Aniline and Beyond: A Multifaceted Case Study for a Bildung-Focused Chemical Education
Abstract
1. Introduction
2. Macroscopic Level
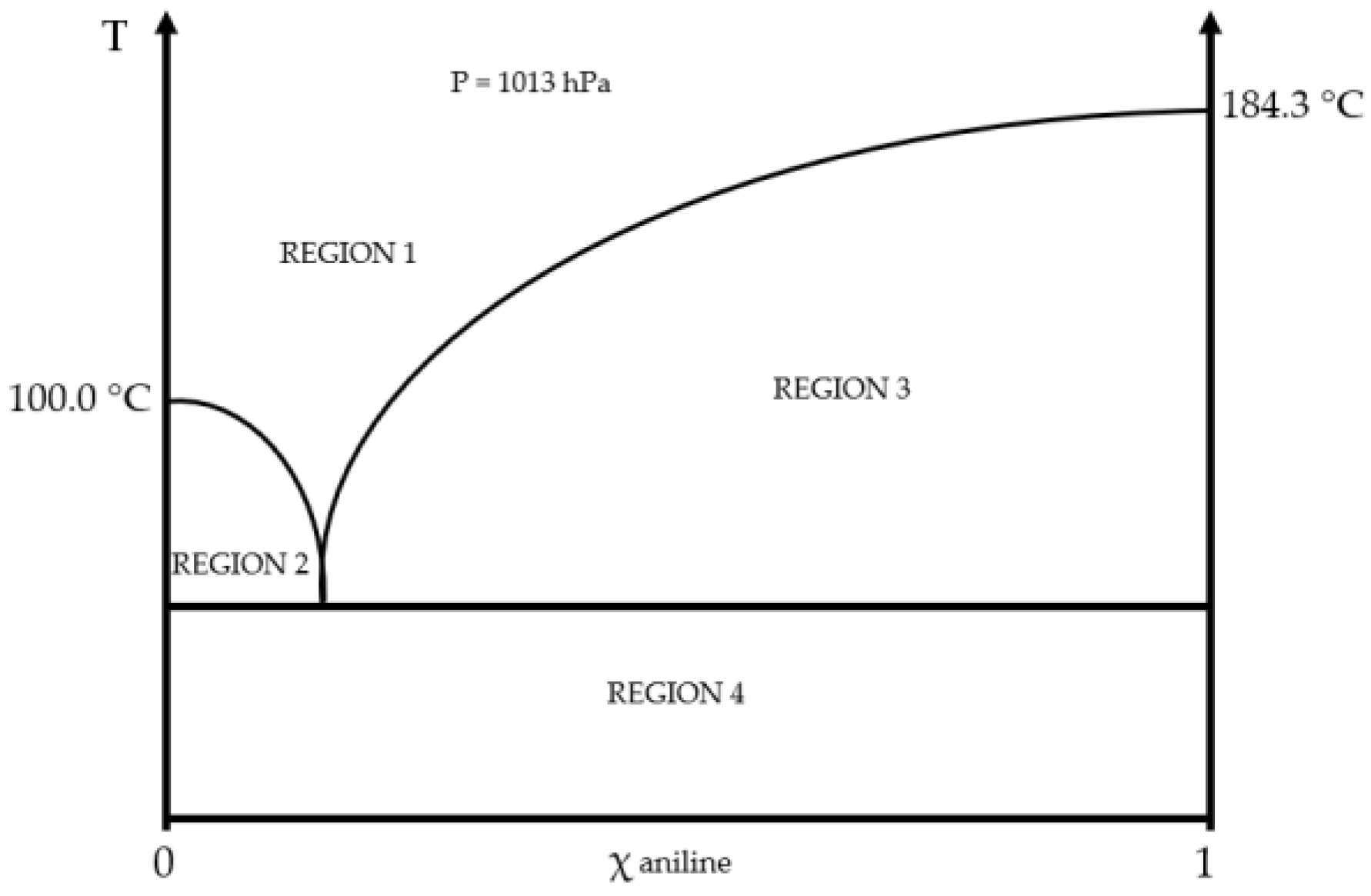
2.1. Physical and Chemical Properties
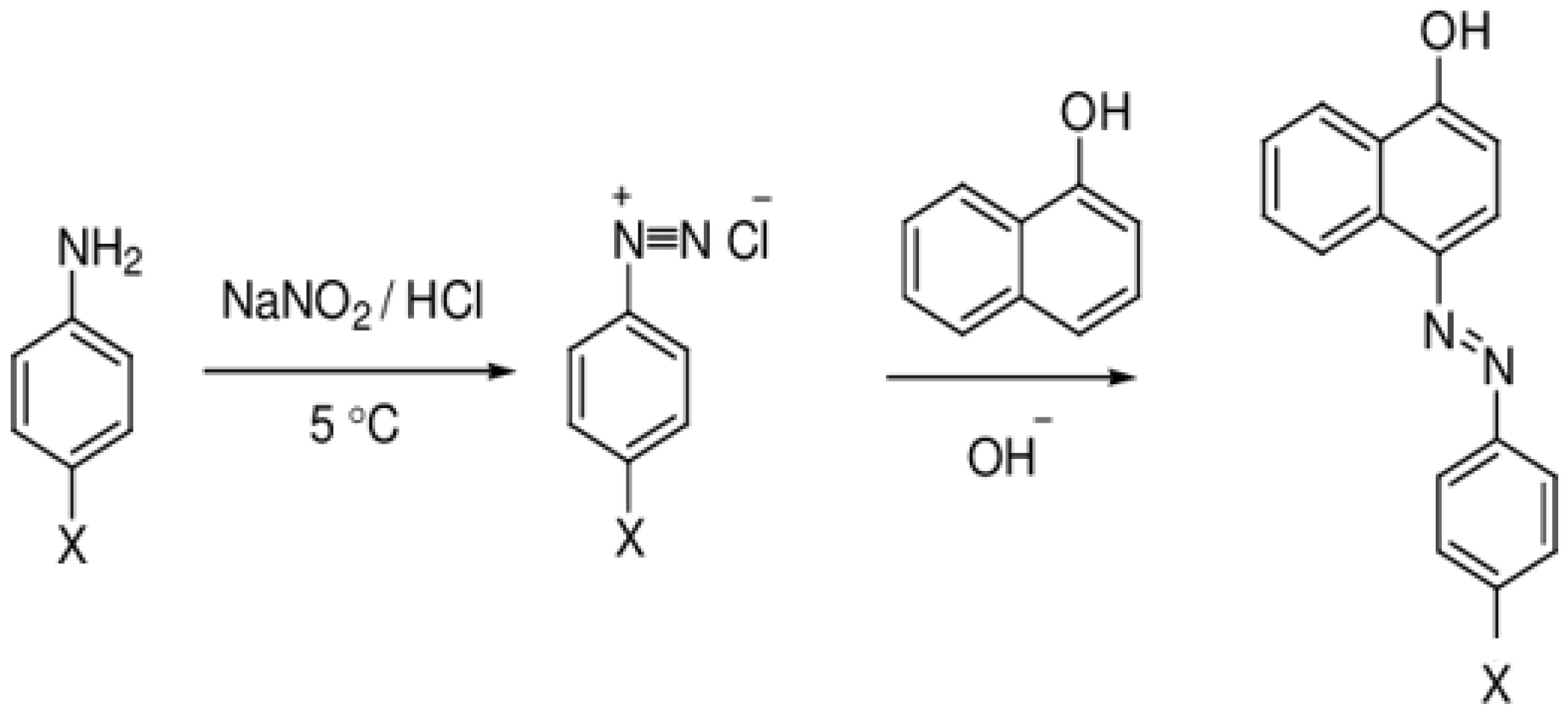
2.2. Some Typical Reactions and Analytical Methods
2.3. Methods of Preparation
- (a)
- reduction of nitrobenzene with iron and aqueous hydrochloric acid, according to the method of Pierre J. A. Béchamp (1816–1908) [22] (in industrial practice, only 1/50 of the quantity of hydrochloric acid stoichiometrically required is used, since ferrous chloride—one of the products of the reaction—contributes to the reduction of nitrobenzene);
- (b)
- reaction of nitrobenzene with concentrated hydrochloric acid and tin [23];
- (c)
- hydrogenation of nitrobenzene in the gas phase by passing it with hydrogen over copper-based catalysts at 200–350 °C [24];
- (d)
- ammonolysis of phenol at 300–600 °C on a vanadium oxide catalyst supported on alumina-silica [24];
- (e)
- ammonolysis of chlorobenzene under pressure at 200 °C with concentrated ammonia solution in the presence of cuprous salts [24];
- (f)
- reaction at low temperatures (−33 °C) of bromobenzene with potassium amide [25].
2.4. Some Historical Notes
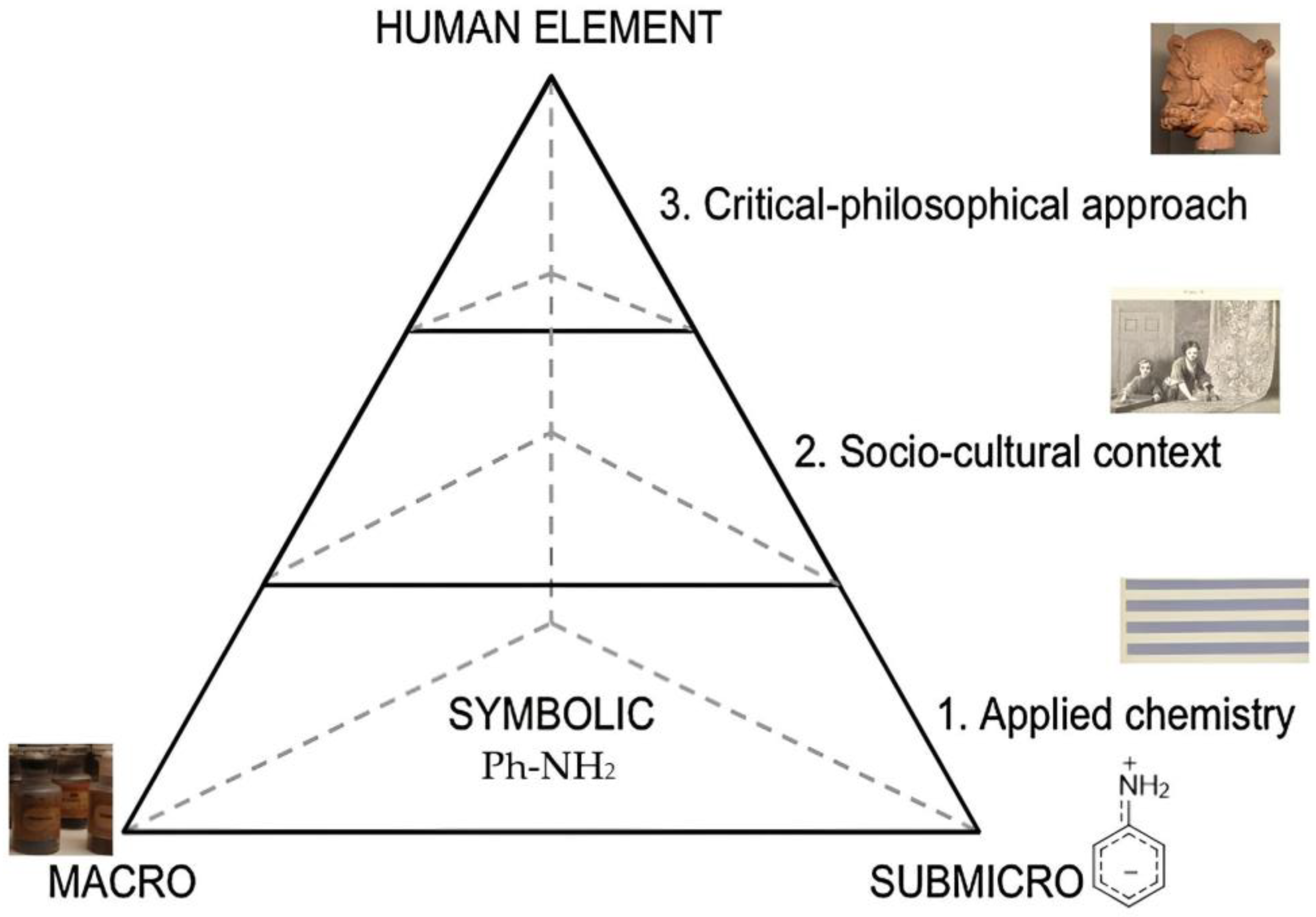
3. Symbolic Level
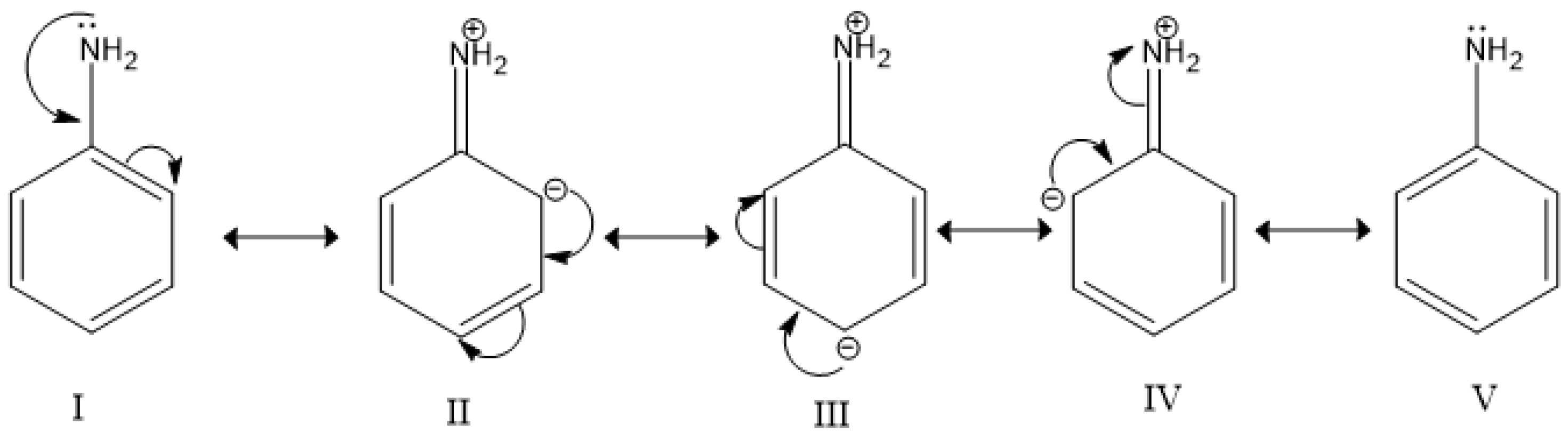
3.1. Formula, Basic Character, Diagrams
3.2. Reactions
3.3. Synthesis
with HCl/CH3COOH/H2SO4/HCOOH
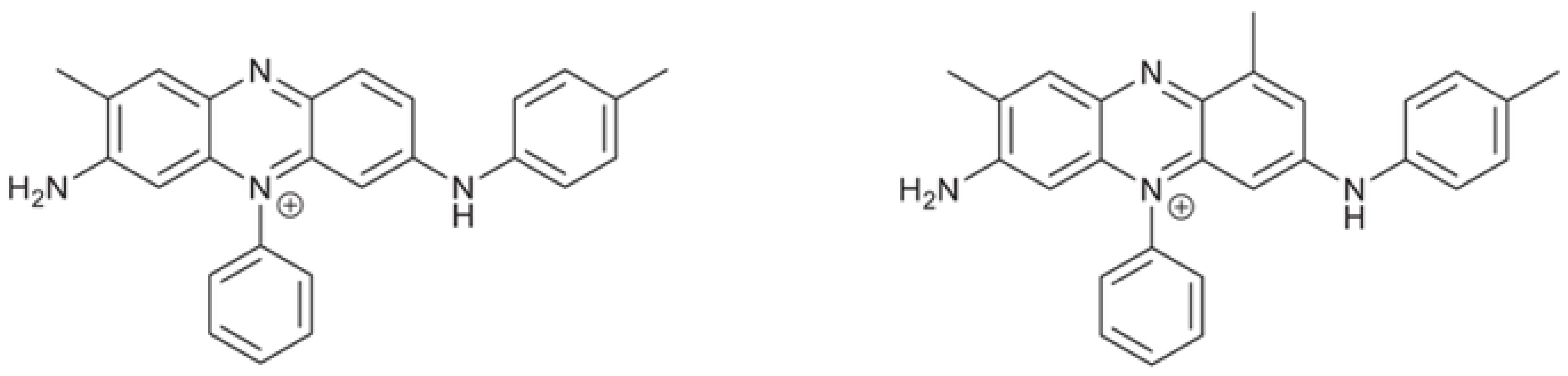
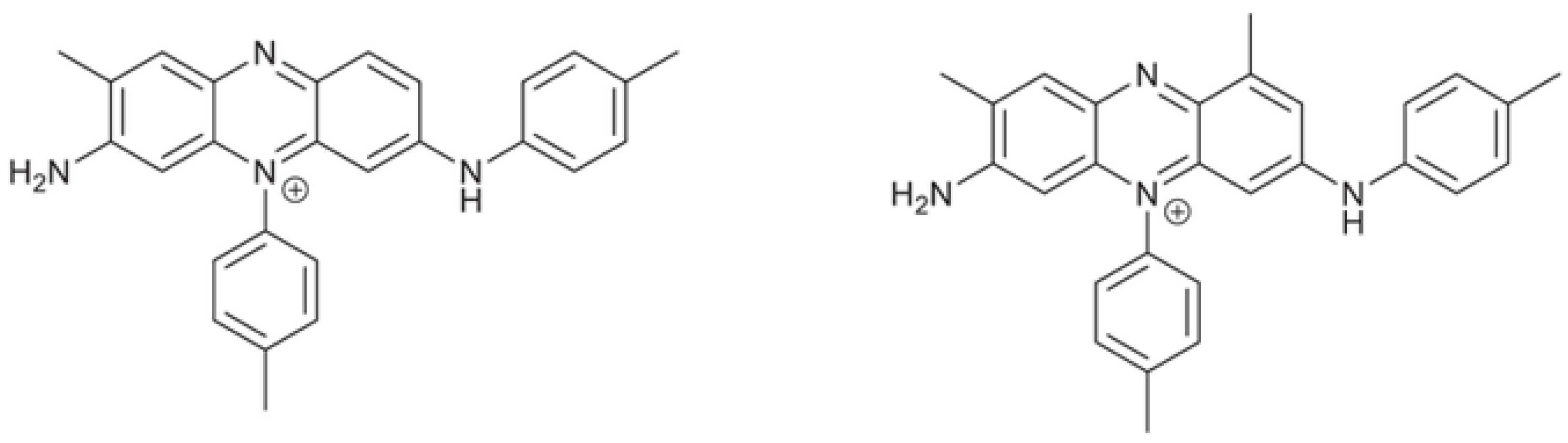
3.4. The Mauveine
4. Sub-Microscopic Level
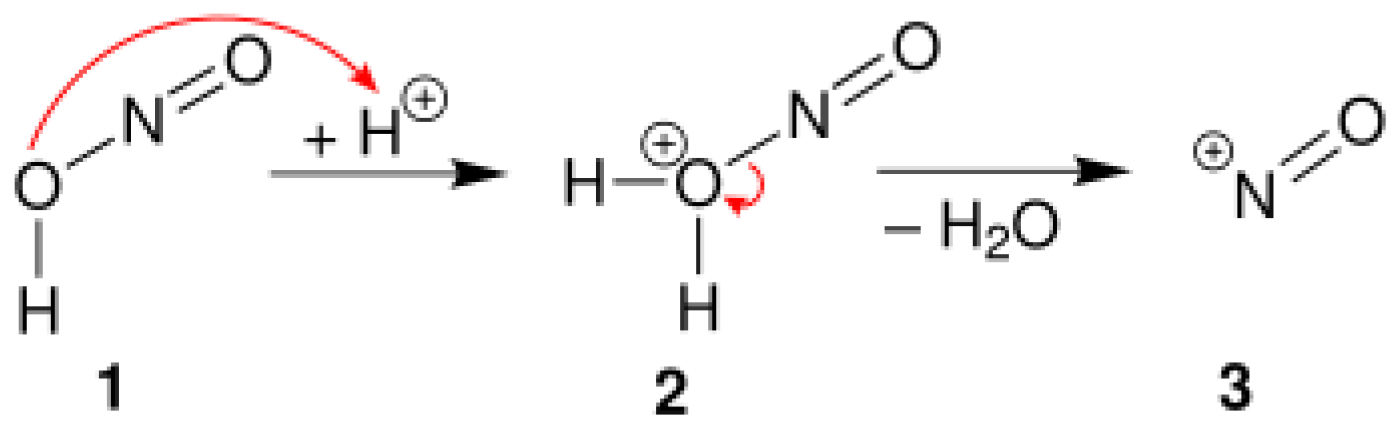
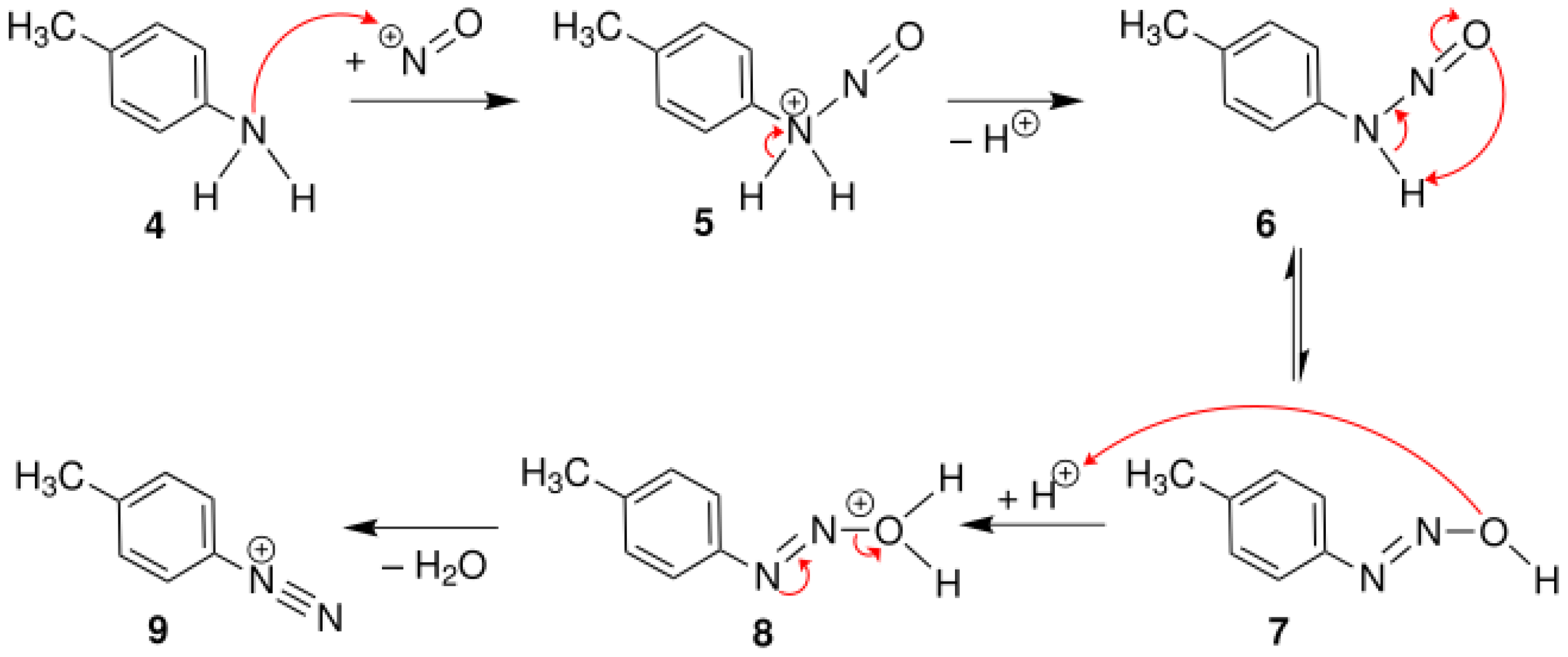
4.1. Diazotization Reaction
4.2. Preparation of Ammonium Salts
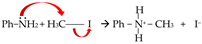
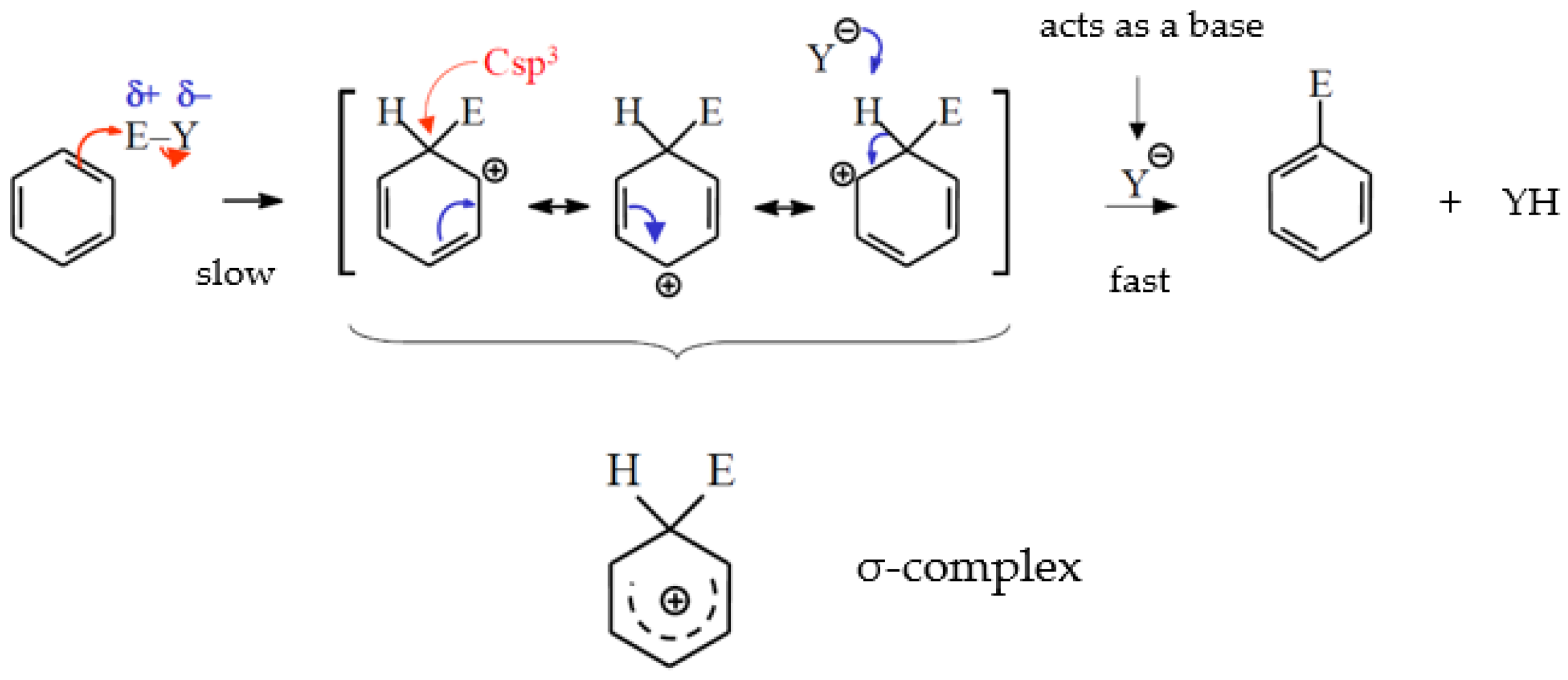
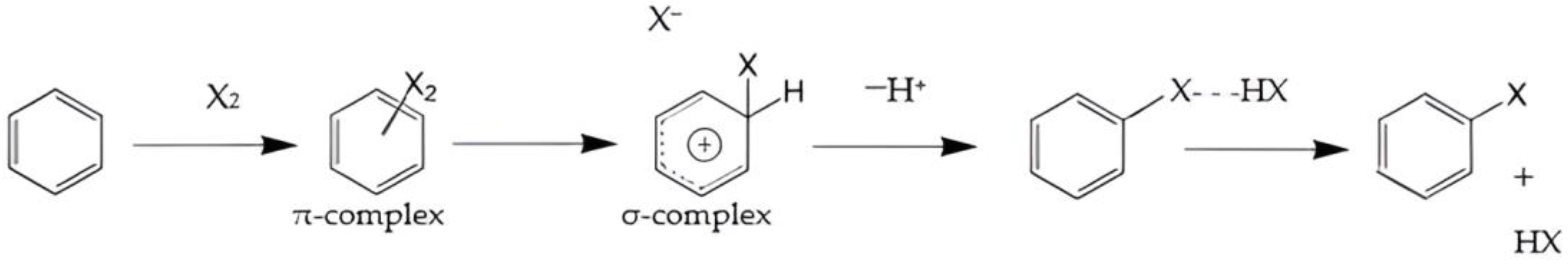
4.3. Electrophilic Aromatic Substitutions
5. Applied Chemistry
6. Socio-Cultural Context
7. Critical–Philosophical Approach
8. Conclusions
Funding
Conflicts of Interest
References
- Scerri, E.R.; McIntyre, L. The Case for the Philosophy of Chemistry. Synthese 1997, 111, 213–232. [Google Scholar] [CrossRef]
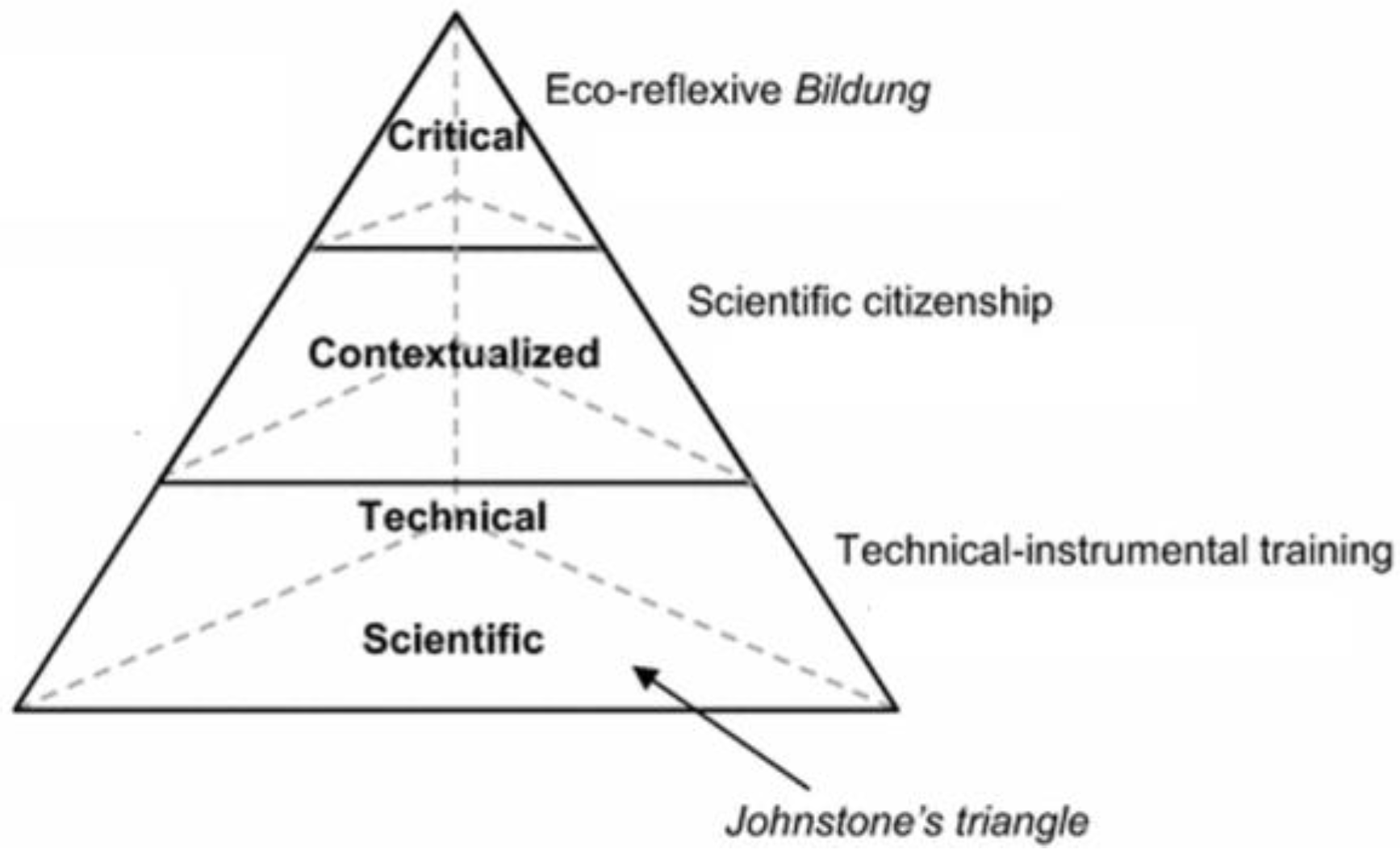
- Johnstone, A.H. Thinking about Thinking. Int. Newsl. Chem. Educ. 1991, 36, 7–10. [Google Scholar]
- Johnstone, A.H. Why Is Science Difficult to Learn? Things Are Seldom What They Seem. J. Comput. Assist. Learn. 1991, 7, 75–83. [Google Scholar] [CrossRef]
- Petillion, R.J.; McNeil, W.S. Johnstone’s Triangle as a Pedagogical Framework for Flipped-Class Instructional Videos in Introductory Chemistry. J. Chem. Educ. 2020, 97, 1536–1542. [Google Scholar] [CrossRef]
- Santos, V.C.; Arroıo, A. The Representational Levels: Influences and Contributions to Research in Chemical Education. J. Turk. Sci. Educ. 2016, 13, 3–18. [Google Scholar]
- Mahaffy, P. The Future Shape of Chemistry Education. Chem. Educ. Res. Pract 2004, 5, 229–245. [Google Scholar] [CrossRef]
- Sjöström, J. Towards Bildung-Oriented Chemistry Education. Sci. Educ. 2013, 22, 1873–1890. [Google Scholar] [CrossRef]
- Hagan, E.; Poulin, J. Statistics of the Early Synthetic Dye Industry. Herit. Sci. 2021, 9, 33. [Google Scholar] [CrossRef]
- Sjöström, J. The Discourse of Chemistry (and Beyond). Hyle 2007, 2, 83–97. Available online: https://www.hyle.org/journal/issues/13-2/sjostrom.pdf (accessed on 27 April 2025).
- Horlacher, R. What Is Bildung? The Everlasting Attractiveness of a Fuzzy Concept in German Educational Theory. PEL 2014, 51, 35–45. [Google Scholar] [CrossRef]
- Sjöström, J.; Eilks, I. The Bildung Theory—From von Humboldt to Klafki and Beyond. In Science Education in Theory and Practice. Springer Texts in Education; Akpan, B., Kennedy, T.J., Eds.; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Schummer, J. Why Chemists Need Philosophy, History, and Ethics. Substantia 2018, 2, 5–6. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6115, Aniline. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Aniline (accessed on 13 March 2025).
- Griswold, J.; Andres, D.; Arnett, E.F.; Garland, F.M. Liquid–Vapor Equilibrium of Aniline–Water. Ind. Eng. Chem. 1940, 32, 878–880. [Google Scholar] [CrossRef]
- Duarte, B.P.M.; Coelho Pinheiro, M.N.; da Silva, D.C.M.; Moura, M.J. Validating the Equilibrium Stage Model for an Azeotropic System in a Laboratorial Distillation Column. Chem. Eng. Educ. 2006, 40, 195–202. [Google Scholar]
- Ahmad, N. The Oxidation of Aromatic Amines by Sodium Hypochlorite; University of Surrey: Guildford, UK, 1970. [Google Scholar]
- Friedemann, T.E.; Keegan, P.K.; Witt, N.F. Determination of Furan Aldehydes. Reaction with Aniline in Acetic and Hydrochloric Acid Solutions. Anal. Biochem. 1964, 8, 300–311. [Google Scholar] [CrossRef]
- BMH Learning. Dichromate Test for Aniline/Acidified Potassium Dichromate Reaction with Aniline. Available online: https://www.youtube.com/watch?v=X181RA8G4sM (accessed on 13 March 2025).
- Meyer, A.S.; Boyd, C.M. Determination of Water by Titration with Coulometrically Generated Karl Fischer Reagent. Anal. Chem. 1959, 31, 215–219. [Google Scholar] [CrossRef]
- Merkel, M.; Steffens, F.; Kalem, M.; Lehner, P. Aniline Purification Process. U.S. Patent 10,889,539 B2, 12 January 2021. Available online: https://patentimages.storage.googleapis.com/9e/c7/c5/727e2d0969fec9/US10889539.pdf (accessed on 22 April 2025).
- Yates, E.; Yates, A. Johann Peter Griess FRS (1829–1888): Victorian Brewer and Synthetic Dye Chemist. Notes Rec. 2016, 70, 65–81. [Google Scholar] [CrossRef]
- Wisniak, J. Pierre Jacques Antoine Béchamp. Contributions to Chemistry. Rev. CENIC Cienc. Quím. 2020, 51, 114–125. [Google Scholar]
- Clark, J. Preparation of Phenylamine Compounds. Available online: https://chem.libretexts.org/@go/page/3993?pdf (accessed on 14 March 2025).
- Anilina. Chimica; Le garzantine; Garzanti: Milano, Italy, 2002; p. 77. [Google Scholar]
- Farmer, S.; Kennepohl, D.; Kabrhel, J.; Roberts, J.; Caserio, M.C. Benzyne. Available online: https://chem.libretexts.org/@go/page/31580?pdf (accessed on 14 March 2025).
- Maar, J.H. Friedlieb Ferdinand Runge (1794-1867)—An Unusual Chemist. Substantia 2025, 9, 73–87. [Google Scholar] [CrossRef]
- Califano, S. Storia della chimica. Volume II. Dalla chimica fisica alle molecole della vita; Digital ed.; Bollati Boringhieri: Torino, Italy, 2011. [Google Scholar]
- Plater, M.J.; Raab, A. Who Made Mauveine First: Runge, Fritsche, Beissenhirtz or Perkin? J. Chem. Res. 2016, 40, 758–762. [Google Scholar] [CrossRef]
- Crookes, W. A Practical Handbook of Dyeing and Calico-Printing; Longmans, Green and Co.: London, UK, 1874. [Google Scholar]
- Iuliano, A. The Dye that Revolutionised Chemistry: Perkin and the Discovery of Mauveine. Research for Cultural Heritage. Available online: https://researcheritage-eng.blogspot.com/2019/07/Perkin-and-the-discovery-of-mauveine.html (accessed on 14 March 2025).
- Cova, T.F.G.G.; Pais, A.A.C.C.; Seixas de Melo, J.S. Reconstructing the Historical Synthesis of Mauveine from Perkin and Caro: Procedure and Details. Sci. Rep. 2017, 7, 6806. [Google Scholar] [CrossRef]
- Garfield, S. Il malva di Perkin. Storia del colore che ha cambiato il mondo; Garzanti: Milano, Italy, 2002. [Google Scholar]
- Gmehling, J.; Menke, J.; Krafczyk, J.; Fischer, K.; Fontaine, J.-C.; Kehiaian, H.V. Azeotropic Data for Binary Mixtures. Available online: https://is.muni.cz/el/1431/podzim2016/C4020/um/pom/azeotropic_data.pdf (accessed on 22 April 2025).
- Sella, A. Karl Fischer’s Titrator. Chemistry World, 3 December 2012. Available online: https://www.chemistryworld.com/opinion/karl-fischers-titrator/5695.article (accessed on 14 March 2025).
- Heines, S.V. Peter Griess—Discoverer of Diazo Compounds. J. Chem. Educ. 1958, 35, 187. [Google Scholar] [CrossRef]
- Firth, J.D.; Fairlamb, I.J.S. A Need for Caution in the Preparation and Application of Synthetically Versatile Aryl Diazonium Tetrafluoroborate Salts. Org. Lett. 2020, 22, 7057–7059. [Google Scholar] [CrossRef]
- Excess Aniline Undergoes Alkylation with Methyl Iodide to Yield Which of the Following? Doubtbut by Allen. Available online: https://www.doubtnut.com/qna/256666655 (accessed on 13 March 2025).
- Kennepohl, D.; Farmer, S.; Reusch, W.; Nguyen, C. Nitration of Benzene (an EAS Reaction). Available online: https://chem.libretexts.org/Courses/Nassau_Community_College/Organic_Chemistry_I_and_II/17%3A_Reactions_of_Aromatic_Compounds/17.03%3A_Nitration_of_Benzene_(an_EAS_Reaction) (accessed on 21 April 2025).
- D’Auria, M. L’evoluzione del pensiero nello studio delle reazioni di sostituzione elettrofila aromatica. Thought evolution in the study of electrophilic aromatic substitution reactions. Chim. Sc. 2002, 2, 53–56. Available online: https://www.soc.chim.it/sites/default/files/cns/pdf/2002-2.pdf (accessed on 27 April 2025).
- Hubig, S.M.; Kochi, J.K. Structure and Dynamics of Reactive Intermediates in Reaction Mechanisms. σ- and π-Complexes in Electrophilic Aromatic Substitutions. J. Org. Chem. 2000, 65, 6807–6818. [Google Scholar] [CrossRef]
- Stamenković, N.; Ulrih, N.P.; Cerkovnik, J. An Analysis of Electrophilic Aromatic Substitution: A “Complex Approach”. Phys. Chem. Chem. Phys. 2021, 23, 5051–5068. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, L.; D’Auria, M. The Use of Heterocyclic Azo Dyes on Different Textile Materials: A Review. Organics 2024, 5, 277–289. [Google Scholar] [CrossRef]
- Chauhan, N.P.S.; Mozafari, M. Chapter 1—Polyaniline: An Introduction and Overview. In Fundamentals and Emerging Applications of Polyaniline; Mozafari, M., Chauhan, N.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–15. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y. Chemical Oxidative Polymerization of Polyaniline: A Practical Approach for Preparation of Smart Conductive Textiles. J. Chem. Educ. 2016, 93, 1606–1611. [Google Scholar] [CrossRef]
- Martinsen, H.E.H. Fashionable Chemistry: The History of Printing Cotton in France in the Second Half of the Eighteenth and First Decades of the Nineteenth Century; University of Toronto: Toronto, CA, USA, 2015. [Google Scholar]
- Gillispie, C.C. The Natural History of Industry. Isis 1957, 48, 398–407. [Google Scholar] [CrossRef]
- Celestino, T. A Practical Handbook of Dyeing and Calico-Printing: Il Secolo del Colore si Mostra al Mondo. Rend. Accad. Naz. Sci. Detta dei XL. Mem. E Rend. Chim. Fis. Mat. E Sci. Nat. 2024, V, 81–87. [Google Scholar]
- Crookes, W. A Recent Triumph of Synthetical Chemistry. Quartely J. Sci. 1870, 360–362. Available online: https://ia800200.us.archive.org/12/items/quarterlyjournal71870lond/quarterlyjournal71870lond.pdf (accessed on 27 April 2025).
- Tamburini, D.; Sabatini, F.; Berbers, S.; van Bommel, M.R.; Degano, I. An Introduction and Recent Advances in the Analytical Study of Early Synthetic Dyes and Organic Pigments in Cultural Heritage. Heritage 2024, 7, 1969–2010. [Google Scholar] [CrossRef]
- Romagnoli, A. William Perkin e il color malva: la prima tinta sintetica. Available online: https://www.missdarcy.it/william-perkin-e-il-color-malva-la-prima-tinta-sintetica/ (accessed on 14 March 2025).
- Beer, T. The Mauve Decade: American Life at the End of the Nineteenth Century; Alfred A. Knopf: New York, NY, USA, 1926. [Google Scholar]
- Daniel, G. Decorative Arts. In Art Nouveau: Art and Design at the Turn of the Century; Selz, P., Constantine, M., Eds.; The Museum of Modern Art: New York, NY, USA, 1959. [Google Scholar]
- Dickens, C.J.H. Oliver Twist; Richard Bentley: London, UK, 1838. [Google Scholar]
- Di Martiis, M.S. Lavoro e salute in Europa prima della rivoluzione industriale. RIMP 2010, 97, 119–162. Available online: https://biblio.liuc.it/scripts/essper/fascicolo.asp?codice=20942010$$97$$$$1 (accessed on 27 April 2025).
- Franchini, E. Manifesti e fogli volanti del movimento operaio del primo Novecento. 2013. Available online: https://filstoria.hypotheses.org/9924 (accessed on 14 March 2025).
- Polanská, K. The Legacy of Emmeline Pankhurst in the British Society; Univerzita Palackého v Olomouci: Olomouc, Sweden, 2016. [Google Scholar]
- Hoffmann, R. The Same and Not the Same; Columbia University Press: New York, NY, USA, 1995. [Google Scholar]
- Celestino, T. High School Sustainable and Green Chemistry: Historical–Epistemological and Pedagogical Considerations. Sustain. Chem. 2023, 4, 304–320. [Google Scholar] [CrossRef]
- Schummer, J.; Børsen, T. (Eds.) Ethics of Chemistry from Poison Gas to Climate Engineering; Digital Ed.; World Scientific Publishing: Singapore; Hackensack, UK, 2021. [Google Scholar]
- Riesmeier, M. Can Chemical Substances Be Natural? Ambix 2025, 72, 58–76. [Google Scholar] [CrossRef]
- Wilson, E.O. The Biophilia Hypothesis; Island Press: Washington, DC, USA, 1993. [Google Scholar]



| Properties | Values |
|---|---|
| Molar mass | 93.14 g/mol |
| d (25 °C) | 1.022 g/cm3 |
| Teb (1013 hPa) | 184.3 °C |
| Tfus (1013 hPa) | −6.2 °C |
| Solubility in water (25 °C) | 36 g/L |
| pKa (25 °C) | 4.6 |
| pKb (25 °C) | 9.3 |
| Flame point | 76 °C |
| Explosion limits | 1.2–11% vol. |
| Autoignition temperature | 540 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celestino, T. Aniline and Beyond: A Multifaceted Case Study for a Bildung-Focused Chemical Education. Organics 2025, 6, 20. https://doi.org/10.3390/org6020020
Celestino T. Aniline and Beyond: A Multifaceted Case Study for a Bildung-Focused Chemical Education. Organics. 2025; 6(2):20. https://doi.org/10.3390/org6020020
Chicago/Turabian StyleCelestino, Teresa. 2025. "Aniline and Beyond: A Multifaceted Case Study for a Bildung-Focused Chemical Education" Organics 6, no. 2: 20. https://doi.org/10.3390/org6020020
APA StyleCelestino, T. (2025). Aniline and Beyond: A Multifaceted Case Study for a Bildung-Focused Chemical Education. Organics, 6(2), 20. https://doi.org/10.3390/org6020020






