Abstract
Electric arc furnaces (EAFs) are used for steel production, particularly when recycling scrap material. In EAFs, carbonaceous material is charged with other raw materials or injected into molten slag to generate foam on top of liquid metal to increase energy efficiency. However, the consumption of fossil carbon leads to greenhouse gas emissions (GHGs). To reduce net GHG emissions from EAF steelmaking, the substitution of fossil carbon with sustainable biogenic carbon can be applied. This study explores the possibility of the substitution of fossil material with biogenic material produced by different pyrolysis methods and from various raw materials in EAF steelmaking processes. Experimental work was performed to study the effect of biogenic material utilization on steel and slag composition using an induction melting furnace with 50 kg of steel capacity. The interaction of biogenic material derived from different raw materials and pyrolysis processes with molten synthetic slag was also investigated using a tensiometer. Relative to other biogenic materials tested, a composite produced with densified softwood had higher intensity interfacial reactions with slag, which may be attributed to the rougher surface morphology of the densified biogenic material.
1. Introduction
Electric arc furnace (EAF) steelmaking is primarily used for recycling of scrap steel. An electric current passes through graphite electrodes, creating the arcs that provide the primary energy source for the melting of the scrap metal. Carbonaceous material is used in the EAF steelmaking process for different purposes, as shown in Table 1. Currently, anthracite and coke are the main carbon sources used in EAFs. Due to the utilization of fossil material, EAF steelmaking contributes to greenhouse gas (GHG) emissions in steel production.

Table 1.
Direct GHG emissions of EAF steelmaking (values taken from a study by Thomson et al. [1]).
Carbonaceous material is charged along with scrap as fuel to provide supplementary energy in addition to electrical energy for melting. Carbonaceous material is also injected into molten slag to generate a foam layer to protect the interior walls of the furnace against serious damage from intense arc radiation and to improve energy efficiency [2,3,4]. The charge carbon and injection carbon typically contribute over 50% of the total carbon input for EAF operation.
For a conventional EAF, replacement of charge carbon and injection carbon by biogenic carbon from a sustainable source has the potential to reduce the direct GHG emissions by 78%, from ~98 to ~22 kg CO2-eq/t steel (Table 1).
In fact, it was estimated that 19% of the total CO2 emissions from EAFs could be reduced by the replacement of 60% fossil charge and injection carbon by biogenic carbon [2]. According to another assessment, the GHG reductions from using biogenic material in a mini-mill/EAF were 28–56 kg CO2-eq/t steel without by-product credits, and 37–75 kg CO2-eq/t steel with by-product credits [5]. Yet another study identified multiple applications for biogenic material in Australian EAF operations, and the GHG emission reduction potential was estimated to be around 8–12% [6,7]. A summary of estimated CO2 emissions reductions is listed in Table 2.

Table 2.
Estimated reduction of GHG emissions in EAF steelmaking via utilization of biogenic carbon.
The effect of biogenic material on the resultant steel and slag composition, as well as the slag foaming behavior, are important process aspects to consider when evaluating the use of biogenic material. The GREENEAF project in Europe studied the potential of substituting fossil carbon with biogenic carbon derived from different sources (forestry and agriculture residues) as charge and injection carbon for EAF steelmaking in pilot-scale and industrial trials [2]. For this project, it was reported that steel and slag compositions were not significantly affected by replacing coal with biogenic material. However, it was also reported that biogenic material utilization in EAF steelmaking is limited due to its physical properties, high cost and inadequate supply.
Nonetheless, a deep understanding of the interaction between slag and different biogenic materials is still lacking. The interfacial interaction between slag and biogenic material, as well as chemical reactions, influences the chemical composition of the resultant steel and slag, and determines the extent of slag foaming and the effectiveness of biogenic material in EAF steelmaking. The reduction reactions and slag foaming behavior depend not only on the physical and chemical properties of slag, but also on the carbon source [2]. The properties of biogenic material can vary depending on the parent raw material and treatment. In addition, the availability of biogenic material in North America, especially in Canada, differs dramatically from that in Europe.
In the present work, the feasibility of using biogenic material as charge carbon and foaming agents to reduce GHG emissions associated with EAF steelmaking was explored. The effect of substituting biogenic material for coke on EAF steel and slag compositions was studied. The performance of biogenic material as charge carbon and as a foaming agent was examined in a 50 kg capacity steel melting furnace. The biogenic materials used in the experiments were derived from woody biomass. Lab-scale experiments were performed to examine the interfacial interaction between bio-char and molten slag. Results were validated in 25 kg steel-slag melting tests.
2. Materials and Methods
2.1. Material Characterization
Proximate analyses were carried out following ASTM standard D7582 and ISO 562, while ultimate analyses were performed following ASTM D 5373, ASTM D4239 and ISO 1928. Ash composition was measured following ASTM D4326. All the analyses were carried out at CanmetENERGY’s characterization laboratory in Ottawa.
2.2. Charge Carbon Substitution with Torrefied Wood Pellets
Experimental work was performed to examine the effect of substituting fossil charge carbon with biogenic charge carbon on steel and slag compositions, and on the electrical heating requirements. In the experiments, 25 kg of low-carbon steel was melted with approximately 0.5 kg of charge carbon (coke or torrefied wood pellets, Figure 1) in an induction furnace. The furnace power consumption and sample temperature were recorded and compared for the two cases charged with different materials.

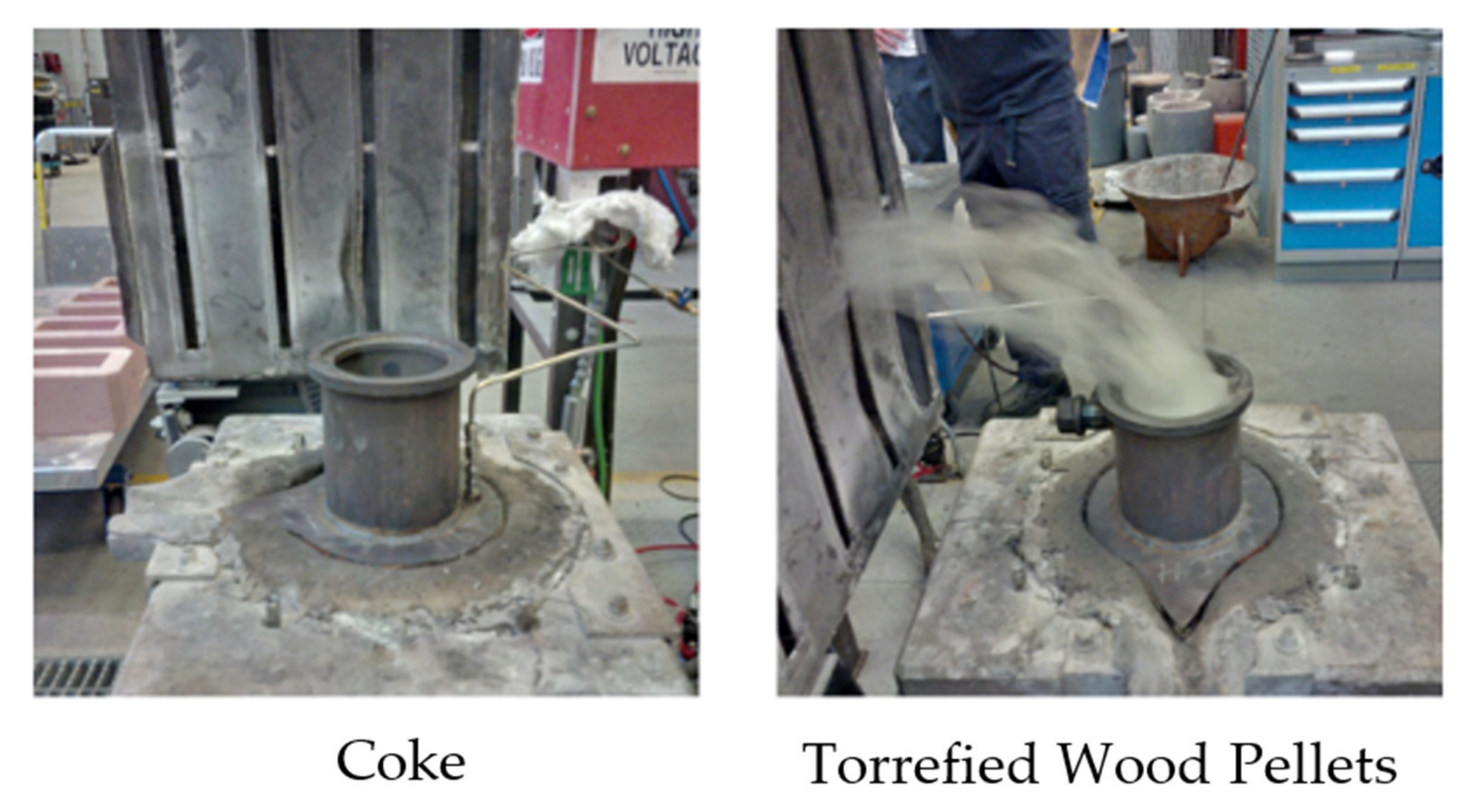
Figure 1.
Appearance of nut coke (left) and torrefied wood pellets (right).
The compositions of the charge carbon materials tested in this work are shown in Table 3. The coke sample was produced by carbonization of metallurgical coal using a pilot-scale oven. The coke that was used is called “nut” coke due to the size of the coke (6–35 mm). This coke is not directly manufactured per se, but is a by-product of blast furnace coke handling and screening. For this paper, nut coke will be called coke.

Table 3.
Charge carbon composition.
The torrefied wood pellets were supplied by an industrial partner and were produced by densification of wood torrefied at a low temperature. The densification procedure cannot be divulged due to a confidentiality agreement. As a result of the pyrolysis conditions, which are relatively mild at a temperature of 260 °C, the volatile matter content is significantly higher than that of coke. Moreover, the ash content of the torrefied wood pellets is much lower than that of coke, as is the carbon content due to the torrefaction time and temperature.
In the experiments, the charge carbon was added with steel at room temperature. The sample temperature and the electrical power consumption of the furnace were continuously recorded to investigate the effect of torrefied wood on melting energy consumption. When the temperature of the liquid steel reached 1630 °C, 1.13 kg of solid slag obtained from industry was added to the molten steel. The steel temperature was increased to 1660 °C and held there for 5 min to complete the melting of the slag.
2.3. Interfacial Interaction between Slag and Bio-Char
Besides charge carbon, biogenic material can also be used to replace injection carbon for slag foaming in EAF steelmaking. The interaction between the carbonaceous material and slag will determine its suitability for slag foaming. Therefore, a detailed study on the interfacial interaction between bio-char and slag was conducted.
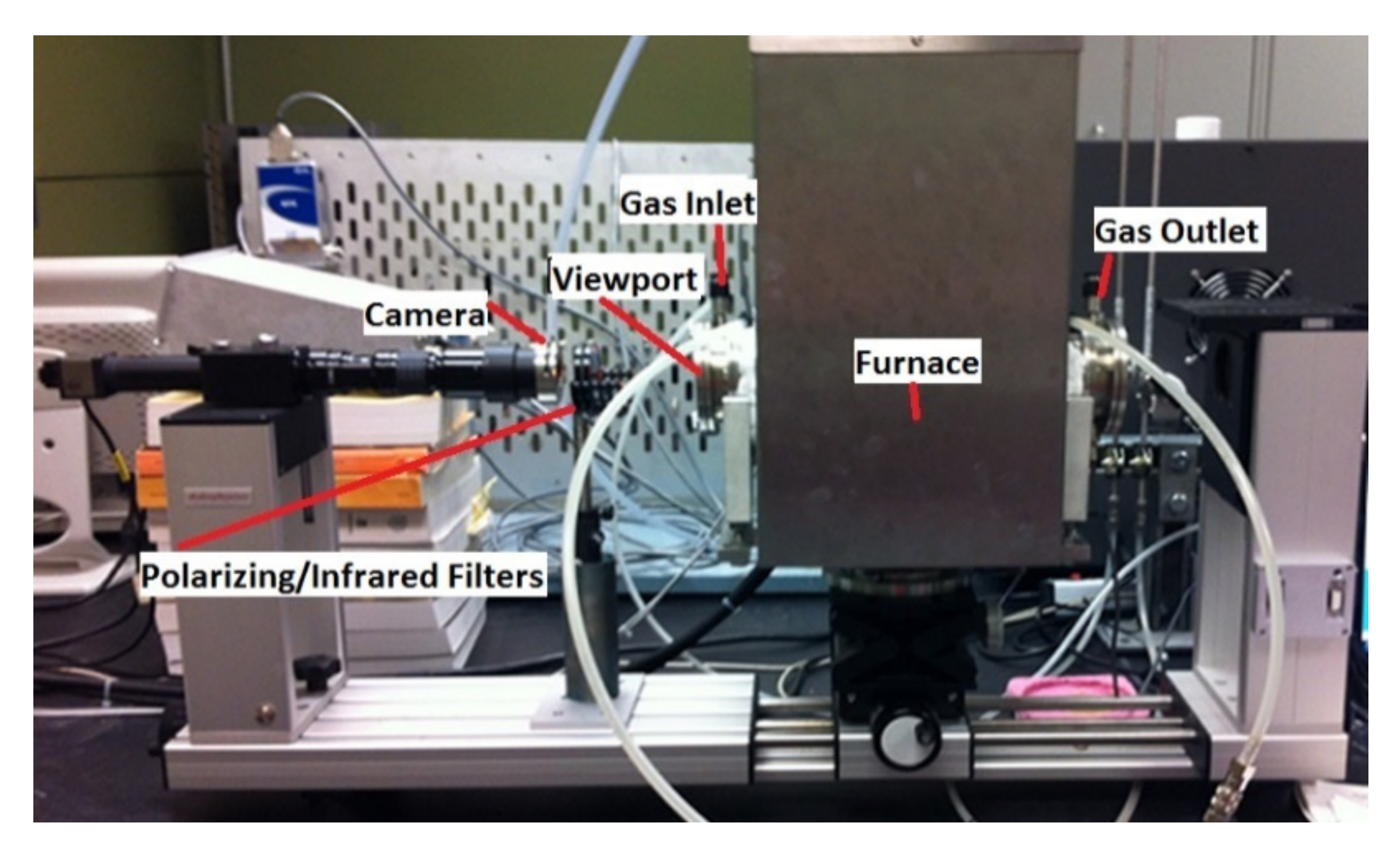
The interaction between slag and carbonaceous material was observed using a sessile drop system in a tensiometer [8]. The method involves placing a pellet of slag on a carbonaceous material bed [9]. From this, the shape of the slag pellet and the contact angle can be measured. The tensiometer consists of a horizontal tube furnace (Dataphysics OCA 15 LHT with HTFC 1700) allowing direct observation of a molten slag droplet sitting on the chosen carbonaceous substrate at up to 1600 °C. The setup is shown in Figure 2.
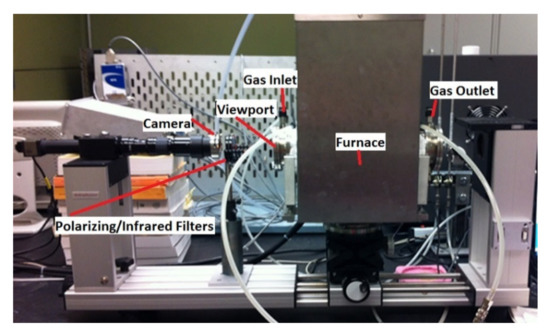
Figure 2.
Tensiometer setup.
The carbonaceous materials examined were ground to 100% less than 63 μm, and 0.50 ± 0.01 g of the crushed carbonaceous sample was packed and leveled in a graphite sample holder with an inner diameter of 20 mm to produce a smooth substrate surface. The bulk density of the substrate bed should be similar for all the cases, since the mass and crucible volume are the same for all the cases. In order to investigate the interaction between different bio-chars and slag, the biogenic materials were prepared with different pyrolysis techniques. Table 4 lists sample information such as pyrolysis technique, raw biomass type, as well as post-processing for densification. Some details have been omitted due to a confidentiality agreement. All biogenic materials were kept at 900 °C for 1 h prior to the tensiometer tests in order to release volatile matter and convert them to bio-char. Different approaches to enhance the interaction between bio-char and slag were tried. Details are presented in Section 3.

Table 4.
Description of bio-char substances and coke.
The chemical compositions of the bio-char substrates can be found in Table 5. As shown, the chemical compositions and ash compositions of the five bio-char substrates selected for analysis in this work are significantly different in order to better examine the effect of chemical composition of the bio-char on the interfacial interaction with the slag. Ash compositions were calculated based on a total material basis. Additionally, there are other minor components as well as the loss of fusion not listed in Table 5.

Table 5.
Chemical composition of biogenic materials, prior to heating at 900 °C, and coke used for interfacial interactions experiments.
Table 6 shows the chemical composition of slag used in this work: 0.20 ± 0.01 g of synthetic slag prepared using analytical grade chemicals was pressed into a cylindrical disc with a 3 mm height and 6 mm diameter. The slag disc was placed onto the substrate and heated in the tensiometer. The entire heating was carried out under a reducing gas atmosphere of 4 vol% H2 and 96 vol% Ar gas. Mixtures of oxides were used to avoid the compositional variability of industrial slag. Although results from a single test with each material are presented, a minimum of two tests with each material were conducted and produced similar results.

Table 6.
Chemical composition of synthetic slag.
It was estimated from thermochemical modeling using FactSage (an integrated thermochemistry database and computing system) that the liquidus temperature of the slag examined in this work is about 1450 °C. To ensure the slag is fully molten and to use a temperature similar to the industrial process, experiments were conducted by heating the sample up to 1600 °C for observing the interaction between the molten slag and the carbonaceous substrate.
In each experiment, the sample was preheated to 750 °C and then heated to 1300 °C at 12 °C/min. After reaching 1300 °C, the sample temperature was further increased by 5 °C/min until reaching 1600 °C. During this final heating period, the shape of the slag sample was continuously recorded at a frame rate of 1.5 frames/second to capture its physical evolution. The recording was started when the slag shape began to change and continued until the sample had been at 1600 °C for 30 min.
2.4. Slag Foaming by Bio-Char
In these experiments, 23 kg of steel was melted using an induction furnace of 100 kW. When the temperature of the liquid steel reached 1630 °C, approximately 1.13 kg of industrial slag was charged in the furnace crucible and the sample temperature was increased to 1660 °C to melt the slag. Sixty grams of coke or bio-char (HSBC sample, as shown in Table 5) samples with a controlled size (~0.6 mm) were added on top of the molten slag. The slag and carbonaceous material were stirred and mixed thoroughly and quickly. The slag heights before and after adding carbonaceous materials were measured for comparison. The slag heights after adding carbonaceous materials were measured at 5 min intervals for 30 min. The maximum measured slag height was recorded as the slag foaming height.
3. Results and Discussion
3.1. Effect of Charge Carbon Substitution by Torrefied Wood Pellets on EAF Operation
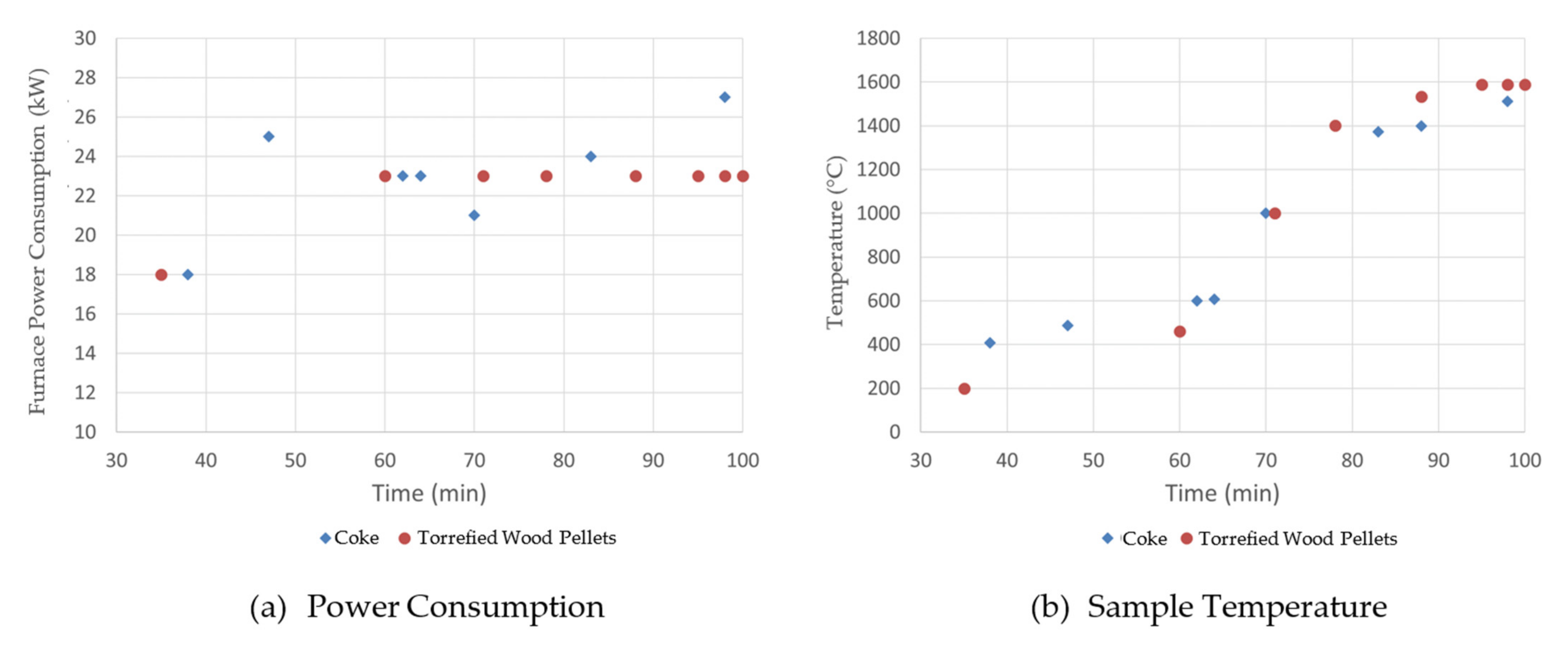
Figure 3 shows the furnace power consumption and sample temperature during steel melting tests. As shown, the power consumption for the coke case was not as stable as for the bio-char case. However, the total energy supplied, i.e., the integration of power over time (which is the area under the Figure 3a curves), is very similar for each case. The molten steel temperature profiles are also similar between the two cases. This indicates that the substitution of coke by torrefied wood pellets has no obvious impact on energy demand to melt the steel.
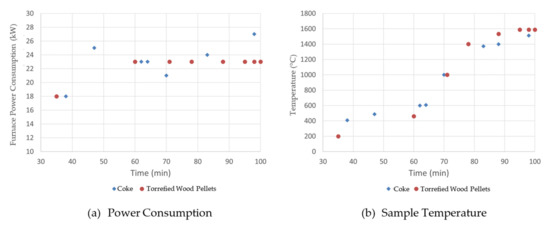
Figure 3.
Effect of torrefied wood pellets on steel melting power consumption.
Samples of steel and slag were collected for chemical composition analysis at the end of the experiments. The results are shown in Table 7 and Table 8. The replacement of coke with torrefied wood pellets as charge carbon results in decreased carbon, sulfur and phosphorus contents in the resultant steel. This is due to the fixed carbon, sulfur and phosphorus content in the torrefied wood tested in this work, which is significantly lower than that of coke. The observation is in agreement with findings reported in the literature [2]. As the ash content in the torrefied wood pellets is significantly lower than that of coke (1.9% vs. 9.8%, see Table 3), its influence on slag composition is small (Table 8). The contents of SiO2 and Al2O3 in slag after the addition of coke are slightly higher than in the torrefied wood pellets case, which is due to their larger contents in the coke. Note that only a small amount of carbon was charged on top of the slag, with a carbon-to-slag mass ratio of 0.4. Therefore, the ash contained in charged carbon is small compared to the charged slag amount. Thus, the experimental results obtained from this work reveal that the replacement of coke with torrefied wood pellets as charge carbon in EAF operation has no negative impacts on both steel and slag compositions. It should be noted that the Al2O3 crucible was eroded by slag during the melting process within a range of 8 to 12 mm/h. The corrosion of the crucible impacted the composition of the slag.

Table 7.
Effect of torrefied wood pellets on steel composition.

Table 8.
Effect of torrefied wood pellets on slag composition.
During the melting experiments, it was observed that intense smoke was generated in the test with torrefied wood pellets when the temperature of the sample reached about 400 °C, as illustrated in Figure 4. This is due to the high volatile matter content in torrefied wood pellets, which is significantly higher than that of coke. It is hypothesized that upon reaching 400 °C, the torrefied wood pellets started to devolatilize, resulting in intense smoke generation. The rapid release of volatile matter may impose challenges in an industrial operation. Another possible cause for the smoke may be vaporization of alkalis from torrefied wood or combustion near the top of the furnace. Nonetheless, the challenges may be overcome by adjusting torrefied wood pyrolysis conditions, i.e., opting for higher idle temperatures and longer residence times. As the pyrolysis severity increases, the volatile matter content in the resultant torrefied wood decreases, and this should reduce the amount of volatile matter that can be released when used in the EAF. This should also delay the volatile matter release, due to a higher temperature that is needed to initiate the devolatilization, and produce a torrefied wood with a higher carbon content. Furthermore, using densified torrefied wood might delay the volatile matter release due to its higher density and larger particle size, which can reduce operational risks. However, this could also influence the interaction of the torrefied wood with slag.
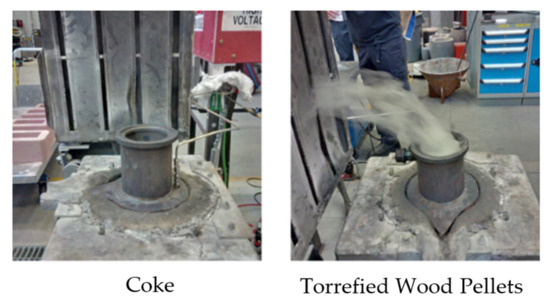
Figure 4.
Observations of smoke production.
3.2. Interfacial Interaction between Slag and Bio-Char
3.2.1. Enhancement of Interaction between Bio-Char and Slag by Ash Composition Modification
Our previous research demonstrated that the interaction between bio-char and slag was poor compared to the interaction between coke and slag [9]. It was reported that the content and chemical composition of minerals in the carbonaceous substrate can change the chemical composition of slag and affect interactions [4]. Hence, the content and chemical composition of ash in bio-char has been modified in some of our tensiometer experiments to explore a proper approach for improving interactions between slag and bio-char (see Table 9). The slag used in HSBC-slag is the same slag used to prepare the slag disc. Ash content in typical metallurgical coke is about 10 wt.%, and that of the studied slow pyrolysis bio-char (HSBC) is approximately 2 wt.% (see Table 5). Thus, adding 10 wt.% of Fe(NO3)3 or slag to the HSBC sample will result in an ash content value closer to that of coke.

Table 9.
Modification of the substrate’s chemical composition.
Our previous study also showed that the crystallinity and graphitization degrees of coke are higher than those of bio-char [9]. The greater interaction of coke may be due to its higher crystallinity. Note that in order to make the reactive properties of bio-char similar to metallurgical coke, the degree of crystallinity and structural order of bio-char may have to be increased. It was reported that the carbon’s structural order in woody material can be increased by Fe catalysts during high-temperature pyrolysis [10,11]. Since a high temperature was employed during our tensiometer tests, Fe may induce crystallization of bio-char and consequently enhance its reaction with slag. Iron nitrate (Fe(NO3)3) was selected as an additive to mix with bio-char, since it will not introduce impurities to the system.
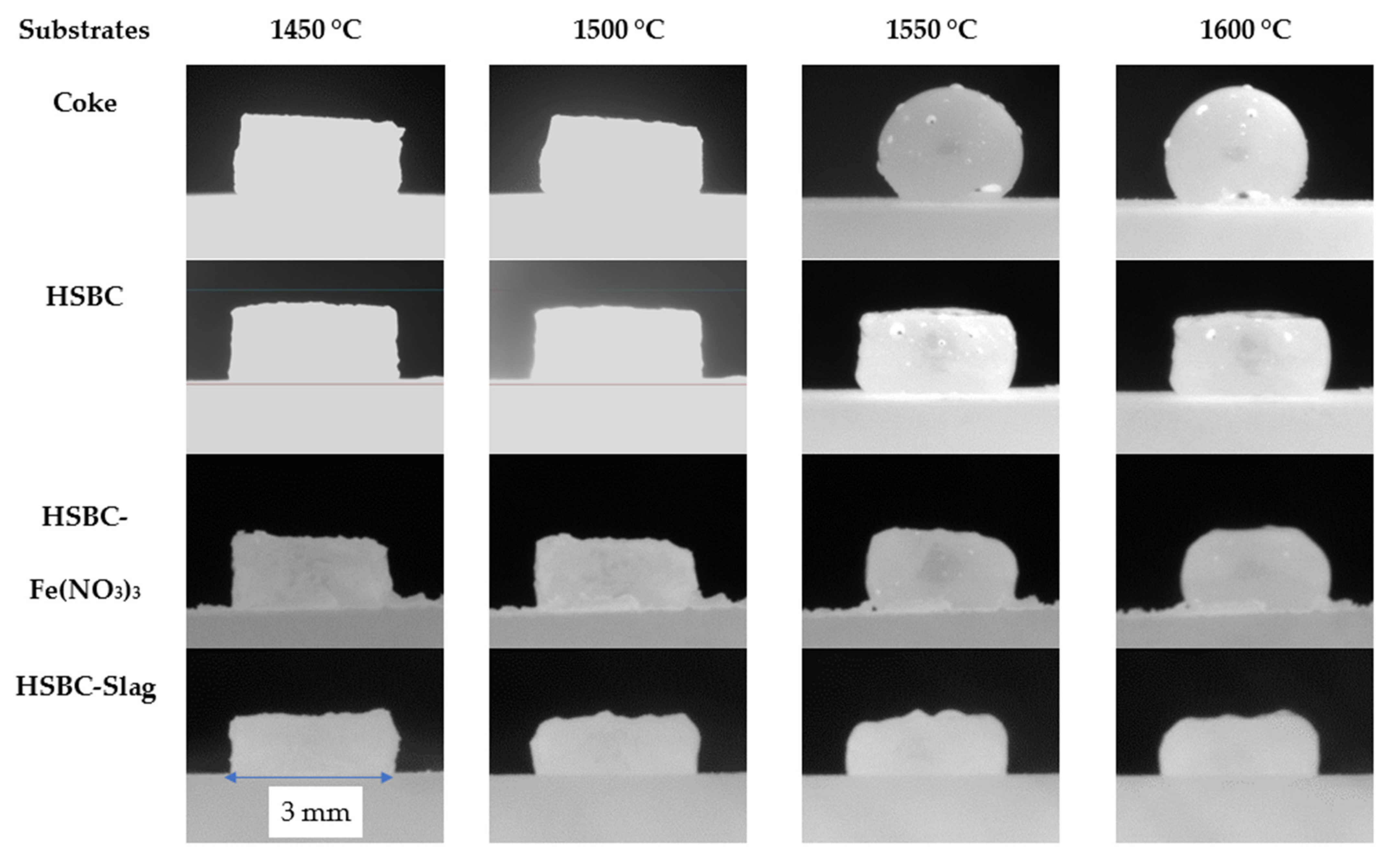
Figure 5 compares the appearance of the slag pellet after the sample temperature reached 1450 °C to highlight the effect of the substrate on the behavior of the molten slag.
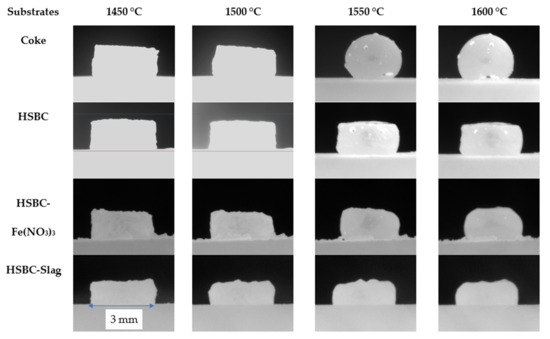
Figure 5.
Comparison of the interaction between slag and carbonaceous substrates, with a focus on bio-char ash modification.
With reference to Figure 5, the observed interaction between the slag and different substrates is summarized in Table 10.

Table 10.
Summary of interfacial interaction observations.
Due to the fact that the slag pellet did not form a droplet on the three bio-char substrates, the contact angle between the molten slag and the substrates could not be measured for these materials. The entrapment of gas in the slag causes the slag to foam in an industrial EAF operation. In the above experiments, it is assumed that part of the Fe2O3 placed in the slag mix had already been reduced to FeO [12,13]. The gas formation at the slag/substrate interface arises from the reaction between carbon and FeO in the slag to form CO2 or CO gas. As observed, the interaction between the hardwood bio-char substrate is drastically weaker than that of coke examined in this work. It signifies that the foaming capacity of bio-char is relatively much weaker [9]. By blending Fe(NO3)3 or slag with bio-char, an improvement in interactions with slag can be observed compared to the original bio-char. However, the slag shapes on both modified bio-char substrates are still close to the pellet shape, indicating that the blends resulted in limited enhancement of this interaction. Moreover, bio-char with added slag or Fe(NO3)3 possesses a higher ash content, and the amount of additional elements other than carbon in bio-char materials will introduce impurities to the steelmaking process, which in turn can increase operation issues and costs. Further investigation is required to determine why the slag does not appear to melt in the case of bio-char.
3.2.2. Enhancement of the Interaction between Bio-Char and Slag by Raw Biomass Selection
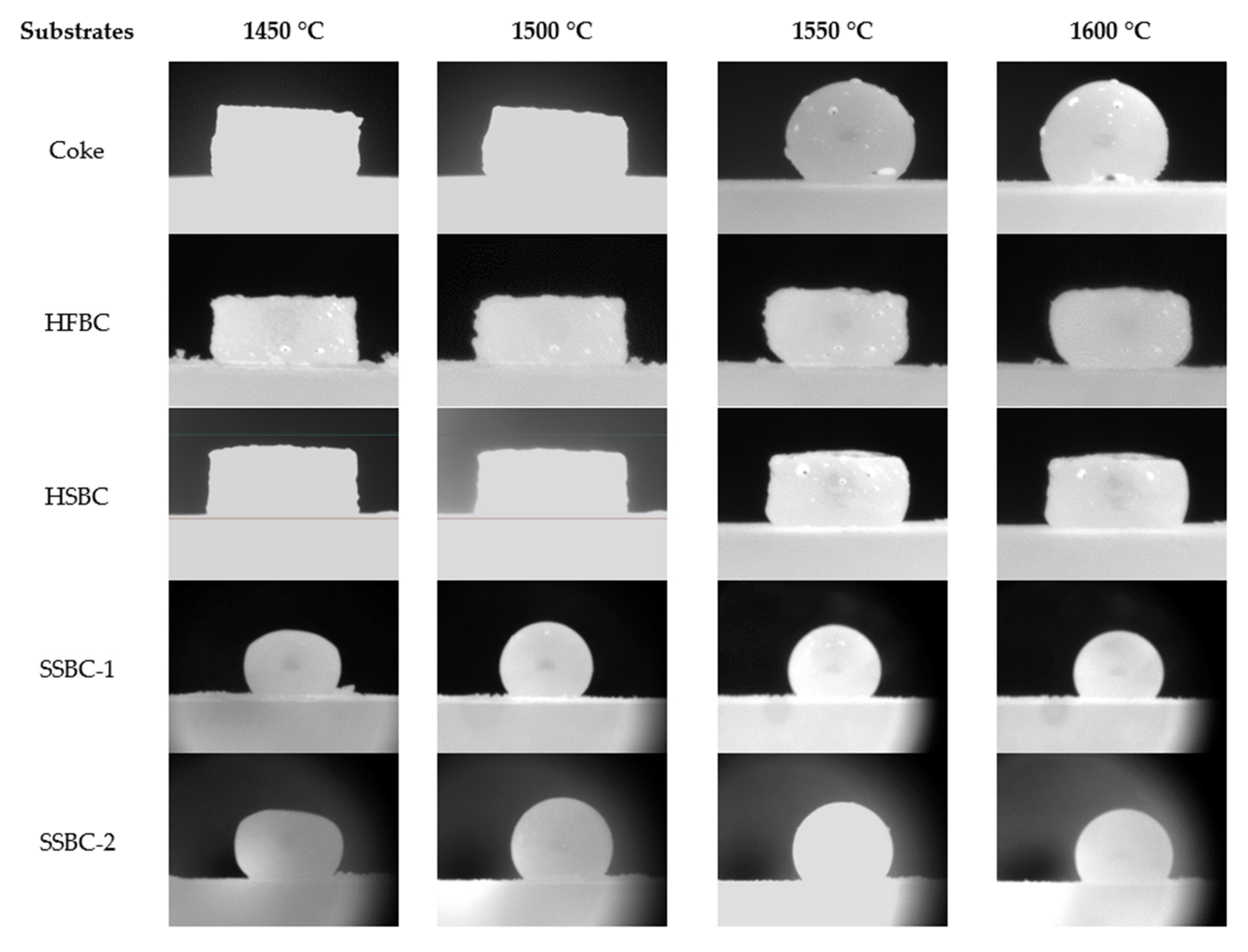
Using the tensiometer, the interaction between the synthetic slag and the different bio-char substrates was examined (Table 4). The results were compared with coke as the reference case. Figure 6 compares the appearance of the slag pellet after the sample temperature reached 1450 °C to highlight the effect of the substrate on the behavior of the molten slag.
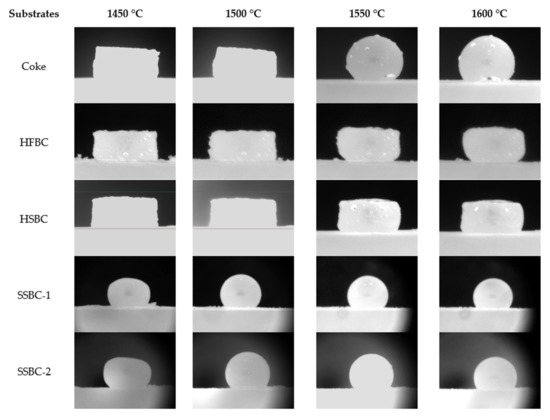
Figure 6.
Interaction between slag and coke, and between slag and different bio-char substrates.
Although the slag’s liquidus temperature was estimated to be about 1450 °C according to thermodynamic calculations, the actual liquidus temperature appeared to be different with different carbonaceous substrates. The reason is still unclear and is under investigation. It is possible that the reduction of FeOx within the slag pellets raised the liquidus temperature. Comparing the evaluation of slag droplets at different moments shown in Figure 6, the observed interaction between the slag and different substrates is summarized in Table 11.

Table 11.
Summary of interfacial interaction observations.
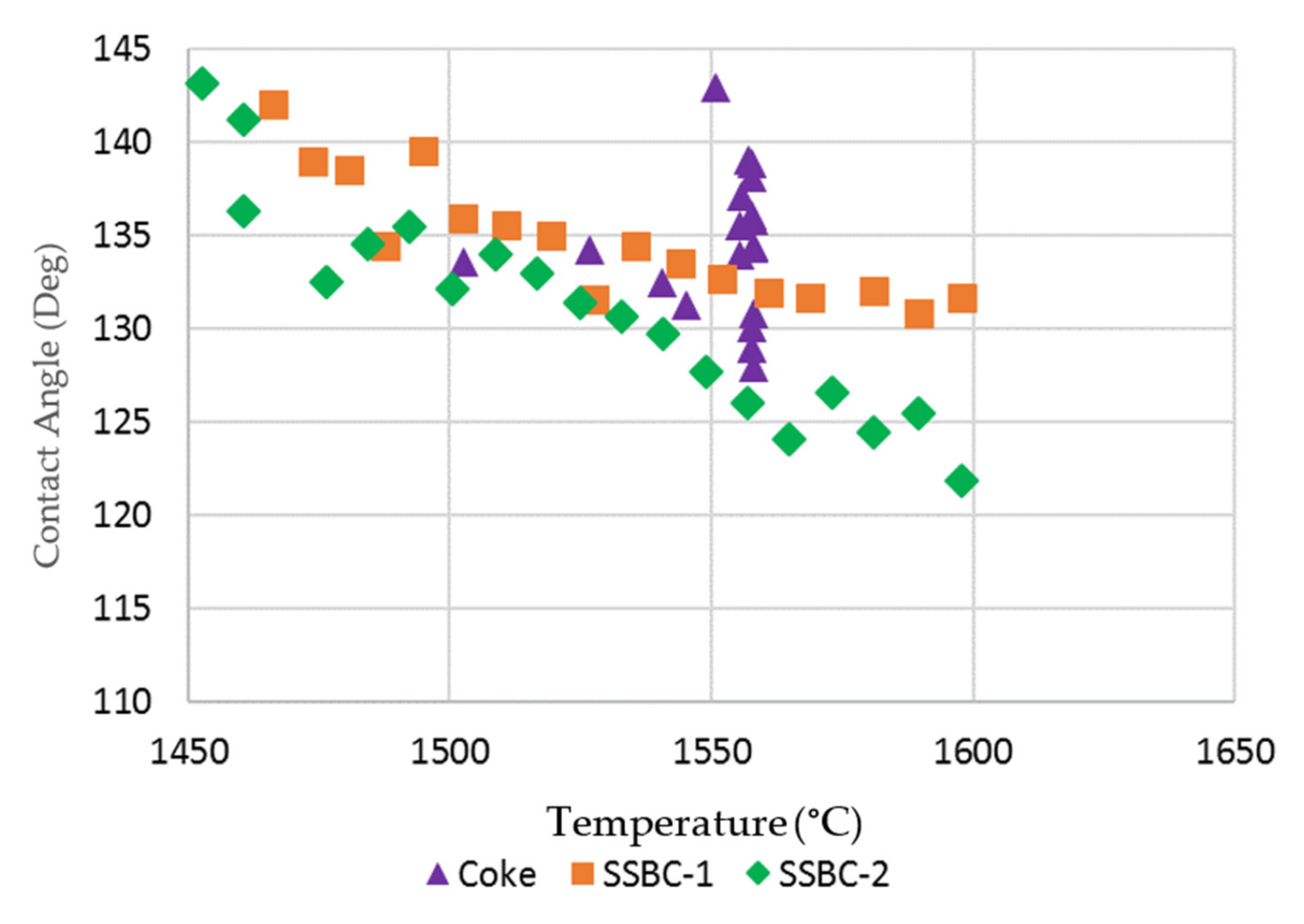
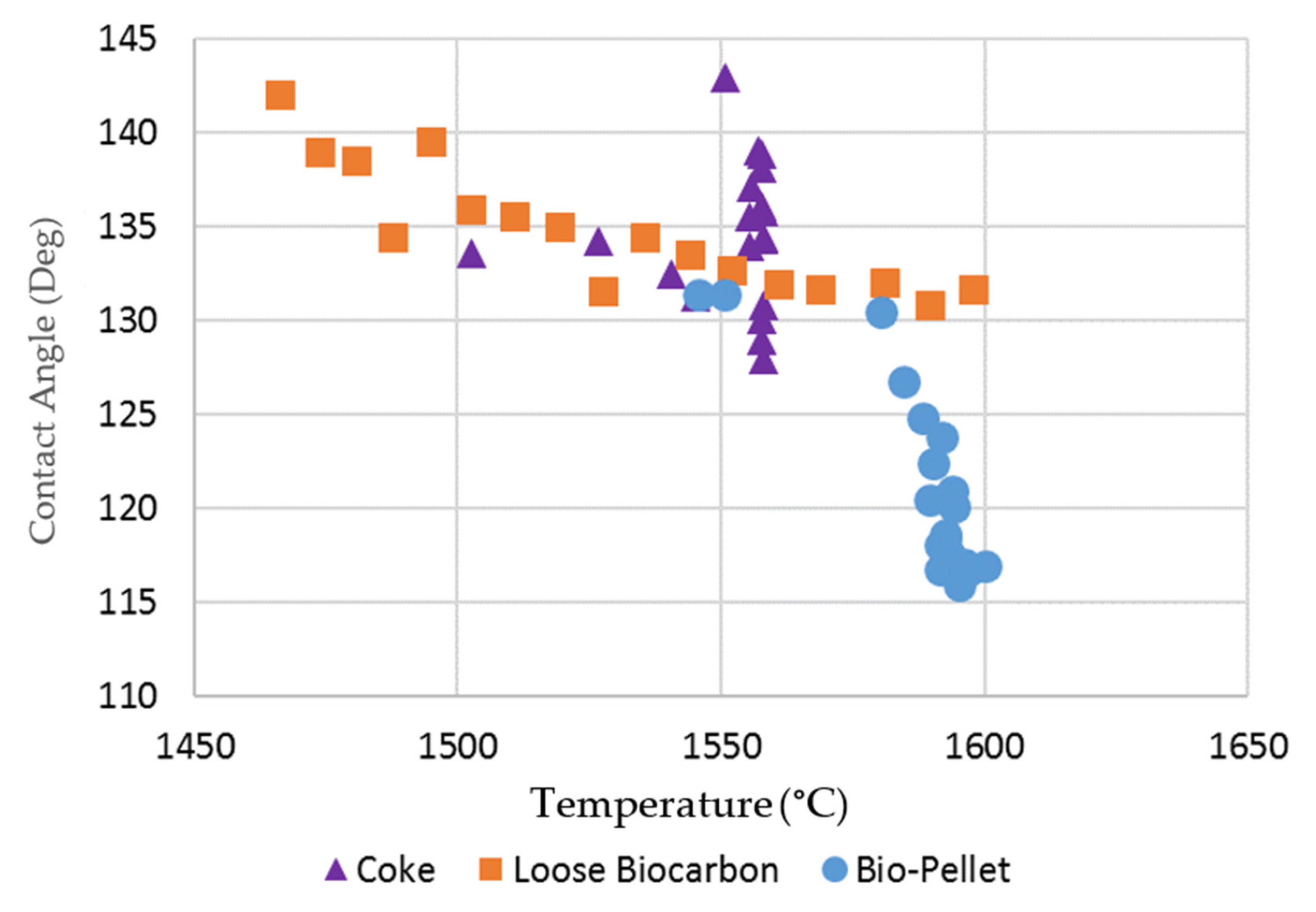
As the slag pellet did not form a droplet on the two hardwood bio-char substrates, the contact angle between the molten slag and the substrates could not be measured. The contact angles for the softwood bio-char substrates at temperatures > 1450 °C were measured by image analysis, and are presented in Figure 7 and compared to the contact angle on the coke substrate. Due to the continuous rolling movement of the slag droplet on the coke bed surface, the contact angle of the slag droplet varies between measurements. For the coke substrate, there was no obvious trend for contact angle as a function of temperature. For the slag on the softwood bio-char samples, as the temperature increased, the contact angles were reduced. Smaller contact angles indicate increased wetting. Our experiments indicate that the wettability of molten slag using softwood bio-char is somewhat similar to that on coke at temperatures greater than 1550 °C.
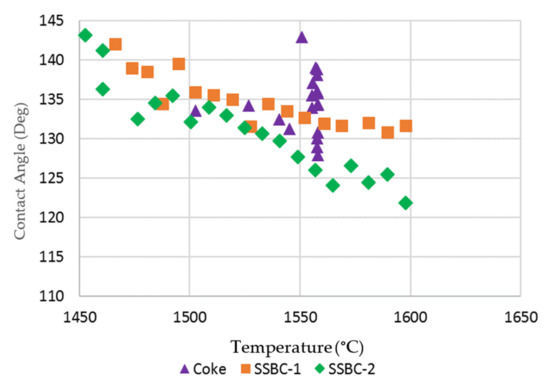
Figure 7.
Contact angle of molten slag on coke and loose bio-char substrates.
In these experiments, the gas formation (CO2 or CO) at the slag/substrate interface arises from the reaction between carbon and FeO in the slag. As observed, the degree of slag interaction with the hardwood bio-char substrate is drastically weaker than the interaction with coke. The slag shapes on both hardwood substrates are close to the initial slag pellet’s shape, signifying that the foaming capacity of bio-char is relatively weaker than that of coke [14,15]. Using softwood bio-char, there was an improvement in wettability with slag in comparison to the hardwood bio-char. However, as the concentration of gas bubbles formed in the slag on softwood bio-char is still very low, the softwood bio-char may have limited interaction with the slag.
3.2.3. Enhancement of Interaction between Bio-Char and Slag by Densification of Bio-Char
A possible factor influencing the slag–carbon interaction is the morphology of the substrate surface [9]. The roughness of the particle surface appears to affect the interaction between liquid and solid phases. The gas is formed at the slag/substrate interface from the reduction of FeO in the slag by carbon in the substrate. The progress of this reaction is determined by the heterogeneous gas bubble nucleation, followed by growth and eventually detachment of gas bubbles at the interface. Heterogeneous gas bubble nucleation depends on the wetting between the liquid and solid phase, as well as the roughness of the solid surface. It is reported in the literature that bubble formation in water is enhanced by a hydrophobic surface [16]. However, the enhancement in bubble nucleation is only observed on rough hydrophobic surfaces [17].
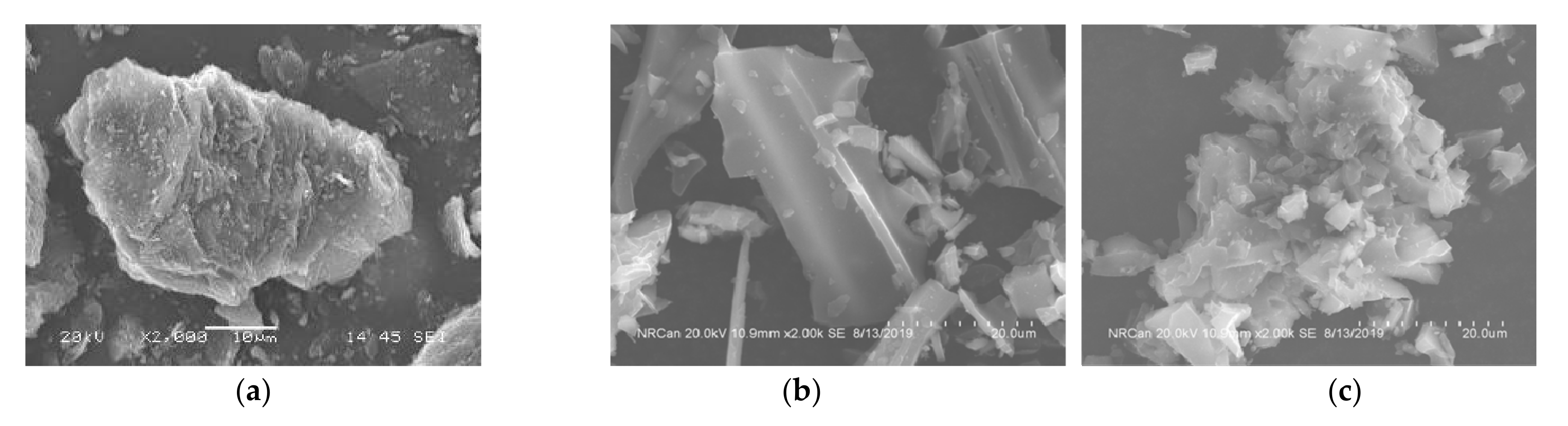
The surface morphologies of loose bio-char and densified bio-char were studied using scanning electron microscopy (SEM) (Figure 8). Based on the visual examination of the SEM images, the surfaces of the loose bio-char are smooth in comparison to densified bio-char substrates examined in this work.

Figure 8.
Surface morphology of: (a) coke, (b) loose softwood bio-char and (c) densified softwood bio-char.
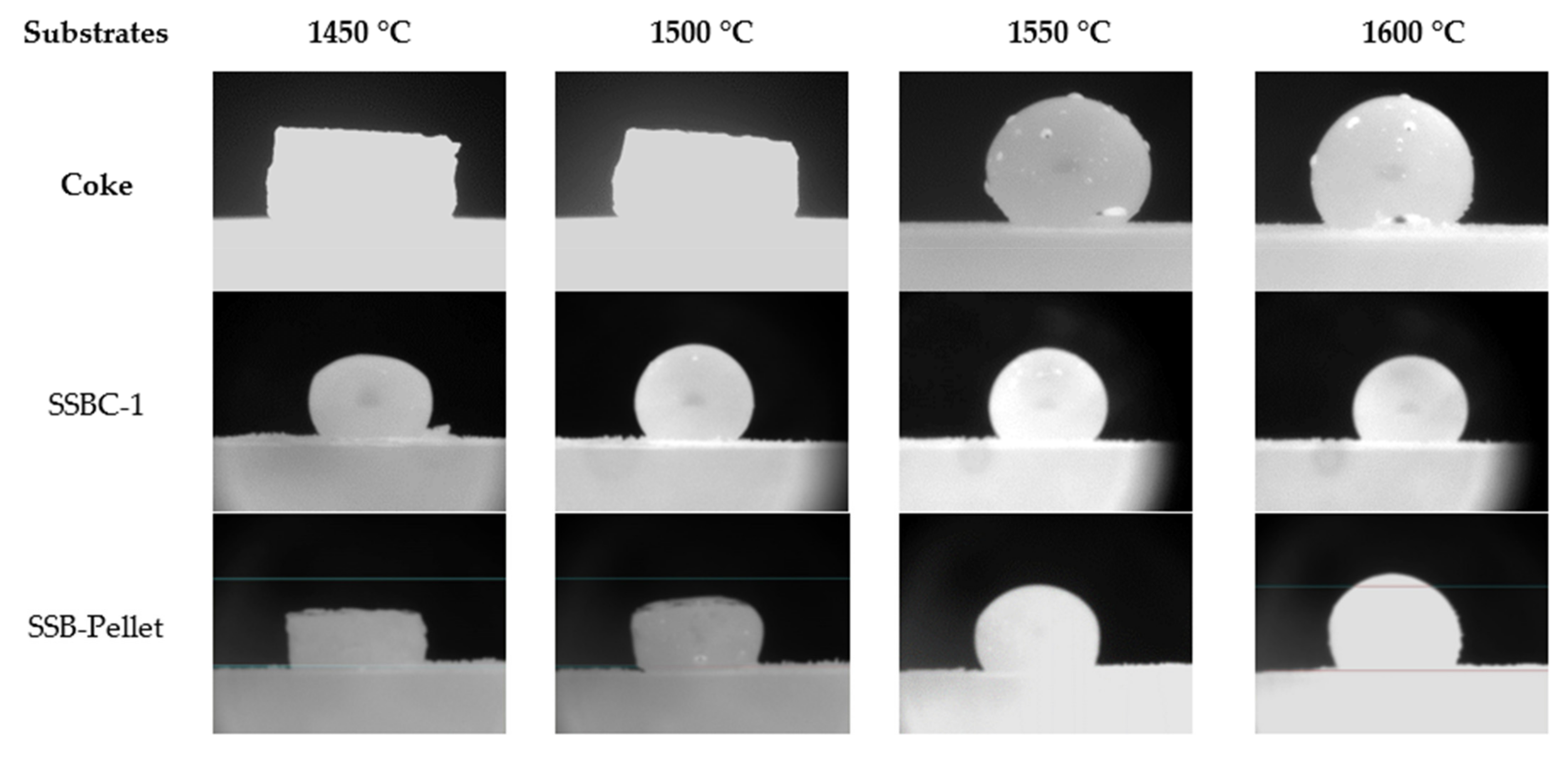
The rough surface of densified bio-char may provide more bubble nucleation sites for gas bubbles to be formed, grow and then detach. The formation of gas bubbles at the interface allows the reduction of FeO in slag to progress. As indicated in Table 3, SSB-Pellet, produced by densification of SSBC-1 and bio-oil, was also used in the tensiometer tests. It can lead to a more intense initial interaction between the slag and substrate, as observed in tensiometer experiments, i.e., refer to the photo for the SSB-Pellet at 1550 °C in Figure 9. Consequently, the contact angle of molten slag on the densified bio-char substrate is relatively smaller compared to those on coke and loose bio-char (Figure 10).
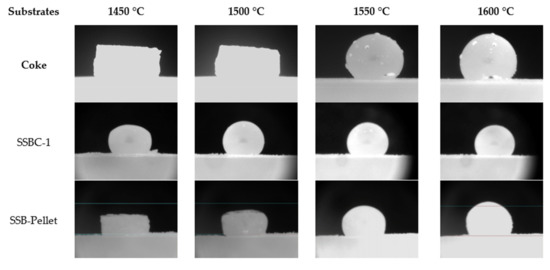
Figure 9.
Slag droplets on loose (SSBC-1) and densified (SSB-Pellet) bio-char substrates.
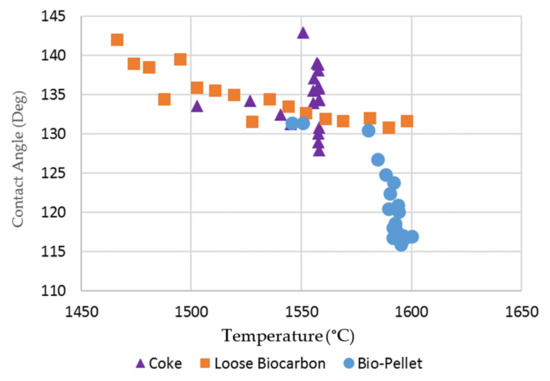
Figure 10.
Contact angle of molten slag on coke, loose bio-char (SSBC-1) and densified bio-char (SSB-Pellet) substrates.
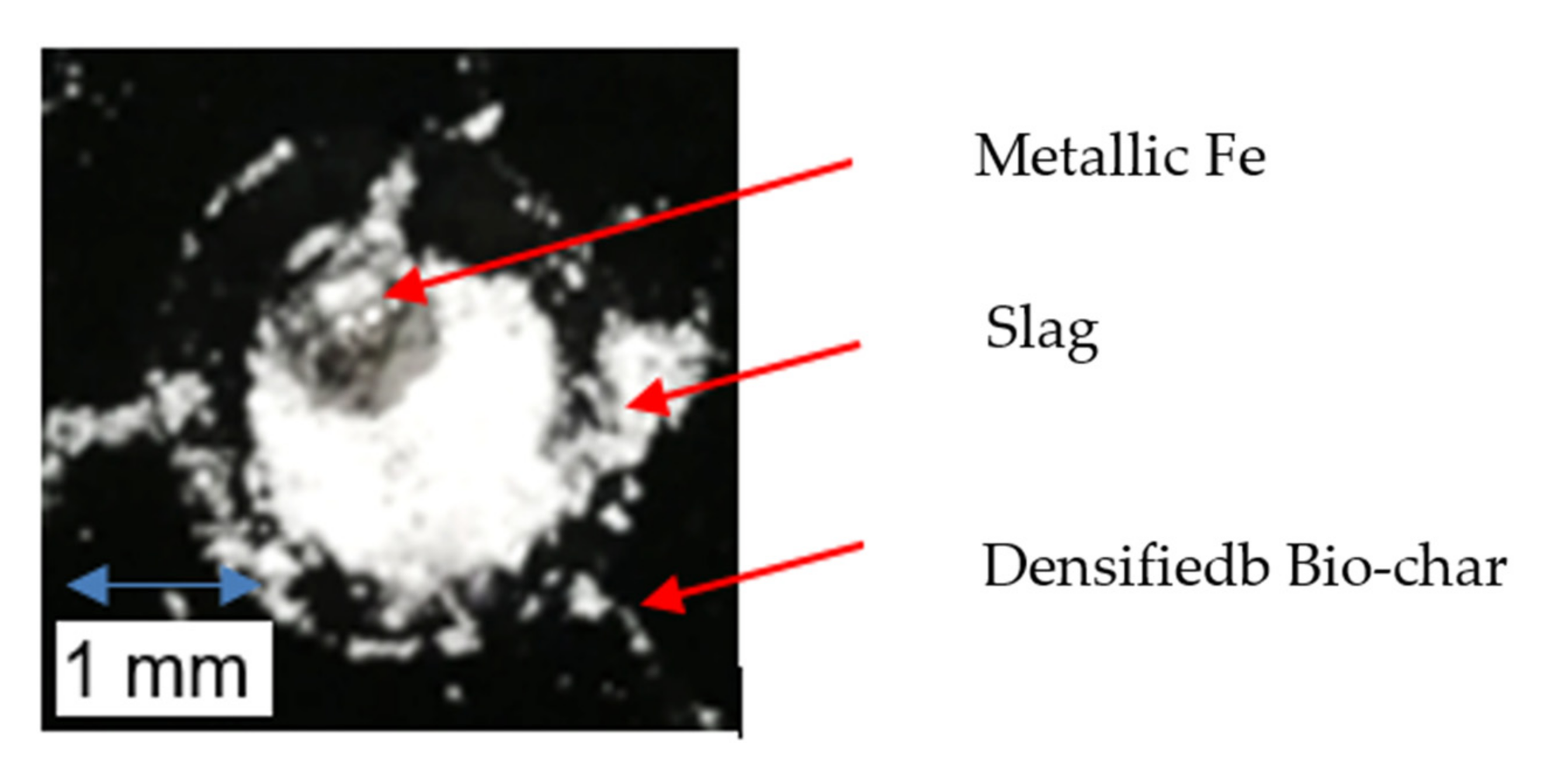
Metallic Fe is also produced during the interaction between slag and densified bio-char, as seen in Figure 11. According to thermodynamic simulations using FactSage, it is possible for solid metallic Fe to be formed at the carbon–slag interface for this system at 900 °C. However, solid Fe is unlikely to form at the temperatures of interest in this study as the Fe produced at the substrate–slag interface is likely to be near saturation with carbon. The eutectic for the Fe-C system is 1147 °C at 4.3 wt.% C, so the iron should be in a liquid state. Nonetheless, the metallic Fe may act as a barrier to reaction. The lack of rolling movement of the slag droplet on the SSB-Pellet substrate may impede contact between active slag and bio-char, thus restricting the reduction reaction of FeO. This can lead to limited gas bubble generation and weak interactions between slag and the densified bio-char substrate at late stages of the tensiometer experiment, as seen in Figure 9. In actual EAF operation, the interaction between injected carbonaceous material and the slag could be more dynamic than in the static system in the tensiometer, with the interface being constantly renewed, as in the case of the rolling liquid slag on coke. Perhaps the bio-chars would not be as disadvantaged by metallic iron in this case.
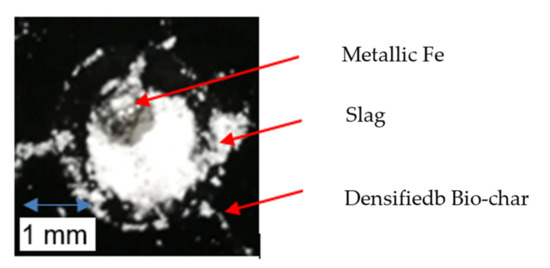
Figure 11.
Metallic Fe from the reaction between slag and densified bio-char.
To determine whether the metallic Fe acts as a barrier to reaction, or rather as an accelerator to promote it, additional research is required.
It can be speculated that once the slag melts and reacts with the carbon in the substrate, a layer of molten metallic iron-carbon alloy would form and settle between the slag and substrate. Thus, further reaction could depend on the wetting of the substrate by the alloy, dissolution of carbon by the liquid iron alloy and then reduction of the FeOx in slag by the alloy via CO/CO2 at the interface [6,7,18]. The presence of a higher level of surface-active sulfur in the coke tends to affect the wetting of coke by the molten iron alloy and may be a key reason for the observed behavior. The C-to-slag ratio is much higher in the tensiometer experiments than in an EAF, so the degree of reduction could be much greater. Nonetheless, the focus of this study was on the initial interaction between the slag and carbonaceous material. Furthermore, it would be useful to know the iron content of the slags formed in the experiments, and to measure the relative amounts of Fe3+, Fe2+ and Fe0 via titration or Mossbauer spectroscopy; unfortunately, the system and procedure used did not allow us to collect the slag drops after experiments.
3.2.4. Enhancement of Interaction between Bio-Char and Slag by Blending with Coke
As discussed above, the lack of interaction between bio-char and slag may arise from the smooth bio-char particle surface. In order to enhance the behavior of bio-char in slag foaming, the effect of blending bio-char with coke was explored.
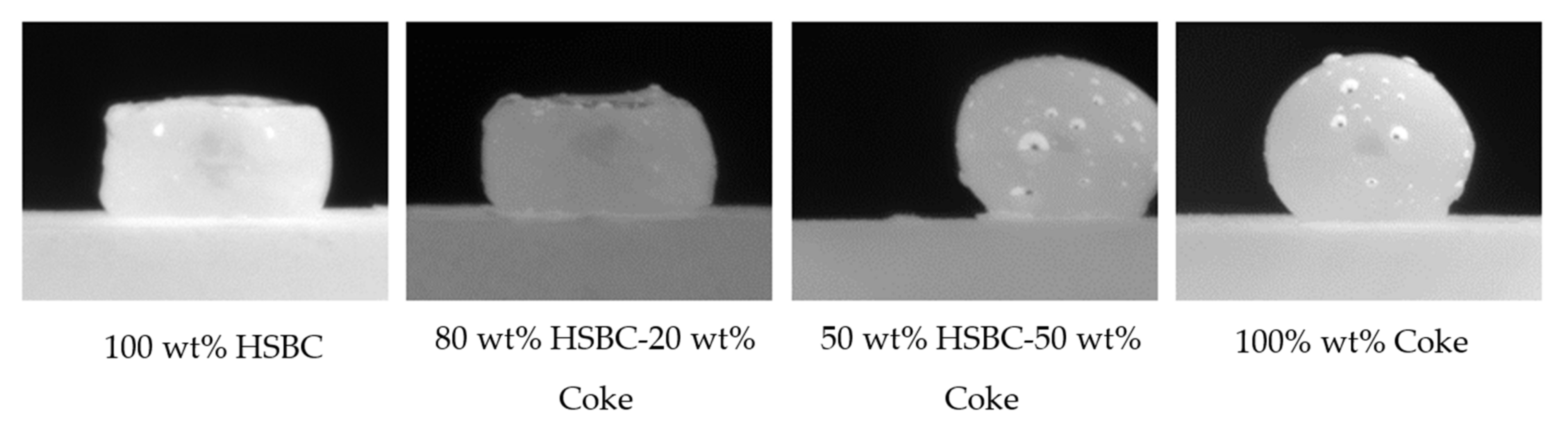
A series of tensiometer experiments was conducted to examine the interaction between slag and HSBC/coke mixtures with different compositions. Figure 12 summarizes the experimental observations. By increasing the coke content in the HSBC/coke substrate from 0 to 50 wt.%, the interaction between slag and the substrate is significantly improved, and similar to 100 wt.% coke.
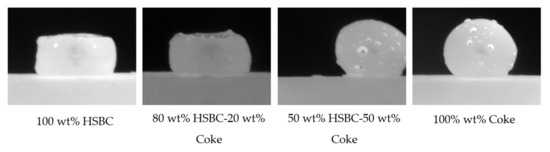
Figure 12.
Enhancement of substrate interaction with slag by blending with coke.
The small-scale tensiometer experiments revealed that blending bio-char with coke has the potential to improve slag foaming reactions. In order to validate the small-scale experimental observations, larger-scale experiments, described in Section 2.3, were conducted to measure foam height. Softwood bio-char SSBC-2, as shown in Table 5, was selected for the larger-scale experiments.
3.3. Slag Foaming at a Larger Scale
The measured increase in slag layer thickness with various coke and bio-char mixtures is summarized in Table 12. By blending the bio-char with coke, the slag layer height does increase due to foaming compared to the case with 100 wt.% bio-char. However, the slag layer thickness is significantly lower than the 100 wt.% coke case. This implies that the enhancement in foaming capacity of bio-char by blending with coke may be limited.

Table 12.
Slag layer thickness measurements.
The difference in behavior between the large-scale melting tests and small-scale tensiometer tests likely arises from the low density of bio-char. It was observed during the large-scale tests that the added bio-char floated on the slag surface even when the bio-char and coke were pre-mixed before addition. The floating of bio-char on the slag surface partially prevents contact between coke and slag from occurring. As a result, the foaming capacity of carbonaceous material is not fully utilized.
4. Conclusions
In this investigation, different effects were observed by replacing fossil carbon material with biogenic carbon materials.
- 1.
- Substitution of charge material with bio-char:
- ○
- No detrimental effect was found on molten steel and slag chemical composition as well as furnace efficiency.
- 2.
- Substitution of injection material with bio-char:
- ○
- The addition of slag or an Fe-additive in bio-char substrates was not effective for enhancing the interaction between bio-char and slag.
- ○
- Bio-chars derived from softwood and hardwood had very different interactions with slag.
- ○
- Wettability of bio-char with slag improved upon modifying the surface morphology of bio-char through densification, likely due to a smaller contact angle for slag on densified bio-char. A hypothesis was proposed that highly reactive bio-char leads to metallic Fe formation, preventing further interactions and slag foaming.
- ○
- Mixing bio-char with coke improved the slag foaming capability relative to 100 wt.% bio-char, but did not improve its slag foaming capacity to near what coke delivers.
Overall, the enhancement of interactions between slag and bio-char requires further study.
Author Contributions
Conceptualization, X.H. and K.W.N.; methodology, X.H.; investigation, X.H.; resources, K.W.N.; writing—original draft preparation, X.H.; writing—review and editing, L.G., M.D. and T.T.; visualization, D.L.; supervision, K.W.N.; project administration, X.H.; funding acquisition, K.W.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Resources Canada under its Program for Energy Research and Development (OERD), grant number CEO-20-03. The Carbon Carbonization Research Association (CCRA) also funded the research under the program of CCRA 70.
Institutional Review Board Statement
Not applicable. The study does not involve humans or animals.
Informed Consent Statement
Not applicable. The study does not involve humans.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, x. Huang, upon reasonable request.
Acknowledgments
Assistance of technologists at CanmetMATERIALS in conducting steel melting test and Ami Patel (Coop student from the University of Waterloo) in experimental work related to tensiometer tests is much appreciated. The authors also appreciate the financial support of this project from Natural Resources Canada under its Program for Energy Research and Development.
Conflicts of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Thomson, M.J.; Evenson, E.J.; Kempe, M.J.; Goodfellow, H.D. Control of Greenhouse Gas Emissions from Electric Arc Furnace Steelmaking: Evaluation Methodology with Case Studies. Ironmak. Steelmak. 2000, 27, 273–279. [Google Scholar] [CrossRef]
- Bianco, L.; Baracchini, G.; Cirilli, F.; Moriconi, A.; Moriconi, E.; Marcos, M.; Demus, T.; Echterhof, T.; Pfeifer, H.; Beiler, C.; et al. Sustainable EAF Steel Production (GREENEAF); European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Sahajwalla, V.; Rahman, M.; Hong, L.; Saha-Chaudhury, N.; Spencer, D. Influence of Carbonaceous Materials on Slag Foaming Behavior during EAF Steelmaking. Iron Steel Technol. 2006, 3, 54–63. [Google Scholar]
- Corbari, R.; Matsuura, H.; Halder, S.; Walker, M.; Fruehan, R.J. Foaming and the Rate of the Carbon-Iron Oxide Reaction in Slag. Metall. Mater. Trans. B 2009, 40, 940. [Google Scholar] [CrossRef]
- Norgate, T.; Haque, N.; Somerville, M.; Jahanshahi, S. Biomass as a Source of Renewable Carbon for Iron and Steelmaking. ISIJ Int. 2012, 52, 1472–1481. [Google Scholar] [CrossRef] [Green Version]
- Mathieson, J.; Rogers, H.; Somerville, M.; Ridgeway, P.; Jahanshahi, S. Use of Biomass in the Iron and Steel Industry—An Australian Perspective. In Proceedings of the 1st International Conference on Energy Efficiency and CO2 Reduction in the Steel Industry (EECR Steel 2011), Dusseldorf, Germany, 27 June–1 July 2011. [Google Scholar]
- Jahanshahi, S.; Mathieson, J.G.; Somerville, M.A.; Haque, N.; Norgate, T.E.; Deev, A.; Pan, Y.; Xie, D.; Ridgeway, P.; Zulli, P. Development of Low-Emission Integrated Steelmaking Process. J. Sustain. Metall. 2015, 1, 94–114. [Google Scholar] [CrossRef] [Green Version]
- Duchesne, M.A.; Hughes, R.W. Slag Density and Surface Tension Measurements by the Constrained Sessile Drop Method. Fuel 2017, 188, 173–181. [Google Scholar] [CrossRef]
- Huang, X.-A.; Ng, K.W.; Giroux, L.; Duchesne, M. Carbonaceous Material Properties and Their Interactions with Slag during Electric Arc Furnace Steelmaking. Metall. Mater. Trans. B 2019, 50, 1387–1398. [Google Scholar] [CrossRef]
- Ramirez-Rico, J.; Gutierrez-Pardo, A.; Martinez-Fernandez, J.; Popov, V.V.; Orlova, T.S. Thermal Conductivity of Fe Graphitized Wood Derived Carbon. Mater. Design 2016, 99, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Nettelroth, D.; Schwarz, H.-C.; Burblies, N.; Guschanski, N.; Behrens, P. Catalytic Graphitization of Ordered Mesoporous Carbon CMK-3 with Iron Oxide Catalysts: Evaluation of Different Synthesis Pathways. Phys. Status Solidi 2016, 213, 1395–1402. [Google Scholar] [CrossRef]
- Teasdale, S.L.; Hayes, P.C. Observations of the Reduction of FeO from Slag by Graphite, Coke and Coal Char. ISIJ Int. 2005, 45, 634–641. [Google Scholar] [CrossRef]
- Kongkarat, S.; Cherdhirunkorn, B.; Thongreang, R. Utilization of Waste HDPE for Sustainable EAF Steelmaking: Carbon Dissolution into Liquid Steel. Steel Res. Int. 2017, 88, 1600168. [Google Scholar] [CrossRef]
- Funke, A.; Demus, T.; Willms, T.; Schenke, L.; Echterhof, T.; Niebel, A.; Pfeifer, H.; Dahmen, N. Application of Fast Pyrolysis Char in an Electric Arc Furnace. Fuel Process. Technol. 2018, 174, 61–68. [Google Scholar] [CrossRef]
- Demus, T.; Reichel, T.; Schulten, M.; Echterhof, T.; Pfeifer, H. Increasing the Sustainability of Steel Production in the Electric Arc Furnace by Substituting Fossil Coal with Biochar Agglomerates. Ironmak. Steelmak. 2016, 43, 564–570. [Google Scholar] [CrossRef]
- Yang, J.; Duan, J.; Fornasiero, D.; Ralston, J. Very Small Bubble Formation at the Solid−Water Interface. J. Phys. Chem. B 2003, 107, 6139–6147. [Google Scholar] [CrossRef]
- Ryan, W.L.; Hemmingsen, E.A. Bubble Formation in Water at Smooth Hydrophobic Surfaces. J. Colloid Interface Sci. 1993, 157, 312–317. [Google Scholar] [CrossRef]
- Meier, T.; Hay, T.; Echterhof, T.; Pfeifer, H.; Rekersdrees, T.; Schlinge, L.; Elsabagh, S.; Schliephake, H. Process Modeling and Simulation of Biochar Usage in an Electric Arc Furnace as a Substitute for Fossil Coal. Steel Res. Int. 2017, 88, 1600458. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).