Biogas Dry Reforming for Hydrogen through Membrane Reactor Utilizing Negative Pressure
Abstract
1. Introduction
2. Experimentation
2.1. Experimental Set-Up
2.2. Performance Evaluation of Proposed Reactor
3. Results and Discussion
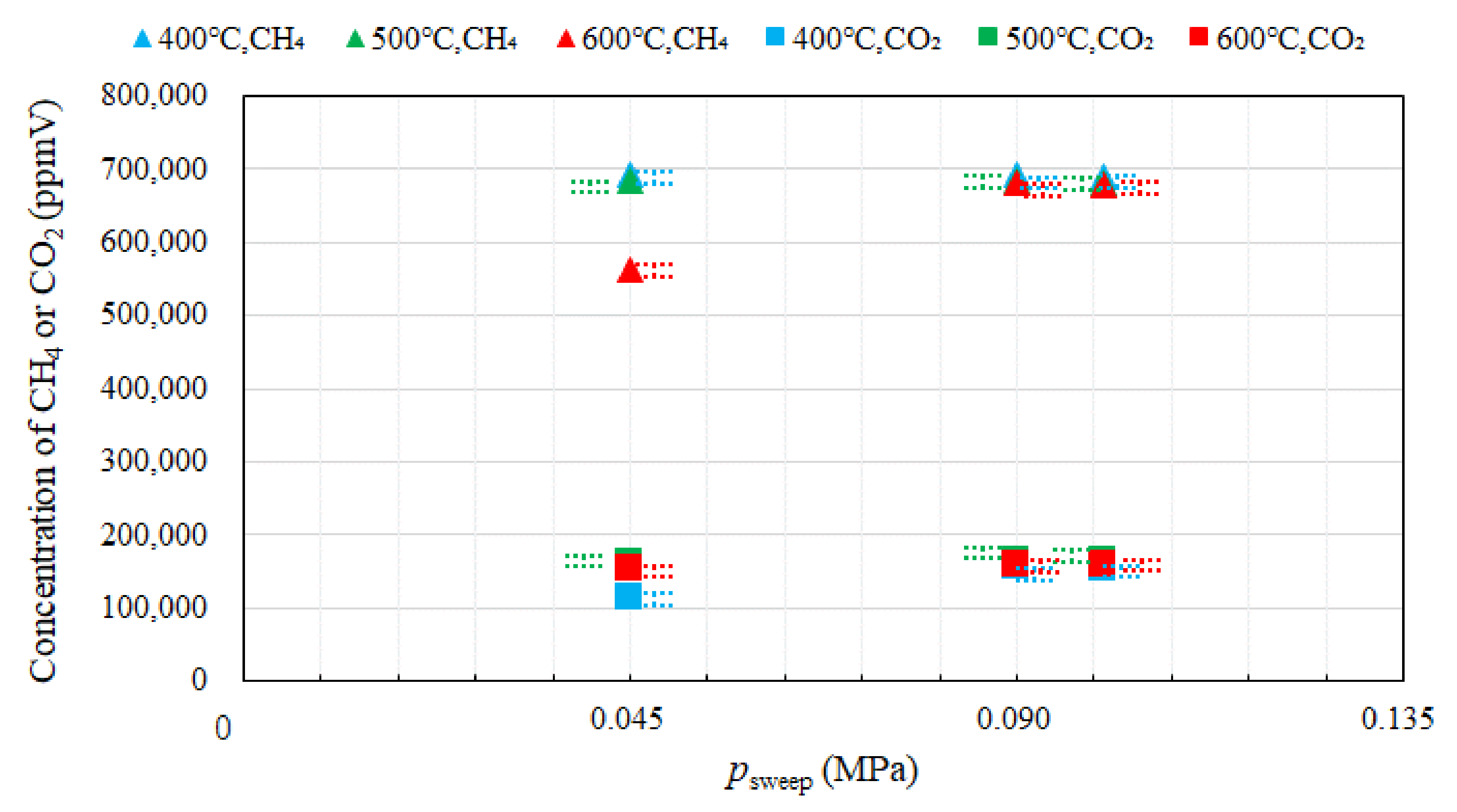
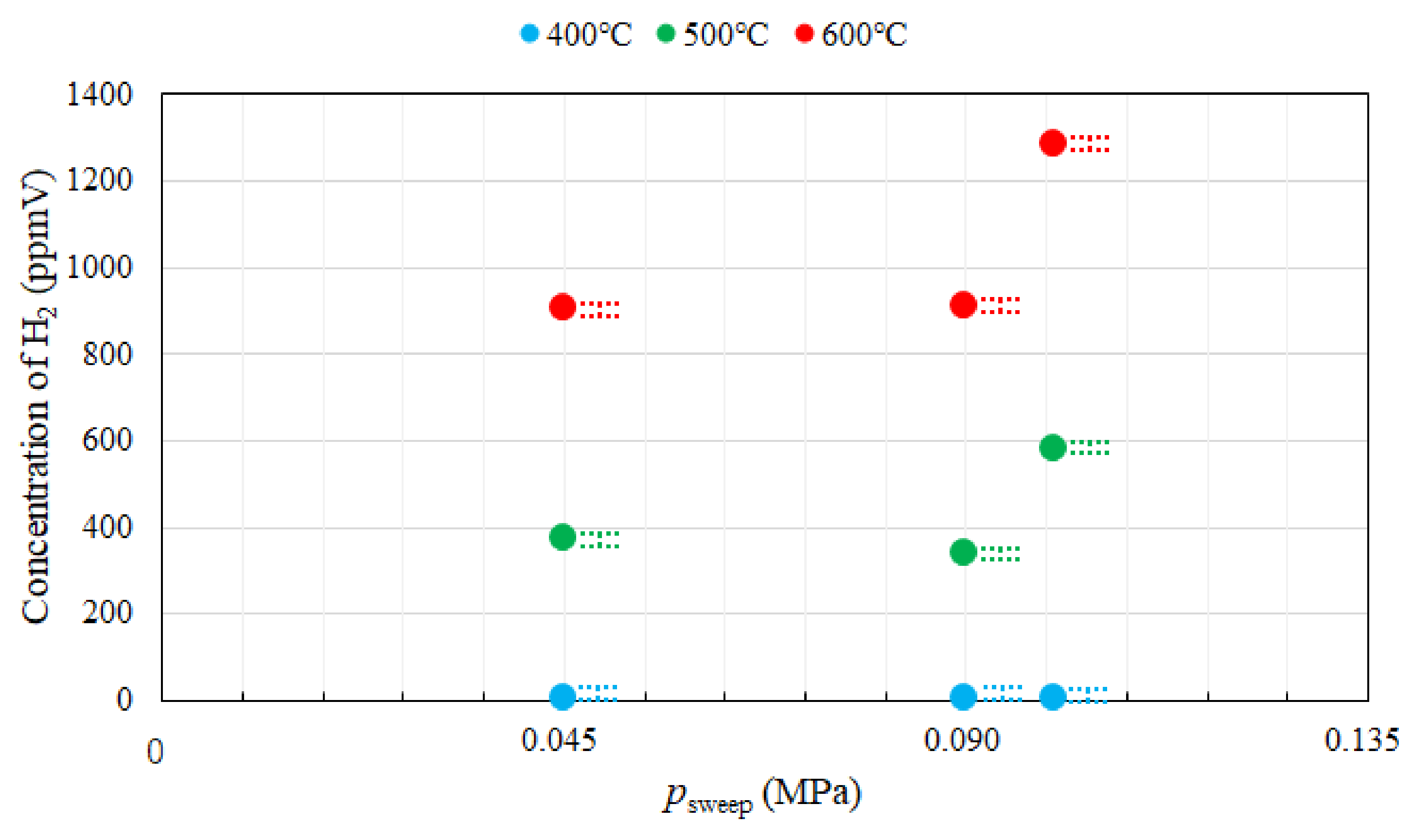
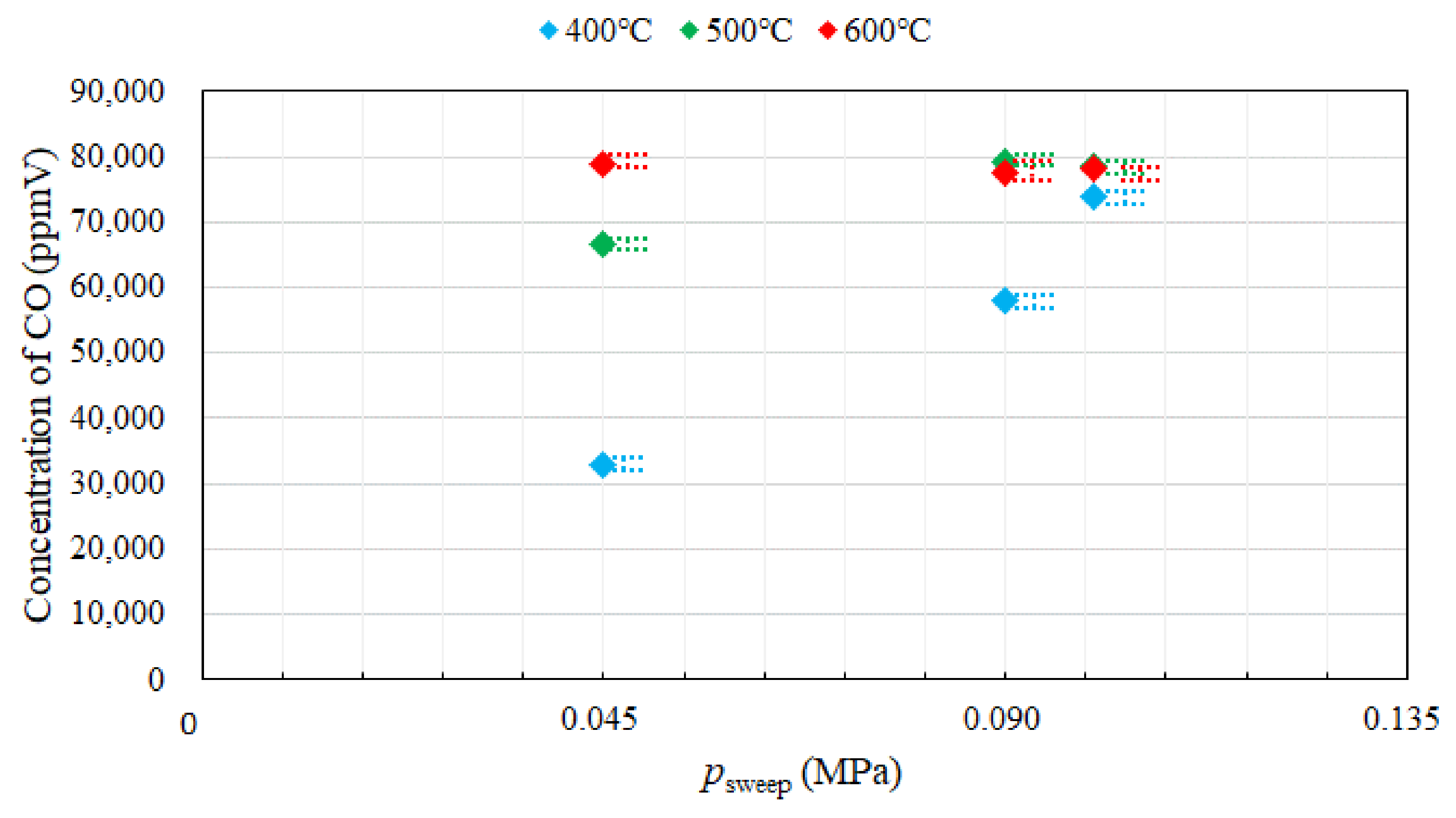
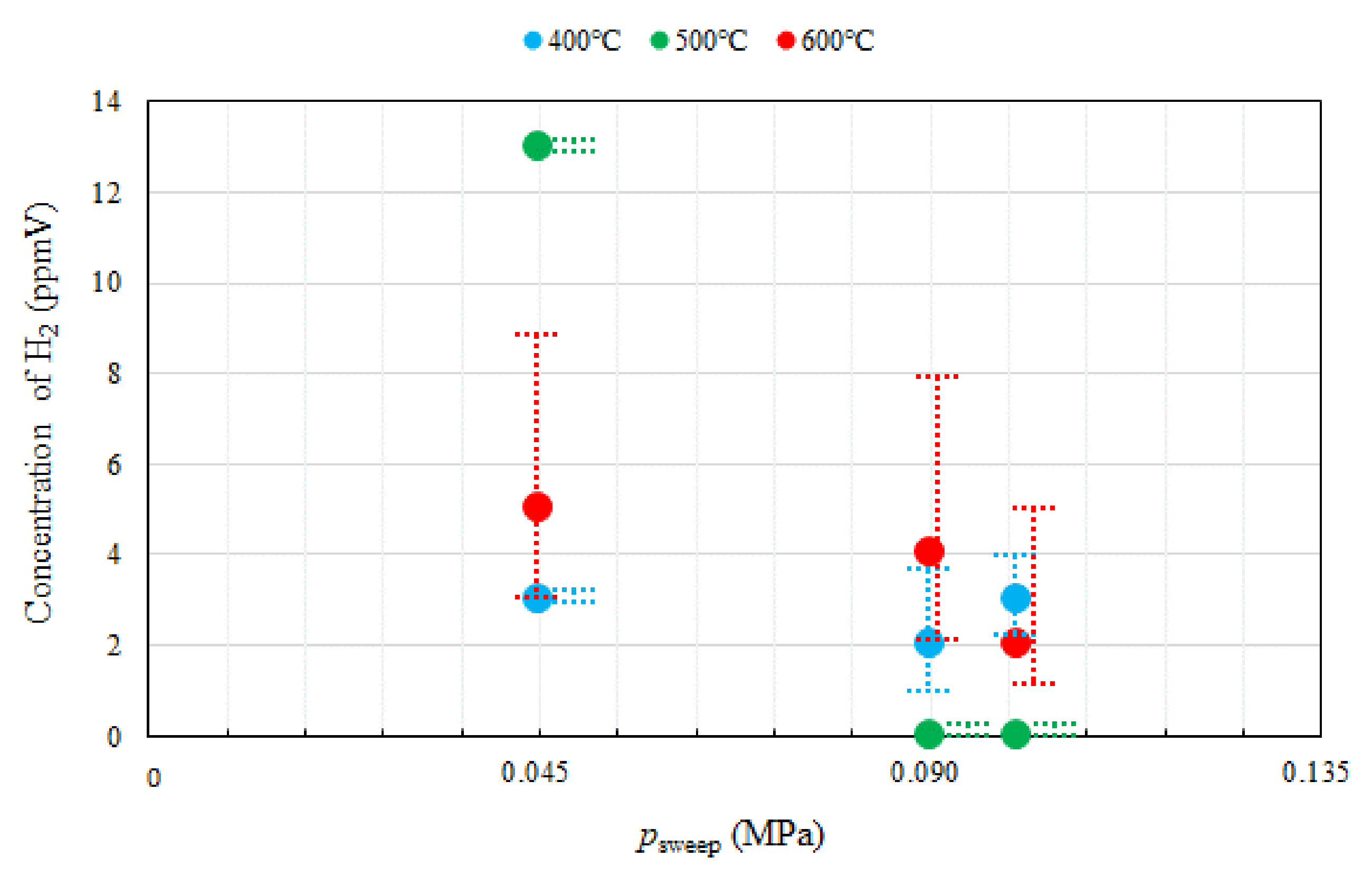
3.1. Effect of Pressure of Sweep Gas on the Performance of Dry Reforming under Different Reaction Temperatures
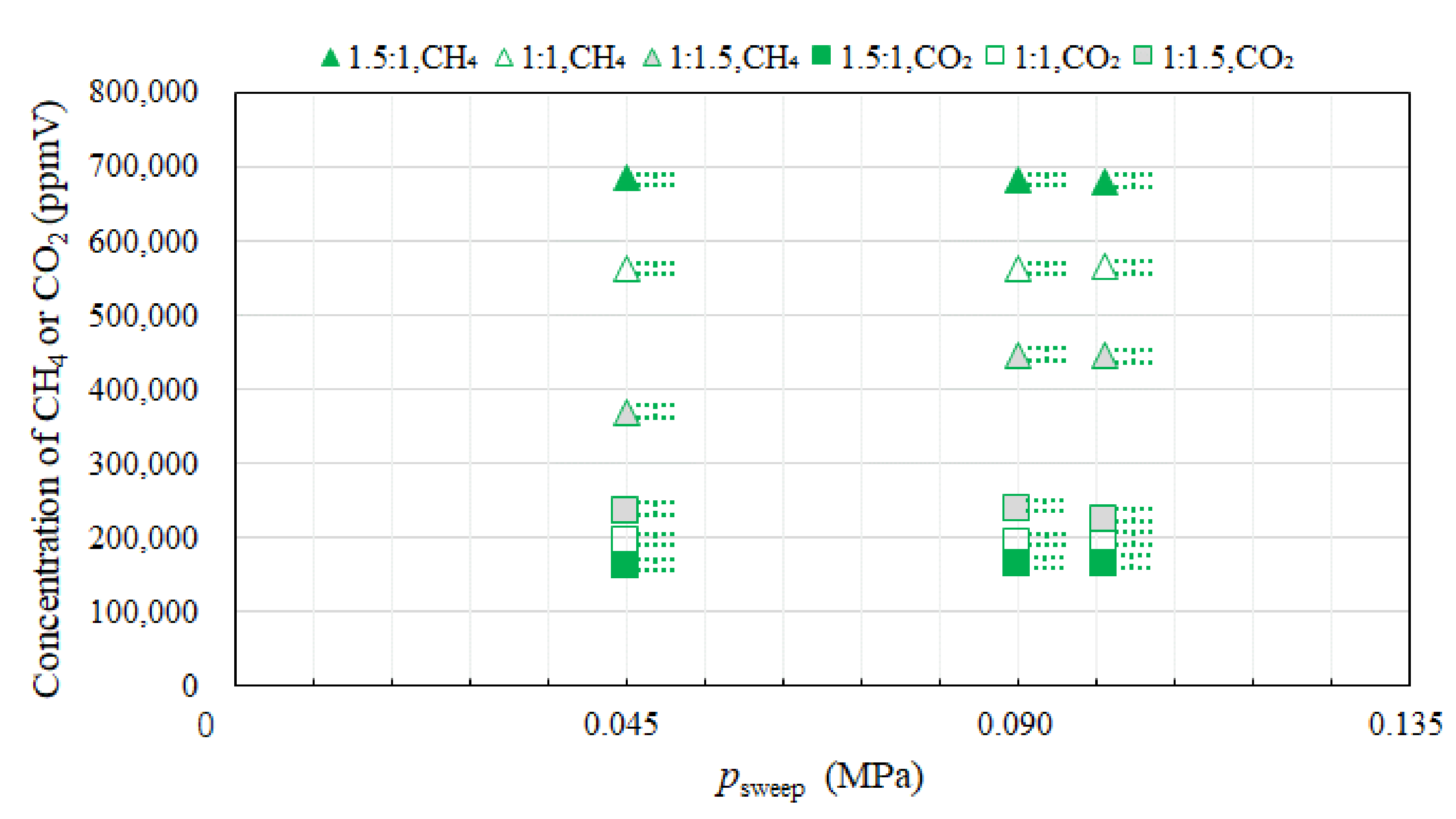
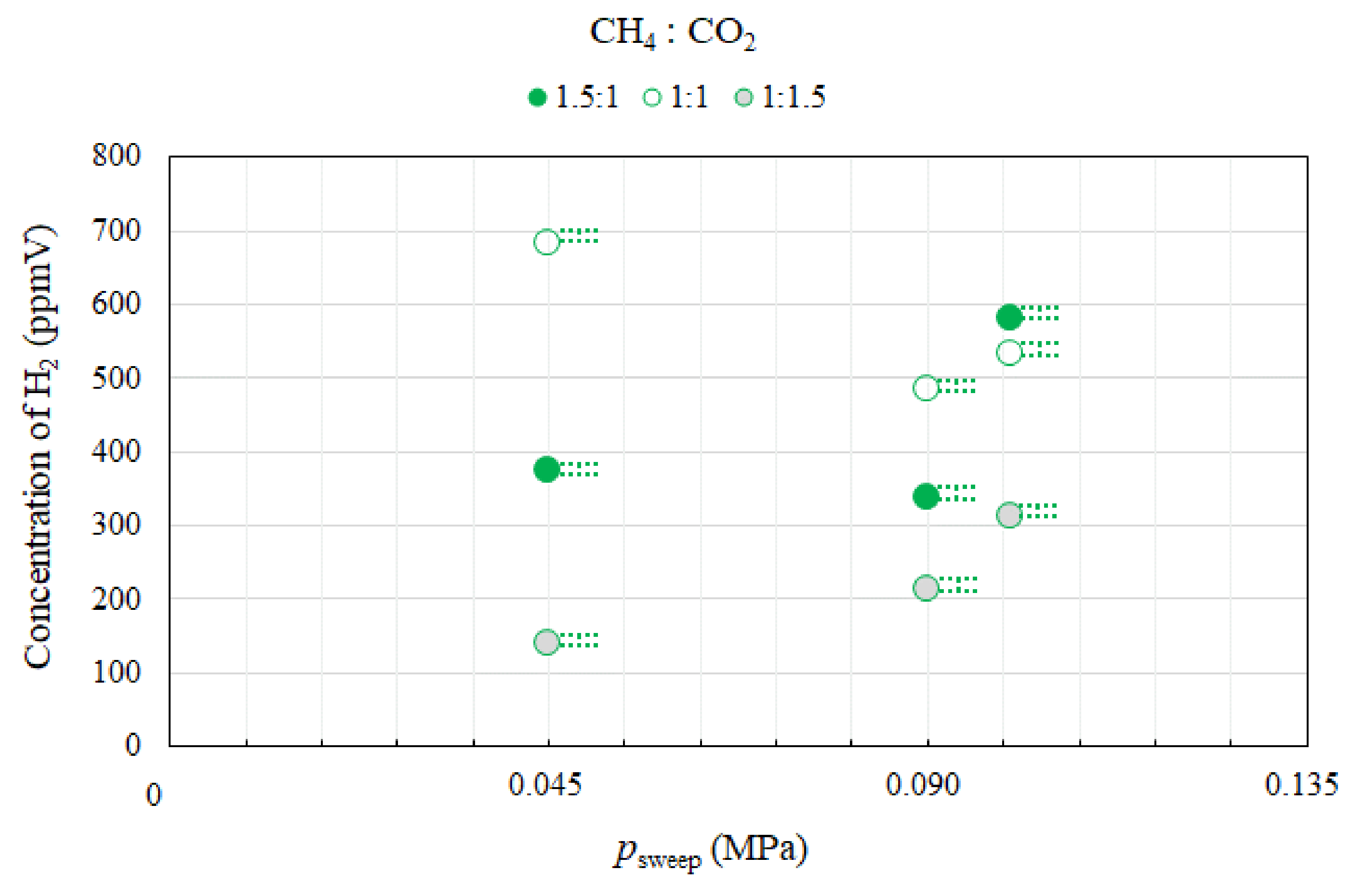
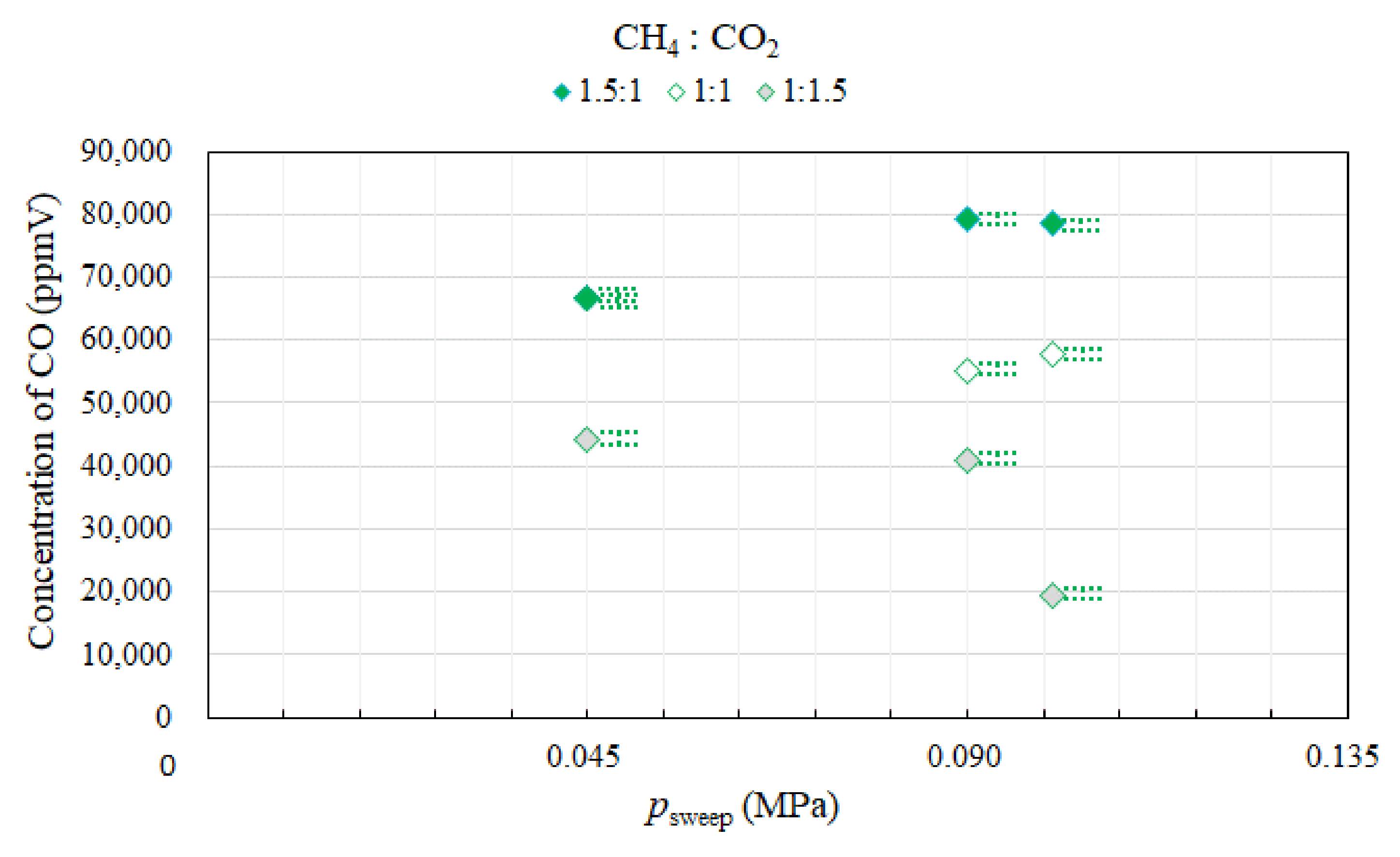
3.2. Effect of Pressure of Sweep Gas on the Performance of Dry Reforming under Different Molar Ratios
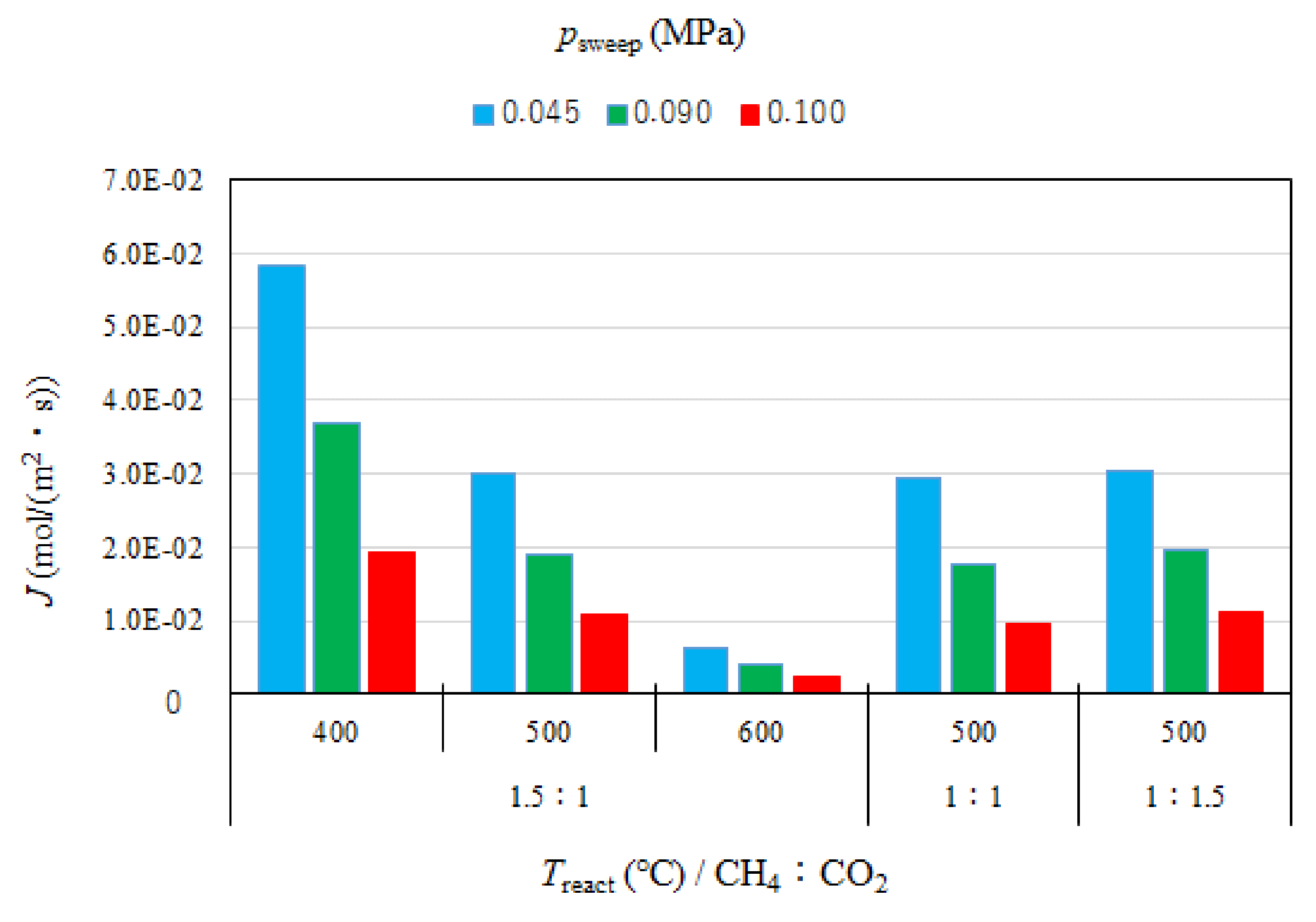
3.3. Effect of Pressure of Sweep Gas on H2 Flux from Reaction Chamber to Sweep Chamber
4. Conclusions
- (i)
- Under the condition of the molar ratio of CH4:CO2 = 1.5:1 changing the reaction temperature by 400 °C, 500 °C, 600 °C, the impact of psweep on concentrations of CH4 and CO at the outlet of reaction chamber is negligible. It is concluded that the concentrations of H2 and CO at the outlet of reaction chamber increase with incase in the reaction temperature. It is revealed that the concentration of H2 at the outlet of reaction chamber, under negative pressure conditions, is smaller than that under the atmosphere pressure condition. High CO selectivity is obtained, while H2 selectivity is low.
- (ii)
- Under the condition of the reaction temperature of 500 °C changing molar ratio of CH4:CO2, the highest concentration of H2 at the outlet of reaction chamber is obtained at the molar ratio of CH4:CO2 = 1:1. In addition, the concentrations of H2 at the outlet of reaction chamber under negative pressure conditions are lower than those under the atmosphere pressure condition, except for the case of the molar ratio of CH4:CO2 = 1:1. The concentration of CO at the outlet of reaction chamber is the highest in the case of the molar ratio of CH4:CO2 = 1.5:1. High CO selectivity is obtained, while H2 selectivity is low.
- (iii)
- CO2 conversion is larger compared to CH4 conversion irrespective of psweep, reaction temperature and molar ratio of CH4:CO2. CH4 conversion and H2 yield is the highest in the case of CH4:CO2 = 1:1, respectively.
- (iv)
- The concentration of H2 at the outlet of sweep chamber is the highest at the reaction temperature of 500 °C and psweep of 0.045 MPa. It is due to the balance between the permeation flux and reaction kinetics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tinnirello, M.; Papurello, D.; Santarelli, M.; Fiorilli, S. Thermal activation of digested sewage sludges for carbon dioxide removal from biogas. Fuels 2020, 1, 30–46. [Google Scholar] [CrossRef]
- Kuan, C.Y.; Neng, M.L.Y.; Chan, Y.B.; Sim, Y.L.; Strothers, J.; Pratt, L.M. Thermal transformation of plan waste to high-quality hydrocarbon fuel. Fuels 2020, 1, 2–14. [Google Scholar] [CrossRef]
- Karatza, D.; Konstantopoulos, C.; Chianese, S.; Diplas, S.; Svec, P.; Hristoforou, E.; Musmarra, D. Hydrogen production through water splitting at low temperature over Fe3O4 pellet: Effects of electric power, magnetic field, and temperature. Fuel Process. Technol. 2021, 211, 106606. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Li, J.; Wei, B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Vinvent, I.; Lee, E.C.; Kim, H.M. Comprehensive impedance investigation of low-cost anion exchange membrane electrolysis for large-scale hydrogen production. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Kalai, D.Y.; Stangeland, K.; Jin, Y.; Tucho, W.M.; Yu, Z. Biogas dry reforming for syngas production on La promoted hydrotalcite-derived Ni catalyst. Int. J. Hydrog. Energy 2018, 43, 19438–19450. [Google Scholar] [CrossRef]
- World Bioenergy Association. Global Bioenergy Statistics. Available online: https://worldbioenergy.org./global-bioenergy-statistics (accessed on 23 March 2021).
- The Japan Gas Association. Available online: https://www.gas.or.jp/gas-life/biomass/ (accessed on 23 March 2021).
- Foo, S.Y.; Cheng, C.K.; Nguyen, T.H.; Adesina, A.A. Kinetic study of methane CO2 reforming on Co-Ni/Al2O3 and Ce-Co-Ni/Al2O3 catalysts. Catal. Today 2011, 164, 221–226. [Google Scholar] [CrossRef]
- Bobrova, L.N.; Bobin, A.S.; Mezentseva, N.V.; Sadykov, V.A.; Thybaut, J.W.; Marin, G.B. Kinetic assessment of dry reforming of methane on Pt + Ni containing composite of fluorite-like structure. Appl. Catal. B Environ. 2016, 182, 513–524. [Google Scholar] [CrossRef]
- Park, N.; Park, M.J.; Baek, S.C.; Ha, K.S.; Lee, Y.J.; Kwak, G.; Park, H.G.; Jun, K.W. Modeling and optimization of the mixed reforming of methane: Maximizing CO2 utilization for non-equilibrated reaction. Fuel 2014, 115, 357–365. [Google Scholar] [CrossRef]
- Ozkara-Aydinoglu, S.; Aksoylu, A.E. A comparative study on the kinetics of carbon dioxide reforming of methane over Pt-Ni/Al2O3 catalyst: Effect of Pt/Ni ratio. Chem. Eng. J. 2013, 215–216, 542–549. [Google Scholar] [CrossRef]
- Li, K.; He, F.; Yu, H.; Wang, Y.; Wu, Z. Theoretical study on the reaction mechanism of carbon dioxide reforming of methane on La and La2O3 modified Ni (1 1 1) surface. J. Catal. 2018, 364, 248–261. [Google Scholar] [CrossRef]
- Elsayed, N.H.; Roberts, N.R.M.; Joseph, B.; Kuhn, J.N. Comparison of Pd-Ni-Mg/Ceria-Zirconia and Pt-Ni-Mg/Ceria-Zirconia catalysts for syngas production via low temperature forming of model biogas. Top. Catal. 2016, 59, 138–146. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Omoregbe, O.; Danh, H.T.; Nguyen-Huy, C.; Setiabudi, H.D.; Abidin, S.Z.; Truong, Q.D.; Vo, D.V.N. Syngas production from methane dry reforming over Ni/SBA-15 catalyst: Effect of operating parameters. Int. J. Hydrog. Energy 2017, 42, 11283–11294. [Google Scholar] [CrossRef]
- Nataj, S.M.M.; Alavi, S.M.; Mazloom, G. Catalytic performance of Ni supported on ZnO-Al2O3 composites with different Zn content in methane dry reforming. J. Chem. Technol. Biotechnol. 2019, 94, 1305–1314. [Google Scholar] [CrossRef]
- Yan, X.; Hu, T.; Liu, P.; Li, S.; Zhao, B.; Zhang, Q.; Jiao, W.; Chen, S.; Wang, P.; Lu, J.; et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2. Appl. Catal. B Environ. 2019, 246, 221–231. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Wu, Y.; Pan, J.; Zhang, Q.; Tan, Y.; Han, Y. Insight into the effects of the oxygen species over Ni/ZrO2 catalyst surface on methane reforming with carbon dioxide. Appl. Catal. B Environ. 2019, 244, 427–437. [Google Scholar] [CrossRef]
- Park, J.H.; Heo, I.; Chang, T.S. Dry reforming of methane over Ni-substituted CaZrNiOx catalyst prepared by the homogeneous deposition method. Catal. Commun. 2019, 120, 1–5. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ayodele, B.V.; Cheng, C.K.; Khan, M.R. Optimization of renewable hydrogen-rich syngas production from catalytic reforming of greenhouse gases (CH4 and CO2) over calcium iron oxide supported nickel catalyst. J. Energy Inst. 2019, 92, 177–194. [Google Scholar] [CrossRef]
- Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Biogas reforming for hydrogen enrichment by ceria decorated over nickel catalyst supported on titania and alumina. Int. J. Hydrog. Energy 2018, 43, 21246–21255. [Google Scholar] [CrossRef]
- Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Hydrogen enrichment of biogas via dry and authothermal-dry reforming with pure nickel (Ni) nanoparticle. Energy 2019, 172, 733–739. [Google Scholar] [CrossRef]
- Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Catalytic reforming of synthetic biogas for hydrogen enrichment over Ni supported on ZnO-CeO2 mixed catalyst. Biomass Bioenergy 2019, 125, 70–78. [Google Scholar] [CrossRef]
- Schiaroli, N.; Lucarelli, C.; Luna, G.S.; Fornasari, G.; Vaccari, A. Ni-based catalysts to produce synthesis gas by combined reforming of clean biogas. Appl. Catal. A Gen. 2019, 582, 117087. [Google Scholar] [CrossRef]
- Lyu, L.; Han, Y.; Ma, Q.; Makpal, S.; Sun, J.; Gao, X.; Zhang, J.; Fan, H.; Zhao, T.S. Fabrication of Ni-based bimodal porous catalyst for dry reforming of methane. Catalysts 2020, 10, 1220. [Google Scholar] [CrossRef]
- Omran, A.; Yoon, S.H.; Khan, M.; Ghouri, M.; Chatla, A.; Elbashir, N. Mechanistic insights for dry reforming of methane on Cu/Ni bimetallic catalysts: DFT-assisted microkinetic analysis for coke resistance. Catalysts 2020, 10, 1043. [Google Scholar] [CrossRef]
- Anzelmo, B.; Wilcox, J.; Liguori, S. Natural gas stream reforming reaction at low temperature and pressure conditions for hydrogen production via Pd/PSS methane reactor. J. Membr. Sci. 2017, 522, 343–350. [Google Scholar] [CrossRef]
- Yan, Y.; Cui, Y.; Zhang, J.; Chen, Y.; Tang, Q.; Lin, C. Experimental investigation of methane auto-thermal reforming in hydrogen-permeable membrane reactor for pure hydrogen production. Int. J. Hydrog. Energy 2016, 41, 13069–13076. [Google Scholar] [CrossRef]
- Dolan, M.; Beath, A.; Hla, S.; Way, J.; Abu El Hawa, H. An experimental and techno-economic assessment of solar reforming for H2 production. Int. J. Hydrog. Energy 2016, 41, 14583–14595. [Google Scholar] [CrossRef]
- Brunetti, A.; Fontananova, E. CO2 conversion by membrane reactors. J. Nanosci. Nanotechnol. 2019, 19, 3124–3134. [Google Scholar] [CrossRef]
- Sumrunronnasak, S.; Tantayanon, S.; Kiatgamolchai, S.; Sukonket, T. Improved hydrogen production from dry reforming reaction using a catalytic packed-bed membrane reactor with Ni-based catalyst and dense PdAgCu alloy membrane. Int. J. Hydrog. Energy 2016, 41, 2621–2630. [Google Scholar] [CrossRef]
- Duran, P.; Sanz-Martinez, A.; Soler, J.; Manendez, M.; Herguido, J. Pure hydrogen from biogas: Intensified methane dry reforming in a two-zone fluidized bed reactor using permselective membranes. Chem. Eng. J. 2019, 370, 772–781. [Google Scholar] [CrossRef]
- Bosko, M.L.; Munera, J.F.; Lombardo, E.A.; Cornaglia, L.M. Dry reforming of methane in membrane reactors using Pd and Pd-Ag composite membranes on a NaA zeolite modified porous stainless steel support. J. Membr. Sci. 2010, 364, 17–26. [Google Scholar] [CrossRef]
- Munera, J.; Faroldi, B.; Fruits, E.; Lombardo, E.; Cornaglia, L.; Carrazan, S.G. Supported Rh nanoparticles on CaO-SiO2 binary systems for the reforming of methane by carbon dioxide in membrane reactors. Appl. Catal. A Gen. 2014, 474, 114–124. [Google Scholar] [CrossRef]
- Simakov, D.S.A.; Roman-Leshkov, Y. Highly efficient methane reforming over a low-loading Ru/-Al2O3 catalyst in a Pd-Ag membrane reactor. AICHE J. 2018, 64, 3101–3108. [Google Scholar] [CrossRef]
- Liu, J.; Bellini, S.; Nooijer, N.C.A.; Sun, Y.; Tanaka, D.A.P.; Tang, C.; Li, H.; Gallucci, F.; Caravella, A. Hydrogen permeation and stability in ultra-thin Pd-Ru supported membrane. Int. J. Hydrog. Energy 2020, 45, 7455–7467. [Google Scholar] [CrossRef]
- Fontana, A.D.; Faroldi, B.; Cornaglia, L.M.; Tarditi, A.M. Development of catalytic membranes over PdAu selective films for hydrogen production through the dry reforming of methane. Mol. Catal. 2020, 481, 100643. [Google Scholar] [CrossRef]
- Jia, H.; Xu, H.; Sheng, X.; Yang, X.; Shen, W.; Goldbach, A. High-temperature ethanol steam reforming in PdCu membrane reactor. J. Membr. Sci. 2020, 605, 118083. [Google Scholar] [CrossRef]
- Roa, F.; Way, D. Influence of alloy composition and membrane fabrication on the pressure dependence of the hydrogen flux of palladium-copper membranes. Ind. Eng. Chem. Res. 2003, 42, 5827–5835. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.R.; Soria, M.A.; Mateos-Pedero, C.; Guerrero-Ruiz, A.; Odriguez-Ramos, I.; Li, K. Dry reforming of methane using Pd-based membrane reactors fabricated from different substrates. J. Membr. Sci. 2013, 435, 218–225. [Google Scholar] [CrossRef]
- Ugarte, P.; Duran, P.; Lasobras, J.; Soler, J.; Menendez, M.; Herguido, J. Dry reforming of biogas in fluidized bed; process intensification. Int. J. Hydrog. Energy 2017, 42, 13589–13597. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Kumar, B.; Kumar, S.; Jilani, S. Comparative modeling study of catalytic membrane reactor configurations for syngas production by CO2 reforming of methane. J. CO2 Util. 2017, 20, 336–346. [Google Scholar] [CrossRef]
- Leimert, J.M.; Karl, J.; Dillig, M. Dry reforming of methane using a nickel membrane reactor. Processes 2017, 5, 82. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Abuelyamen, A.; Habib, M.A. CFD modeling of hydrogen separation through Pd-based membrane. Int. J. Hydrog. Energy 2020, 45, 23006–23019. [Google Scholar] [CrossRef]
- Li, H.; Goldbach, A.; Li, W.; Xu, H. PdC formation in ultra-thin Pd membrane during separation of H2/CO mixtures. Int. J. Hydrog. Energy 2016, 41, 10193–10201. [Google Scholar] [CrossRef]
- Nishimura, A.; Ohata, S.; Okukura, K.; Hu, E. The Impact of Operating Conditions on the Performance of a CH4 Dry Reforming Membrane Reactor for H2 Production. J. Energy Power Technol. 2020, 2, 1–19. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Kinetic studies of carbon dioxide reforming of methane over Ni-Co/Al-Mg-O bimetallic catalyst. Ind. Eng. Chem. Res. 2009, 48, 677–684. [Google Scholar] [CrossRef]
- Avetisov, A.K.; Rostrup-Nielsen, J.R.; Kuchaev, V.L.; Hansen, J.H.B.; Zyskin, A.G.; Shapatina, E.N. Steady-state kinetics and mechanism of methane reforming with steam and carbon dioxide over Ni catalyst. J. Mol. Catal. A Chem. 2010, 315, 155–162. [Google Scholar] [CrossRef]
- Richardson, J.T.; Paripatyadar, S.A. Carbon dioxide reforming of methane with supported rhodium. Appl. Catal. 1990, 61, 293–309. [Google Scholar] [CrossRef]
- Quiroga, M.M.B.; Luna, A.E.C. Kinetic analysis of rate data for dry reforming of methane. Ind. Eng. Chem. Res. 2007, 46, 5265–5270. [Google Scholar] [CrossRef]
- Benguerba, Y.; Virginie, M.; Dumas, C.; Ernst, B. Computational fluid dynamics study of the dry reforming of methane over Ni/Al2O3 catalyst in membrane reactor. Coke deposition. Kinet. Catal. 2017, 58, 328–338. [Google Scholar] [CrossRef]
- Tsuneki, T.; Shirasaki, Y.; Yasuda, I. Hydrogen permeability of palladium-copper alloy membranes. J. Jpn. Inst. Met. 2006, 70, 658–661. [Google Scholar] [CrossRef][Green Version]
- Fan, M.S.; Abdullah, A.Z.; Bhatia, S. Utilization of greenhouse gases through dry reforming: Screening of nickel-based bimetallic catalysts and kinetic studies. ChemSusChem 2011, 4, 1643–1653. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Lee, B.; Lim, H. Parametric studies for CO2 reforming of methane in a membrane reactor as a new CO2 utilization process. Korean J. Chem. Eng. 2017, 34, 199–205. [Google Scholar] [CrossRef]
- Neni, A.; Benguerba, Y.; Balsamo, M.; Erto, A.; Ernst, B.; Benachour, D. Numerical study of sorption-enhanced methane steam reforming over Ni/Al2O3 catalyst in a fixed-bed reactor. Int. J. Heat Mass Transf. 2021, 165, 120635. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, Y.; Zhang, W.; Feng, D.; Sun, S. The intrinsic kinetics of methane steam reforming over a nickel-based catalyst in a micro fluidized bed reaction system. Int. J. Hydrog. Energy 2020, 45, 1615–1628. [Google Scholar] [CrossRef]
- Marcoberardino, G.D.; Foresti, S.; Binotti, M.; Manzolini, G. Potentiality of a biogas membrane reformer for decentrailized hydrogen production. Chem. Eng. Process. Process Intensif. 2018, 129, 131–141. [Google Scholar] [CrossRef]

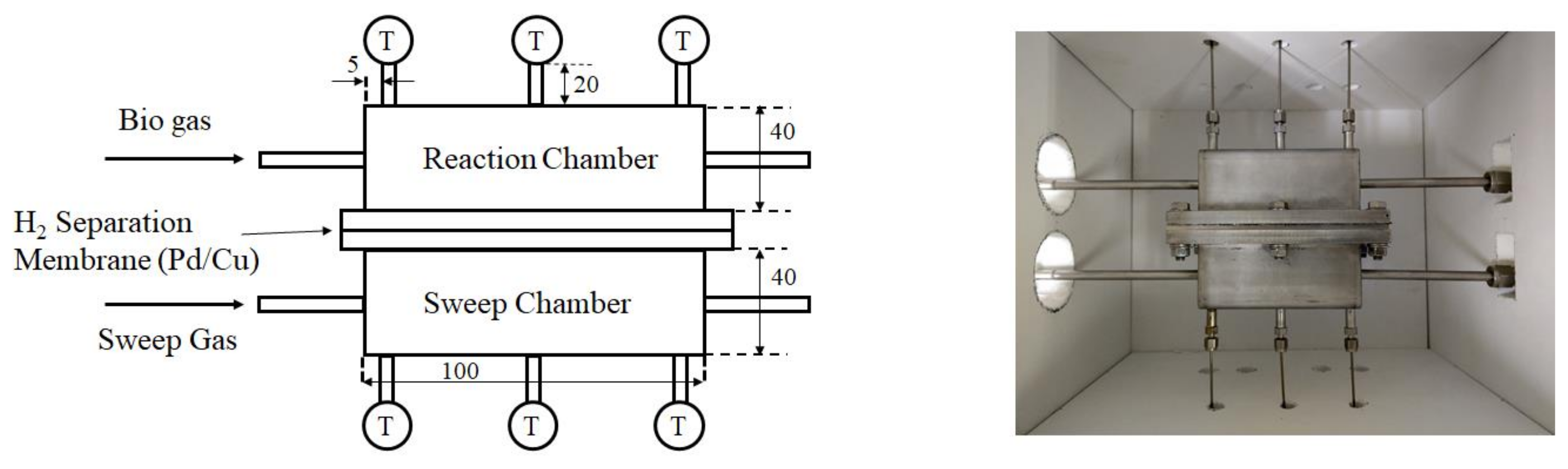
| Reaction temperature (°C) | 400, 500, 600 |
| Pressure of supply gas (MPa) | 0.10 |
| Pressure of sweep gas, psweep (MPa) | 0.10, 0.09, 0.045 |
| Temperature of supply gas (°C) | 25 |
| Molar ratio of supplied CH4:CO2 (Flow rate of CH4 and CO2 (NL/min)) | 1.5:1, 1:1, 1:1.5 (1.088:0.725, 0.725:0.725, 0.725:1.088) |
| Feed ratio of sweep gas | 1.0 |
| psweep (MPa) | H2 Selectivity (%) | CO Selectivity (%) | ||||
|---|---|---|---|---|---|---|
| 400 °C | 500 °C | 500 °C | 600 °C | |||
| 0.045 | 0 | 0.5 | 1.1 | 100 | 99.5 | 98.9 |
| 0.090 | 0 | 0.4 | 1.2 | 100 | 99.6 | 98.8 |
| 0.101 | 0 | 0.7 | 1.6 | 100 | 99.3 | 98.4 |
| psweep (MPa) | 400 °C (%) | 500 °C (%) | 600 °C (%) |
|---|---|---|---|
| 0.045 | 0.002 | 0.129 | 0.303 |
| 0.090 | 0.002 | 0.112 | 0.304 |
| 0.101 | 0.003 | 0.193 | 0.429 |
| psweep (MPa) | 400 °C (%) | 500 °C (%) | 600 °C (%) |
|---|---|---|---|
| 0.045 | 16.5 | 35.2 | 39.4 |
| 0.090 | 29.3 | 40.1 | 38.9 |
| 0.101 | 37.0 | 39.4 | 39.2 |
| psweep (MPa) | 400 °C (%) | 500 °C (%) | 600 °C (%) |
|---|---|---|---|
| 0.045 | 0.0006 | 0.0322 | 0.0758 |
| 0.090 | 0.0005 | 0.0281 | 0.0761 |
| 0.101 | 0.0007 | 0.0483 | 0.1072 |
| psweep (MPa) | H2 Selectivity (%) | CO Selectivity (%) | ||||
|---|---|---|---|---|---|---|
| CH4:CO2 = 1.5:1 | CH4:CO2 = 1:1 | CH4:CO2 = 1:1.5 | CH4:CO2 = 1.5:1 | CH4:CO2 = 1:1 | CH4:CO2 = 1:1.5 | |
| 0.045 | 0.5 | 1.0 | 0.3 | 99.5 | 99.0 | 99.7 |
| 0.090 | 0.4 | 0.9 | 0.5 | 99.6 | 99.1 | 99.5 |
| 0.101 | 0.7 | 0.9 | 1.4 | 99.3 | 99.1 | 98.6 |
| Psweep (MPa) | CH4 Conversion (%) | CO2 Conversion (%) | ||||
|---|---|---|---|---|---|---|
| CH4:CO2 = 1.5:1 | CH4:CO2 = 1:1 | CH4:CO2 = 1:1.5 | CH4:CO2 = 1.5:1 | CH4:CO2 = 1:1 | CH4:CO2 = 1:1.5 | |
| 0.045 | 0.129 | 0.231 | 0.047 | 35.2 | 33.5 | 22.2 |
| 0.090 | 0.112 | 0.162 | 0.072 | 40.1 | 27.8 | 20.6 |
| 0.101 | 0.193 | 0.178 | 0.105 | 39.4 | 29.0 | 9.85 |
| Psweep (MPa) | H2 Yield (%) | ||
|---|---|---|---|
| CH4:CO2 = 1.5:1 | CH4:CO2 = 1:1 | CH4:CO2 = 1:1.5 | |
| 0.045 | 0.0322 | 0.0578 | 0.0117 |
| 0.090 | 0.0281 | 0.0405 | 0.0180 |
| 0.101 | 0.0483 | 0.0444 | 0.0262 |
| Temperature (°C) | Pe (mol/(m·s·Pa0.5)) |
|---|---|
| 400 | 1.0 × 10−8 |
| 500 | 5.0 × 10−9 |
| 600 | 1.0 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, A.; Takada, T.; Ohata, S.; Kolhe, M.L. Biogas Dry Reforming for Hydrogen through Membrane Reactor Utilizing Negative Pressure. Fuels 2021, 2, 194-209. https://doi.org/10.3390/fuels2020012
Nishimura A, Takada T, Ohata S, Kolhe ML. Biogas Dry Reforming for Hydrogen through Membrane Reactor Utilizing Negative Pressure. Fuels. 2021; 2(2):194-209. https://doi.org/10.3390/fuels2020012
Chicago/Turabian StyleNishimura, Akira, Tomohiro Takada, Satoshi Ohata, and Mohan Lal Kolhe. 2021. "Biogas Dry Reforming for Hydrogen through Membrane Reactor Utilizing Negative Pressure" Fuels 2, no. 2: 194-209. https://doi.org/10.3390/fuels2020012
APA StyleNishimura, A., Takada, T., Ohata, S., & Kolhe, M. L. (2021). Biogas Dry Reforming for Hydrogen through Membrane Reactor Utilizing Negative Pressure. Fuels, 2(2), 194-209. https://doi.org/10.3390/fuels2020012








