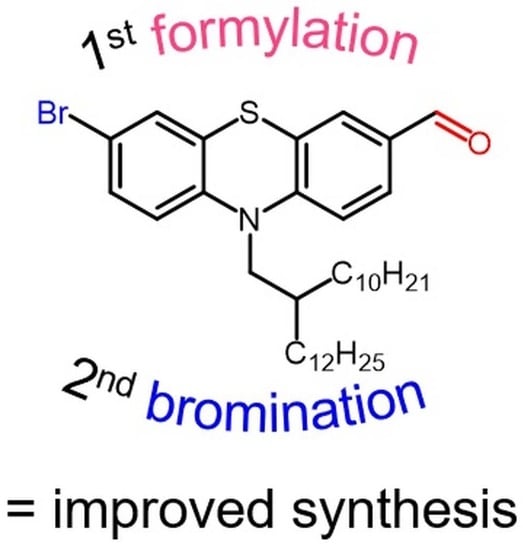
Efficient Synthesis of a 2-Decyl-tetradecyl Substituted 7-Bromophenothiazine-3-carbaldehyde Building Block for Functional Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. General Considerations and Instrumentation
2.2. 10-(2-Decyl-tetradecyl)-10H-phenothiazine-3-carbaldehyde (2)
2.3. 7-Bromo-10-(2-decyl-tetradecyl)-10H-phenothiazine-3-carbaldehyde (3)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer, T.; Krug, R.; Müller, T.J.J. Three-Component Suzuki–Knoevenagel Synthesis of Merocyanine Libraries and Correlation Analyses of Their Oxidation Potentials and Optical Band Gaps. Molecules 2021, 26, 5149. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Ogermann, D.; Pankrath, A.; Kleinermanns, K.; Müller, T.J.J. Phenothiazinyl Rhodanylidene Merocyanines for Dye-Sensitized Solar Cells. J. Org. Chem. 2012, 77, 3704–3715. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Müller, T.J.J. Consecutive Three-Component Synthesis of Donor-Substituted Merocyanines by a One-Pot Suzuki-Knoevenagel Condensation Sequence. Org. Mater. 2020, 2, 64–70. [Google Scholar] [CrossRef]
- Hua, Y.; Chang, S.; Huang, D.; Zhou, X.; Zhu, X.; Zhao, J.; Chen, T.; Wong, W.-Y.; Wong, W.-K. Significant Improvement of Dye-Sensitized Solar Cell Performance Using Simple Phenothiazine-Based Dyes. Chem. Mater. 2013, 25, 2146–2153. [Google Scholar] [CrossRef]
- Hauck, M.; Stolte, M.; Schönhaber, J.; Kuball, H.-G.; Müller, T.J.J. Synthesis, Electronic, and Electro-Optical Properties of Emissive Solvatochromic Phenothiazinyl Merocyanine Dyes. Chem. Eur. J. 2011, 17, 9984–9998. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-J.; Chang, Y.J.; Watanabe, M.; Hon, Y.-S.; Chow, T.J. Phenothiazine derivatives as organic sensitizers for highly efficient dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 4040–4049. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.-Y.; Chi, Z.; Xu, B.; Zhang, X.; Kuang, D.-B.; Zhang, Y.; Liu, S.; Xu, J. Metal-free organic dyes derived from triphenylethylene for dye-sensitized solar cells: Tuning of the performance by phenothiazine and carbazole. J. Mater. Chem. 2012, 22, 8994–9005. [Google Scholar] [CrossRef]
- Iqbal, Z.; Wu, W.-Q.; Zhang, H.; Hua, P.-L.; Fang, X.; Kuang, D.-B.; Wang, L.; Meier, H.; Cao, D. Impact of hydroxy and octyloxy substituents of phenothiazine based dyes on the photovoltaic performance. Dyes Pigm. 2013, 99, 299–307. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Guo, J.; Tang, H.; Zhao, J. Influence of the terminal electron donor in D–D–π–A phenothiazine dyes for dye-sensitized solar cells. Dyes Pigm. 2014, 109, 96–104. [Google Scholar] [CrossRef]
- Dai, X.-X.; Feng, H.-L.; Chen, W.-J.; Yang, Y.; Nie, L.-B.; Wang, L.; Kuang, D.-B.; Meier, H.; Cao, D. Synthesis and photovoltaic performance of asymmetric di-anchoring organic dyes. Dyes Pigm. 2015, 122, 13–21. [Google Scholar] [CrossRef]
- Eiamprasert, U.; Sudchanham, J.; Surawatanawong, P.; Pakawatpanurut, P.; Kiatisevi, S. Additional donor bridge as a design approach for multi-anchoring dyes for highly efficient dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2018, 352, 86–97. [Google Scholar] [CrossRef]
- Jiao, Y.; Mao, L.; Liu, S.; Tan, T.; Wang, D.; Cao, D.; Mi, B.; Gao, Z.; Huang, W. Effects of meta or para connected organic dyes for dye-sensitized solar cell. Dyes Pigm. 2018, 158, 165–174. [Google Scholar] [CrossRef]
- Revoju, S.; Biswas, S.; Eliasson, B.; Sharma, G.D. Asymmetric triphenylamine–phenothiazine based small molecules with varying terminal acceptors for solution processed bulk-heterojunction organic solar cells. Phys. Chem. Chem. Phys. 2018, 20, 6390–6400. [Google Scholar] [CrossRef]
- Sachdeva, T.; Milton, M.D. Logic gate based novel phenothiazine-pyridylhydrazones: Halochromism in solid and solution state. Dyes Pigm. 2019, 164, 305–318. [Google Scholar] [CrossRef]
- Sachdeva, T.; Milton, M.D. Fluorescent dyes for moisture detection in organic solvents: Push-pull based phenothiazine aldehydes with large Stokes shifts. J. Photochem. Photobiol. A Chem. 2020, 402, 112804. [Google Scholar] [CrossRef]
- Chen, H.-W.; Hakeim, O.A.; Song, Q.-H.; Xia, H.-C. Phenothiazine and semi-cyanine based colorimetric and fluorescent probes for detection of sulfites in solutions and in living cells. RSC Adv. 2021, 11, 34643–34651. [Google Scholar] [CrossRef]
- Krämer, C.S.; Zeitler, K.; Müller, T.J.J. Synthesis of Functionalized Ethynylphenothiazine Fluorophores. Org. Lett. 2000, 2, 3723–3726. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, H.; Shao, J.; Zhang, X.; Ji, S.; Zhao, J. Synthesis of Ethynylated Phenothiazine Based Fluorescent Boronic Acid Probes. J. Fluoresc. 2011, 21, 1143–1154. [Google Scholar] [CrossRef]
- Nagarajan, B.; Kushwaha, S.; Elumalai, R.; Mandal, S.; Ramanujam, K.; Raghavachari, D. Novel ethynyl-pyrene substituted phenothiazine based metal free organic dyes in DSSC with 12% conversion efficiency. J. Mater. Chem. A 2017, 5, 10289–10300. [Google Scholar] [CrossRef]
- Han, W.; Shi, Y.; Xue, T.; Wang, T. Synthesis and electrochemical, linear and third-order nonlinear optical properties of ferrocene-based D-π-A dyes as novel photoredox catalysts in photopolymerization under visible LED irradiations. Dyes Pigm. 2019, 166, 140–148. [Google Scholar] [CrossRef]
- Slodek, A.; Zych, D.; Maroń, A.; Gawecki, R.; Mrozek-Wilczkiewicz, A.; Malarz, K.; Musioł, R. Phenothiazine derivatives—Synthesis, characterization, and theoretical studies with an emphasis on the solvatochromic properties. J. Mol. Liq. 2019, 285, 515–525. [Google Scholar] [CrossRef]
- Slodek, A.; Zych, D.; Kotowicz, S.; Szafraniec-Gorol, G.; Zimosz, S.; Schab-Balcerzak, E.; Siwy, M.; Grzelak, J.; Maćkowski, S. “Small in size but mighty in force”—The first principle study of the impact of A/D units in A/D-phenyl-p-phenothiazine-p-dicyanovinyl systems on photophysical and optoelectronic properties. Dyes Pigm. 2021, 189, 109248. [Google Scholar] [CrossRef]
- Franz, A.W.; Popa, L.N.; Rominger, F.; Müller, T.J.J. First synthesis and electronic properties of diphenothiazine dumbbells bridged by heterocycles. Org. Biomol. Chem. 2009, 7, 469–475. [Google Scholar] [CrossRef]
- Stalindurai, K.; Gokula Krishnan, K.; Nagarajan, E.R.; Ramalingan, C. Experimental and theoretical studies on new 7-(3,6-di-tert-butyl-9H-carbazol-9-yl)-10-alkyl-10H-phenothiazine-3-carbaldehydes. J. Mol. Struct. 2017, 1130, 633–643. [Google Scholar] [CrossRef]
- Stalindurai, K.; Karuppasamy, A.; Peng, J.-D.; Ho, K.-C.; Tamilselvan, A.; Ramalingan, C. Fused heterocycles possessing novel metal-free organic dyes for dye-sensitized solar cells. Tetrahedron 2017, 73, 278–289. [Google Scholar] [CrossRef]
- Stalindurai, K.; Karuppasamy, A.; Peng, J.-D.; Ho, K.-C.; Ramalingan, C. Azafluorene Ornamented Thiazine Based Novel Fused Heterocyclic Organic Dyes for Competent Molecular Photovoltaics. Electrochim. Acta 2017, 246, 1052–1064. [Google Scholar] [CrossRef]
- Arai, T.; Kubo, Y. Chemical stimulus-responsive tricyanopyrroline-based ICT chromophore as a potential environment-sensitive probe. Dyes Pigm. 2021, 185, 108927. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, M.; Wang, Y.; Zhou, H.P.; Zheng, J.; Zhang, X.; Wu, J.; Tiana, Y.; Wu, Z. Aggregation-induced and crystallization-enhanced emissions with time-dependence of a new Schiff-base family based on benzimidazole. J. Mater. Chem. C 2014, 2, 3686–3694. [Google Scholar] [CrossRef]
- Mondal, B.; Mukherjee, P.S. Cage Encapsulated Gold Nanoparticles as Heterogeneous Photocatalyst for Facile and Selective Reduction of Nitroarenes to Azo Compounds. J. Am. Chem. Soc. 2018, 39, 12592–12601. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Börüsah, B.K.; Müller, T.J.J. Efficient Synthesis of a 2-Decyl-tetradecyl Substituted 7-Bromophenothiazine-3-carbaldehyde Building Block for Functional Dyes. Organics 2022, 3, 502-506. https://doi.org/10.3390/org3040033
Börüsah BK, Müller TJJ. Efficient Synthesis of a 2-Decyl-tetradecyl Substituted 7-Bromophenothiazine-3-carbaldehyde Building Block for Functional Dyes. Organics. 2022; 3(4):502-506. https://doi.org/10.3390/org3040033
Chicago/Turabian StyleBörüsah, Burak Kürsat, and Thomas J. J. Müller. 2022. "Efficient Synthesis of a 2-Decyl-tetradecyl Substituted 7-Bromophenothiazine-3-carbaldehyde Building Block for Functional Dyes" Organics 3, no. 4: 502-506. https://doi.org/10.3390/org3040033
APA StyleBörüsah, B. K., & Müller, T. J. J. (2022). Efficient Synthesis of a 2-Decyl-tetradecyl Substituted 7-Bromophenothiazine-3-carbaldehyde Building Block for Functional Dyes. Organics, 3(4), 502-506. https://doi.org/10.3390/org3040033