Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies
Abstract
1. Introduction
2. Fundamentals of SOFCs
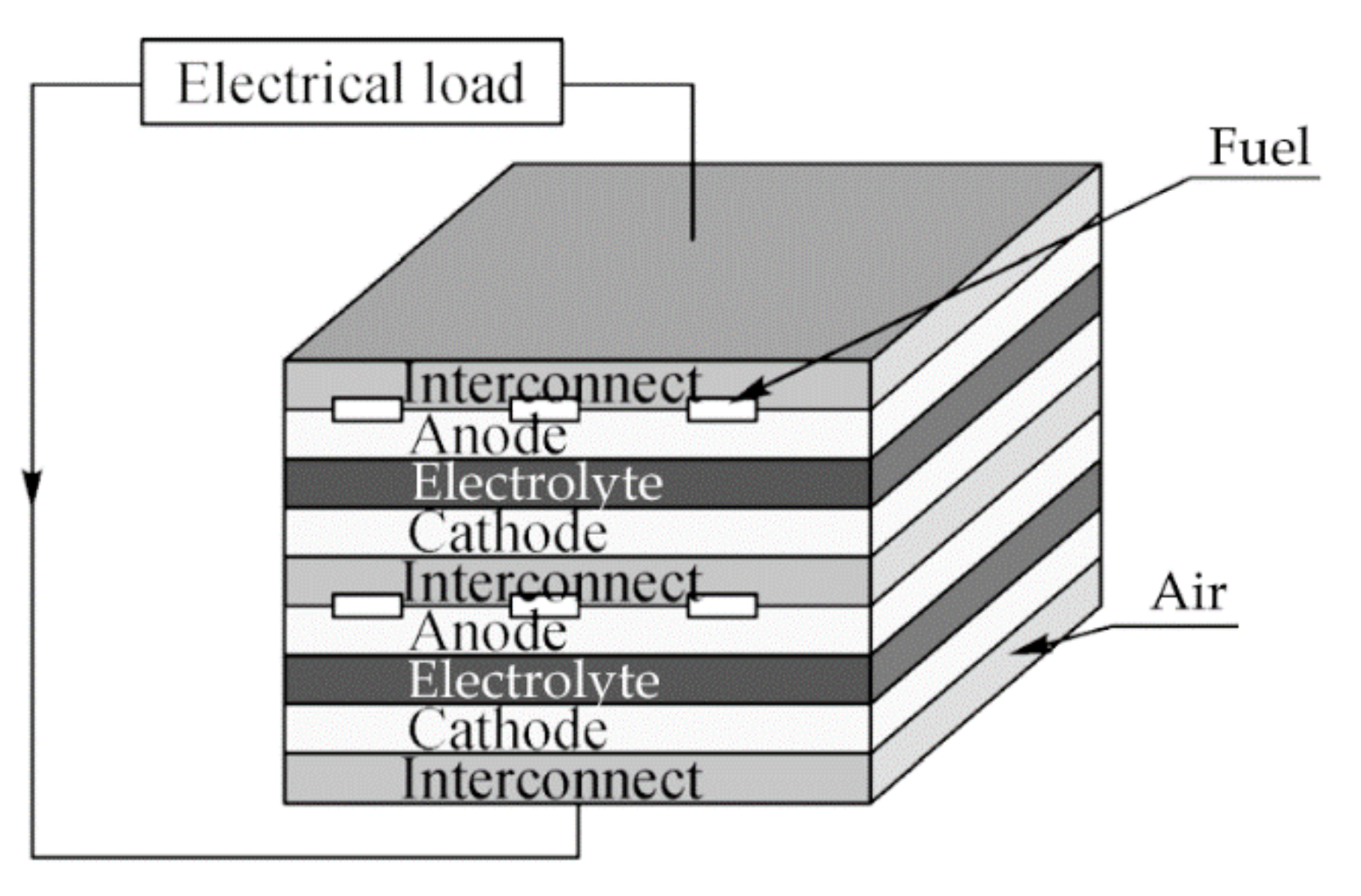
2.1. Working Principles

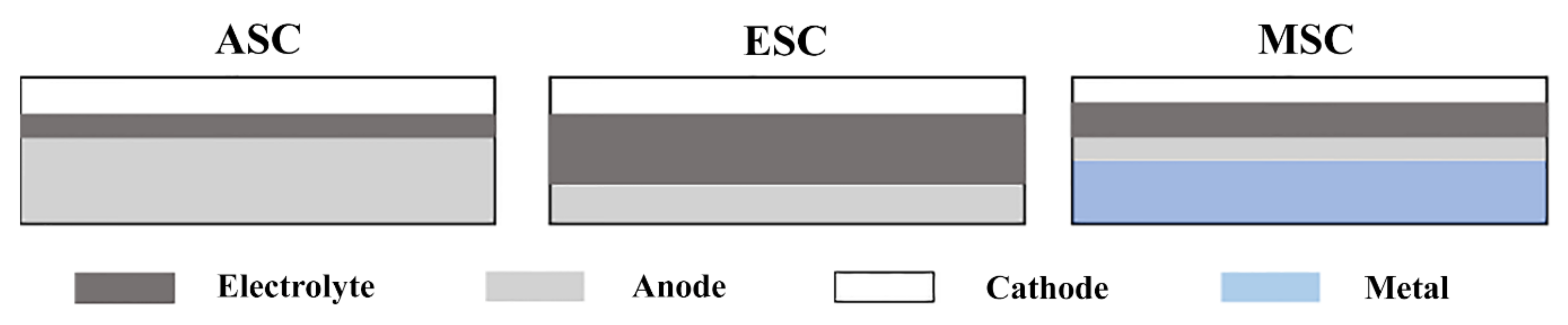
2.2. Cell Design of SOFCs
2.3. Stack Design of SOFCs
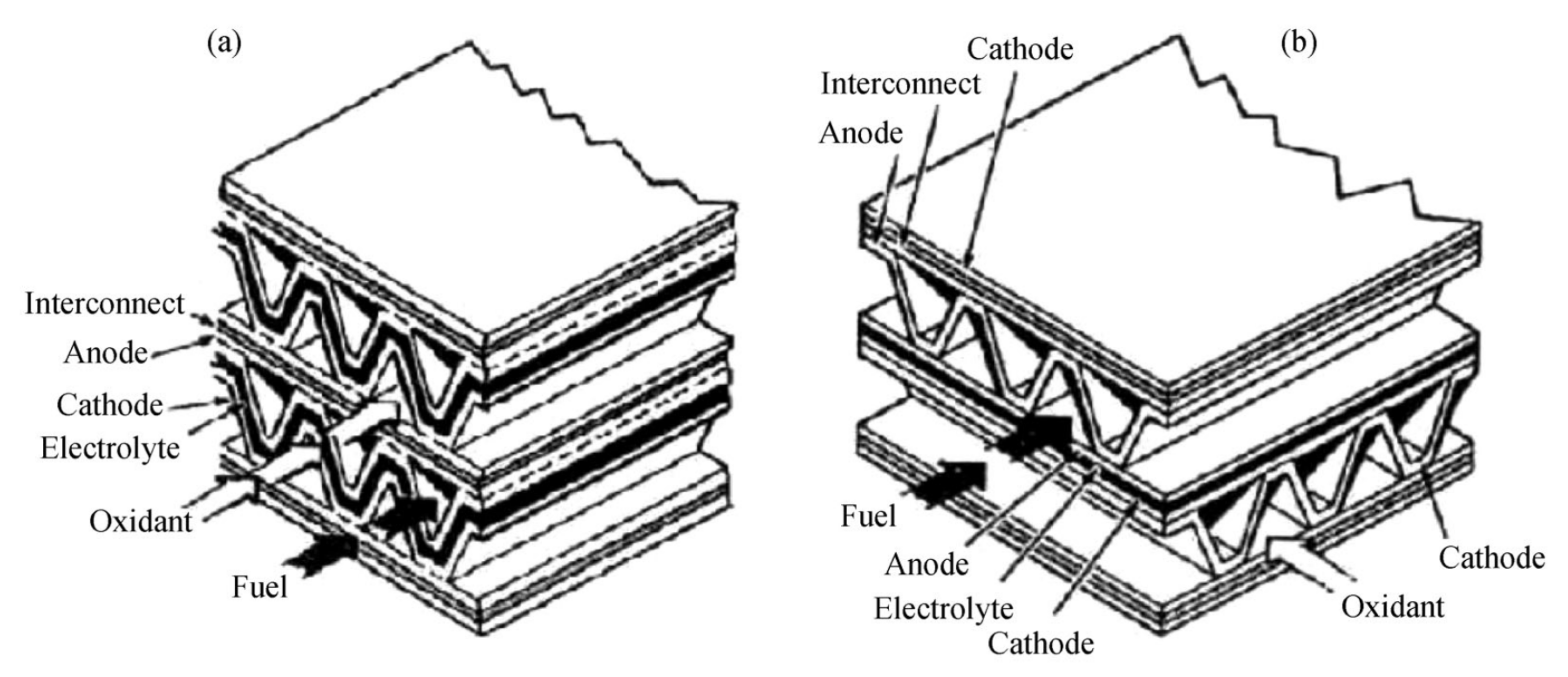
2.3.1. Planar Design
2.3.2. Monolithic Design
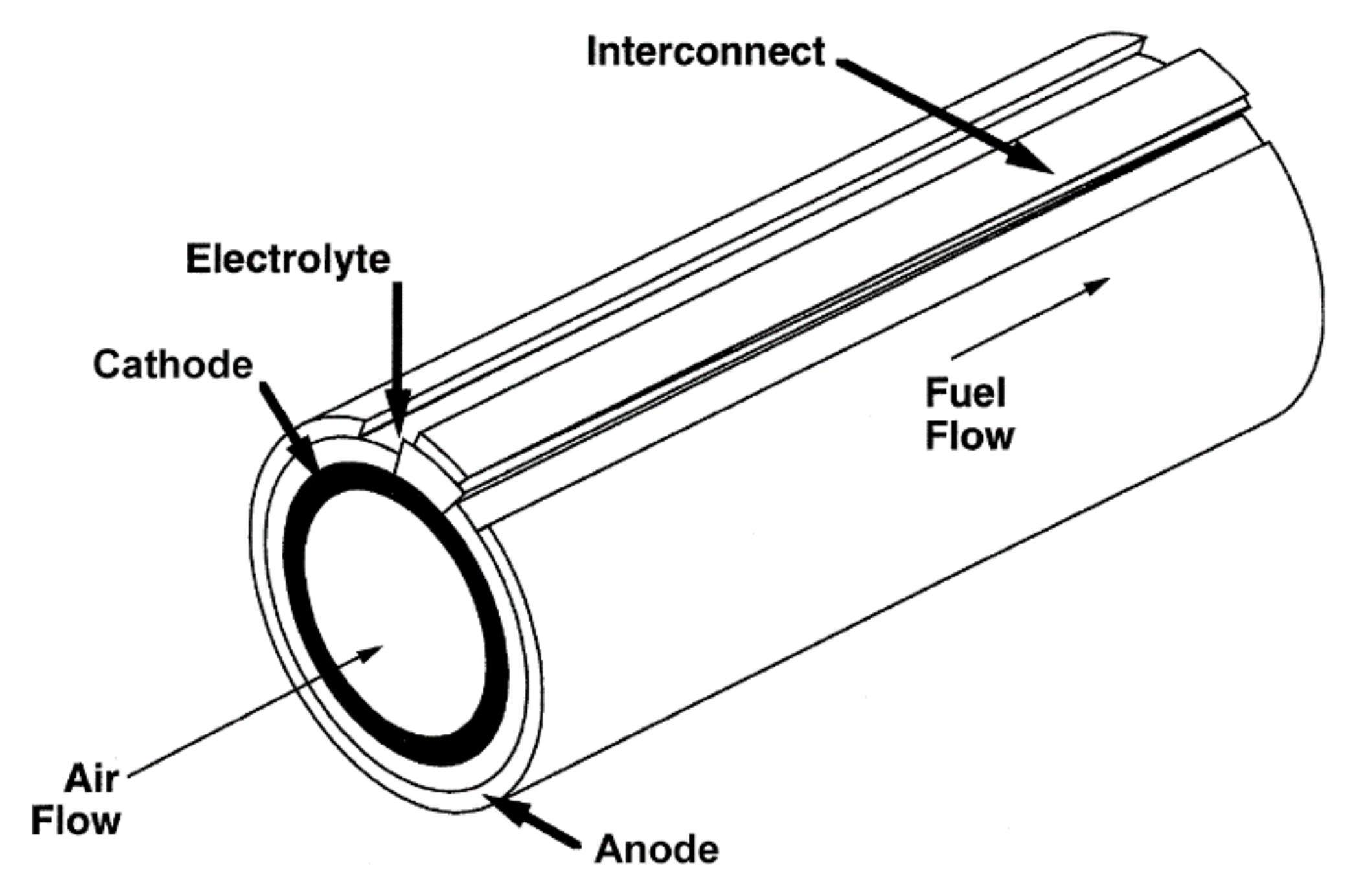
2.3.3. Tubular Design
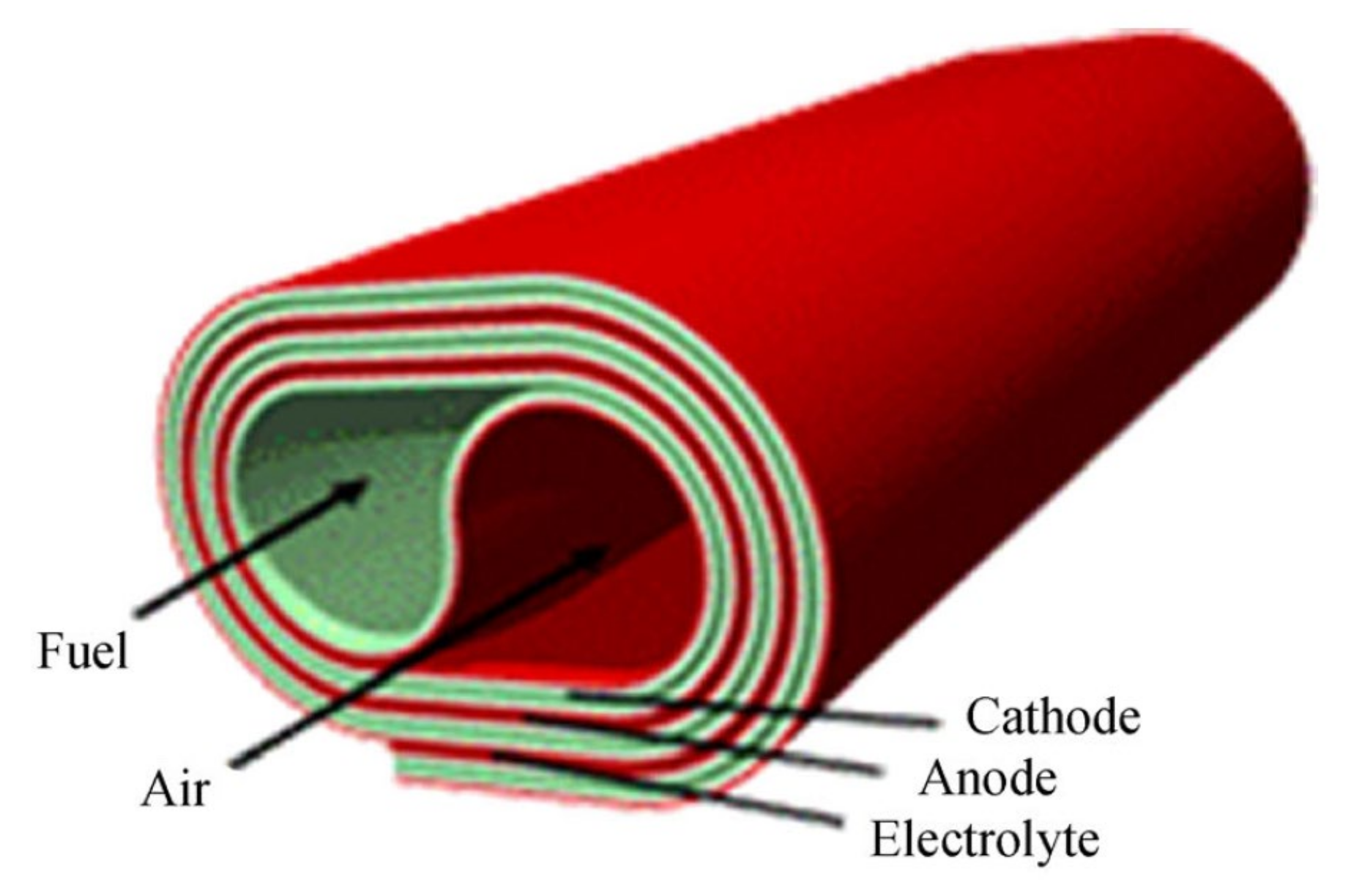
2.3.4. Roll Design
2.4. Fuel Processing in SOFC
3. Material Components of SOFCs
3.1. Electrolyte
3.1.1. Oxygen-Ion-Conducting Electrolyte Materials for SOFCs
3.1.2. Proton-Conducting Electrolyte Materials for SOFCs
3.1.3. Mixed-Ion-Conducting Electrolyte Materials for SOFCs
3.1.4. Fabrication Methods for Electrolytes
3.2. Interconnect
3.3. Anode
3.3.1. Ni-Based Cermet
3.3.2. Perovskite Oxides
- Producing composite with redox-active transition metals, such as Cu and Ni;
- Changing the chemical composition of conventional LSCr-based anode;
- Doping A-site or/and B-site with different metal cations; and
- Mixing other electrical conductors or exsoluted nanoparticles from doped LSCr to create a composite anode.
- Partial substitution of La, Sm, and Ba for Sr;
- Mg substitution with transition metal elements, such as Mn, Fe, Co, Ni, Ti, and Cr; and
- Mo substitution with V and Nb.
3.4. Cathode
- Be highly electronic conductive;
- Be chemically compatible and thermally stable;
- The microstructure should be in high percentages of porosity;
- Give a high catalytic activity for the ORR;
- Should not show any tendency to react with the electrolyte; and
- Be easy to process and reliable cost manufacturing.
3.4.1. Cathodes on Oxygen-Ion-Conducting Electrolyte
3.4.2. Cathodes on Proton-Ion-Conducting Electrolyte
3.4.3. Composite Cathodes
4. SOFC Applications
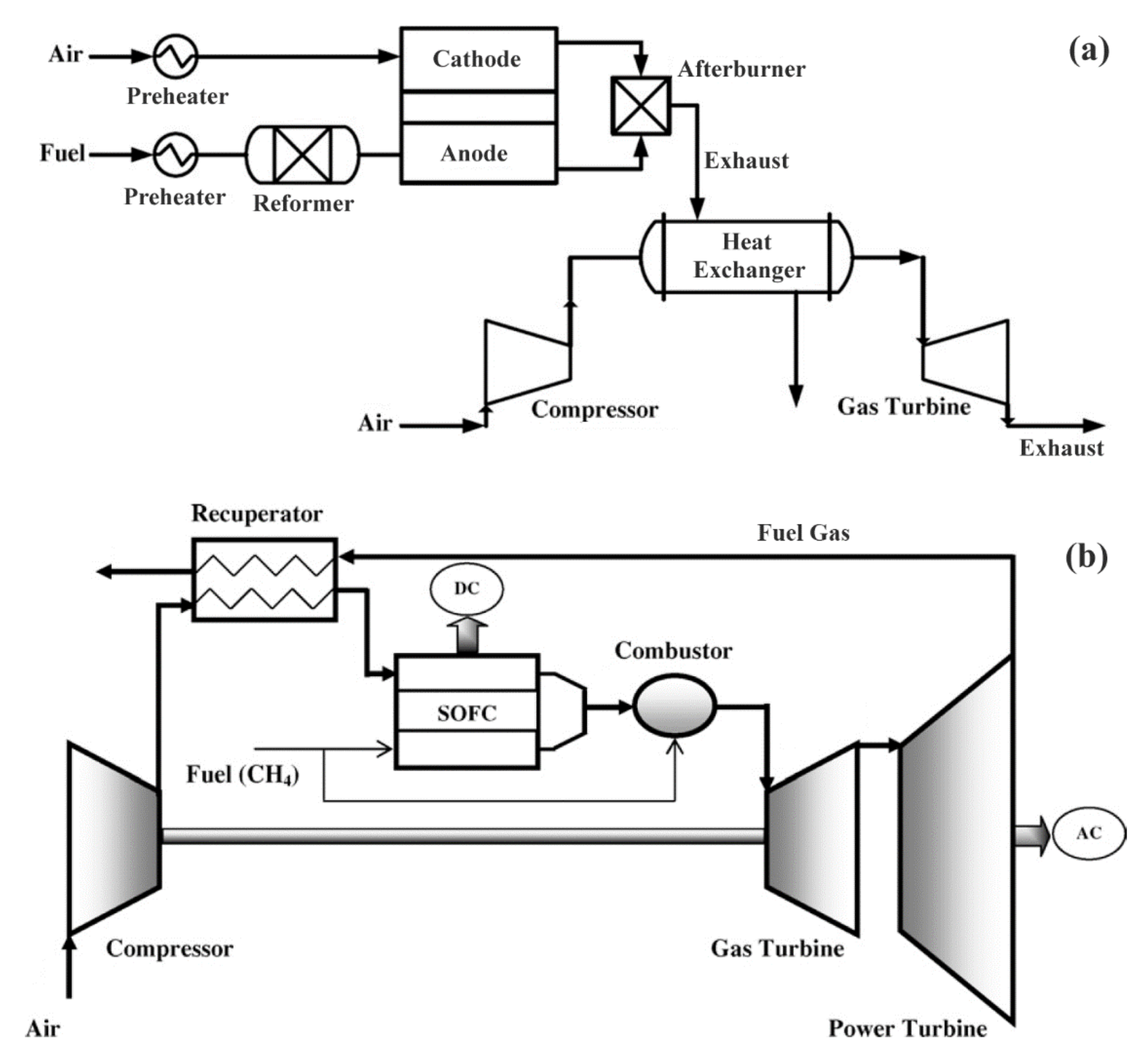
4.1. Combined Gas Turbine (GT) Power System with SOFC
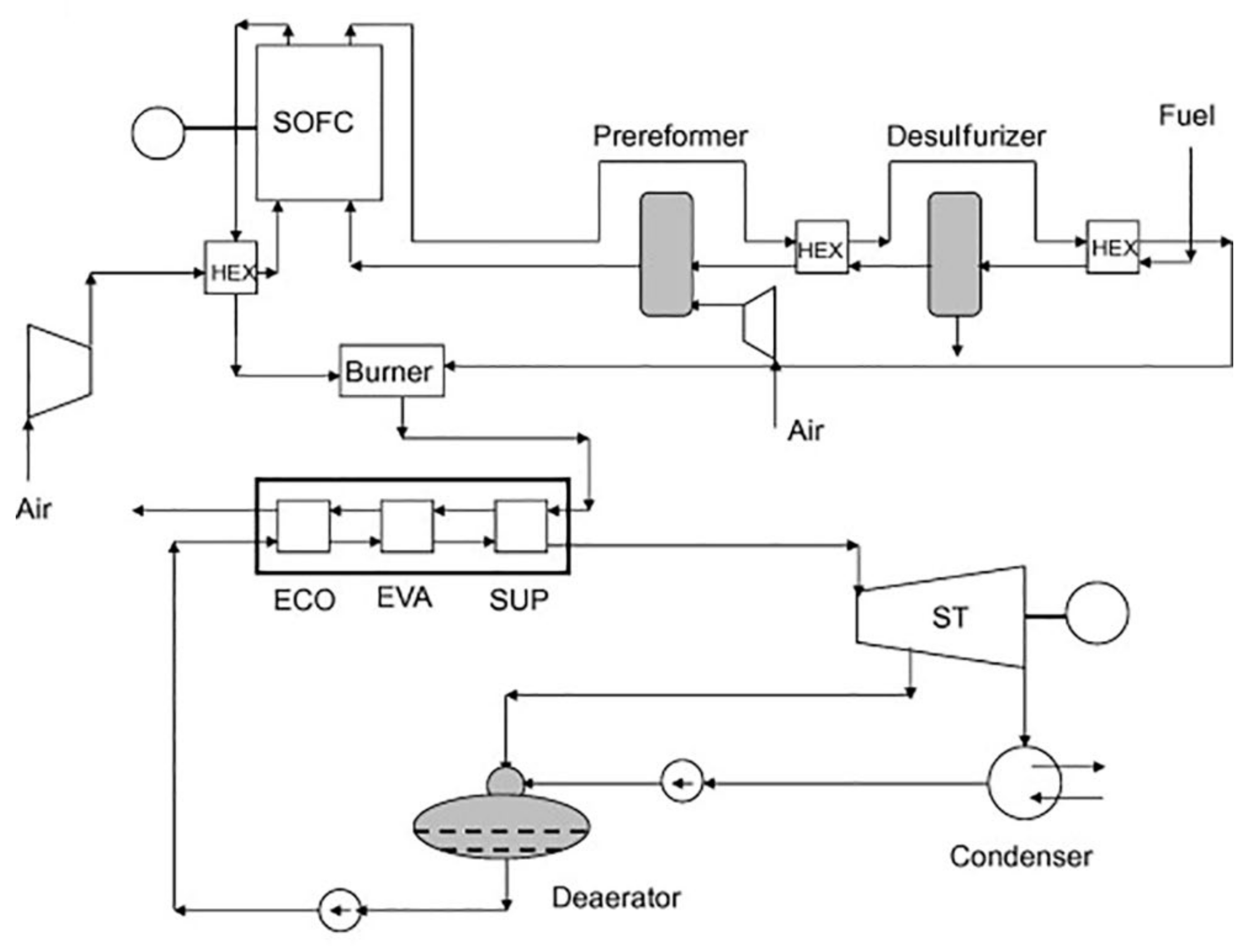
4.2. SOFC Integrated with Rankine Cycle
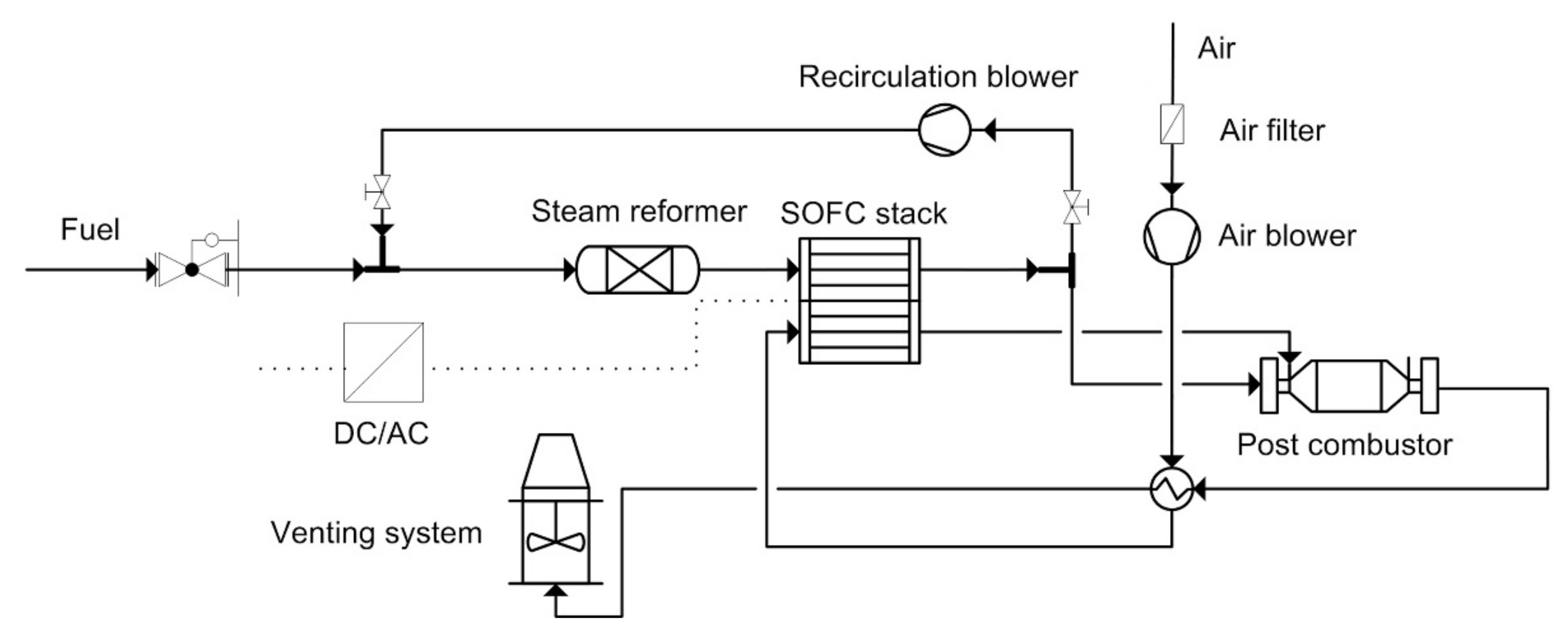
4.3. Combined Heat and Power with SOFC
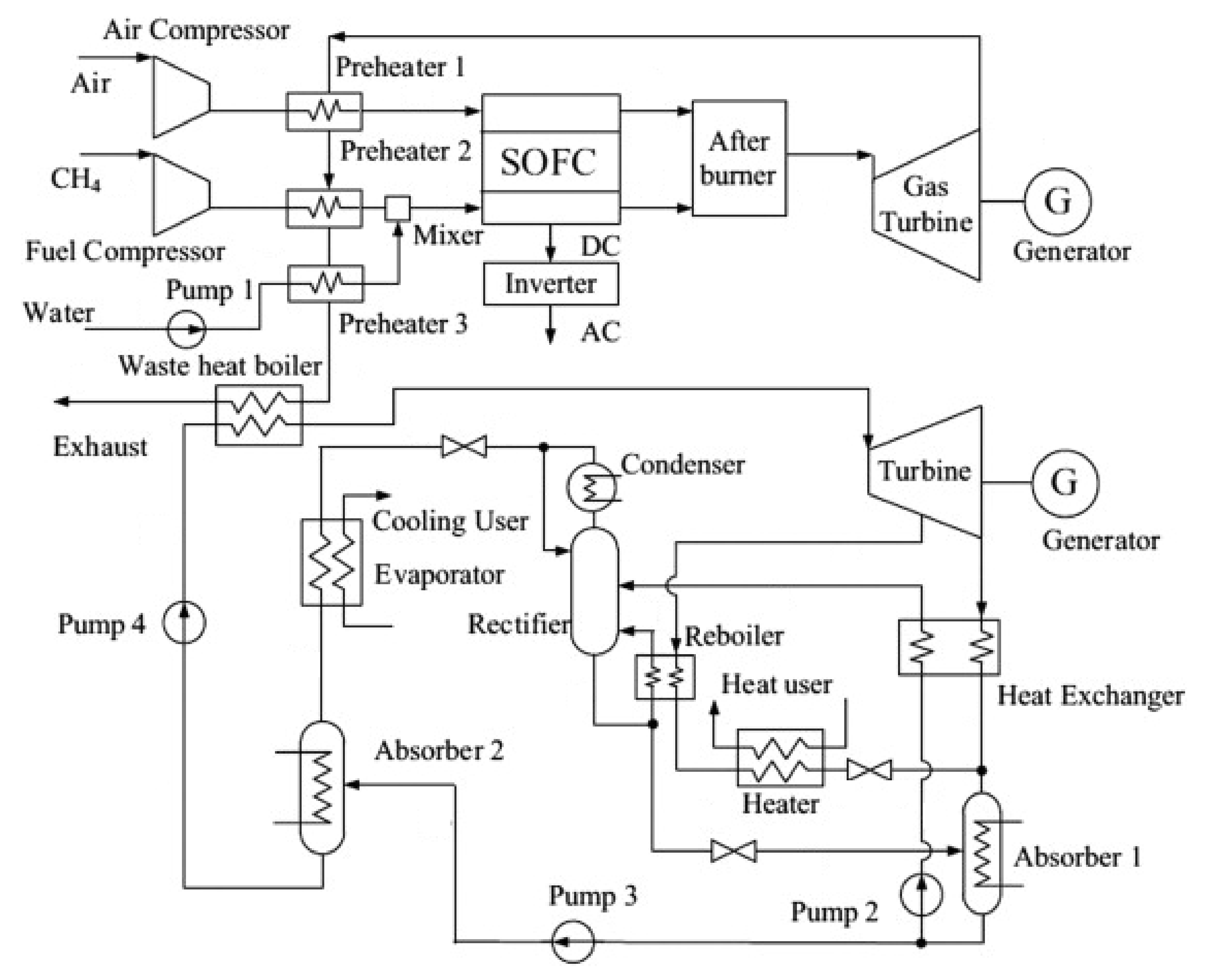
4.4. Trigeneration with SOFC
5. Commercialization
5.1. History
5.1.1. 250 kW Atmospheric CHP System
5.1.2. 1 MW SOFC/GT Hybrid System
5.1.3. 125 kW CHP System
5.2. Latest Projects and Developments
5.2.1. Kyocera
5.2.2. Elcogen
5.2.3. Sunfire
5.2.4. Bloom Energy
5.2.5. Mitsubishi Power
5.2.6. FCH-JU
| Company | Country | Electrolyte | Anode | Cathode | SOFC System Nominal Size (kW) | Electrical Efficiency (%) | Total Efficiency (%) | Stack Technical Lifetime (h) | Working Temperature (°C) | NOx Emissions | Applications | References |
| Kyocera | Japan | YSZ | Ni-YSZ | LSCF | 0.8 | 87 | 55 | 90,000 | 750 | - | Households, stores, and restaurants | [29,106] |
| Elcogen | Estonia | GDC | NiO-YSZ | LSC | 1–3 | 74 | >90 | - | 650 | - | CHP | [122,123,124] |
| Convion | Finland | - | - | - | 60 | 60 | 81 | 40,000 | 700 | <2 mg m−3 | Distributed power generation | [110] |
| Sunfire | Germany | YSZ | NiO-GDC | LSCF | 0.35–20 | >50 | >80 | >(Target) 45,000 | 850 | <10 mg/kWh | Micro-CHP for private homes and off-grid power supply | [125,126] |
| Bloom Energy | USA | Sc-YSZ | - | - | 300 | 53–65 | - | - | - | 0.0017 lbs/MWh | Distributed power generation | [113,127,128,129] |
| Mitsubishi Power | Japan | LSGMC | Ni-SDC | SSC | 250 | 55 | 73 | - | >900 | 15 ppm | CHP | [116,117,118,130] |
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bush, G.W. National Energy Policy: Report of the National Energy Policy Development Group; Executive Office of the President of Washington DC: Washington, DC, USA, 2001.
- Johnson, I. Environment Matters at the World Bank: Annual Review 2001. Available online: https://openknowledge.worldbank.org/handle/10986/13987.
- Zhang, Y.; Knibbe, R.; Sunarso, J.; Zhong, Y.; Zhou, W.; Shao, Z.; Zhu, Z. Recent Progress on Advanced Materials for Solid-Oxide Fuel Cells Operating below 500 °C. Adv. Mater. 2017, 29, 1700132. [Google Scholar] [CrossRef]
- Zhang, X.; Chan, S.H.; Li, G.; Ho, H.K.; Li, J.; Feng, Z. A review of integration strategies for solid oxide fuel cells. J. Power Sources 2010, 195, 685–702. [Google Scholar] [CrossRef]
- Sreedhar, I.; Agarwal, B.; Goyal, P.; Singh, S.A. Recent advances in material and performance aspects of solid oxide fuel cells. J. Electroanal. Chem. 2019, 848, 113315. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Petra, P.M.I.; Ghasemi, M.; Azad, A.K. Achievements and trends of solid oxide fuel cells in clean energy field: A perspective review. Front. Energy 2020, 14, 359–382. [Google Scholar] [CrossRef]
- Ormerod, R.M. Solid oxide fuel cells. Chem. Soc. Rev. 2003, 32, 17–28. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Lyu, Y.; Xie, J.; Wang, D.; Wang, J. Review of cell performance in solid oxide fuel cells. J. Mater. Sci. 2020, 55, 7184–7207. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N. Membranes for direct ethanol fuel cells: An overview. Appl. Energy 2016, 163, 334–342. [Google Scholar] [CrossRef]
- Zakaria, Z.; Awang Mat, Z.; Abu Hassan, S.H.; Boon Kar, Y. A review of solid oxide fuel cell component fabrication methods toward lowering temperature. Int. J. Energy Res. 2020, 44, 594–611. [Google Scholar] [CrossRef]
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrog. Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- Cowin, P.I.; Petit, C.T.G.; Lan, R.; Irvine, J.T.S.; Tao, S. Recent progress in the development of anode materials for solid oxide fuel cells. Adv. Energy Mater. 2011, 1, 314–332. [Google Scholar] [CrossRef]
- Chao, C.-C.; Hsu, C.-M.; Cui, Y.; Prinz, F.B. Improved solid oxide fuel cell performance with nanostructured electrolytes. ACS Nano 2011, 5, 5692–5696. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Azad, A.T.; Petra, P.M.I.; Begum, F.; Eriksson, S.G.; Azad, A.K. Nanomaterials for solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Ran, R.; Cao, J.; Shao, Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 764–774. [Google Scholar] [CrossRef]
- Technology—Convion, (n.d.). Available online: https://convion.fi/technology/ (accessed on 17 May 2021).
- Tesfi, A. Irvine JTS. solid oxides fuel cells: Theory and material. Compr. Renew. Energy 2012, 4, 241–256. [Google Scholar]
- Sarantaridis, D.; Atkinson, A. Mechanical modeling of redox cycling damage in solid oxide fuel cells. In Proceedings of the 7th European Solid Oxide Fuel Cell Forum, EFCF, Lucerne, Swizerland, 3–7 July 2006; p. P0728. [Google Scholar]
- Months, E.P.; Date, A.S.; Gandiglio, M.; Hakala, T.; Fontell, E. ComSos “Commercial-scale SOFC systems”. 2019, pp. 1–11. Available online: https://www.comsos.eu/com18/com18-cont/uploads/2020/02/Market-analysis-of-CHP-solutions-applied-in-commercial-applications.pdf (accessed on 17 September 2021).
- Faes, A.; Hessler-Wyser, A.; Zryd, A. A review of RedOx cycling of solid oxide fuel cells anode. Membranes 2012, 2, 585–664. [Google Scholar] [CrossRef]
- Singhal, S.C. Advances in solid oxide fuel cell technology. Solid State Ion. 2000, 135, 305–313. [Google Scholar] [CrossRef]
- Tesfai, A.; Connor, P.; Nairn, J.; Irvine, J.T.S. Thermal cycling evaluation of rolled tubular solid oxide fuel cells. J. Fuel Cell Sci. Technol. 2011, 8, 061001. [Google Scholar] [CrossRef]
- Hussain, A.M.; Wachsman, E.D. Liquids-to-Power Using Low-Temperature Solid Oxide Fuel Cells. Energy Technol. 2019, 7, 20–32. [Google Scholar] [CrossRef]
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef]
- Patil, K.C. Chemistry of Nanocrystalline Oxide Materials: Combustion Synthesis, Properties and Applications; World Scientific: Singapore, 2008; ISBN 9812793143. [Google Scholar]
- Jiang, Y.; Virkar, A. V A high performance, anode-supported solid oxide fuel cell operating on direct alcohol. J. Electrochem. Soc. 2001, 148, A706. [Google Scholar] [CrossRef]
- Satardekar, P.; Montinaro, D.; Sglavo, V.M. Fe-doped YSZ electrolyte for the fabrication of metal supported-SOFC by co-sintering. Ceram. Int. 2015, 41, 9806–9812. [Google Scholar] [CrossRef]
- Prakash, B.S.; Pavitra, R.; Kumar, S.S.; Aruna, S.T. Electrolyte bi-layering strategy to improve the performance of an intermediate temperature solid oxide fuel cell: A review. J. Power Sources 2018, 381, 136–155. [Google Scholar] [CrossRef]
- Yashiro, K.; Suzuki, T.; Kaimai, A.; Matsumoto, H.; Nigara, Y.; Kawada, T.; Mizusaki, J.; Sfeir, J. Electrical properties and defect structure of niobia-doped ceria. Solid State Ion. 2004, 175, 341–344. [Google Scholar] [CrossRef]
- Shimonosono, T.; Hirata, Y.; Sameshima, S.; Horita, T. Electronic Conductivity of La-Doped Ceria Ceramics. J. Am. Ceram. Soc. 2005, 88, 2114–2120. [Google Scholar] [CrossRef]
- Van Herle, J.; Seneviratne, D.; McEvoy, A.J. Lanthanide co-doping of solid electrolytes: AC conductivity behaviour. J. Eur. Ceram. Soc. 1999, 19, 837–841. [Google Scholar] [CrossRef]
- Yahiro, H.; Eguchi, K.; Arai, H. Electrical properties and microstructure in the system ceria-alkaline earth oxide. J. Mater. Sci. 1988, 23, 1036–1041. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Shipilova, A.V.; Ionov, I.V.; Kovalchuk, A.N.; Rabotkin, S.V.; Oskirko, V.O. Magnetron-sputtered YSZ and CGO electrolytes for SOFC. J. Electron. Mater. 2016, 45, 3921–3928. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tanwar, K.; Suman, R.; Kumar, D.; Uppadhya, S.; Parkash, O. A brief review on ceria based solid electrolytes for solid oxide fuel cells. J. Alloys Compd. 2019, 781, 984–1005. [Google Scholar] [CrossRef]
- Tealdi, C.; Malavasi, L.; Ritter, C.; Flor, G.; Costa, G. Lattice effects in cubic La2Mo2O9: Effect of vacuum and correlation with transport properties. J. Solid State Chem. 2008, 181, 603–610. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Doped LaGaO3 perovskite type oxide as a new oxide ionic conductor. J. Am. Chem. Soc. 1994, 116, 3801–3803. [Google Scholar] [CrossRef]
- Morales, M.; Roa, J.J.; Tartaj, J.; Segarra, M. A review of doped lanthanum gallates as electrolytes for intermediate temperature solid oxides fuel cells: From materials processing to electrical and thermo-mechanical properties. J. Eur. Ceram. Soc. 2016, 36, 1–16. [Google Scholar] [CrossRef]
- Sarat, S.; Sammes, N.; Smirnova, A. Bismuth oxide doped scandia-stabilized zirconia electrolyte for the intermediate temperature solid oxide fuel cells. J. Power Sources 2006, 160, 892–896. [Google Scholar] [CrossRef]
- Ito, N.; Iijima, M.; Kimura, K.; Iguchi, S. New intermediate temperature fuel cell with ultra-thin proton conductor electrolyte. J. Power Sources 2005, 152, 200–203. [Google Scholar] [CrossRef]
- Ranran, P.; Yan, W.; Lizhai, Y.; Zongqiang, M. Electrochemical properties of intermediate-temperature SOFCs based on proton conducting Sm-doped BaCeO3 electrolyte thin film. Solid State Ion. 2006, 177, 389–393. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hernandez-Sanchez, R.; Haile, S.M. High total proton conductivity in large-grained yttrium-doped barium zirconate. Chem. Mater. 2009, 21, 2755–2762. [Google Scholar] [CrossRef]
- Pergolesi, D.; Fabbri, E.; D’Epifanio, A.; Di Bartolomeo, E.; Tebano, A.; Sanna, S.; Licoccia, S.; Balestrino, G.; Traversa, E. High proton conduction in grain-boundary-free yttrium-doped barium zirconate films grown by pulsed laser deposition. Nat. Mater. 2010, 9, 846–852. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chang, I.; Cho, G.Y.; Park, J.; Yu, W.; Tanveer, W.H.; Cha, S.W. Thin film solid oxide fuel cells operating below 600 C: A review. Int. J. Precis. Eng. Manuf. Technol. 2018, 5, 441–453. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.; Xie, F.; Wang, X.; Mao, Z.; Liu, Q.; Zhan, Z. Ionic transport mechanism of La0.9Sr0.1Ga0.8Mg0.2O2.85-(Li/Na)2CO3 composite electrolyte for low temperature SOFCs. Int. J. Hydrogen Energy 2016, 41, 16275–16281. [Google Scholar] [CrossRef]
- Vielstich, W.; Lamm, A.; Gasteiger, H. Handbook of Fuel Cells. Fundamentals, Technology, Applications. U.S. Department of Energy: Chichester, UK, 2003. [Google Scholar]
- Badwal, S.P.S.; Foger, K. Materials for Solid Oxide Fuel Cells. Mater. Forum 1997, 21, 187–224. Available online: http://hdl.handle.net/102.100.100/222691?index=1 (accessed on 17 September 2021).
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Haile, S.M. Materials for fuel cells. Mater. Today 2003, 6, 24–29. [Google Scholar] [CrossRef]
- Mah, J.C.W.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Metallic interconnects for solid oxide fuel cell: A review on protective coating and deposition techniques. Int. J. Hydrog. Energy 2017, 42, 9219–9229. [Google Scholar] [CrossRef]
- Yang, Z.; Weil, K.S.; Paxton, D.M.; Stevenson, J.W. Selection and evaluation of heat-resistant alloys for SOFC interconnect applications. J. Electrochem. Soc. 2003, 150, A1188. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, G.-G.; Li, X.-H.; Stevenson, J.W. (Mn, Co)3O4 spinel coatings on ferritic stainless steels for SOFC interconnect applications. Int. J. Hydrog. Energy 2007, 32, 3648–3654. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X. Recent development of SOFC metallic interconnect. J. Mater. Sci. Technol. 2010, 26, 293–305. [Google Scholar] [CrossRef]
- Chan, S.H. A review of anode materials development in solid oxide fuel cells. J. Mater. Sci. 2004, 39, 4405–4439. [Google Scholar]
- Ai, N.; Lü, Z.; Chen, K.; Huang, X.; Du, X.; Su, W. Effects of anode surface modification on the performance of low temperature SOFCs. J. Power Sources 2007, 171, 489–494. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Qiao, J.; Sun, K.; Xu, P. Improved SOFC performance with continuously graded anode functional layer. Electrochem. Commun. 2009, 11, 1120–1123. [Google Scholar] [CrossRef]
- Lee, J.-J.; Moon, H.; Park, H.-G.; Yoon, D.I.; Hyun, S.-H. Applications of nano-composite materials for improving the performance of anode-supported electrolytes of SOFCs. Int. J. Hydrog. Energy 2010, 35, 738–744. [Google Scholar] [CrossRef]
- Chen, Y.; Bu, Y.; Zhao, B.; Zhang, Y.; Ding, D.; Hu, R.; Wei, T.; Rainwater, B.; Ding, Y.; Chen, F. A durable, high-performance hollow-nanofiber cathode for intermediate-temperature fuel cells. Nano Energy 2016, 26, 90–99. [Google Scholar] [CrossRef]
- Afroze, S.; Karim, A.H.; Cheok, Q.; Eriksson, S.; Azad, A.K. Latest development of double perovskite electrode materials for solid oxide fuel cells: A review. Front. Energy 2019, 13, 770–797. [Google Scholar] [CrossRef]
- Shu, L.; Sunarso, J.; Hashim, S.S.; Mao, J.; Zhou, W.; Liang, F. Advanced perovskite anodes for solid oxide fuel cells: A review. Int. J. Hydrog. Energy 2019, 44, 31275–31304. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J.T.S. Catalytic properties of the perovskite oxide La0.75Sr0.25Cr0.5Fe0.5O3−δ in relation to its potential as a solid oxide fuel cell anode material. Chem. Mater. 2004, 16, 4116–4121. [Google Scholar] [CrossRef]
- Aliotta, C.; Liotta, L.F.; Deganello, F.; La Parola, V.; Martorana, A. Direct methane oxidation on La1−xSrxCr1−yFeyO3−δ perovskite-type oxides as potential anode for intermediate temperature solid oxide fuel cells. Appl. Catal. B Environ. 2016, 180, 424–433. [Google Scholar] [CrossRef]
- Vincent, A.L.; Luo, J.-L.; Chuang, K.T.; Sanger, A.R. Promotion of activation of CH4 by H2S in oxidation of sour gas over sulfur tolerant SOFC anode catalysts. Appl. Catal. B Environ. 2011, 106, 114–122. [Google Scholar] [CrossRef]
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.-W.; Jeong, H.Y.; Shin, J.; Irvine, J.T.S.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X.; Xiao, G.; Zhao, F.; Chen, F. A novel electrode material for symmetrical SOFCs. Adv. Mater. 2010, 22, 5478–5482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, T. Performance of double-perovskite Sr2−xSmxMgMoO6−δ as solid-oxide fuel-cell anodes. J. Power Sources 2011, 196, 8352–8359. [Google Scholar] [CrossRef]
- Howell, T.G.; Kuhnell, C.P.; Reitz, T.L.; Sukeshini, A.M.; Singh, R.N. A2MgMoO6 (A = Sr, Ba) for use as sulfur tolerant anodes. J. Power Sources 2013, 231, 279–284. [Google Scholar] [CrossRef]
- Jin, C.; Yang, Z.; Zheng, H.; Yang, C.; Chen, F. La0.6Sr1.4MnO4 layered perovskite anode material for intermediate temperature solid oxide fuel cells. Electrochem. Commun. 2012, 14, 75–77. [Google Scholar] [CrossRef]
- Shen, J.; Yang, G.; Zhang, Z.; Zhou, W.; Wang, W.; Shao, Z. Tuning layer-structured La0.6Sr1.4MnO4+δ into a promising electrode for intermediate-temperature symmetrical solid oxide fuel cells through surface modification. J. Mater. Chem. A 2016, 4, 10641–10649. [Google Scholar] [CrossRef]
- Chang, H.; Chen, H.; Shao, Z.; Shi, J.; Bai, J.; Li, S.-D. In situ fabrication of (Sr, La) FeO4 with CoFe alloy nanoparticles as an independent catalyst layer for direct methane-based solid oxide fuel cells with a nickel cermet anode. J. Mater. Chem. A 2016, 4, 13997–14007. [Google Scholar] [CrossRef]
- Xu, L.; Yin, Y.-M.; Zhou, N.; Wang, Z.; Ma, Z.-F. Sulfur tolerant redox stable layered perovskite SrLaFeO4−δ as anode for solid oxide fuel cells. Electrochem. Commun. 2017, 76, 51–54. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kim, J.-H.; Huq, A.; Paranthaman, M.P.; Manthiram, A. (Y0.5In0.5) Ba (Co, Zn)4O7 cathodes with superior high-temperature phase stability for solid oxide fuel cells. J. Power Sources 2012, 214, 7–14. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- McCarthy, B.P.; Pederson, L.R.; Chou, Y.S.; Zhou, X.-D.; Surdoval, W.A.; Wilson, L.C. Low-temperature sintering of lanthanum strontium manganite-based contact pastes for SOFCs. J. Power Sources 2008, 180, 294–300. [Google Scholar] [CrossRef]
- Meixner, D.L.; Cutler, R.A. Sintering and mechanical characteristics of lanthanum strontium manganite. Solid State Ion. 2002, 146, 273–284. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Lion, S.S.; Worrell, W.L. Electrical properties of novel mixed-conducting oxides. Appl. Phys. A 1989, 49, 25–31. [Google Scholar] [CrossRef]
- Gholizadeh, A. The effects of A/B-site substitution on structural, redox and catalytic properties of lanthanum ferrite nanoparticles. J. Mater. Res. Technol. 2019, 8, 457–466. [Google Scholar] [CrossRef]
- Hou, S.; Alonso, J.A.; Goodenough, J.B. Co-free, iron perovskites as cathode materials for intermediate-temperature solid oxide fuel cells. J. Power Sources 2010, 195, 280–284. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C.; Dong, F.; Shao, Z.; Ciucci, F. Cobalt-free polycrystalline Ba0.95La0.05FeO3−δ thin films as cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 2014, 250, 188–195. [Google Scholar] [CrossRef]
- Zhou, W.; Jin, W.; Zhu, Z.; Shao, Z. Structural, electrical and electrochemical characterizations of SrNb0.1Co0.9O3−δ as a cathode of solid oxide fuel cells operating below 600 °C. Int. J. Hydrog. Energy 2010, 35, 1356–1366. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, H.; Liu, X.; Meng, J.; Zhang, X.; Meng, F.; Meng, J. Investigation of layered perovskite NdBa0.5Sr0.25Ca0.25Co2O5+δ as cathode for solid oxide fuel cells. Ceram. Int. 2018, 44, 12048–12054. [Google Scholar] [CrossRef]
- Wiff, J.P.; Jono, K.; Suzuki, M.; Suda, S. Improved high temperature performance of La0.8Sr0.2MnO3 cathode by addition of CeO2. J. Power Sources 2011, 196, 6196–6200. [Google Scholar] [CrossRef]
- Hashim, S.S.; Liang, F.; Zhou, W.; Sunarso, J. Cobalt-Free Perovskite Cathodes for Solid Oxide Fuel Cells. ChemElectroChem 2019, 6, 3549–3569. [Google Scholar] [CrossRef]
- Bachina, A.; Ivanov, V.A.; Popkov, V.I. Peculiarities of LaFeO3 nanocrystals formation via glycine-nitrate combustion. Нанoсистемы: физика, химия, математика 2017, 8, 647–653. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Wang, S.; Blinn, K.; Liu, M.; Liu, Z.; Cheng, Z.; Liu, M. Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2–xYbxO3–δ. Science 2009, 326, 126–129. [Google Scholar] [CrossRef]
- Fan, L.; Su, P.-C. Layer-structured LiNi0.8Co0.2O2: A new triple (H+/O2−/e−) conducting cathode for low temperature proton conducting solid oxide fuel cells. J. Power Sources 2016, 306, 369–377. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A.; McDonald, M.S. Fuel Cell Systems Explained; J. Wiley: Chichester, UK, 2003. [Google Scholar]
- Haseli, Y.; Dincer, I.; Naterer, G.F. Thermodynamic analysis of a combined gas turbine power system with a solid oxide fuel cell through exergy. Thermochim. Acta 2008, 480, 1–9. [Google Scholar] [CrossRef]
- Massardo, A.F.; Lubelli, F. Internal reforming solid oxide fuel cell-gas turbine combined cycles (IRSOFC-GT): Part A—Cell model and cycle thermodynamic analysis. J. Eng. Gas Turbines Power 2000, 122, 27–35. [Google Scholar] [CrossRef]
- Rao, A.D.; Samuelsen, G.S. A thermodynamic analysis of tubular solid oxide fuel cell based hybrid systems. J. Eng. Gas Turbines Power 2003, 125, 59–66. [Google Scholar] [CrossRef]
- Rokni, M. Thermodynamic analysis of an integrated solid oxide fuel cell cycle with a rankine cycle. Energy Convers. Manag. 2010, 51, 2724–2732. [Google Scholar] [CrossRef]
- Chan, S.H.; Low, C.F.; Ding, O.L. Energy and exergy analysis of simple solid-oxide fuel-cell power systems. J. Power Sources 2002, 103, 188–200. [Google Scholar] [CrossRef]
- Kupecki, J.; Jewulski, J.; Motylinski, K. Parametric evaluation of a micro-CHP unit with solid oxide fuel cells integrated with oxygen transport membranes. Int. J. Hydrog. Energy 2015, 40, 11633–11640. [Google Scholar] [CrossRef]
- Fontell, E.; Kivisaari, T.; Christiansen, N.; Hansen, J.-B.; Pålsson, J. Conceptual study of a 250 kW planar SOFC system for CHP application. J. Power Sources 2004, 131, 49–56. [Google Scholar] [CrossRef]
- Zink, F.; Lu, Y.; Schaefer, L. A solid oxide fuel cell system for buildings. Energy Convers. Manag. 2007, 48, 809–818. [Google Scholar] [CrossRef]
- Al-Sulaiman, F.A.; Dincer, I.; Hamdullahpur, F. Energy analysis of a trigeneration plant based on solid oxide fuel cell and organic Rankine cycle. Int. J. Hydrog. Energy 2010, 35, 5104–5113. [Google Scholar] [CrossRef]
- Al-Sulaiman, F.A.; Dincer, I.; Hamdullahpur, F. Exergy analysis of an integrated solid oxide fuel cell and organic Rankine cycle for cooling, heating and power production. J. Power Sources 2010, 195, 2346–2354. [Google Scholar] [CrossRef]
- Weber, C.; Koyama, M.; Kraines, S. CO2-emissions reduction potential and costs of a decentralized energy system for providing electricity, cooling and heating in an office-building in Tokyo. Energy 2006, 31, 3041–3061. [Google Scholar] [CrossRef]
- Behling, N.H. Chapter 6—History of Solid Oxide Fuel Cells. In Fuel Cells: Current Technology Challenges and Future Research Needs; Behling, N.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 223–421. [Google Scholar] [CrossRef]
- SOFC (Solid Oxide Fuel Cell) Stack—Energy Conversion Devices—KYOCERA, (n.d.). Available online: https://global.kyocera.com/prdct/ecd/sofc/ (accessed on 19 April 2021).
- KYOCERA Develops Industry’s First 3-Kilowatt Solid-Oxide Fuel Cell for Institutional Cogeneration; Most Efficient SOFC on the Market Using Proprietary Ceramic Technology|News Releases|KYOCERA, (n.d.). Available online: https://global.kyocera.com/news-archive/2017/0702_bnfo.html (accessed on 19 April 2021).
- Elcogen—Solid Oxide Cells and Stacks. 2021. Available online: https://elcogen.com/ (accessed on 3 May 2021).
- Power System in Lempäälä|Case Studies|Elcogen. 2021. Available online: https://elcogen.com/case_study/lemene-fuel-cell-supported-chp-smart-grid-in-finland/ (accessed on 19 April 2021).
- Shiratori, Y.; Sakamoto, M.; Nguyen, T.G.H.; Yamakawa, T.; Kitaoka, T.; Orishima, H.; Matsubara, H.; Watanabe, Y.; Nakatsuka, S.; Doan, T.C.D.; et al. Biogas Power Generation with SOFC to Demonstrate Energy Circulation Suitable for Mekong Delta, Vietnam. Fuel Cells 2019, 19, 346–353. [Google Scholar] [CrossRef]
- Products—Convion. 2021. Available online: https://convion.fi/products/ (accessed on 19 April 2021).
- Elcogen and Magnex Sign LOI on Solid Oxide Fuel Cell (SOFC) Commercialization—Fuel Cells Works. 2021. Available online: https://fuelcellsworks.com/news/elcogen-and-magnex-signed-loi-of-sofc-commercialization/ (accessed on 19 April 2021).
- Applications—Sunfire Remote. 2021. Available online: https://remote.sunfire.de/en/applications (accessed on 19 April 2021).
- Powering the Future of Clean Energy|Bloom Energy, (n.d.). Available online: https://www.bloomenergy.com/company/ (accessed on 5 June 2021).
- Andersson, M.; Froitzheim, J. Technology Review—Solid Oxide Cells 2019. 2019. Available online: https://energiforsk.se/media/26740/technology-review-solid-oxide-cells-2019-energiforskrapport-2019-601.pdf (accessed on 19 April 2021).
- Zhang, X. Current status of stationary fuel cells for coal power generation. Clean. Energy 2018, 2, 126–139. [Google Scholar] [CrossRef]
- Mitsubishi Power, Ltd. System Overview, (n.d.). Available online: https://power.mhi.com/products/sofc/overview (accessed on 19 April 2021).
- Mitsubishi Power, Ltd. MHPS Receives First Order for Integrated Fuel Cell and Gas Turbine Hybrid Power Generation System. 2021. Available online: https://power.mhi.com/news/20180131.html (accessed on 19 April 2021).
- Mitsubishi Power, Ltd. Second Commercial-USE MEGAMIE System Begins Operations at HAZAMA ANDO Technical Research Institute, Supplying Clean Power with Low CO2 Emissions. 2021. Available online: https://power.mhi.com/news/20200409.html (accessed on 19 April 2021).
- Who We Are|www.fch.europa.eu. 2021. Available online: https://www.fch.europa.eu/page/who-we-are (accessed on 19 April 2021).
- ComSos—Comsos, (n.d.). Available online: https://www.comsos.eu/about/the-comsos-concept/ (accessed on 19 April 2021).
- Workshop Industrial Application of SOFC Systems-DEMOSOFC Project. Available online: https://www.fch.europa.eu/event/workshop-industrial-application-sofc-systems-demosofc-project (accessed on 3 May 2021).
- Noponen, M.; Torri, P.; Goos, J.; Chade, D.; Hallanoro, P.; Temmo, A.; Koit, A.; Ounpuu, E. Status of Solid Oxide Fuel Cell Development at Elcogen. ECS Trans. 2015, 68, 151–156. [Google Scholar] [CrossRef]
- McPhail, S.J.; Kiviaho, J.; Conti, B. The Yellow Pages of SOFC Technology. International Status of SOFC deployment 2017, IEA Implementing Agreement Advanced Fuel Cells Annex 32-SOFC Energy and Sustainable Economic Development DOSSIER, ISBN 978-951-38-8602-8 (printed); ISBN 978-951-38-8603-5 (online). Available online: https://www.ieafuelcell.com/fileadmin/publications/2017_The_yellow_pages_of_SOFC_technology.pdf (accessed on 3 May 2021).
- Solid Oxide Cells & Stacks|Products|Elcogen, (n.d.). Available online: https://elcogen.com/products/ (accessed on 21 April 2021).
- Renewables Everywhere—Sunfire, (n.d.). Available online: https://www.sunfire.de/en/ (accessed on 21 April 2021).
- Sunfire-Remote 900—Fuel Cell Systems, (n.d.). Available online: https://www.fuelcellsystems.co.uk/sunfire-remote-store/p/style-01-6mfpa-ppzjl/ (accessed on 21 April 2021).
- Bloom Energy Server ES5-300kW|Bloom Energy, (n.d.). Available online: https://www.bloomenergy.com/resource/bloom-energy-server-es5-300kw/ (accessed on 5 June 2021).
- Clean, Reliable and Affordable: The Bloom Energy Server | Bloom Energy, (n.d.). Available online: https://www.bloomenergy.com/technology/ (accessed on 5 June 2021).
- Sumi, H.; Shimada, H.; Yamaguchi, Y.; Yamaguchi, T. Metal-supported microtubular solid oxide fuel cells with ceria-based electrolytes. J. Ceram. Soc. Jpn. 2017, 125, 208–212. [Google Scholar] [CrossRef]
- What’s SOFC?, (n.d.). Available online: https://power.mhi.com/products/sofc/ (accessed on 3 May 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendonça, C.; Ferreira, A.; Santos, D.M.F. Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies. Fuels 2021, 2, 393-419. https://doi.org/10.3390/fuels2040023
Mendonça C, Ferreira A, Santos DMF. Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies. Fuels. 2021; 2(4):393-419. https://doi.org/10.3390/fuels2040023
Chicago/Turabian StyleMendonça, Catarina, António Ferreira, and Diogo M. F. Santos. 2021. "Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies" Fuels 2, no. 4: 393-419. https://doi.org/10.3390/fuels2040023
APA StyleMendonça, C., Ferreira, A., & Santos, D. M. F. (2021). Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies. Fuels, 2(4), 393-419. https://doi.org/10.3390/fuels2040023






