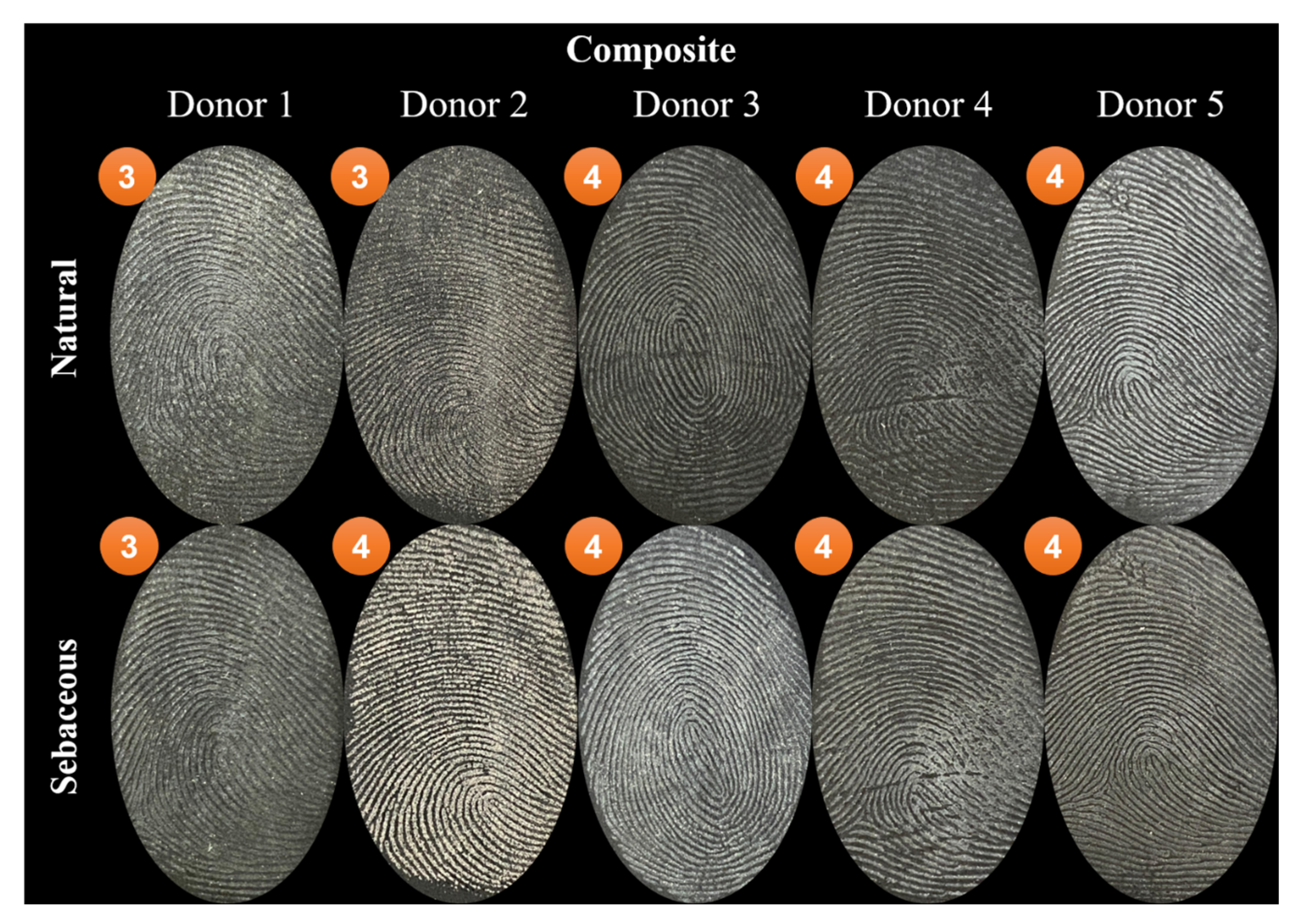

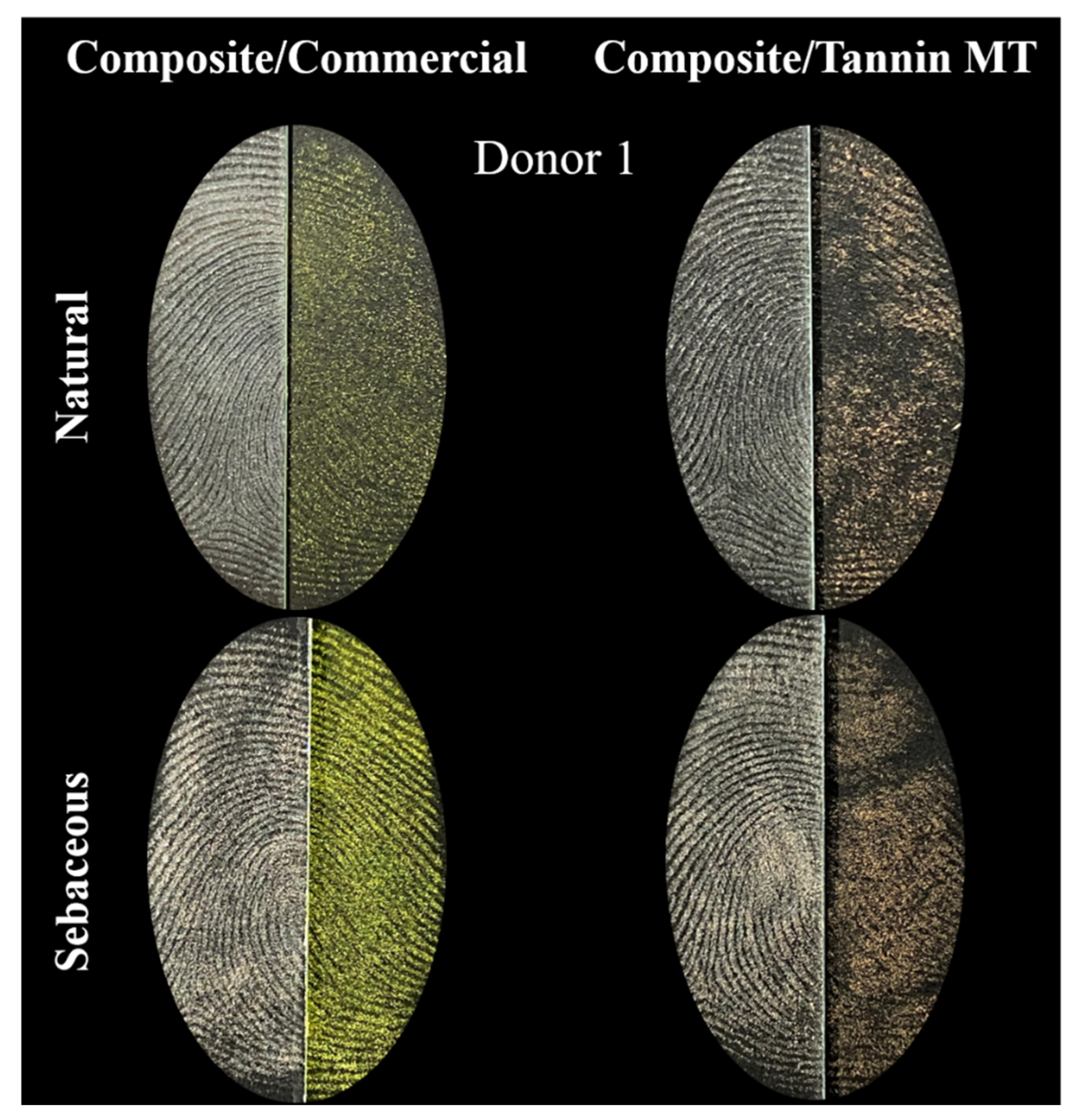
| Score | Level of Detail |
|---|---|
| 0 | No evidence of a mark |
| 1 | Weak development, evidence of contact but no ridge details |
| 2 | Limited development, about 1/3 of ridge details are presented but probably cannot be used for identification purposes |
| 3 | Strong development, between 1/3 and 2/3 of ridge details, identifiable fingermark |
| 4 | Very strong development, full ridge details, identifiable fingermark |
| Elements (%) | MT | MTH | SH | SG |
|---|---|---|---|---|
| Cl | 87.391 | 80.339 | 81.419 | 86.129 |
| K | 8.794 | 8.376 | 4.388 | 5.586 |
| Ca | 2.596 | 2.440 | 1.353 | 1.254 |
| Na | - | - | 5.151 | 6.380 |
| Al | - | 2.249 | 2.198 | - |
| S | 0.845 | 6.045 | 5.213 | 0.404 |
| Fe | 0.162 | 0.168 | 0.064 | 0.084 |
| P | - | 0.167 | - | - |
| Ag | 0.094 | 0.098 | 0.112 | 0.070 |
| Mn | 0.069 | 0.072 | 0.039 | - |
| Cu | 0.049 | 0.045 | 0.042 | 0.025 |
| Br | - | - | 0.020 | - |
| Cr | - | - | - | 0.069 |
| Elements | Concentration (mg kg−1) |
|---|---|
| Cr | 32.5 ± 0.9 |
| Cu | 46.3 ± 0.3 |
| Fe | 2329 ± 207 |
| K | 2957 ± 127 |
| Mn | 10.8 ± 0.2 |
| Na | 10,636 ± 1010 |
| Ni | 22.8 ± 1.16 |
| Zn | 97.4 ± 8.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno, D.T.; Leitzke, A.F.; Martins, R.B.; Bonemann, D.H.; Bertizzolo, E.G.; Sejanes, G.Q.; da Silva, J.P.; Gonçalves, L.M.; Carreno, N.L.V.; Pereira, C.M.P.d. Tannins from Acacia mearnsii De Wild as a Sustainable Alternative for the Development of Latent Fingerprints. Organics 2025, 6, 27. https://doi.org/10.3390/org6020027
Bueno DT, Leitzke AF, Martins RB, Bonemann DH, Bertizzolo EG, Sejanes GQ, da Silva JP, Gonçalves LM, Carreno NLV, Pereira CMPd. Tannins from Acacia mearnsii De Wild as a Sustainable Alternative for the Development of Latent Fingerprints. Organics. 2025; 6(2):27. https://doi.org/10.3390/org6020027
Chicago/Turabian StyleBueno, Danielle Tapia, Amanda Fonseca Leitzke, Rayane Braga Martins, Daisa Hakbart Bonemann, Emanuel Gomes Bertizzolo, Gabrielly Quartieri Sejanes, Juliana Porciúncula da Silva, Lucas Minghini Gonçalves, Neftali Lenin Villarreal Carreno, and Claudio Martin Pereira de Pereira. 2025. "Tannins from Acacia mearnsii De Wild as a Sustainable Alternative for the Development of Latent Fingerprints" Organics 6, no. 2: 27. https://doi.org/10.3390/org6020027
APA StyleBueno, D. T., Leitzke, A. F., Martins, R. B., Bonemann, D. H., Bertizzolo, E. G., Sejanes, G. Q., da Silva, J. P., Gonçalves, L. M., Carreno, N. L. V., & Pereira, C. M. P. d. (2025). Tannins from Acacia mearnsii De Wild as a Sustainable Alternative for the Development of Latent Fingerprints. Organics, 6(2), 27. https://doi.org/10.3390/org6020027






