Characterization of Perfluoro Sulfonic Acid Membranes for Potential Electrolytic Hydrogen Production and Fuel Cell Applications for Local and Global Green Hydrogen Economy
Abstract
1. Introduction
2. Materials and Methodology
2.1. Procedure
2.2. The Solvent System
2.3. Characterization
3. Results and Discussion
3.1. Impact of Mixed Solvents via Partial Dissolution of the Nafion N−115 Membrane
3.2. Evaluation of Mechanical Strength
3.3. AFM Investigation of the N−115 Membrane Under Hydration and Thermal Processing
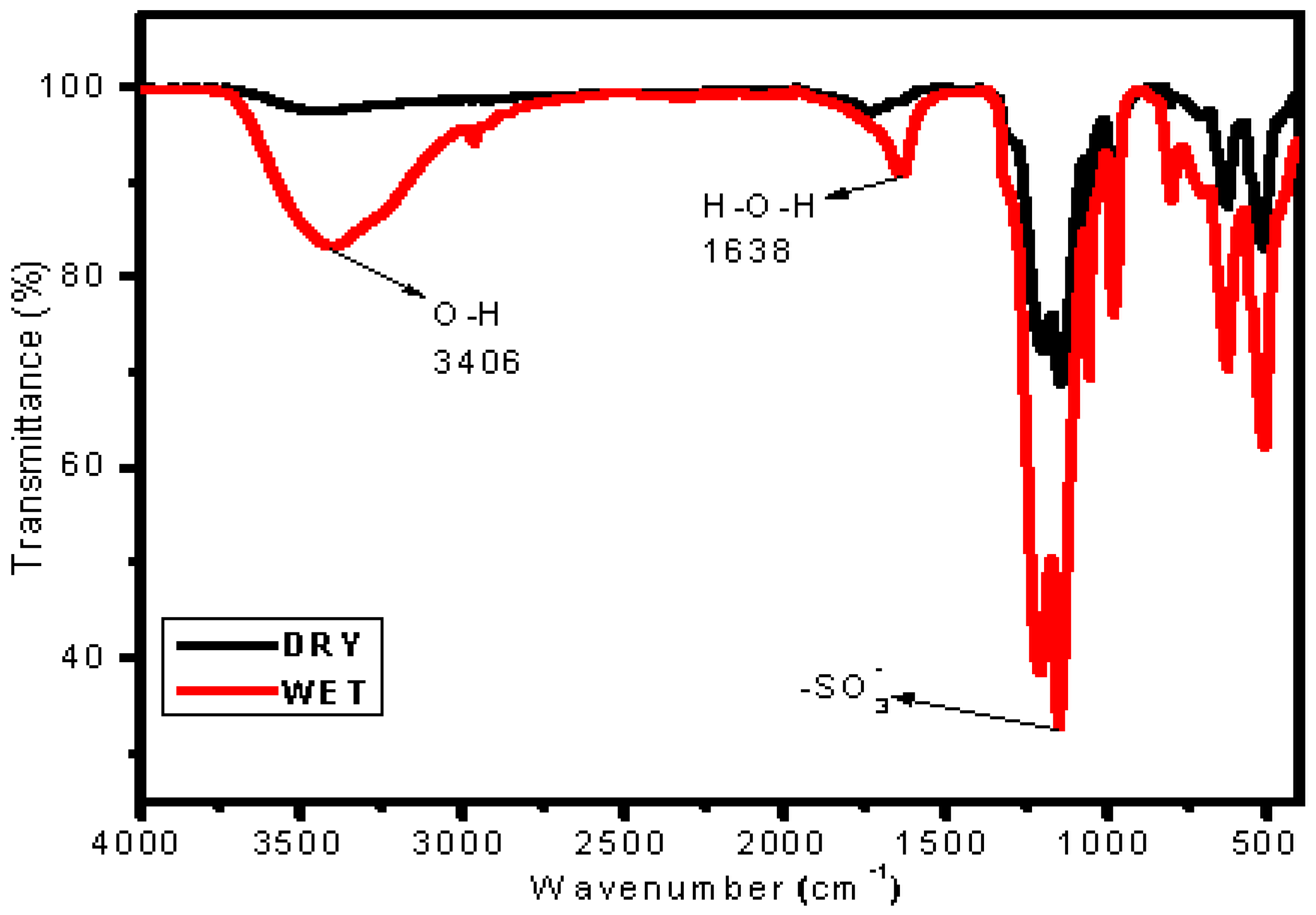
3.4. Assessment of Water Uptake
3.5. Investigation of Chemical Stability
3.6. Characterization of Proton Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheekatamarla, P. Hydrogen and the global energy transition—Path to sustainability and adoption across all economic sectors. Energies 2024, 17, 807. [Google Scholar] [CrossRef]
- Hassan, Q.; Azzawi, I.D.; Sameen, A.Z.; Salman, H.M. Hydrogen fuel cell vehicles: Opportunities and challenges. Sustainability 2023, 15, 11501. [Google Scholar] [CrossRef]
- Seck, G.S.; Hache, E.; Sabathier, J.; Guedes, F.; Reigstad, G.A.; Straus, J.; Wolfgang, O.; Ouassou, J.A.; Askeland, M.; Hjorth, I.; et al. Hydrogen and the decarbonization of the energy system in europe in 2050: A detailed model-based analysis. Renew. Sustain. Energy Rev. 2022, 167, 112779. [Google Scholar] [CrossRef]
- de Sá, M.H. Electrochemical devices to power a sustainable energy transition—An overview of green hydrogen contribution. Appl. Sci. 2024, 14, 2168. [Google Scholar] [CrossRef]
- Meda, U.S.; Rajyaguru, Y.V.; Pandey, A. Generation of green hydrogen using self-sustained regenerative fuel cells: Opportunities and challenges. Int. J. Hydrogen Energy 2023, 48, 28289–28314. [Google Scholar] [CrossRef]
- Baroutaji, A.; Arjunan, A.; Robinson, J.; Abdelkareem, M.A.; Olabi, A.-G. Additive manufacturing for Proton Exchange Membrane (PEM) hydrogen technologies: Merits, challenges, and prospects. Int. J. Hydrogen Energy 2024, 52, 561–584. [Google Scholar] [CrossRef]
- Elegbeleye, I.; Oguntona, O.; Elegbeleye, F. Green Hydrogen: Pathway to Net Zero Green House Gas Emission and Global Climate Change Mitigation. Hydrogen 2025, 6, 29. [Google Scholar] [CrossRef]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton exchange membrane fuel cells (PEMFCs): Advances and challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef]
- da Costa, G.P.; Garcia, D.M.; Van Nguyen, T.H.; Lacharmoise, P.; Simão, C.D. Advancements in printed components for proton exchange membrane fuel cells: A comprehensive review. Int. J. Hydrogen Energy 2024, 69, 710–728. [Google Scholar] [CrossRef]
- Chibac-Scutaru, A.L.; Coseri, S. Advances in the use of cellulose-based proton exchange membranes in fuel cell technology: A review. Int. J. Biol. Macromol. 2023, 247, 125810. [Google Scholar] [CrossRef]
- Jayakumar, A.; Madheswaran, D.K.; Kumar, N.M. A critical assessment on functional attributes and degradation mechanism of membrane electrode assembly components in direct methanol fuel cells. Sustainability 2021, 13, 13938. [Google Scholar] [CrossRef]
- Ng, W.W.; Thiam, H.S.; Pang, Y.L.; Chong, K.C.; Lai, S.O. A State-of-Art on the development of Nafion-based membrane for performance improvement in direct methanol fuel cells. Membranes 2022, 12, 506. [Google Scholar] [CrossRef]
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New perspectives on fuel cell technology: A brief review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Dubelley, F.; Paul, A.; Svecova, L.; Bas, C. Investigation of Membrane–Electrode Separation Processes for the Recycling of Ionomer Membranes in End-of-Life PEM Fuel Cells. Energy Fuels 2025, 39, 2758–2771. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Lysova, A.A.; Voropaeva, D.Y.; Yaroslavtsev, A.B. Approaches to the modification of perfluorosulfonic acid membranes. Membranes 2023, 13, 721. [Google Scholar] [CrossRef]
- Bhowmik, S.; Phukan, S.J.; Sah, N.K.; Roy, M.; Garai, S.; Iyer, P.K. Review of graphitic carbon nitride and its composite catalysts for selective reduction of CO2. ACS Appl. Nano Mater. 2021, 4, 12845–12890. [Google Scholar] [CrossRef]
- Palanisamy, G.; Oh, T.H.; Thangarasu, S. Modified cellulose proton-exchange membranes for direct methanol fuel cells. Polymers 2023, 15, 659. [Google Scholar] [CrossRef]
- Wang, K.Y.; Weber, M.; Chung, T.-S. Polybenzimidazoles (PBIs) and state-of-the-art PBI hollow fiber membranes for water, organic solvent and gas separations: A review. J. Mater. Chem. A 2022, 10, 8687–8718. [Google Scholar] [CrossRef]
- Gao, X.; Yamamoto, K.; Hirai, T.; Ohta, N.; Uchiyama, T.; Watanabe, T.; Imai, H.; Sugawara, S.; Shinohara, K.; Uchimoto, Y. Impact of the Composition of Alcohol/Water Dispersion on the Proton Transport and Morphology of Cast Perfluorinated Sulfonic Acid Ionomer Thin Films. ACS Omega 2021, 6, 14130–14137. [Google Scholar] [CrossRef]
- Li, S.; Gu, R.; Luo, R.; Cheng, X.; Li, X. Enhanced properties of Nafion nanofibrous proton exchange membranes by altering the electrospinning solvents. J. Polym. Eng. 2024, 44, 449–456. [Google Scholar] [CrossRef]
- Aburabie, J.; Lalia, B.; Hashaikeh, R. Proton conductive, low methanol crossover cellulose-based membranes. Membranes 2021, 11, 539. [Google Scholar] [CrossRef]
- Song, C.-H.; Park, J.-S. Effect of Dispersion Solvents in Catalyst Inks on the Performance and Durability of Catalyst Layers in Proton Exchange Membrane Fuel Cells. Energies 2019, 12, 549. [Google Scholar] [CrossRef]
- Madhuranthakam, C.M.R.; Abudaqqa, W.S.; Fowler, M. Advances in polyvinyl alcohol-based membranes for fuel cells: A comprehensive review on types, synthesis, modifications, and performance optimization. Polymers 2024, 16, 1775. [Google Scholar] [CrossRef]
- Solhi, L.; Guccini, V.; Heise, K.; Solala, I.; Niinivaara, E.; Xu, W.; Mihhels, K.; Kroger, M.; Meng, Z.; Wohlert, J. Understanding nanocellulose–water interactions: Turning a detriment into an asset. Chem. Rev. 2023, 123, 1925–2015. [Google Scholar] [CrossRef]
- Oxley, A.; Gaffney, P.R.; Kim, D.; Marchetti, P.; Livingston, A.G. Graft modification of polybenzimidazole membranes for organic solvent ultrafiltration with scale up to spiral wound modules. J. Membr. Sci. 2022, 647, 120199. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Ning, X.; Tian, M.; Long, Y.; Ramakrishna, S. Recent advances in designing and tailoring nanofiber composite electrolyte membranes for high-performance proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 25225–25251. [Google Scholar] [CrossRef]
- Prykhodko, Y.; Fatyeyeva, K.; Hespel, L.; Marais, S. Progress in hybrid composite Nafion®-based membranes for proton exchange fuel cell application. Chem. Eng. J. 2021, 409, 127329. [Google Scholar] [CrossRef]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An overview of proton exchange membranes for fuel cells: Materials and manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Rahim, A.; Shamsuri, N.; Adam, A.; Aziz, M.; Hamsan, M.; Rusdi, H.; Siong, S.O.J.; Noor, I.; Kadir, M.; Shukur, M. Characterization of nanocomposite polyvinyl alcohol/cellulose acetate blend gel polymer electrolytes for supercapacitor application. J. Energy Storage 2024, 97, 112964. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Mu, Y.; Wu, B.; Jiang, Y.; Zeng, L.; Zhao, T. Recent advances in the anode catalyst layer for proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 2023, 176, 113182. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, S.; Liang, Y.; Li, H.; Xu, Z.; Xu, Q.; Yin, J.; Shen, S.; Yan, X.; Zhang, J. Engineering the catalyst layers towards enhanced local oxygen transport of Low-Pt proton exchange membrane fuel cells: Materials, designs, and methods. Int. J. Hydrogen Energy 2023, 48, 4389–4417. [Google Scholar] [CrossRef]
- Kumar, A.G.; Singh, A.; Komber, H.; Voit, B.; Tiwari, B.R.; Noori, M.T.; Ghangrekar, M.M.; Banerjee, S. Novel sulfonated Co-poly (ether imide) s containing trifluoromethyl, fluorenyl and hydroxyl groups for enhanced proton exchange membrane properties: Application in microbial fuel cell. ACS Appl. Mater. Interfaces 2018, 10, 14803–14817. [Google Scholar] [CrossRef]
- Santhosh Kumar, K.; Vijayalakshmi, K.; Sivanath, S.; Jayalatha, T.; Mohanty, S.; Shaneeth, M. Interaction of Nafion ionomers toward various solvents. J. Appl. Polym. Sci. 2013, 128, 3710–3719. [Google Scholar] [CrossRef]
- Barik, B.; Yun, Y.; Kumar, A.; Bae, H.; Namgung, Y.; Park, J.-Y.; Song, S.-J. Highly enhanced proton conductivity of single-step-functionalized graphene oxide/nafion electrolyte membrane towards improved hydrogen fuel cell performance. Int. J. Hydrogen Energy 2023, 48, 11029–11044. [Google Scholar] [CrossRef]
- Wei, J.; Ma, Y.; Qin, Z.; Jin, Z.; Jin, Y.; Yang, L.; Yao, L.; Jiang, W.; Deng, Y.; Huang, Y. Membrane fabricated via a facile non-solvent induced microstructure rearrangement with superior CO2 separation performances. Sep. Purif. Technol. 2023, 320, 124182. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, T.; Liu, Q.; Lei, J.; Fang, J. A distributed energy system integrating SOFC-MGT with mid-and-low temperature solar thermochemical hydrogen fuel production. Int. J. Hydrogen Energy 2021, 46, 19846–19860. [Google Scholar] [CrossRef]
- LaVan, D.; Yi, F.; Adams, T.; Tao, R.; Pelczar, E.; Xia, H.; Hu, X.; Sauerbrunn, S.; Matisons, J. Abstracts of the 2023 49th Annual NATAS Conference. Polymers 2023, 15, 3250. [Google Scholar] [CrossRef]
- Chitra, S.; Balakumar, S. Insight into the impingement of different sodium precursors on structural, biocompatible, and hemostatic properties of bioactive materials. Mater. Sci. Eng. C 2021, 123, 111959. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, M.; Tang, Y.; Zang, R.; Zhang, X.; Huang, X.; Chen, Y.; Yamauchi, Y.; Kaskel, S.; Pang, H. Understanding synthesis–structure–performance correlations of nanoarchitectured activated carbons for electrochemical applications and carbon capture. Adv. Funct. Mater. 2022, 32, 2204714. [Google Scholar] [CrossRef]
- Li, D.; Yu, Y.; Ning, C.-Z. Super-stable high-quality few-layer black phosphorus for photonic applications. ACS Appl. Nano Mater. 2021, 4, 4746–4753. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Liu, D.; An, J.; Zhang, M.; Li, J.H.; Jiang, C. Plasmonic AgNPs reinforced flexible hydrogel Surface-Enhanced Raman scattering (SERS) sensor for in-situ detection of curved samples. Chem. Eng. J. 2024, 494, 153082. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, N.; Yuen, G.; de Lannoy, C.-F. Cross-linked iron nanoparticle-doped reduced graphene oxide membranes for micropollutant removal from water. Chem. Eng. J. 2023, 455, 140624. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Belgacem, I.B.; Emori, W.; Uzoma, P.C. Nafion degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrogen Energy 2021, 46, 27956–27973. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Ramakrishnan, S.; Yu, Y.-T.; Yoo, D.J. Advanced Nafion nanocomposite membrane embedded with unzipped and functionalized graphite nanofibers for high-temperature hydrogen-air fuel cell system: The impact of filler on power density, chemical durability and hydrogen permeability of membrane. Compos. Part B Eng. 2021, 215, 108828. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.; Yang, Z.; Fan, J.; Li, H.; Xu, S. Low water swelling polyaromatic proton exchange membranes. J. Membr. Sci. 2023, 684, 121879. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Li, J.; Liang, X.; Zhou, W.; Peng, S. Strategies and techniques for improving heat resistance and mechanical performances of poly (lactic acid) (PLA) biodegradable materials. Int. J. Biol. Macromol. 2022, 218, 115–134. [Google Scholar] [CrossRef]
- Stenina, I.; Yaroslavtsev, A. Prospects for the Development of Hydrogen Energy. Polymer Membranes for Fuel Cells and Electrolyzers. Membr. Membr. Technol. 2024, 6, 15–26. [Google Scholar] [CrossRef]
- Yan, X.; Xu, Z.; Yuan, S.; Han, A.; Shen, Y.; Cheng, X.; Liang, Y.; Shen, S.; Zhang, J. Structural and transport properties of ultrathin perfluorosulfonic acid ionomer film in proton exchange membrane fuel cell catalyst layer: A review. J. Power Sources 2022, 536, 231523. [Google Scholar] [CrossRef]
- Zakertabrizi, M.; Hosseini, E.; Korayem, A.H.; Razmjou, A.; Fane, A.G.; Chen, V. Insight from perfectly selective and ultrafast proton transport through anhydrous asymmetrical graphene oxide membranes under Grotthuss mechanism. J. Membr. Sci. 2021, 618, 118735. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Emori, W.; Uzoma, P.C.; Mansir, I.B.; Radwan, A.B.; Ige, O.O.; Abdullah, A.M. A review of bipolar plates materials and graphene coating degradation mechanism in proton exchange membrane fuel cell. Int. J. Energy Res. 2022, 46, 3766–3781. [Google Scholar] [CrossRef]
- Demchenko, A.P. Proton transfer reactions: From photochemistry to biochemistry and bioenergetics. BBA Adv. 2023, 3, 100085. [Google Scholar] [CrossRef]
- Wang, G.; Kang, J.; Yang, S.; Lu, M.; Wei, H. Influence of structure construction on water uptake, swelling, and oxidation stability of proton exchange membranes. Int. J. Hydrogen Energy 2024, 50, 279–311. [Google Scholar] [CrossRef]
- Lufrano, E.; Simari, C.; Di Vona, M.L.; Nicotera, I.; Narducci, R. How the morphology of nafion-based membranes affects proton transport. Polymers 2021, 13, 359. [Google Scholar] [CrossRef]
- Asghar, M.R.; Zhang, W.; Su, H.; Zhang, J.; Liu, H.; Xing, L.; Yan, X.; Xu, Q. A review of proton exchange membranes modified with inorganic nanomaterials for fuel cells. Energy Adv. 2025, 4, 185–223. [Google Scholar] [CrossRef]
- Freger, V.; Ramon, G.Z. Polyamide desalination membranes: Formation, structure, and properties. Prog. Polym. Sci. 2021, 122, 101451. [Google Scholar] [CrossRef]
- Zupančič, B.; Grdadolnik, J. Solute-induced changes in the water H-bond network of different alcohol-aqueous systems. J. Mol. Liq. 2021, 341, 117349. [Google Scholar] [CrossRef]
- Singh, J.; White, R.L. A variable temperature infrared spectroscopy study of NaY zeolite dehydration. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118142. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Liu, J.; Jiang, Z.; Wan, J.; Wang, J.; Chai, S.; Ai, C.; Dang, F.; Albilali, R. Promoting intermediates transformation by boosting H2O dissociation over core-shell Pd@ CoO Janus for acetone efficacious oxidation. Appl. Catal. B Environ. Energy 2024, 354, 124113. [Google Scholar] [CrossRef]
- Ibrahim, A.; Hossain, O.; Chaggar, J.; Steinberger-Wilckens, R.; El-Kharouf, A. GO-nafion composite membrane development for enabling intermediate temperature operation of polymer electrolyte fuel cell. Int. J. Hydrogen Energy 2020, 45, 5526–5534. [Google Scholar] [CrossRef]
- Peressut, A.B.; Latorrata, S.; Stampino, P.G.; Dotelli, G. Development of self-assembling sulfonated graphene oxide membranes as a potential proton conductor. Mater. Chem. Phys. 2021, 257, 123768. [Google Scholar] [CrossRef]
- Pathak, R.B.; Kumar, P.; Mishra, A.P.; Verma, V. Fabrication of nanocomposite membrane composed of sulfonated PVDF and thermo-mechanically modified fly ash for application in direct methanol fuel cells. J. Polym. Res. 2023, 30, 396. [Google Scholar] [CrossRef]
- Costa, L.A.T.; de Aguiar, L.C.V.; Gomes, A.d.S.; Brum, E.J.B.A. Characterization of PVA and phenol salt modified tin dioxide cationic membranes. Int. J. Hydrog. Energy 2022, 47, 7415–7431. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, J.; Zhang, C.; Wang, W.; Qu, W.; Wang, W.; Li, W.; Wu, H. Preparation of mechanically strong and active composite films based on fish myofibrillar proteins: The dual effects of oxidized polyphenol crosslinking and layered double hydroxide reinforcement. Food Hydrocoll. 2022, 129, 107616. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, C.; Zeng, Y.; Spendelow, J.S.; Wu, G. Advanced nanocarbons for enhanced performance and durability of platinum catalysts in proton exchange membrane fuel cells. Small 2021, 17, 2006805. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Li, N.; Hu, Z.; Chen, S. Sulfonated graphitic carbon nitride nanosheets as proton conductor for constructing long-range ionic channels proton exchange membrane. J. Membr. Sci. 2020, 601, 117908. [Google Scholar] [CrossRef]
- Nandi, S.; Wang, S.; Wahiduzzaman, M.; Yadav, V.; Taksande, K.; Maurin, G.; Serre, C.; Devautour-Vinot, S. Multivariate Sulfonic-Based Titanium Metal–Organic Frameworks as Super-protonic Conductors. ACS Appl. Mater. Interfaces 2021, 13, 20194–20200. [Google Scholar] [CrossRef]
- Zhang, T.; Ju, J.; Zhang, Z.; Su, D.; Wang, Y.; Kang, W. Wearable flexible zinc-ion batteries based on electrospinning technology. J. Energy Chem. 2024, 98, 562–587. [Google Scholar] [CrossRef]
- Shi, C.; Liu, T.; Chen, W.; Cui, F.; Liu, L.; Cai, Y.; Li, Y. Interaction, structure and tensile property of swollen Nafion® membranes. Polymer 2021, 213, 123224. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Xu, W.; Long, J.; Huang, W.; He, Z.; Liu, S.; Zhang, Y. A sulfonated polyimide/nafion blend membrane with high proton selectivity and remarkable stability for vanadium redox flow battery. Membranes 2021, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Liu, L.; Min, L.; Xu, L.; Zhang, W.; Wang, Y. Enhanced proton conductivity of Nafion membrane with electrically aligned sulfonated graphene nanoplates. Int. J. Hydrogen Energy 2021, 46, 17784–17792. [Google Scholar] [CrossRef]
- Mehrazi, S. Understanding the Microstructure and Interaction Between Nanoparticles and Polymers Through the Study of Precursor Complex Fluid in a PEMFC. Ph.D. Thesis, University of California, Merced, CA, USA, 2022. [Google Scholar]
- Nguyen, A.T.; Weigle, A.T.; Shukla, D. Functional regulation of aquaporin dynamics by lipid bilayer composition. Nat. Commun. 2024, 15, 1848. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.; Mayerhöfer, B.; Trinke, P.; Bensmann, B.; Hanke-Rauschenbach, R.; Krajinovic, K.; Thiele, S.; Kerres, J. Conducting Aromatic Multiblock Copolymer and Blend Membranes and Their Application in PEM Electrolysis. Polymers 2021, 13, 3467. [Google Scholar] [CrossRef]
- Bakr, A.M.; Darwish, A.; Azab, A.; Makram, B.; Elzwawy, A. Influence of Ce and Ti oxides doping on the PMMA polymer: Structural, optical, THz, dielectric, and antimicrobial characteristics. Polym. Bull. 2024, 81, 13703–13727. [Google Scholar] [CrossRef]
- Azab, N.A.; Mohamed, M.M. Graphene foam-carbon nitride based composites as photoelectrocatalysts for overall water splitting in acidic and neutral media. J. Photochem. Photobiol. A Chem. 2023, 443, 114831. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, W.; Zheng, W. Proton batteries shape the next energy storage. Energy Storage Mater. 2023, 61, 102913. [Google Scholar] [CrossRef]
- Hegde, S.; Ravindrachary, V.; Praveena, S.; Ismayil; Guruswamy, B.; Sagar, R.N. Microstructural, dielectric, and transport properties of proton-conducting solid polymer electrolyte for battery applications. Ionics 2020, 26, 2379–2394. [Google Scholar] [CrossRef]
- Rao, A.S.; Rashmi, K.; Manjunatha, D.; Jayarama, A.; Prabhu, S.; Pinto, R. Pore size tuning of Nafion membranes by UV irradiation for enhanced proton conductivity for fuel cell applications. Int. J. Hydrogen Energy 2019, 44, 23762–23774. [Google Scholar] [CrossRef]
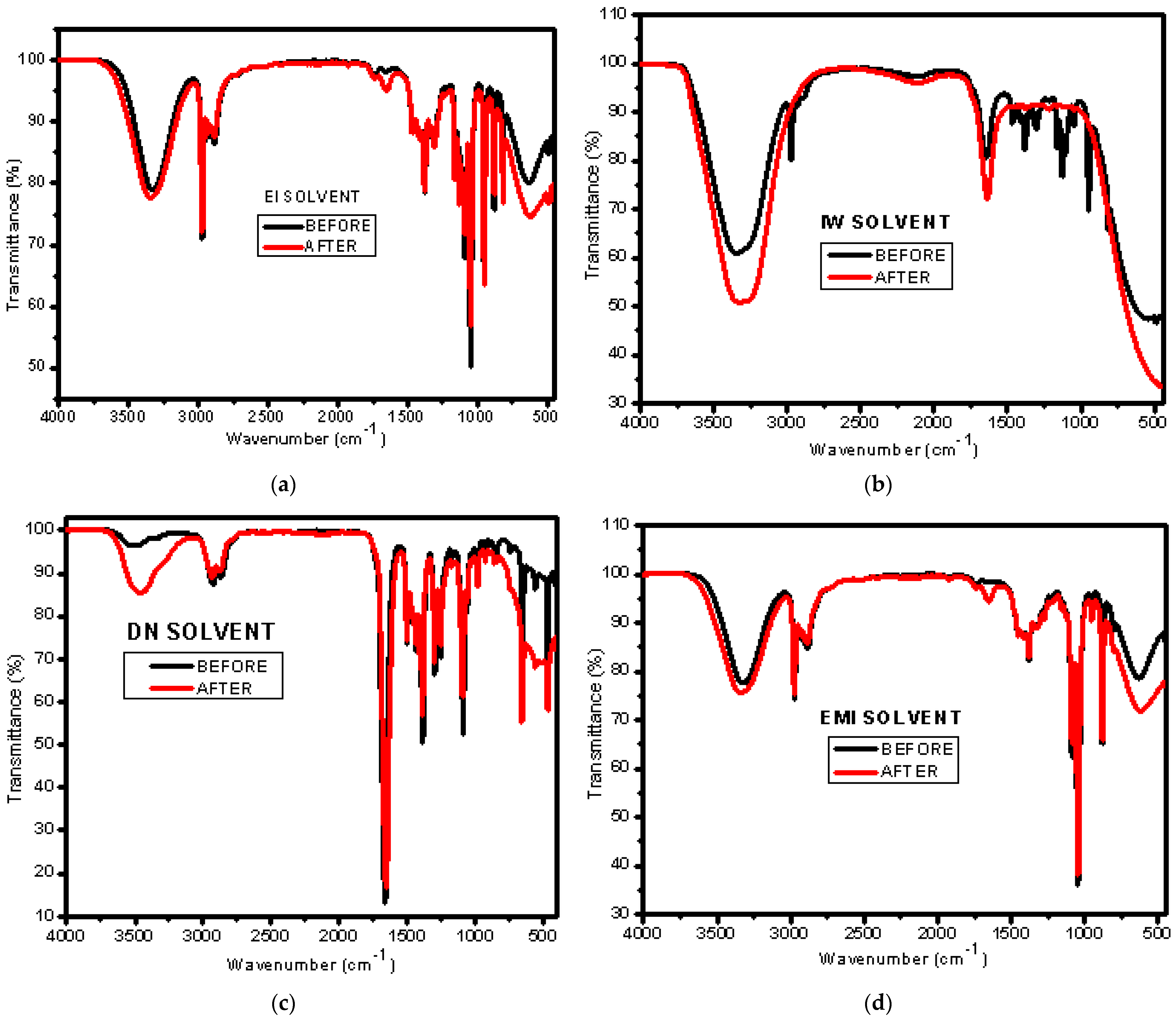
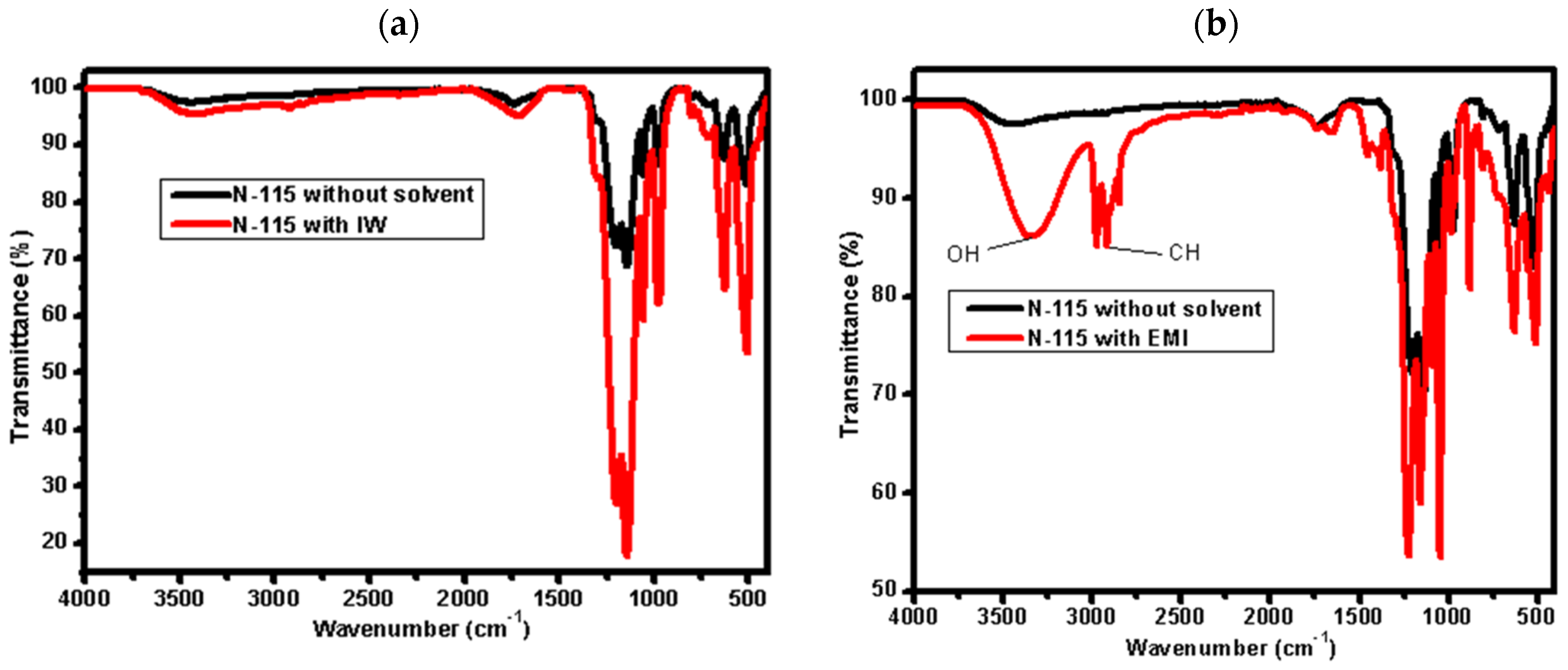
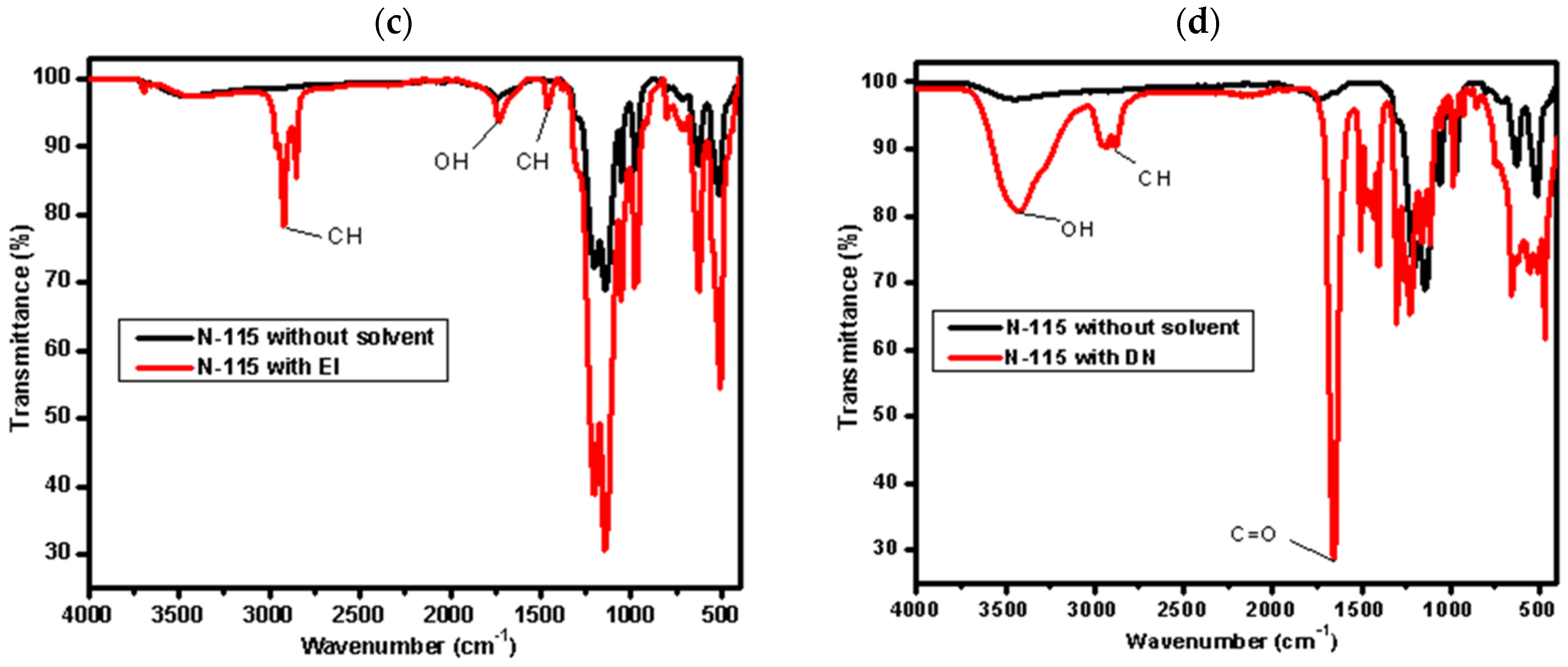


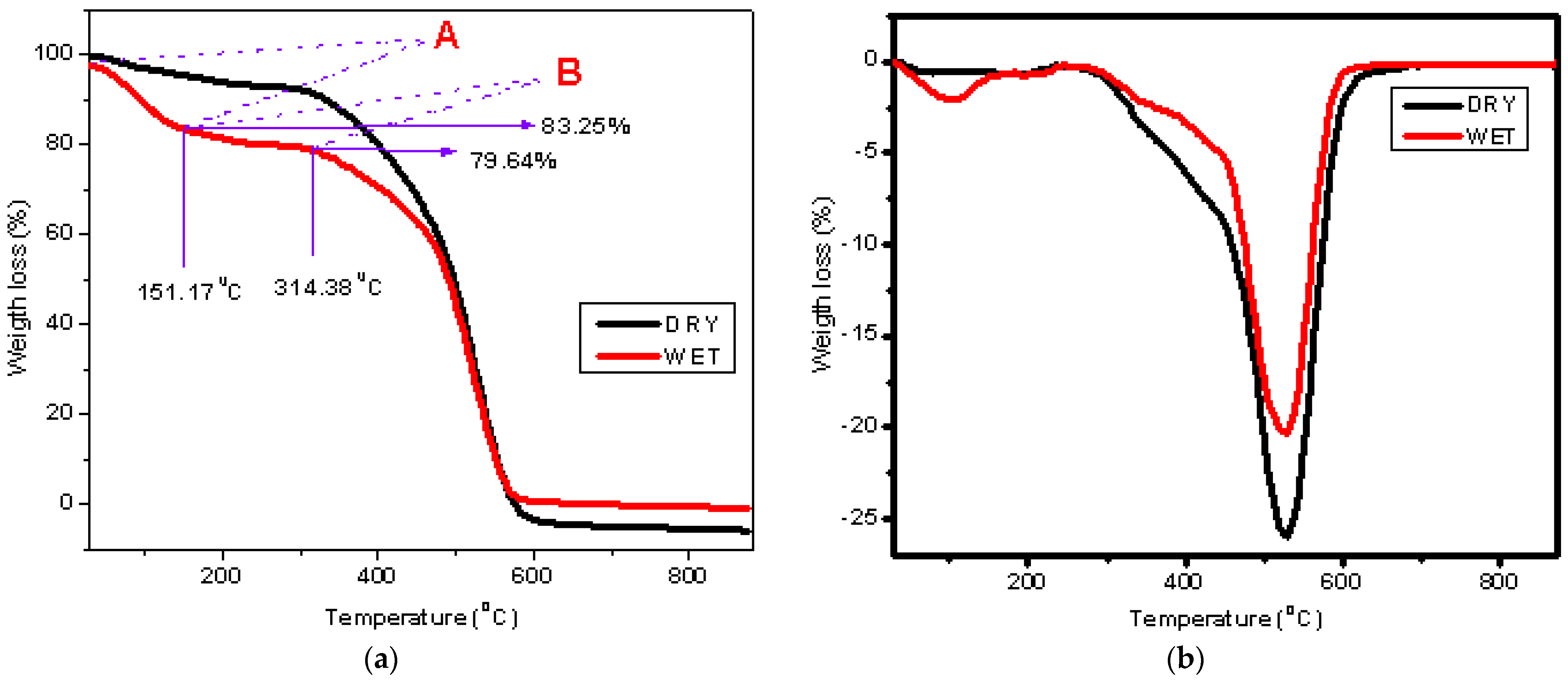
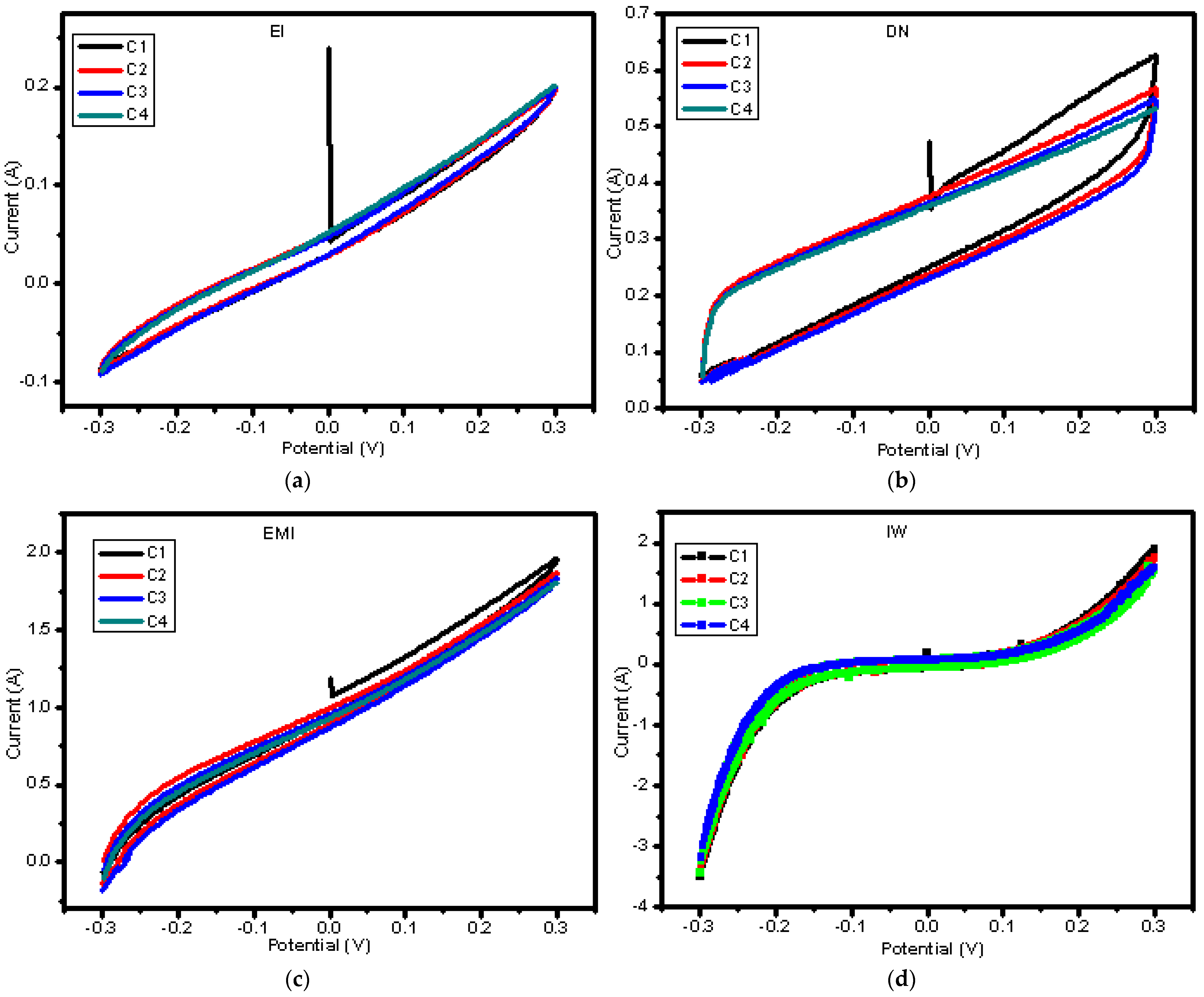
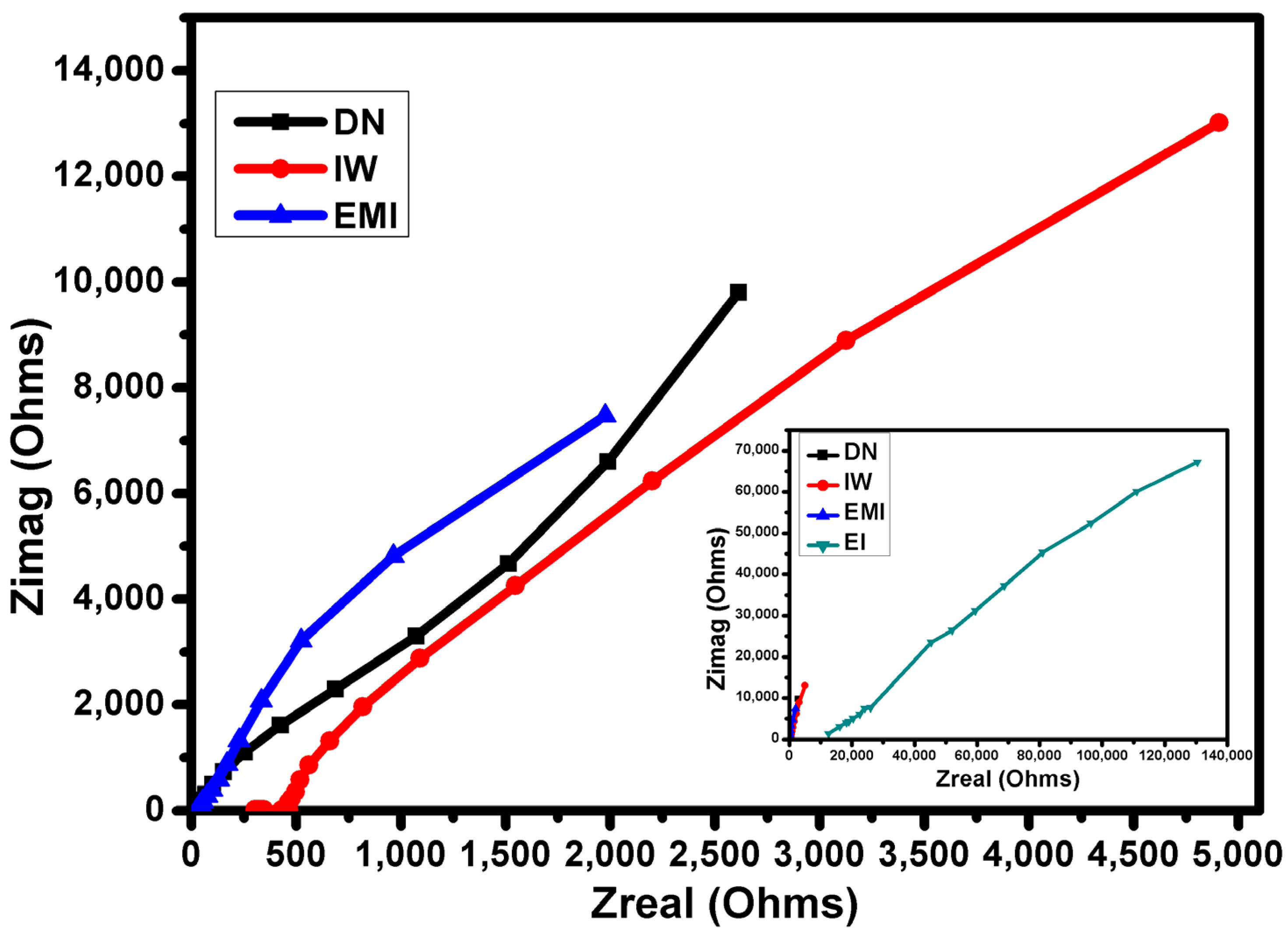
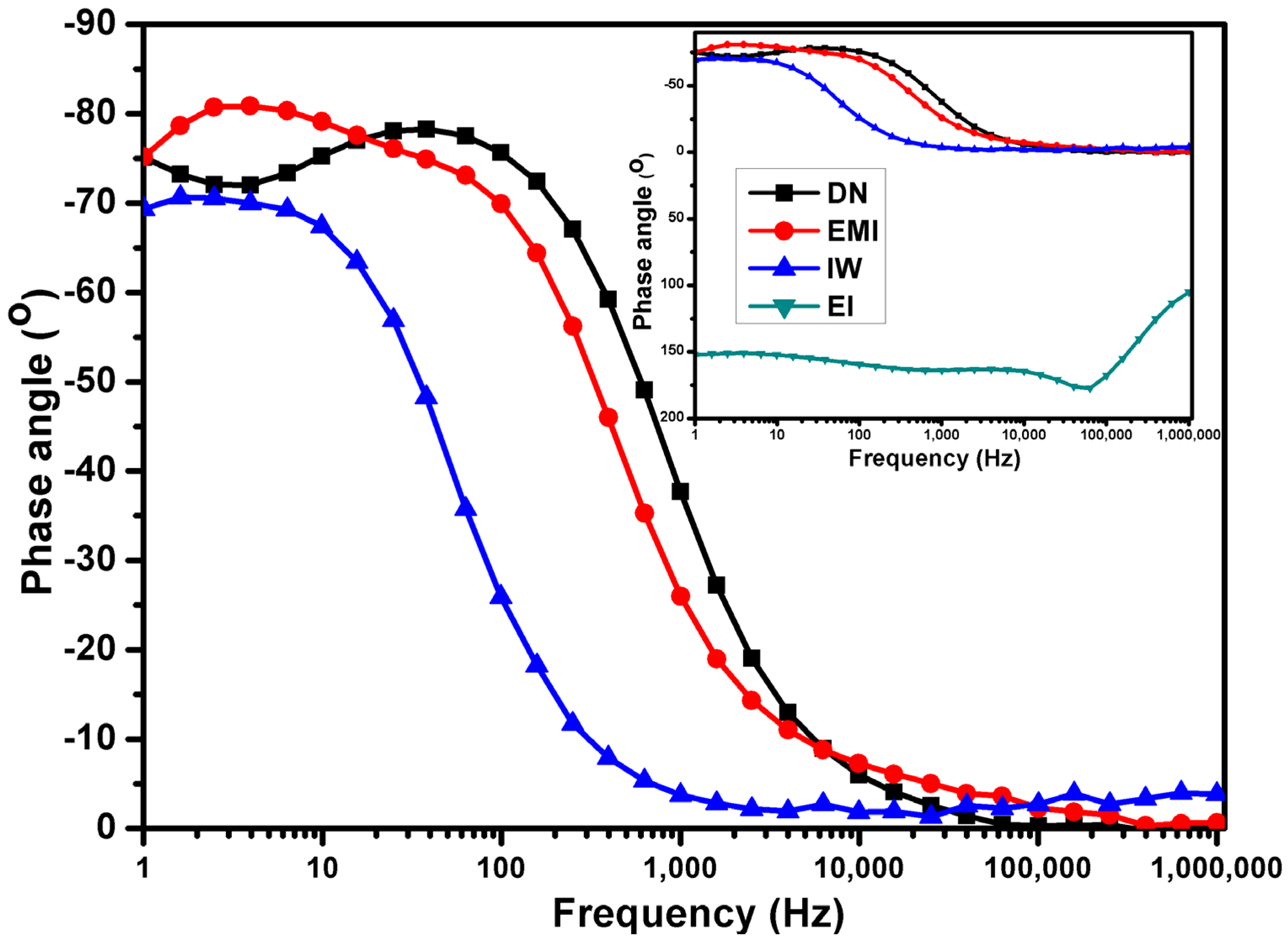
| Mixed Solvent | pH | Temperature (°C) | Electric Potential (mV) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| EI | 6.23 | 2.57 | 20.3 | 20.6 | 39 | 245 |
| IW | 6.29 | 2.83 | 19.08 | 21.3 | 35 | 231 |
| DN | 9.62 | 6.74 | 21.8 | 22.9 | −156 | 9 |
| EMI | 6.58 | 2.81 | 19.7 | 21.2 | 17 | 232 |
| Solvent System | Resistance (Ω) | Conductivity (S·cm−1) |
|---|---|---|
| EMI | 1978 | 6.42 × 10−6 |
| EI | 130400 | 9.74 × 10−8 |
| DN | 2613 | 4.86 × 10−6 |
| IW | 4901 | 2.59 × 10−6 |
| Solvent System | Solvent Type | Proton Conductivity (S·cm−1) | Structural Stability | Electrochemical Behavior |
|---|---|---|---|---|
| EMI | Polar protic | Highest | Good | Stable CV response; low EIS impedance |
| EI | Polar protic | Lowest | Moderate | High impedance; poor CV response |
| IW | Polar protic | Low | Degraded | Moderate CV; resistive EIS behavior |
| DN | Polar aprotic | Moderate | Good | Moderate impedance; decent CV performance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mdleleni, L.; Mlala, S.; Naki, T.; Meyer, E.L.; Agoro, M.A.; Rono, N. Characterization of Perfluoro Sulfonic Acid Membranes for Potential Electrolytic Hydrogen Production and Fuel Cell Applications for Local and Global Green Hydrogen Economy. Fuels 2025, 6, 63. https://doi.org/10.3390/fuels6030063
Mdleleni L, Mlala S, Naki T, Meyer EL, Agoro MA, Rono N. Characterization of Perfluoro Sulfonic Acid Membranes for Potential Electrolytic Hydrogen Production and Fuel Cell Applications for Local and Global Green Hydrogen Economy. Fuels. 2025; 6(3):63. https://doi.org/10.3390/fuels6030063
Chicago/Turabian StyleMdleleni, Lihle, Sithenkosi Mlala, Tobeka Naki, Edson L. Meyer, Mojeed A. Agoro, and Nicholas Rono. 2025. "Characterization of Perfluoro Sulfonic Acid Membranes for Potential Electrolytic Hydrogen Production and Fuel Cell Applications for Local and Global Green Hydrogen Economy" Fuels 6, no. 3: 63. https://doi.org/10.3390/fuels6030063
APA StyleMdleleni, L., Mlala, S., Naki, T., Meyer, E. L., Agoro, M. A., & Rono, N. (2025). Characterization of Perfluoro Sulfonic Acid Membranes for Potential Electrolytic Hydrogen Production and Fuel Cell Applications for Local and Global Green Hydrogen Economy. Fuels, 6(3), 63. https://doi.org/10.3390/fuels6030063











