Abstract
The water hyacinth (WH), also known as Eichhornia crassipes, is Bangladesh’s fast-growing and rapidly expanding sustainable aquatic bioenergy feedstock. The WH, as an energy crop, has been harnessed as a phytoremediation agent to purify contaminated water and produce fuel and environmentally friendly products. A country’s economy relies on the availability of raw materials for energy production, cleaning life-supporting abiotic resources for consumption, and the innovation of cost-effective, eco-friendly products. The present study focuses on a three-in-one nexus using the WH to purify polluted water, the (post-purification) biomass to produce clean energy fuels (biogas and bioethanol), and for the manufacture of daily-use products. The ability of the WH, an aquatic macrophyte, to act as a phytoremediator to improve the quality of eutrophic lake water in a laboratory setting was investigated. Water samples were collected from four lakes surrounding the urban community in Dhaka, Bangladesh. The potential to remove salts and solutes and improve the physio-chemical properties of water, including pH, dissolved oxygen (DO), electrical conductivity (EC), total dissolved solids (TDSs), turbidity, and NaCl concentration, were assessed. During the aquatic macrophyte treatment, a 100% WH survival rate was shown, with no visible toxicity symptoms observed in the biomass. The WH improved water quality after one week, as determined by a significant decrease in turbidity, EC, NaCl, and TDSs, and improved pH and DO levels. Here, we establish the WH’s proficiency in removing nutrients/solutes and improving water quality. In addition, we discuss the utilization of this invasive aquatic biomass to produce energy after remediation of water including cost-effective and eco-friendly products to incur daily life with environmental and socioeconomic benefits in Bangladesh.
1. Introduction
Increasing water consumption and the rising demand for safe, drinkable water are global concerns. Although water makes up over three-quarters of Earth’s surface, just a tiny fraction is fit for human use. Water is a crucial non-living environmental resource for humans and all life on Earth. Unfortunately, in recent years, urbanization and industrialization have contaminated water sources, making them unsafe for consumption.
Water quality has declined globally due to rapid urbanization, rising populations, industrialization, human activities, and the inefficient use of this precious natural resource. Therefore, other than modernization, overpopulation causes problems, such as waste management and contamination of surface water, particularly rivers, lakes, and other bodies of freshwater [1]. Furthermore, with the increase in urbanization, industrialization, and population growth, the demand for aquatic resources is increasing daily, resulting in severe surface water pollution. Therefore, there is an urgent requirement for efficient biological solutions to reduce the contamination of eutrophic water and preserve aquatic ecosystems. In this way, aquatic macrophytes help clean up contaminated bodies of water that the public uses by absorbing pollutants such as organic compounds, salts, sediments, and metals [2].
Both wealthy and poor countries may benefit from phytoremediation, a green biotechnological tool and a novel environmentally friendly approach. Water pollution may be effectively filtered out by wastewater treatment plants, according to [3]. This method eliminates or neutralizes harmful environmental toxins by use of green plants. The authors of [4,5,6,7,8] all praised aquatic macrophytes like water lettuce, duckweed, and WH (Eichhornia crassipes) for their ability to absorb pollutants from wastewaters in environments such as lakes, rivers, and ponds. The authors of [9] and [10] found that these macrophytes can also generate biobased energy as biofuel.
Among aquatic plants, the WH is a highly productive and effective floating macrophyte commonly used in phytoremediation systems due to its ease of cultivation under various stress conditions, including solid/salt/metal stress and sporadic reproductive capacity [11,12]. According to many studies [13,14], this organism’s strong reproductive capability allows it to create large amounts of biomass in aquatic habitats, regardless of the levels of contaminants. But it is an invasive weed that can damage ecosystems, human health, and economic growth because of how quickly it grows and how well it survives in harsh environments.
Technological development of macrophytes for the accumulation and phytoextraction/absorption of contaminants by phytoremediation from aquatic bodies, fertilizer production from slurry or composting, production of animal feed, and many more environmentally beneficial utilities support socioeconomic aspects [15]. In addition, it was recently determined that the biomass in its natural ecosystem could be sustainably managed and used to produce biofuels, such as bioethanol and biogas [16] via fermentation and digestion/decomposition, similar to other aquatic energy crops [17]. Sustainably managing the WH would generate sufficient avenues for research, including developing and promoting end products.
Eco-friendly biological processes save energy and are vital for environmental sustainability [18]. Rising energy costs have led to a global search for fossil fuel alternatives. Thus, the WH presents a promising opportunity for the 21st-century biofuel industry [15,19]. Extensive research is currently focused on phytoremediation and biofuel production as an alternative to fossil fuels.
Biofuel, which releases 85% fewer greenhouse gases than gasoline, is a promising fossil fuel replacement due to its renewability [20]. Further research is needed using non-edible second-generation biomasses, such as lignocelluloses and celluloses, to produce biofuel instead of first-generation biomasses, such as starch and sugar [21]. Biofuels produced from WH biomass are considered successful [22,23], as the overreliance on fossil fuels has led to an increasing requirement for alternate fuels [24,25]. Because of its abundance, biodegradability, and high cellulose content, WH is well-suited for use in phytoremediation and bioenergy generation. Biogas, which may power homes in rural areas, and bioethanol, which can power vehicles and factories, are both made from its biomass.
Integrating the WH as both a phytoremediation plant and biofuel feedstock (bioethanol, biogas) is a promising eco-friendly approach for removing water contaminants and developing renewable energy [26].
Thus, it could be utilized as a feedstock or to produce valuable commodities to control the WH’s rapid growth in water bodies. Our investigation aimed to evaluate the efficacy and potential of the WH biomass to eliminate contaminants and improve the quality of the lake water samples by phytoremediation while simultaneously producing fuel and beneficial byproducts. We examine the suitability and effectiveness of this macrophyte in treating eutrophic water and demonstrate the potential of using the remaining plants as feedstock, which would otherwise be considered waste, to produce green energy and eco-friendly, cost-effective goods. Here, we highlight the WH’s multi-faceted advantages and application management.
2. Materials and Methods
The laboratory experiment involving phytoremediation of lake water was conducted at North South University in Dhaka, Bangladesh, at the Department of Environmental Science and Management. Contaminants in eutrophic water were measured before and after treatment with the WH to evaluate the phytoremediation ability of the biomass, where the change in physiochemical characteristics and pollutant removal rates of the water samples were studied. A week of meticulously regulated laboratory conditions by maintaining the temperature and photoperiodism was executed to complete the experiment, where the water quality parameters were investigated and evaluated.
2.1. Collection of Water Samples
The four lakes in Dhaka, Bangladesh, that surround the metropolitan city were studied for their eutrophic water: Uttara Lake (sample A), Dhanmondi Lake (sample B), Gulshan Lake (sample C), and Hatirjheel Lake (sample D) (Figure 1). Four locations on each lake’s surface were sampled using water bottles at a shallow depth. The experiment was conducted with 4 treatments and 3 replications.
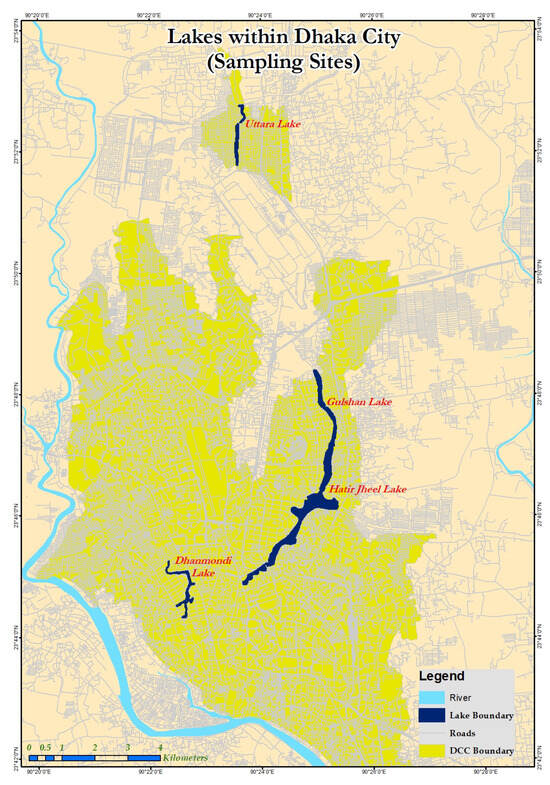
Figure 1.
Water samples were collected from lakes in Dhaka, Bangladesh.
2.2. Collection of Aquatic Macrophytes
To conduct floating-aquatic-macrophyte-based treatment (FAMT) to decontaminate eutrophic water, the water hyacinth (Eichhornia crassipes) was selected as a test crop. The WH belongs to the Kingdom: Plantae, Phylum: Magnoliophyta, Class: Liliopsida, Order: Liliales, Family: Pontederiaceac, Genus: Eichhornia, and Species: Eichhornia crassipes. The plant samples were collected from a pond in Purbachal, Dhaka, a local body of freshwater, and they were free of contaminants found in household water (Figure 2). The WH was then brought to the lab for the phytoremediation trials. The plants were watered, placed in a container with tap water to let them acclimate, and left in the sun for three to five days. Once acclimatized, the plants were placed in 1 L beakers containing the collected lake water samples, maintained at 25 °C, and exposed to a 14/10 h light/dark cycle.
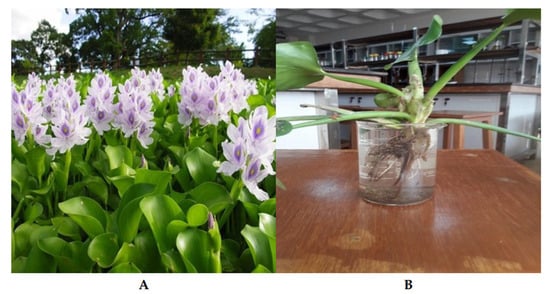
Figure 2.
Collection (A) and cultivation (B) of water hyacinth at North South University’s Department of Environmental Science and Management in Dhaka, Bangladesh.
2.3. Analysis of Eutrophic Lake Water for Physical and Chemical Properties
The pH values of the water samples were recorded using a glass electrode pH meter (Griffin pH meter, Model No. 40). The dissolved oxygen concentration (DO) was measured using a Portuguese-made device called the Hannan Instruments HI 9143-Dissolved Oxygen Meter. The turbidity of the water was measured using a turbidimeter (DRT-100B, HF Scientific Inc., USA). Sodium chloride (NaCl), electrical conductivity (EC), and total dissolved solids (TDSs) were measured using a meter made by Hannan Instruments (HI 9835 Model, 270 Washington Hwy, Smithfield, Rhode Island, 02917, USA). Figure 3 shows pre- and post-treatment water quality.
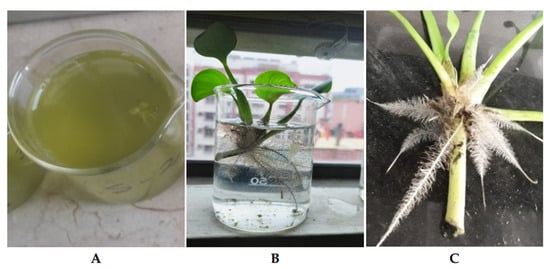
Figure 3.
Water quality before and after treatment. Water sample before remediation (A). Water quality improvement after seven days of treatment (B). (C) Remediation by plants with a deep root system.
Physico-chemical parameters were determined using grab samples collected immediately after being transported to the laboratory; macrophytes were added and left for one week. The eutrophic water sample parameters were determined twice (on the first and seventh days). Pre- and post-treatment, the physio-chemical parameters of the eutrophic water (TDSs, pH, DO, EC, NaCl%, and turbidity) were also assessed.
3. Results
3.1. Water Quality Measurement
The investigation of the effectiveness of WH as a phyto-remediating agent indicated that the plant efficiently lowers or raises all assessed indices of water quality to levels that allow the treated water to be used for various purposes other than drinking.
Figure 4, Figure 5, Figure 6 and Figure 7 illustrate the quality metrics of the four lake water samples pre- and post-treatment, and the percentage removal efficiency of pollutants after seven days of treatment is shown in Table 1, Table 2, Table 3 and Table 4. The experiment was conducted by measuring the water quality parameters using the aforementioned instruments.

Figure 4.
The chemical and physical characteristics of water from Uttara Lake before and after it was treated in water hyacinth cultures.
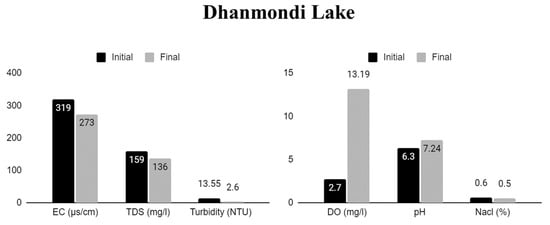
Figure 5.
Physical and chemical properties of Dhanmondi Lake water pre-and post-treatment in cultures with water hyacinth.
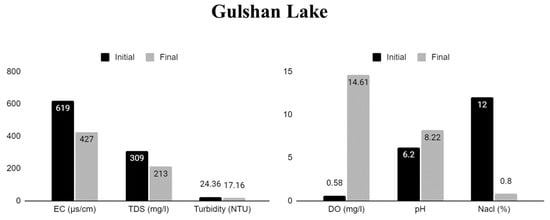
Figure 6.
Physical and chemical properties of Gulshan Lake water pre-and post-treatment in cultures with water hyacinth.
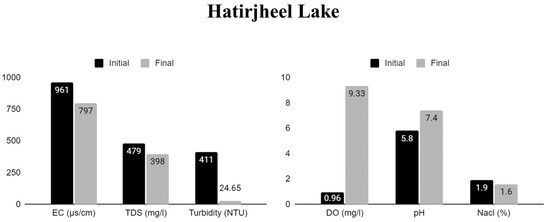
Figure 7.
Physical and chemical properties of Hatirjheel Lake water pre- and post-treatment in cultures with water hyacinth.

Table 1.
Mean percentage reduction/increment of physio-chemical parameters (Uttara Lake or sample A).

Table 2.
Mean percentage reduction/increment of physio-chemical parameters (Dhanmondi Lake or sample B).

Table 3.
Mean percentage reduction/increment of physio-chemical parameters (Gulshan Lake or sample C).

Table 4.
Percent reduction/increment of physio-chemical parameters (Hatirjheel Lake or sample D).
The experiment demonstrated the efficient elimination of salts and solids to improve the quality of eutrophic water, where the samples showed different rates of pollutant reduction including improvement of water quality depending on the load of contamination. Among the tested samples, Gulshan Lake (sample C) showed the most significant increase in DO level, followed by Dhanmondi Lake (sample B), Uttara Lake (sample A), and Hatirjheel Lake (sample D). In the case of TDSs, sample C also showed the highest rate of removal, followed by sample D, and a lower rate of reduction in samples B and A after seven days of treatment.
Also, among the water samples tested, sample C showed the most significant decrease in EC, followed by samples D and B. However, sample A had the most minimal reduction. Thus, samples from Gulshan, Hatirjheel, and Dhanmondi Lake all showed substantial decreases in EC. Similarly, the Nacl reduction was most significant in sample C, followed by D and B, and had the lowest reduction in sample A.
According to Table 1, Table 2, Table 3 and Table 4, the NaCl concentration decreased most in Gulshan Lake, followed by Hatirjheel Lake and Dhanmondi Lake. However, Uttara Lake did not show any change and remained the same before or after remediation. Uttara Lake’s water is safe to use and drink, according to the World Health Organization. The local authorities maintain it well with lower levels of eutrophication compared with lakes B, C and D. The percentage reduction in EC and TDSs was also the lowest in Uttara Lake compared to the others.
We also evaluated the pH of the gathered samples before treatment; they were below neutral. After treatment, all samples, except sample C (Gulshan Lake), exhibited pH values within the required range. Sample C showed a slight increase in pH, with the most significant increase observed in Hatirjheel Lake, followed by Dhanmondi Lake, with the lowest increase in Uttara Lake.
Moreover, after treatment, all cultures appeared much more transparent, while before, they were pale greenish brown hues and had an unpleasant, repulsive smell (Figure 3). The investigation demonstrated the WH’s potential to remove contaminants and improve water quality by reducing the turbidity levels, which shows the highest reduction in sample D and then C and the lowest reduction in B and A. The phytoremediation process showed a significant improvement in all parameters to which the energy crop was applied.
The results indicate that after inoculating the macrophyte, the water sample from Gulshan Lake showed the most significant improvements in most of the parameters including EC, TDSs, NaCl, and DO levels, indicating that WH biomass is the most efficient pollutant remover.
The research showed that WHs can absorb and survive in polluted water by a process that mainly occurs in the root system, where most of the salts and dissolved solids from the contaminated water accumulate. Recent studies by [19,27,28,29,30] confirmed that aquatic macrophyte roots absorbed high levels of metals and solutes from polluted water.
3.2. Removal Efficiencies of Pollutants/Phytoextraction
Table 1, Table 2, Table 3 and Table 4 and Figure 4, Figure 5, Figure 6 and Figure 7 show the percentage decrease in pollutants and improvement of the water samples. In terms of the total suspended solids (TDSs), the percentage removal was found to be the highest in sample C (31%), followed by sample D (17%) and sample B (14%), with the least removal found in sample A (3%). Thus, the total dissolved solids were successfully eliminated by the aquatic macrophyte culture treatment via plant absorption. Additionally, macrophytes eliminated suspended and colloidal particles by flocculation and the release of biopolymers from their roots. The partial removal of suspended particles and decomposition of other organic pollutants were also facilitated by bacterial growth [31].
The pH values of all samples were below neutral prior to phytoremediation. Optimal pH values after treatment were found to be between 7.2 and 7.4, with the exception of sample C, which became somewhat alkaline (8.2). The results indicated that out of the four-sample tested, sample C had the highest pH rise, which was 32%, followed by sample D, 27%, and B, 15%, whereas sample A had the lowest increment rate, which was 11%.
Results for the NaCl concentration were also found to decrease the most in sample C (93%), followed by sample B (17%) and then sample D (16%), remaining the same in sample A, meaning that no reduction was established. In terms of EC reduction, the highest value was found in sample C (31%), followed by D (17%), B (14%), and then A (2%).
Tables and figures also show the dissolved oxygen (DO) levels of the samples, which is a useful indicator for determining water pollution levels. The dissolved oxygen content in the untreated sample C was 0.58 mg/L, but it increased dramatically in the treated experimental water. Following WH treatment, the dissolved oxygen levels in water samples from sample C, sample D, sample A, and sample B rose from 0.58 to 14.61 mg/L, 0.96 to 9.33 mg/L, 0.88 to 10.14 mg/L, and 2.7 to 13.19 mg/L, respectively, in the following order: C > D > A > B, whereas the mentioned samples’ percentage increments were 2419%, 1052%, 871%, and 388%, respectively. Elevated toxicity in sample C may have stimulated phytoplankton photosynthetic activity, which in turn raised dissolved oxygen levels. Reduced oxygen penetration into the water as a result of elevated root respiration rates and increased oxygen consumption by microbes in the water samples may explain why other plant cultures have low DO levels. Oxygen exchange rates from aerial tissues to the root zone varied between plant cultures, which explained why there was a disparity in DO levels [32].
In the case of water turbidity, after treatment, the highest reduction was found in sample D, which was 94%, followed by B (81%), A (72%), and C (29%). The most significant reduction in turbidity levels, also known as water clarity, was seen in the water of sample D.
During the experiments, the water quality in all four lakes improved, as shown by a reduction in turbidity, TDSs, NaCl, and EC, whereas samples C and D showed the highest EC, NaCl, and TDS removal compared with samples A and B. The highest removal percentage was seen in sample C, reaching 31% for EC, 31% for TDSs, and 93% for NaCl after one week or 168 h of culture. In sample C, the highest increases in DO, 2419%, and pH, 32%, were observed.
Due to its vast root system, which offers a wide surface area with a high biofiltration capacity, the WH is able to efficiently eliminate solids and salts. After seeing the buildup of various components inside the plant, [2,29,33,34,35] confirmed what we found: that WH successfully eliminates some particles and salts from water samples.
Therefore, under a wide range of eutrophic water conditions, the WH always survived. After a week, the extensive root system enhanced the water quality, as shown by a significant decrease in turbidity, TDSs, and EC, along with a rise in DO and pH. This improvement also included a decrease in the percentage of NaCl. Over the course of seven days, the dissolved oxygen concentration (DO), a useful measure of water quality, rose substantially in all treated cultures.
3.3. Sample A. Phytoextraction in the Laboratory (Uttara Lake)
The analysis of sample A (Uttara Lake) revealed the plants’ ability to reach and maintain reasonably low levels of turbidity (64–18.05 NTU), EC (417–410 µS/cm), and TDSs (209–203 mg L−1) in all the analyses, whereas NaCl (0.8–0.8%) remained unchanged and the DO (0.88–10.14 mg L−1) and pH (6.5–7.24) values improved.
3.4. Sample B. Phytoextraction in the Laboratory (Dhanmondi Lake)
The WH in sample B (Dhanmondi lake) decreased turbidity levels (13.55–2.6 NTU), with low levels of TDSs (159–136 mg L−1) and EC (319–273 µS/cm), although low levels of NaCl (0.6–0.5%) remained stable, DO (2.7–13.19 mg L−1) improved, and pH levels increased (6.3–7.24).
3.5. Sample C. Phytoextraction in the Laboratory (Gulshan Lake)
Results for sample C (Gulshan Lake) revealed that the plants could attain and maintain relatively low turbidity levels (24.36−17.16 NTU), TDSs (309−213 mg L−1), and EC (619−427 µS/cm), with deficient levels of NaCl (12−0.8%), improved DO (0.58−14.61 mg L−1), and increased pH levels (6.2−8.22).
3.6. Sample D. Phytoextraction at the Laboratory (Hatirjheel Lake)
For sample D (Hatirjheel Lake), results confirmed the plants’ ability to reach and preserve relatively reduced levels of EC (961–797 µS/cm), NaCl (1.9–1.6%), TDSs (479–398 mg L−1) and turbidity (411–24.65 NTU), in every test, and improved DO (0.96–9.33 mg L−1) and pH levels (5.8–7.4).
The findings show that the WH might be a great floating macrophyte in eutrophic lakes, helping to improve the water quality by filtering out salts and particles and raising the pH, oxygen levels, and turbidity. The WH is a versatile plant serving various purposes and functions (Figure 8).
As an essential renewable resource, it can be used in many ways besides water purification, as biofuel production, organic fertilizer, paper, fiberboard and furniture production, yarn and rope (weaving purposes) crafts, floating gardens, mushroom cultivation, charcoal briquettes, animal fodder and fish feed [9,16,36,37,38,39,40,41,42].
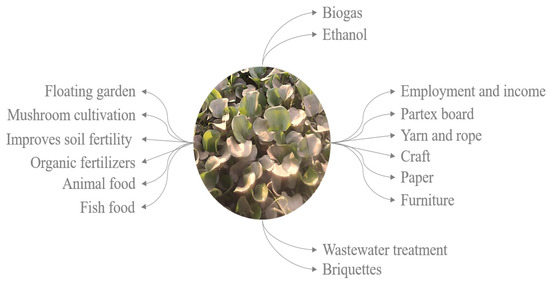
Figure 8.
Various uses of water hyacinth as a renewable source from nature (source: [43]).
3.7. Biofuels from Water Hyacinth
Moreover, given its capacity for phytoremediation and generation of bioproducts, the water hyacinth has been found to have potential use in bioenergy production. Biofuels, which include gaseous fuel biogas and liquid fuel bioethanol, provide a sustainable energy alternative to traditional fossil fuels while lowering emissions of greenhouse gases and air pollution. Biofuels from WH are considered second-generation biofuels as they are nonfood crops, and they also do not require precious land area for cultivation.
WH proliferates rapidly and has a high nitrogen content; this could be utilized to make biogas (Figure 9) [16,44,45]. After application/use for phytoremediation, the leftover slurry or biogas residue can be transported as liquid fertilizer after biogas production. Additionally, as a potential source of lignocellulosic biomass, it could be used in bioethanol fermentation (Figure 10). The waste biomass from phytoremediation may be turned into biogas, and the other byproducts can be turned into organic manure or bioethanol [20] by the breakdown of fermentable saccharides. Bioethanol could be used as a transport fuel to replace gasoline.
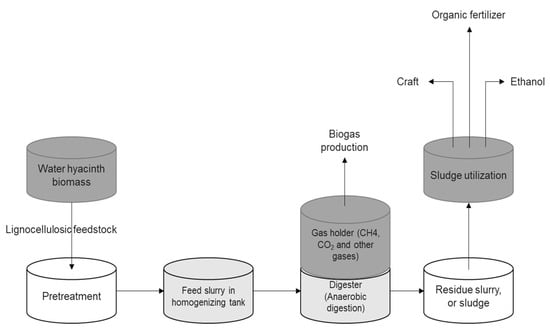
Figure 9.
Schematic flowchart illustrating the production of biogas and bioproducts from water hyacinth feedstock.
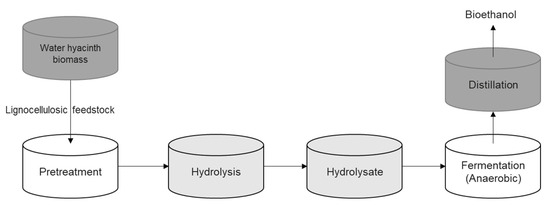
Figure 10.
The schematic flowchart illustrates bioethanol production from water hyacinth feedstock.
Therefore, making biofuels from WHs can help diversify energy sources, slow the spread of WH in subtropical regions, and improve water quality while reducing emissions of carbon dioxide (CO2) [26,46].
An important issue in developing cities like Bangladesh is the phytoremediation of eutrophic waterways; this research sought to explore the potential use of WH for this purpose, with the end goal of cleaning up polluted water and finding a market for the residual biomass. Clean energy sources including biogas, bioethanol, and bioproducts may be made from biomass after phytoremediation. Renewable green energy sources, or biofuels, are a great way to power vehicles without negatively impacting the environment [37,47].
In a reactor or digester, which is an airtight container, usually underground, biogas is produced from WH by means of anaerobic digestion [48,49]. The end product is methane gas, which has several practical applications such as lighting for gas lamps, fueling engines, creating power, and combusting directly in domestic stoves for cooking and in broilers to generate heat. Furthermore, the byproducts of digestion may be used as a fertilizer for crops or crafts, since they are rich in nutrients and cellulosic materials [16,50].
According to [51] active research is being conducted to investigate the WH as a renewable biomass source that can produce alcohol on a commercial scale at a reasonable cost. The WH is a potential raw material for biofuel production due to its bioethanol-producing capabilities, which are comparable to those of agricultural waste [52,53,54,55,56]. The WH is mostly composed of hemicellulose and lignocellulosic biomass, with negligible amounts of lignin. The authors of [25,26,36,57,58] all found that it may be used as a precursor for anaerobic fermentation to produce bioethanol.
Bangladesh, as a riverine country with suitable climatic conditions [59], can produce enormous amounts of aquatic floating macrophyte biomass (Eichhornia crassipes). Such material could be carefully managed to produce biofuel [60]. Moreover, by regulating carbon exportation from natural wetlands, this burgeoning biofuel-based industry is an excellent example of human adaptation to climate change [61].
4. Technological Potential Modeling of Bioenergy Production
The concept of using aquatic plants for conversion to clean energy is gaining attention in tropical and sub-tropical regions of the world where warm climate is conductive to rapid plant growth throughout the year. This biomass availability is an important factor for achieving a good yield of biofuel given the current fuel demand and the need for lower GHG alternative. The high growth rates of WH mean that it can thus be considered as an attractive raw material and indicate its capacity to mobilize and store nutrients in the tissues and assimilate large amounts of carbon dioxide; simultaneously, the plant can stimulate biofuels including biogas and bioethanol in many tropical and subtropical climates of the world among various types of lignocellulosic biomasses [36,62].
As a result, such biobased fuels from water hyacinth are renewable, low-cost, nontoxic, readily available, biodegradable and beneficial overall for the environment [63,64] if enough can be produced annually. Laboratory experiments indicated that the water hyacinth yield is estimated at 22 mL of ethanol per kg of biomass [65], or 12 L of biogas per kg, with a potential yield of 1681.08 m3 per day, or 573,248.28 m3 annually [66]. Compared to Bangladesh’s residential reliance on traditional natural gas, around 500,000 kg per year feedstock can satiate its annual need. With an average biomass yield of 60 kg per m2 [18] or around 1 hectare a year, only a minority of the 150,000 hectares of ponds available in the country [60] would have to be utilized.
This determination of the land or water area usage parameter necessary for fuel production and consumption may be achieved using a simple model that entails dividing the area need for WH-based biomass fuel production by the whole population of the sample. It is important to note that all of the parameters mentioned are yearly values. The formula produces the value AUreq, denoting the area usage requirement for consumption, and it is quantified in terms of acres per person or hectares per person.
where h is the total quantity of fuels, either as ethanol or biogas; the biofuel crop yield can be measured in liters or cubic meters per acre and denoted by CY:
Rv,req is the overall demand for fuel, expressed in volume per person, where is the biomass output in liters per unit area, is the pretreatment conversion percentage yield, and fuel is the equivalent fuel generated in liters per ton.
where Ptotal is Bangladesh’s total population and Re,req is the required fuel consumption for end use (home, industry, transportation, etc.) in liters. Energy/fuel usage for end uses can be calculated as follows:
The letter “i” is used to indicate cases with different kinds of end use of bioenergy. The ratio of biofuel performance yield to replacing fuel, if from diesel to ethanol, or natural gas to biogas, is
where Ygas represents an energy yield (or energy content) of 1 gallon, or another volumetric unit of fuel, such as gasoline at 21.099 kJ/L; Yethanol is the energy yield of 1 gallon of ethanol at 32,163.74 kJ/L; and Ydiesel is the energy yield of 1 gallon of petroleum diesel at 35.174 kJ/L.
If nvi is the total number of homes in Bangladesh, to illustrate how the area use model for ethanol works, consider the following scenario:
where AUreq, ethanol is the area usage required for WH-based ethanol production, in areas or Ha, and AUreq, diesel is the area usage required for conventional production, in acres or Ha, from the following formula:
The theoretical production capacity, Ptheo,c, has to be compared to the maximum production capacity, Pmax,c, of the plants, where the following occurs:
Ptheo,c is the present production capacity theoretical in L, CY is the crop yield in L acres or unit area, Ac,t is the total acres (size), and hi is the number of various plants and crops. The maximum production capacity in L is denoted by the notation CMP,C, whereas the maximum production capacity in L is represented by the notation Pmax,c. The relatively high bioethanol and biogas production potential indicates that water hyacinth can be considered a promising energy crop for supplying sustainable clean and renewable energy for certain end-use sectors with further research and development keeping the feasible capacities in mind.
5. Discussion
The WH’s fast growth rate and large biomass production make it the best aquatic plant for removing pollutants from eutrophic water. Still, factors such as temperature, water quality, and biological constraints may affect how well it works. Low temperature, high salt, and low nutrient levels can negatively affect the plant’s ability to remove contaminants efficiently. The WH absorbs impurities via the roots, which are rapidly transported to other parts of the plant, as confirmed by [39,67]. Therefore, applying aquatic macrophytes, such as the WHs, into eutrophic water is considered highly absorbent, allowing for the remediation of water pollution and acting as a contaminant sink [68]. Our results also confirm the findings of previous research considering floating plants as potential macrophytes for pollution absorption.
In all treatment cultures, WH growth reduced the water EC, TDS, turbidity, and NaCl content by removing salts and particles from the water via plant ingestion or root adsorption. As WH expanded, both the dissolved oxygen and water pH rose. During photosynthesis, the floating macrophyte raised the pH levels by blocking light at the water’s surface. It is widely acknowledged that a rise in pH is accompanied by oxygen deprivation [19,33].
WH enhanced the physio-chemical characteristics of eutrophic water, as shown by an increase in average DO and pH values across all treatment cultures. This finding is in line with the findings of [34]. According to the results of the trial, the floating plant is capable of treating contaminated water and may be used for controlling pollution on a broad scale. DO increased significantly in all plant cultures, and the pH rose from 14% to 32% in all lake water cultures. TDSs, EC, turbidity, and NaCl also decreased in all treated samples [17,35,36]. The results of these studies indicate that floating macrophytes have the potential to be used as efficient phytoremediators in order to address water pollution.
Aquatic macrophytes increased dissolved oxygen and pH levels in polluted water, as shown in other research [8,33,37,38], which is supported by our findings. The process of photosynthesis, which involves the uptake of salts, metal ions, nutrients, and water by plants, results in a pH increase. Our findings support the idea that WH is an appropriate plant for phytoremediation of polluted tropical water structures, which is in line with those of [18,39,40,41].
Improving water quality necessitates the exclusion of surplus nutrients, salts, and dissolved/suspended solids, as well as the adjustment of pH, DO, temperature, and other parameters. The implications of the research are consistent with [7,40,69,70,71], who postulate that the WH could be utilized as an efficient bio-agent for handling contaminated water and improving water quality issues.
The study results showed that sample C, which was more eutrophic, had a more significant decrease in TDSs, NaCl, and turbidity than the other three samples. Furthermore, the maximum purification was attained in the most contaminated water (sample C).
The WH and other aquatic macrophytes are effective at removing pollutants in water, including salts, solids, and metals, as supported by previous research [2,5,7,14,17,19,47,72]. Hence, aquatic plants are considered essential tools in phyto-technologies for managing contaminants in aquatic environments.
According to the findings of this study, the WH is a powerful phytoremediator for removing salts and solids and improving water quality. The described macrophyte is highly resistant to contaminants, such as accumulated solids and salts. There was no indication of injury, tissue toxicity, or a growth decrease. Due to the WH hemofiltration activity, it is a potential candidate for purifying contaminated water. The plant demonstrated a positive effect on all collected samples. Results from [29] and [5] corroborate the feasibility of using the WH for large-scale removal of contaminants from contaminated water bodies. The initial strong odor of pre-treated water samples steadily decreased during purification, while the greenish-brown hue became practically colorless. We also found that our results are in line with those of [9,73]. They postulate that the WH could be used to make bioenergy, various goods, and manure, and to stimulate economic development through increased investments in projects. This would lead to resilient urban systems and contaminant-free environments, not only in aquatic systems but also in general. Here, the WH has shown promising capabilities as a floating macrophyte in aquatic environments, with the ability to improve the water quality of eutrophic lakes by reducing organic solutes and salts, increasing oxygen levels, and decreasing turbidity.
Moreover, after the phytoremediation process, a 100% survival rate of the species was observed without any visible symptom of toxicity in the feedstock. This result also confirms the findings of [10,47], who found no visible symptoms of damage in the energy crop biomass. The high growth rates of the energy crop indicate its capacity to produce biofuel. The plant can also be used to produce ecofriendly products. Biobased fuels from the water hyacinth are renewable, low cost, nontoxic, readily available, biodegradable and ecofriendly [63,64]. Nowadays, biofuels are being promoted as low-carbon alternatives to fossil fuels as they could help to mitigate climate change by reducing greenhouse gas emissions [37,74]. Thus, biofuel production from the water hyacinth has positive environmental effects.
6. Conclusions
It can be concluded that the water hyacinth may be considered as a potential aquatic bioresource that could support the impact of the three-in-one nexus. Due to the climatic conditions of Bangladesh, it is strongly suggested that WHs be considered positively as an abundantly available source of biomass feedstock, as there is currently a global depletion of natural resources. Furthermore, the overabundance of WH can be perceived as an advantage for remedying nutrient-rich water, producing biofuels and eco-friendly products from residual biomass, and providing employment and income. Biofuel has been identified as a key fuel to replace petroleum-based fossil fuel and creates less pollution than conventional fuels. Moreover, extracting biofuel from WH biomass is one important technique to reduce both the use of fossil fuel and environmental pollution. Bioconversion of lignocellulose biomass to biofuel has considerable potential because it is inexpensive and abundant particularly in the context of a warm climate. In recent years, the concept of using WH for the conversion of energy has been gaining attention in tropical and subtropical regions of the world where a warm climate is conductive to plant growth throughout the year.
Our findings suggest that the WH may be an economical and resourceful biological solution, particularly for cleaning up contaminated aquatic systems by reducing the pollution load in developing countries like Bangladesh, as the physical and chemical properties of Dhaka’s eutrophic lake waters could be improved by using WH biomass. This strategy promotes sustainability, effectively treating large volumes of polluted water before being released into fresh water/marine ecosystems. Thus, WH aligns with a green development strategy, producing clean energy, reducing greenhouse gas emissions, and improving water quality, resulting in environmental and socioeconomic benefits.
Author Contributions
Conceptualization, investigation, methodology, K.N.; validation, data curation, formal analysis, editing, S.A.S.; writing, K.N. and S.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhateria, R.; Jain, D. Water quality assessment of lake water: A review. Sustain. Water Resour. Manag. 2016, 2, 161–173. [Google Scholar] [CrossRef]
- Rezania, S.; Ponraj, M.; Talaiekhozani, A.; Mohamad, S.E.; Din, M.F.M.; Taib, S.M.; Sabbagh, F.; Sairan, F.M. Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. J. Environ. Manag. 2015, 163, 125–133. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavaş, I.; Ünay, A.; Abdel-Daim, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of floating aquatic plants in phytoremediation of heavy metals polluted water: A review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Miretzky, P.; Saralegui, A.; Cirelli, A.F. Aquatic macrophytes potential for the simultaneous removal of heavy metals (Buenos Aires, Argentina). Chemosphere 2004, 57, 997–1005. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, Z.; Liu, M.; Liu, H.; Wang, Y.; Wen, X.; Zhang, Y.; Yan, S. Site test of phytoremediation of an open pond contaminated with domestic sewage using water hyacinth and water lettuce. Ecol. Eng. 2016, 95, 753–762. [Google Scholar] [CrossRef]
- Olguín, E.J.; García-López, D.A.; González-Portela, R.E.; Sánchez-Galván, G. Year-round phytofiltration lagoon assessment using Pistia stratiotes within a pilot-plant scale biorefinery. Sci. Total Environ. 2017, 592, 326–333. [Google Scholar] [CrossRef]
- Nabi, A.; Alam, A.; Hoque, S. Treatment of wastewater with free floating aquatic macrophyte—Eichhornia crassipes. Jahangirnagar Univ. Environ. Bull. 2016, 5, 1–9. [Google Scholar]
- Alam, A.R.; Hoque, S. Phytoremediation of industrial wastewater by culturing aquatic macrophytes, Trapa natans L. and Salvinia cucullata Roxb. Jahangirnagar Univ. J. Biol. Sci. 2017, 6, 19–27. [Google Scholar] [CrossRef][Green Version]
- Ali, N.; Chaudhary, B.; Khandelwal, S. Better use of water hyacinth for fuel, manure and pollution free environment. Indian J. Environ. Prot. 2004, 24, 297–303. [Google Scholar]
- Nahar, K. Azolla (Caroliniana): An Aquatic Energy Crop for Remediation of Eutrophic Ecosystems with Prospect of Biofuel Production in Bangladesh. Asia Pac. J. Energy Environ. 2020, 7, 79–86. [Google Scholar]
- Malik, A. Environmental challenge vis a vis opportunity: The case of water hyacinth. Environ. Int. 2007, 33, 122–138. [Google Scholar] [CrossRef]
- Melignani, E.; de Cabo, L.I.; Faggi, A.M. Copper uptake by Eichhornia crassipes exposed at high level concentrations. Environ. Sci. Pollut. Res. 2015, 22, 8307–8315. [Google Scholar] [CrossRef]
- Malar, S.; Sahi, S.V.; Favas, P.J.; Venkatachalam, P. Mercury heavy-metal-induced physiochemical changes and genotoxic alterations in water hyacinths [Eichhornia crassipes (Mart.)]. Environ. Sci. Pollut. Res. 2015, 22, 4597–4608. [Google Scholar] [CrossRef]
- Ansari, A.A.; Naeem, M.; Gill, S.S.; AlZuaibr, F.M. Phytoremediation of contaminated waters: An eco-friendly technology based on aquatic macrophytes application. Egypt. J. Aquat. Res. 2020, 46, 371–376. [Google Scholar] [CrossRef]
- Rai, P.K. Eichhornia crassipes as a potential phytoremediation agent and an important bioresource for Asia Pacific region. Environ. Skept. Crit. 2016, 5, 12. [Google Scholar]
- Nahar, K. Biogas production from water hyacinth (Eichhornia crassipes). Asian J. Appl. Sci. Eng. 2012, 1, 9–13. [Google Scholar]
- Nahar, K.; Sunny, S.A. Duckweed-based clean energy production dynamics (ethanol and biogas) and phyto-remediation potential in Bangladesh. Model. Earth Syst. Environ. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Ganguly, A.; Chatterjee, P.; Dey, A. Studies on ethanol production from water hyacinth—A review. Renew. Sustain. Energy Rev. 2012, 16, 966–972. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, J.; Saini, A.; Kumar, P. Phytoremediation of copper, iron and mercury from aqueous solution by water lettuce (Pistia stratiotes L.). Environ. Sustain. 2019, 2, 55–65. [Google Scholar] [CrossRef]
- Nigam, J. Bioconversion of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to motor fuel ethanol by xylose–fermenting yeast. J. Biotechnol. 2002, 97, 107–116. [Google Scholar] [CrossRef]
- Lewandowski, I.; Scurlock, J.M.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mukherjee, P.; Chatterjee, N. Optimization of enzymatic hydrolysis of water hyacinth by Trichoderma reesei vis-a-vis production of fermentable sugars. Acta Aliment. 2008, 37, 367–377. [Google Scholar] [CrossRef]
- Aswathy, U.; Sukumaran, R.K.; Devi, G.L.; Rajasree, K.; Singhania, R.R.; Pandey, A. Bio-ethanol from water hyacinth biomass: An evaluation of enzymatic saccharification strategy. Bioresour. Technol. 2010, 101, 925–930. [Google Scholar] [CrossRef]
- Zaldivar, J.; Nielsen, J.; Olsson, L. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. 2001, 56, 17–34. [Google Scholar] [CrossRef]
- Idrees, M.; Adnan, A.; Sheikh, S.; Qureshic, F.A. Optimization of dilute acid pretreatment of water hyacinth biomass for enzymatic hydrolysis and ethanol production. EXCLI J. 2013, 12, 30. [Google Scholar]
- Wang, Z.; Zheng, F.; Xue, S. The economic feasibility of the valorization of water hyacinth for bioethanol production. Sustainability 2019, 11, 905. [Google Scholar] [CrossRef]
- Rodrigues, A.C.D.; do Amaral Sobrinho, N.M.B.; dos Santos, F.S.; dos Santos, A.M.; Pereira, A.C.C.; Lima, E.S.A. Biosorption of toxic metals by water lettuce (Pistia stratiotes) biomass. Water Air Soil Pollut. 2017, 228, 156. [Google Scholar] [CrossRef]
- Rodrigues, A.C.D.; Rocha, M.V.d.C.; Lima, E.S.A.; Pinho, C.F.d.; Santos, A.M.d.; Santos, F.S.d.; Amaral Sobrinho, N.M.B.d. Potential of water lettuce (Pistia stratiotes L.) for phytoremediation: Physiological responses and kinetics of zinc uptake. Int. J. Phytoremediation 2020, 22, 1019–1027. [Google Scholar] [CrossRef]
- Gupta, P.; Roy, S.; Mahindrakar, A.B. Treatment of water using water hyacinth, water lettuce and vetiver grass–a review. System 2012, 49, 50. [Google Scholar] [CrossRef]
- Galal, T.M.; Farahat, E.A. The invasive macrophyte Pistia stratiotes L. as a bioindicator for water pollution in Lake Mariut, Egypt. Environ. Monit. Assess. 2015, 187, 701. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.A.; Ramasami, E. Biotechnological Methods of Pollution Control; University Press: Hyderabad, India, 1999. [Google Scholar]
- Sooknah, R.D.; Wilkie, A.C. Nutrient removal by floating aquatic macrophytes cultured in anaerobically digested flushed dairy manure wastewater. Ecol. Eng. 2004, 22, 27–42. [Google Scholar] [CrossRef]
- Mahmood, Q.; Zheng, P.; Islam, E.; Hayat, Y.; Hassan, M.; Jilani, G.; Jin, R. Lab scale studies on water hyacinth (Eichhornia crassipes Marts Solms) for biotreatment of textile wastewater. Casp. J. Environ. Sci. 2005, 3, 83–85. [Google Scholar]
- Lu, Q.; He, Z.L.; Graetz, D.A.; Stoffella, P.J.; Yang, X. Phytoremediation to remove nutrients and improve eutrophic stormwaters using water lettuce (Pistia stratiotes L.). Environ. Sci. Pollut. Res. 2010, 17, 84–96. [Google Scholar] [CrossRef]
- Gaballah, M.; Ismail, K.; Beltagy, A.; Zein Eldin, A.; Ismail, M. Wastewater treatment potential of water lettuce (Pistia stratiotes) with modified engineering design. J. Water Chem. Technol. 2019, 41, 197–205. [Google Scholar] [CrossRef]
- Zhang, Q.; Weng, C.; Huang, H.; Achal, V.; Wang, D. Optimization of bioethanol production using whole plant of water hyacinth as substrate in simultaneous saccharification and fermentation process. Front. Microbiol. 2016, 6, 1411. [Google Scholar] [CrossRef]
- Nahar, K.; Sunny, S.A. Climate Change and State of Renewable Energy in Bangladesh: An Environmental Analysis. In Climate Change in Bangladesh: A Cross-Disciplinary Framework; Springer: Cham, Switzerland, 2021; pp. 25–45. [Google Scholar]
- Dipu, S.; Kumar, A.A.; Thanga, V.S.G. Phytoremediation of dairy effluent by constructed wetland technology. Environmentalist 2011, 31, 263–278. [Google Scholar] [CrossRef]
- Galal, T.M.; Eid, E.M.; Dakhil, M.A.; Hassan, L.M. Bioaccumulation and rhizofiltration potential of Pistia stratiotes L. for mitigating water pollution in the Egyptian wetlands. Int. J. Phytoremediation 2018, 20, 440–447. [Google Scholar] [CrossRef]
- Nassouhi, D.; Ergönül, M.B.; Fikirderşici, Ş. The use of some submersed and free floating aquatic macrophytes in the bioremediation of heavy metal pollution. SDU-JEFF 2018, 14, 148–165. [Google Scholar]
- Goswami, T.; Saikia, C. Water hyacinth—A potential source of raw material for greaseproof paper. Bioresour. Technol. 1994, 50, 235–238. [Google Scholar] [CrossRef]
- Awuah, E.; Oppong-Peprah, M.; Lubberding, H.; Gijzen, H. Comparative performance studies of water lettuce, duckweed, and algal-based stabilization ponds using low-strength sewage. J. Toxicol. Environ. Health Part A 2004, 67, 1727–1739. [Google Scholar] [CrossRef]
- Patel, S. Threats, management and envisaged utilizations of aquatic weed Eichhornia crassipes: An overview. Rev. Environ. Sci. Bio/Technol. 2012, 11, 249–259. [Google Scholar] [CrossRef]
- El-Shinnawi, M.; El-Din, M.A.; El-Shimi, S.; Badawi, M. Biogas production from crop residues and aquatic weeds. Resour. Conserv. Recycl. 1989, 3, 33–45. [Google Scholar] [CrossRef]
- Singhal, V.; Rai, J. Biogas production from water hyacinth and channel grass used for phytoremediation of industrial effluents. Bioresour. Technol. 2003, 86, 221–225. [Google Scholar] [CrossRef]
- Wang, Z.; Calderon, M.M. Environmental and economic analysis of application of water hyacinth for eutrophic water treatment coupled with biogas production. J. Environ. Manag. 2012, 110, 246–253. [Google Scholar] [CrossRef]
- Nahar, K.; Hoque, S. Phytoremediation to improve eutrophic ecosystem by floating macrophyte water lettuce (Pistia stratiotes) at lab scale. Egypt. J. Aquat. Res. 2021, 47, 231–237. [Google Scholar] [CrossRef]
- Chen, B. Ecological Engineering of Water Hyacinth Control and Utilization in River Basins. Ph.D. Thesis, Tongji University, Shanghai, China, 2007. [Google Scholar]
- Polprasert, C.; Mya, S. Anaerobic Digestion: Principles and Practices for Biogas Systems; Gunnerson, C.G., Stuckey, D.C., Eds.; Integrated Resource Recovery Series, UNDP Project Management Report, No. 5; World Bank technical paper; No. 49: The World Bank: Washington, DC, USA, 1986; p. xv, 154. [Google Scholar]
- Bhattacharya, A.; Kumar, P. Water hyacinth as a potential biofuel crop. Electron. J. Environ. Agric. Food Chem. 2010, 9, 112–122. [Google Scholar]
- Magdum, S.; More, S.; Nadaf, A. Biochemical conversion of acid-pretreated water hyacinth (Eichhornia crassipes) to alcohol using Pichia Stipitis NCIM3497. Int. J. Adv. Biotechnol. Res. 2012, 3, 585–590. [Google Scholar]
- Nahar, K.; Sunny, S.A. Jatropha curcas L: A sustainable feedstock for the production of bioenergy and by products. J. Energy Nat. Resour. 2014, 3, 51–57. [Google Scholar] [CrossRef]
- Nahar, K.; Sunny, S.A. Biodiesel, Glycerin and Seed-cake Production from Roof-top Gardening of Jatropha curcas L. Curr. Environ. Eng. 2016, 3, 18–31. [Google Scholar] [CrossRef]
- Manivannan, A.; Narendhirakannan, R. Biodegradation of lignocellulosic residues of water hyacinth (Eichhornia crassipes) and response surface methodological approach to optimize bioethanol production using fermenting yeast Pachysolen tannophilus NRRL Y-2460. Int. J. Bioeng. Life Sci. 2014, 8, 153–158. [Google Scholar]
- Awasthi, M.; Kaur, J.; Rana, S. Bioethanol production through water hyacinth, Eichhornia crassipes via optimization of the pretreatment conditions. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 42–46. [Google Scholar]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar]
- Ma, F.; Yang, N.; Xu, C.; Yu, H.; Wu, J.; Zhang, X. Combination of biological pretreatment with mild acid pretreatment for enzymatic hydrolysis and ethanol production from water hyacinth. Bioresour. Technol. 2010, 101, 9600–9604. [Google Scholar] [CrossRef] [PubMed]
- Narra, M.; Divecha, J.; Shah, D.; Balasubramanian, V.; Vyas, B.; Harijan, M.; Macwan, K. Cellulase production, simultaneous saccharification and fermentation in a single vessel: A new approach for production of bio-ethanol from mild alkali pre-treated water hyacinth. J. Environ. Chem. Eng. 2017, 5, 2176–2181. [Google Scholar] [CrossRef]
- Sunny, S. Green Buildings, Clean Transport and the Low Carbon Economy: Towards Bangladesh’s Vision of a Greener Tomorrow; LAP LAMBERT Academic Publishing: Saarbrucken, Germany, 2011. [Google Scholar]
- Nahar, K.; Sunny, S.A.; Shazi, S.S. Land use requirement and urban growth implications for the production of biofuel in Bangladesh. Forest 2011, 1350, 92. [Google Scholar]
- Bergier, I.; Salis, S.M.; Miranda, C.H.; Ortega, E.; Luengo, C.A. Biofuel production from water hyacinth in the Pantanal wetland. Ecohydrol. Hydrobiol. 2012, 12, 77–84. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, Y.; Han, H.; Weng, C. Enhancing bioethanol production from water hyacinth by new combined pretreatment methods. Bioresour. Technol. 2018, 251, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Balagurumurthy, B.; Prakash, A.; Bhaskar, T. Catalytic hydrothermal liquefaction of water hyacinth. Bioresour. Technol. 2015, 178, 157–165. [Google Scholar] [CrossRef]
- Das, S.; Goswami, S.; Talukdar, A.D. Physiological responses of water hyacinth, Eichhornia crassipes (Mart.) Solms, to cadmium and its phytoremediation potential. Turk. J. Biol. 2016, 40, 84–94. [Google Scholar] [CrossRef]
- Masami, G.O.; Usui, I.; Urano, N. Ethanol production from the water hyacinth Eichhornia crassipes by yeast isolated from various hydrospheres. Afr. J. Microbiol. Res. 2008, 2, 110–113. [Google Scholar]
- Kunatsa, T.; Madiye, L.; Chikuku, T.; Shonhiwa, C.; Musademba, D. Feasibility study of biogas production from water hyacinth. Int. J. Eng. Technol. 2013, 3, 119–128. [Google Scholar]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Leung, H.; Duzgoren-Aydin, N.; Au, C.; Krupanidhi, S.; Fung, K.; Cheung, K.; Wong, Y.; Peng, X.; Ye, Z.; Yung, K.; et al. Monitoring and assessment of heavy metal contamination in a constructed wetland in Shaoguan (Guangdong Province, China): Bioaccumulation of Pb, Zn, Cu and Cd in aquatic and terrestrial components. Environ. Sci. Pollut. Res. 2017, 24, 9079–9088. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.T.; Mahmood, T.; Malik, S.A. Phytoremediation technologies for Ni++ by water hyacinth. Afr. J. Biotechnol. 2010, 9, 8648–8660. [Google Scholar]
- Reyes, A.; Santos, M. Phytoremediation potential of Water Hyacinth (Eichhornia crassipes) in tanks with high organic matter. Int. J. Fish Aquat. Stud. 2019, 7, 107–109. [Google Scholar]
- Zimmels, Y.; Kirzhner, F.; Malkovskaja, A. Application of Eichhornia crassipes and Pistia stratiotes for treatment of urban sewage in Israel. J. Environ. Manag. 2006, 81, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Mardalena, M.; Faizal, M.; Napoleon, A. The Absorption of Iron (Fe) and Manganese (Mn) from Coal Mining Wastewater with Phytoremediation Technique Using Floating Fern (Salvinia natans), Water Lettuce (Pistia stratiotes) and Water Hyacinth (Eichornia crassipes). Biol. Res. J. 2018, 4, 1–7. [Google Scholar]
- Shirinpur-Valadi, A.; Hatamzadeh, A.; Sedaghathoor, S. Study of the accumulation of contaminants by Cyperus alternifolius, Lemna minor, Eichhornia crassipes, and Canna × generalis in some contaminated aquatic environments. Environ. Sci. Pollut. Res. 2019, 26, 21340–21350. [Google Scholar] [CrossRef]
- Sunny, S.A. Globalization and complexity of environmental governance in sustainable development and climate change policy diffusion mechanisms in developing countries-the American response and the case of Bangladesh. J. Sustain. Dev. Stud. 2013, 3, 101–126. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).