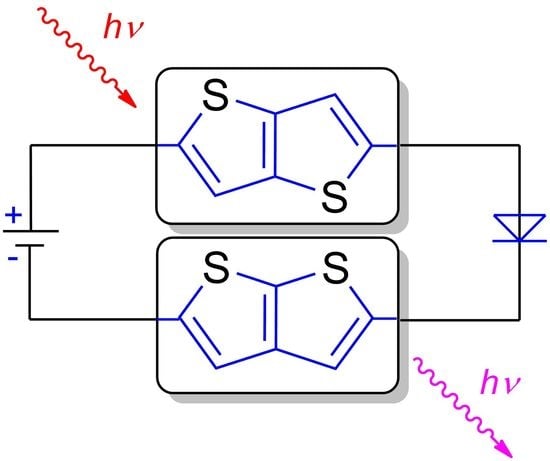
Thienothiophene Scaffolds as Building Blocks for (Opto)Electronics
Abstract
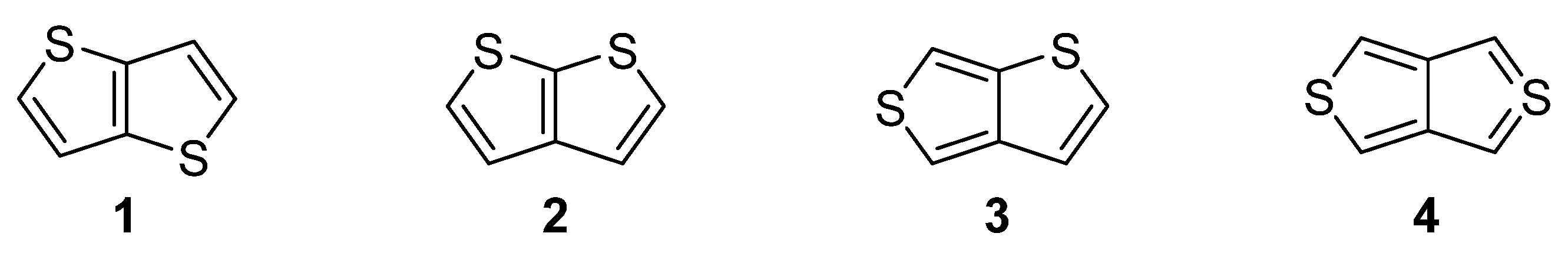
1. Introduction
2. Synthesis
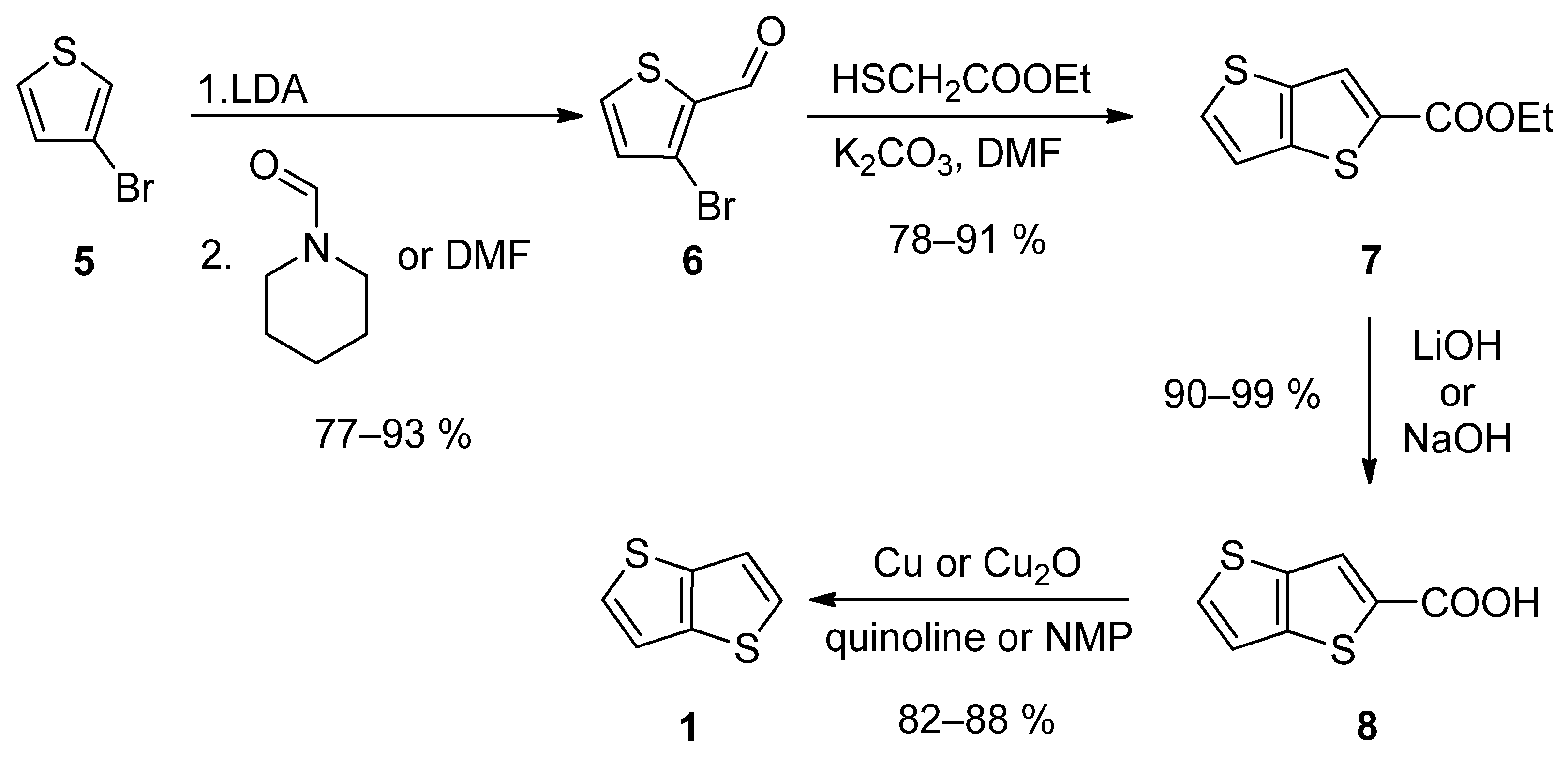
2.1. Synthesis of Thieno[3,2-b]hiophene 1
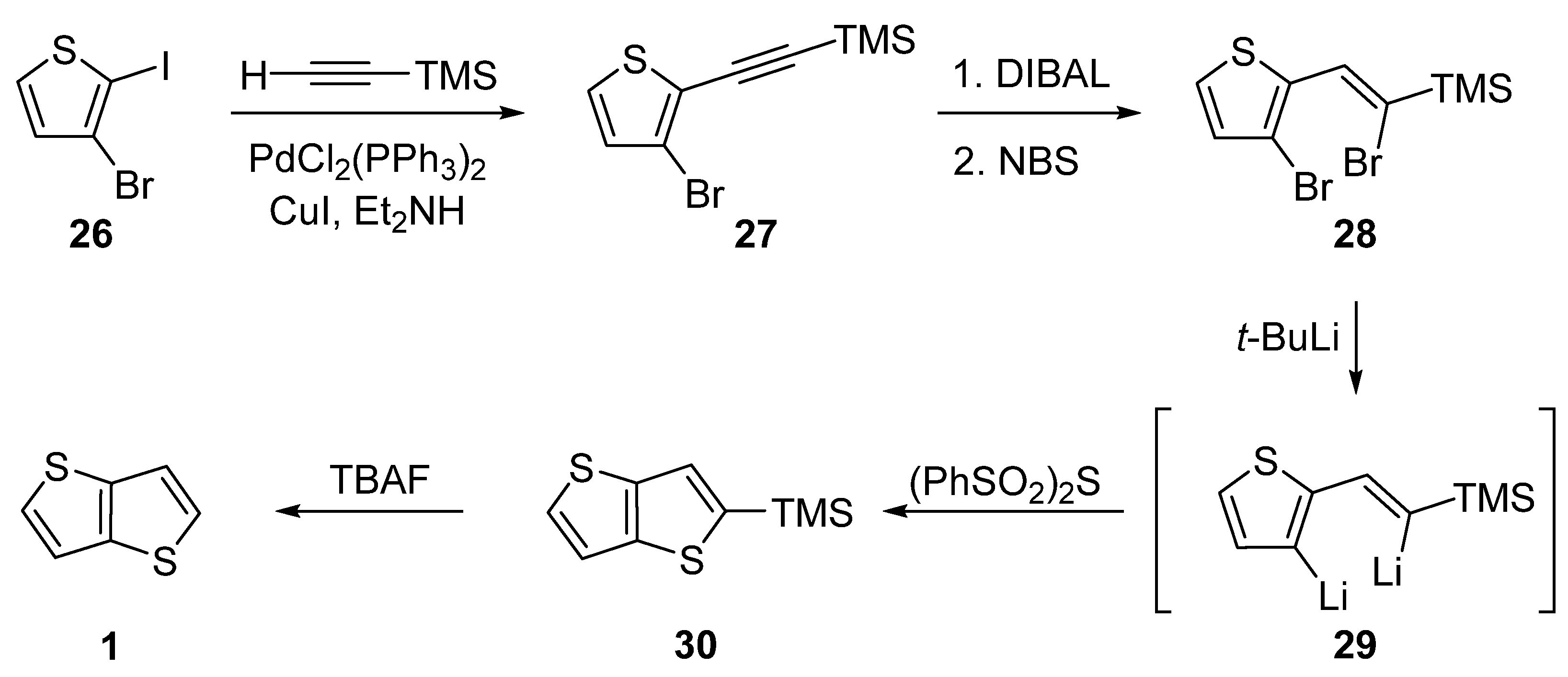
2.1.1. Method 1
2.1.2. Method 2
2.1.3. Method 3
2.1.4. Method 4
2.1.5. Method 5
2.1.6. Method 6
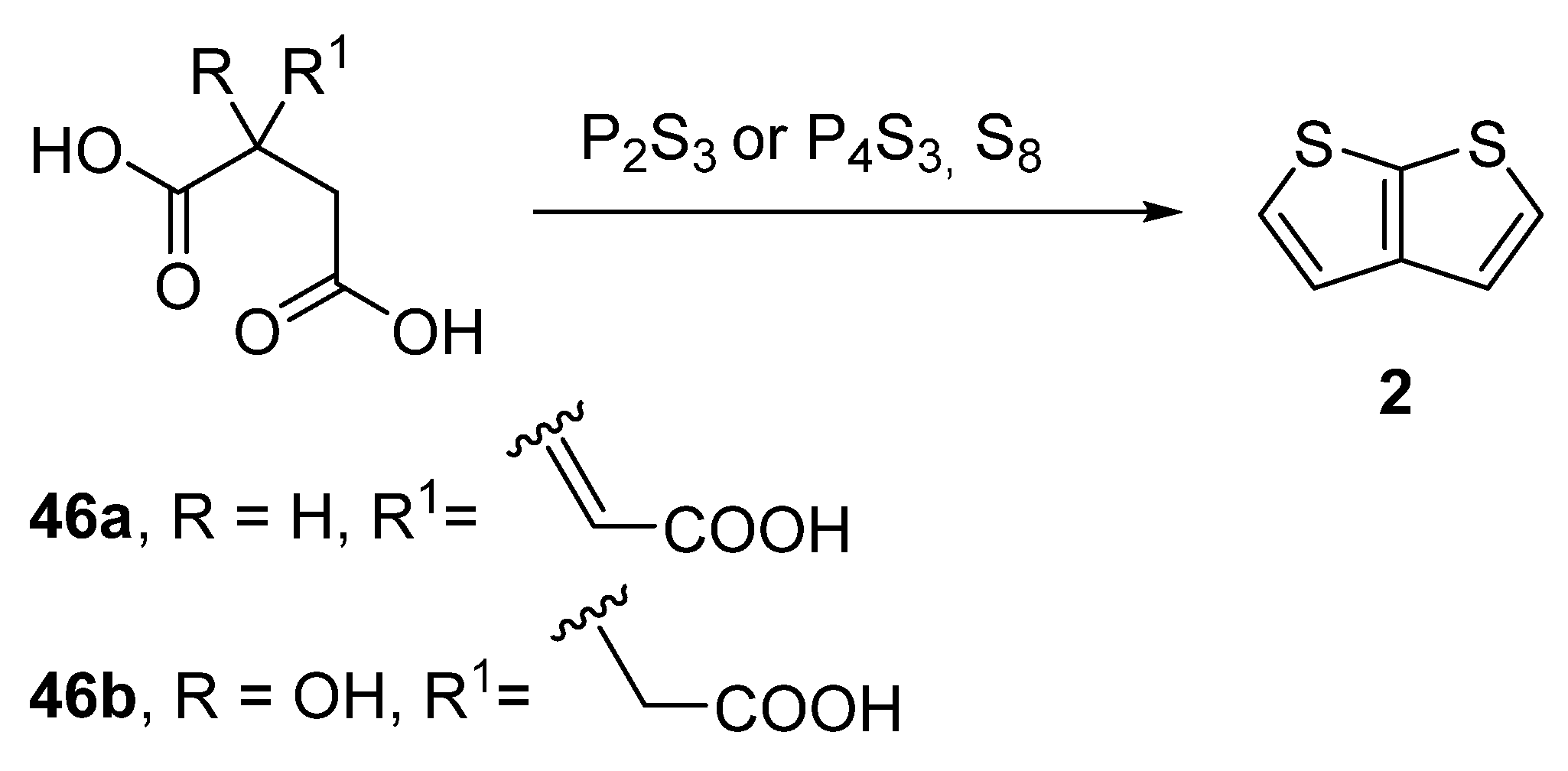
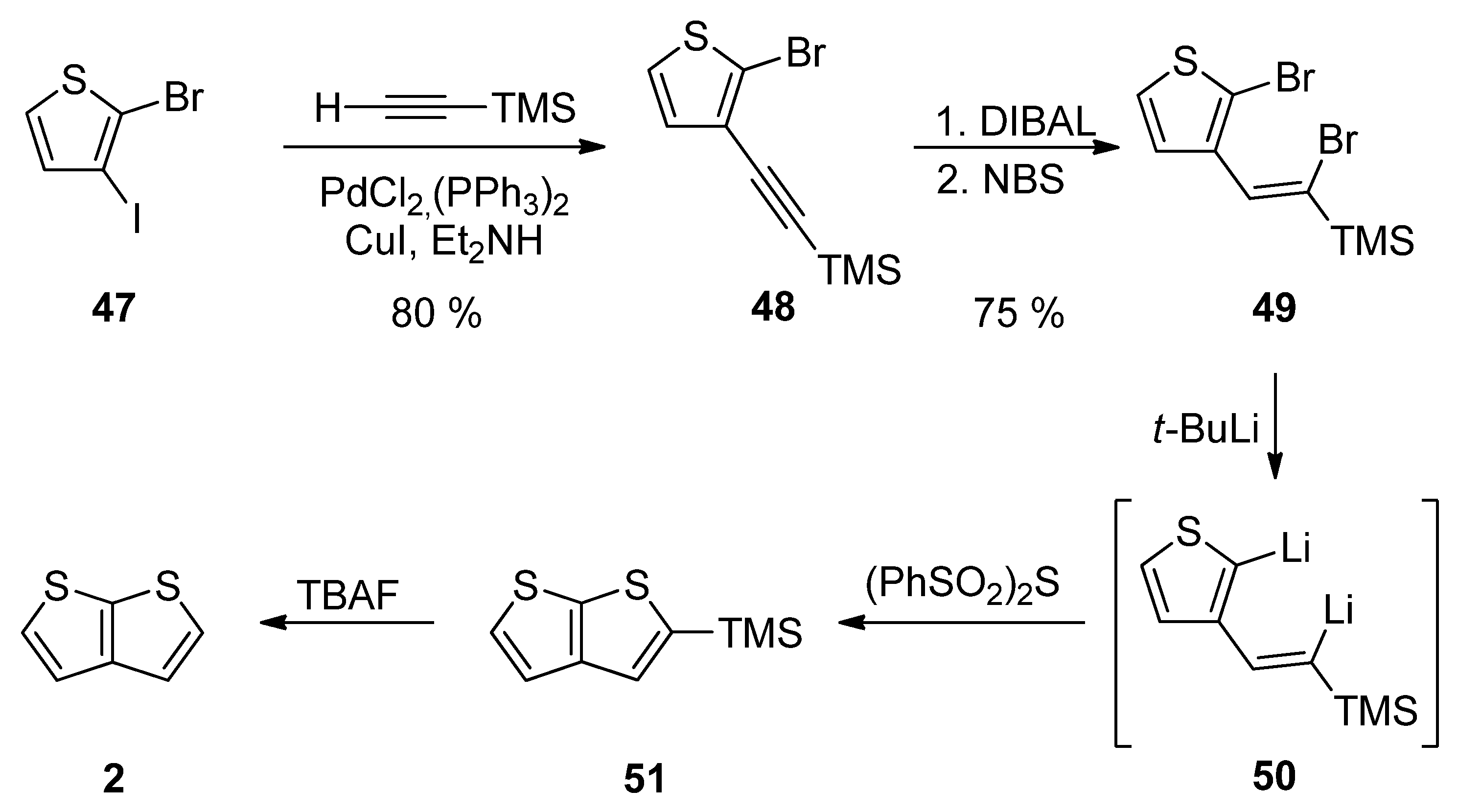
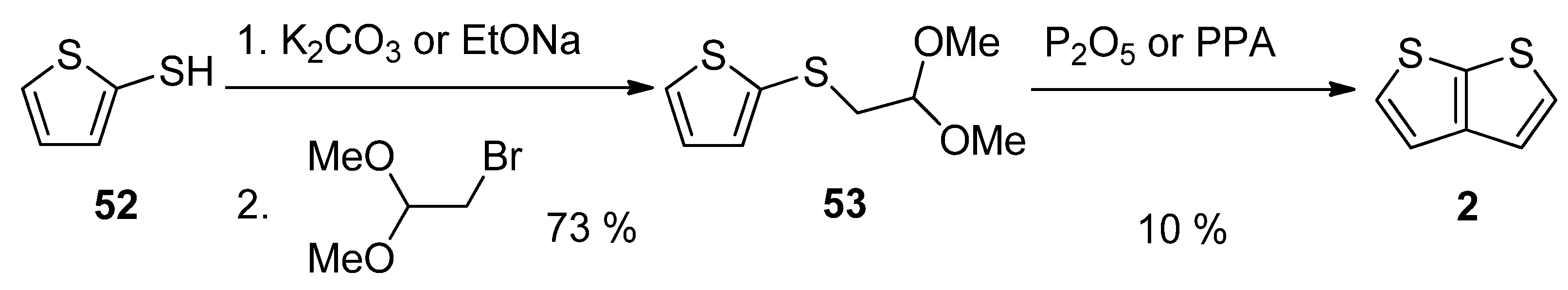
2.2. Synthesis of Thieno[2,3-b]thiophene 2
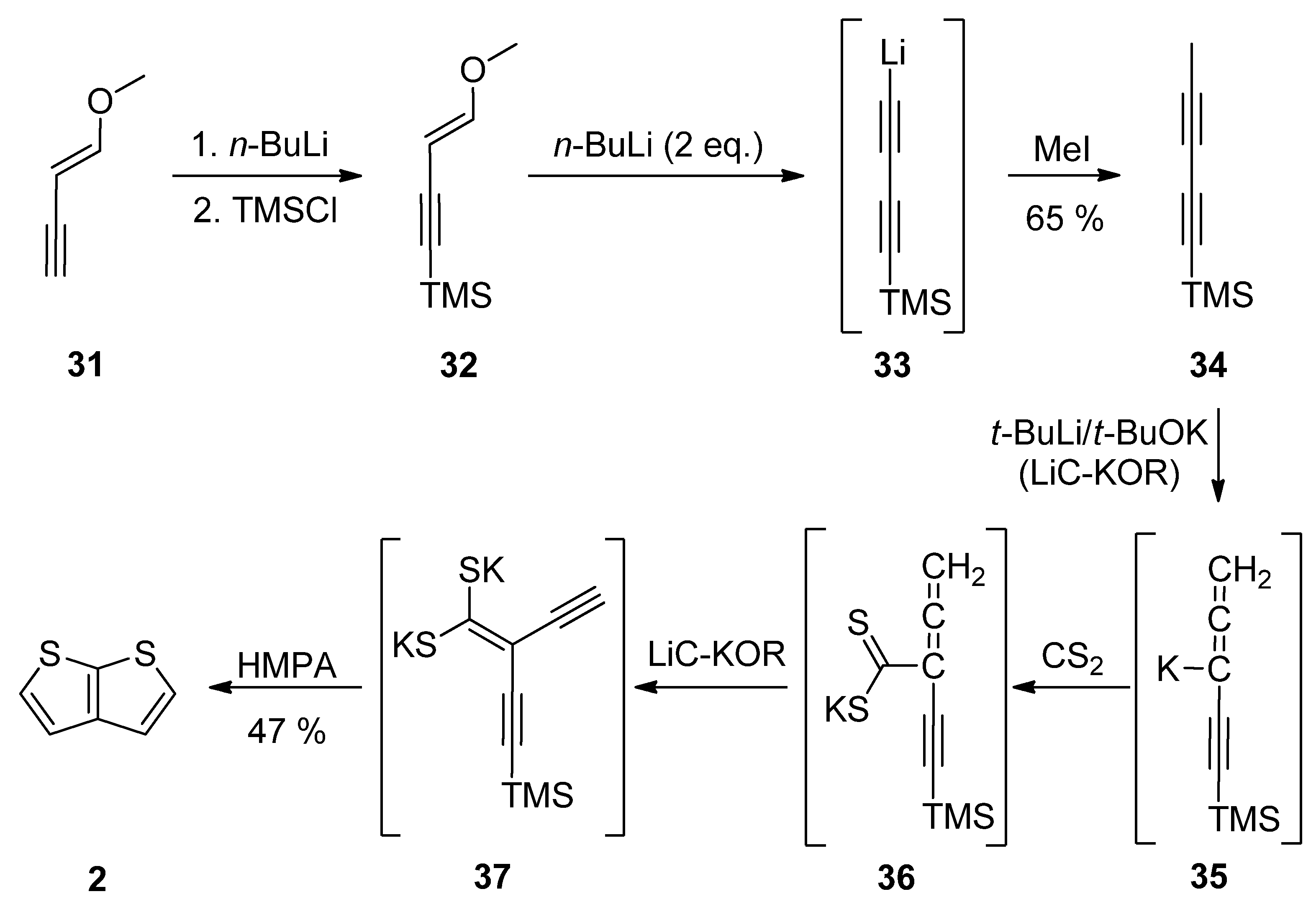
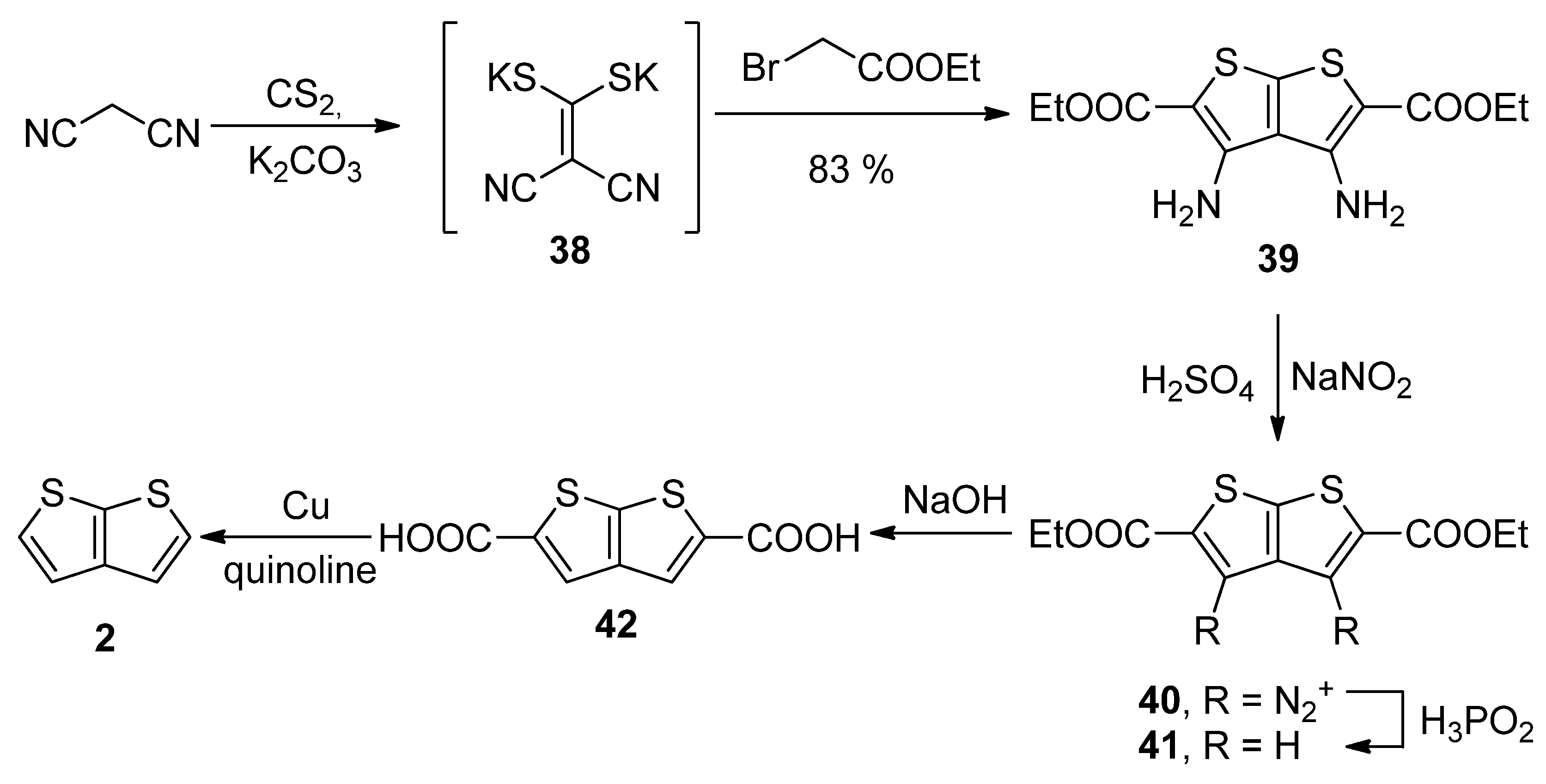
2.2.1. Method 7
2.2.2. Method 8
2.2.3. Method 9
2.2.4. Method 10
2.2.5. Method 11
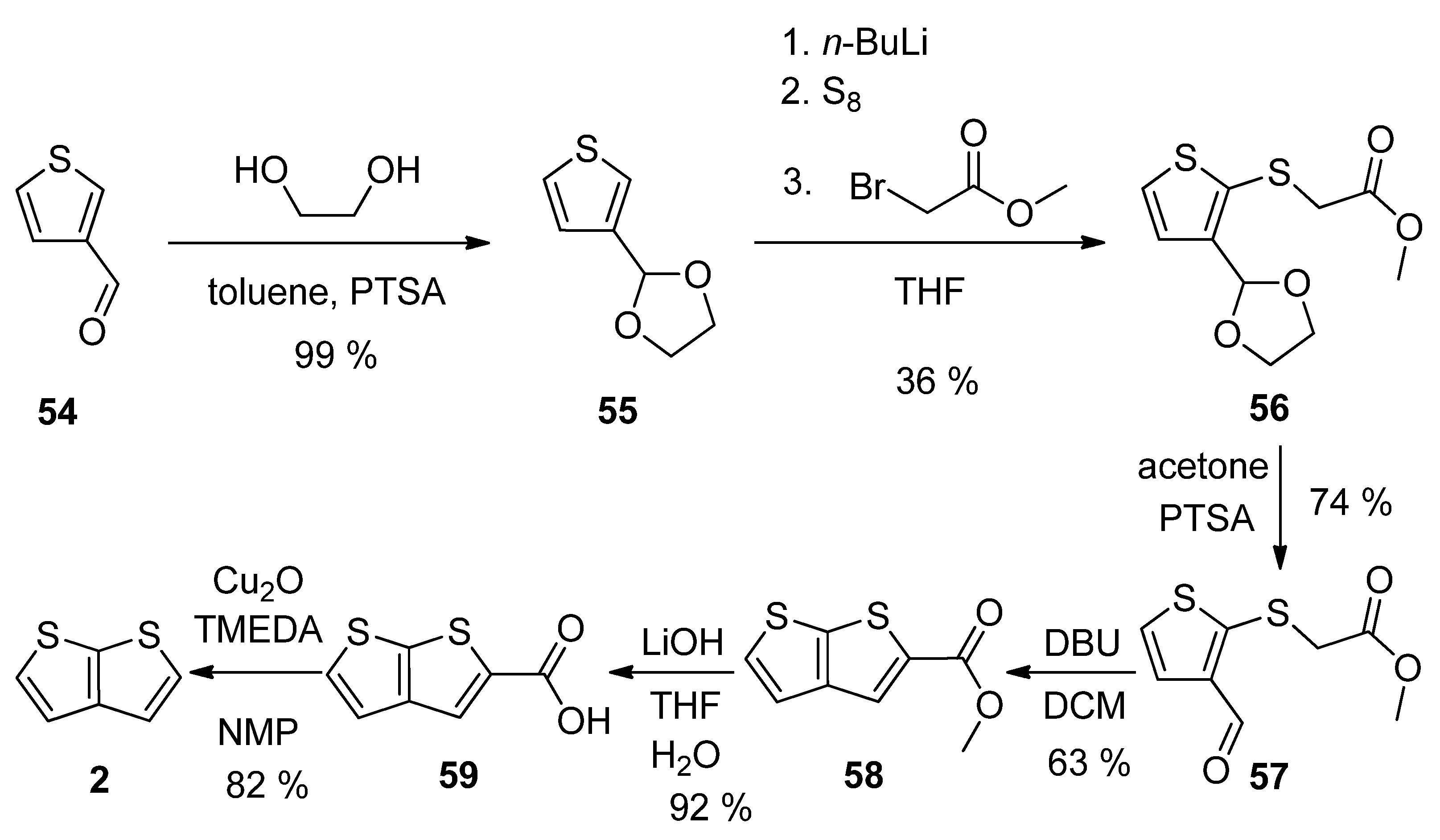
2.2.6. Method 12
2.2.7. Method 13
2.2.8. Method 14
3. Applications of TTs in (Opto)Electronics
3.1. Organic Light Emitting Diodes (OLEDs)
3.2. Organic Solar Cells (OSCs)
3.2.1. Bulk Heterojunction Solar Cells (BHJs)
3.2.2. Dye Sensitized Solar Cells (DSSCs)
3.2.3. Perovskite Solar Cells (PSCs)
3.3. Organic Field-Effect Transistors (OFETs)
3.4. Nonlinear Optics (NLO)
4. Conclusions
Funding
Conflicts of Interest
References
- Kulhánek, J.; Klikar, M.; Pytela, O.; Almonasy, N.; Tydlitát, J.; Bureš, F. Quadrupolar fluorophores with tetrafluorobenzene central electron acceptor. J. Fluor. Chem. 2021, 243, 109735. [Google Scholar] [CrossRef]
- Raposo, M.M.M.; Herbivo, C.; Hugues, V.; Clermont, G.; Castro, M.C.R.; Comel, A.; Blanchard-Desce, M. Synthesis, Fluorescence, and Two-Photon Absorption Properties of Push–Pull 5-Arylthieno [3,2-b]thiophene Derivatives. Eur. J. Org. Chem. 2016, 2016, 5263–5273. [Google Scholar] [CrossRef]
- Novotná, E.; Kityk, I.V.; Pytela, O.; Bureš, F.; Ludwig, M.; Klikar, M.; Ozga, K.; Jedryka, J. ThDione: A Powerful Electron-Withdrawing Moiety for Push–Pull Molecules. Chempluschem 2020, 85, 1549–1558. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, Y.; Zhao, C.; Zhang, J.; Ghadari, R.; Hu, L.; Kong, F. The strategy for high-efficiency hole conductors by engineering short-range intramolecular interactions. Dyes Pigments 2022, 197, 109889. [Google Scholar] [CrossRef]
- Torricelli, F.; Alessandri, I.; Macchia, E.; Vassalini, I.; Maddaloni, M.; Torsi, L. Green Materials and Technologies for Sustainable Organic Transistors. Adv. Mater. Technol. 2022, 7, 2100445. [Google Scholar] [CrossRef]
- D’Orazio-Colman, A.; Son, D.H.; Binti Nasrun, R.F.; Kim, J.H. Novel conjugated polymers based on cyclopenta[c]thiophene-4,6(5H)-dione for efficient polymer solar cells. J. Power Sources 2022, 542, 231737. [Google Scholar] [CrossRef]
- Meyer, V. Zur Kenntniss der Thiophen- und Pyrrolgruppe. Ber. Dtsch. Chem. Ges. 1883, 16, 2968–2975. [Google Scholar] [CrossRef]
- Perepichka, I.F.; Perepichka, D.F. PEDOT—Properties and technical relevance. In Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics, 1st ed.; John Wiley & Sons, Inc.: Chichester, UK, 2009; pp. 549–576. ISBN 9780470745533. [Google Scholar]
- Perepichka, I.F.; Perepichka, D.F. Electroactive oligothiophenes and polythiophenes for organic field effect transistors. In Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics, 1st ed.; John Wiley & Sons, Inc.: Chichester, UK, 2009; pp. 595–646. ISBN 9780470745533. [Google Scholar]
- Perepichka, I.F.; Perepichka, D.F. Photovoltaics Based on Thiophene Polymers: A Short Overview. In Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics, 1st ed.; John Wiley & Sons, Inc.: Chichester, UK, 2009; pp. 673–693. ISBN 9780470745533. [Google Scholar]
- Perepichka, I.F.; Perepichka, D.F. Thiophene-based materials for electroluminescent applications. In Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics, 1st ed.; John Wiley & Sons, Inc.: Chichester, UK, 2009; pp. 695–756. ISBN 9780470745533. [Google Scholar]
- Perepichka, I.F.; Perepichka, D.F. Thienothiophenes. In Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics, 1st ed.; John Wiley & Sons, Inc.: Chichester, UK, 2009; pp. 219–233. ISBN 9780470745533. [Google Scholar]
- Mashraqui, S.H.; Ghadigaonkar, S.; Ashraf, M.; Sri Ranjini, A.; Ghosh, S.; Das, P.K. Optically transparent and thermally stable nonlinear optic chromophores featuring a thieno [2,3-b]thiophene donor. Tetrahedron 2007, 63, 10011–10017. [Google Scholar] [CrossRef]
- Zhang, A.; Xiao, H.; Cong, S.; Zhang, M.; Zhang, H.; Bo, S.; Wang, Q.; Zhen, Z.; Liu, X. A systematic study of the structure–property relationship of a series of nonlinear optical (NLO) julolidinyl-based chromophores with a thieno [3,2-b]thiophene moiety. J. Mater. Chem. C 2015, 3, 370–381. [Google Scholar] [CrossRef]
- Kulhánek, J.; Bureš, F.; Opršal, J.; Kuznik, W.; Mikysek, T.; Růžička, A. 1,4-Phenylene and 2,5-thienylene π-linkers in charge-transfer chromophores. Asian J. Org. Chem. 2013, 2, 422–431. [Google Scholar] [CrossRef]
- Tang, W.; Lin, T.; Ke, L.; Chen, Z.-K. Synthesis, Photophysics, Theoretical Modeling, and Electroluminescence of Novel 2,7-Carbazole-Based Conjugated Polymers with Sterically Hindered Structures. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7725–7738. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Kan, B.; Wan, X.; Liu, F.; Ni, W.; Feng, H.; Russell, T.P.; Chen, Y. A solution-processed high performance organic solar cell using a small molecule with the thieno [3,2-b]thiophene central unit. Chem. Commun. 2015, 51, 15268–15271. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.S.M.; Castro, M.C.R.; Mesquita, I.; Andrade, L.; Mendes, A.; Raposo, M.M.M. Synthesis and characterization of novel thieno [3,2-b]thiophene based metal-free organic dyes with different heteroaromatic donor moieties as sensitizers for dye-sensitized solar cells. Dyes Pigments 2017, 136, 46–53. [Google Scholar] [CrossRef]
- Peng, S.H.; Huang, T.W.; Gollavelli, G.; Hsu, C.S. Thiophene and diketopyrrolopyrrole based conjugated polymers as efficient alternatives to spiro-OMeTAD in perovskite solar cells as hole transporting layers. J. Mater. Chem. C 2017, 5, 5193–5198. [Google Scholar] [CrossRef]
- Shu, Y.; Mikosch, A.; Winzenberg, K.N.; Kemppinen, P.; Easton, C.D.; Bilic, A.; Forsyth, C.M.; Dunn, C.J.; Singh, T.B.; Collis, G.E. N -Alkyl functionalized barbituric and thiobarbituric acid bithiophene derivatives for vacuum deposited n-channel OFETs. J. Mater. Chem. C 2014, 2, 3895–3899. [Google Scholar] [CrossRef]
- Fei, Z.; Pattanasattayavong, P.; Han, Y.; Schroeder, B.C.; Yan, F.; Kline, R.J.; Anthopoulos, T.D.; Heeney, M. Influence of side-chain regiochemistry on the transistor performance of high-mobility, all-donor polymers. J. Am. Chem. Soc. 2014, 136, 15154–15157. [Google Scholar] [CrossRef]
- Mu, S.; Oniwa, K.; Jin, T.; Asao, N.; Yamashita, M.; Takaishi, S. A highly emissive distyrylthieno [3,2-b]thiophene based red luminescent organic single crystal: Aggregation induced emission, optical waveguide edge emission, and balanced ambipolar carrier transport. Org. Electron. 2016, 34, 23–27. [Google Scholar] [CrossRef]
- Blenkle, M.; Boldt, P.; Bräuchle, C.; Grahn, W.; Ledoux, I.; Nerenz, H.; Stadler, S.; Wichern, J.; Zyss, J. Chalcogens as electron donors for selected nonlinear optic phores. J. Chem. Soc. Perkin Trans. 1996, 2, 1377–1384. [Google Scholar] [CrossRef]
- Ahmed, M.O.; Pisula, W.; Mhaisalkar, S.G. Synthesis and characterization of new thieno [3,2-b]thiophene derivatives. Molecules 2012, 17, 12163–12171. [Google Scholar] [CrossRef]
- Fuller, L.S.; Iddon, B.; Smith, K.A. Thienothiophenes Part 2 reactions of thieno [3,2-b]thiophene and its polybromo derivatives. J. Chem. Soc. Perkin Trans. 1997, 1, 3465–3470. [Google Scholar] [CrossRef]
- Kawabata, K.; Takeguchi, M.; Goto, H. Optical activity of heteroaromatic conjugated polymer films prepared by asymmetric electrochemical polymerization in cholesteric liquid crystals: Structural function for chiral induction. Macromolecules 2013, 46, 2078–2091. [Google Scholar] [CrossRef]
- Xue, Y.; Xue, Z.; Zhang, W.; Zhang, W.; Chen, S.; Lin, K.; Xu, J. Enhanced electrochromic performances of Polythieno [3,2-b]thiophene with multicolor conversion via embedding EDOT segment. Polymer 2018, 159, 150–156. [Google Scholar] [CrossRef]
- Podlesný, J.; Pytela, O.; Klikar, M.; Jelínková, V.; Kityk, I.V.; Ozga, K.; Jedryka, J.; Rudysh, M.; Bureš, F. Small isomeric push-pull chromophores based on thienothiophenes with tunable optical (non)linearities. Org. Biomol. Chem. 2019, 17, 3623–3634. [Google Scholar] [CrossRef] [PubMed]
- Archer, W.J.; Taylor, R. Electrophilic Aromatic Substitution. Part 31. I Partial Rate Factors for Detritiation of Thieno [2,3-b]thiophen and Thieno [3,2-b]thiophen: Weak Hydrogen Bonding to Sulphur in Trifluoroacetic Acid. J. Chem. Soc. Perkin Trans. 1982, 2, 295–299. [Google Scholar] [CrossRef]
- Challenger, F.; Holmes, J.L. The Orientation of Substitution in the Isomeric Thiophthens. The Synthesis of Solid Thiophthen [Thiopheno(3′:2′-2:3)thiophen]. J. Chem. Soc. 1953, 1837–1842. [Google Scholar] [CrossRef]
- Leriche, P.; Raimundo, J.-M.; Turbiez, M.; Monroche, V.; Allain, M.; Sauvage, F.-X.; Roncali, J.; Frère, P.; Skabara, P.J. Linearly extended tetrathiafulvalene analogues with fused thiophene units as π-conjugated spacers. J. Mater. Chem. 2003, 13, 1324–1332. [Google Scholar] [CrossRef]
- Ghaisas, V.V.; Tilak, B.D. Thiophenes and Thiapyrans. Proc.-Indian Acad. Sci. Sect. A 1954, 39, 14–19. [Google Scholar] [CrossRef]
- Henssler, J.T.; Matzger, A.J. Facile and Scalable Synthesis of the Fused-Ring Heterocycles Thieno [3,2-b]thiophene and Thieno [3,2-b]furan. Org. Lett. 2009, 11, 3144–3147. [Google Scholar] [CrossRef]
- Schroth, W.; Hintzsche, E.; Jordan, H.; Jende, T.; Spitzner, R.; Thondorf, I. 1,2-Dithiins and precursors, XVII: Synthesis and properties of thieno anellated 1,2-dithiins, structural influence on colour. Tetrahedron 1997, 53, 7509–7528. [Google Scholar] [CrossRef]
- Yamamoto, T.; Katsuta, H.; Toyota, K.; Iwamoto, T.; Morita, N. Preparation of 4,7-Dibromobenzo[b]thiophene as a Versatile Building Block and Synthetic Application to a Bis(ethynylthienyl)oligoarene System. Bull. Chem. Soc. Jpn. 2012, 85, 613–623. [Google Scholar] [CrossRef]
- Yasuike, S.; Kurita, J.; Tsuchiya, T. Syntheses of Novel Group 15 and 16 Thieno [2,3-b]-, Thieno [3,4-b]-, and Thieno [3,2-b]-heteroles. Heterocycles 1997, 45, 1891–1894. [Google Scholar] [CrossRef]
- de Jong, R.L.P.; Brandsma, L. A one-pot procedure for thieno [2,3-b]thiophene and some of its derivatives using derivatives of 1,3-pentadiyne and carbon disulfide as building units. Synth. Commun. 1991, 21, 145–149. [Google Scholar] [CrossRef]
- de Jong, R.L.P.; Brandsma, L. Synthesis of condensed bicyclic thiophene derivatives from diyne systems. J. Chem. Soc. Chem. Commun. 1983, 1056–1057. [Google Scholar] [CrossRef]
- Otsubo, T.; Kono, Y.; Hozo, N.; Miyamoto, H.; Aso, Y.; Ogura, F.; Tanaka, T.; Sawada, M. Syntheses, Structures, and Properties of 2,3,6,7-Tetrathiabenzo [1,3-cd:4,6-c′d′]dipentalene and Its Methyl, Ethyl, Methylthio, and Ethylthio Derivatives: Novel Fused Polynuclear Heteroarenes. Bull. Chem. Soc. Jpn. 1993, 66, 2033–2041. [Google Scholar] [CrossRef]
- Zweifel, G.; Rajagopalan, S. (Z)-1-Methoxybut-1-en-3-yne. A versatile synthon for 1,4-bis(trimethylsilyl)-1,3-butadiyne as well as for nucleophilic aldehyde and butadiyne equivalents. J. Am. Chem. Soc. 1985, 107, 700–701. [Google Scholar] [CrossRef]
- Comel, A.; Kirsch, G. Efficient One Pot Preparation of Variously Substituted Thieno [2,3-b]thiophene. J. Heterocycl. Chem. 2001, 38, 1167–1171. [Google Scholar] [CrossRef]
- Korchevin, N.A.; Sukhomazova, É.N.; Russavskaya, N.V.; Turchaninova, L.P.; Sigalov, M.V.; Klyba, L.V.; Deryagina, É.N.; Voronkov, M.G. Thermal transformations of allyl 2-thienyl sulfide and selenide. Chem. Heterocycl. Compd. 1991, 27, 1049–1052. [Google Scholar] [CrossRef]
- Capelle, G. Bull. Soc. Chim. Fr. 1908, 4, 151. Available online: https://www.reaxys.com/#/results/citations/0/RX014__571082895178774403/UlgwMTQ9QyNIMDI0PVMjSDAyMz1S/list/b4627b9e-e366-49be-8638-996a3c372a45/1/desc/CIT.PREPY/// (accessed on 25 October 2022).
- Meyer, R.; Wesche, H. Pyrogene Acetylen-Kondensationen. IV. Ber. Dtsch. Chem. Ges. 1917, 50, 422–441. [Google Scholar] [CrossRef]
- Meyer, R.; Meyer, W. Pyrogene Acetylen-Kondensationen. V. Ber. Dtsch. Chem. Ges. 1918, 51, 1571–1587. [Google Scholar] [CrossRef]
- Peel, J.B.; Robinson, P.L. CCLXIX.—The reaction between acetylene and sulphur at temperatures up to 650°. J. Chem. Soc. 1928, 2068–2070. [Google Scholar] [CrossRef]
- Hanna, D.C.; Smith, E.F. Observations on Derivatives of Aconitic Acid. J. Am. Chem. Soc. 1899, 21, 381–383. [Google Scholar] [CrossRef]
- Biedermann, A.; Jacobson, P. Ueber eine dem Naphtalin entsprechende Verbindung der Thiophenreihe. Ber. Dtsch. Chem. Ges. 1886, 19, 2444–2447. [Google Scholar] [CrossRef]
- Challenger, F.; Harrison, J.B. Sulphur compounds of technical interest. The isomeric thiophthens. J. Inst. Pet. Technol. 1935, 21, 135–147. [Google Scholar]
- Oster, H. Ueber einige neue Indophenine. Ber. Dtsch. Chem. Ges. 1904, 37, 3348–3352. [Google Scholar] [CrossRef]
- Heeny, M.; McCulloch, I.; Bailey, C. Mono-, Oligo- and Polythieno(2,3-b)thiophenes. Patent EP1510535A1, 30 July 2004. Available online: https://worldwide.espacenet.com/patent/search/family/034400456/publication/EP1510535A1?q=EP04018079A (accessed on 25 October 2022).
- Rodlovskaya, E.N.; Vasnev, V.A.; Naumkin, A.V.; Vashchenko, A.A.; Goriachiy, D.O. The development of hybrid materials that combine polyamides with thienothiophene units and inorganic objects. High Perform. Polym. 2017, 29, 704–707. [Google Scholar] [CrossRef]
- Isci, R.; Rahimi Varzeghani, A.; Kaya, K.; Sütay, B.; Tekin, E.; Ozturk, T. Triphenylamine/Tetraphenylethylene Substituted 4-Thieno [3,2-b]thiophen-3-ylbenzonitriles: Synthesis, Photophysical-Electronic Properties, and Applications. ACS Sustain. Chem. Eng. 2022, 10, 1605–1615. [Google Scholar] [CrossRef]
- Tamilavan, V.; Kim, S.; Sung, J.; Lee, D.Y.; Cho, S.; Jin, Y.; Jeong, J.; Park, S.H.; Hyun, M.H. Enhanced photovoltaic performances of bis(pyrrolo [3,4-c]pyrrole-1,3-dione)-based wide band gap polymer via the incorporation of an appropriate spacer unit between pyrrolo [3,4-c]pyrrole-1,3-dione units. Org. Electron. 2017, 42, 34–41. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, J.S.; Jung, A.R.; Cha, W.; Kim, H.; Son, H.J.; Cho, J.H.; Kim, B.S. Processing temperature control of a diketopyrrolopyrrole-alt-thieno [2,3-b]thiophene polymer for high-mobility thin-film transistors and polymer solar cells with high open-circuit voltages. Polymer 2016, 105, 79–87. [Google Scholar] [CrossRef]
- Abbasriyaludeen, A.R.; Chitra, K.; Ramasamy, S.; Murugan, P.; Chandrasekar, P. Molecular engineering of twisted dipolar chromophores for efficiency boosted BHJ solar cells. J. Mater. Chem. C 2021, 9, 4562–4575. [Google Scholar] [CrossRef]
- Fernandes, S.S.M.; Castro, M.C.R.; Ivanou, D.; Mendes, A.; Raposo, M.M.M. Push-Pull Heterocyclic Dyes Based on Pyrrole and Thiophene: Synthesis and Evaluation of Their Optical, Redox and Photovoltaic Properties. Coatings 2022, 12, 34. [Google Scholar] [CrossRef]
- Zhu, S.; An, Z.; Chen, X.; Chen, P.; Liu, Q. Cyclic thiourea functionalized dyes with binary π-linkers: Influence of different π-conjugation segments on the performance of dye-sensitized solar cells. Dyes Pigments 2015, 116, 146–154. [Google Scholar] [CrossRef]
- Massin, J.; Ducasse, L.; Abbas, M.; Hirsch, L.; Toupance, T.; Olivier, C. Molecular engineering of carbazole-fluorene sensitizers for high open-circuit voltage DSSCs: Synthesis and performance comparison with iodine and cobalt electrolytes. Dyes Pigments 2015, 118, 76–87. [Google Scholar] [CrossRef]
- Paek, S.; Zimmermann, I.; Gao, P.; Gratia, P.; Rakstys, K.; Grancini, G.; Nazeeruddin, M.K.; Malik, A.R.; Samia, A.K.; Khalid, A.A.; et al. Donor-π-donor type hole transporting materials: Marked π-bridge effects on optoelectronic properties, solid-state structure, and perovskite solar cell efficiency. Chem. Sci. 2016, 7, 6068–6075. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, L.; You, G.; Li, L.; Yao, L.; Zhou, Z.; Yang, E.; Cai, W.; Ling, Q.; Zhen, H. Dopant-free, hole-transporting polymers containing benzotriazole acceptor unit for perovskite solar cells. Dyes Pigments 2022, 200, 110170. [Google Scholar] [CrossRef]
- Yang, X.; Xi, J.; Sun, Y.; Zhang, Y.; Zhou, G.; Wong, W.Y. A dopant-free twisted organic small-molecule hole transport material for inverted planar perovskite solar cells with enhanced efficiency and operational stability. Nano Energy 2019, 64, 103946. [Google Scholar] [CrossRef]
- Noh, J.H.; Jeon, N.J.; Choi, Y.C.; Nazeeruddin, M.K.; Grätzel, M.; Seok, S. Il Nanostructured TiO2/CH3NH3PbI3 heterojunction solar cells employing spiro-OMeTAD/Co-complex as hole-transporting material. J. Mater. Chem. A 2013, 1, 11842–11847. [Google Scholar] [CrossRef]
- Durso, M.; Gentili, D.; Bettini, C.; Zanelli, A.; Cavallini, M.; De Angelis, F.; Grazia Lobello, M.; Biondo, V.; Muccini, M.; Capelli, R.; et al. π-Core tailoring for new high performance thieno(bis)imide based n-type molecular semiconductors. Chem. Commun. 2013, 49, 4298–4300. [Google Scholar] [CrossRef]
- Yue, J.; Sun, S.; Liang, J.; Zhong, W.; Lan, L.; Ying, L.; Huang, F.; Yang, W.; Cao, Y. Effects of pyridyl group orientations on the optoelectronic properties of regio-isomeric diketopyrrolopyrrole based π-conjugated polymers. J. Mater. Chem. C 2016, 4, 2470–2479. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Y.; Chen, X.; Yu, L.; Huang, Y.; Wang, Z.; Wang, S.; Lou, Y.; Ma, X.; Sun, Y.; et al. A Centrosymmetric Organic Semiconductor with Donor–Acceptor Interaction for Highly Photostable Organic Transistors. Adv. Funct. Mater. 2022, 32, 2111705. [Google Scholar] [CrossRef]
- Rawcliffe, R.; Shkunov, M.; Heeney, M.; Tierney, S.; McCulloch, I.; Campbell, A. Organic field-effect transistors of poly(2,5-bis(3-dodecylthiophen-2-yl)thieno [2,3-b]thiophene) deposited on five different silane self-assembled monolayers. Chem. Commun. 2008, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Song, P.; Ma, F.; Yang, Y. Second-Order Nonlinear Optical Switch Manipulation of Photosensitive Layer by an External Electric Field Coupled with Graphene Quantum Dots. J. Phys. Chem. A 2019, 123, 7401–7407. [Google Scholar] [CrossRef] [PubMed]
- Marco, A.B.; Gindre, D.; Iliopoulos, K.; Franco, S.; Andreu, R.; Canevet, D.; Sallé, M. (Super)gelators derived from push-pull chromophores: Synthesis, gelling properties and second harmonic generation. Org. Biomol. Chem. 2018, 16, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Marco, A.B.; Andreu, R.; Franco, S.; Garín, J.; Orduna, J.; Villacampa, B.; Alicante, R. Efficient second-order nonlinear optical chromophores based on dithienothiophene and thienothiophene bridges. Tetrahedron 2013, 69, 3919–3926. [Google Scholar] [CrossRef]
- Jen, A.K.-Y.; Rao, V.P.; Drost, K.J.; Cai, Y.; Mininni, R.M.; Kenney, J.T.; Binkley, E.S.; Dalton, L.R. The Development of Highly Active, Thermally Stable Chromophores and Polymers for Electrooptic Applications. Proc. SPIE-Int. Soc. Opt. Eng. 1994, 2285, 49–57. [Google Scholar] [CrossRef]
- Mashraqui, S.H.; Sangvikar, Y.S.; Meetsma, A. Synthesis and structures of thieno [2,3-b]thiophene incorporated [3.3]dithiacyclophanes. Enhanced first hyperpolarizability in an unsymmetrically polarized cyclophane. Tetrahedron Lett. 2006, 47, 5599–5602. [Google Scholar] [CrossRef]
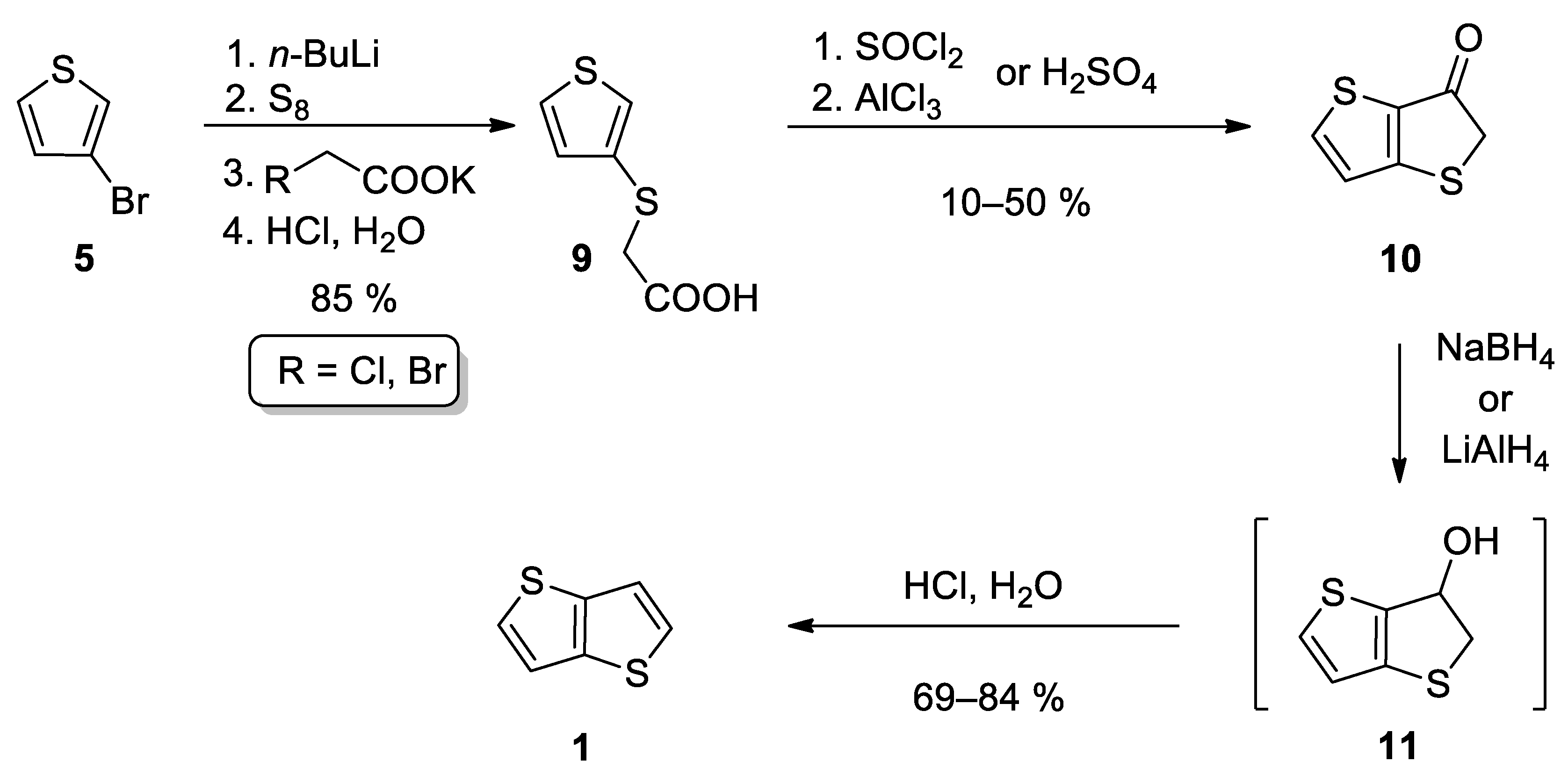
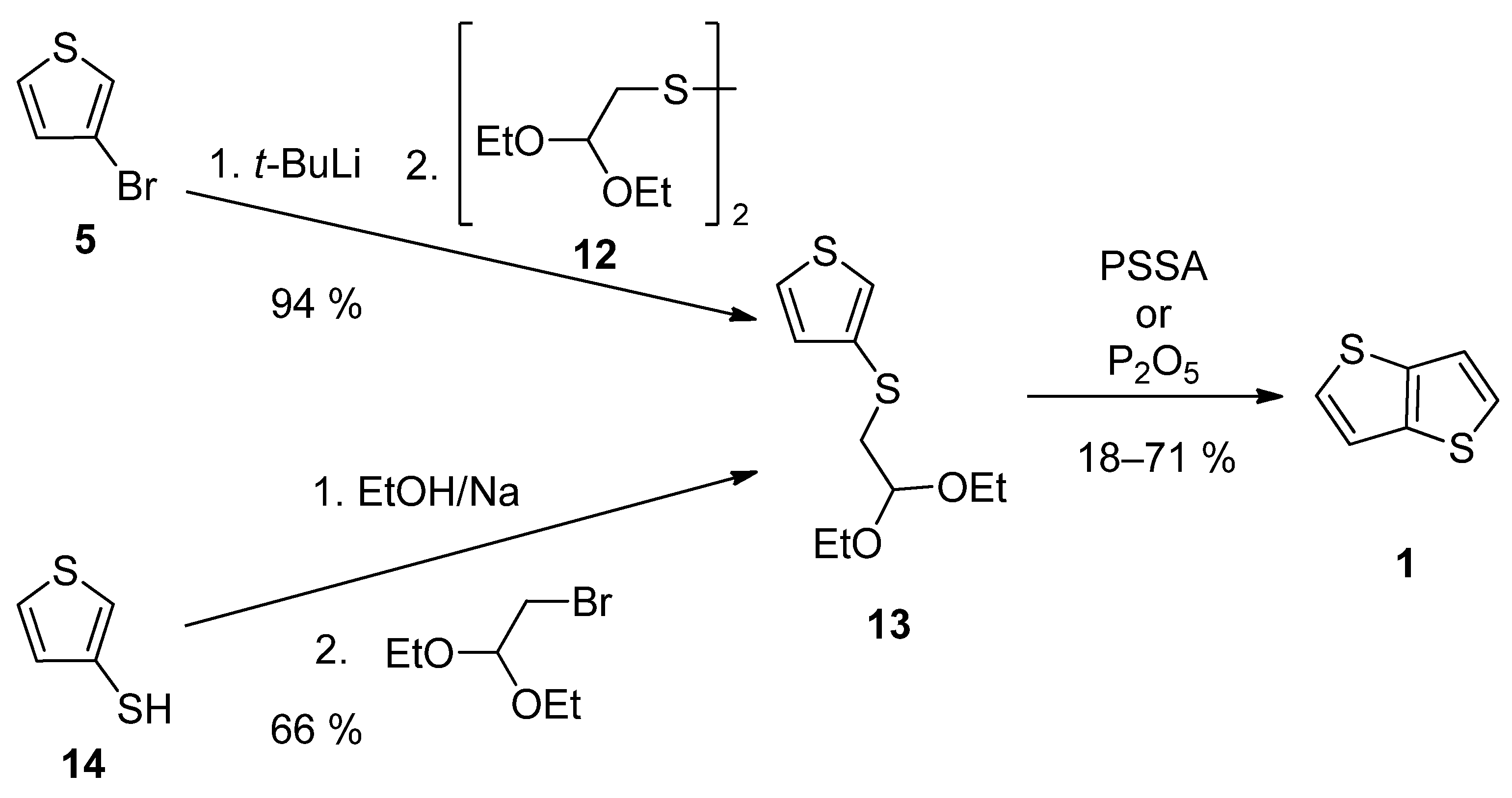
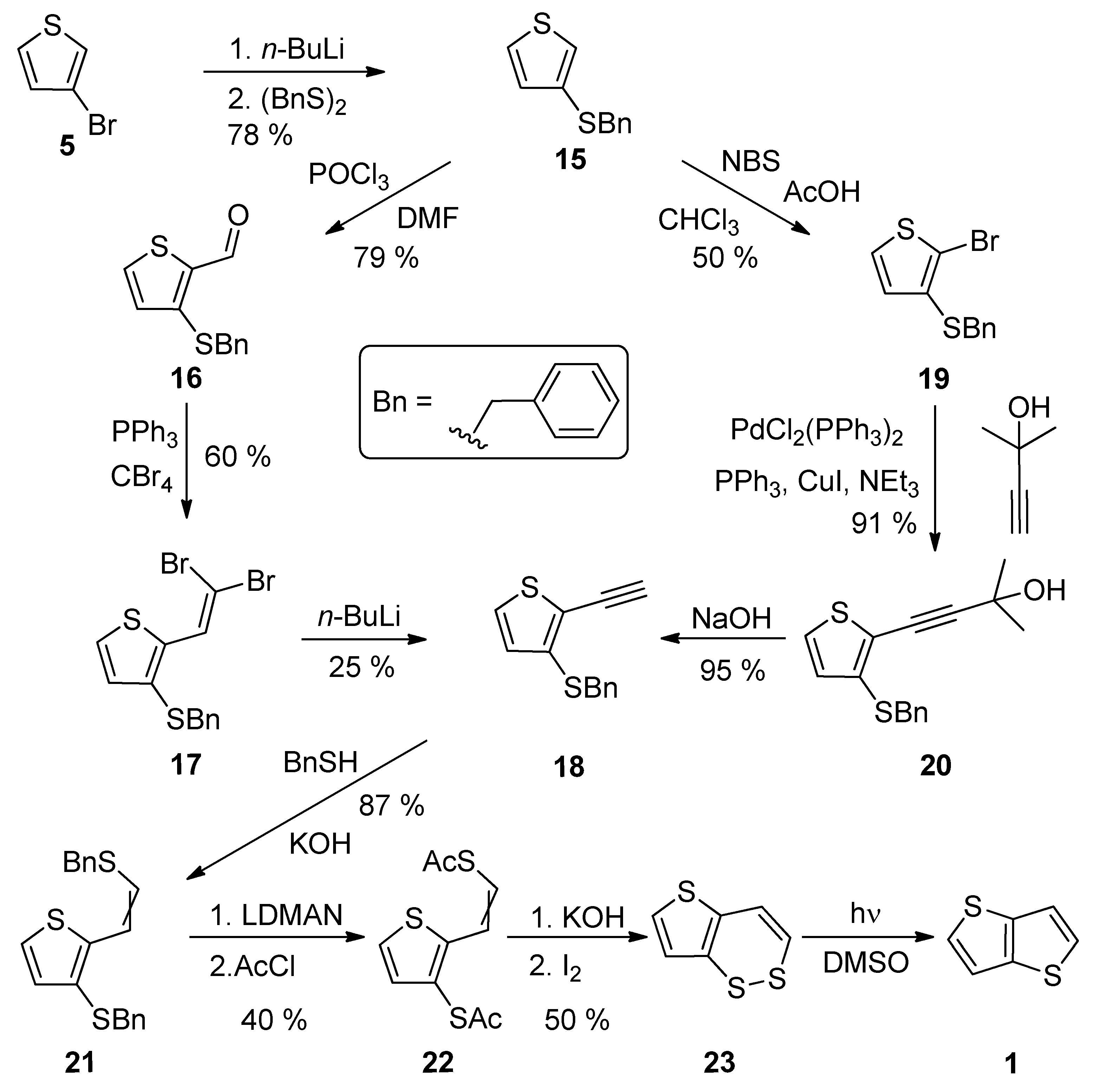
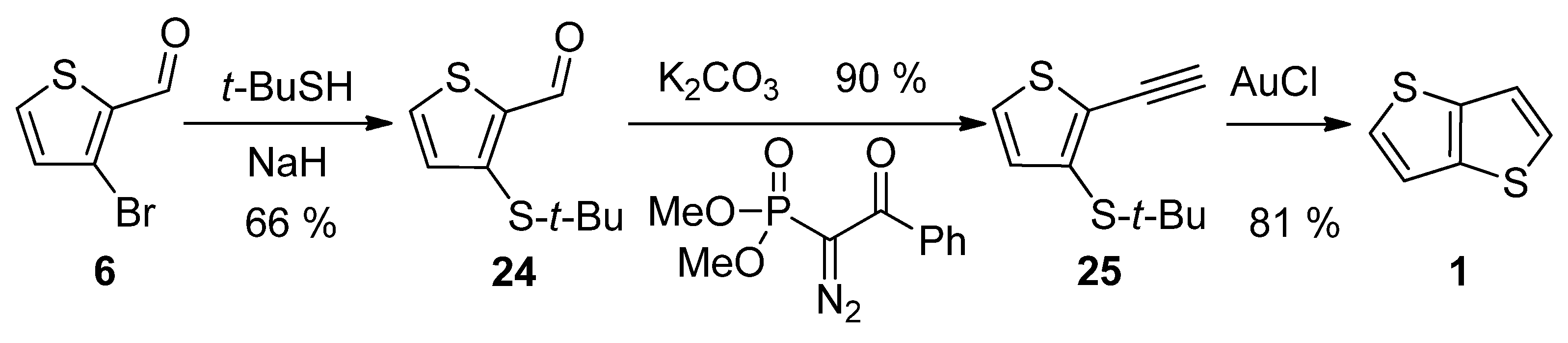
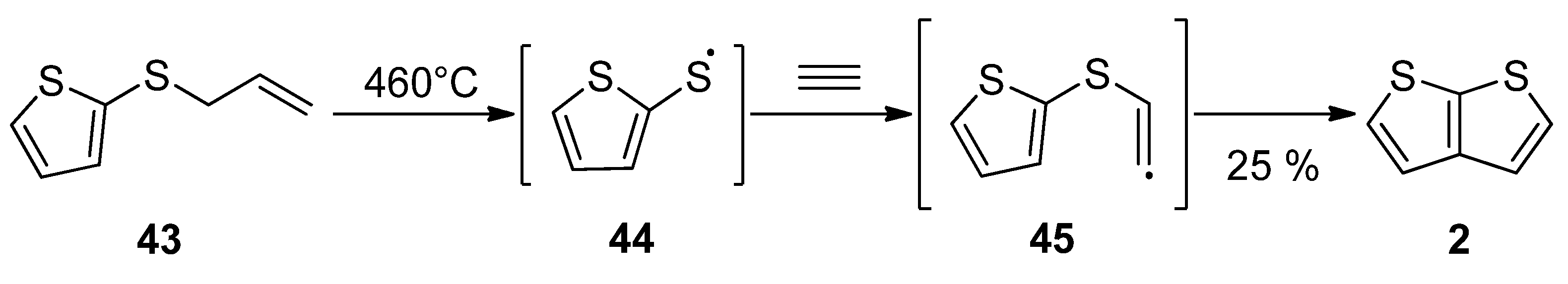
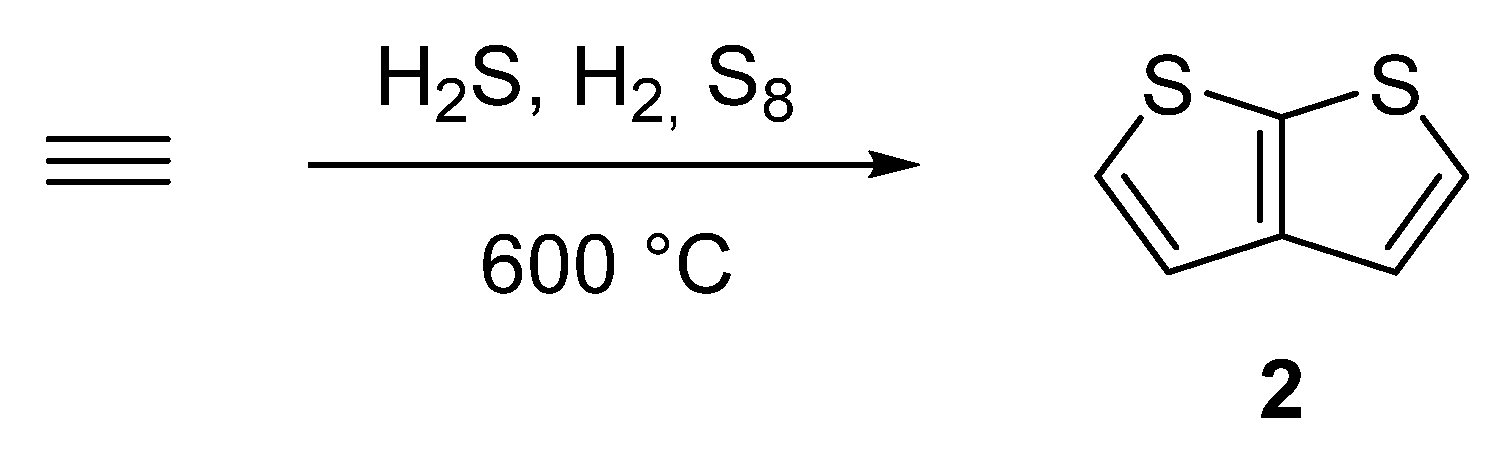



| Method | Target TT Molecule | Number of Steps | Overall Yield [%] | References |
|---|---|---|---|---|
| 1 | 1 | 4 | 50 | [24,25,26,27,28] |
| 2 | 1 | 4 | 36 | [29,30,31] |
| 3 | 1 | 2 | 12/67 | [32,33] |
| 4 | 1 | 8 | - | [34] |
| 5 | 1 | 3 | 48 | [35] |
| 6 | 1 | 4 | - | [36] |
| 7 | 2 | 4 | 40 | [37,38,39,40] |
| 8 | 2 | 4 | 30 | [41] |
| 9 | 2 | 1 | 25 | [42] |
| 10 | 2 | 1 | - | [43,44,45,46] |
| 11 | 2 | 1 | - | [47,48,49,50] |
| 12 | 2 | 4 | 18 | [36] |
| 13 | 2 | 2 | 7 | [32,51] |
| 14 | 2 | 6 | 13 | [28] |
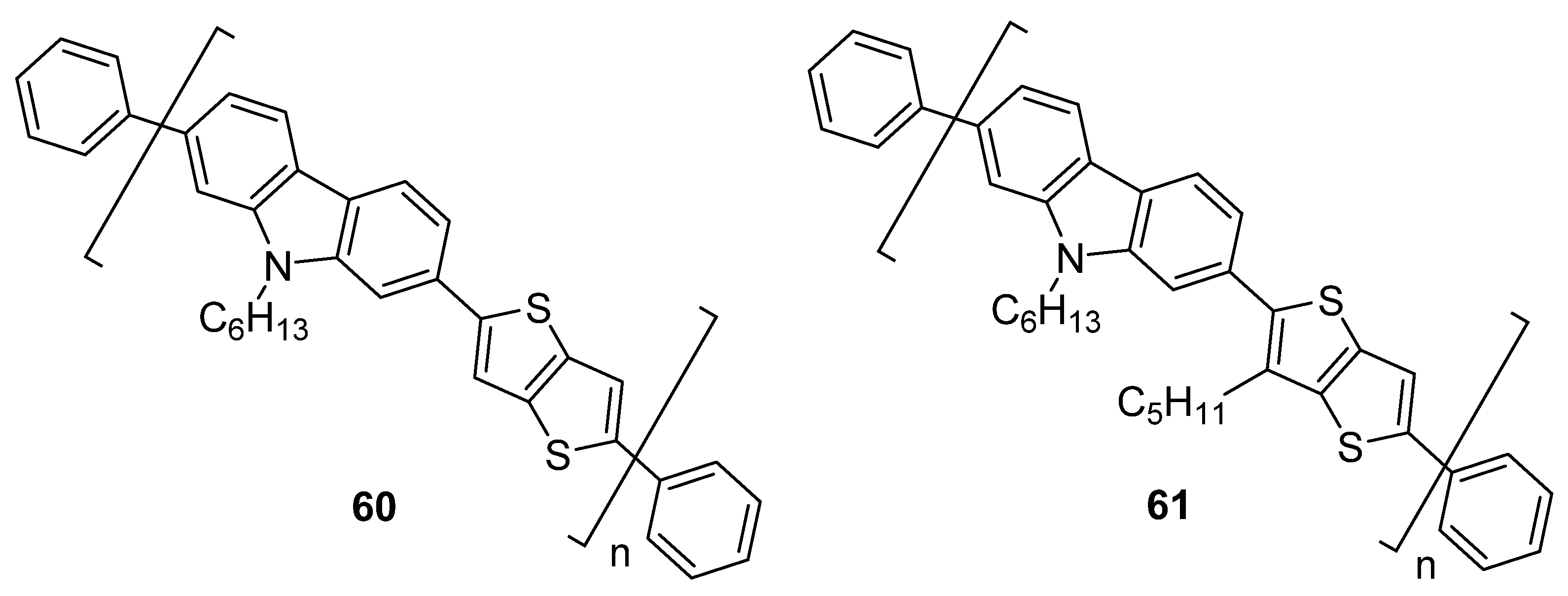
| Compound | EHOMOa [eV] | ELUMOa [eV] | ΔE [eV] | λmax(abs) b [nm] | λmax(em) b [nm] | ηl [cd·A−1] |
|---|---|---|---|---|---|---|
| 60 | −5.18 | −2.71 | 2.47 | 411 | 492 | 1.01 |
| 61 | −5.24 | −2.73 | 2.51 | 409 | 475 | 0.37 |
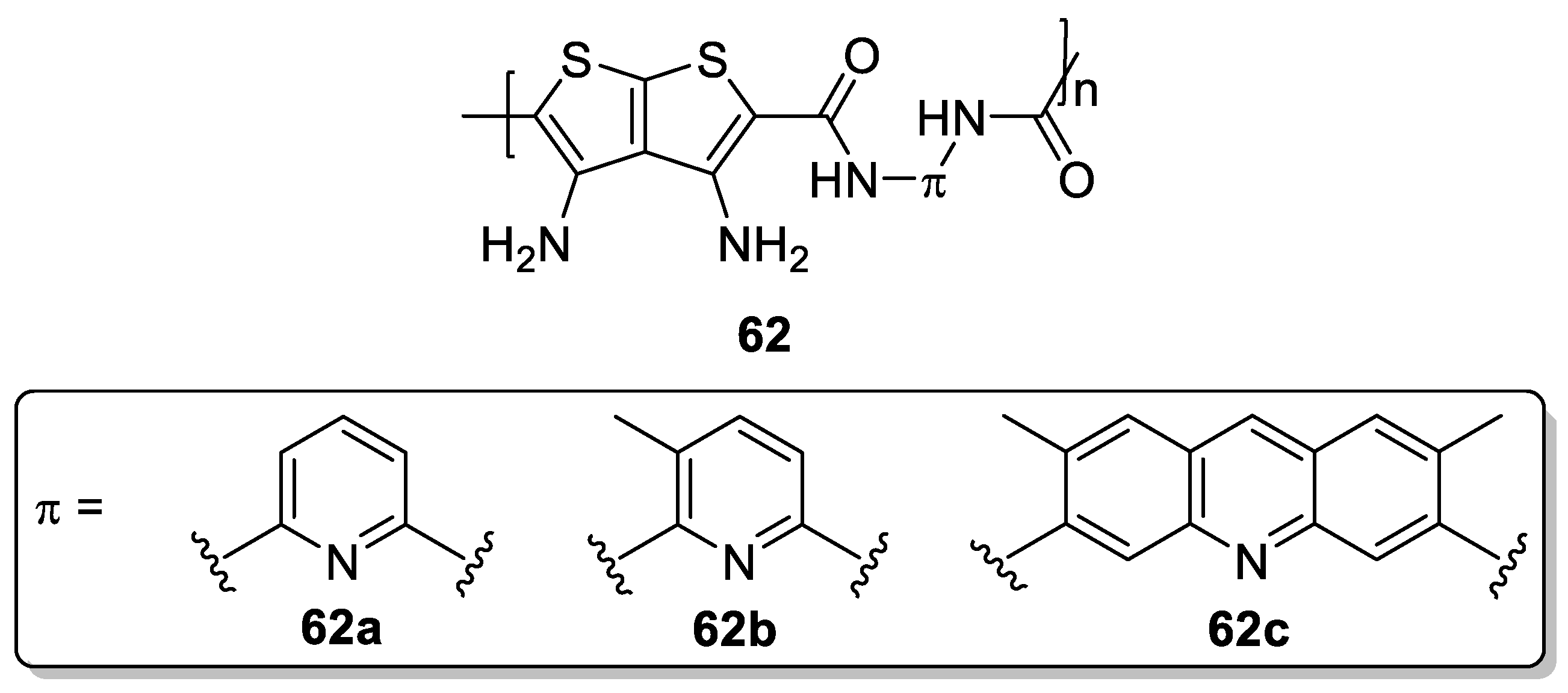
| Compound | λmax(em) [nm] | L [cd·m−2] |
|---|---|---|
| 62a | 635 | 14 |
| 62b | 638 | 11 |
| 62c | 641 | 11 |
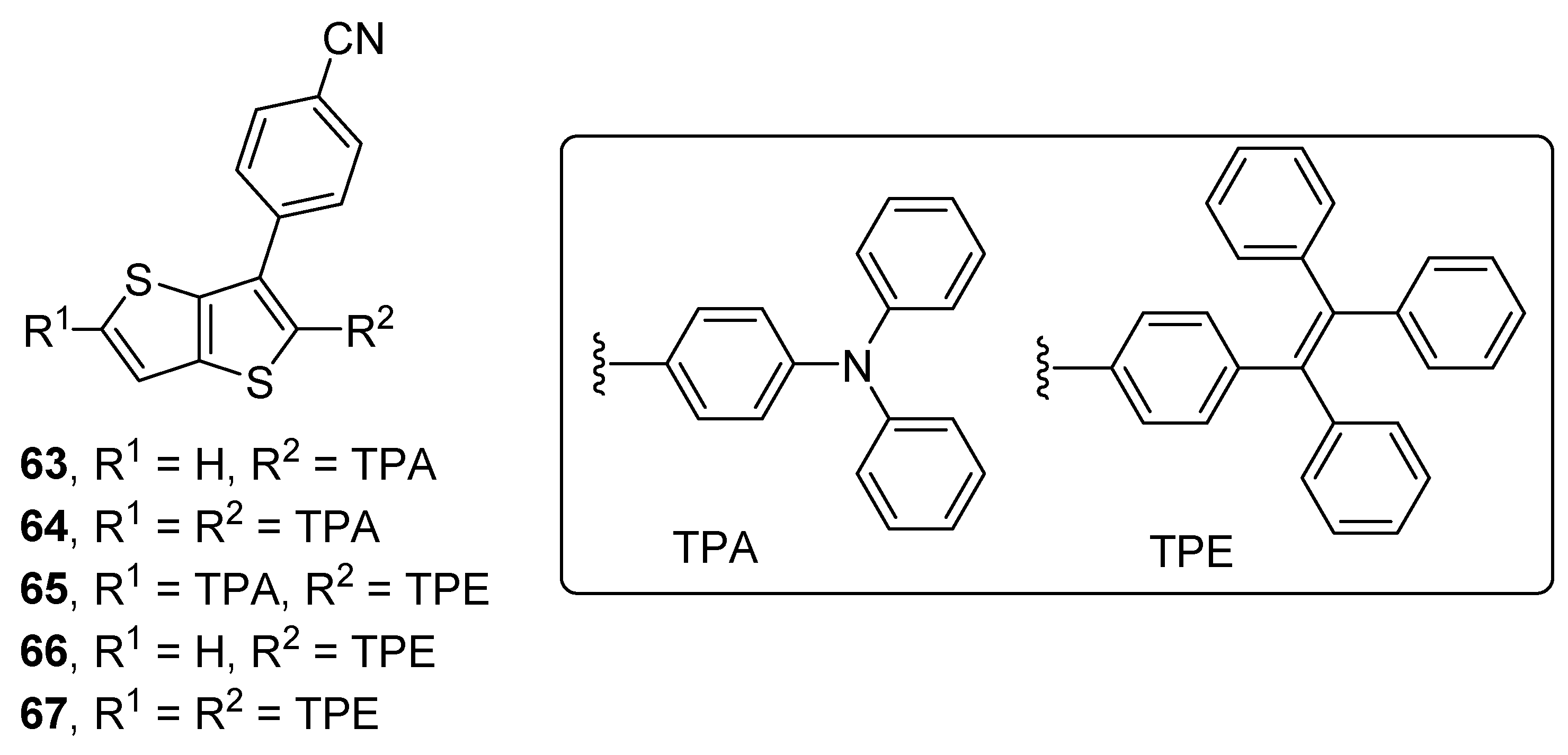
| Com. | λmax(abs) a [nm] | λmax(em) a [nm] | Lb [cd·m−2] | ηl [cd·A−1] | EHOMOc [eV] | ELUMOd [eV] | ΔEopt e [eV] |
|---|---|---|---|---|---|---|---|
| 63 | 365 | 523 | 2790 | 4.70 | −5.50 | −2.62 | 2.88 |
| 64 | 396 | 511 | 1190 | 0.90 | −5.24 | −2.61 | 2.63 |
| 65 | 391 | 526 | 1710 | 1.30 | −5.22 | −2.52 | 2.70 |
| 66 | 352 | - | 280 | 1.70 | −5.80 | −2.78 | 3.02 |
| 67 | 381 | 501 | 400 | 1.30 | −5.53 | −2.74 | 2.79 |
| Com. | λmax(abs) a [nm] | EHOMO [eV] | ELUMO [eV] | ΔE [eV] | µh [cm2V−1s−1] | PCE [%] | FF [%] |
|---|---|---|---|---|---|---|---|
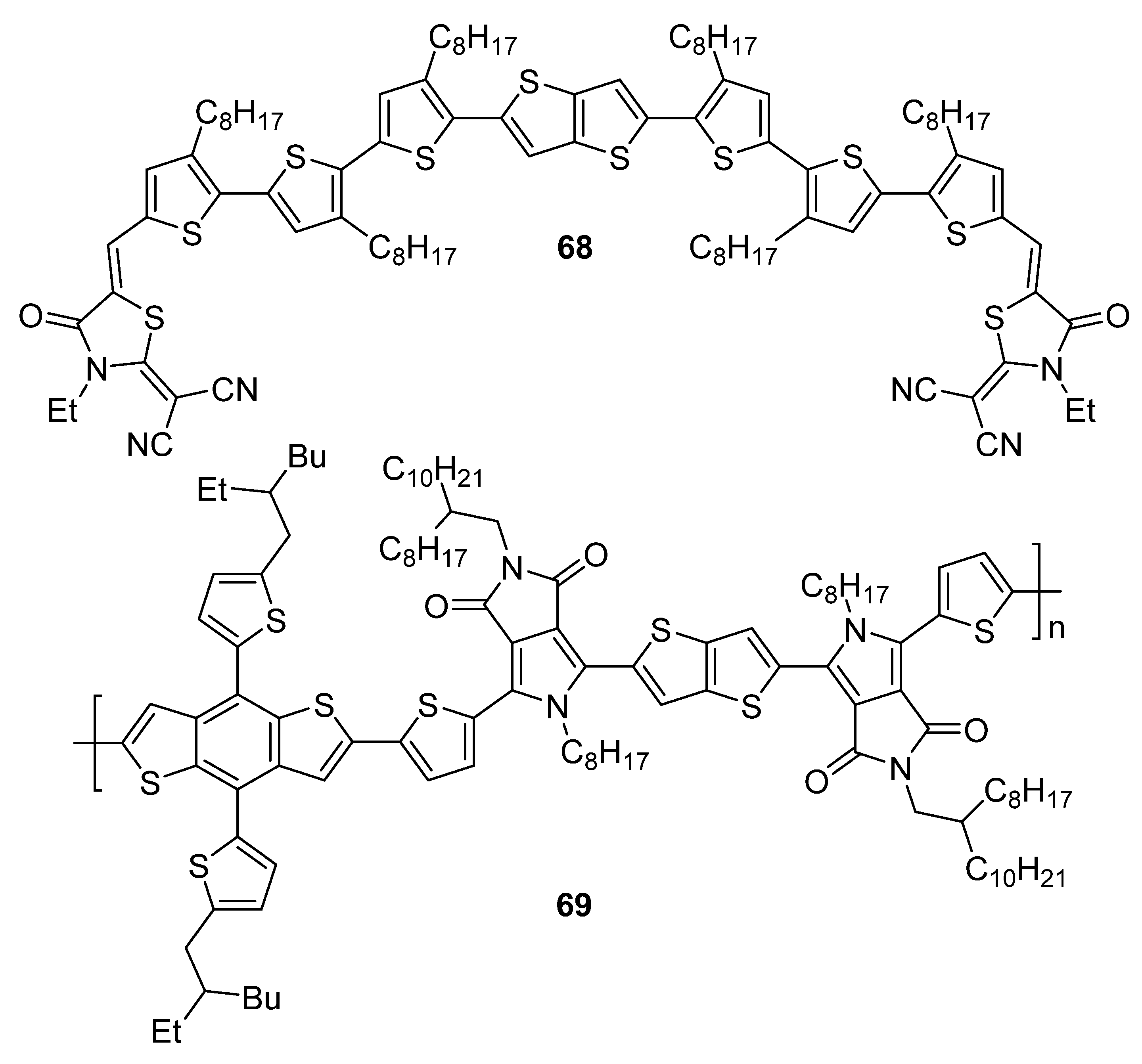
| 68 | 608 | −5.05 b | −3.46 b | 1.62 | 6.40 × 10−4 | 7.91 | 65.5 |
| 69 | 505 | −5.42 c | −3.36 d | 2.06 e | 6.21 × 10−4 f | 5.21 g | 67.0 |
| 70 | 723 | −5.40 c | −3.74 c | 1.66 | 1.60 × 10−1 f | 2.90 | 51.0 |
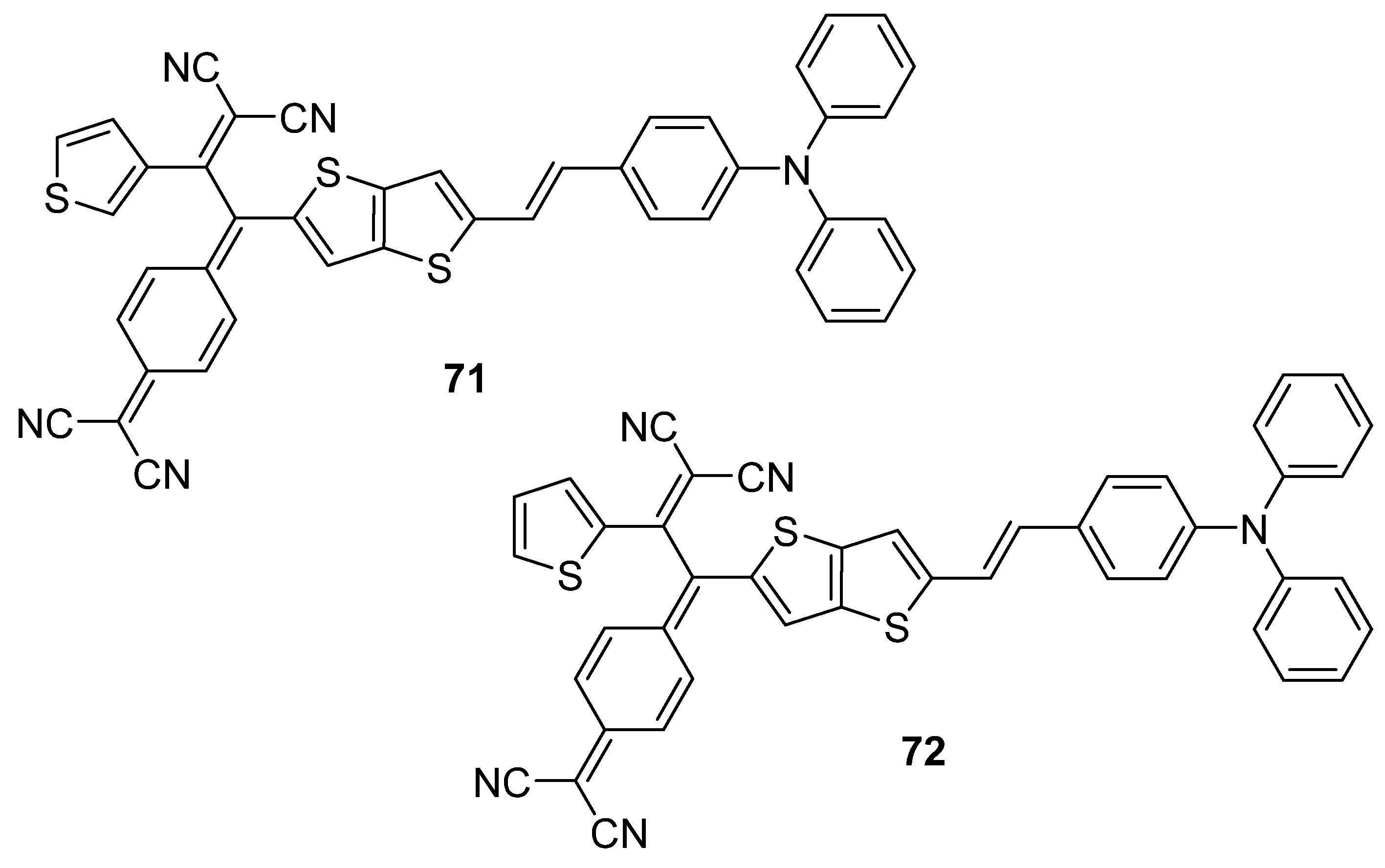
| 71 | 818 | −5.19 h | −3.85 i | 1.34 j | - | 6.62 | 59.7 |
| 72 | 819 | −5.33 h | −3.98 i | 1.35 j | - | 6.92 | 58.5 |
| Com. | λmax(abs) [nm] | EHOMO [eV] | ELUMO [eV] | ΔE [eV] | PCE [%] | FF [%] |
|---|---|---|---|---|---|---|
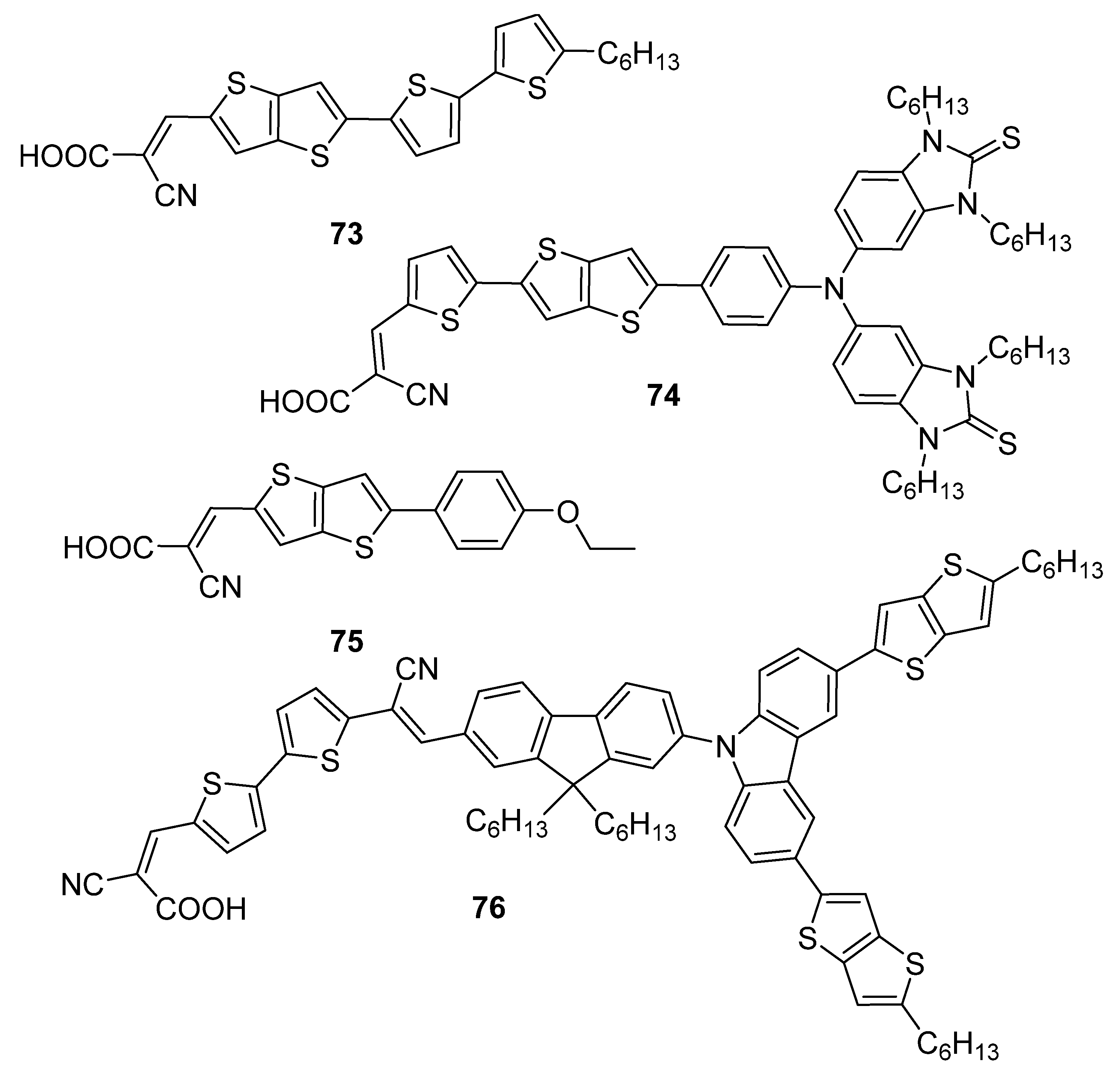
| 73 | 433 a | −5.04 b | −2.34 b | 2.70 | 2.49 | 65.3 |
| 74 | 488 c | −4.95 d | −3.75 d | 1.20 | 7.38 | 66.0 |
| 75 | 399 a | −5.22 b | −2.74 b | 2.48 | 2.21 | 63.0 |
| 76 | 474 e | −5.41 f | −3.59 f | 1.82 | 6.50 | 74.0 |
| Com. | λmax(abs) [nm] | EHOMO [eV] | ELUMO [eV] | ΔE [eV] | µh [cm2V−1s−1] | PCE [%] | FF [%] |
|---|---|---|---|---|---|---|---|
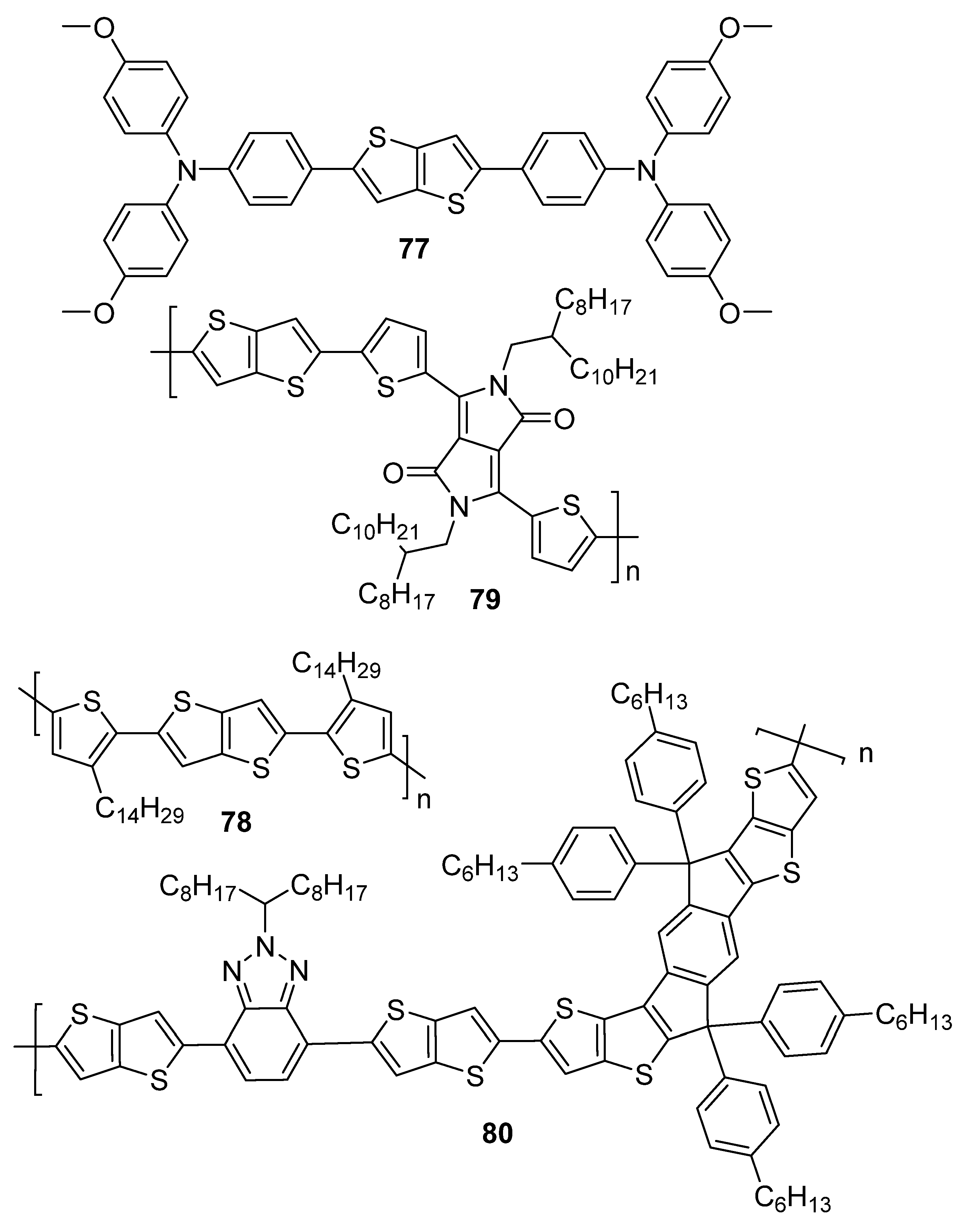
| 77 | 410 a | −5.20 b | −2.49 b | 2.71 | 4.05 × 10−5 | 11.11 | 64.2 |
| 78 | - | −5.26 b | −3.10 b | 2.16 | 1.64 × 10−6 | 4.40 | 51.0 |
| 79 | - | −5.30 b | −3.40 b | 1.90 | 1.11 | 9.31 | 67.2 |
| 80 | - | −5.36 b | −3.33 c | 2.03 d | 1.24 × 10−4 | 15.80 | 66.6 |
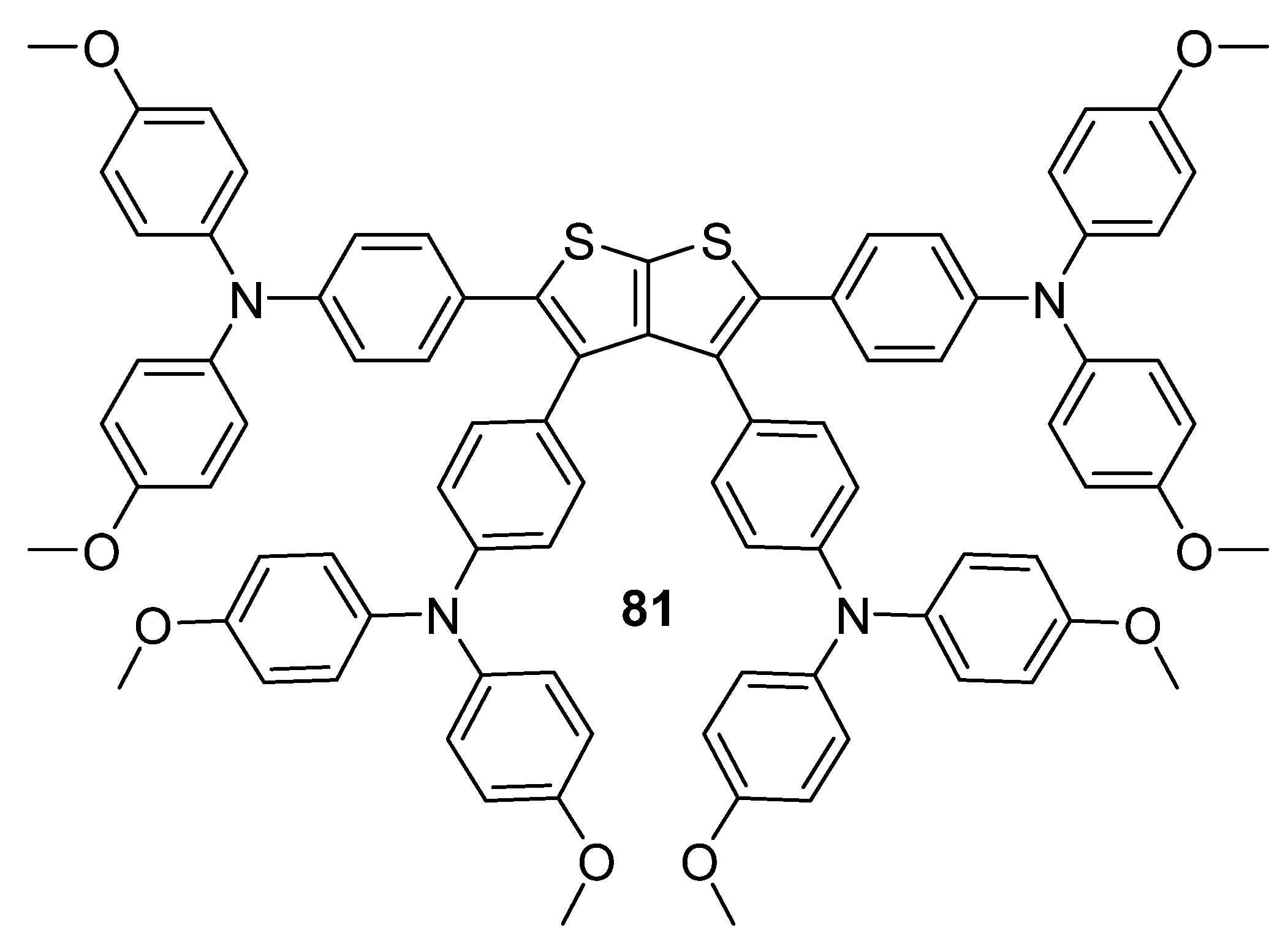
| 81 | - | −5.44 e | −2.42 c | 3.02 d | 3.76 × 10−4 | 18.78 | 76.2 |
| Compound | λmax(abs) [nm] | EHOMO [eV] | ELUMO [eV] | ΔE [eV] | µ [cm2V−1s−1] |
|---|---|---|---|---|---|
| 82 | 443 a | −5.69 b | −3.20 c | 2.49 d | (e) 3.00 × 10−1 |
| 83 | 445 e | −6.29 f | −4.23 g | 2.06 d | (e) 2.00 × 10−1 |
| 84 | 552 h | −4.51 i | −2.70 i | 1.78 d | (h) 28.00 × 10−1 |
| 85 | 423 j | −5.91 i | −3.16 i | 2.75 | (e) 1.30 × 10−1 (h) 0.85 × 10−1 |
| 86 | 626 k | −5.32 l | −3.09 l | 2.23 | (h) 3.50 × 10−1 |
| 87 | 389 a | −4.92 c | −2.30 c | 2.62 | (N/S) 7.2 × 10−1 |
| Compound | λmax(abs) [nm] | EHOMO [eV] | ELUMO [eV] | ΔE [eV] | µh [cm2V−1s−1] |
|---|---|---|---|---|---|
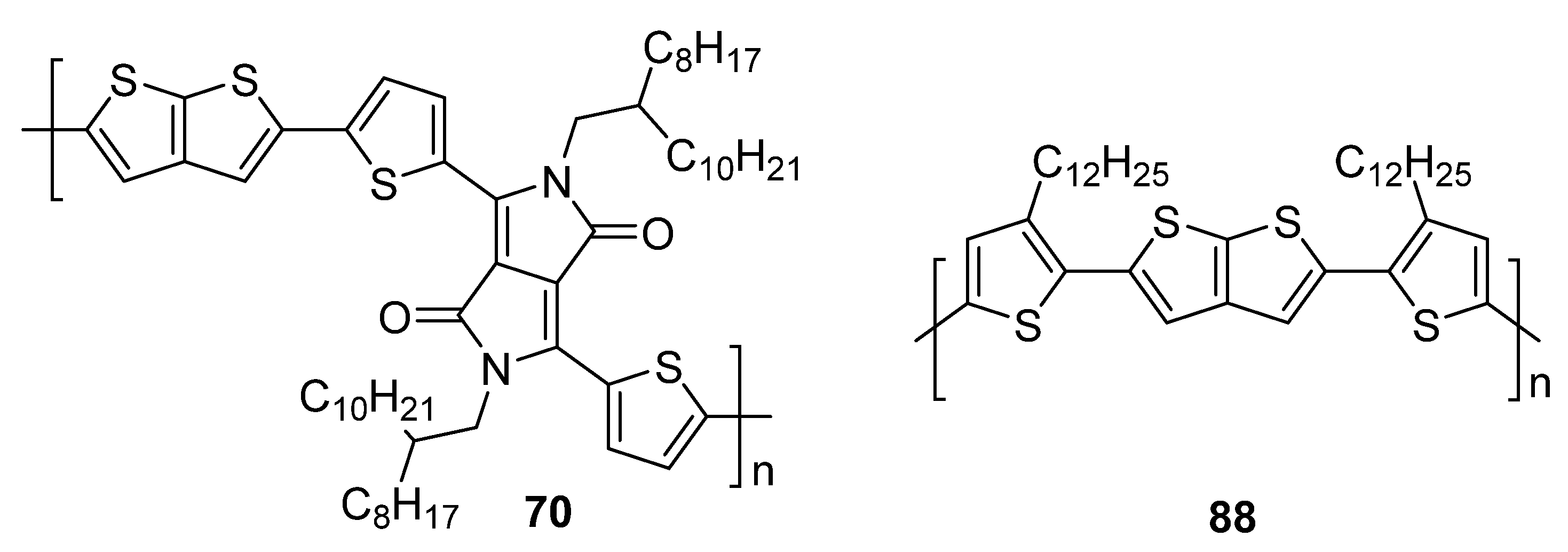
| 70 | 706 a | −5.40 b | −3.74 b | 1.66 | 1.60 × 10−1 |
| 88 | - | - | - | - | 0.21 × 10−1 |
| Com. | λmax(abs) [nm] | EHOMOb [eV] | ELUMOb [eV] | ΔE [eV] | μβ [10−48 esu] | μβd [10−48 esu] | μb [D] |
|---|---|---|---|---|---|---|---|
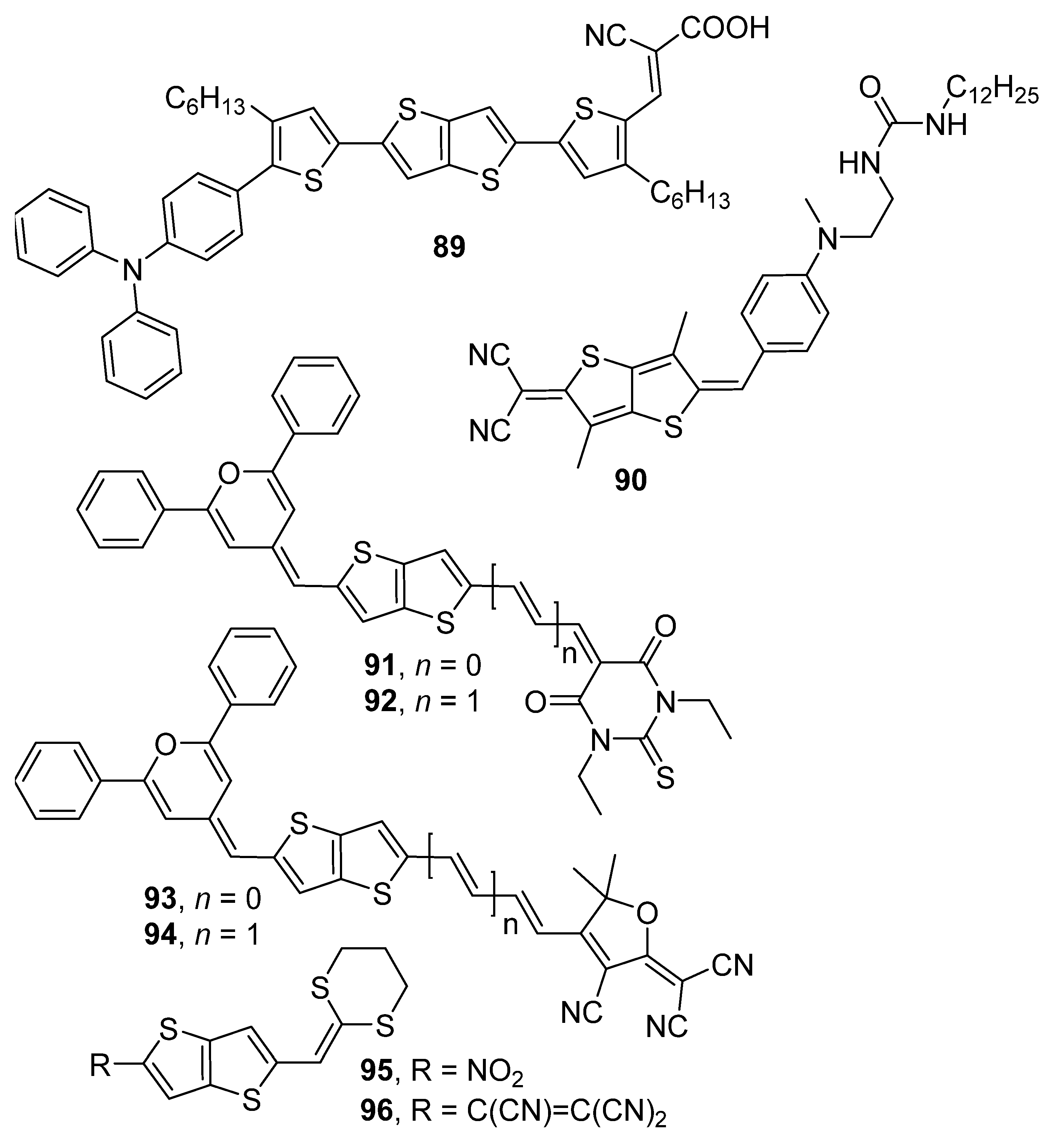
| 91 | 653 a | −5.68 | −3.42 | 2.26 | 2800 c | 1310 | 13.6 |
| 92 | 677 a | −5.62 | −3.55 | 2.07 | 5400 c | 2340 | 14.6 |
| 93 | 711 a | −5.84 | −3.85 | 1.99 | 14,900 c | 5670 | 24.1 |
| 94 | 708 a | −5.75 | −3.90 | 1.85 | 21,900 c | 8500 | 25.1 |
| 95 | 450 e | - | - | - | 380 f | 280 | - |
| 96 | 570 e | - | - | - | 2200 | 1287 | - |
| Compound | X Atom | λmax(abs) a [nm] | β [10−30 esu] | βc [10−30 esu] | μ [D] |
|---|---|---|---|---|---|
| 97a | S | 350 | 26 b | 17 | 4.6 |
| 97b | Se | 350 | 22 b | 15 | 4.6 |
| 98a | O | 502 | 65 b | 25 | 9.0 |
| 98b | S | 516 | 124 b | 43 | 9.5 |
| 98c | Se | 518 | 115 b | 39 | 10.0 |
| 98d | Te | 538 | 125 d | 37 | 10.0 |
| Compound | λmax(abs) a [nm] | βb [10−30 esu] | βc [10−30 esu] |
|---|---|---|---|
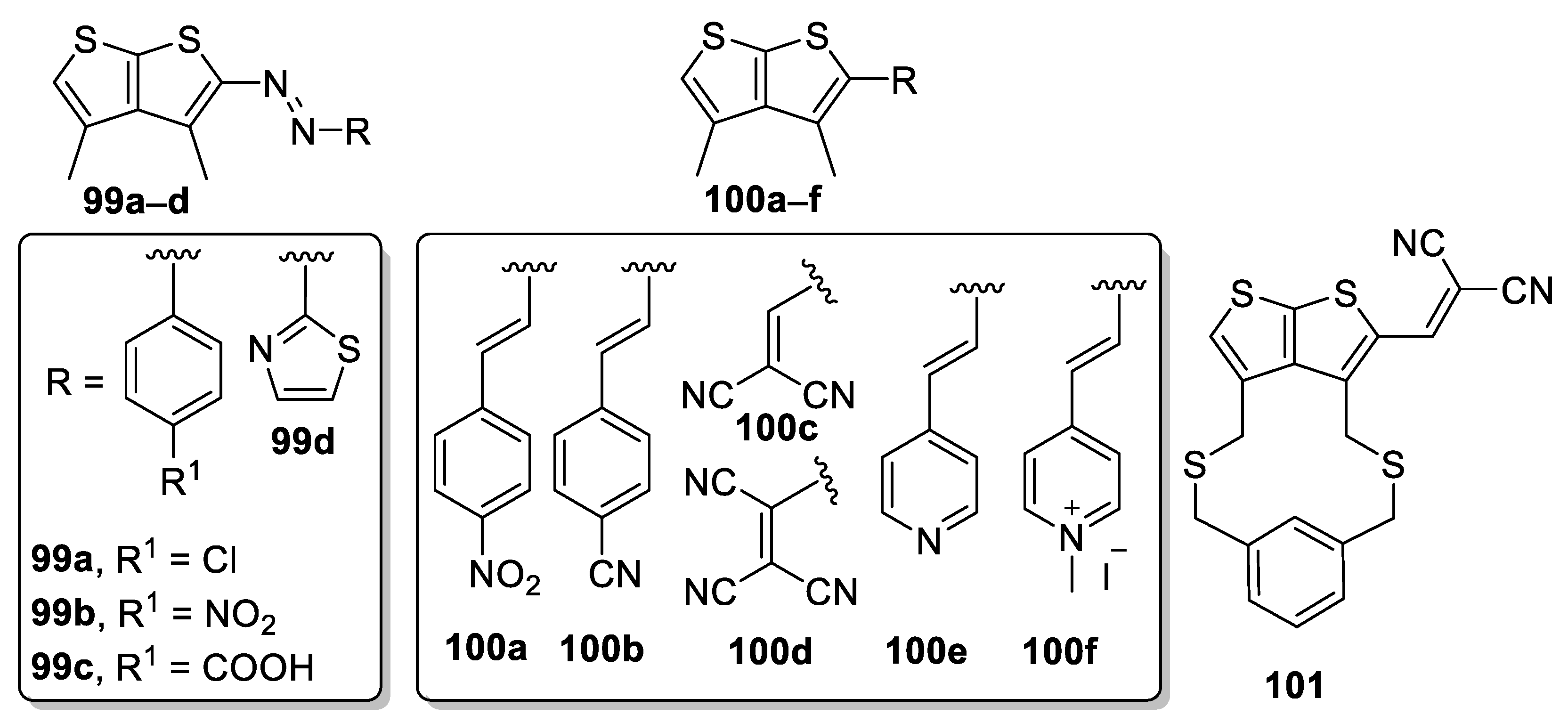
| 99a | 382 | 35 | 14 |
| 99b | 422 | 40 | 10 |
| 99c | 404 | 48 | 17 |
| 99d | 431 | 47 | 13 |
| 100a | 407 | 40 | 14 |
| 100b | 372 | 10 | 4 |
| 100c | 381 | 10 | 4 |
| 100d | 408 | 44 | 16 |
| 100e | 368 | 10 | 5 |
| 100f | 442 | 29 | 7 |
| 101 | 390 | 22 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podlesný, J.; Bureš, F. Thienothiophene Scaffolds as Building Blocks for (Opto)Electronics. Organics 2022, 3, 446-469. https://doi.org/10.3390/org3040029
Podlesný J, Bureš F. Thienothiophene Scaffolds as Building Blocks for (Opto)Electronics. Organics. 2022; 3(4):446-469. https://doi.org/10.3390/org3040029
Chicago/Turabian StylePodlesný, Jan, and Filip Bureš. 2022. "Thienothiophene Scaffolds as Building Blocks for (Opto)Electronics" Organics 3, no. 4: 446-469. https://doi.org/10.3390/org3040029
APA StylePodlesný, J., & Bureš, F. (2022). Thienothiophene Scaffolds as Building Blocks for (Opto)Electronics. Organics, 3(4), 446-469. https://doi.org/10.3390/org3040029