Abstract
Bipyridine ethers are commonly occurring structural motifs in supramolecular chemistry. The herein reported efforts aim to extend the synthetic platform of bipyridino-precursors with new bifunctional intermediates and to improve some previously reported synthetic strategies for structural analogues, like bipyridine-diols as common macrocycle precursors. In addition, their optimized and highly efficient oxidation to the corresponding dialdehydes is reported to obtain further reactive intermediates with wide modifiability. Furthermore, methylations of pyridine-carbaldehydes were carried out alongside different synthetic strategies to introduce chirality centers. Synthetic difficulties and some unsuccessful approaches are also reported to help in focusing future efforts.
1. Introduction
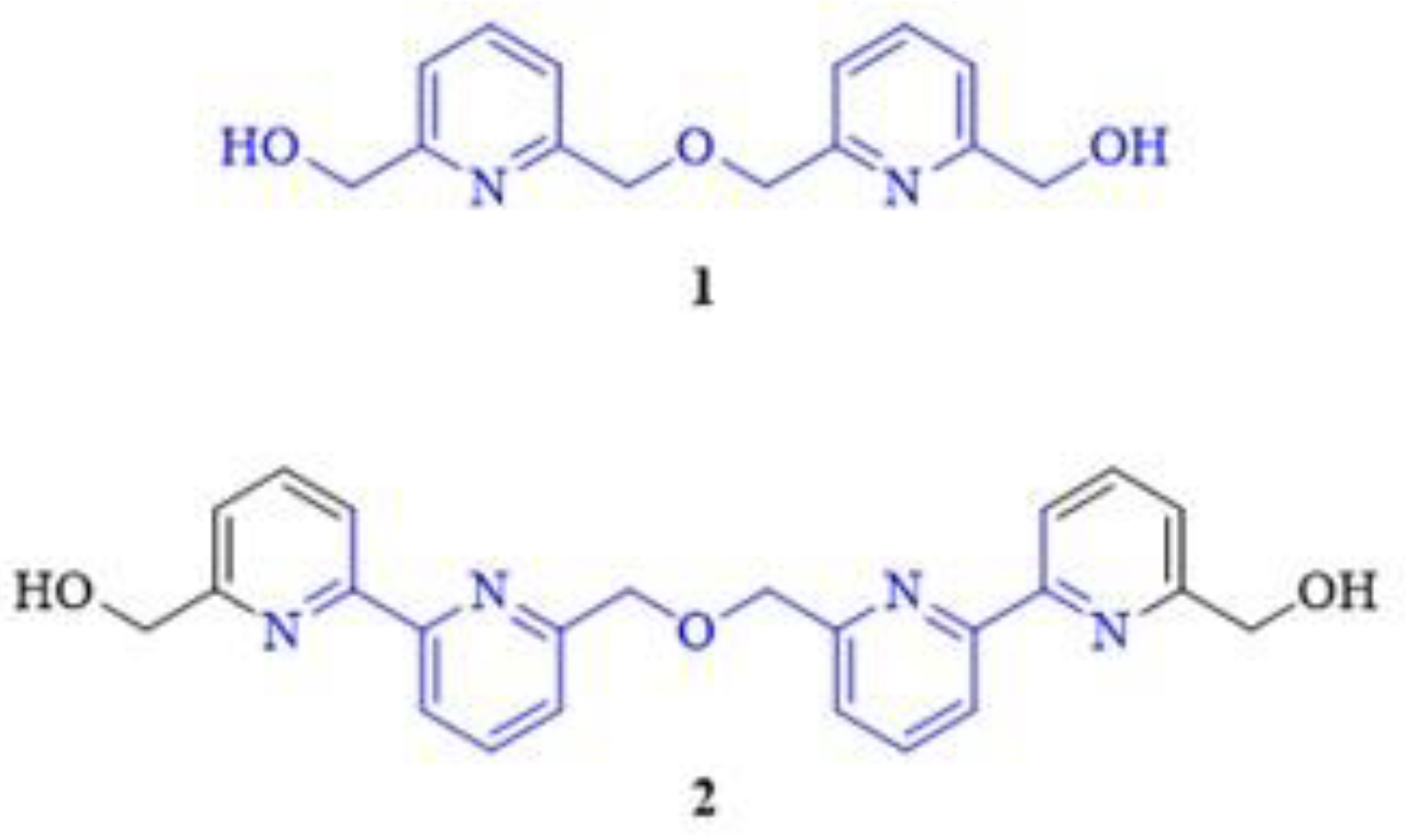
Bipyridine ethers derived from 2,6-pyridinedimethanol and its derivatives have already been reported by several research groups focusing on the synthesis of pyridino-macrocycles [1,2,3,4,5,6,7,8,9,10,11,12] or functional materials for supramolecular chemistry applications [13]. Two examples of these useful intermediates (1, 2) are shown in Figure 1.
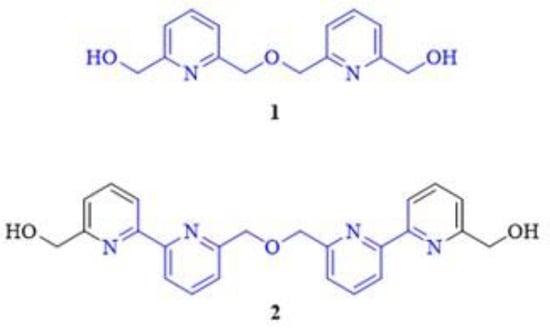
Figure 1.
Bis(pyridine)-dimethanol ether (1) and another 6,6′-disubstituted 2,2′-bipyridine (2) as commonly applied building blocks of multiheteroaromatic macrocycles, functional materials and supramolecular devices (the highlighted common structural motifs are in the focus of the current synthetic efforts).
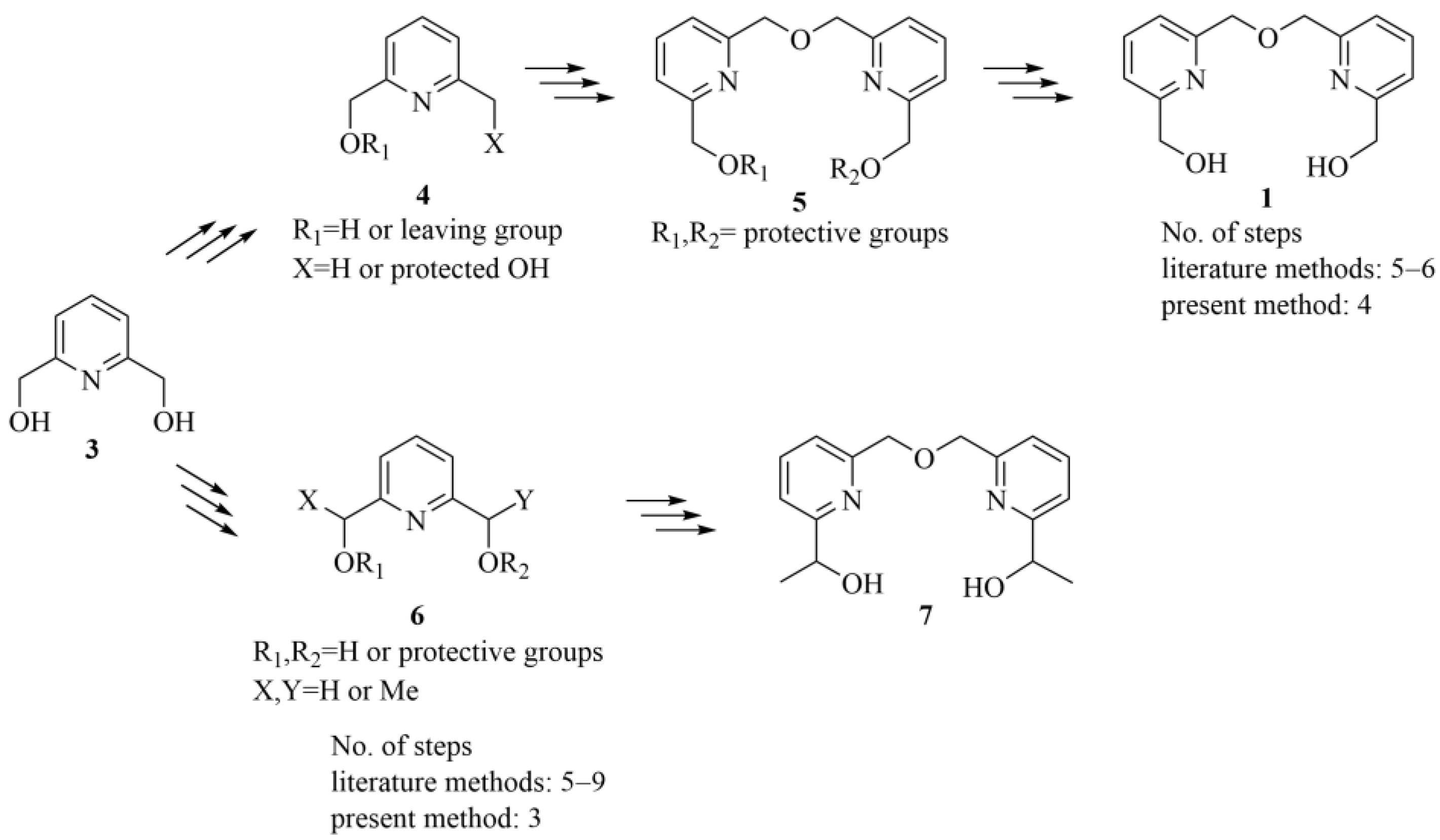
Recently, we reported an improved synthetic procedure for the preparation of intermediate 1 (Scheme 1) [14], which simplified the previously published multistep methods [1,2,6,15,16].
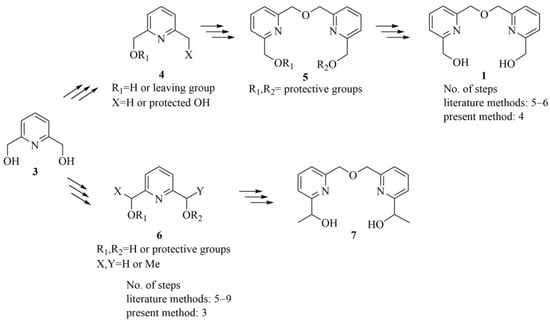
Scheme 1.
General synthetic approaches for the preparation of bifunctional bipyridine intermediates (1, 7). We aimed to reduce the number of steps while providing a good overall yield (53%) for intermediate 1 by starting from pyridine dimethanol 3 [14]. Continuing these efforts, we aimed to further reduce the required number of reaction steps while ensuring a similarly high overall yield and improving atom efficiency by replacing the previously applied [14] trityl protective groups.
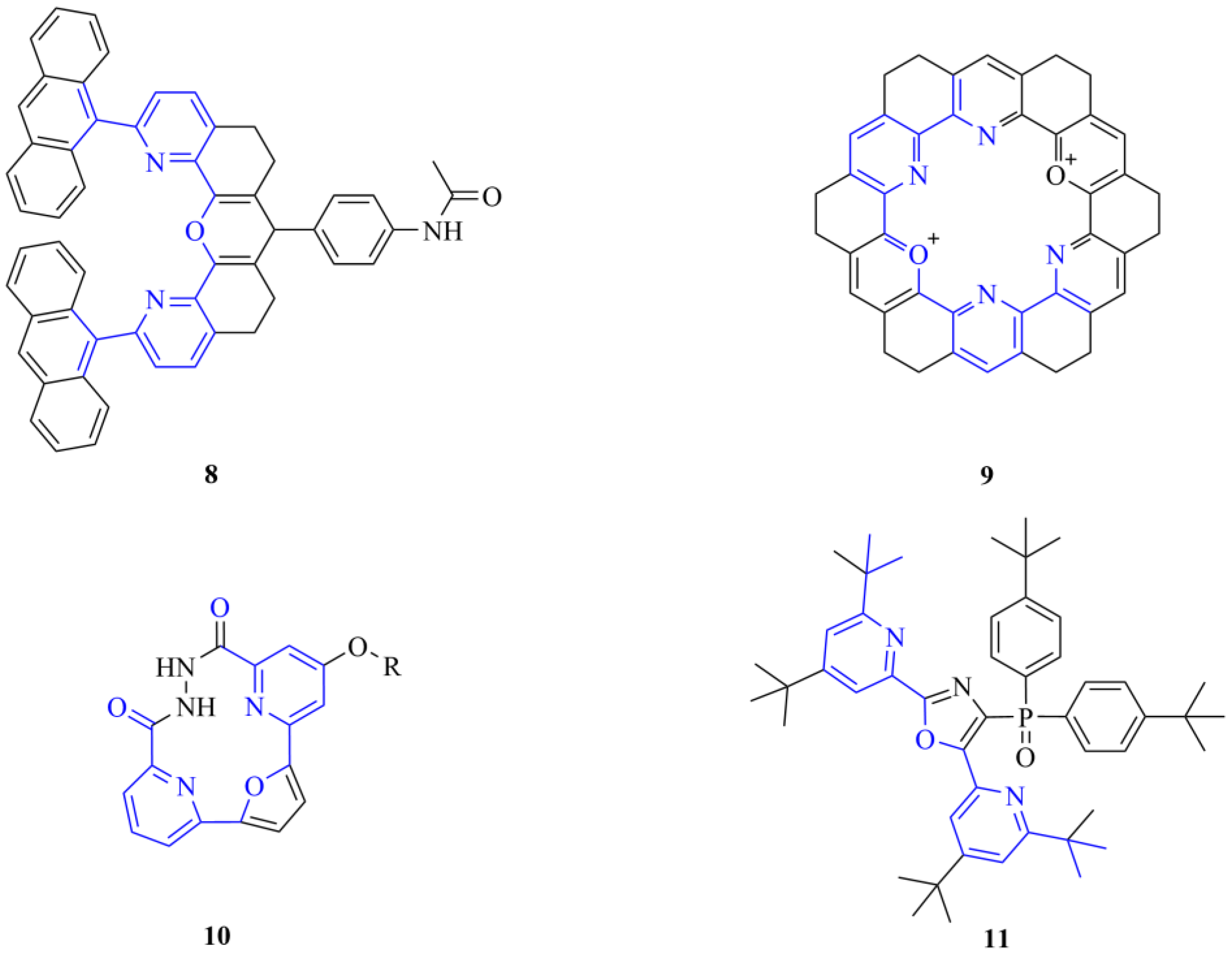
Although we used these intermediates as macrocycle precursors, these types of bifunctional compounds can be valuable in alternative uses as well, e.g., the production of polymers [17]. Moreover, intermediates with a similar structure can also be applied as noncyclic coordinating ligands (8) [18,19,20] or occur as common subunits of kekulenes (9) [21], supramolecular polymers [22], molecular tweezers (10) [23,24,25] and organic electroluminescent devices (11) [26], as shown in Figure 2.
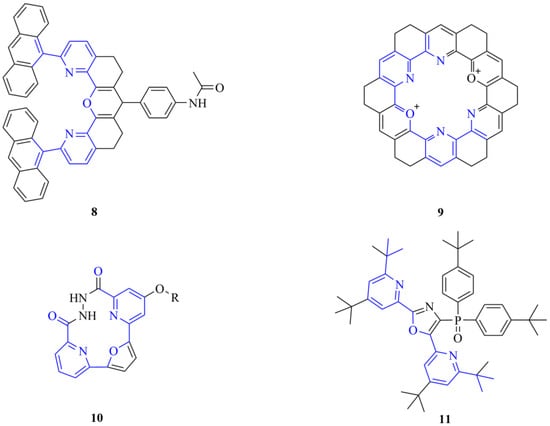
Figure 2.
Examples (8–11) of the diverse applicability of the common 6,6′-disubstituted 2,2′-bipyridine analogue structural motifs in supramolecular chemistry (the highlighted common structural motifs are in the focus of the current synthetic efforts).
Although the first pyridino-macrocycles were synthesized in the early 1970s [1], the scientific interest in these host molecules is still very intense today [27,28,29,30,31,32,33].
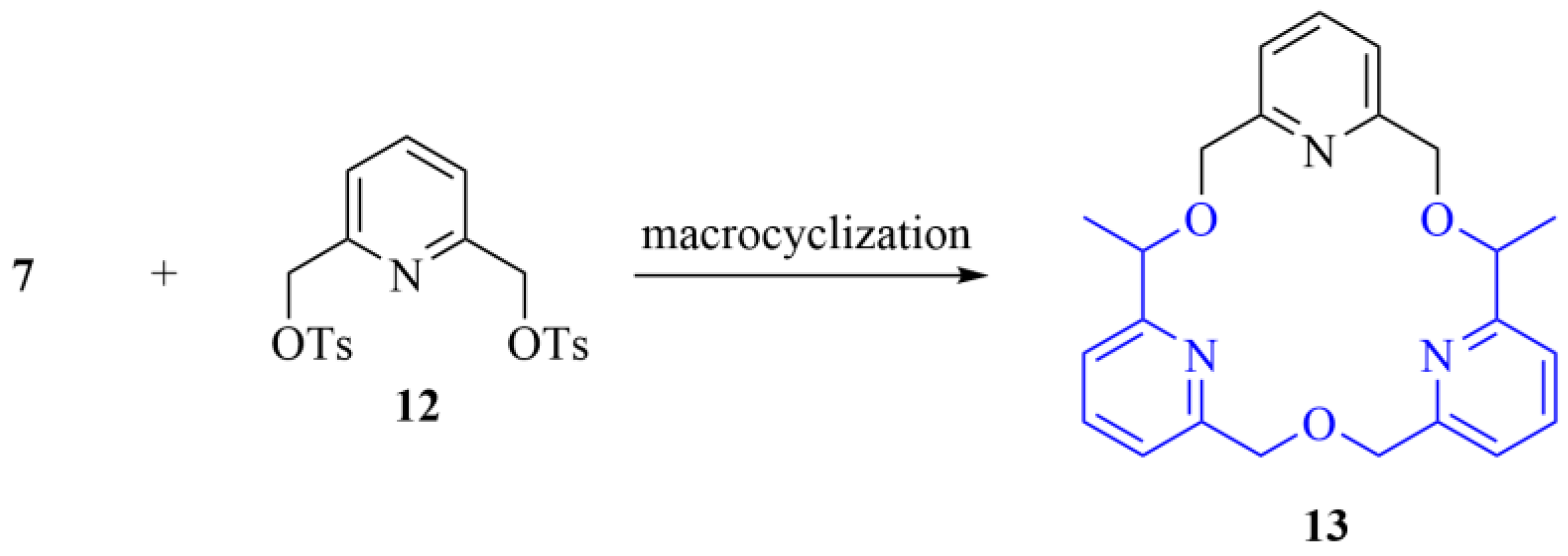
As an extension of our recent study on a tris(pyridino)-crown ether—which showed unique applicability in the selective molecular separation of amines with different degrees of N-substitution [14]—we aimed to prepare the chiral analogue of this macrocycle for the enantiomeric recognition of biologically active amines Scheme 2.
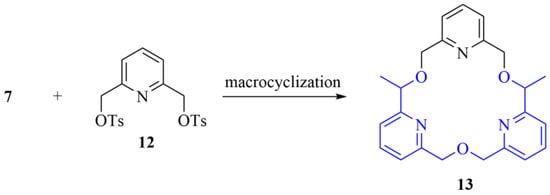
Scheme 2.
One potential application of the synthesized bipyridine key intermediate as a precursor for chiral crown ethers (The highlighted common structural motif is in the focus of the current synthetic efforts).
Herein, we report the most effective and simplest procedure for the preparation of bipyridine diol 1 by further improving our recent work on the same synthetic chemical platform [1,2,6,14,15,16]. Three synthetic strategies were attempted. Among these efforts, oxidation of diol 1 was carried out by several methods to gain the corresponding dialdehyde as the achiral precursor of the desired chiral building blocks. Subsequently, reactions were attempted to introduce chiral centers, exemplified by the synthesis of a dimethyl derivative in a racemic form.
Based on the common occurrence of the above-discussed bipyridino-motifs in diverse applications, we believe that the reported new bipyridine intermediates carry potential for broad applications and future development, especially in supramolecular chemistry.
2. Experimental Section
2.1. General
Starting materials and reagents were purchased from Sigma-Aldrich Corporation (Merck, Darmstadt, Germany) and used without further purification unless otherwise noted. Solvents were dried and purified according to well-established methods [34]. Silica Gel 60 F254 (Merck, Germany) and aluminum oxide 60 F254 neutral type E (Merck, Germany) plates were used for thin-layer chromatography (TLC). All reactions were monitored by TLC and visualized under a UV lamp. Aluminum oxide (neutral, activated, Brockman I) and Silica Gel 60 (70–200 mesh, Merck) were used for column chromatography. The ratios of solvents for the eluents are given in volumes (mL/mL). Evaporations were carried out under reduced pressure unless otherwise stated.
The new compounds were characterized by their physical constants, such as melting points, and by thin-layer chromatography retention factors (Rf), IR, 1H-NMR and 13C-NMR spectroscopy and HRMS spectrometry. Melting points were recorded on a Boetius micro-melting point apparatus. Infrared spectra were recorded on a Bruker AlphaT FT-IR spectrometer (Bruker Corporation, Billerica, MA, USA) using KBr pastilles. 1H- (500 MHz) and 13C- (125 MHz) NMR spectra were recorded on a Bruker DRX-500 Avance spectrometer (Bruker Corporation, USA). HRMS analysis was carried out on a Thermo Velos Pro Orbitrap Elite system (Thermo Fisher Scientific, Dreieich, Germany). The ionization method was ESI and was operated in positive ion mode. The protonated molecular ion peak was fragmented by CID at a normalized collision energy of 35–45%. The sample was dissolved in methanol. Data acquisition and analysis were accomplished with Xcalibur software version 2.2 (Thermo Fisher Scientific, Germany).
2.2. Synthesis
2.2.1. Preparation of {6-[(benzyloxy)methyl]pyridin-2-yl}methanol (15, Scheme 3)
Procedure ‘A’
Pyridinedimethanol 14 (28.70 g, 0.21 mol) was dissolved in a mixture of 300 mL THF and 40 mL DMF and cooled down to 0 °C under an argon atmosphere. Then, sodium hydride (16.60 g, 60 w/w% dispersion in mineral oil, 0.42 mol) was added to this solution in small portions. This mixture was stirred for 15 min, and then benzyl bromide (24.5 mL, 35.23 g, 0.21 mol) was added to it dropwise. The mixture was stirred at room temperature for 24 h. Distilled water (5 mL) was added to the reaction mixture, and the volatile components were evaporated. Water (400 mL) and ethyl acetate (400 mL) were added to the residue, and the aqueous phase was extracted with ethyl acetate (4 × 100 mL). The combined organic phase was dried with magnesium sulfate, filtered and evaporated. The crude product was purified by column chromatography on a silica gel adsorbent using a mixture of ethyl acetate/hexane = 3:2 as an eluent to produce title compound 15.
Monobenzylated pyridine derivative 15 is a pale-yellow oil, 32.5 g (69%). The product was identical in every aspect to that reported in [6]. Rf: 0.40 (SiO2, ethyl acetate/hexane = 3:2).
Procedure ‘B’
Diol 14 (1.50 g, 10.78 mmol) was added to a stirred mixture of sodium hydride (0.69 g, 17.25 mmol) and DMSO (10 mL) under an argon atmosphere. Benzyl bromide (2.21 g, 12.94 mmol) in DMSO (10 mL) was added dropwise over 30 min and stirred for 1 day at room temperature. The reaction mixture was poured into ice-water (50 mL), and the mixture was extracted with diethyl ether (3 × 50 mL). The combined organic phase was dried with magnesium sulfate, filtered and evaporated. The crude product was purified by column chromatography on a silica gel adsorbent using a mixture of propan-2-ol/cyclohexane = 1:10 as an eluent to produce title compound 15.
Product 15 was a pale-yellow oil (0.67 g, 27%). The product was identical in every aspect to that reported in [6].
2.2.2. Preparation of 2,6-bis[(benzyloxy)methyl]pyridine (16, Scheme 3)
The dibenzylated pyridine derivative 16 is a major product; its synthesis is described above in the preparation of the monobenzylated derivative 8 (Section 2.2.1). The dibenzylated derivative 16 from procedure ‘B’, 2.41 g (70%), is a white crystalline solid.
M.p.: 81 °C. Rf: 0.35 (SiO2, propane-2-ol/cyclohexane = 1:10). 1H-NMR (500 MHz, CDCl3) δ 7.71 (t, J = 7.7 Hz, 1H), 7.42–7.30 (m, 12H), 4.68 (s, 4H), 4.66 (s, 4H). 13C-NMR (125 MHz, CDCl3) δ 157.9, 138.0, 137.3, 128.4, 127.8, 127.7, 120.0, 73.1, 72.9. IR: νmax [cm−1] (KBr): 3074, 3061, 3027, 2921, 2881, 2819, 1592, 1580, 1469, 1454, 1443, 1389, 1344, 1305, 1229, 1075, 1027, 1018, 992, 916, 804, 787, 730, 694, 629, 465. HRMS (m/z): [MH+]: 320.1606, calculated [M+]: 319.1572.
2.2.3. Preparation of {6-[(benzyloxy)methyl]pyridin-2-yl}methyl 4-methylbenzenesulfonate (17, Scheme 3)
Monobenzyl derivative 15 (8.55 g, 37.30 mmol) was dissolved in 90 mL dichloromethane (DCM), and the solution was cooled to 0 °C. Potassium hydroxide (41.50 g, 0.73 mol) in 65 mL water was added to this solution under an argon atmosphere, and the mixture was stirred for 10 min at 0 °C. Tosyl chloride (7.11 g, 37.30 mol) in 90 mL DCM was added dropwise to the reaction mixture, and it was stirred for 1 day at room temperature. DCM (150 mL) and water (150 mL) were added to the mixture, and the phases were separated. The aqueous phase was extracted with DCM (3 × 100 mL). The combined organic phase was dried with anhydrous magnesium sulfate, filtered and evaporated. The crude product was purified by column chromatography on a silica gel adsorbent using a mixture of ethyl acetate/hexane = 1:3 as an eluent to give tosylate 17 (13.73 g, 97%) as a colorless oil.
Rf: 0.85 (SiO2, ethyl acetate/hexane = 3:2). 1H-NMR (500 MHz, CDCl3) δ 7.81 (d, J = 7.9 Hz, 2H), 7.68 (t, J = 8.1 Hz, 1H), 7.43–7.27 (m, 9H), 5.12 (s, 2H), 4.61 (s, 2H), 4.59 (s, 2H), 2.40 (s, 3H). 13C-NMR (125 MHz, CDCl3) δ 158.4, 153.0, 145.1, 137.6, 132.8, 129.9, 128.5, 128.1, 127.8, 121.0, 120.5, 73.0, 72.7, 71.8, 21.7. IR: νmax [cm−1]: 3086, 3062, 3031, 2943, 2869, 2827, 2584, 2033, 1722, 1654, 1624, 1541, 1496, 1453, 1362, 1228, 1159, 1117, 1030, 1008, 815, 742, 679, 561. HRMS (m/z): [MH+]: 384.1225, calculated [M+]: 383.1191.
2.2.4. Preparation of 6,6′-[oxybis(methylene)]bis{2-[(benzyloxy)methyl]pyridine} (18, Scheme 3)
Pyridinemethanol 15 (7.15 g, 31.00 mmol) was dissolved in THF (70 mL), and this solution was cooled to 0 °C under an argon atmosphere. Sodium hydride (60 w/w% dispersion in oil, 3.12 g, 78.00 mmol) was added to this solution in small portions, and the mixture was stirred for 15 min. Tosylate 17 (11.89 g, 31.00 mmol) in THF (70 mL) was added dropwise to this mixture, and it was stirred for 72 h at room temperature. Water (1.5 mL) was added to the reaction mixture. The solvent was then removed, and the solid residue was dissolved in a mixture of ethyl acetate (100 mL) and water (100 mL). The aqueous phase was extracted with ethyl acetate (4 × 70 mL). The combined organic phase was dried with magnesium sulfate, filtered and evaporated. The crude product was recrystallized from ethyl acetate (200 mL) to give bipyridine derivative 18 (12.36 g, 90%) as a white crystalline solid.
M.p.: 104–106 °C. Rf: 0.50 (SiO2, ethyl acetate/hexane = 3:2). 1H-NMR (500 MHz, CDCl3) δ 7.71 (t, J = 7.8 Hz, 2H), 7.42–7.28 (m, 14H), 4.75 (s, 4H), 4.67 (s, 4H), 4.64 (s, 4H). 13C-NMR (125 MHz, DEPTQ-135, CDCl3) δ 158.1, 157.6, 138.0, 137.3, 128.4, 127.8, 127.8, 120.1, 120.0, 73.7, 73.1, 73.0. IR: νmax [cm−1]: 3086, 3058, 3030, 3006, 2924, 2887, 2873, 2852, 2782, 1591, 1581, 1496, 1468, 1445, 1381, 1345, 1331, 1307, 1239, 1207, 1131, 1071, 1028, 993, 913, 823, 785, 729, 695, 634, 508, 468. HRMS (m/z): [MH+]: 441.2133, calculated [M+]: 440.2100.
2.2.5. Preparation of [6-({[6-(hydroxymethyl)pyridin-2-yl]methoxy}methyl)pyridin-2-yl]methanol (1, Scheme 3)
Ethyl acetate (130 mL) was added to a mixture of dibenzyl derivative 18 (12.36 g, 28.00 mmol) and Pd/C catalyst (1.86 g, 15 w/w% Pd) under argon. Argon was replaced by hydrogen, and the mixture was stirred at 40 °C for 6 h under a hydrogen atmosphere (atm pressure). The mixture was filtered through folded filter paper, and the filtrate was evaporated. The crude product was purified by column chromatography on a silica gel adsorbent using a mixture of methanol/toluene = 1:5 as an eluent to give bipyridine diol 1 (6.13 g, 84%) as a pale-yellow crystalline solid. Rf: 0.25 (SiO2, methanol/toluene = 1:4).
This product was identical in every aspect to that reported in [1,14].
2.2.6. Preparation of 6,6′-[oxybis(methylene)]dipyridine-2-carbaldehyde (19, Schemes 3 and 5)
Procedure ‘A’
Bipyridine diol 1 (100 mg, 0.38 mmol) and manganese dioxide (134 mg, 1.54 mmol) were added to chloroform (1 mL). The mixture was stirred for 3 h at 80 °C in a pressure flask. The mixture was filtered and evaporated. The crude product was purified by column chromatography on a silica gel absorbent using a mixture of ethanol/toluene = 1:10 as an eluent. Bipyridine dialdehyde 19 (39 mg, 40%) is a white crystalline solid.
M.p.: 120–121 °C. Rf: 0.5 (SiO2, ethanol/toluene = 1:3), 0.3 (SiO2, ethanol/toluene = 1:10). 1H-NMR (500 MHz, CDCl3) δ 10.04 (s, 2H), 7.94–7.85 (m, 4H), 7.76 (dd, J = 7.1, 1.8 Hz, 2H), 4.89 (s, 4H). 13C-NMR (125 MHz, DEPTQ-135, CDCl3) δ 193.3, 158.9, 152.3, 137.8, 125.7, 120.7, 73.5. IR: νmax [cm−1]: 2847, 2372, 1707, 1624, 1592, 1522, 1445, 1390, 1350, 1263, 1243, 1212, 1169, 1112, 1039, 869, 802, 764, 664. HRMS (m/z): [MH+]: 257.0881, calculated [M+]: 256.0848.
Procedure ‘B’
Oxalyl chloride (410 mg, 3.23 mmol) was dissolved in DCM (1.4 mL) and cooled to 0 °C under an argon atmosphere. DMSO (417 mg, 5.08 mmol) was added to this solution dropwise. Then, diol 1 (100 mg, 0.39 mmol) in Hünig’s base (1.49 g, 11.50 mmol) and DCM (0.5 mL) was added dropwise, and the mixture was stirred for 45 min at 0 °C. The solution was evaporated, and the crude product was purified by column chromatography on a silica gel absorbent using a mixture of ethanol/toluene = 1:10 as an eluent. Bipyridine 19 (30 mg, 30%) is a white crystalline solid.
This product was identical in every aspect to that reported in Procedure ‘A’ in Section 2.2.6.
Procedure ‘C’
Bipyridine diol 1 (100 mg, 0.38 mmol) was dissolved in DMSO (2 mL). The solution of CuCl2/TMEDA (30 µL, 0.003 mmol, 0.1 M in MeCN) and the solution of TEMPO (300 µL, 0.03 mmol, 0.1 M in DMSO) were added under an oxygen atmosphere (atm pressure). The mixture was stirred for 3 h at 100 °C and then evaporated, and the crude product was purified by column chromatography on a silica gel absorbent using a mixture of ethanol/toluene = 1:10 as an eluent to give bipyridine dialdehyde 19 (20 mg, 20%).
This product was identical in every aspect to that reported in Procedure ‘A’ in Section 2.2.6.
Procedure ‘D’
Bipyridine diol 1 (100 mg, 0.38 mmol) was dissolved in methanol (2 mL). The solution of CuCl2/TMEDA (30 µL, 0.003 mmol, 0.1 M in MeCN) and the solution of TEMPO (300 µL, 0.03 mmol, 0.1 M in DMSO) were added under an oxygen atmosphere (atm pressure). The mixture was stirred for 3 h at room temperature. The solution was evaporated, and the crude product was purified by column chromatography on a silica gel absorbent using a mixture of ethanol/toluene = 1:10 as an eluent to give bipyridine dialdehyde 19 (30 mg, 30%).
This product was identical in every aspect to that reported in Procedure ‘A’ in Section 2.2.6.
Procedure ‘E’
Chloroform (40 mL) was added to a mixture of bipyridine diol 1 (2.00 g, 7.68 mmol) and selenium dioxide (3.41 g, 30.70 mmol). A catalytic amount of water was added to this mixture, and it was stirred at 80 °C for 3 h in a pressure flask. The mixture was filtered, and the filtrate was extracted with aqueous hydrochloric acid solution (50 mL, 10 w/w%). The pH of the aqueous phase was set to 11, and it was extracted with ethyl acetate (3 × 50 mL). The combined organic phase was dried with magnesium sulfate, filtered and evaporated to give bipyridine dialdehyde 19 (1.40 g, 71%).
This product was identical in every aspect to that reported in Procedure ‘A’ in Section 2.2.6.
Procedure ‘F’
Pyridinium chlorochromate (332 mg, 1.54 mmol) was dissolved in chloroform (2 mL) and cooled to 0 °C under an argon atmosphere. Diol 1 (100 mg, 0.38 mmol) was added to this solution, and it was stirred for 3 h at 0 °C. The reaction was monitored by TLC, and it showed that the desired product did not form in this case.
2.2.7. Preparation of 6-({[6-(hydroxymethyl)pyridin-2-yl]methoxy}methyl)pyridine-2-carbaldehyde (20, Schemes 3 and 5)
Bipyridine derivative 20 is a byproduct; its synthesis is described above in Section 2.2.6. Bipyridine derivative 20 is a white crystalline solid, and it had the highest yields in procedures ‘A’ (60 mg, 40%) and ‘B’ (40 mg, 60%).
M.p.: 83–84 °C. Rf: 0.44 (SiO2, ethanol/toluene = 1:4). 1H-NMR (500 MHz, CDCl3) δ 10.03 (s, 1H), 7.93–7.82 (m, 2H), 7.79–7.64 (m, 2H), 7.44–7.36 (m, 1H), 7.18–7.13 (m, 1H), 4.84 (s, 2H), 4.78 (s, 2H), 4.73 (s, 2H), 3.92 (broad s, 1H). 13C-NMR (125 MHz, DEPTQ-135, CDCl3) δ 193.3, 159.1, 158.6, 156.8, 152.2, 137.7, 137.4, 125.7, 120.6, 120.0, 119.4, 73.7, 73.2, 64.0. IR: νmax [cm−1]: 3174, 2955, 2918, 2884, 2850, 1711, 1596, 1581, 1458, 1435, 1340, 1309, 1262, 1209, 1113, 1081, 1050, 1023, 991, 896, 831, 776, 760, 668, 633, 554. HRMS (m/z): [MH+]: 259.1038, calculated [M+]: 258.1004.
2.2.8. Preparation of 1-[6-({[6-(1-hydroxyethyl)pyridin-2-yl]methoxy}methyl)pyridin-2-yl]ethan-1-ol (21, Scheme 3)
Methyl iodide (605 μL, 9.77 mmol) was added to activated magnesium (284 mg, 11.70 mmol) in diethyl ether (1 mL), and this mixture was refluxed under argon. After the reaction was completed, dialdehyde 19 (250 mg, 0.96 mmol) in dioxane (1 mL) was added dropwise, and the mixture was stirred for 48 h at room temperature. Water (10 mL) and ethyl acetate (10 mL) were added, and the aqueous phase was extracted with ethyl acetate (3 × 5 mL). The combined organic phase was dried with magnesium sulfate, filtered and evaporated. The crude product was purified by column chromatography on a silica gel absorbent using a mixture of ethanol/toluene = 1:10 as an eluent to give diol 21 (40 mg, 14%) as a pale-yellow crystalline solid.
M.p.: >300 °C (decomposition). Rf: 0.12 (SiO2, ethanol/toluene = 1:3), 0.20 (SiO2, ethanol/toluene = 1:3). 1H-NMR (500 MHz, CDCl3) δ 7.66 (t, J = 7.6 Hz, 2H), 7.13 (d, J = 7.7 Hz, 2H), 7.05 (d, J = 7.7 Hz, 2H), 5.26 (s, 2H), 4.92 (s, 2H), 4.91–4.89 (m, 2H), 4.88 (s, 2H), 1.27–1.22 (m, 6H). 13C-NMR (125 MHz, DMSO-d6) δ 148.5, 146.4, 142.4, 128.7, 127.9, 72.5, 61.1, 20.7. IR: νmax [cm−1]: 3445, 3339, 2962, 2925, 2853, 1735, 1597, 1582, 1450, 1410, 1365, 1259, 1085, 1014, 864, 794, 703, 661, 399. HRMS (m/z): [MH+]: 289.1507, calculated [M+]: 288.1474.
2.2.9. Preparation of 6-(((6-(1-hydroxyethyl)pyridin-2-yl)methoxy)methyl)picolinaldehyde (22, Scheme 3)
Bipyridine 22 is a byproduct; its synthesis is described in Section 2.2.8. It is a white crystalline solid, and its yield was 60 mg (23%).
M.p.: >300 °C (decomposition). Rf: 0.58 (SiO2, ethanol/toluene = 1:3). 1H-NMR (500 MHz, CDCl3) δ 9.99 (s, 1H), 7.88 (d, J = 7.3 Hz, 1H), 7.76 (dd, J = 7.5, 1.8 Hz, 1H), 7.71 (t, J = 7.7 Hz, 2H), 7.42 (d, J = 7.7 Hz, 1H), 7.16 (d, J = 7.8 Hz, 1H), 4.86 (s, 2H), 4.85–4.84 (m, 1H), 4.81 (s, 2H), 1.21–1.16 (m, 3H). 13C-NMR (125 MHz, DMSO-d6) δ 192.4, 148.2, 146.2, 142.6, 128.7, 128.3, 128.2, 128.0, 127.6, 127.1, 81.0, 72.7, 60.7, 21.6. IR: νmax [cm−1]: 3262, 2922, 2850, 1711, 1594, 1578, 1465, 1341, 1311, 1261, 1209, 1113, 1083, 1057, 1023, 992, 908, 791, 776, 759, 668, 633, 554. HRMS (m/z): [MH+]: 273.3097, calculated [M+]: 272.3040.
2.2.10. Preparation of 6-((benzyloxy)methyl)pyridine-2-carbaldehyde (23, Scheme 5)
The monobenzyl derivative of pyridinedimethanol (15, 2.35 g, 10.25 mmol) was dissolved in a mixture of DCM (65 mL), pyridine (2.56 mL) and water (2.75 mL). Dess–Martin reagent (5.65 g, 13.32 mmol) was added in small portions to this mixture, and it was stirred for 1 day at room temperature under an argon atmosphere. Silica gel (5.0 g) was added to the mixture, and the volatile components were evaporated. The crude product was purified by column chromatography on a silica gel adsorbent using a mixture of propane-2-ol/hexane = 1:10 as an eluent to give aldehyde 23 (1.91 g, 80%) as a pale-yellow viscous oil. Rf: 0.6 (SiO2, propane-2-ol/hexane = 1:10).
This product was identical in every aspect to that reported in [35].
2.2.11. Preparation of 1-{6-[(benzyloxy)methyl]pyridin-2-yl}ethan-1-ol (24, Scheme 5)
Aldehyde 23 (500 mg, 2.20 mmol) in diethyl ether (1 mL) was added dropwise to a stirred solution of methylmagnesium iodide (498 mg, 3.00 mmol) in 10 mL diethyl ether under an argon atmosphere for 10 min at room temperature. The mixture was refluxed for 2 h and then cooled to room temperature. Saturated aqueous NH4Cl solution (20 mL) was added to the mixture, and then the aqueous phase was extracted with diethyl ether (3 × 30 mL). The combined organic phase was dried with magnesium sulfate, filtered and evaporated. The crude product was purified by column chromatography on a silica gel adsorbent using a mixture of propan-2-ol/cyclohexane = 1:10 as an eluent to give alcohol 24 (476 mg, 89%) as a pale-yellow viscous oil.
Rf: 0.3 (SiO2, propane-2-ol/cyclohexane = 1:10). 1H-NMR (500 MHz, CDCl3) δ 7.62 (t, J = 7.7 Hz, 1H), 7.34–7.21 (m, 6H), 7.09 (d, J = 7,7 Hz, 1H), 4.79 (q, J = 6.6 Hz, 1H), 4.61 (s, 2H), 4.56 (s, 2H), 4.42 (broad s, 1H), 1.41 (d, J = 6.6 Hz, 3H). 13C-NMR (125 MHz, CDCl3) δ 162.3, 157.1, 137.9, 137.7, 128.5, 127.8, 127.8, 119.8, 118.5, 73.0, 72.7, 68.5, 24.2. IR: νmax [cm−1]: 3402 (broad), 3074, 3061, 3027, 2921, 2882, 2817, 1590, 1470, 1454, 1443, 1389, 1344, 1305, 1230, 1075, 1027, 916, 804, 735, 464. HRMS (m/z): [MH+]: 244.1293, calculated [M+]: 243.1259.
2.2.12. Preparation of 2-[(benzyloxy)methyl]-6-{1-[(trimethylsilyl)oxy]ethyl}pyridine (25, Scheme 5)
Alcohol 24 (500 mg, 2.06 mmol) was dissolved in a mixture of DCM (10 mL) and DIPEA (1.07 g, 8.28 mmol). Trimethylsilyl chloride (448 mg, 4.12 mmol) in 1 mL DCM was added dropwise to the mixture for 5 min at 0 °C under an argon atmosphere. The mixture was allowed to warm up to room temperature and stirred for 1 day. Water (50 mL) was added to the reaction mixture, and the aqueous phase was extracted with DCM (3 × 50 mL). The combined organic phase was dried with magnesium sulfate, filtered and evaporated. The crude product was purified by column chromatography on a silica gel adsorbent using a mixture of acetone/hexane = 1:30 as an eluent to give 25 (350 mg, 54%) as a pale-yellow viscous oil.
Rf: 0.3 (SiO2, acetone/hexane = 1:30). 1H-NMR (500 MHz, CDCl3) δ 7.70 (t, J = 7.8 Hz, 1H), 7.45–7.27 (m, 7H), 4.95 (q, J = 6.5 Hz, 1H), 4.68 (s, 2H), 4.65 (s, 2H), 1.47 (d, J = 6.5 Hz, 3H), 0.12 (s, 9H). 13C-NMR (125 MHz, CDCl3) δ 164.9, 157.2, 138.1, 137.5, 128.5, 127.9, 127.8, 119.6, 118.1, 73.2, 73.0, 71.8, 25.6, 0.1. IR: νmax [cm−1] (film): 3079, 3057, 3030, 2917, 2874, 2825, 1592, 1578, 1453, 1397, 1360, 1346, 1250, 1152, 1115, 1099, 1078, 1028, 992, 958, 890, 841, 800, 736, 697, 622. HRMS (m/z): [MH+]: 316.1688, calculated [M+]: 315.1655.
2.2.13. Preparation of 2-[(benzyloxy)methyl]-6-{1-[(tert-butyldimethylsilyl)oxy]ethyl}pyridine (27, Scheme 5)
Alcohol 24 (110 mg, 0.45 mmol) was dissolved in a mixture of THF (2.5 mL) and DIPEA (234 mg, 1.81 mmol). Tert-butyldimethylsilyl chloride (136 mg, 0.90 mmol) in 0.5 mL THF was added dropwise to the mixture, and it was heated to 60 °C and stirred at this temperature for 1 day. After the reaction was complete, the volatile components were evaporated. The crude product was purified by PTLC on a silica gel adsorbent using a mixture of acetone/hexane = 1:10 as an eluent to give 27 (30 mg, 19%) as a pale-yellow viscous oil.
Rf: 0.6 (SiO2, acetone/hexane = 1:10). 1H-NMR (500 MHz, CDCl3) δ 7.76 (t, J = 7.7 Hz, 1H), 7.49 (d, J = 7.8 Hz, 1H), 7.42–7.27 (m, 6H), 5.03–4.99 (m, 1H), 4.73 (s, 2H), 4.68 (s, 2H), 1.48 (d, J = 6,4 Hz, 3H), 0.94 (s, 9H), 0.11 (s, 3H), 0.04 (s, 3H). 13C-NMR (125 MHz, CDCl3) δ 165.1, 156.8, 138.0, 137.7, 128.4, 127.9, 127.8, 119.6, 118.1, 73.0, 72.7, 71.7, 25.9, 25.8, 18.2, −4.8, −4.9. IR: νmax [cm−1]: 2953, 2928, 2884, 1594, 1578, 1455, 1390, 1361, 1253, 1115, 1080, 1020, 736, 622, 606. HRMS (m/z): [MH+]: 358.2158, calculated [M+]: 357.2124.
2.2.14. Preparation of (6-{[(tert-butyldimethylsilyl)oxy]methyl}pyridin-2-yl)methanol (29, Scheme 7)
Diol 14 (3.78 g, 27.20 mmol) was dissolved in DMF (112 mL). Tert-butyldimethylsilyl chloride (4.52 g, 30.00 mmol) and dibutyltin oxide (6.72 g, 27.00 mmol) were added to the solution, and then the resulting mixture was heated to 80 °C and stirred under argon at this temperature. After 2 days, the volatile components were evaporated. The residue was taken up in DCM (200 mL) and extracted with aqueous NaOH solution (100 mL, 10 w/w%). The precipitate was filtered, and the organic phase was dried with magnesium sulfate, filtered and evaporated. The crude product was purified by column chromatography on a silica gel absorbent using a gradient elution of a propan-2-ol/cyclohexane mixture (1→20 V/V% propane-2-ol) to give monoprotected pyridinemethanol 29 (1.24 g, 18%) as a pale-yellow viscous oil. Rf: 0.35 (SiO2, propane-2-ol/hexane = 1:10).
This product was identical in every aspect to that reported in [36].
2.2.15. Preparation of 2,6-bis{[(tert-butyldimethylsilyl)oxy]methyl}pyridine (30, Scheme 7)
Double-protected pyridine derivative 30 is a byproduct; its synthesis is described in Section 2.2.14. Its yield was 4.29 g (43%). It is a pale-yellow viscous oil.
Rf: 0.65 (SiO2, propane-2-ol/hexane = 1:10), 0.6 (SiO2, propane-2-ol/hexane = 1:20), 0.4 (SiO2, propane-2-ol/hexane = 1:50). 1H-NMR (500 MHz, CDCl3) δ 7.72 (t, J = 7.7 Hz, 1H), 7.37 (d, J = 7.7 Hz, 2H), 4.80 (s, 4H), 0.96 (s, 18H), 0.12 (s, 12H). 13C-NMR (125 MHz, CDCl3) δ 160.2, 137.3, 118.0, 66.1, 25.9, 18.4, −5.3, −5.4. IR: νmax [cm−1]: 2954, 2929, 2885, 2856, 1593, 1579, 1471, 1461, 1450, 1389, 1362, 1254, 1112, 1006, 909, 835, 776, 736, 668, 606. HRMS (m/z): [MH+]: 368.2396, calculated [M+]: 367.2363.
2.2.16. Preparation of 6-{[(tert-butyldimethylsilyl)oxy]methyl}pyridine-2-carbaldehyde (31, Scheme 7)
Alcohol 29 (1.22 g, 4.81 mmol) was dissolved in a mixture of DCM (65 mL), pyridine (2.6 mL) and water (2.8 mL). Dess–Martin reagent (3.06 g, 7.22 mmol) was added to this solution in small portions, and the resulting mixture was stirred for 1 day at room temperature under an argon atmosphere. When the reaction was complete, hexane (25 mL) was added to the reaction mixture, and it was filtered. The filtrate was evaporated, and the crude product was purified by column chromatography on a silica gel adsorbent using a mixture of propane-2-ol/cyclohexane = 1:50 as an eluent to give 31 (0.85 g, 70%) as a pale-yellow viscous oil. Rf: 0.1 (SiO2, propane-2-ol/cyclohexane = 1:100), 0.4 (SiO2, propane-2-ol/cyclohexane = 1:50).
This product was identical in every aspect to that reported in [15].
2.2.17. Preparation of 1-(6-{[(tert-butyldimethylsilyl)oxy]methyl}pyridin-2-yl)ethan-1-ol (32, Scheme 7)
Aldehyde 31 (815 mg, 3.24 mmol) in diethyl ether (1.5 mL) was added dropwise to a stirred solution of methylmagnesium iodide (747 mg, 4.50 mmol in 10 mL diethyl ether) under an argon atmosphere at room temperature for 10 min. The mixture was refluxed for 30 min and then cooled to room temperature. Saturated aqueous NH4Cl solution (20 mL) was added to the mixture, and the aqueous phase was extracted with diethyl ether (3 × 20 mL). The combined organic phase was dried with magnesium sulfate, filtered and evaporated. The product did not need purification. Alcohol 32 (550 mg, 63%) is a pale-yellow viscous oil. Rf: 0.3 (SiO2, propane-2-ol/cyclohexane = 1:20).
This product was identical in every aspect to that reported in [15].
2.2.18. Preparation of 2-{[(tert-butyldimethylsilyl)oxy]methyl}-6-[1-(methoxymethoxy)ethyl]pyridine (33, Scheme 7)
Alcohol 32 (490 mg, 1.83 mmol) was added to a mixture of DCM (10 mL) and DIPEA (1040 mg, 8.05 mmol). Methoxymethylene chloride (440 mg, 5.47 mmol) in 10 mL DCM was added dropwise to this solution for 20 min at 0 °C under an argon atmosphere. The mixture was warmed up to room temperature and stirred for 3 days. The volatile components were evaporated, and the crude product was purified by column chromatography on a silica gel adsorbent using a mixture of propane-2-ol/hexane = 1:70 as an eluent to give 33 (480 mg, 84%) as a pale-yellow viscous oil.
Rf: 0.5 (SiO2, propane-2-ol/hexane = 1:70). 1H-NMR (500 MHz, CDCl3) δ 7.69 (t, J = 7.7 Hz, 1H), 7.40 (d, J = 7.5 Hz, 1H), 7.27 (d, J = 7.8 Hz, 1H), 4.84–4.76 (m, 3H), 4.64 (dd, J = 43.7, 28.1 Hz, 2H), 3.35 (s, 3H), 1.48 (d, J = 6.6 Hz, 3H), 0.94 (s, 9H), 0.11 (s, 6H). 13C-NMR (125 MHz, CDCl3) δ 161.7, 160.7, 137.3, 118.5, 118.2, 94.9, 75.5, 66.1, 55.5, 25.9, 18.4, −5.3, −5.4. IR: νmax [cm−1]: 2954, 2929, 2887, 2857, 1593, 1579, 1462, 1362, 1255, 1216, 1156, 1102, 1081, 1035, 1005, 993, 920, 851, 835, 815, 798, 777, 671, 599. HRMS (m/z): [MH+]: 312.1950, calculated [M+]: 311.1917.
2.2.19. Preparation of {6-[1-(methoxymethoxy)ethyl]pyridin-2-yl}methanol (34, Scheme 7)
Tetrabutylammonium fluoride (604 mg, 2.31 mmol) in 2.5 mL acetonitrile was added dropwise to diprotected pyridine derivative 33 (480 mg, 1.54 mmol) in acetonitrile (7.5 mL) for 10 min. The mixture was stirred for 2 days at room temperature, and then the volatile components were evaporated. The residue was taken up in diethyl ether (20 mL) and water (20 mL). The aqueous phase was extracted with ether (3 × 15 mL). The combined organic phase was dried with magnesium sulfate, filtered and evaporated. The crude product was purified by column chromatography on a silica gel adsorbent using a mixture of propane-2-ol/hexane = 1:6 as an eluent to give 34 (280 mg, 92%) as a pale-yellow viscous oil.
Rf: 0.4 (SiO2, propane-2-ol/hexane = 1:6), 0.15 (SiO2, propane-2-ol/hexane = 1:10). 1H-NMR (500 MHz, CDCl3) δ 7.67 (t, J = 7.7 Hz, 1H), 7.32 (d, J = 7.7 Hz, 1H), 7.14 (d, J = 7.7 Hz, 1H), 4.83 (q, J = 6.6 Hz, 1H), 4.72 (s, 2H), 4.63 (dd, J = 45.7, 32.0 Hz, 2H), 3.34 (s, 3H), 1.48 (d, J = 6.5 Hz, 3H). 13C-NMR (125 MHz, CDCl3) δ 161.9, 158.5, 137.6, 119.4, 118.9, 95.0, 75.2, 64.0, 55.6, 22.2. IR: νmax [cm−1]: 3389 (wide), 2931, 2890, 2824, 1595, 1578, 1460, 1370, 1217, 1157, 1099, 1073, 1030, 993, 918, 841, 800, 754, 733, 611. HRMS (m/z): [MH+]: 198.1085, calculated [M+]: 197.1052.
2.2.20. Preparation of {6-[1-(methoxymethoxy)ethyl]pyridin-2-yl}methyl 4-methylbenzenesulfonate (35, Scheme 7)
Alcohol 34 (100 mg, 0.51 mmol) was added to a mixture of DCM (8 mL) and aqueous KOH solution (8 mL, 40 w/w%). Tosyl chloride (156 mg, 0.82 mmol) in 2 mL DCM was added dropwise to this mixture with vigorous stirring at 0 °C. The mixture was warmed up to room temperature and stirred for 3 days. After the reaction was complete, water (10 mL) and DCM (10 mL) were added to this mixture. The phases were separated, and the aqueous phase was extracted with DCM (3 × 15 mL). The combined organic phase was dried with magnesium sulfate, filtered and evaporated. Tosylate 35 (159 mg, 89%) is a pale-yellow viscous oil.
Rf: 0.4 (SiO2, propane-2-ol/hexane = 1:10). 1H-NMR (500 MHz, CDCl3) δ 7.82 (d, J = 8.3 Hz, 2H), 7.68 (t, J = 7.8 Hz, 1H), 7.36–7.28 (m, 4H), 5.12 (s, 2H), 4.74 (q, J = 6.6 Hz, 1H), 4.61 (dd, J = 55.1, 41.7 Hz, 2H), 3.32 (s, 3H), 2.43 (s, 3H), 1.43 (d, J = 6.6 Hz, 3H). 13C-NMR (125 MHz, CDCl3) δ 162.7, 153.2, 145.1, 137.7, 132.9, 130.0, 128.2, 120.4, 119.9, 95.0, 75.3, 71.9, 55.6, 22.2, 21.7. IR: νmax [cm−1]: 2930, 2888, 2823, 1596, 1578, 1494, 1461, 1361, 1308, 1213, 1189, 1175, 1158, 1097, 1074, 1032, 991, 953, 917, 836, 813, 753, 668, 545. HRMS (m/z): [MH+]: 352.1174, calculated [M+]: 351.1140.
2.2.21. Preparation of 6,6′-[oxybis(methylene)]bis{2-[1-(methoxymethoxy)ethyl]pyridine} (36, Scheme 7)
Alcohol 34 (86 mg, 0.44 mmol) in THF (3 mL) was added dropwise to a stirred suspension of sodium hydride (105 mg, 2.62 mmol) in THF (1.5 mL) under an argon atmosphere. The mixture was stirred for 30 min at room temperature and for 2 h at 60 °C. Tosylate 35 (153 mg, 0.44 mmol) in a mixture of THF (3 mL) and DMF (1 mL) was added dropwise to the reaction mixture, and it was stirred for 3 days at 60 °C. After the reaction was complete, the volatile components were evaporated. The residue was taken up in ice-water (20 mL) and DCM (20 mL). The phases were separated, and the aqueous phase was shaken with DCM (3 × 15 mL). The combined organic phase was dried with magnesium sulfate, filtered and evaporated. The crude product was purified by column chromatography on a silica gel adsorbent using a mixture of propane-2-ol/hexane = 1:15 as an eluent to give dipyridine 36 (50 mg, 30%) as a pale-yellow viscous oil.
Rf: 0.1 (SiO2, propane-2-ol/hexane = 1:15). 1H-NMR (500 MHz, CDCl3) δ 7.71 (t, J = 7.7 Hz, 2H), 7.43 (d, J = 7.7 Hz, 2H), 7.34 (d, J = 7.7 Hz, 2H), 4.83 (q, J = 6.7 Hz, 2H), 4.77 (s, 4H), 4.66 (dd, J = 42.8, 29.1 Hz, 4H), 3.37 (s, 6H), 1.51 (d, J = 6.6 Hz, 6H). 13C-NMR (125 MHz, CDCl3) δ 162.4, 157.7, 137.3, 119.9, 118.8, 94.9, 75.5, 73.8, 55.5, 22.3. IR: νmax [cm−1]: 2928, 2887, 2822, 1592, 1577, 1458, 1400, 1366, 1341, 1260, 1215, 1155, 1099, 1032, 992, 918, 843, 801, 753, 599. HRMS (m/z): [MH+]: 377.2032, calculated [M+]: 376.1998.
2.2.22. Preparation of 1,1′-{[oxybis(methylene)]bis(pyridine-6,2-diyl)}bis(ethan-1-ol) (21, Scheme 7)
Aqueous HCl solution (2 mL, 1.0 M) was added dropwise to a solution of MOM-protected dipyridine derivative 36 (100 mg, 0.27 mmol) in 2 mL dioxane. The resulting reaction mixture was stirred at room temperature for 24 h, and then the volatile components were removed. The residue was taken up in water (20 mL) and ethyl acetate (20 mL). The aqueous phase was saturated with sodium chloride, and the phases were separated. The aqueous phase was extracted with ethyl acetate (3 × 15 mL). The combined organic phase was dried over anhydrous sodium sulfate, filtered and evaporated. The residue was triturated with methanol to give the compound dipyridine diol 21 as yellow crystals (67 mg, 0.27 mmol, 88%).
The product was identical in every aspect to that reported in Section 2.2.8.
3. Results and Discussion
The first attempted synthetic pathway started with the commercially available 2,6-pyridinedimethanol (14 in Scheme 3). It is important to note that some pyridino-intermediates have already been reported, but they were obtained through a more difficult synthetic route, for example, the ones obtained by Tsukube and Newcomb [1,6].
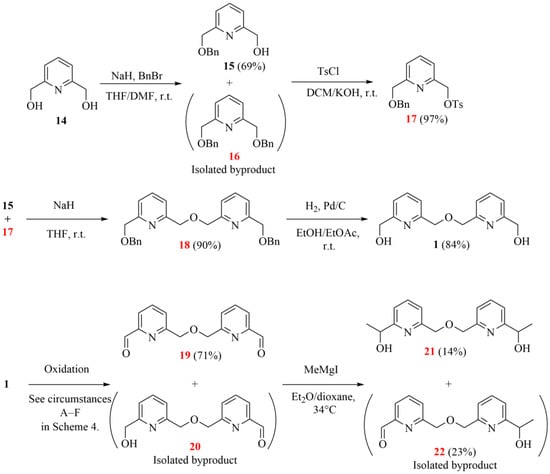
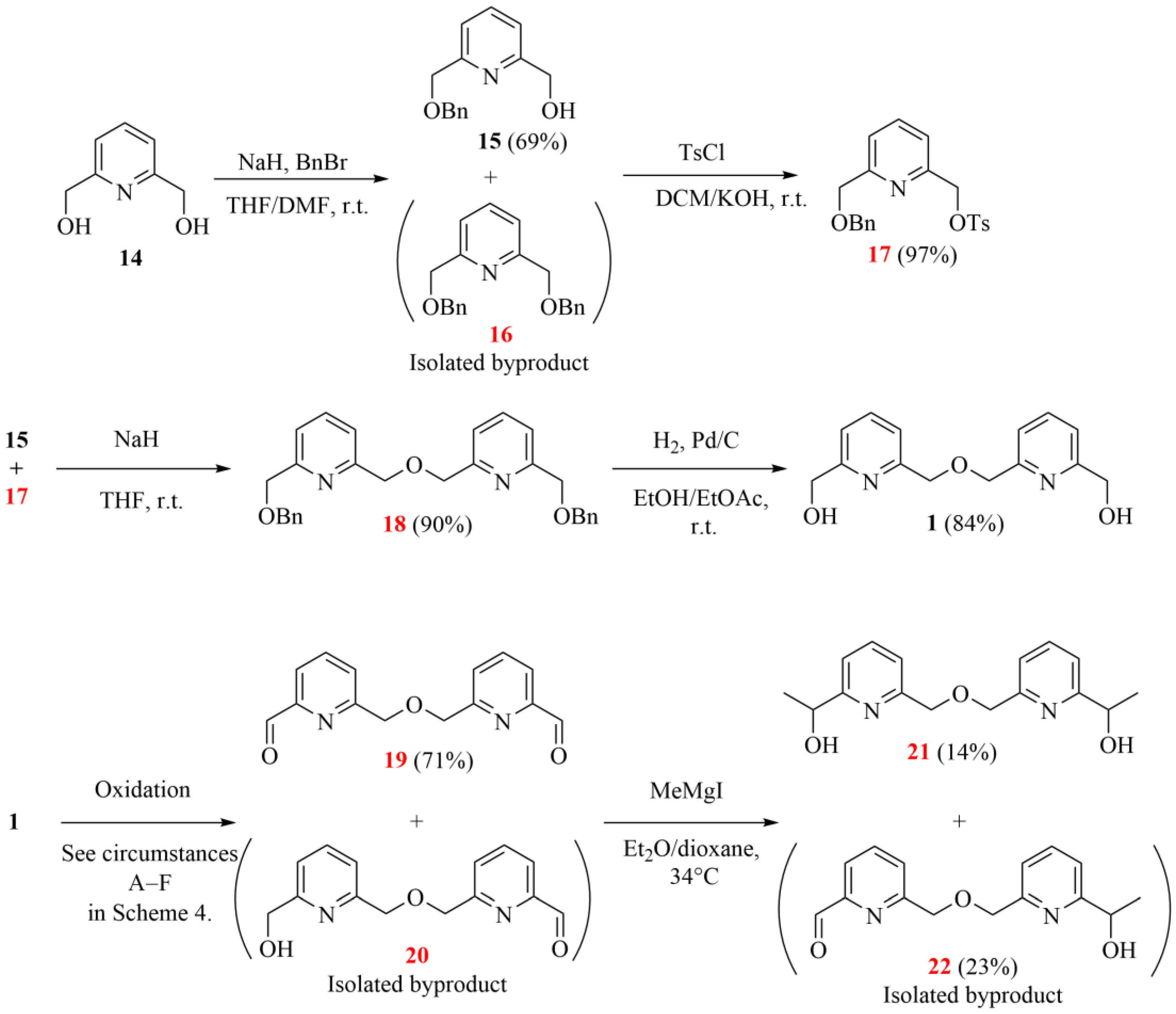

Scheme 3.
The initially attempted synthetic strategy to obtain the desired chiral bipyridine diol intermediate (the herein characterized new compounds are numbered in red).
Scheme 3.
The initially attempted synthetic strategy to obtain the desired chiral bipyridine diol intermediate (the herein characterized new compounds are numbered in red).

The benzylation in the first step resulted in the monoprotected derivative (15) as the major product with an acceptable yield, besides the formation of the expected dibenzylated derivative (16), which could be removed easily by normal-phase flash chromatography. (Replacing NaH with NaOH resulted in a higher proportion of the dibenzylated product (16), while it was the major product (70%) when using DMSO instead of a THF/DMF solvent mixture.) The free hydroxyl group of 15 was then tosylated to provide a suitable monofunctionalized intermediate (17) for coupling with a second pyridine unit. After coupling these two pyridine intermediates (15 and 17), the resulting bipyridine (18) was deprotected by catalytic hydrogenation under generally applied conditions. Subsequently, the oxidation of bipyridine diol 1 was carried out in several ways to give the corresponding symmetric dialdehyde (19) (more information can be found in Scheme 4), which could be methylated by a Grignard reaction in the last step.
Unfortunately, the methylation only resulted in a low yield due to the poor solubility of the starting dial (19) in the generally applied ether-type solvents. Additionally, the resulting mono- and diol (22 and 21 and their salts) were also poorly soluble in the reaction mixture. The yield could not be significantly increased even under harsher conditions. Thus, further strategies were also attempted to improve the overall efficiency of the intermediate synthesis.
The reactions for the oxidation of diol 1 are shown in Scheme 3.
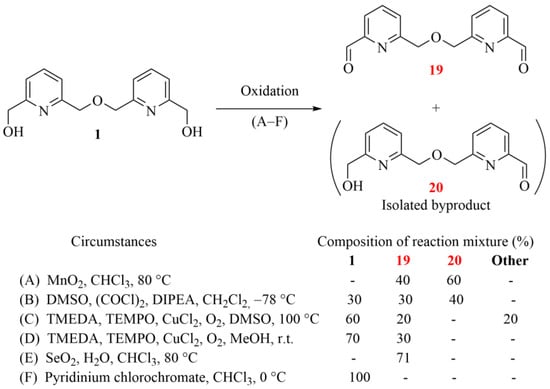
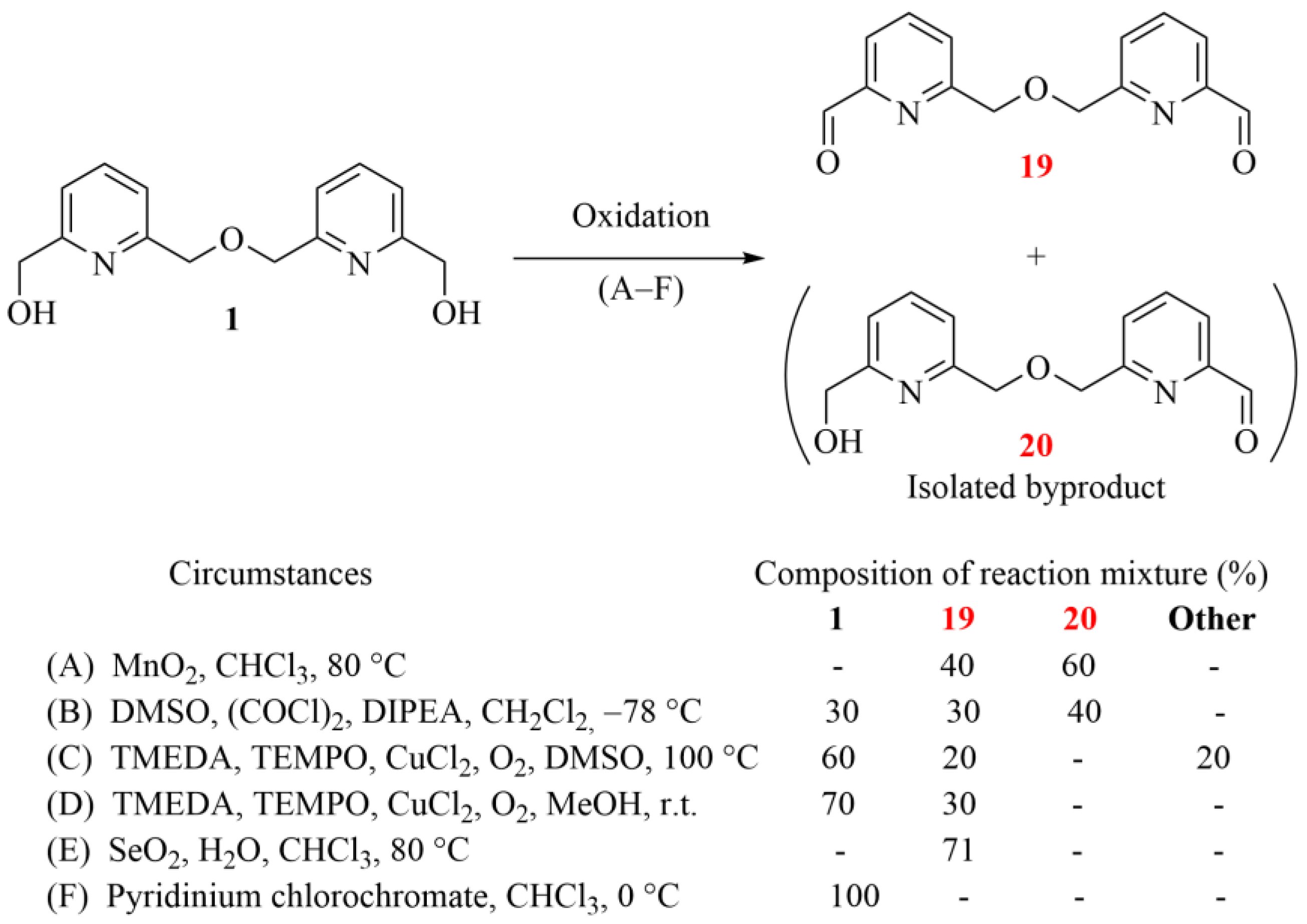

Scheme 4.
Reactions for the oxidation of bipyridine diol 1 to the corresponding dialdehyde (19), the intermediate for the subsequent Grignard reaction (yields are indicated by ‘-’ when the ratio of the corresponding component did not reach 5%; the herein characterized new compounds are numbered in red).
Scheme 4.
Reactions for the oxidation of bipyridine diol 1 to the corresponding dialdehyde (19), the intermediate for the subsequent Grignard reaction (yields are indicated by ‘-’ when the ratio of the corresponding component did not reach 5%; the herein characterized new compounds are numbered in red).

Conventional oxidation methods and some recent alternatives were also attempted. Since we aimed to synthesize symmetric bifunctional precursors for macrocyclization, the target product was only dialdehyde 19; monoaldehyde 20 was obtained as an undesired byproduct. Other components could not be isolated—only the two aldehydes (19, 20) and the starting diol (1)—in each test reaction.
The conventional method with MnO2 (see method (A) in Scheme 4) mainly resulted in the mono-oxidation of the diol (1), besides obtaining the desired dialdehyde (19) in parallel, but only as a minor product. Swern oxidation (see method (B) in Scheme 4 resulted in reduced conversion under the applied conditions while still giving the undesired 20 as the major product. The TMEDA-mediated radical reaction under an oxygen atmosphere proved to be less effective, but showed selectivity for the dialdehyde (19) at a low-conversion stage. Finally, diol 1 was most effectively oxidized to dialdehyde 19 by using SeO2 with a catalytic amount of water under harsh conditions. Moreover, this method showed selectivity toward dialdehyde 19. Surprisingly, the commonly applied pyridinium chlorochromate gave no conversion in the range between 0 °C and 25 °C. Although oxidation could be induced under harsher conditions (>60 °C), in these cases, several side reactions were also observed, including the cleavage of the ether bond.
A second synthetic pathway was carried out to improve the overall yield of the first one (Scheme 5).
The synthesis started from the same pyridinedimethanol (14), but the oxidation was carried out on its monobenzylated derivative 15 instead of building the bipyridine skeleton first. As in the case of oxidizing 15, the previously successful SeO2-based method showed lower conversion under the same conditions, so we looked for other alternatives. The Dess–Martin reaction proved to be especially advantageous, providing a high conversion and a pure product profile at the same time. Aldehyde 23 was subjected to the Grignard reaction, which could be effectively performed, but the silyl ethers (25 and 27) showed poor stability during their chromatographic purification in the next steps. (Silyl-ethers were chosen based on their stability in the hydrogenation step for the planned debenzylation. However, slight decomposition was observed even under an inert atmosphere and at low temperature.)
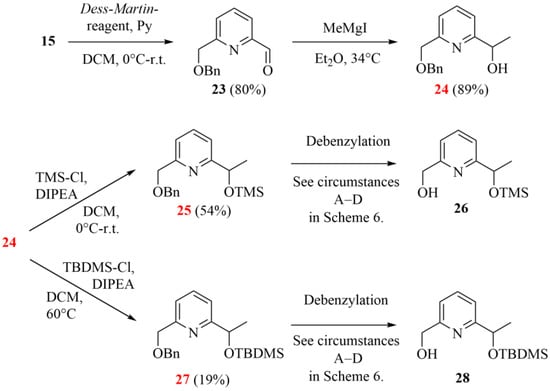
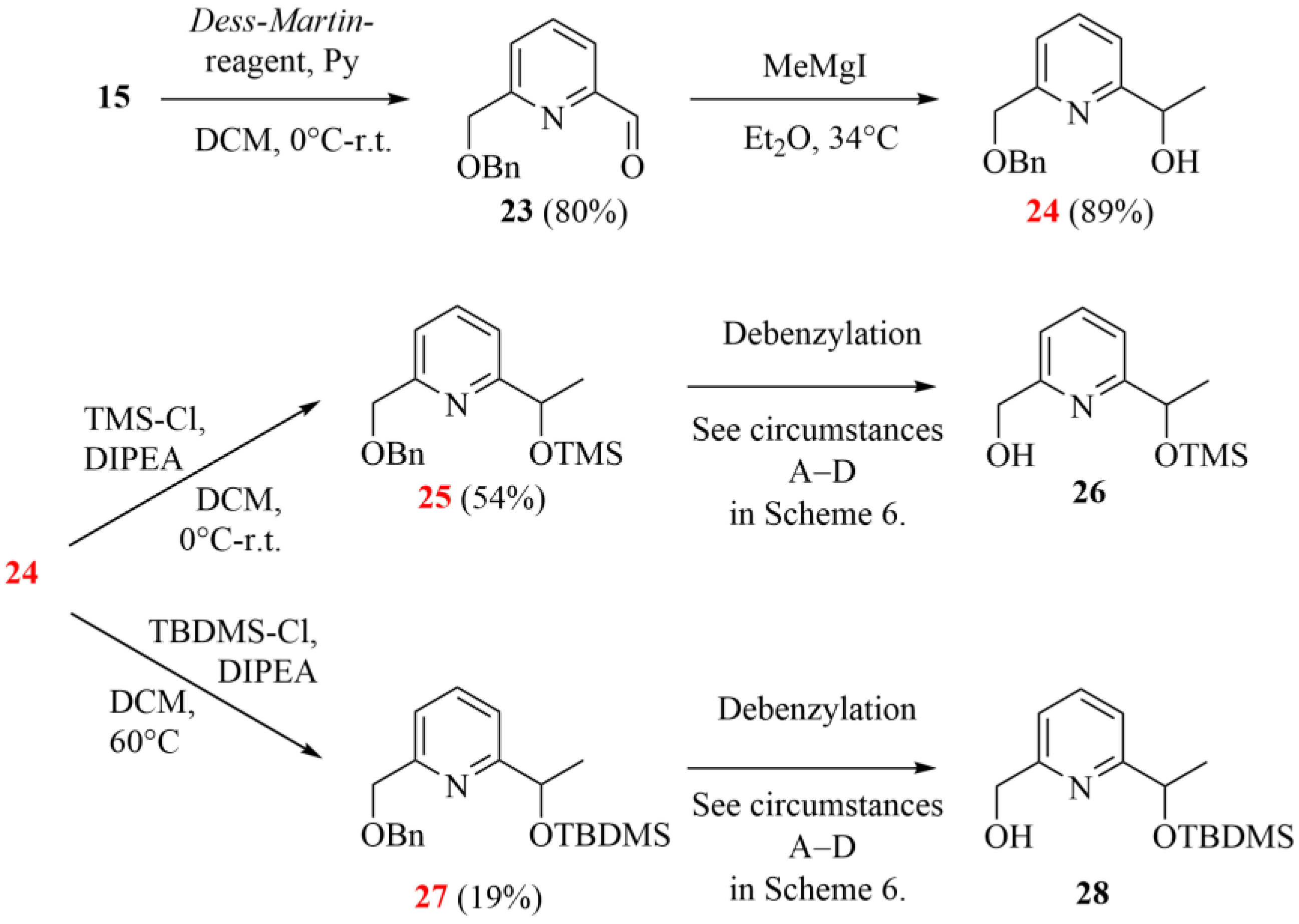

Scheme 5.
Second synthetic strategy to gain monoprotected chiral pyridine precursors (24, 26 and 27; the herein characterized new compounds are numbered in red).
Scheme 5.
Second synthetic strategy to gain monoprotected chiral pyridine precursors (24, 26 and 27; the herein characterized new compounds are numbered in red).

During the final debenzylation step providing the chiral monopyridine building blocks (see 26 and 28 in Scheme 5), we faced additional difficulties. Surprisingly, the conventional catalytic hydrogenations did not work at ambient temperature, and the addition of catalytic amounts of strong acids or raising the temperature resulted in the cleavage of the Si-O-C ether groups, too. Moreover, the expected product was present only in a negligible proportion in the reaction mixtures. It is important to highlight that there are three near-equivalent possibilities to break the bond as both ethereal oxygens are directly connected to a benzyl-type carbon, which reduces the selectivity of the debenzylations. The applied conditions for the attempted debenzylation step are summarized in Scheme 6.
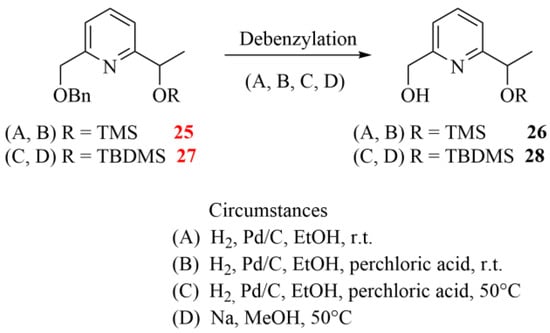
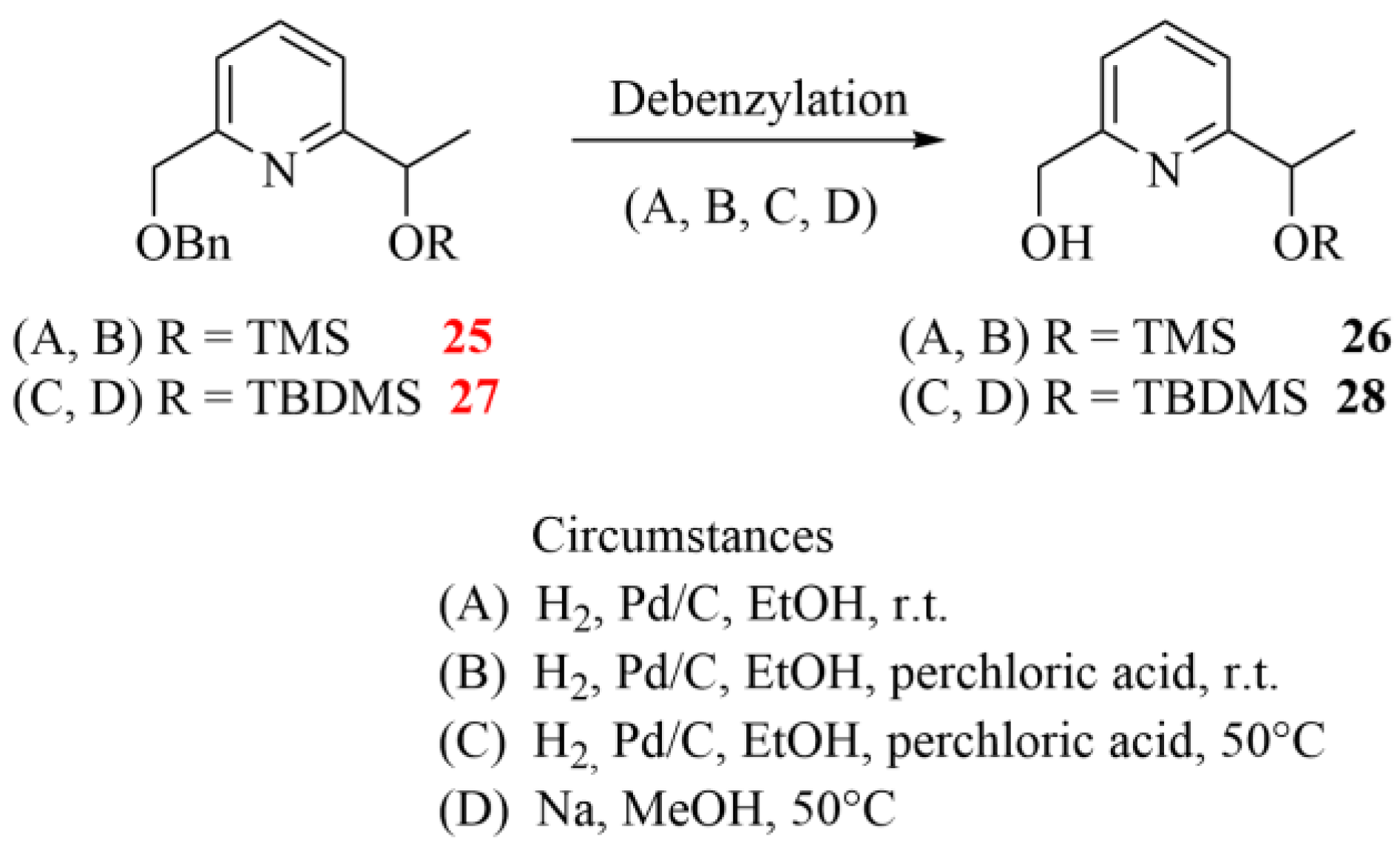

Scheme 6.
Attempted debenzylation of pyridine diethers 25 and 27 under different conditions (the herein characterized new compounds are numbered in red).
Scheme 6.
Attempted debenzylation of pyridine diethers 25 and 27 under different conditions (the herein characterized new compounds are numbered in red).

Finally, a longer synthetic route—reported in Scheme 7—provided the highest overall yield with less difficulties.
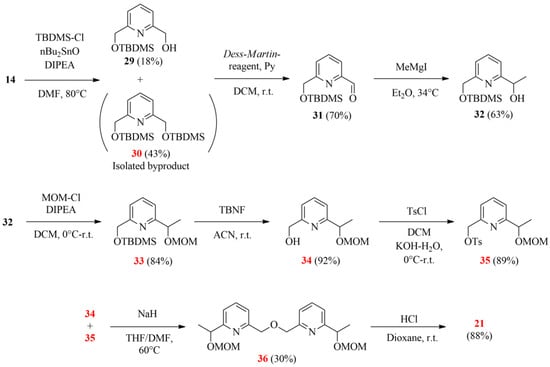
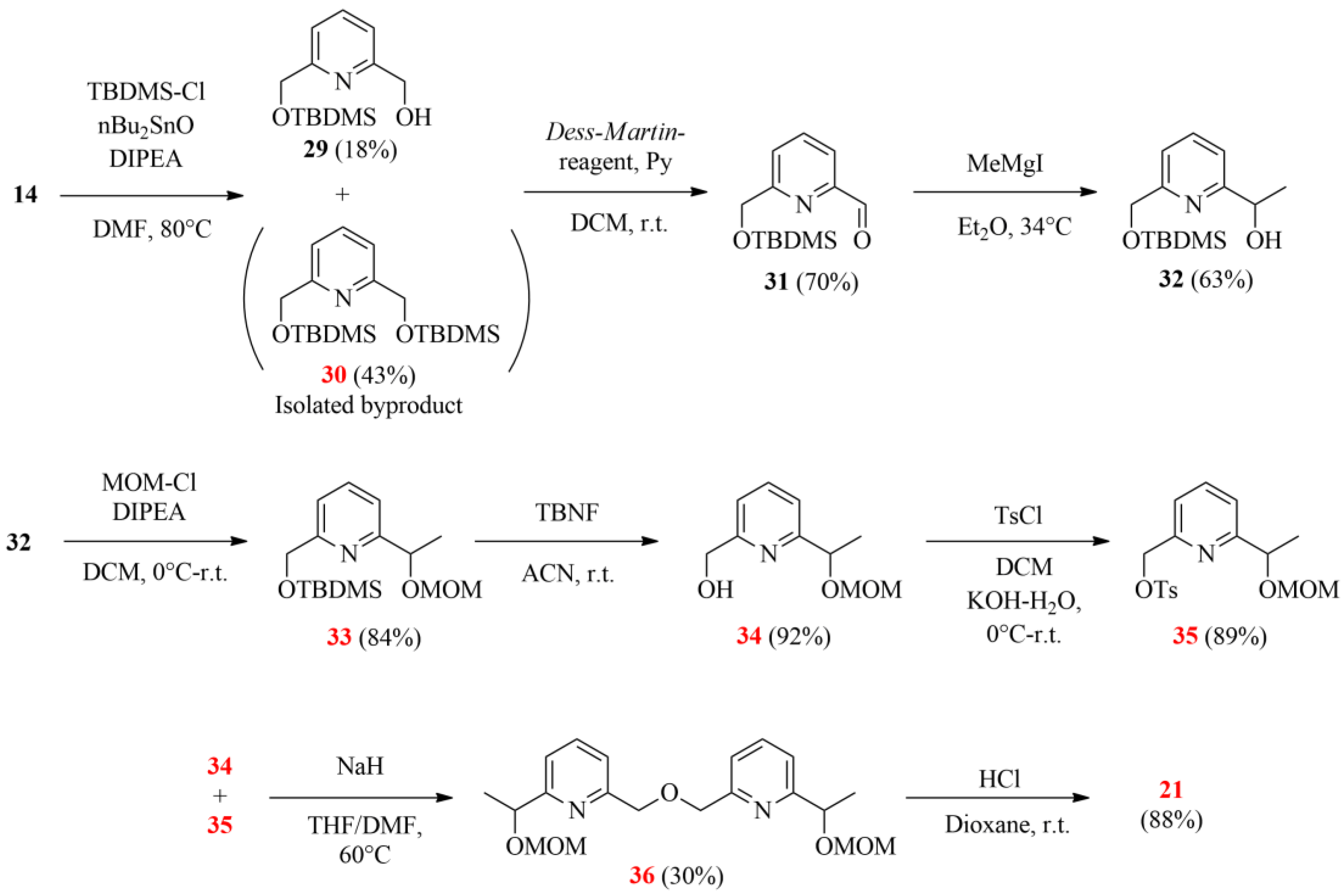

Scheme 7.
The suggested improved strategy for synthesizing the desired bipyridine diol (21; the herein characterized new compounds are numbered in red).
Scheme 7.
The suggested improved strategy for synthesizing the desired bipyridine diol (21; the herein characterized new compounds are numbered in red).

In the initial step, the monoprotection of 14 was carried out by introducing the O-TBDMS group as it showed higher stability compared to O-TMS in our previous experiments. Despite adding dibutyltin oxide, only poor selectivity was reached, probably due to solubility reasons. The poor yield was attributed to the low efficiency of the necessary chromatographic purification. The synthesis was continued with a Dess–Martin oxidation followed by a Grignard reaction, similar to the previous pathways. For the protection of secondary alcohol 32, the methoxymethyl group (MOM) was chosen, the removal of which is favored under acidic conditions, and it remains stable during the deblocking of the TBDMS protecting group. After obtaining the orthogonally double-protected 33, the O-silyl protecting group was selectively removed by using tetrabutylammonium fluoride (TBNF) at ambient temperature. Half of the resulting monohydroxy derivative 34 was then converted to tosylate 35, and they were coupled to gain bipiridine 36. Although the formation of the latter bipyridine resulted in a low yield, the deprotection in acidic conditions was quite effective, giving 14.
4. Conclusions
We reported the synthesis and characterization of 15 new pyridine derivatives (16–22, 24, 25, 27, 30, 33–36), which can serve as potential building blocks of chiral multiheteroaromatic macrocycles. A simplified and improved procedure was described for the preparation of bipyridine diols, while possible synthetic difficulties of the attempted synthetic strategies were also discussed.
According to our experience, it is suggested that the oxidation of pyridine methanols to the corresponding aldehydes be performed through a Dess–Martin reaction, which greatly surpasses conventional oxidizing agents. Benzylation is favored for selective O-monoprotection supplemented with orthogonal MOM protection, if possible. However, despite the good feasibility of selective O-benzylations, the effective cleavage of the O-benzyl groups is not possible due to the copresence of additional benzyl-like carbon in the molecule. Thus, benzyl protection is not recommended overall. Silyl ether-type O-protective groups showed poor stability in general besides the low yields of the protection. TBDMS is obviously favored over TMS. Moreover, it is better to build the chirality centers on monofunctional derivatives due to selectivity reasons.
In summary, despite the obtained moderate yields and synthetic difficulties, the reported methods provide improved and simplified alternatives to the previously reported ones while producing several widely applicable new pyridine intermediates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org6020018/s1, Figure S1: 1H-NMR spectrum of 16; Figure S2: 13C-NMR spectrum of 16; Figure S3: 1H-NMR spectrum of 17; Figure S4: 13C-NMR spectrum of 17; Figure S5: 1H-NMR spectrum of 18; Figure S6: 13C-NMR spectrum of 18; Figure S7: 1H-NMR spectrum of 19; Figure S8: 13C-NMR spectrum of 19; Figure S9: 1H-NMR spectrum of 20; Figure S10: 13C-NMR spectrum of 20; Figure S11: 1H-NMR spectrum of 21; Figure S12: 13C-NMR spectrum of 21; Figure S13: 1H-NMR spectrum of 22; Figure S14: 13C-NMR spectrum of 22; Figure S15: 1H-NMR spectrum of 24; Figure S16: 13C-NMR spectrum of 24; Figure S17: 1H-NMR spectrum of 25; Figure S18: 13C-NMR spectrum of 25; Figure S19: 1H-NMR spectrum of 27; Figure S20: 13C-NMR spectrum of 27; Figure S21: 1H-NMR spectrum of 30; Figure S22: 13C-NMR spectrum of 30; Figure S23: 1H-NMR spectrum of 33; Figure S24: 13C-NMR spectrum of 33; Figure S25: 1H-NMR spectrum of 34; Figure S26: 13C-NMR spectrum of 34; Figure S27: 1H-NMR spectrum of 35; Figure S28: 13C-NMR spectrum of 35; Figure S29: 1H-NMR spectrum of 36; Figure S30: 13C-NMR spectrum of 36.
Author Contributions
Conceptualization, P.H. and Á.G.; formal analysis, P.V.; investigation, B.J., A.A., P.K., B.F., M.T. and P.V.; methodology, P.H. and Á.G.; project administration, Á.G.; resources, P.H.; supervision, T.T., P.H. and Á.G.; visualization, B.J. and A.A.; writing—original draft, B.J., A.A. and Á.G.; writing—review and editing, P.V., T.T. and P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the EKÖP-24-4-II-BME-63 University Research Fellowship Programme and by the Doctoral Excellence Fellowship Programme (DCEP-25-1-BME-3) of the Ministry for Culture and Innovation through the National Research, Development, and Innovation Fund.

Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors express their thanks to Dániel Ster and Rita Molnárné Bernáth for their valuable technical assistance during this work. Thanks to György Tibor Balogh, Balázs Simon and András Marton for their help in structure elucidation. Thanks are also due to Viktória Fábos for enabling measurements at X-Chem, Inc.
Conflicts of Interest
The authors declare no conflicts of interest. The funding institution had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Newcomb, M.; Gokel, G.W.; Cram, D.J. Pyridyl unit in host compounds. J. Am. Chem. Soc. 1974, 96, 6810–6811. [Google Scholar] [CrossRef]
- Newcomb, M.; Timko, J.M.; Walba, D.M.; Cram, D.J. Host-guest complexation. 3. Organization of pyridyl binding sites. J. Am. Chem. Soc. 1977, 99, 6392–6398. [Google Scholar] [CrossRef]
- Voegtle, F.; Oepen, G.; Rasshofer, W. Complexes between neutral molecules. V. Urea, thiourea and malonodinitrile complexes of noncyclic crown-type polyethers with pyridine N-oxide subunits. Liebigs Ann. Chem. 1979, 10, 1577–1584. [Google Scholar] [CrossRef]
- Weber, E.; Josel, H.P.; Puff, H.; Franken, S. Solid-state inclusion compounds of new host macrocycles with uncharged organic molecules. Host synthesis, inclusion properties, and x-ray crystal structure of an inclusion compound with n-propanol. J. Org. Chem. 1985, 50, 3125–3132. [Google Scholar] [CrossRef]
- Diebold, A.; Fischer, J.; Weiss, R. Synthesis and spectroscopic and structural characterization of potentially heterodinucleating ligands containing a meso-diphenylporphyrin. New J. Chem. 1996, 20, 959–970. [Google Scholar]
- Tsukube, H.; Shinoda, S.; Uenishi, J.I.; Hiraoka, T.; Imakoga, T.; Yonemitsu, O. Ag+-specific pyridine podands: Effects of ligand geometry and stereochemically controlled substitution on cation complexation and transport functions. J. Org. Chem. 1998, 63, 3884–3894. [Google Scholar] [CrossRef]
- Onozawa, T.; Sakakura, T.; Tanaka, M. Preparation of Novel Crown Compounds by Cyclization of Aromatic 1,3-Dialdehydes. Patent No. JP 07247282, 26 September 1995. [Google Scholar]
- He, Y.-B.; Cai, H.-B.; Meng, L.-Z.; Wu, C.-T. Syntheses of azacrown compounds containing pyridine ring and central functional group. Gaodeng Xuexiao Huaxue Xuebao 1997, 18, 1974–1977. [Google Scholar]
- Newkome, G.R.; Lee, H.W.; Fronczek, F.R. A New route to macrocycles possessing the 2,6-pyridino subunit via bis-1,3-dithianes [1]. Isr. J. Chem. 1986, 27, 87–95. [Google Scholar] [CrossRef]
- Eisenbach, C.D.; Schubert, U.S.; Baker, G.R.; Newkome, G.R. A general synthetic strategy for functionalized oligo(bipyridines): New building blocks for supramolecular chemistry and their first application in macromolecules. J. Chem. Soc. Chem. Commun. 1995, 1, 69–70. [Google Scholar] [CrossRef]
- He, Y.B.; Lehn, J.-M. Synthesis of a novel macrocyclic ligand containing bipyridine units. Chin. J. Chem. 2000, 18, 384–387. [Google Scholar] [CrossRef]
- He, Y.-b.; Xiao, Y.-J.; Meng, L.-z. Syntheses of Macrocyclic Ligand Containing Bipyridine Subunit. J.-Wuhan Univ. Nat. Sci. Ed. 1999, 45, 795–798. [Google Scholar]
- Schubert, U.S.; Kersten, J.L.; Pemp, A.E.; Eisenbach, C.D.; Newkome, G.R. A New Generation of 6,6′-Disubstituted 2,2′-Bipyridines: Towards Novel Oligo(bipyridine) Building Blocks for Potential Applications in Materials Science and Supramolecular Chemistry. Eur. J. Org. Chem. 1998, 1998, 2573–2581. [Google Scholar] [CrossRef]
- Vezse, P.; Benda, B.; Fekete, A.; Golcs, Á.; Tóth, T.; Huszthy, P. Covalently immobilizable tris (pyridino)-crown ether for separation of amines based on their degree of substitution. Molecules 2022, 27, 2838. [Google Scholar] [CrossRef] [PubMed]
- Uenishi, J.; Nishiwaki, K.; Hata, S.; Nakamura, K. An optical resolution of pyridyl and bipyridylethanols and a facile preparation of optically pure oligopyridines. Tetrahedron Lett. 1994, 35, 7973–7976. [Google Scholar] [CrossRef]
- Uenishi, J.; Hiraoka, T.; Hata, S.; Nishiwaki, K.; Yonemitsu, O.; Nakamura, K.; Tsukube, H. Chiral Pyridines: Optical Resolution of 1-(2-Pyridyl)-and 1-[6-(2,2′-Bipyridyl)] ethanols by Lipase-Catalyzed Enantioselective Acetylation. J. Org. Chem. 1998, 63, 2481–2487. [Google Scholar] [CrossRef]
- Mehdipour-Ataei, S.; Heidari, H. Preparation of novel pyridine-based, thermally stable poly(ether imide)s. J. Appl. Polym. Sci. 2004, 91, 22–26. [Google Scholar] [CrossRef]
- Jung, O.S.; Jo, D.H.; Lee, Y.A.; Conklin, B.J.; Pierpont, C.G. Bistability and molecular switching for semiquinone and catechol complexes of cobalt. Studies on redox isomerism for the bis(pyridine) ether series Co(py2X)(3,6-DBQ)2, X = O, S, Se, and Te. Inorg. Chem. 1997, 36, 19–24. [Google Scholar] [CrossRef]
- Uenishi, J.; Tsukibe, H. Preparation of Three Coordination-Type Pyridylpodand Derivatives as Separation and Recovery Agents for Silver Ion. Patent No. JP 08231511, 10 September 1996. [Google Scholar]
- Oepen, G.; Voegtle, F. Ligand structure and complexation. LIII. Noncyclic neutral ligands with two central pyridine units and rigid donor end groups. Liebigs Ann. Chem. 1980, 4, 512–517. [Google Scholar] [CrossRef]
- Ransohoff, J.E.B.; Staab, H.A. En route to hexaaza-kekulene. Tetrahedron Lett. 1985, 26, 6179–6182. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Min, J.; Liu, C.; Mao, S.; Wang, L.; Yang, B.; Dong, Z. A General Approach for Synthesis of Circularly Assembled Supramolecular Polymers by Means of Region-confined Amphiphilic Supramolecular Polymerization. Chem. Res. Chin. Univ. 2023, 39, 736–740. [Google Scholar] [CrossRef]
- Shimazawa, R.; Hashimoto, Y.; Iwasaki, S. Water-soluble Zimmerman’s molecular tweezer analogs: Dextran-coupling method for solubilization. Tetrahedron Lett. 1992, 33, 7197–7200. [Google Scholar] [CrossRef]
- Zimmerman, S.C.; Wu, W. A rigid molecular tweezers with an active site carboxylic acid: Exceptionally efficient receptor for adenine in an organic solvent. J. Am. Chem. Soc. 1989, 111, 8054–8055. [Google Scholar] [CrossRef]
- Zimmerman, S.C.; Zeng, Z.; Wu, W.; Reichert, D.E. Synthesis and structure of molecular tweezers containing active site functionality. J. Am. Chem. Soc. 1991, 113, 183–196. [Google Scholar] [CrossRef]
- Liang, B.; Wang, D.; Chen, L.; Chen, X.; Zhang, D. Preparation of Compounds Containing Five-Membered Heterocyclic Structure for Organic Electroluminescent Devices and Display Devices. Patent CN116854682, 10 October 2023. [Google Scholar]
- Lin, N.; Huang, L.; Ding, H.H.; Zhang, Y.; Dong, W.J.; Xia, B.Y.; Ren, W.S.; Zhao, D. Synthesis of para-linked azacalix[n]pyridine[n]pyrazines and their uranyl ion binding properties. Org. Chem. Front. 2021, 8, 6657–6662. [Google Scholar] [CrossRef]
- Zahradníková, E.; Císařová, I.; Drahoš, B. Triple M as Manganese: Medicine, magnetism and macrocycles. Seven-coordinate Mn(II) complexes with pyridine-based macrocyclic ligands. Polyhedron 2021, 203, 115231–115239. [Google Scholar] [CrossRef]
- Knighton, R.C.; Beer, P.D. Sodium cation-templated synthesis of an ion-pair binding heteroditopic[2]catenane. Org. Chem. Front. 2021, 8, 2468–2472. [Google Scholar] [CrossRef]
- Li, D.; Qin, T.; Liu, X.; Zhang, B.; Zhong, F.; Ma, C.; Gan, Q. Macrocyclic aromatic amide foldamer: Synthesis, twisted-to-boxlike conformational switching, and molecular recognition. Chem. Plus Chem. 2021, 86, 920–923. [Google Scholar] [CrossRef]
- Thierer, L.M.; Wang, Q.; Brooks, S.H.; Cui, P.; Qi, J.; Gau, M.R.; Manor, B.C.; Carroll, P.J.; Tomson, N.C. Pyridyldiimine macrocyclic ligands: Influences of template ion, linker length and imine substitution on ligand synthesis, structure and redox properties. Polyhedron 2021, 198, 115044–115058. [Google Scholar] [CrossRef]
- Leygue, N.; Picard, C.; Faure, P.; Bourrier, E.; Lamarque, L.; Zwier, J.M.; Galaup, C. Design of novel tripyridinophane-based Eu(III) complexes as efficient luminescent labels for bioassay applications. Org. Biomol. Chem. 2022, 20, 182–195. [Google Scholar] [CrossRef]
- Gibson, H.W.; Price, T.L., Jr.; Pederson, A.M.P.; Niu, Z.; Nellipalli, P. Chelidamic acid derivatives: Precursors to functionalized pyridyl cryptands & functionalized metal ligands. Tetrahedron 2021, 94, 132333–132343. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents: Physical Properties and Methods of Purification. In Techniques of Chemistry, 4th ed.; Wiley: New York, NY, USA, 1998; ISBN 978-0-471-08467-9. [Google Scholar]
- Jung, B.; Sung, H.K. Chiral imine copper chloride-catalyzed enantioselective desymmetrization of 2-substituted 1,2,3-propanetriols. Proc. Natl. Acad. Sci. USA 2007, 104, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Tsukube, H.; Uenishi, J.; Higaki, H.; Kikkawa, K.; Tanaka, T.; Wakabayashi, S.; Oae, S. Side arm effects on cation binding, extraction, and transport functions of oligopyridine-functionalized aza-crown ethers. J. Org. Chem. 1993, 58, 4389–4397. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).