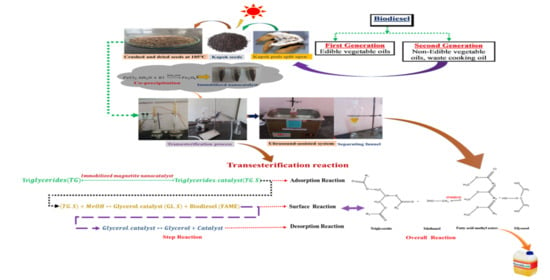
Ultrasonication Assisted Catalytic Transesterification of Ceiba Pentandra (Kapok) Oil Derived Biodiesel Using Immobilized Iron Nanoparticles
Abstract
:1. Introduction
2. Experimental Setup
2.1. Materials
2.2. Procedure for Synthesis of Magnetite Nanoparticles
2.3. Immobilization of Rhizopus-Oryzae Enzyme on Iron Nanoparticles
2.4. Oil Extraction from the Kapok Oilseeds
2.5. Physiochemical Properties of Kapok Oil
2.6. Catalytic Transesterification Assisted by Ultrasonication
3. Results and Discussion
3.1. Textural Characterization
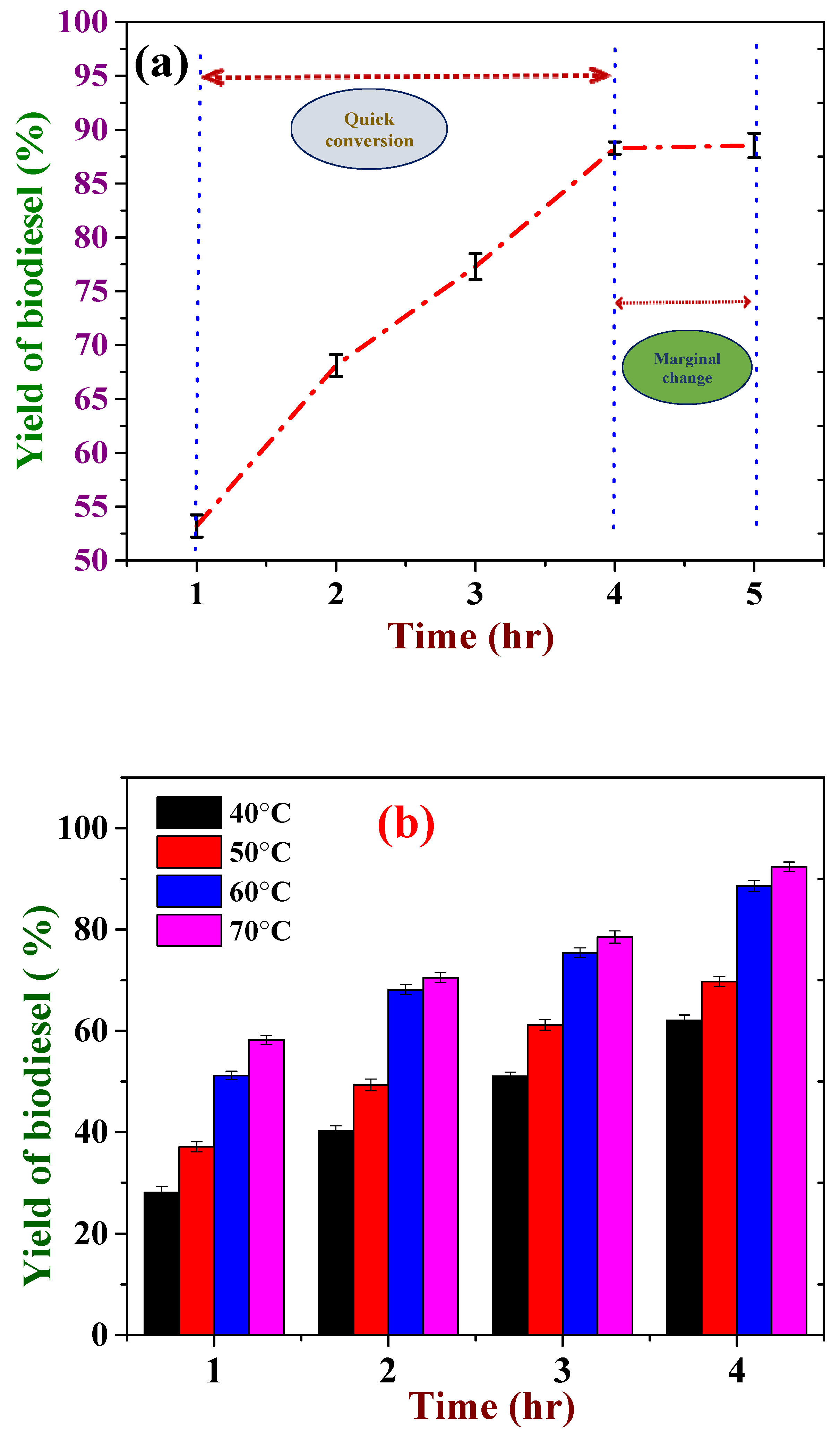
3.2. Temperature and Reaction Time Affecting the Yield of Biodiesel
3.3. Effect of Catalysts Loading on the Yield of Biodiesel
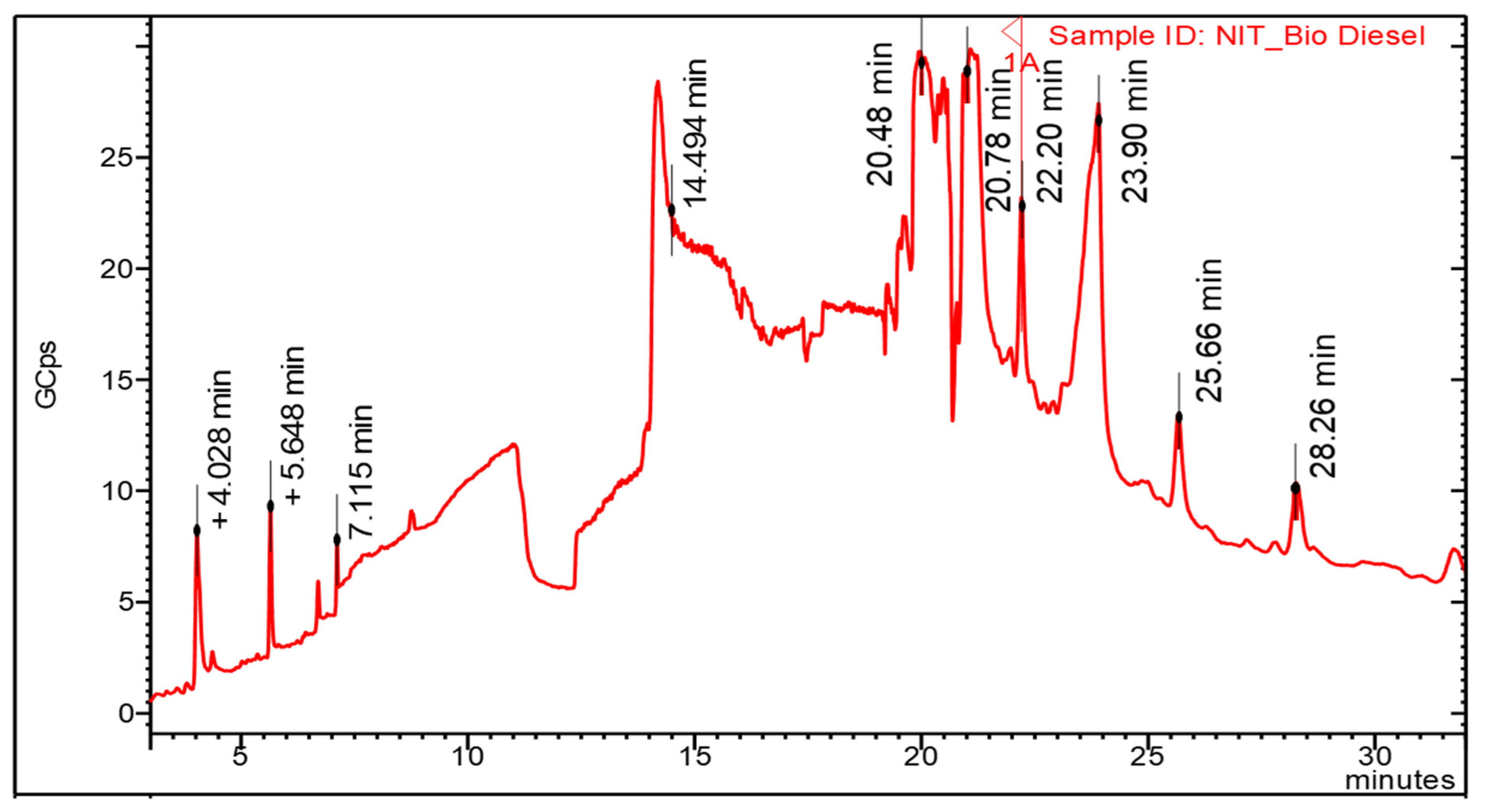
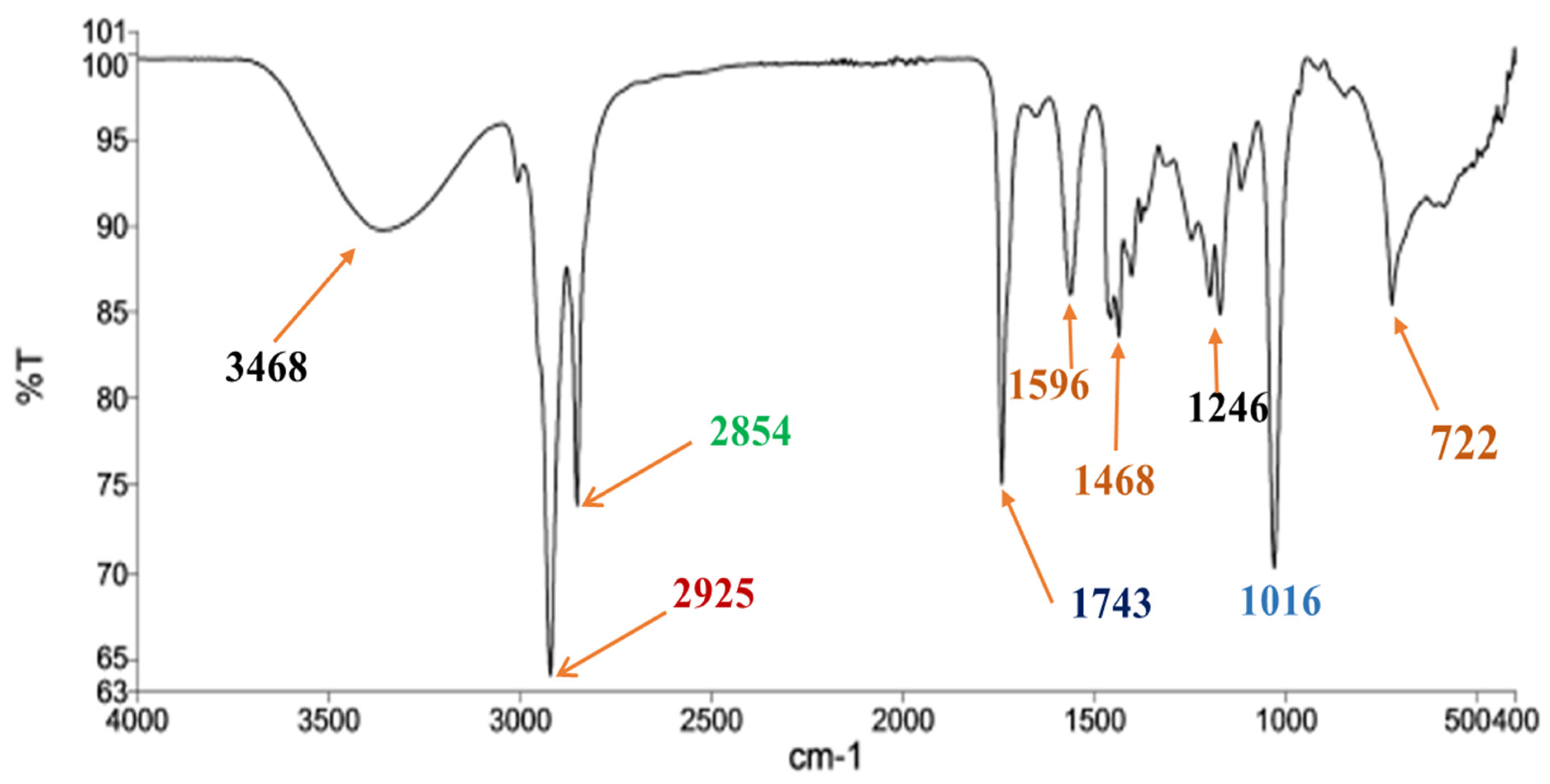
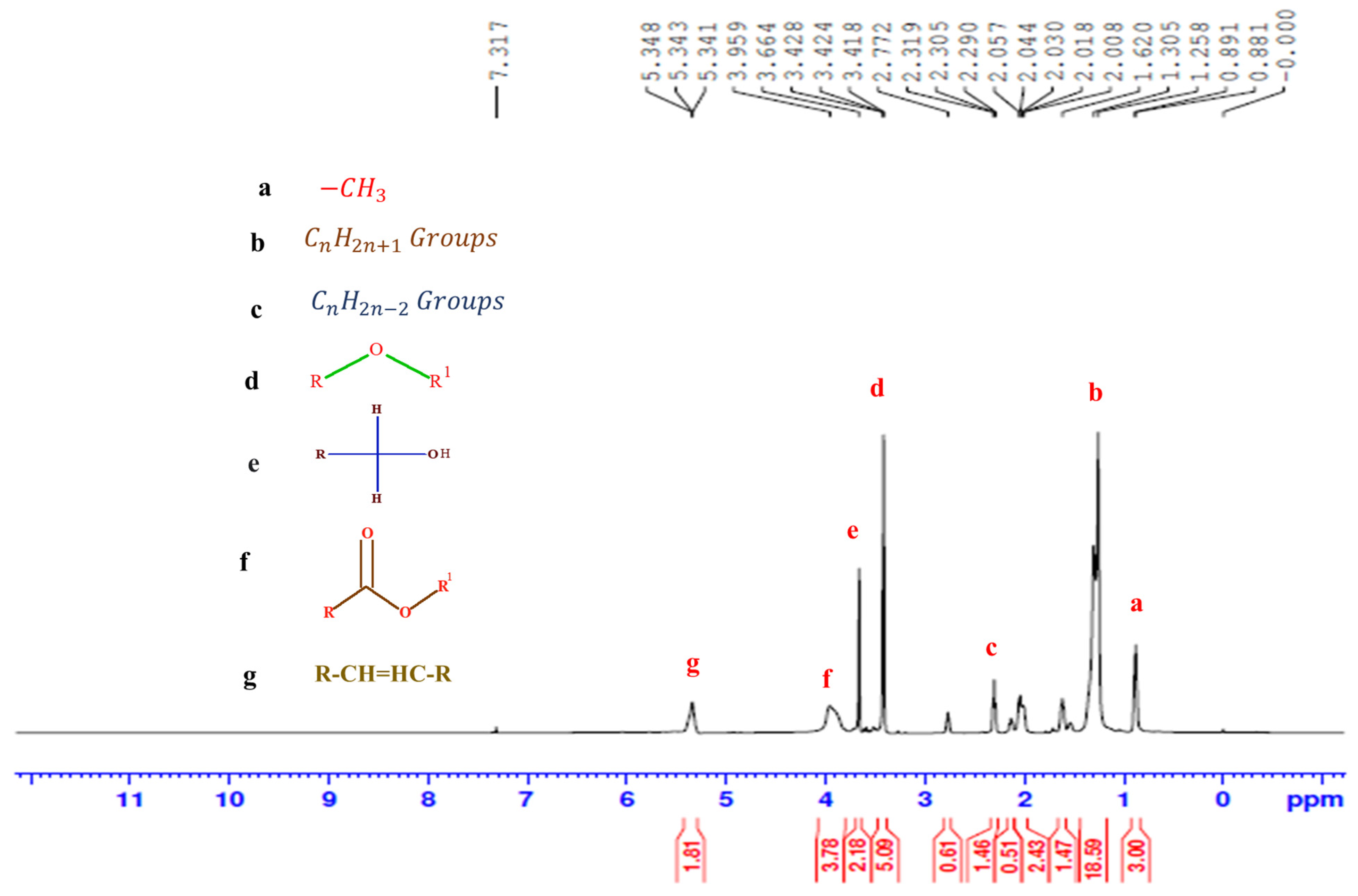
3.4. Characterizations of Kapok Biodiesel
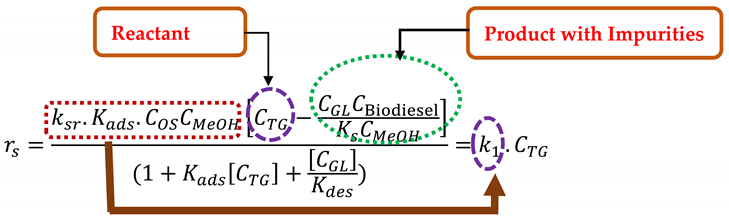
3.5. Kinetic and Thermodynamic Modeling of the Transesterification Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loo, D.L.; Teoh, Y.H.; How, H.G.; Teh, J.S.; Andrei, L.C.; Starčević, S.; Sher, F. Applications Characteristics of Different Biodiesel Blends in Modern Vehicles Engines: A Review. Sustainability 2021, 13, 9677. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.; Bhatia, S. Feasibility of Edible Oil vs. Non-Edible Oil vs. Waste Edible Oil as Biodiesel Feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Atabani, A.; Silitonga, A.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.; Mekhilef, S. A Comprehensive Review on Biodiesel as an Alternative Energy Resource and its Characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Shahid, E.M.; Jamal, Y. Production of Biodiesel: A Technical Review. Renew. Sustain. Energy Rev. 2011, 15, 4732–4745. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sindhanaiselvan, S.; Gandhi, N.N.; Devi, S.S.; Renganathan, S. Optimization and Kinetic Studies on Biodiesel Production from Underutilized Ceiba Pentandra Oil. Fuel 2013, 103, 693–698. [Google Scholar] [CrossRef]
- Jamaluddin, N.; Riayatsyah, T.M.I.; Silitonga, A.S.; Mofijur, M.; Shamsuddin, A.H.; Ong, H.C.; Mahlia, T.M.I.; Rahman, S.A. Techno-Economic Analysis and Physicochemical Properties of Ceiba Pentandra as Second-Generation Biodiesel Based on ASTM D6751 and EN 14214. Processes 2019, 7, 636. [Google Scholar] [CrossRef] [Green Version]
- Teoh, Y.; How, H.; Sher, F.; Le, T.; Nguyen, H.; Yaqoob, H. Fuel Injection Responses and Particulate Emissions of a CRDI Engine Fueled with Cocos Nucifera Biodiesel. Sustainability 2021, 13, 4930. [Google Scholar] [CrossRef]
- Vedharaj, S.; Vallinayagam, R.; Yang, W.; Chou, S.; Chua, K.J.; Lee, P. Experimental Investigation of Kapok (Ceiba pentandra) Oil Biodiesel as an Alternate Fuel for Diesel Engine. Energy Convers. Manag. 2013, 75, 773–779. [Google Scholar] [CrossRef]
- Cui, F.-J.; Zhao, H.-X.; Sun, W.-J.; Wei, Z.; Yu, S.-L.; Zhou, Q.; Dong, Y. Ultrasound-Assisted Lipase-Catalyzed Synthesis of D-isoascorbyl Palmitate: Process Optimization and Kinetic Evaluation. Chem. Cent. J. 2013, 7, 180. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kodgire, P.; Kachhwaha, S.S. Investigation of Ultrasound-Assisted KOH and CaO Catalyzed Transesterification for Biodiesel Production from Waste Cotton-Seed Cooking Oil: Process Optimization and Conversion Rate Evaluation. J. Clean. Prod. 2020, 259, 120982. [Google Scholar] [CrossRef]
- Veljković, V.B.; Avramović, J.M.; Stamenković, O. Biodiesel Production by Ultrasound-Assisted Transesterification: State of the Art and the Perspectives. Renew. Sustain. Energy Rev. 2011, 16, 1193–1209. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Activity of Solid Catalysts for Biodiesel Production: A Review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Biodiesel Production from Renewable Feedstocks: Status and Opportunities. Renew. Sustain. Energy Rev. 2012, 16, 4763–4784. [Google Scholar] [CrossRef]
- Batistella, L.; Lerin, L.A.; Brugnerotto, P.; Danielli, A.J.; Trentin, C.M.; Popiolski, A.; Treichel, H.; Oliveira, J.V.; de Oliveira, D. Ultrasound-Assisted Lipase-Catalyzed Transesterification of Soybean Oil in Organic Solvent System. Ultrason. Sonochem. 2012, 19, 452–458. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Ribeiro, D.S.; Couteiro, P.P.; Bastos, C.M.B.; de Queiroz, D.S.; Parreira, J.M.; Langone, M.A.P. Investigation of the Reuse of Immobilized Lipases in Biodiesel Synthesis: Influence of Different Solvents in Lipase Activity. Appl. Biochem. Biotechnol. 2016, 179, 485–496. [Google Scholar] [CrossRef]
- Amini, Z.; Ong, H.C.; Harrison, M.; Kusumo, F.; Mazaheri, H.; Ilham, Z. Biodiesel Production by Lipase-Catalyzed Transesterification of Ocimum basilicum L. (Sweet Basil) Seed Oil. Energy Convers. Manag. 2017, 132, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A Review on FAME Production Processes. Fuel 2010, 89, 1–9. [Google Scholar] [CrossRef]
- Gharat, N.; Rathod, V.K. Ultrasound Assisted Enzyme Catalyzed Transesterification of Waste Cooking Oil with Dimethyl Carbonate. Ultrason. Sonochem. 2012, 20, 900–905. [Google Scholar] [CrossRef]
- Santin, C.M.; Michelin, S.; Scherer, R.P.; Valério, A.; di Luccio, M.; Oliveira, D.; Oliveira, J.V. Comparison of Macauba and Soybean Oils as Substrates for the Enzymatic Biodiesel Production in Ultrasound-Assisted System. Ultrason. Sonochem. 2017, 35, 525–528. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, G.; Poonam; Singh, C. Ultrasonic-Assisted Transesterification of Jatropha Curcus Oil Using Solid Catalyst, Na/SiO2. Ultrason. Sonochem. 2010, 17, 839–844. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential Applications of Enzymes Immobilized on/in Nano Materials: A Review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef]
- Lerin, L.A.; Loss, R.A.; Remonatto, D.; Zenevicz, M.C.; Balen, M.; Netto, V.O.; Ninow, J.L.; Trentin, C.M.; Oliveira, J.V.; De Oliveira, D. A Review on Lipase-Catalyzed Reactions in Ultrasound-Assisted Systems. Bioprocess Biosyst. Eng. 2014, 37, 2381–2394. [Google Scholar] [CrossRef] [PubMed]
- Jegannathan, K.R.; Abang, S.; Poncelet, D.; Chan, E.S.; Ravindra, P. Production of Biodiesel Using Immobilized Lipase—A Critical Review. Crit. Rev. Biotechnol. 2008, 28, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, O.K.; Lee, E.Y. Nano-Immobilized Biocatalysts for Biodiesel Production from Renewable and Sustainable Resources. Catalysts 2018, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Al-Zuhair, S.; Ling, F.W.; Jun, L.S. Proposed Kinetic Mechanism of the Production of Biodiesel from Palm Oil Using Lipase. Process Biochem. 2007, 42, 951–960. [Google Scholar] [CrossRef]
- Moreira, K.D.S.; De Oliveira, A.L.B.; Lourembergue, S.d.M., Jr.; Monteiro, R.R.C.; Da Rocha, T.N.; Menezes, F.L.; Fechine, L.M.U.D.; Denardin, J.C.; Michea, S.; Freire, R.M.; et al. Lipase from Rhizomucor Miehei Immobilized on Magnetic Nanoparticles: Performance in Fatty Acid Ethyl Ester (FAEE) Optimized Production by the Taguchi Method. Front. Bioeng. Biotechnol. 2020, 8, 693. [Google Scholar] [CrossRef]
- Santos, S.; Puna, J.; Gomes, J.; Marchetti, J. A Review on the Use of Bio/Nanostructured Heterogeneous Catalysts in Biodiesel Production. Nano-Biocatal. Biodiesel Prod. 2021, 59–91. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Xie, W.; Ma, N. Immobilized Lipase on Fe3O4 Nanoparticles as Biocatalyst for Biodiesel Production. Energy Fuels 2009, 23, 1347–1353. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Immobilization of Candida Rugosa Lipase onto Graphene Oxide Fe3O4 Nanocomposite: Characterization and Application for Biodiesel Production. Energy Convers. Manag. 2018, 159, 42–53. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and Characterization of Biocompatible Fe3O4 Nanoparticles. J. Biomed. Mater. Res. Part A 2007, 80, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Ashkevarian, S.; Badraghi, J.; Mamashli, F.; Delavari, B.; Saboury, A.A. Covalent Immobilization and Characterization of Rhizopus Oryzae Lipase on Core-Shell Cobalt Ferrite Nanoparticles for Biodiesel Production. Chin. J. Chem. Eng. 2020, 37, 128–136. [Google Scholar] [CrossRef]
- Zhao, J.-F.; Lin, J.-P.; Yang, L.-R.; Wu, M.-B. Enhanced Performance of Rhizopus oryzae Lipase by Reasonable Immobilization on Magnetic Nanoparticles and Its Application in Synthesis 1,3-Diacyglycerol. Appl. Biochem. Biotechnol. 2019, 188, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.; Rasul, M.; Khan, M.; Sharma, S.C.; Mofijur, M.; Bhuiya, M. Prospects, Feedstocks and Challenges of Biodiesel Production from Beauty Leaf Oil and Castor Oil: A Nonedible Oil Sources in Australia. Renew. Sustain. Energy Rev. 2016, 61, 302–318. [Google Scholar] [CrossRef]
- Khalil, M.I. Co-Precipitation in Aqueous Solution Synthesis of Magnetite Nanoparticles Using Iron (III) Salts as Precursors. Arab. J. Chem. 2015, 8, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Azhar, O.; Jahan, Z.; Sher, F.; Niazi, M.B.K.; Kakar, S.J.; Shahid, M. Cellulose Acetate-Polyvinyl Alcohol Blend Hemodialysis Membranes Integrated with Dialysis Performance and High Biocompatibility. Mater. Sci. Eng. C 2021, 126, 112127. [Google Scholar] [CrossRef]
- Gholami, A.; Pourfayaz, F.; Maleki, A. Techno-Economic Assessment of Biodiesel Production from Canola Oil through Ultrasonic Cavitation. Energy Rep. 2020, 7, 266–277. [Google Scholar] [CrossRef]
- Yasvanthrajan, N.; Sivakumar, P.; Muthukumar, K.; Murugesan, T.; Arunagiri, A. Production of Biodiesel from Waste Bio-Oil through Ultrasound Assisted Transesterification Using Immobilized Lipase. Environ. Technol. Innov. 2020, 21, 101199. [Google Scholar] [CrossRef]
- Adewale, P.; Dumont, M.-J.; Ngadi, M. Enzyme-Catalyzed Synthesis and Kinetics of Ultrasonic-Assisted Biodiesel Production from Waste Tallow. Ultrason. Sonochem. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Karimi, M.; Keyhani, A.; Akram, A.; Rahman, M.; Jenkins, B.; Stroeve, P. Hybrid Response Surface Methodology-Genetic Algorithm Optimization of Ultrasound-Assisted Transesterification of Waste Oil Catalysed by Immobilized Lipase on Mesoporous Silica/Iron Oxide Magnetic Core-Shell Nanoparticles. Environ. Technol. 2013, 34, 2201–2211. [Google Scholar] [CrossRef]
- Kumar, G.; Kumar, D.; Johari, R.; Singh, C.P. Enzymatic Transesterification of Jatropha Curcas Oil Assisted by Ultrasonication. Ultrason. Sonochem. 2011, 18, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Mahapatra, P.; Garlapati, V.K.; Banerjee, R. Enzymatic Transesterification of Jatropha Oil. Biotechnol. Biofuels 2009, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-Lipase as Surface Functionalized Nano Biocatalyst for the Production of Biodiesel Using Waste Cooking Oil as Feedstock: Characterization and Process Optimization. Energies 2019, 13, 177. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Qin, X.; Li, D.; Yang, B.; Wang, Y. One-Step Synthesis of High-Yield Biodiesel from Waste Cooking Oils by a Novel and Highly Methanol-Tolerant Immobilized Lipase. Bioresour. Technol. 2017, 235, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Subhedar, P.B.; Gogate, P.R. Ultrasound Assisted Intensification of Biodiesel Production Using Enzymatic Interesterification. Ultrason. Sonochem. 2016, 29, 67–75. [Google Scholar] [CrossRef]
- Suslick, K. Applications of Ultrasound to Materials Chemistry. MRS Bull. 1995, 20, 29–34. [Google Scholar] [CrossRef]
- Karthickeyan, V. Effect of Thermal Barrier Coating on Performance and Emission Characteristics of Kapok Oil Methyl Ester in Diesel Engine. Aust. J. Mech. Eng. 2018, 18, 467–480. [Google Scholar] [CrossRef]
- Silva, L.M.; Filho, E.A.; Simpson, A.J.; Monteiro, M.R.; Venâncio, T. Comprehensive Multiphase NMR Spectroscopy: A New Analytical Method to Study the Effect of Biodiesel Blends on the Structure of Commercial Rubbers. Fuel 2015, 166, 436–445. [Google Scholar] [CrossRef]
- Norjannah, B.; Ong, H.C.; Masjuki, H. Effects of Methanol and Enzyme Pretreatment to Ceiba Pentandra Biodiesel Production. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 1548–1555. [Google Scholar] [CrossRef]
- Asokan, M.A.; Vijayan, R. Effective Conversion of Kapok Seed (Ceiba Pentandra) Oil into Biodiesel and Investigation of Effects of Catalyst Concentrations and Chromatographic Characterization. Int. J. Chem. Tech. Res. 2014, 6, 5709–5715. [Google Scholar]
- Rashid, U.; Knothe, G.; Yunus, R.; Evangelista, R.L. Kapok Oil Methyl Esters. Biomass-Bioenergy 2014, 66, 419–425. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Hu, J.; Zhou, H.; Adeleye, A.S.; Keller, A.A. 1H NMR and GC-MS Based Metabolomics Reveal Defense and Detoxification Mechanism of Cucumber Plant under Nano-Cu Stress. Environ. Sci. Technol. 2016, 50, 2000–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, A.; Lohan, P.; Jha, P.N.; Mehrotra, R. Biodiesel Production through Lipase Catalyzed Transesterification: An Overview. J. Mol. Catal. B Enzym. 2010, 62, 9–14. [Google Scholar] [CrossRef]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Inorganic Heterogeneous Catalysts for Biodiesel Production from Vegetable Oils. Biomass-Bioenergy 2011, 35, 3787–3809. [Google Scholar] [CrossRef]
- Feyzi, M.; Shahbazi, Z. Preparation, Kinetic and Thermodynamic Studies of Al–Sr Nanocatalysts for Biodiesel Production. J. Taiwan Inst. Chem. Eng. 2017, 71, 145–155. [Google Scholar] [CrossRef]
- Teixeira, R.A.; Lima, E.C.; Benetti, A.D.; Thue, P.S.; Cunha, M.R.; Cimirro, N.F.; Sher, F.; Dehghani, M.H.; dos Reis, G.S.; Dotto, G.L. Preparation of Hybrids of Wood Sawdust with 3-Aminopropyl-Triethoxysilane. Application as an Adsorbent to Remove Reactive Blue 4 Dye from Wastewater Effluents. J. Taiwan Inst. Chem. Eng. 2021, 125, 141–152. [Google Scholar] [CrossRef]
- Arun, G.; Ayoub, M.; Zulqarnain, Z.; Deshannavar, U.; Yusoff, M.H.M.; Farrukh, S.; Sher, F. Valorization of Solketal Synthesis from Sustainable Biodiesel Derived Glycerol Using Response Surface Methodology. Catalysts 2021, 11, 1537. [Google Scholar] [CrossRef]



| Oil extraction yield | 23% |
| Acid value | 18.541 mg KOH/g oil |
| Free fatty acid | 9.317% |
| Saponification Value | 186.813 mg KOH/g Fat |
| Specific gravity (at 20 °C) | 0.850 |
| pH | 5.0 |
| Kinematic viscosity (at 30 °C) | 40 cSt |
| Feedstock | Bio-Catalyst | Alcohol/Oil | Reaction Condition | Yield | Ref. |
|---|---|---|---|---|---|
| Waste cottonseed oil | Immobilized Rhizopus oryzae lipase | Ethanol/oil molar ratio: 4.5:1 | Temperature: 45 °C, Reaction time: 6 h Frequency: 20 kHz | 98.7% | [38] |
| Waste cooking oil | Immobilized enzyme Novozym 435 | Dimethyl carbonate/ oil molar ratio: 6:1 | Temperature: 70 °C, Reaction time: 4 h Frequency: 25 kHz | 86.61% | [18] |
| Waste tallow | Immobilized Candida antarctica lipase B (CALB) | Methanol/ fat molar ratio:8:1 | Temperature: 50 °C, Reaction time: 20 min Frequency: 20 kHz | 85.6 ± 0.08% | [39] |
| Canola oil | T. lanuginose lipase immobilized on mesoporous silica/superparamagnetic iron oxide | Methanol/oil molar ratio:4.34 | Reaction time: 6 h Frequency: 40 kHz | 83.77% | [40] |
| Jatropha curcas oil | Chromobacterium viscosum lipase on immobilized silica activated | Methanol/oil molar ratio:4:1 | Reaction time: 30 min Frequency: 30 kHz | 84.5 ±0.5% | [41] |
| Jatropha oil | E. aerogenes and Rhizopus oryzae3562 lipase immobilized on silica activated | Methanol/oil molar ratio:4:1 | Temperature: 55 °C, Reaction time: 48 h | 94% | [42] |
| Cooking oil | PDA-lipase immobilized on Fe3O4- | Methanol/oil molar ratio:6:1 | Temperature: 37 °C, Reaction time: 30 h | 92% | [43] |
| Kapok oil | Rhizopus-oryzae lipase immobilization on magnetic Fe3O4-NPs | Methanol/oil molar ratio:6:1 | Temperature: 60 °C, Reaction time: 4 h Frequency: 25 kHz | 93 ± 1.04% | This work |
| Retention Time (min) | Component Name | Molecularweight | Composition (%) |
|---|---|---|---|
| 4.028 | 13-Tetradecynoic acid, methyl ester | 238 | 1.283 |
| 5.648 | 13,16-Octadecadiynoic acid, methyl ester | 290 | 10.275 |
| 7.115 | Nonanoic acid, methyl ester | 172 | 2.209 |
| 14.494 | Pentadecanoic acid, methyl ester | 270 | 16.984 |
| 20.480 | 7-Hexadecenoic acid, methyl ester | 268 | 20.439 |
| 20.78 | Docosanoic acid, methyl ester | 354 | 1.607 |
| 22.20 | Tricosanoic acid, methyl ester | 368 | 3.992 |
| 23.90 | Tetracosanoic acid, methyl ester | 382 | 15.862 |
| 25.66 | Pentacosanoic acid, methyl ester | 396 | 1.988 |
| 28.26 | Hexacosanoic acid, methyl ester | 410 | 1.501 |
| Consecutive Reversible Reactions of Triglyceride | Equation | ||
|---|---|---|---|
 | (5a) | ||
| Step Reactions | Rate Equations | Equilibrium Constants | |
| Adsorption reaction | , | (5b) | |
| Surface reaction | , | (5c) | |
| Desorption reaction | (5d) | ||
| Overall reaction | (5e) | ||
| Temperature (°C) | Regression Equation | R2 | Rate Constant k1 (h−1) |
|---|---|---|---|
| 40 | y = 0.2124x + 0.1006 | 0.994 | 0.212 |
| 50 | y = 0.2459x + 0.2062 | 0.998 | 0.246 |
| 60 | y = 0.4622x + 0.2029 | 0.953 | 0.466 |
| 70 | y = 0.5423x + 0.1965 | 0.908 | 0.542 |
| Terms | Description | Value |
|---|---|---|
| k0 | Frequency factor (h−1) | 27,446.66 |
| Activation Energy (kJ mol−1) | 30.79 | |
| Enthalpy (kJ mol−1) | 28.06 | |
| Entropy (J mol−1 K−1) | −237.12 | |
| Gibbs free energy | (kJmol−1) | |
| 313 K | 102.28 | |
| 323 K | 104.65 | |
| 333 K | 107.02 | |
| 343 K | 109.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasawan, M.; Chen, S.-S.; Das, B.; Chang, H.-M.; Chang, C.-T.; Nguyen, T.X.Q.; Ku, H.-M.; Chen, Y.-F. Ultrasonication Assisted Catalytic Transesterification of Ceiba Pentandra (Kapok) Oil Derived Biodiesel Using Immobilized Iron Nanoparticles. Fuels 2022, 3, 113-131. https://doi.org/10.3390/fuels3010008
Pasawan M, Chen S-S, Das B, Chang H-M, Chang C-T, Nguyen TXQ, Ku H-M, Chen Y-F. Ultrasonication Assisted Catalytic Transesterification of Ceiba Pentandra (Kapok) Oil Derived Biodiesel Using Immobilized Iron Nanoparticles. Fuels. 2022; 3(1):113-131. https://doi.org/10.3390/fuels3010008
Chicago/Turabian StylePasawan, Mithileth, Shiao-Shing Chen, Bhanupriya Das, Hau-Ming Chang, Chang-Tang Chang, Thi Xuan Quynh Nguyen, Hong-Ming Ku, and Yue-Fang Chen. 2022. "Ultrasonication Assisted Catalytic Transesterification of Ceiba Pentandra (Kapok) Oil Derived Biodiesel Using Immobilized Iron Nanoparticles" Fuels 3, no. 1: 113-131. https://doi.org/10.3390/fuels3010008
APA StylePasawan, M., Chen, S.-S., Das, B., Chang, H.-M., Chang, C.-T., Nguyen, T. X. Q., Ku, H.-M., & Chen, Y.-F. (2022). Ultrasonication Assisted Catalytic Transesterification of Ceiba Pentandra (Kapok) Oil Derived Biodiesel Using Immobilized Iron Nanoparticles. Fuels, 3(1), 113-131. https://doi.org/10.3390/fuels3010008