Quantitative and Sensitive Mid-Infrared Frequency Modulation Detection of HCN behind Shock Waves
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
3.1. Pressure Broadening Coefficient
3.2. HCN Detection Limit
3.3. Kinetic HCN Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Schmid, D.; Karlström, O.; Yrjas, P. Release of NH3, HCN and NO during devolatilization and combustion of washed and torrefied biomass. Fuel 2020, 280, 118583. [Google Scholar] [CrossRef]
- Stuhr, M.; Hesse, S.; Friedrichs, G. Quantitative and Sensitive Mid-Infrared Frequency Modulation Detection of HCN behind Shock Waves. In Proceedings of the European Combustion Meeting, Online. 14–15 April 2021; 10, pp. 767–772. [Google Scholar]
- Lamoureux, N.; Desgroux, P.; Olzmann, M.; Friedrichs, G. The story of NCN as a key species in prompt-NO formation. Prog. Energ. Combust. 2021, 87, 100940. [Google Scholar] [CrossRef]
- Moskaleva, L.; Lin, M. The spin-conserved reaction CH + N2→ H + NCN: A major pathway to prompt NO studied by quantum/statistical theory calculations and kinetic modeling of rate constant. Proc. Combust. Inst. 2000, 28, 2393–2401. [Google Scholar] [CrossRef]
- Teng, W.S.; Moskaleva, L.V.; Chen, H.L.; Lin, M.C. Ab Initio Chemical Kinetics for H + NCN: Prediction of NCN Heat of Formation and Reaction Product Branching via Doublet and Quartet Surfaces. J. Phys. Chem. A 2013, 117, 5775–5784. [Google Scholar] [CrossRef]
- Faßheber, N.; Dammeier, J.; Friedrichs, G. Direct measurements of the total rate constant of the reaction NCN+ H and implications for the product branching ratio and the enthalpy of formation of NCN. Phys. Chem. Chem. Phys. 2014, 16, 11647–11657. [Google Scholar] [CrossRef][Green Version]
- Klippenstein, S.J.; Pfeifle, M.; Jasper, A.W.; Glarborg, P. Theory and modeling of relevance to prompt-NO formation at high pressure. Combust. Flame 2018, 195, 3–17. [Google Scholar] [CrossRef]
- Faßheber, N.; Lamoureux, N.; Friedrichs, G. The rate constant of the reaction NCN + H2 and its role in NCN and NO modeling in low pressure CH4/O2/N2-flames. Phys. Chem. Chem. Phys. 2015, 17, 15876–15886. [Google Scholar] [CrossRef]
- Dammeier, J.; Faßheber, N.; Friedrichs, G. Direct measurements of the high temperature rate constants of the reactions NCN + O, NCN + NCN, and NCN + M. Phys. Chem. Chem. Phys. 2012, 14, 1030–1037. [Google Scholar] [CrossRef]
- Lamoureux, N.; Merhubi, H.E.; Pillier, L.; de Persis, S.; Desgroux, P. Modeling of NO formation in low pressure premixed flames. Combust. Flame 2016, 163, 557–575. [Google Scholar] [CrossRef]
- Dammeier, J.; Friedrichs, G. Thermal Decomposition of NCN3 as a High-Temperature NCN Radical Source: Singlet-Triplet Relaxation and Absorption Cross Section of NCN(3Σ). J. Phys. Chem. A 2010, 114, 12963–12971. [Google Scholar] [CrossRef]
- Lamoureux, N.; Western, C.M.; Mercier, X.; Desgroux, P. Reinvestigation of the spectroscopy of the transition of the NCN radical at high temperature: Application to quantitative NCN measurement in flames. Combust. Flame 2013, 160, 755–765. [Google Scholar] [CrossRef]
- Faßheber, N.; Bornhorst, L.; Hesse, S.; Sakai, Y.; Friedrichs, G. The Reaction NCN + H2: Quantum Chemical Calculations, Role of 1NCN Chemistry, and 3NCN Absorption Cross Section. J. Phys. Chem. A 2020, 124, 4632–4645. [Google Scholar] [CrossRef]
- Lamoureux, N.; Merhubi, H.E.; Mercier, X.; Pauwels, J.; Desgroux, P. HCN quantitative measurement in a laminar low pressure flame at 1036 nm using pulsed CRDS technique. Proc. Combust. Inst. 2013, 34, 3557–3564. [Google Scholar] [CrossRef]
- Lamoureux, N.; Merhubi, H.E.; Gasnot, L.; Schoemaecker, C.; Desgroux, P. Measurements and modelling of HCN and CN species profiles in laminar CH4/O2/N2 low pressure flames using LIF/CRDS techniques. Proc. Combust. Inst. 2015, 35, 745–752. [Google Scholar] [CrossRef]
- Gersen, S.; Mokhov, A.; Levinsky, H. Diode laser absorption measurement and analysis of HCN in atmospheric-pressure, fuel-rich premixed methane/air flames. Combust. Flame 2008, 155, 267–276. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Z.; Konnov, A.; Aldén, M. Quantitative HCN measurements in CH4/N2O/O2/N2 flames using mid-infrared polarization spectroscopy. Combust. Flame 2011, 158, 1898–1904. [Google Scholar] [CrossRef]
- Hot, D.; Pedersen, R.L.; Weng, W.; Zhang, Y.; Aldén, M.; Li, Z. Spatially and temporally resolved IR-DFWM measurement of HCN released from gasification of biomass pellets. Proc. Combust. Inst. 2019, 37, 1337–1344. [Google Scholar] [CrossRef]
- Varghese, P.L.; Hanson, R.K. Tunable diode laser measurements of spectral parameters of HCN at room temperature. J. Quant. Spectrosc. Radiat. Transf. 1984, 31, 545–559. [Google Scholar] [CrossRef]
- Chang, A.; Hanson, R. Shock-tube study of HCN self-broadening and broadening by argon for the P(10) line of the ν1 band at 3 µm. J. Quant. Spectrosc. Radiat. Transf. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Friedrichs, G. Sensitive Absorption Methods for Quantitative Gas Phase Kinetic Measurements. Part 1: Frequency Modulation Spectroscopy. Z. Phys. Chem. 2008, 222, 1–30. [Google Scholar] [CrossRef]
- Deppe, J.; Friedrichs, G.; Ibrahim, A.; Römming, H.J.; Wagner, H.G. The thermal decomposition of NH2 and NH radicals. Ber. Bunsenges. Phys. Chemi 1998, 102, 1474–1485. [Google Scholar] [CrossRef]
- Friedrichs, G.; Wagner, H.G. Quantitative FM spectroscopy at high temperatures: The detection of 1CH2 behind shock waves. Z. Phys. Chem. 2000, 214, 1723–1746. [Google Scholar] [CrossRef]
- Friedrichs, G.; Herbon, J.T.; Davidson, D.F.; Hanson, R.K. Quantitative detection of HCO behind shock waves: The thermal decomposition of HCO. Phys. Chem. Chem. Phys. 2002, 4, 5778–5788. [Google Scholar] [CrossRef]
- Wang, S.; Hanson, R.K. Ultra-sensitive spectroscopy of OH radical in high-temperature transient reactions. Opt. Lett. 2018, 43, 3518–3521. [Google Scholar] [CrossRef]
- Stuhr, M.; Faßheber, N.; Friedrichs, G. Single-tone mid-infrared frequency modulation spectroscopy for sensitive detection of transient species. Opt. Express 2019, 27, 26499–26512. [Google Scholar] [CrossRef]
- Glarborg, P.; Marshall, P. Importance of the Hydrogen Isocyanide Isomer in Modeling Hydrogen Cyanide Oxidation in Combustion. Energy Fuels 2017, 31, 2156–2163. [Google Scholar] [CrossRef]
- Colberg, M.; Friedrichs, G. Room temperature and shock tube study of the reaction HCO + O2 using the photolysis of glyoxal as an efficient HCO source. J. Phys. Chem. A 2006, 110, 160–170. [Google Scholar] [CrossRef]
- Gordon, I.; Rothman, L.; Hill, C.; Kochanov, R.; Tan, Y.; Bernath, P.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.; et al. The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- Devi, V.M.; Benner, D.; Smith, M.; Rinsland, C.; Sharpe, S.W.; Sams, R. A multispectrum analysis of the ν1 band of H12C14N: Part I. Intensities, self-broadening and self-shift coefficients. J. Quant. Spectrosc. Radiat. Transf. 2003, 82, 319–341. [Google Scholar] [CrossRef]
- Rinsland, C.; Devi, V.M.; Smith, M.; Benner, D.C.; Sharpe, S.W.; Sams, R.L. A multispectrum analysis of the ν1 band of H12C14N: Part II. Air- and N2-broadening, shifts and their temperature dependences. J. Quant. Spectrosc. Radiat. Transf. 2003, 82, 343–362. [Google Scholar] [CrossRef]
- Rao, D.R.; Oka, T. Dicke narrowing and pressure broadening in the infrared fundamental band of HCl perturbed by Ar. J. Mol. Spectrosc. 1987, 122, 16–27. [Google Scholar] [CrossRef]
- Appleton, J.P. Shock-Tube Study of the Vibrational Relaxation of Nitrogen Using Vacuum-Ultraviolet Light Absorption. J. Chem. Phys. 1967, 47, 3231–3240. [Google Scholar] [CrossRef]
- Riley, W.J. Handbook of Frequency Stability Analysis; US Department of Commerce, National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2008.
- Votsmeier, M.; Song, S.; Davidson, D.; Hanson, R. Sensitive detection of NH2 in shock tube experiments using frequency modulation spectroscopy. Int. J. Chem. Kinet. 1999, 31, 445–453. [Google Scholar] [CrossRef]
- Silver, J.A. Frequency-modulation spectroscopy for trace species detection: Theory and comparison among experimental methods. Appl. Opt. 1992, 31, 707–717. [Google Scholar] [CrossRef] [PubMed]

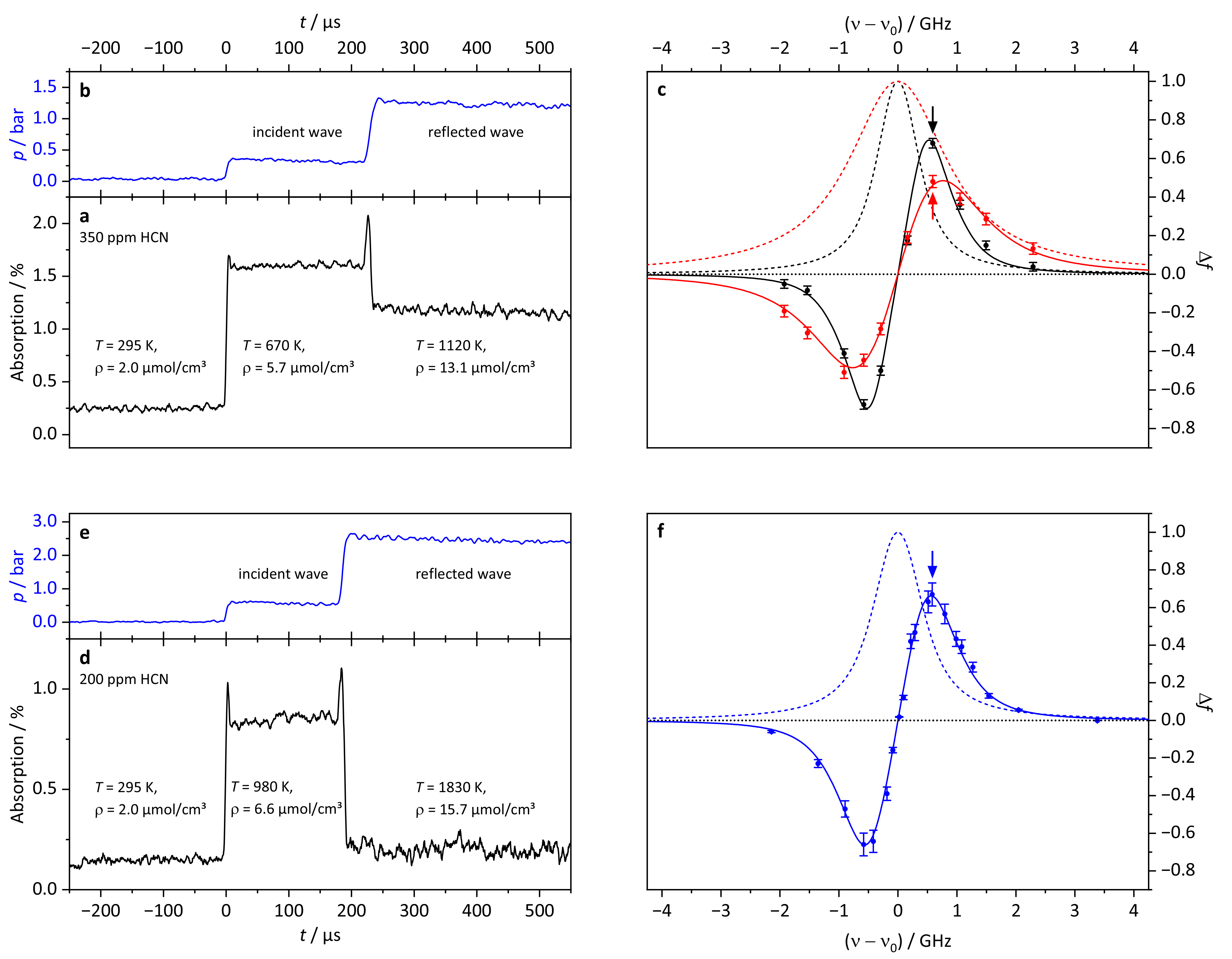
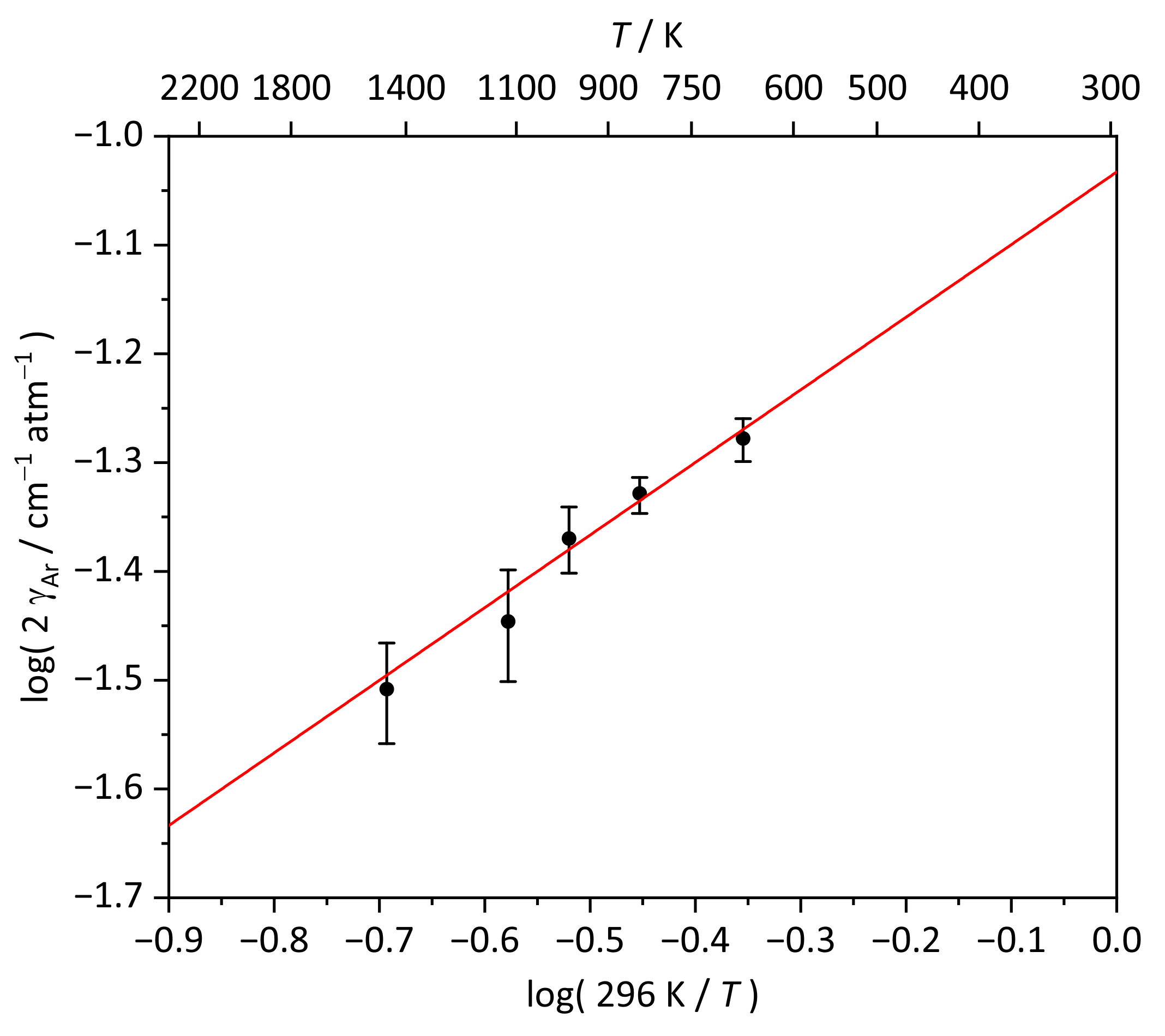
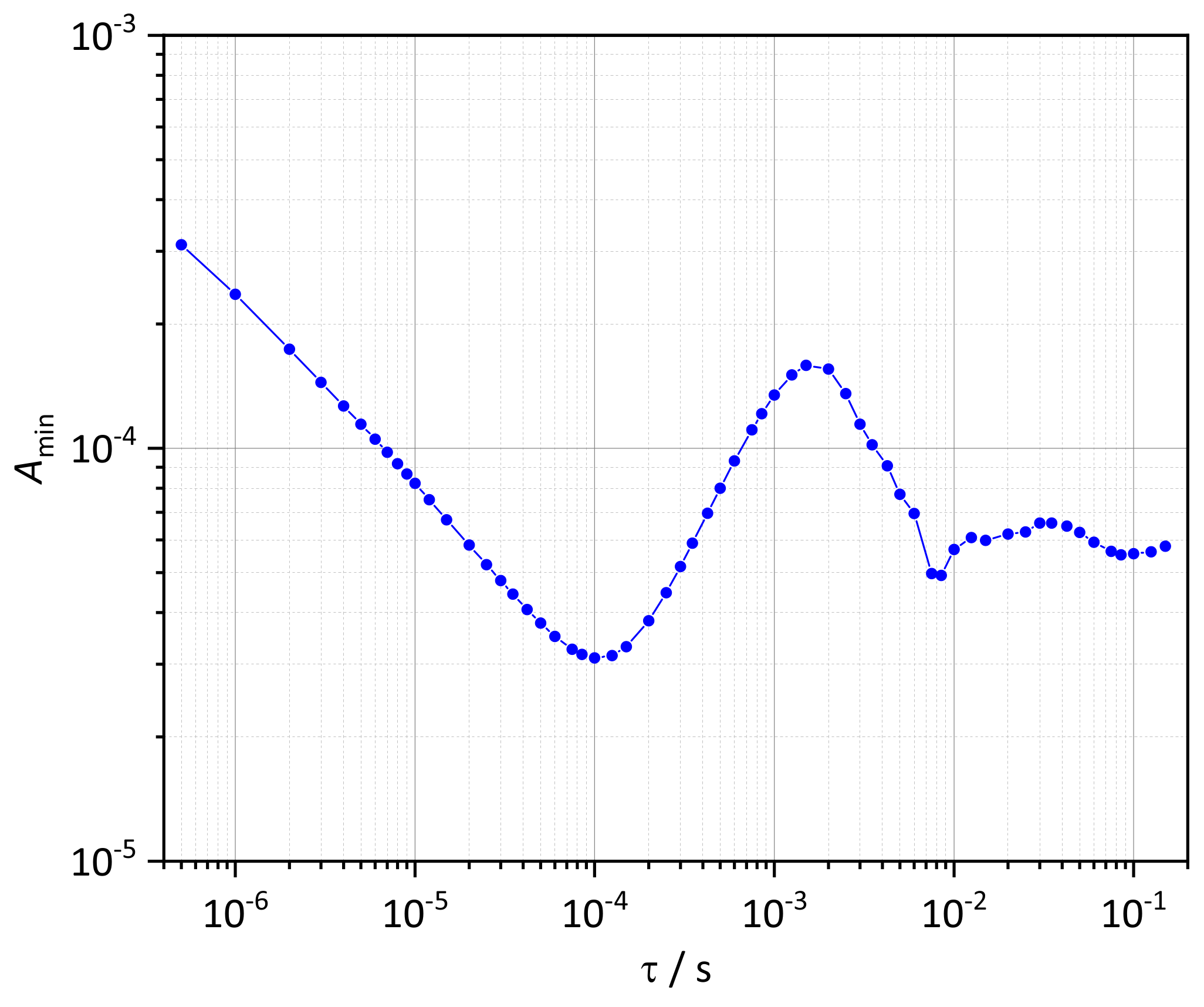
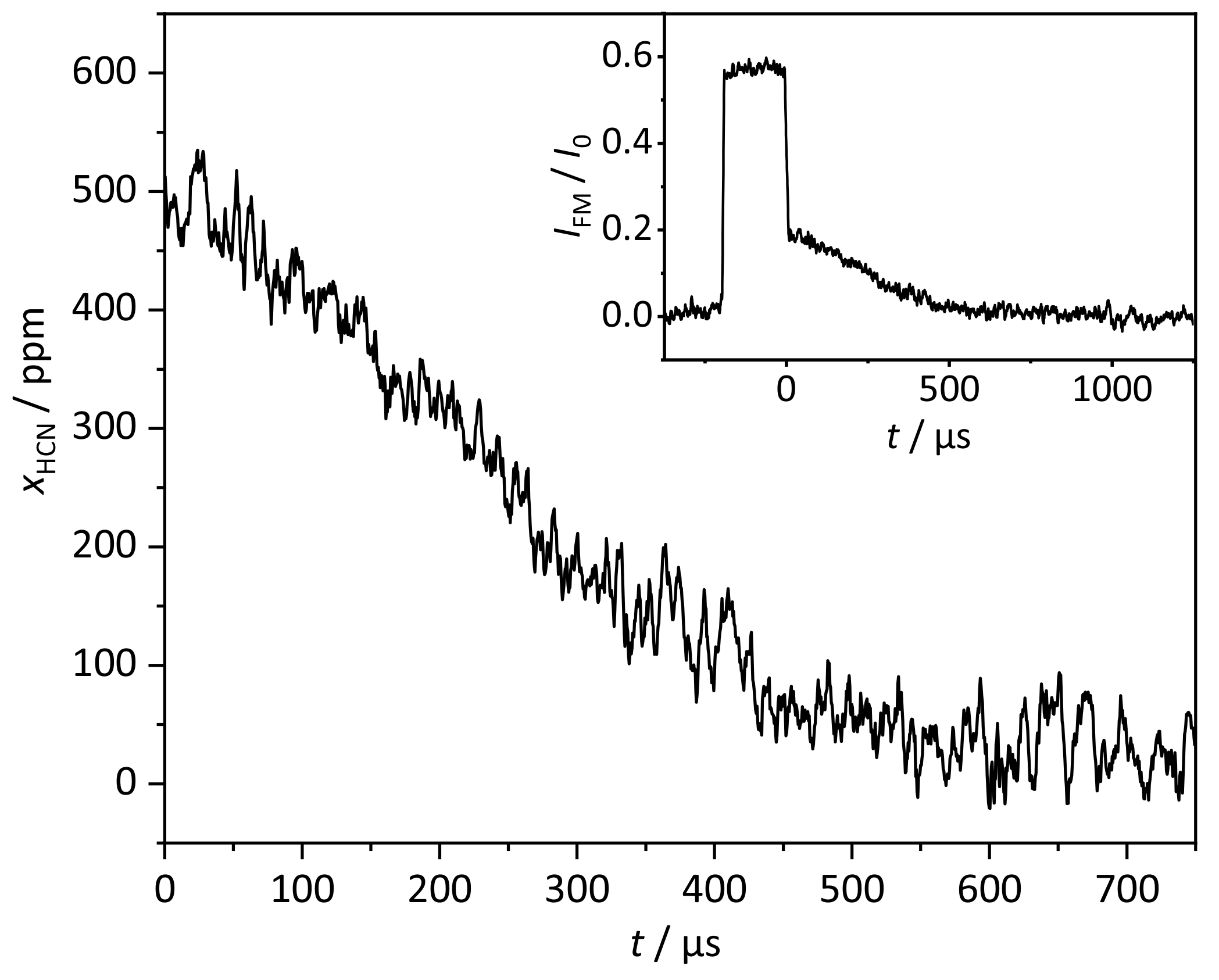
| Data Set | /ppm | T/K | p/bar | /cm | |
|---|---|---|---|---|---|
| 1 | inc. | 200 | 980 | 0.54 | 0.0279 |
| refl. | 200 | 1830 | 2.39 | — * | |
| 2 | inc. | 350 | 670 | 0.32 | 0.0240 |
| refl. | 350 | 1120 | 1.22 | 0.0631 | |
| 3 | inc. | 500 | 840 | 0.21 | 0.0254 |
| refl. | 500 | 1460 | 0.98 | 0.0484 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuhr, M.; Hesse, S.; Friedrichs, G. Quantitative and Sensitive Mid-Infrared Frequency Modulation Detection of HCN behind Shock Waves. Fuels 2021, 2, 437-447. https://doi.org/10.3390/fuels2040025
Stuhr M, Hesse S, Friedrichs G. Quantitative and Sensitive Mid-Infrared Frequency Modulation Detection of HCN behind Shock Waves. Fuels. 2021; 2(4):437-447. https://doi.org/10.3390/fuels2040025
Chicago/Turabian StyleStuhr, Michael, Sebastian Hesse, and Gernot Friedrichs. 2021. "Quantitative and Sensitive Mid-Infrared Frequency Modulation Detection of HCN behind Shock Waves" Fuels 2, no. 4: 437-447. https://doi.org/10.3390/fuels2040025
APA StyleStuhr, M., Hesse, S., & Friedrichs, G. (2021). Quantitative and Sensitive Mid-Infrared Frequency Modulation Detection of HCN behind Shock Waves. Fuels, 2(4), 437-447. https://doi.org/10.3390/fuels2040025






