MCR under Microwave Irradiation: Synthesis in Water of New 2-Amino-bis(2-phosphonoacetic) Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedure for Amines
2.2. Octylamino-2-diphosphonic Acetic Acid
2.3. Decylamino-2-diphosphonic Acetic Acid
2.4. Dodecylamino-2-diphosphonic Acetic Acid
2.5. Dodecyldiamino-2-tetraphosphonic Acetic Acid
2.6. P-xylenediamino-2-tetraphosphonic Acetic Acid
2.7. Dibutylaminophosphonic Acetic Acid
2.8. General Procedure for Amino Acids
2.9. L-amino-3-phenylpropanoic Diphosphonic Acetic Acid
2.10. L-amino-2 Methyl-4 Pentanoic Diphosphonic Acetic Acid
2.11. L-amino-2 p-Hydroxyphenyl-3 Propanoic Diphosphonic Acetic Acid
2.12. N-phosphonic Acetic Acid of Glutathione
2.13. Ethyleneimine N-phosphonoacetic Acid Polymer
2.14. (3.S)-2,3,4,9-Tetrahydro-1h-pyrido[3,4-b]indole-1,3-dicarboxylic Acid
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Didi, M.A.; Villemin, D. Phosphonic and Aminophosphonic acids. Synthesis and Applications, Editions Universitaires Européennes; OmniScriptum Publising: Saarbrücken, Germany, 2018; ISBN 978-620-2-27586-6. [Google Scholar]
- Troev, K.D. Chemistry and Application of H-Phosphonates; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 9780444527370. [Google Scholar]
- Kukhar, V.P.; Hudson, H.R. Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; John Wiley: Chichester, UK, 2000; ISBN 0-471-89149-1. [Google Scholar]
- Kafarski, P. Phosphonopeptides containing free phosphonic groups: Recent advances. RSC Adv. 2020, 10, 25898–25910. [Google Scholar] [CrossRef]
- Grembecka, J.; Mucha, A.; Cierpicki, T.; Kafarski, P. The most potent organophosphorus inhibitors of leucine aminopeptidase. Structure-based design, chemistry, and activity. J. Med Chem. 2003, 46, 2641–2655. [Google Scholar] [CrossRef]
- Hiratake, J.; Oda, J. Aminophosphonic and aminoboronic acids as key elements of a transition state analogue inhibitor of enzymes. Biosci. Biotechnol. Biochem. 1997, 61, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Cameron, D.G.; Hudson, H.R.; Pianka, M. Aminoalkynephosphonic acids in agricultural fungicides: A new development in crop protection chemicals. Phosphorus Sulfur Silicon Relat. Elem. 1990, 51, 391. [Google Scholar] [CrossRef]
- Hsieh-Wilson, L.C.; Schultz, P.G.; Stevens, R.C. Insights into antibody catalysis: Structure of an oxygenation catalyst at 1.9-angstrom resolution. Proc. Natl. Acad. Sci. USA 1996, 93, 5363–5367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabachnik, M.I.; Medved, T.Y.; Dyatlova, N.M.; Arkhipova, O.G.; Rudomino, M.V. Organophosphorus complexones. Russ. Chem. Rev. 1968, 37, 503–518. [Google Scholar] [CrossRef]
- Kabachnik, M.I.; Medved, T.Y.; Dyatlova, N.M.; Rudomino, M.V. Organophosphorus complexones. Russ. Chem. Rev. 1974, 43, 733–744. [Google Scholar] [CrossRef]
- Amar, H.; Benzakour, J.; Derja, A.; Villemin, D.; Moreau, B. A corrosion inhibition study of iron by phosphonic acids in sodium chloride solution. J. Electroanal. Chim. 2003, 558, 131–139. [Google Scholar] [CrossRef]
- Amar, H.; Benzakour, J.; Derja, A.; Villemin, D.; Moreau, B. Investigation of the inhibitive effect of phosphonic acids on corrosion of iron in sodium chloride 3% media. Corros. Eng. Sci. Technol. 2006, 41, 291–296. [Google Scholar] [CrossRef]
- Didi, M.A.; Kaid, M.; Baghdad, M.; Villemin, D. Aminododecyldiphosphonic acid for solvent extraction of bismuth ions. Int. J. Nonferrous Metal. 2012, 1, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Mutin, P.H.; Guerrero, G.; Vioux, A. Organic-inorganic hybrid materials based on organophosphorus coupling molecules: From metal phosphonates to surface modification of oxides. Comptes Rendus Chim. 2003, 6, 1153–1164. [Google Scholar] [CrossRef]
- Monteil, C.; Bar, N.; Retoux, R.; Henry, J.; Bernay, B.; Villemin, D. Partially phosphonated polyethylenimine-coated nanoparticles as convenient support for enzyme immobilization in bioprocessing. Sens. Actuators B 2014, 192, 269–274. [Google Scholar] [CrossRef]
- Rosario, M.P.; Colodrero, R.M.P.; Olivera-Pastor, P.; Losilla, E.R.; Hernández-Alonso, D.; Aranda, M.A.G.; Leon-Reina, L.; Rius, J.; Demadis, K.D.; Moreau, B.; et al. High proton conductivity in a flexible, cross-linked, ultramicroporous magnesium tetraphosphonate hybrid framework. Inorg. Chem. 2012, 51, 7689–7698. [Google Scholar] [CrossRef]
- Turner, A.; Jaffrés, P.-A.; MacLean, E.J.; Villemin, D.; McKee, V.; Hix, G.B. Hydrothermal synthesis and crystal structure of two Co phosphonates containing trifunctional phosphonate anions: Co3(O3PCH2NH2CH2PO3)2 and Co3(O3PCH2-NC4H7-CO2)2.5H2O. J. Chem. Soc. Dalton Trans. 2003, 1314–1319. [Google Scholar] [CrossRef]
- Moedritzer, K.; Irani, R. The direct synthesis of α-aminomethylphosphonic acids. Mannich-type reactions with orthophosphorous acid. J. Org. Chem. 1966, 31, 1603–1607. [Google Scholar] [CrossRef]
- Siméon, F.; Saint-Clair, J.F.; Villemin, D. Alkylmethyleneamino phosphonic acids synthesis under microwave irradiation. In Proceedings of the First International Conference on Microwave Chemistry, Prague, Czeck Republic, 7–11 September 1998; p. 186. [Google Scholar]
- Villemin, D.; Moreau, B.; Didi, M.A.; Kaid, M.; Jaffrès, P.-A. Synthesis in Water under Focussed Microwave Irradiation: A Rapid and Convenient Synthesis of Polyaminopolymethylene Phosphonic Acids. In Proceedings of the 12th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-12), sciforum.net, MDPI. 1–30 November 2008; [E0002]. Available online: https://sciforum.net/paper/view/conference/1252 (accessed on 1 May 2021).
- Villemin, D.; Moreau, B.; Elbilali, A.; Didi, M.A.; Kaid, M.; Jaffrès, P.-A. Green synthesis of poly(aminomethylene phosphonic) acids. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1–9. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Galkin, V.I. The Kabachnik–Fields reaction: Synthetic potential and the problem of the mechanism. Russ. Chem. Rev. 1998, 67, 857–882. [Google Scholar] [CrossRef]
- Fields, E.K. The synthesis of esters of substituted aminophosphonic acids. J. Am. Chem. Soc. 1952, 74, 1528–1531. [Google Scholar] [CrossRef]
- Zefirov, N.S.; Matveeva, E.A. Catalytic Kabachnik-Fields reaction: New horizons for old reaction. Arkivoc 2008, 1, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Villemin, D.; Didi, M.A. Aminomethylenephosphonic Acids Syntheses and Applications (A Review). Orient. J. Chem. 2015, 31, 1–12. Available online: http://www.orientjchem.org/?p=10779 (accessed on 1 May 2021). [CrossRef]
- Justyna, K.; Małolepsza, J.; Kusy, D.; Maniukiewicz, W.; Błażewska, K.M. The McKenna reaction—Avoiding side reactions in phosphonate deprotection. Beilstein J. Org. Chem. 2020, 16, 1436–1446. [Google Scholar] [CrossRef]
- Heinicke, J.; Lach, J.; Peulecke, N.; Jones, P.G.; Dix, I. Phosphinoglycines and Phosphinoglycolates. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 783–786. [Google Scholar] [CrossRef]
- Heinicke, J.; Lach, J.; Basvani, K.R.; Peulecke, N.; Jones, P.G.; Köckerling, M. Phosphino amino acids: Synthesis, structure and reactivity. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 666–677. [Google Scholar] [CrossRef]
- Villemin, D.; Monteil, C.; Bar, N.; Didi, M.A. Phosphonated polyethyleneimines (PEIP) as multi-use polymers. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 879–890. [Google Scholar] [CrossRef]
- Didi, M.A.; Miraoui, A.; Villemin, D. Neodymium (III) removal by functionalized magnetic nanoparticles. J. Rad. Nucl. Chem. 2016, 307, 963–971. [Google Scholar] [CrossRef]
- Pictet, A.; Spengler, T. Über die Bildung von Isochinolin-derivaten durch Einwirkung von Methylal auf Phenyl-äthylamin, Phenyl-alanin und Tyrosin. Ber. Dtsch. Chem. Ges. 1911, 44, 2030–2036. [Google Scholar] [CrossRef] [Green Version]
- Bobbitt, J.M.; Willis, M.P. Electrochemistry of natural products. 7. Oxidative decarboxylation of some tetrahydro-.beta.-carbolinecarboxylic acids. J. Org. Chem. 1980, 45, 1978–1984. [Google Scholar] [CrossRef]




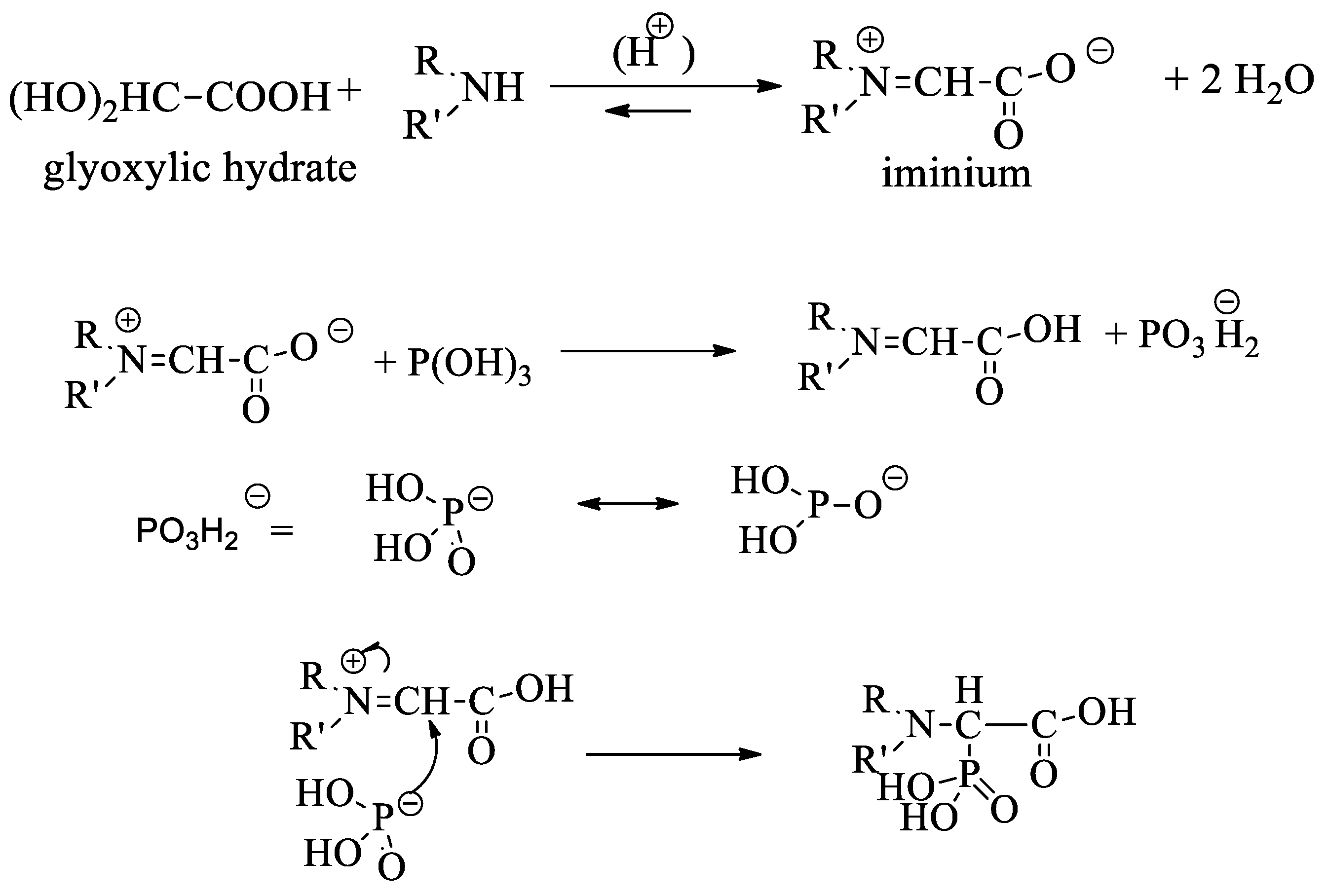
| Amine | Product | Yield a | Yield b |
|---|---|---|---|
| 1-butylamine | CH3(CH2)3-N[CH(COOH)(PO(OH)2) ]2 | 92 | – |
| 1-hexylamine | CH3(CH2)5-N[CH(COOH)(PO(OH)2) ]2 | 92 | – |
| 1-octylamine | CH3(CH2)7-N[CH(COOH)(PO(OH)2) ]2 | 90 | 39 |
| 1-decylamine | CH3(CH2)9-N[CH(COOH)(PO(OH)2) ]2 | 90 | 40 |
| 1-dodecylamine | CH3(CH2)11-N[CH(COOH)(PO(OH)2) ]2 | – | 50 |
| dibutylamine | [CH3(CH2)2]2 NCH(COOH)(PO(OH)2) | – | 41 |
| dicyclohexylamine | (C6H11)2 NCH(COOH)(PO(OH)2) | 0 | – |
| t-butylamine | (CH3)3C-N[CH(COOH)(PO(OH)2 ]2 | 0 | – |
| t-octylamine | C4H9 CH2(CH3)2C N[CH(COOH)[PO(OH)2 ]2 | 0 | – |
| 1-adamantanamine | C10H17 N[CH(COOH)(PO(OH)2 ]2 | 0 | – |
| 1,2-diaminoethane | [(HOOC)[(HO)2 PO]2 CH]2 N(CH2)2N[CH(COOH)[PO(OH)2] ]2 | 82 | – |
| 1,12-diaminododecane | [(HOOC)[(HO)2 PO]2 CH]2 N(CH2)12N[CH(COOH)[PO(OH)2] ]2 | – | 66 |
| p-xylenediamine | [(HOOC)[(HO)2 PO]2 CH]2NCH2C6H4CH2N[CH(COOH)[(PO(OH)2] ]2 | – | 65 |
| Starting Reactant | Isolated Products |
|---|---|
| L-Phenylalanine |  |
| L-Leucine |  |
| L-Tyrosine |  |
| L-Glutathione |  |
| L-Tryptophan |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villemin, D.; Moreau, B.; Bar, N. MCR under Microwave Irradiation: Synthesis in Water of New 2-Amino-bis(2-phosphonoacetic) Acids. Organics 2021, 2, 98-106. https://doi.org/10.3390/org2020009
Villemin D, Moreau B, Bar N. MCR under Microwave Irradiation: Synthesis in Water of New 2-Amino-bis(2-phosphonoacetic) Acids. Organics. 2021; 2(2):98-106. https://doi.org/10.3390/org2020009
Chicago/Turabian StyleVillemin, Didier, Bernard Moreau, and Nathalie Bar. 2021. "MCR under Microwave Irradiation: Synthesis in Water of New 2-Amino-bis(2-phosphonoacetic) Acids" Organics 2, no. 2: 98-106. https://doi.org/10.3390/org2020009
APA StyleVillemin, D., Moreau, B., & Bar, N. (2021). MCR under Microwave Irradiation: Synthesis in Water of New 2-Amino-bis(2-phosphonoacetic) Acids. Organics, 2(2), 98-106. https://doi.org/10.3390/org2020009






