Dihydrooxazine Byproduct of a McMurry–Melton Reaction en Route to a Synthetic Bacteriochlorin
Abstract
:1. Introduction
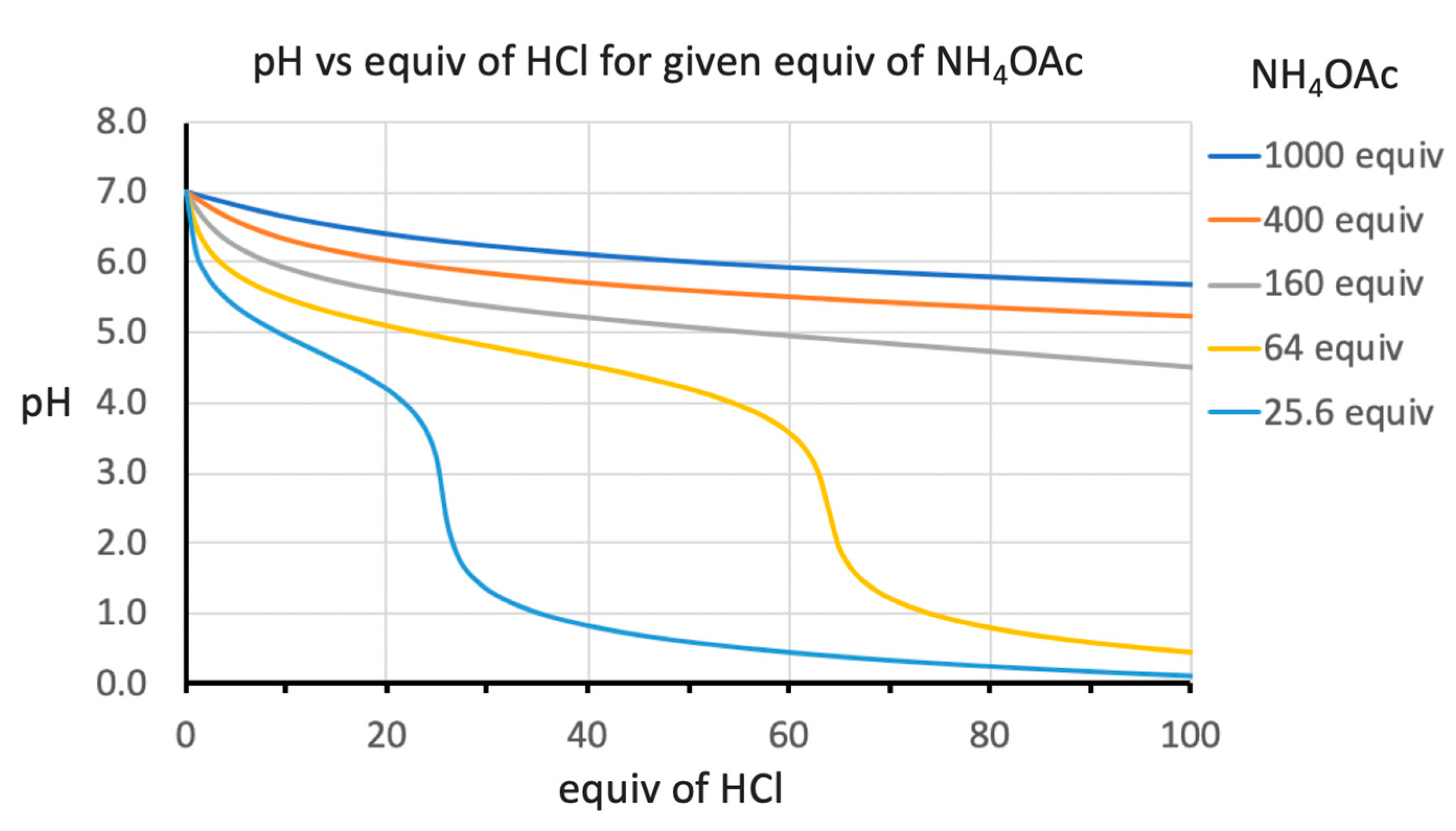
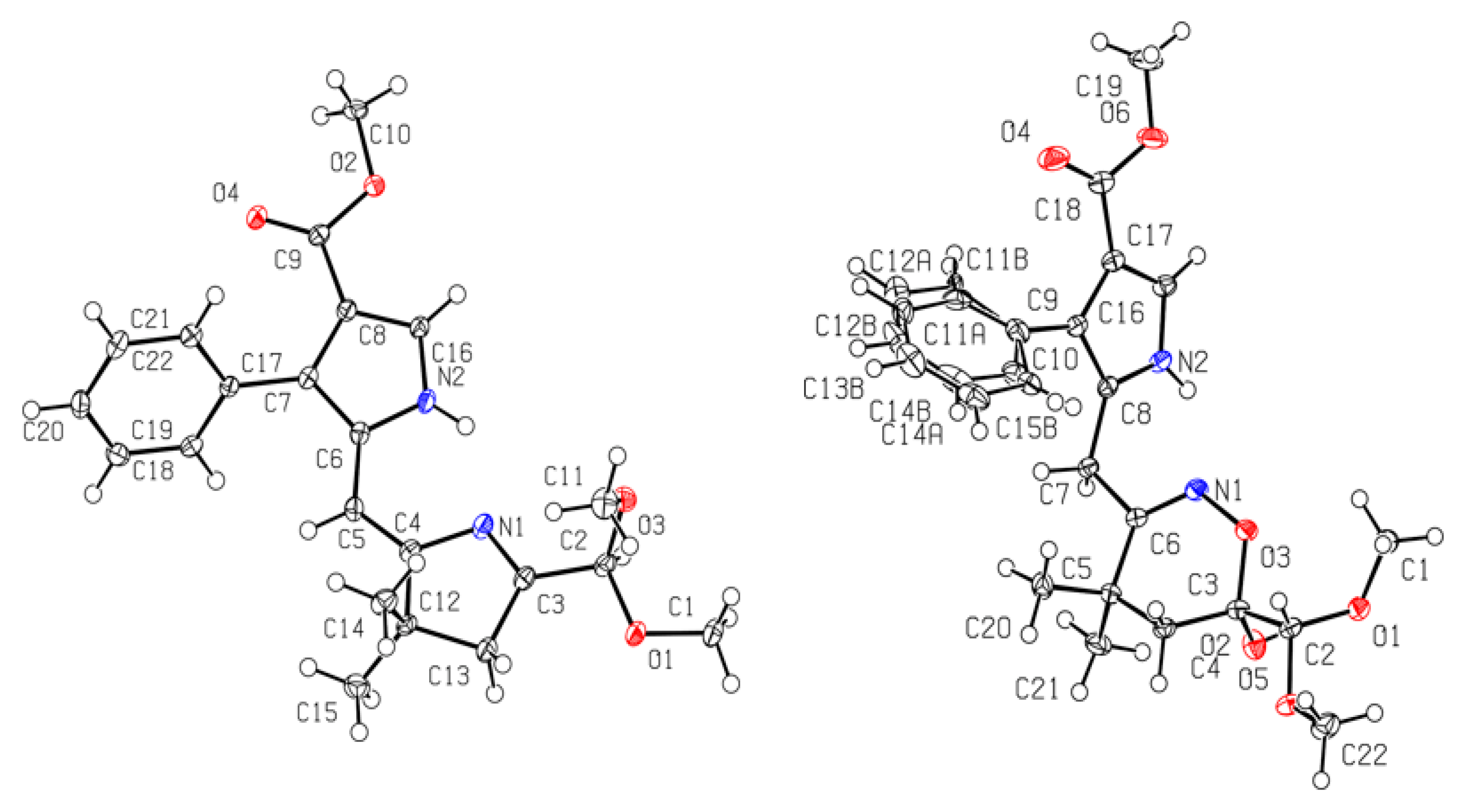
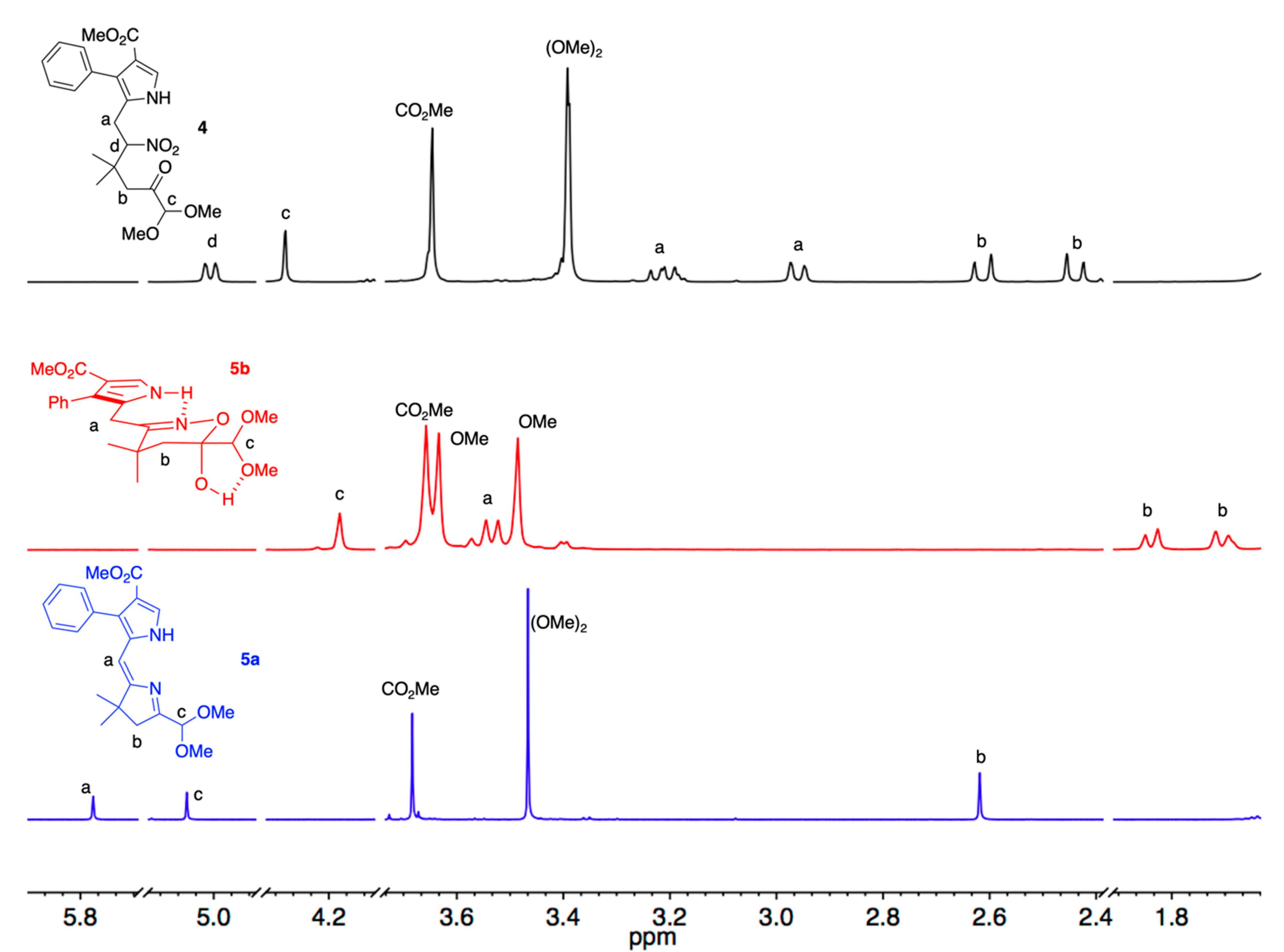
2. Results and Discussion
3. Discussion
4. Conclusions
5. Experimental Section
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| TiCl3 | HCl | HCl/TiCl3 Molar Ratio | References |
|---|---|---|---|
| 8.6 wt % | 28 wt % | 13.8 | [2,3,23,24] |
| solid | - | - | [3,25,26] |
| 20 wt % | 3 wt % | 0.63 | [3,9,27,30] |
| 12 wt % | 5–10 wt % | 1.76–3.53 | [29] |
| 20 wt % | 2 N (~7 wt %) | 1.48 | [30,31,32] |
| 12 wt % | unspecified | - | [31] |
| 10–15 wt % | unspecified | - | this work |
References
- Scheer, H. An Overview of Chlorophylls and Bacteriochlorophylls. Biochemistry, Biophysics, Functions and Applications. In Chlorophylls and Bacteriochlorophylls. Biochemistry, Biophysics, Functions and Applications; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 25, pp. 1–26. [Google Scholar]
- Kim, H.-J.; Lindsey, J.S. De Novo Synthesis of Stable Tetrahydroporphyrinic Macrocycles: Bacteriochlorins and a Tetradehydrocorrin. J. Org. Chem. 2005, 70, 5475–5486. [Google Scholar] [CrossRef] [PubMed]
- Krayer, M.; Ptaszek, M.; Kim, H.-J.; Meneely, K.R.; Fan, D.; Secor, K.; Lindsey, J.S. Expanded Scope of Synthetic Bacteriochlorins via Improved Acid Catalysis Conditions and Diverse Dihydrodipyrrin-Acetals. J. Org. Chem. 2010, 75, 1016–1039. [Google Scholar] [CrossRef] [PubMed]
- van Leusen, A.M.; Siderius, H.; Hoogenboom, B.E.; van Leusen, D. A New and Simple Synthesis of the Pyrrole Ring System from Michael Acceptors and Tosylmethylisocyanides. Tetrahedron Lett. 1972, 13, 5337–5340. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, Z.; Zhang, D. Synthesis of Multi-Substituted Pyrrole Derivatives Through [3 + 2] Cycloaddition with Tosylmethyl Isocyanides (TosMICs) and Electron-Deficient Compounds. Molecules 2018, 23, 2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G.; Stanforth, S.P. The Vilsmeier Reaction of Fully Conjugated Carbocycles and Heterocycles. Org. React. 1997, 49, 1–330. [Google Scholar]
- Luzzio, F.A. The Henry Reaction: Recent Examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Tokoroyama, T. Discovery of the Michael Reaction. Eur. J. Org. Chem. 2010, 2009–2016. [Google Scholar] [CrossRef]
- Mass, O.; Lindsey, J.S. A trans-AB-Bacteriochlorin Building Block. J. Org. Chem. 2011, 76, 9478–9487. [Google Scholar] [CrossRef]
- Kobayashi, M.; Akiyama, M.; Kano, H.; Kise, H. Spectroscopy and Structure Determination. In Chlorophylls and Bacteriochlorophylls. Biochemistry, Biophysics, Functions and Applications; Springer: Dordrecht, The Netherlands, 2006; Volume 25, pp. 79–94. [Google Scholar]
- Noland, W.E. The Nef Reaction. Chem. Rev. 1955, 55, 137–155. [Google Scholar] [CrossRef]
- Wolfrom, M.L. John Ulric Nef 1862–1915 (Biographical Memoir). Natl. Acad. Sci. 1960, 34, 204–227. [Google Scholar]
- Pinnick, H.W. The Nef Reaction. Org. React. 1990, 38, 655–792. [Google Scholar]
- Ballini, R.; Petrini, M. Recent Synthetic Developments in the Nitro to Carbonyl Conversion (Nef Reaction). Tetrahedron 2004, 60, 1017−1047. [Google Scholar] [CrossRef]
- Ballini, R.; Petrini, M. The Nitro to Carbonyl Conversion (Nef Reaction): New Perspectives for a Classical Transformation. Adv. Synth. Catal. 2015, 357, 2371−2402. [Google Scholar] [CrossRef]
- McMurry, J.E.; Melton, J. A New Method for the Conversion of Nitro Groups into Carbonyls. J. Org. Chem. 1973, 38, 4367–4373. [Google Scholar] [CrossRef]
- Lindsey, J.S. De Novo Synthesis of Gem-Dialkyl Chlorophyll Analogues for Probing and Emulating our Green World. Chem. Rev. 2015, 115, 6534–6620. [Google Scholar] [CrossRef] [Green Version]
- McMurry, J.E. Carbonyl-Coupling Reactions Using Low-Valent Titanium. Chem. Rev. 1989, 89, 1513–1524. [Google Scholar] [CrossRef]
- McMurry, J.E.; Fleming, M.P. A New Method for the Reductive Coupling of Carbonyls to Olefins. Synthesis of β-Carotene. J. Am. Chem. Soc. 1974, 96, 4708–4709. [Google Scholar] [CrossRef]
- Harrison, P.J.; Fookes, C.J.R.; Battersby, A.R. Synthesis of the Isobacteriochlorin Macrocycle: A Photochemical Approach. J. Chem. Soc. Chem. Commun. 1981, 797–799. [Google Scholar] [CrossRef]
- Battersby, A.R.; Dutton, C.J.; Fookes, C.J.R. Synthetic Studies Relevant to Biosynthetic Research on Vitamin B12. Part 7. Synthesis of (±)-Bonellin Dimethyl Ester. J. Chem. Soc. Perkin Trans. 1988, 1, 1569–1576. [Google Scholar] [CrossRef]
- Marrec, O.; Christophe, C.; Billard, T.; Langlois, B.; Vors, J.-P.; Pazenok, S. Synthesis of β-Trifluoromethylated Δ1-Pyrrolines. Adv. Synth. Catal. 2010, 352, 2825–2830. [Google Scholar] [CrossRef]
- Strachan, J.-P.; O’Shea, D.F.; Balasubramanian, T.; Lindsey, J.S. Rational Synthesis of Meso-Substituted Chlorin Building Blocks. J. Org. Chem. 2000, 65, 3160–3172. [Google Scholar] [CrossRef]
- Kim, H.-J.; Dogutan, D.K.; Ptaszek, M.; Lindsey, J.S. Synthesis of Hydrodipyrrins Tailored for Reactivity at the 1- and 9-Positions. Tetrahedron 2007, 63, 37–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, M.; Cramer, D.L.; Bhise, A.D.; Kee, H.L.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Accessing the Near-Infrared Spectral Region with Stable, Synthetic, Wavelength-Tunable Bacteriochlorins. New J. Chem. 2008, 32, 947–958. [Google Scholar] [CrossRef]
- Borbas, K.E.; Ruzié, C.; Lindsey, J.S. Swallowtail Bacteriochlorins. Lipophilic Absorbers for the Near-Infrared. Org. Lett. 2008, 10, 1931–1934. [Google Scholar] [CrossRef]
- Aravindu, K.; Krayer, M.; Kim, H.-J.; Lindsey, J.S. Facile Synthesis of a B,D-Tetradehydrocorrin and Rearrangement to Bacteriochlorins. New J. Chem. 2011, 35, 1376–1384. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Bocian, D.F.; Lindsey, J.S. Synthesis of 24 Bacteriochlorin Isotopologues, Each Containing a Symmetrical Pair of 13C or 15N Atoms in the Inner Core of the Macrocycle. J. Org. Chem. 2014, 79, 1001–1016. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, H.-J.; Tang, Q.; Yang, E.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Synthesis and Photophysical Characteristics of 2,3,12,13-Tetraalkylbacteriochlorins. New J. Chem. 2016, 40, 5942–5956. [Google Scholar] [CrossRef]
- Fujita, H.; Jing, H.; Krayer, M.; Allu, S.; Veeraraghavaiah, G.; Wu, Z.; Jiang, J.; Diers, J.R.; Magdaong, N.C.M.; Mandal, A.K.; et al. Annulated Bacteriochlorins for Near-Infrared Photophysical Studies. New J. Chem. 2019, 43, 7209–7232. [Google Scholar] [CrossRef]
- Jing, H.; Wang, P.; Chen, B.; Jiang, J.; Vairaprakash, P.; Liu, S.; Rong, J.; Chen, C.-Y.; Tran, V.-P.; Nalaoh, P.; et al. Synthesis of Bacteriochlorins Bearing Diverse β-Substituents. New J. Chem. 2022, 46, 5534–5555. [Google Scholar] [CrossRef]
- Sun, R.; Liu, M.; Wang, P.; Qin, Y.; Schnedermann, C.; Maher, A.G.; Zheng, S.-L.; Liu, S.; Chen, B.; Zhang, S.; et al. Synthesis and Properties of Metalated Tetradehydrocorrins. Inorg. Chem. 2022, in press. [Google Scholar] [CrossRef]
- Tabolin, A.A.; Lesiv, A.V.; Khomutova, Y.A.; Nelyubina, Y.V.; Ioffe, S.L. Rearrangement of 3-Alkylidene-2-siloxy-tetrahydro-1,2-oxazines (ASENA). A New Approach Toward the Synthesis of 3-α-Hydroxyalkyl-5, 6-dihydro-4H-1,2-oxazines. Tetrahedron 2009, 65, 4578–4592. [Google Scholar] [CrossRef]
- Tabolin, A.A.; Leviv, A.V.; Khomutova, Y.A.; Ioffe, S.L. Synthesis of α-Prolinols and 2-Amino-1,5-diols from Primary Nitroalkanes and Other Simple Precursors via Intermediacy of 5,6-Dihydro-4H-1,2-oxazines. Synthesis 2012, 44, 1898–1906. [Google Scholar] [CrossRef] [Green Version]
- Malykhin, R.S.; Golovanov, I.S.; Nelyubina, Y.V.; Ioffe, S.L.; Sukhorukov, A.Y. Construction of Saturated Oxazolo[3,2-b]oxazines via Tandem [3+2]-Cycloaddition/[1,3]-Rearrangement of Cyclic Nitronates and Ketenes. J. Org. Chem. 2021, 86, 16337–16348. [Google Scholar] [CrossRef] [PubMed]
- Tabolin, A.A.; Sukhorukov, A.Y.; Ioffe, S.L.; Dilman, A.D. Recent Advances in the Synthesis and Chemistry of Nitronates. Synthesis 2017, 49, 3255–3268. [Google Scholar] [CrossRef]
- Weast, R.C. (Ed.) CRC Handbook of Chemistry and Physics, 52nd ed.; The Chemical Rubber Co.: Cleveland, OH, USA, 1971–1972; p. B-64. [Google Scholar]
- Bruker (2009) APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2002.
- Available online: https://onlinelibrary.wiley.com/iucr/doi/10.1107/S2053229614024218 (accessed on 2 June 2022).
- Available online: http://scripts.iucr.org/cgi-bin/paper?S0021889808042726 (accessed on 2 June 2022).
- Available online: https://docs.scipy.org/doc/scipy/reference/generated/scipy.optimize.fsolve.html (accessed on 2 June 2022).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, V.-P.; Matsumoto, N.; Nalaoh, P.; Jing, H.; Chen, C.-Y.; Lindsey, J.S. Dihydrooxazine Byproduct of a McMurry–Melton Reaction en Route to a Synthetic Bacteriochlorin. Organics 2022, 3, 262-274. https://doi.org/10.3390/org3030019
Tran V-P, Matsumoto N, Nalaoh P, Jing H, Chen C-Y, Lindsey JS. Dihydrooxazine Byproduct of a McMurry–Melton Reaction en Route to a Synthetic Bacteriochlorin. Organics. 2022; 3(3):262-274. https://doi.org/10.3390/org3030019
Chicago/Turabian StyleTran, Vy-Phuong, Nobuyuki Matsumoto, Phattananawee Nalaoh, Haoyu Jing, Chih-Yuan Chen, and Jonathan S. Lindsey. 2022. "Dihydrooxazine Byproduct of a McMurry–Melton Reaction en Route to a Synthetic Bacteriochlorin" Organics 3, no. 3: 262-274. https://doi.org/10.3390/org3030019






