Abstract
Plugs of lignite coal from multiple formations were subjected to a series of tests to determine the amount of rare-earth elements (REEs) to be extracted from coal in an in situ mining operation. These tests were used to determine if extraction of REEs and other critical minerals in an in situ environment would be possible for future attempts as an alternative to extraction mining. The tests involved subjecting whole lignite coal plugs from the Twin Butte coal seams in North Dakota to flow-through tests of water, and concentrations of 1.0 M ammonium nitrate, 1.0 M and 1.5 M sulfuric acid, and 1.0 M and 1.5 M hydrochloric acid (HCl) solvents at different concentrations and combinations. The flow-through testing was conducted by alternating the solvent and water flow-through to simulate an in situ mining scenario. The samples were analyzed for their concentrations of REEs (lanthanum [La], cerium [Ce], praseodymium [Pr], neodymium [Nd], samarium [Sm], europium [Eu], gadolinium [Gd], terbium [Tb], dysprosium [Dy], holmium [Ho], erbium [Er], thulium [Tm], ytterbium [Yb], lutetium [Lu], yttrium [Y], and scandium [Sc], as well as germanium [Ge] and cobalt [Co], manganese [Mn], nickel [Ni], and barium [Ba]). Results from the testing showed that REEs were extracted in concentrations that were on average higher using sulfuric acid (8.9%) than with HCl (5.8%), which had a higher recovery than ammonium nitrate. Tests were performed over a standard time interval for comparison between solvents, while a second set of testing was done to determine recovery rates of REEs and critical minerals under certain static and constant flow-through times to determine extraction in relation to time. Critical minerals had a higher recovery rate than the REEs across all tests, with a slightly higher recovery of light REEs over heavy REEs.
1. Introduction
Rare-earth elements (REEs) are crucial materials in an incredible array of consumer goods, energy system components, and military defense applications because of their unique properties. Global production and the entire value chain of REEs are dominated by China [1], with the United States currently mining approximately 12% of the global supply of mineral concentrates, all of which are exported. Further, the supply of REEs is majority produced in China from a single resource (ion-adsorbed clays) that is projected by some experts to last only another 10 to 20 years [2,3]. The United States currently considers the REE market an issue of national security. As such, the U.S. Department of Energy (DOE) and several other federal agencies are funding research to identify alternative domestic sources of REEs along with developing the methods to extract and produce them. As part of this effort, the DOE has determined that coal and coal by-products are potential alternative REE sources.
Previous work by the University of North Dakota (UND) and the Energy & Environmental Research Center (EERC) to extract REEs from coal has shown that there is a significant amount of potentially recoverable REEs and other critical metals in coal [4,5,6,7,8]. REEs have been shown to be authigenic minerals in coal. These minerals are typically concentrated in the clay matter of coal seams or within seam partings and margins [9]. Work conducted by Laudal [9] has shown low pH solutions to have high recovery rates of REEs in processed lignite coals. Their data combined with inferred evidence and literature indicates that the REEs in North Dakota lignite appear to be primarily associated with the organic matrix. They determined that the primary mode of occurrence of REEs in the lignite coal was present as ion-exchangeable cations, as organic complexes, in acid-soluble mineral forms, and in non-soluble inorganic forms. Their work utilizing chemical fractionation showed that about 80–95% of the REEs in North Dakota lignite are extractable through HCl leaching in the process, with the remaining REEs associated in non-soluble minerals, such as clays, silicates, sulfides or others.
To date, the explored methods of extraction require the coal to be mined from the ground and processed further through crushing, grinding, and preparation prior to leaching to extract the REEs. However, many coal seams that contain high levels of REEs occur in areas that may either be too deep for conventional surface mining or have other mitigating factors that prohibit conventional mining. Additionally, the cost and time required to open a new coal mine may be prohibitive. New mines tend to be severely limited by the economic restrictions on recovering deep coal seams or environmental restrictions on surface mining and processing. The present study seeks to optimize extraction approaches for North Dakota lignite, and one alternative REE extraction technique that may address both limitations is in situ leaching (ISL).
ISL, also known as in situ recovery (ISR) or solution mining, is a process by which a solution, also known as a lixiviant in the hydrometallurgical processing of ores, is injected into an ore body that leaches out the target mineral (s) from the ore, and the mineral-laden solution is brought to the surface from a production well. The mineral-laden solution, also known as a pregnant lixiviant, is then processed to remove the targeted minerals. ISL may be a desirable alternative to other mining methods such as open-pit and shaft mining because it only recovers the minerals from the ore and does not produce tailings. ISL is sometimes referred to as “green mining” because it results in minimal disturbance to environmental structures [10]. The potential benefit to using ISL as opposed to leaching surface-mined coal is the ability to reach coal seams that may be too deep to be economically minable and the ability to extract REEs in coal when surface mining is not desirable for environmental reasons. Outside of managing waste streams, ISL also requires remediation of the groundwater in the ore body to bring the groundwater back to prior ISL conditions. The goal of this research is to develop the understanding of the potential for ISL to be used in the extraction of REEs from lignite coal as opposed to conventional surface mining methods to collect coal and extract coal through leaching processes, as with Laudal [9].
2. Materials and Methods
2.1. Coal Samples for Bench-Scale Extraction
Coal samples were collected for utilization in a bench-scale extraction system to test the potential of ISL for REE recovery from lignite coal. A large sample block of the Twin Butte coal seam was acquired from the North American Coal Corporation Freedom Mine near Beulah, North Dakota.
The Twin Butte seam is in the Sentinel Butte Formation, Fort Union Group. The Twin Butte is a multiseam coal bed that occurs in isolated deposits under only the highest elevations and is currently mined at the Freedom Mine, operated by North American Coal, in Mercer County, North Dakota. The concentration of REEs in the Twin Butte comes from the uppermost portion of the coal seam and shows REE concentrations >300 parts per million (ppm) [4].
To simulate conditions for in situ extraction of REEs and critical minerals from lignite coal seams, the EERC modified a Hoek cell to accommodate fluid flow-through. Hoek cells comprise a steel body and two steel end caps, which are screwed to the body of the cell when in use and a coupling used to provide confining pressure to samples. Hoek cells are used to measure the strength of cylindrical rock specimens, which are subjected to triaxial compression. In this project, the Hoek cell was used to confine the coal sample while allowing solution to flow through the permeable sample at the desired rates. A generalized conceptual illustration of the procedure utilizing gravity is found in Figure 1. This project system had to be under pressure and gravity flow was not sufficient. A Hoek cell designed to provide high-pressure confinement using a nonreactive silicone gasket to hold the coal plug was used to ensure flow through the coal plug. Modifications were made to the Hoek cell by machining and installing polyetheretherketone (PEEK) platens, allowing fluid to flow through the sample plug and into a collection vessel for mass balance calculations. This is shown schematically in Figure 2 and Figure 3.
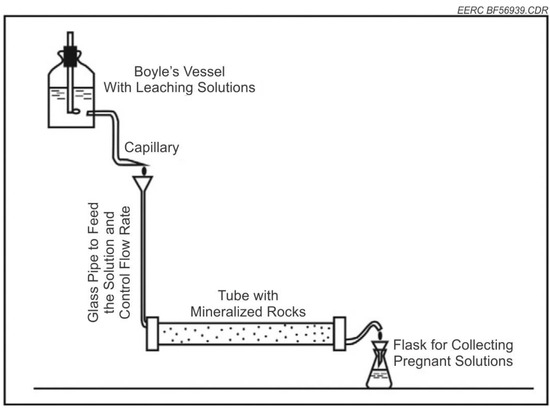
Figure 1.
Conceptual scheme of laboratory testing [11].
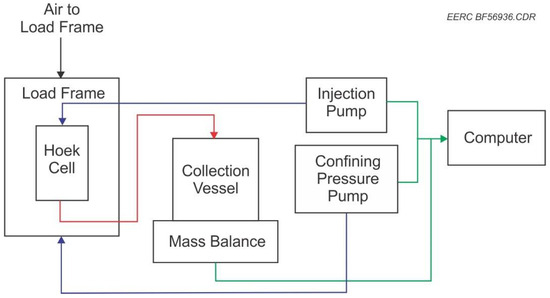
Figure 2.
Simple diagram of flow-through system designed and built.
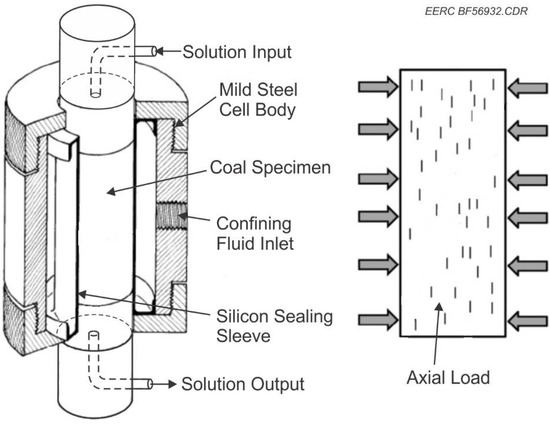
Figure 3.
Schematic of modified Hoek cell.
Confining pressure was applied to the silicone sealing sleeve inside the Hoek cell by a pump using distilled water. A computer measured and controlled the pressure in the pump applying confining pressure, as well as the flow rate for the pump injecting the solution into the coal plug. The computer also recorded the mass balance of collected fluid and axial load. Axial load is needed to be applied to ensure proper flow through the coal plug. This was accomplished by applying a load on the platens using compressed nitrogen. To control the flow rate of solution through the coal plugs, a pump designed to handle low-pH solutions was used. The use of a pressure release system was incorporated into the testing system in the case of backpressure buildup which would trigger at 500 psi (3447 kpa) to reduce the risk of failure and release of solvents from the system during testing.
2.2. Sample Preparation
For testing in the flow-through system, 30 mm diameter coal plugs were taken from the coal samples. Horizontal plugs were taken from the larger sample blocks of coal from the Twin Butte seam using a drill press and 30 mm coring bit. Because of the frangible nature of the coal, the number of samples taken from an individual sample block was limited to four to five plugs before the plugs would lose integrity.
Using a drill press, the coal plugs were drilled from the sample blocks of coal using a low flow of distilled water as cutting fluid. The 30 mm plugs tended to hold integrity during the drilling phase but would become detached and lodged in the 30 mm drill bit, requiring extra steps to remove the plugs. At times, this led to damage of the plug and potential loss of volume of the plug for testing. The drilled plug could also stay attached to the coal sample block after drilling and, in the process of breaking the plug free from the sample block, would result in fracture, causing damage and potential loss of the plug or plug volume.
After the plugs were taken from the larger piece of coal, they were covered in PVC shrink wrap to maintain sample integrity before testing. Careful consideration is given when applying the shrink wrap as it is activated using heat and too much exposure to heat when applying the shrink wrap can cause the coal plug to dehydrate, which causes dehydration fractures. The shrink-wrapped plugs were then placed inside a sealed container with a few milliliters of distilled water to keep the humidity of the coal sample high. Without proper humidity, dehydration cracks develop quickly, making the sample plug questionable for testing.
To prepare the plugs for testing, the ends of the plugs were cut perpendicular to the plug axis and sanded down manually to sit flush with the platens in the Hoek cell. The shrink wrap around the sample was removed just prior to inserting the plug into the Hoek cell. Measurements of the plug were taken to determine both its bulk volume and pore volume, to calculate how much fluid in milliliters the sample can contain. The average length of the prepared coal plugs was 25 mm with an average weight of approximately 23 g. Porosity testing of the coal plugs causes multiple dehydration cracks to form in the plugs, making them unusable for a flow-through test. Due to the susceptibility of dehydration cracks during porosity measurement, porosity measurements were taken from a single plug for each of the main coal sample blocks and used as a representative porosity measurement for the remaining coal plugs. The porosity was then measured using a helium porosimeter. Porosity measurements for the Twin Butte sample were ~8.5%. To account for potential variability on the porosity in coal plugs a porosity value of 10.0%, rounded up from ~8.5%, was used to calculate the pore volume and amount of solution used for testing in the Twin Butte samples to account for potential variability in porosity.
The silicone sealing sleeve was loaded into the Hoek cell and the end caps screwed into place. The assembled Hoek cell was then filled with distilled water that filled the void space between the gasket and the Hoek cell and provided the confining pressure. The prepared coal plug was then inserted into the silicone sealing sleeve in the Hoek cell with the upper and lower platens placed at either end. Once the platens were in place, the Hoek cell was then loaded into the load frame. The platens and Hoek cell were then centered so that the axial load was applying pressure directly through the center of the platens. The axial load was then applied to the platens using nitrogen gas, which pushed a plunger down to the platens. A load sensor on the end of the plunger accurately measured the pressure being applied to the cell in the form of psi. Next, confining pressure was applied to the sample by pumping water into the Hoek cell from the confining pressure pump. Once the confining pressure stabilized, the flow rate on the solution pump was manually set. The confining pump was controlled by a computer, and the injection pump and mass balance data were recorded through the computer.
2.3. Methods
Testing of bench-scale simulated in situ extraction of REEs and metals from lignite coal focused on varying the acid strength and type as well as variations in contact time and pressure. Testing was generally performed in three stages:
- Stage 1: 2 pore volumes of distilled water passed through coal samples;
- Stage 2: 3 pore volumes of solution passed through coal samples;
- Stage 3: 3 pore volumes of distilled water passed through coal samples.
One test was performed in five stages:
- Stage 1: 2 pore volumes of distilled water passed through coal samples;
- Stage 2: 3 pore volumes of 1.0 M ammonium acetate passed through coal samples;
- Stage 3: 3 pore volumes of distilled water passed through coal samples;
- Stage 4: 3 pore volumes of 1.0 M hydrochloric acid (HCl) passed through coal samples;
- Stage 5: 3 pore volumes of distilled water passed through coal samples.
For static residence time tests, Stage 2 would have 1 pore volume passed through the coal, the second pore volume would flow into the sample and then be held static for the residence time frame, and a third pore volume to flow through the plug after the residence time had been reached.
Different acids under different strengths were tested with one test per acid and strength to see what, if any, recovery could be achieved with different acids and strengths. The acids and their strengths were chosen based on the work by Laudal [9]. They determined the REEs in lignites were easily extracted by diluting HCl in their processes with H2SO4 having the best REE recovery per volume over HCl also showing large increases in recovery between 0.5 M and 1.0 M concentrations. HCl varied between 1.0 and 1.5 M solution, and sulfuric acid (H2SO4) was tested using 0.5 and 1.0 M solutions. A 1.0 M ammonium acetate was tested as a preflush to 1.0 M HCl to see the effects of an alkaline solution on the coal before flowing an acid through the sample. This ammonium acetate preflush was chosen to determine if results from Laudal [9] would show similar changes in the distribution of REEs extractions between the heavy and light REEs.
Static testing of the 1 M HCl as a solvent in contact with the sample was conducted over intervals of 18, 24, 42, and 72 h of static residence time with the coal. An additional test with a constant flow of HCl over an 18 h period was tested for comparison to the static test. For this test, the effective flow time was created by programming the injection pump to pulse on and off to have an effective flow rate of 0.0045 mL/min, which kept the HCl in contact with the coal for approximately 18 h but still moved the fluid through the coal sample.
A test was also performed to evaluate the effects of solutions flowing through coal under a confining pressure representative of a depth of ~2000 feet. Using a confining pressure of 850 psi (5860 kpa), a continuous flow-through test was conducted at 0.03 mL/min of fluid with Stage 1 flowing 2 pore volumes of H2O, Stage 2 flowing 5 pore volumes of 1 M HCl, and Stage 3 flowing 3 pore volumes of H2O. Table 1 lists the tests conducted, which include a test with water only as a solution and tests of the floor and roof material of the Twin Butte coal seam.

Table 1.
Bench-scale in situ test matrix.
REE concentrations in the fluid collected after the flow-through of each coal plug as well as the REE concentration remaining in the coal plug were analyzed using inductively couple plasma– mass spectrometry (ICP–MS). Before running the coal plugs through the ICP–MS after in situ testing, they were dried and washed. The samples were analyzed for REEs (lanthanum [La], cerium [Ce], praseodymium [Pr], neodymium [Nd], samarium [Sm], europium [Eu], gadolinium [Gd], terbium [Tb], dysprosium [Dy], holmium [Ho], erbium [Er], thulium [Tm], ytterbium [Yb], lutetium [Lu], yttrium [Y], and scandium [Sc], as well as germanium [Ge] and cobalt [Co]. The elements manganese [Mn], nickel [Ni], and barium [Ba] were added to the list of elements analyzed in later samples for the purpose of the high economic interest.
3. Results
3.1. Varying Solutions
Figure 4 shows the percent recovery of metals in the coal from the pregnant lixiviant on a whole coal basis as tested. The 0.5 M H2SO4 solution averaged a recovery rate of 8.9% for REEs. This was higher than the 1.5 M HCl solution test, with a recovery rate of 5.8% for REEs, but due to the limited number of tested conducted the true significance of this difference cannot be realized. Laudal [5] notes that tests on lignite coal showed a higher recovery rate of REEs from coal using 1.0 M HCl compared to 0.5 M H2SO4. So the differences here would need further testing for understanding of the difference in recovery. It was also shown that the ash content of the coal samples after testing dropped by 32% with the 1.0 M HCl compared to 2% with the 1.0 M H2SO4. Notable differences in recovery between the acids and the use of an ammonium acetate preflush are shown. While the ammonium acetate preflush sample yielded less REEs than the other HCl tests, it showed a noticeable increase in recovery in Sc, which had very poor recovery with the rest of the tests.
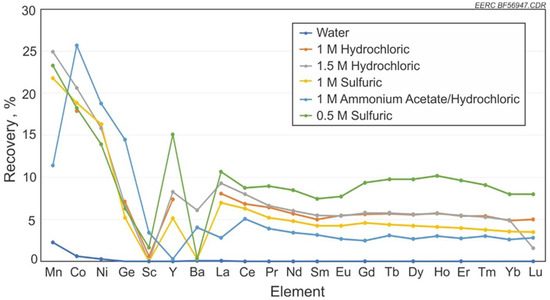
Figure 4.
Recovery rates of REEs and other metals with varying acids and strengths, and alkaline solutions.
The ammonium acetate preflush and HCl sample also showed significant decreases in recovery of Mn and Y but increased recovery of Co, Ni, and Ge when compared to the other solutions. The recovery rates between each solution show a similar pattern of recovery across all the lanthanides. The largest variation came from recovery of Mn, Y, and Ba. Only one sample was tested per solution, which could factor in the possibility that the change in recovery rates may be due to variation in surface area, permeability, or other factors of individual samples.
3.2. Residence Time Testing
The test results for recovery versus residence time of solution in contact with the coal are shown in Figure 5 for the individual elements and an average of all elements recovered versus time in Figure 6. Results show that under a static residence scenario, the recovery rate reaches a high at 18 h and then steadily drops with longer residence time. REE extraction from coals and coal by-products and subsequent retention of the REEs in the aqueous phase are sensitive to the pH of the solution.
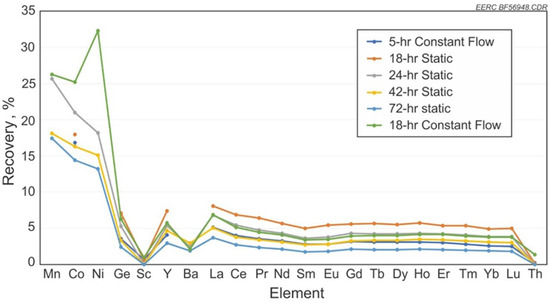
Figure 5.
Static contact, flow-through contact, and time using HCl.
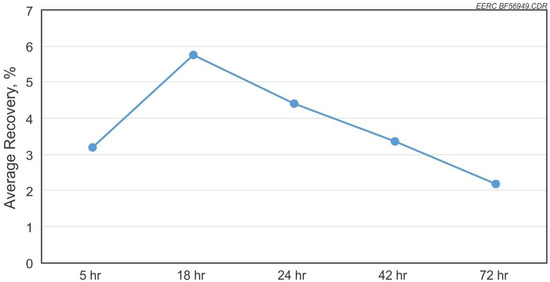
Figure 6.
The average recovery rate for REEs versus change in residence time.
Test results for static contact of the solution 1 M HCl compared to flow-through contact and time are shown in Figure 5. As discussed previously, the best recovery was at 18 h at a static contact. Constant flow of HCl for 18 h shows slightly less recovery of REEs but an increase in Co and Ni recovery compared to the static tests.
3.3. High Confining Pressure
During all of the tests, the confining pressure was held at 60 psi (413 kpa) and axial pressure kept slightly higher to keep the silicone sealing sleeve in place. A test was performed with an increased confining pressure to simulate the pressure expected at approximately a 2000-foot (610 m) depth representing a deep unminable coal seam. As described earlier, this was a three-stage test, with the solution flow rate set to 0.03 mL/min. At the confining pressure of 850 psi (5860 kpa) during Stage 1, the system was measuring a backpressure of 220 psi (1516 kpa) and held constant at that pressure through the beginning of Stage 2. When the HCl began to contact the coal, the backpressure began to increase to the point where the flow rate had to be dropped to 0.01 mL/min to keep from hitting the safety pressure release system which triggers at 500 psi (3447 kpa) of backpressure. The backpressure on the system stabilized around ~140 psi (965 kpa) at this reduced flow rate. The flow rate continued at 0.01 mL/min until the flush water from Stage 3 was flowing through the coal. At this point, the flow rate was increased to determine if water flow could be maintained at the original rate of 0.03 mL/min without exceeding the 500 psi (3447 kpa) limit. However, at flow rates of 0.03 mL/min, system backpressure rose to near the limit, and flow rates had to be reduced. This reduction in permeability in the coal plug indicates that the HCl causes some swelling or damage to the permeability, and this will need to be considered in a full-scale scenario. More work needs to be done to ascertain the effect of other solvents to extract the REEs as they may also have a negative effect on coal permeability.
4. Discussion
The results of the testing do show promising potential for REE recovery from coal using ISL. The observed variability in recovery rates among different acids and concentrations highlights additional potential through optimizing concentration levels, which could enhance recovery efficiency. Furthermore, the differences in acid types influencing the distribution of recovered REEs suggest that exploring alternative lixiviants may further increase the overall recovery of REEs in a potential ISL operation.
The amount of research, however, is limited due to the scope of the testing done as it was only meant to show if any recovery could be possible from lignite coal using in situ methods. To that end there is limited data on varying other circumstances in recovery. It has been shown through the work by Murphy and others [12] that other coal seams in the Fort Union group have high concentrations of REEs in coal and we do not know how in situ extraction would work on other coal seams as only one sample from one other coal seam was tested. More testing would then need to be conducted on different lignite coal seams to see the variability in extraction of REEs using in situ methods. Variabilities in these seams in extraction could come from numerous different factors, such as petrophysics, depositional environment, or REE distribution within the coal seams.
Along with the limits in coal seam testing we also are limited on the mechanisms of extraction through different lixiviants. Difference in the extraction between different concentrations of acids and types is shown in testing, but only a limited number of tests were able to be performed for this initial research. More testing on the lixiviants used in this research plus potentially other lixiviants outside of the ones used here would need to be tested more thoroughly to understand their extraction mechanisms and see what could be improved upon to increase extraction rates and efficiencies.
While this work focuses on the recovery of rare earth elements (REEs) from unminable seams, there is potential in applying ISL to minable coal seams. This approach yields benefits by reducing ash content in coal samples post-testing. Laudal [9] observed that HCl leachate effectively removes high levels of non-REE elements compared to H2SO4 in lignite coal which was replicated in this work where both acid tests saw decrease in ash content. By implementing ISL in coal seams prior to coal extraction for fuel, it could result in lower ash content and enhance the quality of the coal for fuel. This presents opportunities to expand the use of ISL beyond unminable lignite seams, broadening its application in the coal industry.
5. Conclusions
This work was a study of the potential of ISL for the recovery of REEs and critical metals from lignite coals. While preliminary, it illustrates the potential that ISL holds for extracting critical metals from coal seams that may be unminable because of depth or the potential of environmental damage from traditional mining techniques.
Stronger acid is not necessarily the key to recovery of REEs and minerals from coal, as shown by the higher recovery percentage from a lower-strength 0.5 M H2SO4 compared to stronger 1.5 M HCl. The use of the 0.5 M H2SO4 solution was able to recover the most out of the solvents tested, with an average recovery of the REEs of 8.9%. While this recovery average was less than the >50% recovery of REEs in the work done by Laudal [9] with 0.5 M H2SO4, it shows that it could hold potentially economic amounts of recoverable REEs and other critical minerals. The limited number of tested samples using H2SO4 and HCl as solvents leaves to be determined the true significance in the differences in recovery. Sc was the only element that did not have any significant recovery under any of the testing matrix parameters, except for the ammonium acetate preflush prior to HCl. Sc is one of the highest-value REEs. Alternatively, the recovery rate of the remaining REEs was much lower with the ammonium acetate preflush as opposed to the single-solution tests. REE extraction from coals and coal by-products and subsequent retention of the REEs in the aqueous phase are sensitive to the pH of the solution, and each coal system will need a tailored approach to determine acid type and strength for best results.
Author Contributions
Conceptualization, I.K.F. and B.C.F.; methodology, I.K.F.; validation, I.K.F.; formal analysis, I.K.F. and I.K.F.; investigation, I.K.F. and B.C.F.; writing—original draft preparation, I.K.F. and B.C.F.; writing—review and editing, I.K.F. and B.C.F.; funding acquisition, I.K.F. and B.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Industrial Commission of North Dakota for financial support of these investigations via the State Energy Research Center of the Energy & Environmental Research Center. The views and opinions of authors expressed herein do not necessarily state or reflect those of the Industrial Commission of North Dakota. The authors declare no competing financial interest.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Special thanks to I-Hsuan Ho at UND for his assistance in preparing this article. The Energy & Environmental Research Center, nor any of its subcontractors nor the Industrial Commission of North Dakota nor any person acting on behalf of either: (A) Makes any warranty or representation, express or implied, with respect to the accuracy, completeness, or usefulness of the information contained in this report, or that the use of any information, apparatus, method, or process disclosed in this report may not infringe privately owned rights; or (B) Assumes any liabilities with respect to the use of, or for damages resulting from the use of, any information, apparatus, method or process disclosed in this report. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the Industrial Commission of North Dakota. The views and opinions of authors expressed herein do not necessarily state or reflect those of the Industrial Commission of North Dakota.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| REE | Rare Earth Elements |
| HCl | Hydrochloric acid |
| DOE | Department of Energy |
| UND | University of North Dakota |
| EERC | Energy & Environmental Research Center |
| ISL | In Situ Leaching |
| ISR | In Situ Recovery |
| ppm | Parts per million |
| PEEK | Polyetheretherketone |
| H2SO4 | Sulfuric acid |
| ICP-MS | Inductively Couple Plasma– Mass Spectrometry |
References
- U.S. Geological Survey, 2020, Rare Earths—Mineral Commodity Summaries 2020. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-rare-earths.pdf (accessed on 15 September 2021).
- Bao, Z.; Zhao, Z. Geochemistry of mineralization with exchangeable REY in the weathering crusts of granitic rocks in South China. Ore Geol. Rev. 2008, 33, 519–535. [Google Scholar] [CrossRef]
- Chi, R.; Tian, J. Weathered Crust Elution-Deposited Rare Earth Ores; Nova Science Publishers: New York, NY, USA, 2008. [Google Scholar]
- Zygarlicke, C.J.; Folkedahl, B.C.; Nyberg, C.M.; Feole, I.K.; Kurz, B.A.; Theakar, N.L.; Benson, S.A.; Hower, J.; Eble, C. Rare-Earth Elements (REEs) in U.S. Coal-Based Resources—Sampling, Characterization, and Round-Robin Interlaboratory Study; Final Report for U.S. Department of Energy National Energy Technology Laboratory Cooperative Agreement No. DE-FE0029007, EERC Publication 2019-EERC-09-08; Energy & Environmental Research Center: Grand Forks, ND, USA, 2019. [Google Scholar]
- Laudal, D.A.; Benson, S.A.; Palo, D.; Addleman, R.S. Rare earth elements in North Dakota lignite coal and lignite-related materials. J. Energy Resour. Technol. 2018, 140, 062205. [Google Scholar] [CrossRef]
- Critical Minerals Sustainability Program, 2021, netl.doe.gov. Available online: https://www.netl.doe.gov/coal/rare-earth-elements/program-overview/background (accessed on 30 November 2021).
- Kruger, N.W.; Moxness, L.D.; Murphy, E.C. Rare Earth Element Concentrations in Fort Union and Hell Creek Strata in Western North Dakota; North Dakota Geological Survey Report of Investigation, no. 117; Department of Mineral Resources: Williston, ND, USA, 2017. [Google Scholar]
- Lin, R.; Soong, Y.; Granite, E.J. Evaluation of trace elements in U.S. coals using the USGS COALQUAL database version 3.0. Part I: Rare earth elements and yttrium (REY). Int. J. Coal Geol. 2018, 192, 1–13. [Google Scholar] [CrossRef]
- Laudal, D.A. Evaluation of Rare-Earth Element Extraction from North Dakota Coal-Related Feed Stocks. Ph.D. Thesis, University of North Dakota, Grand Forks, ND, USA, 2017. [Google Scholar]
- Haschke, M.; Ahmadian, J.; Zeidler, L.; Hubrig, T. In-situ recovery of critical technology elements. Procedia Eng. 2016, 138, 248–257. [Google Scholar] [CrossRef]
- Seredkin, M.; Zobolotsky, A.; Jeffres, G. In situ recovery, an alternative to conventional methods of mining—Exploration, resource estimation, environmental issues, project evaluation and economics. Ore Geol. Rev. 2016, 79, 500–514. [Google Scholar] [CrossRef]
- Murphy, E.C.; Moxness, L.D.; Kruger, N.W.; Maike, C.A. Rare Earth Element Concentrations in the Harmon, Hanson, and H Lignites in Slope County, North Dakota; Report of Investigation No. 119; North Dakota Geological Survey: Bismarck, ND, USA, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).