Abstract
Photodynamic therapy (PDT) is a clinically approved therapeutic option for the treatment of various types of cancer. PDT calls for the application of photosensitizers (PSs) and photoactivation with a particular light wavelength while tissue oxygen is present. Anticancer efficacy depends on the combination of these three substrates leading to the generation of cytotoxic reactive oxygen species (ROS) that promote apoptosis, necrosis, and autophagy of cancer cells. However, one of the biggest problems with conventional PDT is the poor accumulation and targeting of PSs to tumor tissues, resulting in undesirable side effects and unfavorable therapeutic outcomes. To overcome this, new photosensitizers have been developed through bioconjugation and encapsulation with targeting molecules, such as peptides, allowing a better accumulation and targeting in tumor cells. Several studies have been conducted to test the efficacy of several peptide-conjugated photosensitizers and improve PDT efficacy. This review aims to present current insights into various types of peptide-conjugated photosensitizers, with the goal of enhancing cancer treatment efficacy, addressing the limitations of conventional PDT, and expanding potential applications in medicine.
1. Photodynamic Therapy (PDT): An Overview
Photodynamic therapy (PDT) is one of the clinically approved treatments for various cancer types and other conditions such as inflammation, bacterial infections, and dermatologic diseases [1,2,3,4,5]. PDT is based on a photochemical reaction between three substrates: light, molecular oxygen, and a light-activatable molecule or photosensitizer (PS) [6]. There are several types of light sources, with the primary ones being lamps, lasers, and light-emitting diodes (LEDs), each differing in cost and characteristics. Therefore, choosing one of them depends on the sources available and the desired characteristics [7,8,9,10,11,12,13,14].
When the cells that have absorbed photosensitizers are exposed to a specific wavelength of light, reactive oxygen species (ROS), which can induce the selective destruction of the target tissue, are produced [15,16]. Upon light absorption, the photosensitizer (PS) transitions from its ground state (singlet state, 1PS) to an excited singlet state (1PS*). This excited singlet state is very unstable and, consequently, can progress to a more stable, long-lived, electronically excited state: the triplet state (3PS*) [8,14,15]. Moreover, the photosensitization process encompasses two processes that occur in parallel, and the ratio depends on the PS used, the concentrations of oxygen and substrate, and the affinity of the PS with the substrate. Specifically, in the Type I photosensitization process, the triplet state directly engages with a substrate (such as a cell membrane or molecule) and undergoes reactions involving hydrogen atom abstraction or electron transfer, leading to the generation of free radicals and radical ions. When these radicals react with molecular oxygen, ROS are produced, inducing oxidative damage and cell death. In Type II reactions, the PS in its excited triplet state transfers its energy to molecular oxygen leading to the formation of singlet oxygen (1O2), its highly reactive and cytotoxic singlet state [7,13,17]. Figure 1 shows the principle of PDT and a schematic illustration of PS activation for treating a solid tumor.
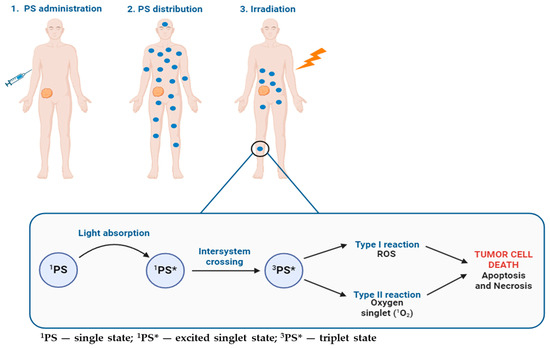
Figure 1.
Clinical application of PDT for the treatment of a solid tumor after PS administration until irradiation, with a detailed representation of the photosensitization process.
Photodynamic Therapy in Cancer
Despite the advances in recent years in cancer therapy and the consequent increase in overall and progression-free survival, this is an area that needs to be studied further. The most traditional cancer therapies include chemotherapy and radiotherapy; however, these kinds of treatments are associated with poor disease outcomes and recurrence due to several conditions, such as drug resistance, poor drug absorption, inability to address tumor heterogeneity, off-target effects, and serious systemic adverse effects [1,4,18,19]. In this context, PDT appears as a unique and promising approach within this landscape of cancer treatments since it provides a high degree of specificity in targeting cancer cells without harming normal tissues. This specificity is obtained through the selective uptake of PSs by cancer cells and the localized application of light to activate these PSs [2,20]. Photosensitizers are crucial for therapeutic success and must fulfill some requirements, namely be able to generate singlet oxygen efficiently, have a high absorption coefficient in the long-wavelength region (600 nm to 800 nm), allow deeper tissue penetration, be non-toxic in the absence of light, have tumor specificity or selective accumulation at tumor sites, have light-dependent high cytotoxicity, and be rapidly excreted from the patient’s body [15,16,21,22,23,24]. Nonetheless, one of the main limitations of PDT is the low water solubility of PSs, which results in poor permeability and penetration in tumor cells. As their accumulation in the tumor is limited, this leads to an increase in the dosage of PS used, damaging normal and healthy cells and causing unfavorable side effects [25]. Three generations of PSs were successively created to improve PS characteristics. First-generation PSs have some limitations such as skin photosensitivity, low absorption, and poor tissue selectivity and penetration of light. To solve these problems, the second generation of PSs has emerged; however, the problem of low solubility persisted. Consequently, the third generation emerged with the intent to improve PS solubility, pharmacokinetic properties, and the selectivity and accumulation in the targeted tumor sites, reducing the administered dose of the drug. These were achieved by conjugating them with targeting entities or moieties, such as carbohydrates, antibodies, peptides, and amino acids, or by encapsulation into carriers [1,6,15,24,26]. Moreover, these conjugates can cause PSs to be activated only when they are at the target location, known as activatable. Examples of these types of PSs are BODIPY and Cyanine derivative-based PSs [5,25,27,28,29,30].
Table 1 shows the photosensitizers most used in clinics for cancer treatment and diagnosis, as well as the cancers where clinical trials are being carried out [31].

Table 1.
Photosensitizers that are most used in clinics and clinical trials, with the respective activation wavelength and the type of cancer(s) where they are used/tested [31].
Consequently, enhancing certain characteristics of photosensitizers, such as their solubility and permeability in cancer cells through conjugation with other molecules, is crucial for photodynamic therapy to be genuinely effective against cancer. Among several entities that can be used, peptides are one of the most studied ones to be conjugated with PSs acting as transporters or altering the physicochemical properties of PSs.
Peptides are small molecules composed of a short chain of 2 to 50 amino acids, formed by a condensation reaction and linked together by a covalent bond. Sequential peptide bonds with additional amino acids form a peptide chain and the building block of proteins [32]. These molecules offer significant advantages in clinical practice because they exhibit greater target specificity, enhanced cell penetration, and fewer side effects due to reduced interactions with off-target sites. Consequently, they are applied to a wide range of diseases such as diabetes mellitus, cardiovascular diseases, gastrointestinal diseases, cancer, infectious diseases, and vaccine development [33,34,35,36,37]. Specifically in cancer, anticancer peptides (ACPs) have emerged as a potential therapeutic approach having the capability of inducing apoptosis of cancer cells, destroying the structure of the cell membrane, inhibiting angiogenesis, and inducing the production of cytokines [38]. Cell-penetrating peptides (CPPs), a type of ACPs, are extensively researched as carriers for delivering therapeutic drugs across physiological barriers due to their minimal toxicity and quick ability to enter cells [39,40]. Therefore, all these characteristics make peptides an asset to improve the action of PSs.
Nonetheless, peptides often suffer from membrane impermeability, poor in vivo stability due to the intrinsic limitations of amino acids, and easy hydrolysis by enzymes in the stomach and intestine. Consequently, peptide modifications and alternative routes of administration are being studied to improve their activity and stability [35,37,41,42,43].
In this article, we will review the capability of peptides to improve PS characteristics and, consequently, optimize PDT results in cancer treatment. The characteristics of PS–peptide conjugates and examples under investigation and already implemented to improve this kind of therapy will also be discussed.
2. Peptide-Conjugated Photosensitizers for PDT
As stated before, peptides have been explored as PS carriers due to their biocompatible and biodegradable characteristics and their easy design, synthesis, and purification. In addition, peptide–PS conjugates allow better accumulation of the PS at the site of action, ensuring in situ generation of ROS and enhancing therapeutic efficacy. This reduces the dose of PS used, decreasing its toxicity and reducing side effects [44]. However, these peptide-based conjugates have an inherently low resistance to enzyme-mediated degradation and, therefore, need to be modified. This modification could be achieved using D-amino acids, pseudo-amino acids, and peptide cyclization, increasing the stability of the peptide. The peptide could be attached directly to the PS via a linker or incorporated into the composition of more complex delivery systems, such as binding to nanoparticles (NPs) (Figure 2) [31,44,45,46,47]. Below, several peptide–PS conjugate approaches will be reviewed.
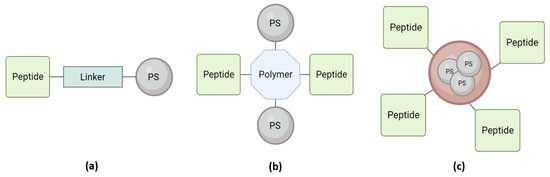
Figure 2.
Peptide–PS conjugates approach for PDT. (a) Monomeric peptides directly conjugated to the PS; (b) Multimeric peptides and photosensitizers with a polymeric scaffold; (c) PS-loaded liposome decorated with homing peptides.
2.1. Porphyrin–Peptide Conjugates
Porphyrin–peptide conjugates have been employed in PDT; however, many exhibit limited accumulation or short retention times within tumors. To overcome these problems, researchers designed a pH-responsive PEGylated porphyrin–peptide conjugate (PHHPEG6) in which the PS was derived from histidine dipeptides and short PEG chains. Contrary to non-PEGylated porphyrin–histidine–histidine (PHH), which was shown to be unstable under physiological environments, PHHPEG6 demonstrated good stability and a strong response to pH changes, forming stable aggregates in acidic scenarios which can promote cellular uptake, improve singlet molecular oxygen releasing, and enhance PDT therapeutic efficacy when internalized into cells via the lysosomal pathway [48]. Another study evaluated five different porphyrin–peptide conjugates and compared them with the FDA-approved purified hematoporphyrin derivative in prostate cancer cell lines. The results revealed that the most promising conjugate (porphyrin-HIV-1 Tat (48–60) conjugate 2) is less toxic than non-conjugated porphyrin, being more selective to prostate tumor cells because their uptake is 5 to 8 times higher than FDA-approved hematoporphyrin [49].
Synthetic peptide nucleic acids (PNAs) are self-organized structures including DNA bases and a peptide backbone. Their propensity to self-assemble into supramolecular structures has led several researchers to study their interaction with PSs to improve the efficacy of PDT. A study from 2019 synthesized a conjugate through a covalent attachment of Fmoc-PNA-G-(Bhoc)-OH (PNA) with 5-(4-aminophenyl)-10,15,20-triphenyl porphyrin (TPP-NH2), demonstrating that the PNA-TPP conjugate can readily produce NPs with well-defined nanostructures and uniform sizes by self-assembly. In addition, the PNA-TPP nanoparticles demonstrated stability in biological solutions and were successfully absorbed by the cells, inducing selective cytotoxicity when irradiated with light. While they have demonstrated great efficacy as nanoagents for in vitro PDT, incorporating tumor-targeting groups or adding tumor-responsive sequences could enhance tumor targeting [50].
5-Aminolevulinic acid (5-ALA) is an FDA-approved PS, a protoporphyrin IX (PpIX) precursor, used to treat several cancers. 5-ALA is characterized by low solubility, difficulty in penetrating cell membranes, and poor stability. Therefore, several studies have been made to improve its efficacy. In one study, the peptide (HS-EK5-RGD) targeting the ανβ3 integrin receptor was used as a vector for the delivery of 5-ALA conjugated to gold NPs. 5-ALA prodrug nanocarriers could stop undesired leakage in a physiological environment since 5-ALA was coupled to AuNPs via a pH-sensitive hydrazone bond. Additionally, 5-ALA is promptly released once it is internalized by tumor cells. Furthermore, 5-ALA prodrug nanoparticles showed increased ROS formation and, after 10 min of irradiation, 90% of the cells were killed; this was more effective than free 5-ALA and suggested higher PDT efficacy [51].
2.2. Pyropheophorbide-a (Pyroa)–Peptide Conjugates
Pyropheophorbide-a (Pyro) is a promising molecule for clinical use, being useful both in tumor imaging and photodynamic therapy. Recently, a study demonstrated that a molecule combining Pyro, an RGD dimer peptide (3PRGD2), and 64Cu (64Cu-Pyro-3PRGD2), targeting the tumor-specific αvβ3 integrin, has a high potential for tumor imaging and is helpful in tumor diagnosis. Beyond imaging features, αvβ3 integrin can also be helpful in therapy, helping novel drugs or other target peptides to kill cancer cells [52,53,54]. Furthermore, additional research has demonstrated that the integration of many iRGD-mediated actions by the iRGD, in conjunction with pyropheophor-bide-a (Ppa) and a quencher (BK01) in the cyclic iRGD backbone (Ppa-iRGDC-BK01), permits persistent tumor reloading with the activated PS. An interesting discovery showed that Ppa-iRGDC-BK01 may thermally accelerate supramolecular assembly by reorganizing interpeptide hydrogen bonds and hydrophobic contact between Pyroa pendants when exposed to body temperature. The Pyroa-iRGDC-BK01 depot, created by local injection near the tumor, allowed for safe and precise repeated PDT through a straightforward process involving a single injection and multiple irradiations. This allows a lower dose of PS to be administered, resulting in less toxicity and lower treatment costs, thereby improving the efficacy of PDT. In addition, a study demonstrated that RGD-L-Glu-Pyro 2 was stable and had high cytotoxicity in αvβ3-positive cancer cells showing a 23-fold different PDT activity against tumor cells compared to Pyroa alone which does not demonstrate any difference [55,56].
Another study used a specific binding peptide targeting CD133 (LQNAPRS) conjugated to Pyroa to overcome the problem of tumor non-selectivity by attempting to target CD133, which is highly expressed in colorectal cancer stem cells (CRC CSCs). A hydrophilic linker with a polyethylene glycol (PEG) chain was inserted between the CD133 peptide and Pyroa to increase the water solubility of the CD133–Pyroa conjugate, and the results showed an increase in the target and inhibition of CD133 CSCs by CD133–Pyroa when compared to unconjugated Pyroa, by promoting the production of ROS and inducing autophagic cell death. Additionally, in vivo studies have revealed that CD133–Pyroa exhibits a strong inhibitory effect on CRC CSC-derived xenograft tumors in nude mice, with significant accumulation in tumor tissue and demonstrating high therapeutic effectiveness [57,58].
2.3. Chlorin–Peptide Conjugates
Chlorin e6 (Ce6) is a hydrophobic anionic porphyrin derivative used in PDT to treat tumors. Recent studies suggest that a self-assembling short peptide derived from diphenylalanine (CDP) and an amino acid derivative (Fmoc-L-Lys, F) can induce the self-assembly of Ce6 to form well-ordered NPs. Specifically, these studies showed that CDP-Ce6 and F-Ce6 NPs are responsive to each microenvironment tumor’s pH, detergents, and enzymes, exhibiting on-demand release. Moreover, in vitro studies using MCF-7 breast cancer cells proved that CDP-Ce6 and F-Ce6 NPs improved NP internalization, exhibiting approximately a 4-fold increased photocytotoxicity compared to the control group of Ce6 alone. In vivo tests demonstrated that, after PDT treatment, no significant changes in body weight or organ damage were observed in the mice, providing preliminary evidence that the NPs are a safe anticancer treatment [59,60,61,62].
In other studies, TPC (5-(4-carboxyphenyl)-10,15,20-triphenyl chlorin) was used as a PS, coupled to two peptides—DKPPR and TKPRR—to activate the neuropilin-1 (NRP-1) receptor. Studies showed that TPC conjugated with DKPPR or TKPRR could bind to the NRP-1 receptor, while free TPC showed no significant binding to the same receptor. Therefore, the new proposed TPC–peptide conjugates showed to have potential for further development as future PDT agents targeting NRP-1, having more favorable IC50 values—30 μM ≤ IC50 ≤ 39 μM in DKPPR conjugates and 51 μM ≤ IC50 ≤ 55 μM—compared with the previously synthesized TPC–Ahx–ATWLPPR conjugate (IC50 = 171 μM) and exhibiting the enhanced affinity of these new conjugates. In particular, the DKPPR conjugates showed good levels of distribution in an animal model, demonstrating that the conjugation enhances the targeting of PSs to cancer cells, thereby improving the performance levels of PDT [63,64].
2.4. Phthalocyanine (Pc)–Peptide Conjugates
Phthalocyanines (Pcs) are a group of promising PSs for use in PDT. Recently, a group of researchers prepared a new peptide-conjugated PS using silicon Pc (SiPc) as the light-activating moiety and the cRGDfK peptide (or simply cRGD) as the peptide moiety. A PEG linker and an additional carboxylic acid group were also tested for incorporation into the conjugates to optimize the conjugate structure, increase water solubility, and avoid direct linkage of the two moieties. PS linked to the peptide via two PEG linkers and glutamate, RGD-(Linker)2-Glu-SiPc, showed the highest activity eradicating the U87-MG brain tumor cells in the tumor model after one dose of photodynamic treatment, and no tumor growth was observed for up to 35 days. These results demonstrated the great potential of using PDT based on the compound RGD-(Linker)2-Glu-SiPc in the treatment of tumors [65,66,67].
2.5. Fluorophore–Peptide Conjugates
A new approach to PS delivery was recently tested by conjugating the CPP protamine (Pro) with a typical fluorescent dye, rhodamine (Rho). Photodynamic cell death experiments showed the effectivity of using Rho and Pro attached (RhoPro) in PDT. In an in vitro study using colon cancer cells (HT-29), the percentage of death cells increased by 36.2% when compared to Rho and Pro alone. Moreover, in the RhoPro assay, morphological changes typical of necrosis were seen. In fact, this attachment provides the membrane-internalizing activity and induces fast PD cell death, due to light-induced cell membrane rupture, suggesting a good candidate as a PS for solid tumor treatment [68].
In addition, Pro conjugates with methylene blue (MB) (a fluorescent dye) were tested for being preferentially internalized into lysosomes, where they exhibit necrosis-inducing lysosomal damage. In vitro assays showed that MB–Pro had a more efficient photodynamic activity than MB alone against colon cancer cells. The results showed significant differences in cell viability on the third day of testing, with a viability of 75.5% in the MB-alone group and 52.6% in the conjugated group at the same concentration. By causing necrotic cell death, the conjugates concentrated in the lysosomes improved PDT efficiency, whereas PDT with non-coupled MB only produced apoptotic processes. [69].
2.6. Rose Bengal (RB)–Peptide Conjugates
Rose Bengal (RB) covalently linked to the amphipathic peptide (AMP) C(KLAKLAK)2 was recently tested on malignant melanoma cells. In vivo experiments revealed that the conjugate could significantly inhibit the growth of B16-F10-Luc2 melanoma tumors without evident side effects, achieving a tumor 479% smaller compared to the mice treated with RB alone. This encouraging outcome was explained by the fact that AMP increases the sensitivity of cells to ROS, increasing their vulnerability to ROS-induced oxidative stress. This allowed for the activation of RB-C(KLAKLAK)2 at low concentrations using a low-cost, low-intensity white light source. These findings suggested that RB-C(KLAKLAK)2-mediated PDT may be useful in the treatment of metastatic melanoma, particularly in those individuals whose tumors are not amenable to surgical excision [47,70].
Truly, all the peptide conjugate approaches seem to be excellent alternative options to improve photodynamic therapeutic action. Nonetheless, is important to note that they still have weaknesses as therapeutic approaches. Solubility problems appear to be one of the main issues to overcome, especially in porphyrin–peptide conjugates. However, this conjugate was shown to be capable of generating a high quantity of ROS, also showing a positive impact in improving PDT. Also, fluorophore–peptide conjugates look to be highly versatile conjugates that can act with lysosomes and are capable of inducing cell death. Still, ROS generation seems to be more limited when compared to the other conjugates. RB– and pyroa–peptide conjugates also showed to be a good option for PDT development because they showed a high capacity to produce singlet oxygen [71] and ROS, respectively. On the other hand, (Pc)–peptide conjugates appear to be among the most promising peptide conjugates, as results demonstrated significant improvements in water solubility and strong efficacy in inhibiting tumor growth. Nevertheless, all these conjugates were tested and showed promising results in different cancer types, which could suggest that depending on the cancer cell features and tumor microenvironment, different peptide conjugates may have a higher therapeutic efficacy.
3. The Impact of CPPs in the Context of Therapeutic Advancements
The evolution of CPPs traces back to the discovery of the human immunodeficiency virus (HIV) trans-activator of transcription (TAT) protein. In 1988, two independent groups, Frankel and Pabo and Green and Loewenstein, discovered that the HIV TAT could enter cells and trigger the replication of the viral genome. However, after the demonstration that only certain portions of the TAT protein are required for cellular uptake (residues 1–72 and 37–72) [72], investigations into CPPs advanced swiftly, leading to a substantial increase in the research field of synthetic CPPs [73]. The TAT CPP, which consists of the residues 48 to 60, is used as a cell delivery tool and is extensively employed in the transport of DNA, proteins, and drug molecules across membranes [74]. In 1991, Alain Prochiantz and colleagues synthesized the 60-amino acid homeoprotein found in Drosophila, originally named pAntennapedia (pAntp) and now named penetratin. Studies have shown that the antennapedia protein entered nerve cells grown in culture, moved to the nucleus, and caused notable alterations in the neuron [72,75,76].
Despite extensive scientific research and numerous published studies on CPPs, many questions are emerging among scientists regarding how these peptides are taken up by cells [77]. The challenges in understanding how these peptides enter cells and the rate of efficiency of cellular entry are primarily attributed to variations in their physicochemical properties, concentrations, types, sizes, and CPP–cargo combinations [78]. Endocytosis is a fundamental and energy-dependent cellular process that takes place in all cells [79]. This type of cell penetration is the most common and includes a wide range of cellular processes, such as phagocytosis, caveolae or clathrin-mediated endocytosis, clathrin and caveolin-independent endocytosis, and micropinocytosis [80]. Otherwise, direct penetration is a method of crossing biological membranes that does not rely on energy or receptor involvement, encompassing diverse mechanisms, namely pore formation, inverted micelle, membrane thinning, and carpet-like model [80,81].
Over the past few years, NP-based diagnostic techniques and therapeutic delivery systems have evolved in the area of cancer. CPPs have been demonstrated to be effective carriers and deliver cytotoxic drugs into tumor cells to trigger apoptosis, showing no toxicity at concentrations below hundreds of micromoles (μmol) [40]. Specifically, a notable advancement in peptide technology involves tumor-homing CPPs, which are peptides capable of differentiating between cancerous and normal cells and targeting specific types of tumor cells. These molecules can attach to tumor cells through surface antigens or receptors that are typically overexpressed in certain types of tumors [82]. Examples of these peptides are Angiopep-2 (glioblastoma); iRGD (breast cancer melanoma); CREKA (breast cancer); PL3 (glioblastoma prostatic cancer); gHo (glioma); LyP-1 (breast cancer); TT1 (breast cancer); and iNGR (breast cancer) [40]. Moreover, several studies have demonstrated lipid change composition, high levels of anionic molecules in tumor cell membranes, and overexpression of tumor antigens or receptors that are interesting targets for a more directed cancer treatment and potential targets for CPPs [82,83,84]. Truly, the rapid advancement in the medical field is based on specialized nanotechnologies, such as CPPs combined with diverse cargoes. In addition, peptide modifications such as altering the amino acid sequence, utilizing peptide-loaded nanoparticles, and conjugating peptide drugs to polymers were also made to enhance the effectiveness and specificity of therapeutic peptide delivery [85].
Table 2 demonstrates examples of CPPs and cargoes used in cancer research.

Table 2.
Examples of cargo–CPP conjugates in different experimental models for cancer treatment.
4. Conclusions
PDT is a non-invasive treatment approach primarily used in cancer treatment. This therapeutic approach consists of the combination of a PS, tissue oxygen, and visible light, leading to the production of cytotoxic ROS causing localized oxidative damage to target cells and leading to apoptosis, necrosis, and autophagy of cancer cells. However, the non-specific distribution of PSs is one of the most significant challenges, leading to undesirable side effects and suboptimal therapeutic outcomes. To address these limitations, contemporary research has focused on developing actively targeted photosensitizing molecules that ensure higher cellular uptake and targeted delivery at tumor sites. The use of peptides conjugated with PSs (peptide–PS conjugates) is currently under investigation to improve PDT efficacy for cancer treatment and demonstrates substantial potential, suggesting a more reliable and patient-friendly therapeutic strategy. Moreover, the development of third-generation PSs showed to be an effective receptor-mediated targeting strategy for PDT. Therefore, incorporating all these targeted approaches could significantly improve the clinical application of PDT, giving an effective and safer treatment option for cancer patients. In addition, despite the promising results of the experiments, is essential to promote the continued exploration and refinement of these strategies to understand their full potential in clinical applications.
Author Contributions
Conceptualization, N.V.; methodology R.R., I.C., M.P. and N.V.; formal analysis, R.R., I.C., M.P. and N.V.; investigation, R.R., I.C.,and M.P.; writing—original draft preparation, R.R., I.C. and M.P.; writing—review and editing, R.R., M.P. and N.V.; supervision, N.V.; project administration, N.V.; funding acquisition, N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by Fundo Europeu de Desenvolvimento Regional (FEDER) funds through the COMPETE 2020 Operational Programme for Competitiveness and Internationalisation (POCI), Portugal 2020 and by Portuguese funds through Fundação para a Ciência e a Tecnologia (FCT) in the framework of projects IF/00092/2014/CP1255/CT0004 and CHAIR in Onco-Innovation.
Data Availability Statement
Not applicable.
Acknowledgments
M.P. acknowledges FCT for funding her Ph.D. grant (2021.07450.BD).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bartusik-Aebisher, D.; Woźnicki, P.; Dynarowicz, K.; Aebisher, D. Photosensitizers for Photodynamic Therapy of Brain Cancers—A Review. Brain Sci. 2023, 13, 1299. [Google Scholar] [CrossRef]
- Gil-Pallares, P.; Navarro-Bielsa, A.; Almenara-Blasco, M.; Gracia-Cazaña, T.; Gilaberte, Y. Photodynamic Therapy, a successful treatment for granular parakeratosis. Photodiagnosis Photodyn Ther. 2023, 42, 103562. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, A.M.C.; Aguiar, E.M.G.; Ferreira, C.B.R.; Siqueira, J.M.; Corrêa, L.; Nunes, F.D.; Franco, A.L.; Cecatto, R.B.; Hamblin, M.R.; Rodrigues, M. Photodynamic therapy in cancer stem cells-state of the art. Lasers Med. Sci. 2023, 38, 251. [Google Scholar] [CrossRef] [PubMed]
- Kolarikova, M.; Hosikova, B.; Dilenko, H.; Barton-Tomankova, K.; Valkova, L.; Bajgar, R.; Malina, L.; Kolarova, H. Photodynamic therapy: Innovative approaches for antibacterial and anticancer treatments. Med. Res. Rev. 2023, 43, 717–774. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Mahootchi, P.; Rastegar, Z.; Abbasi, B.; Alam, M.; Abbasi, K.; Fani-Hanifeh, S.; Amookhteh, S.; Sadeghi, S.; Soufdoost, R.S.; et al. Photodynamic Therapy in Oral Cancer: A Narrative Review. Photobiomodulation Photomed. Laser Surg. 2023, 41, 248–264. [Google Scholar] [CrossRef]
- Van Straten, D.; Mashayekhi, V.; De Bruijn, H.; Oliveira, S.; Robinson, D. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Calixto, G.; Bernegossi, J.; De Freitas, L.; Fontana, C.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—Cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Hempstead, J.; Jones, D.P.; Ziouche, A.; Cramer, G.M.; Rizvi, I.; Arnason, S.; Hasan, T.; Celli, J.P. Low-cost photodynamic therapy devices for global health settings: Characterization of battery-powered LED performance and smartphone imaging in 3D tumor models. Sci. Rep. 2015, 5, 10093. [Google Scholar] [CrossRef]
- Huis In ‘T Veld, R.V.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 330. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; McKenna, K.E.; Rhodes, L.E. Guidelines for topical photodynamic therapy: Update. Br. J. Dermatol. 2008, 159, 1245–1266. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.-i.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef]
- Sapio, L.; Naviglio, S. Innovation through Tradition: The Current Challenges in Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 5296. [Google Scholar] [CrossRef]
- Kim, T.E.; Chang, J.-E. Recent Studies in Photodynamic Therapy for Cancer Treatment: From Basic Research to Clinical Trials. Pharmaceutics 2023, 15, 2257. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.; Reis, L.V. Photodynamic Therapy: From the Basics to the Current Progress of N-Heterocyclic-Bearing Dyes as Effective Photosensitizers. Molecules 2023, 28, 5092. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef]
- Lovell, J.F.; Liu, T.W.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Bilici, K.; Cetin, S.; Celikbas, E.; Yagci Acar, H.; Kolemen, S. Recent Advances in Cyanine-Based Phototherapy Agents. Front. Chem. 2021, 9, 707876. [Google Scholar] [CrossRef]
- Mallidi, S.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef]
- Shindy, H.A. Fundamentals in the chemistry of cyanine dyes: A review. Dye. Pigment. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, H.; Xu, J.F.; Zhang, X. Activatable Photosensitizer for Smart Photodynamic Therapy Triggered by Reactive Oxygen Species in Tumor Cells. ACS Appl. Mater. Interfaces 2020, 12, 26982–26990. [Google Scholar] [CrossRef]
- Jain, R.; Mohanty, S.; Sarode, I.; Biswas, S.; Singhvi, G.; Dubey, S.K. Multifunctional Photoactive Nanomaterials for Photodynamic Therapy against Tumor: Recent Advancements and Perspectives. Pharmaceutics 2023, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.; Krishnamurthy, K. Biochemistry, Peptide. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Barman, P.; Joshi, S.; Sharma, S.; Preet, S.; Sharma, S.; Saini, A. Strategic Approaches to Improvise Peptide Drugs as Next Generation Therapeutics. Int. J. Pept. Res. Ther. 2023, 29, 61. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.-L.; Harris, J.L.; Khanna, K.K.; Hong, J.-H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef] [PubMed]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial peptides - Advances in development of therapeutic applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Wanjari, U.R.; Gopalakrishnan, A.V.; Bradu, P.; Biswas, A.; Ganesan, R.; Renu, K.; Dey, A.; Vellingiri, B.; El Allali, A.; et al. Evolving strategies and application of proteins and peptide therapeutics in cancer treatment. Biomed Pharmacother 2023, 163, 114832. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Xie, M.; Liu, D.; Yang, Y. Anti-cancer peptides: Classification, mechanism of action, reconstruction and modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Xu, J.; Wang, F.; Ye, L.; Wang, R.; Zhao, L.; Yang, X.; Jianbo, J.; Liu, A.; Zhai, G. Penetrating peptides: Applications in drug delivery. J. Drug Deliv. Sci. Technol. 2023, 84, 104475. [Google Scholar] [CrossRef]
- Zhou, M.; Zou, X.; Cheng, K.; Zhong, S.; Su, Y.; Wu, T.; Tao, Y.; Cong, L.; Yan, B.; Jiang, Y. The role of cell-penetrating peptides in potential anti-cancer therapy. Clin. Transl. Med. 2022, 12, e822. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Lombardi, L.; Williams, D.R.; Albericio, F. Strategies for Improving Peptide Stability and Delivery. Pharmaceuticals 2022, 15, 1283. [Google Scholar] [CrossRef]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and Recent Advances in Peptide and Protein Drug Delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Rossino, G.; Marchese, E.; Galli, G.; Verde, F.; Finizio, M.; Serra, M.; Linciano, P.; Collina, S. Peptides as Therapeutic Agents: Challenges and Opportunities in the Green Transition Era. Molecules 2023, 28, 7165. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, M.; Han, H.; Zhang, B.; Matson, J.B.; Chen, D.; Li, W.; Wang, Y. Peptide-based supramolecular photodynamic therapy systems: From rational molecular design to effective cancer treatment. Chem. Eng. J. 2022, 436, 135240. [Google Scholar] [CrossRef]
- Ivanova-Radkevich, V.I. Biochemical Basis of Selective Accumulation and Targeted Delivery of Photosensitizers to Tumor Tissues. Biochemistry 2022, 87, 1226–1242. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Tirand, L.; Frochot, C.; Vanderesse, R.; Thomas, N.; Gravier, J.; Guillemin, F.; Barberi-Heyob, M. Recent improvements in the use of synthetic peptides for a selective photodynamic therapy. Anticancer Agents Med. Chem. 2006, 6, 469–488. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, X.; Yang, Y.; Li, J. Assembled Photosensitizers Applied for Enhanced Photodynamic Therapy. CCS Chem. 2023, 5, 1043–1060. [Google Scholar] [CrossRef]
- Sun, B.; Guo, X.; Feng, M.; Cao, S.; Yang, H.; Wu, H.; Van Stevendaal, M.H.M.E.; Oerlemans, R.A.J.F.; Liang, J.; Ouyang, Y.; et al. Responsive Peptide Nanofibers with Theranostic and Prognostic Capacity. Angew. Chem. Int. Ed. 2022, 61, e202208732. [Google Scholar] [CrossRef]
- Sehgal, I.; Sibrian-Vazquez, M.; Vicente, M.G. Photoinduced cytotoxicity and biodistribution of prostate cancer cell-targeted porphyrins. J. Med. Chem. 2008, 51, 6014–6020. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; Karikis, K.; Han, J.; Kokotidou, C.; Charisiadis, A.; Folias, F.; Douvas, A.M.; Mitraki, A.; Charalambidis, G.; Yan, X.; et al. A self-assembly study of PNA–porphyrin and PNA–BODIPY hybrids in mixed solvent systems. Nanoscale 2019, 11, 3557–3566. [Google Scholar] [CrossRef]
- Wu, J.; Lin, Y.; Li, H.; Jin, Q.; Ji, J. Zwitterionic stealth peptide-capped 5-aminolevulinic acid prodrug nanoparticles for targeted photodynamic therapy. J. Colloid Interface Sci. 2017, 485, 251–259. [Google Scholar] [CrossRef]
- Fan, D.; Wang, K.; Gao, H.; Luo, Q.; Wang, X.; Li, X.; Tong, W.; Zhang, X.; Luo, C.; Yang, G.; et al. A 64Cu-porphyrin-based dual-modal molecular probe with integrin αvβ3 targeting function for tumour imaging. J. Label. Compd. Radiopharm. 2020, 63, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Lucente, E.; Liu, H.; Liu, Y.; Hu, X.; Lacivita, E.; Leopoldo, M.; Cheng, Z. Novel 64Cu Labeled RGD2-BBN Heterotrimers for PET Imaging of Prostate Cancer. Bioconjugate Chem. 2018, 29, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Marco, R.D. Multi-Targeting Approach of αvβ3 Integrin Ligands and their Applications in Diagnostic Field and Cancer Therapy. Glob. J. Eng. Sci. 2022, 9, 10. [Google Scholar] [CrossRef]
- Cho, H.J.; Park, S.J.; Jung, W.H.; Cho, Y.; Ahn, D.J.; Lee, Y.S.; Kim, S. Injectable Single-Component Peptide Depot: Autonomously Rechargeable Tumor Photosensitization for Repeated Photodynamic Therapy. ACS Nano 2020, 14, 15793–15805. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tan, S.; Xing, Y.; Liu, Q.; Li, S.; Chen, Q.; Yu, M.; Wang, F.; Hong, Z. cRGD Peptide-Conjugated Pyropheophorbide-a Photosensitizers for Tumor Targeting in Photodynamic Therapy. Mol. Pharm. 2018, 15, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Tang, D.; Hong, Z.; Wang, J.; Yao, H.; Lu, L.; Yi, H.; Fu, S.; Zheng, C.; He, G.; et al. CD133 peptide-conjugated pyropheophorbide-a as a novel photosensitizer for targeted photodynamic therapy in colorectal cancer stem cells. Biomater. Sci. 2021, 9, 2020–2031. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, K.; Liu, M.; Hu, P.; Xu, Y.; Yin, D.; Yang, Y.; Dong, X.; Qu, C.; Zhang, L.; et al. Phototherapeutic effect of transformable peptides containing pheophorbide a on colorectal cancer. Drug Deliv. 2022, 29, 1608–1619. [Google Scholar] [CrossRef]
- Abbas, M.; Zou, Q.; Li, S.; Yan, X. Self-Assembled Peptide- and Protein-Based Nanomaterials for Antitumor Photodynamic and Photothermal Therapy. Adv. Mater. 2017, 29, 1605021. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, H.I.; Kim, J.K.; Kim, C.H.; Kim, Y.J. Peptide 18-4/chlorin e6-conjugated polyhedral oligomeric silsesquioxane nanoparticles for targeted photodynamic therapy of breast cancer. Colloids Surf. B Biointerfaces 2020, 189, 110829. [Google Scholar] [CrossRef]
- Liu, K.; Xing, R.; Zou, Q.; Ma, G.; Möhwald, H.; Yan, X. Simple Peptide-Tuned Self-Assembly of Photosensitizers towards Anticancer Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2016, 55, 3036–3039. [Google Scholar] [CrossRef]
- Torkhovskaya, T.I.; Kostryukova, L.V.; Tereshkina, Y.A.; Tikhonova, E.G.; Morozevich, G.E.; Plutinskaya, A.D.; Lupatov, A.Y.; Pankratov, A.A. Chlorin e6 embedded in phospholipid nanoparticles equipped with specific peptides: Interaction with tumor cells with different aminopeptidase N expression. Biomed. Pharmacother. 2021, 134, 111154. [Google Scholar] [CrossRef] [PubMed]
- Kamarulzaman, E.; Gazzali, A.; Acherar, S.; Frochot, C.; Barberi-Heyob, M.; Boura, C.; Chaimbault, P.; Sibille, E.; Wahab, H.; Vanderesse, R. New Peptide-Conjugated Chlorin-Type Photosensitizer Targeting Neuropilin-1 for Anti-Vascular Targeted Photodynamic Therapy. Int. J. Mol. Sci. 2015, 16, 24059–24080. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Bechet, D.; Becuwe, P.; Tirand, L.; Vanderesse, R.; Frochot, C.; Guillemin, F.; Barberi-Heyob, M. Peptide-conjugated chlorin-type photosensitizer binds neuropilin-1 in vitro and in vivo. J. Photochem. Photobiol. B 2009, 96, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Chen, W.; Tan, Y.; Chen, H.; Yin, J. Photodynamic therapy based on organic small molecular fluorescent dyes. Chin. Chem. Lett. 2019, 30, 1689–1703. [Google Scholar] [CrossRef]
- Liu, Q.; Pang, M.; Tan, S.; Wang, J.; Chen, Q.; Wang, K.; Wu, W.; Hong, Z. Potent peptide-conjugated silicon phthalocyanines for tumor photodynamic therapy. J. Cancer 2018, 9, 310–320. [Google Scholar] [CrossRef]
- Ongarora, B.G.; Fontenot, K.R.; Hu, X.; Sehgal, I.; Satyanarayana-Jois, S.D.; Vicente, M.G. Phthalocyanine-peptide conjugates for epidermal growth factor receptor targeting. J. Med. Chem. 2012, 55, 3725–3738. [Google Scholar] [CrossRef]
- Park, C.-K.; Kim, Y.H.; Hwangbo, S.; Cho, H. Photodynamic therapy by conjugation of cell-penetrating peptide with fluorochrome. Int. J. Nanomed. 2017, 12, 8185–8196. [Google Scholar] [CrossRef]
- Ser, J.; Lee, J.Y.; Kim, Y.H.; Cho, H. Enhanced Efficacy of Photodynamic Therapy by Coupling a Cell-Penetrating Peptide with Methylene Blue. Int. J. Nanomed. 2020, 15, 5803–5811. [Google Scholar] [CrossRef]
- Dhillon, S.K.; Porter, S.L.; Rizk, N.; Sheng, Y.; McKaig, T.; Burnett, K.; White, B.; Nesbitt, H.; Matin, R.N.; McHale, A.P.; et al. Rose Bengal-Amphiphilic Peptide Conjugate for Enhanced Photodynamic Therapy of Malignant Melanoma. J. Med. Chem. 2020, 63, 1328–1336. [Google Scholar] [CrossRef]
- Dhaini, B.; Wagner, L.; Moinard, M.; Daouk, J.; Arnoux, P.; Schohn, H.; Schneller, P.; Acherar, S.; Hamieh, T.; Frochot, C. Importance of Rose Bengal Loaded with Nanoparticles for Anti-Cancer Photodynamic Therapy. Pharmaceuticals 2022, 15, 1093. [Google Scholar] [CrossRef]
- Langel, Ü. Cell-Penetrating Peptides and Transportan. Pharmaceutics 2021, 13, 987. [Google Scholar] [CrossRef] [PubMed]
- Raucher, D.; Ryu, J.S. Cell-penetrating peptides: Strategies for anticancer treatment. Trends Mol. Med. 2015, 21, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, Y.C.; Oh, H.; Guo, D.S.; Nau, W.M.; Hennig, A. Cellular Uptake of Cell-Penetrating Peptides Activated by Amphiphilic p-Sulfonatocalix[4]arenes. Chem.–A Eur. J. 2024, 30, e202400174. [Google Scholar] [CrossRef] [PubMed]
- Joliot, A.; Pernelle, C.; Deagostini-Bazin, H.; Prochiantz, A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 1864–1868. [Google Scholar] [CrossRef]
- Park, J.; Ryu, J.; Kim, K.A.; Lee, H.J.; Bahn, J.H.; Han, K.; Choi, E.Y.; Lee, K.S.; Kwon, H.Y.; Choi, S.Y. Mutational analysis of a human immunodeficiency virus type 1 Tat protein transduction domain which is required for delivery of an exogenous protein into mammalian cells. J. Gen. Virol. 2002, 83, 1173–1181. [Google Scholar] [CrossRef]
- Dowaidar, M. Uptake pathways of cell-penetrating peptides in the context of drug delivery, gene therapy, and vaccine development. Cell. Signal. 2024, 117, 111116. [Google Scholar] [CrossRef]
- Mueller, J.; Kretzschmar, I.; Volkmer, R.; Boisguerin, P. Comparison of cellular uptake using 22 CPPs in 4 different cell lines. Bioconjug. Chem. 2008, 19, 2363–2374. [Google Scholar] [CrossRef]
- Koren, E.; Torchilin, V.P. Cell-penetrating peptides: Breaking through to the other side. Trends Mol. Med. 2012, 18, 385–393. [Google Scholar] [CrossRef]
- Durzyńska, J.; Przysiecka, Ł.; Nawrot, R.; Barylski, J.; Nowicki, G.; Warowicka, A.; Musidlak, O.; Goździcka-Józefiak, A. Viral and other cell-penetrating peptides as vectors of therapeutic agents in medicine. J. Pharmacol. Exp. Ther. 2015, 354, 32–42. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Jafari, S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef]
- Rusiecka, I.; Gągało, I.; Kocić, I. Cell-penetrating peptides improve pharmacokinetics and pharmacodynamics of anticancer drugs. Tissue Barriers 2022, 10, 1965418. [Google Scholar] [CrossRef] [PubMed]
- Afratis, N.; Gialeli, C.; Nikitovic, D.; Tsegenidis, T.; Karousou, E.; Theocharis, A.D.; Pavão, M.S.; Tzanakakis, G.N.; Karamanos, N.K. Glycosaminoglycans: Key players in cancer cell biology and treatment. FEBS J. 2012, 279, 1177–1197. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; Ten Hagen, T.L.M. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Haratipour, P.; Lingeman, R.G.; Perry, J.J.P.; Gu, L.; Hickey, R.J.; Malkas, L.H. Novel Peptide Therapeutic Approaches for Cancer Treatment. Cells 2021, 10, 2908. [Google Scholar] [CrossRef] [PubMed]
- Alves, I.D.; Carré, M.; Montero, M.P.; Castano, S.; Lecomte, S.; Marquant, R.; Lecorché, P.; Burlina, F.; Schatz, C.; Sagan, S.; et al. A proapoptotic peptide conjugated to penetratin selectively inhibits tumor cell growth. Biochim. Biophys. Acta 2014, 1838, 2087–2098. [Google Scholar] [CrossRef]
- Aroui, S.; Brahim, S.; De Waard, M.; Bréard, J.; Kenani, A. Efficient induction of apoptosis by doxorubicin coupled to cell-penetrating peptides compared to unconjugated doxorubicin in the human breast cancer cell line MDA-MB 231. Cancer Lett. 2009, 285, 28–38. [Google Scholar] [CrossRef]
- Aroui, S.; Brahim, S.; Hamelin, J.; De Waard, M.; Bréard, J.; Kenani, A. Conjugation of doxorubicin to cell penetrating peptides sensitizes human breast MDA-MB 231 cancer cells to endogenous TRAIL-induced apoptosis. Apoptosis 2009, 14, 1352–1365. [Google Scholar] [CrossRef]
- Aroui, S.; Brahim, S.; Waard, M.D.; Kenani, A. Cytotoxicity, intracellular distribution and uptake of doxorubicin and doxorubicin coupled to cell-penetrating peptides in different cell lines: A comparative study. Biochem. Biophys. Res. Commun. 2010, 391, 419–425. [Google Scholar] [CrossRef]
- Lopes, L.; Pepe, D.; Carvalho, V.; McCall, M.; De Lemos, D. Transportan in nanocarriers improves skin localization and antitumor activity of paclitaxel. Int. J. Nanomed. 2016, 11, 2009–2019. [Google Scholar] [CrossRef]
- Wang, S.; Zhelev, N.Z.; Duff, S.; Fischer, P.M. Synthesis and biological activity of conjugates between paclitaxel and the cell delivery vector penetratin. Bioorg. Med. Chem. Lett. 2006, 16, 2628–2631. [Google Scholar] [CrossRef]
- Lelle, M.; Frick, S.U.; Steinbrink, K.; Peneva, K. Novel cleavable cell-penetrating peptide-drug conjugates: Synthesis and characterization. J. Pept. Sci. 2014, 20, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Konishi, Y.; Ueda, M.; Saji, H.; Futaki, S. Accumulation of arginine-rich cell-penetrating peptides in tumors and the potential for anticancer drug delivery in vivo. J. Control Release 2012, 159, 181–188. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, N.; Liu, X.; Liang, W.; Xu, W.; Torchilin, V.P. siRNA-containing liposomes modified with polyarginine effectively silence the targeted gene. J. Control. Release 2006, 112, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Kang, D.H.; Kim, T.W.; Kong, H.J.; Ryu, J.S.; Jeon, S.; Ahn, T.S.; Jeong, D.; Baek, M.J.; Im, J. Intracellular delivery of oxaliplatin conjugate via cell penetrating peptide for the treatment of colorectal carcinoma in vitro and in vivo. Int. J. Pharm. 2021, 606, 120904. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Deshpande, P.P.; Perche, F.; Dodwadkar, N.S.; Sane, S.D.; Torchilin, V.P. Octa-arginine-modified pegylated liposomal doxorubicin: An effective treatment strategy for non-small cell lung cancer. Cancer Lett. 2013, 335, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ran, R.; Chen, J.; Kuang, Q.; Tang, J.; Mei, L.; Zhang, Q.; Gao, H.; Zhang, Z.; He, Q. Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials 2014, 35, 4835–4847. [Google Scholar] [CrossRef]
- Koshkaryev, A.; Piroyan, A.; Torchilin, V.P. Bleomycin in octaarginine-modified fusogenic liposomes results in improved tumor growth inhibition. Cancer Lett. 2013, 334, 293–301. [Google Scholar] [CrossRef]
- Shi, N.Q.; Gao, W.; Xiang, B.; Qi, X.R. Enhancing cellular uptake of activable cell-penetrating peptide-doxorubicin conjugate by enzymatic cleavage. Int. J. Nanomed. 2012, 7, 1613–1621. [Google Scholar] [CrossRef]
- Gao, W.; Xiang, B.; Meng, T.T.; Liu, F.; Qi, X.R. Chemotherapeutic drug delivery to cancer cells using a combination of folate targeting and tumor microenvironment-sensitive polypeptides. Biomaterials 2013, 34, 4137–4149. [Google Scholar] [CrossRef]
- Leng, X.; Dong, X.; Liu, L.; Zhu, D.; Zhang, H. Transactivator of transcription (TAT) peptide-chitosan functionalized multiwalled carbon nanotubes as a potential drug delivery vehicle for cancer therapy. Int. J. Nanomed. 2015, 10, 3829–3841. [Google Scholar] [CrossRef]
- Fales, A.M.; Yuan, H.; Vo-Dinh, T. Cell-penetrating peptide enhanced intracellular Raman imaging and photodynamic therapy. Mol. Pharm. 2013, 10, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Y.; Zhang, W.; Chen, J.; Yang, X.; Ma, P.; Zhang, B.; Liu, B.; Ni, J.; Wang, R. Cell penetrating peptide TAT can kill cancer cells via membrane disruption after attachment of camptothecin. Peptides 2015, 63, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Taki, H.; Kanazawa, T.; Akiyama, F.; Takashima, Y.; Okada, H. Intranasal Delivery of Camptothecin-Loaded Tat-Modified Nanomicells for Treatment of Intracranial Brain Tumors. Pharmaceuticals 2012, 5, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; Dos Santos Rodrigues, B.; Sun, C.; Singh, J. Biodistribution of TAT or QLPVM coupled to receptor targeted liposomes for delivery of anticancer therapeutics to brain in vitro and in vivo. Nanomedicine 2020, 23, 102112. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.L.; Liu, J.J.; Hong, R.L. Translocation of liposomes into cancer cells by cell-penetrating peptides penetratin and tat: A kinetic and efficacy study. Mol. Pharmacol. 2002, 62, 864–872. [Google Scholar] [CrossRef]
- Fang, B.; Jiang, L.; Zhang, M.; Ren, F.Z. A novel cell-penetrating peptide TAT-A1 delivers siRNA into tumor cells selectively. Biochimie 2013, 95, 251–257. [Google Scholar] [CrossRef]
- Tints, K.; Prink, M.; Neuman, T.; Palm, K. LXXLL Peptide Converts Transportan 10 to a Potent Inducer of Apoptosis in Breast Cancer Cells. Int. J. Mol. Sci. 2014, 15, 5680–5698. [Google Scholar] [CrossRef]
- Yamada, T.; Das Gupta, T.K.; Beattie, C.W. p28-Mediated Activation of p53 in G2–M Phase of the Cell Cycle Enhances the Efficacy of DNA Damaging and Antimitotic Chemotherapy. Cancer Res. 2016, 76, 2354–2365. [Google Scholar] [CrossRef]
- Thomas, E.; Dragojevic, S.; Price, A.; Raucher, D. Thermally Targeted p50 Peptide Inhibits Proliferation and Induces Apoptosis of Breast Cancer Cell Lines. Macromol. Biosci. 2020, 20, 2000170. [Google Scholar] [CrossRef]
- Vale, N.; Ribeiro, E.; Cruz, I.; Stulberg, V.; Koksch, B.; Costa, B. New Perspective for Using Antimicrobial and Cell-Penetrating Peptides to Increase Efficacy of Antineoplastic 5-FU in Cancer Cells. J. Funct. Biomater. 2023, 14, 565. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).