Copper-Content Dependent Structural and Electrical Properties of CZTS Films Formed by “Green” Colloidal Nanocrystals
Abstract
1. Introduction
2. Experimental Section
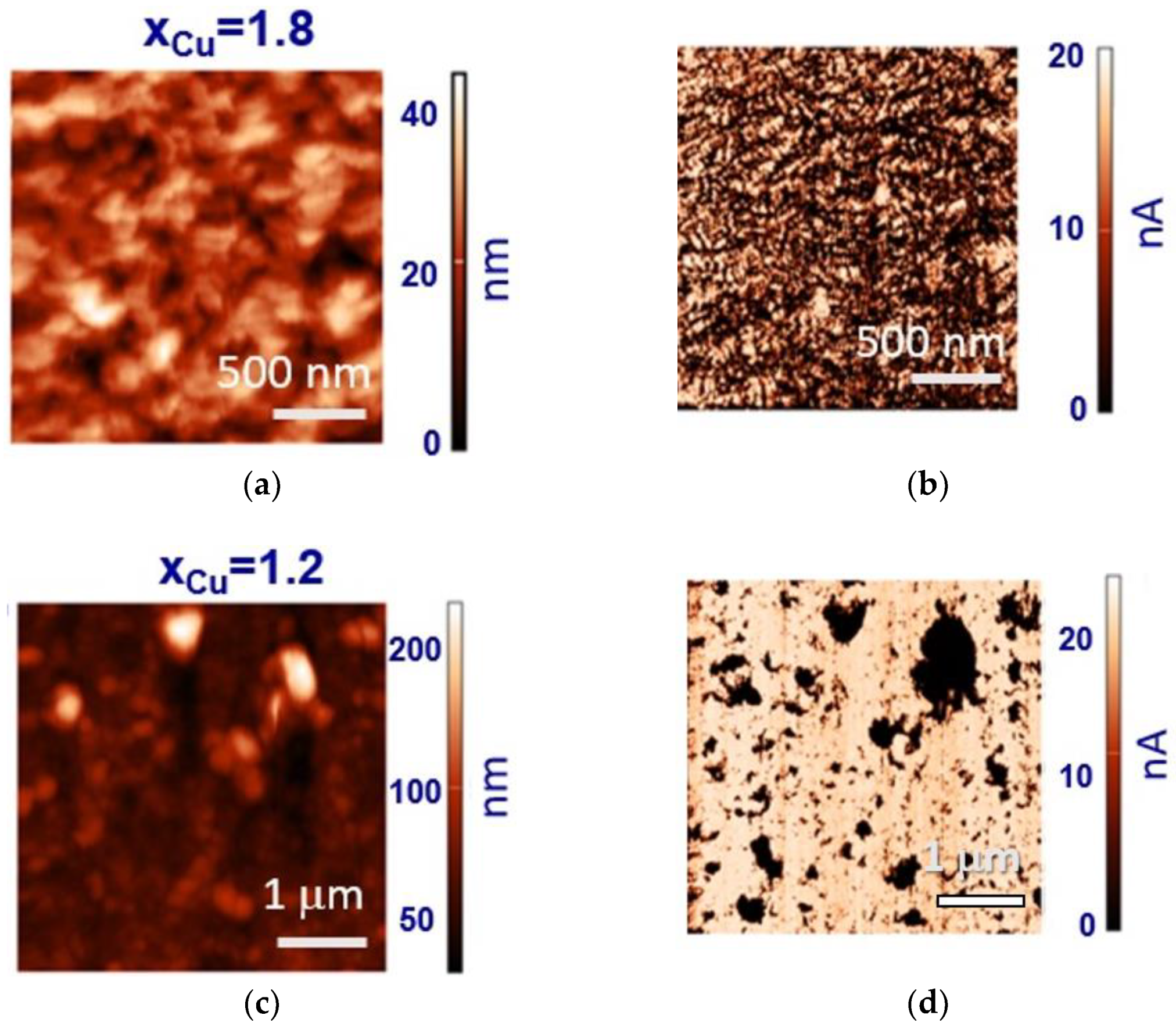
3. Results and Discussion
3.1. X-ray Photoemission Spectroscopy
3.2. Raman Spectroscopy
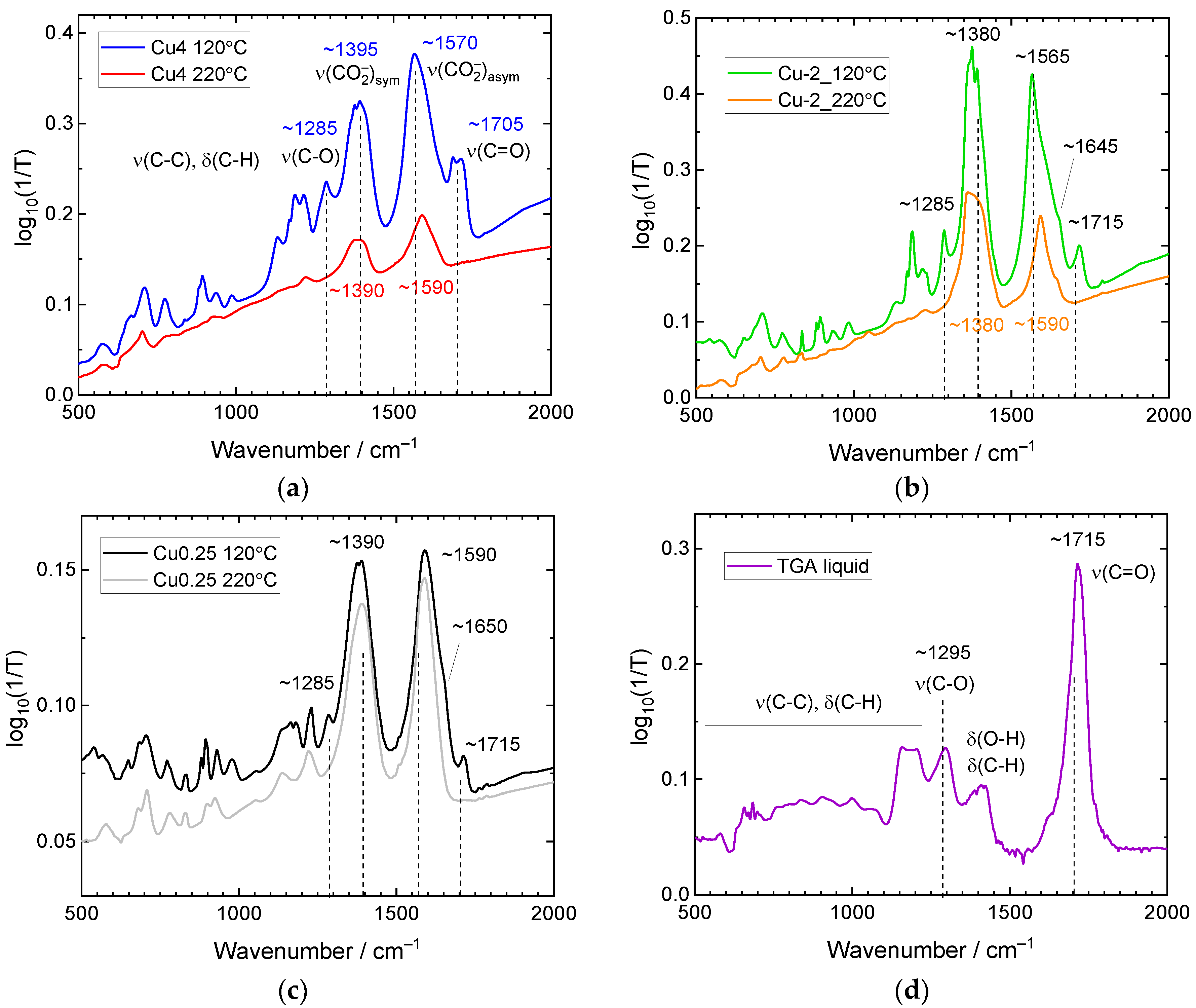
3.3. Infrared Spectroscopy
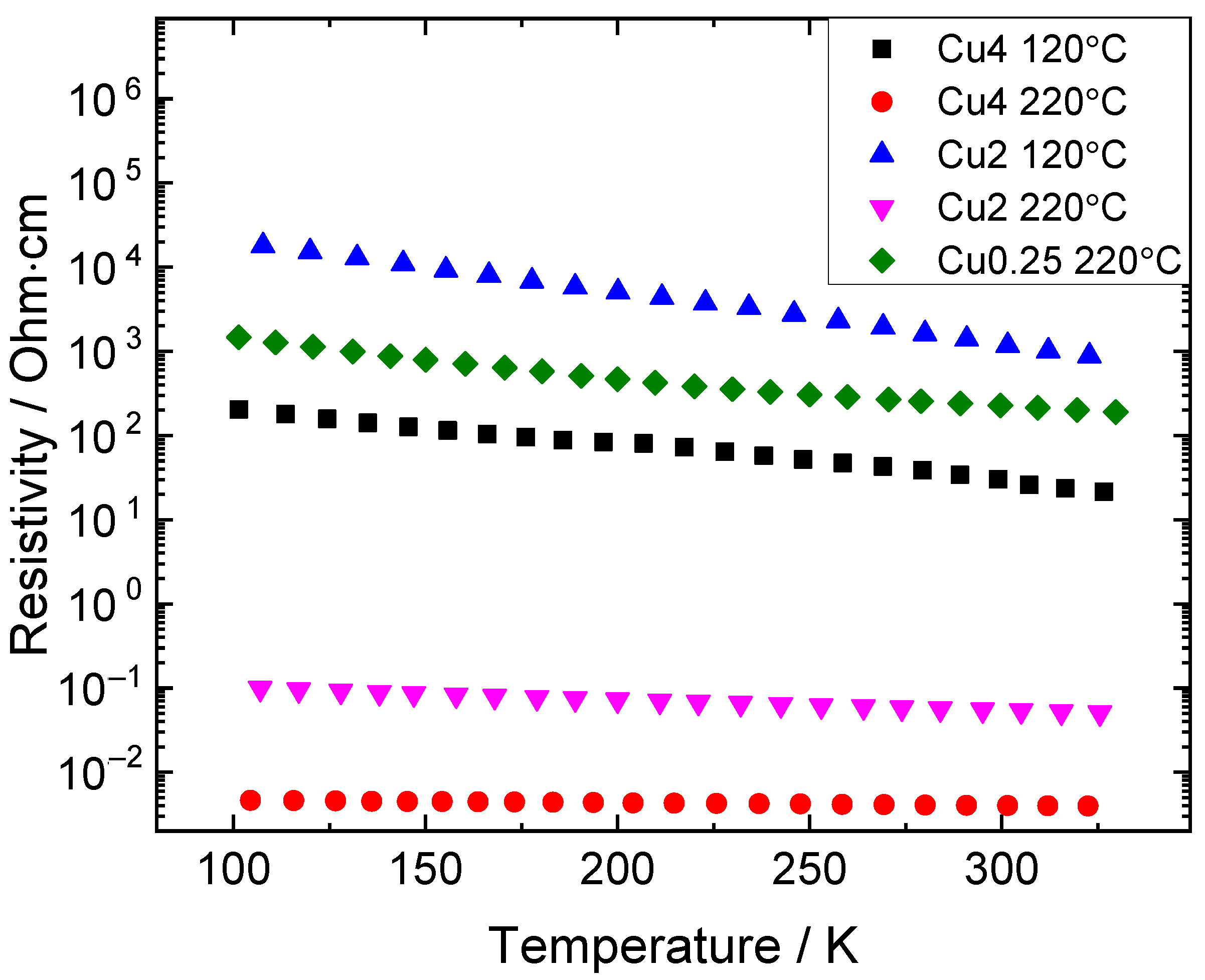
3.4. Electrical Conductivity of the NC Films
3.5. Optical Absorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giraldo, S.; Jehl, Z.; Placidi, M.; Izquierdo-Roca, V.; Pérez-Rodríguez, A.; Saucedo, E. Progress and Perspectives of Thin Film Kesterite Photovoltaic Technology: A Critical Review. Adv. Mater. 2019, 31, 1806692. [Google Scholar] [CrossRef]
- Stroyuk, O.; Raevskaya, A.; Gaponik, N. Solar Light Harvesting with Multinary Metal Chalcogenide Nanocrystals. Chem. Soc. Rev. 2018, 47, 5354. [Google Scholar] [CrossRef] [PubMed]
- Guc, M.; Schorr, S.; Gurieva, G.; Guc, M.; Dimitrievska, M. Point Defects, Compositional Fluctuations, and Secondary Phases in Non-Stoichiometric Kesterites. J. Phys. Energy 2020, 2, 012002. [Google Scholar]
- Fonoll-Rubio, R.; Andrade-Arvizu, J.; Blanco-Portals, J.; Becerril-Romero, I.; Guc, M.; Saucedo, E.; Peiro, F.; Calvo-Barrio, L.; Ritzer, M.; Schnohr, C.S.; et al. Insights into Interface and Bulk Defects in a High Efficiency Kesterite-Based Device. Energy Environ. Sci. 2021, 14, 507. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Kou, D.; Zhou, W.; Zhou, Z.; Yuan, S.; Qi, Y.; Zheng, Z.; Wu, S. Local Cu Component Engineering to Achieve Continuous Carrier Transport for Enhanced Kesterite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 795. [Google Scholar] [CrossRef] [PubMed]
- Khammar, F.Y.M.; Hafdallah, S.G.A.; Aida, N.A.M.S. Copper Concentration Effect on Physical Properties of Ultrasonically Sprayed Cu2ZnSnS4 Absorber Thin Films for Solar Cell Applications. Appl. Phys. A 2021, 127, 148. [Google Scholar]
- Babichuk, I.S.; Golovynskyi, S.; Brus, V. Secondary Phases in Cu2ZnSnS4 Films Obtained by Spray Pyrolysis at Different Substrate Temperatures and Cu Contents. Mater. Lett. 2018, 216, 173. [Google Scholar] [CrossRef]
- Márquez, J.; Neuschitzer, M.; Dimitrievska, M.; Gunder, R.; Haass, S.; Werner, M.; Romanyuk, Y.E.; Schorr, S.; Pearsall, N.M.; Forbes, I. Systematic Compositional Changes and Their Influence on Lattice and Optoelectronic Properties of Cu2ZnSnSe4 Kesterite Solar Cells. Sol. Energy Mater. Sol. Cells 2016, 144, 579. [Google Scholar] [CrossRef]
- Nagaoka, A.; Masuda, T.; Yasui, S.; Taniyama, T.; Nose, Y. The Single-Crystal Multinary Compound Cu2ZnSnS4 as an Environmentally Friendly High-Performance Thermoelectric Material. Appl. Phys. Express 2018, 11, 051203. [Google Scholar] [CrossRef]
- Stroyuk, O. Solar Light Harvesting with Nanocrystalline Semiconductors; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Shavel, A.; Ibáñez, M.; Luo, Z.; De Roo, J.; Carrete, A.; Dimitrievska, M.; Genç, A.; Meyns, M.; Pérez-Rodríguez, A.; Kovalenko, M.V.; et al. Scalable Heating-Up Synthesis of Monodisperse Cu2ZnSnS4 Nanocrystals. Chem. Mater. 2016, 8, 720. [Google Scholar] [CrossRef]
- Akhavan, V.A.; Goodfellow, B.W.; Panthani, M.G.; Steinhagen, C.; Harvey, T.B.; Stolle, C.J.; Korgel, B.A. Colloidal CIGS and CZTS Nanocrystals: A Precursor Route to Printed Photovoltaics. J. Solid State Chem. 2012, 189, 2. [Google Scholar] [CrossRef]
- Peksu, E.; Terlemezoglu, M.; Parlak, M.; Karaagac, H. Characterization of Cu-rich and Zn-poor Cu2ZnSnS4 single crystal grown by vertical Bridgman technique. J. Cryst. Growth 2021, 574, 126336. [Google Scholar] [CrossRef]
- Nagaoka, A.; Yoshino, K.; Taniguchi, H.; Taniyama, T.; Miyake, H. Preparation of Cu2ZnSnS4 single crystals from Sn solutions. J. Cryst. Growth 2012, 341, 38. [Google Scholar] [CrossRef]
- Scragg, J.J.S.; Choubrac, L.; Lafond, A.; Ericson, T.; Platzer-Björkman, C. A low-temperature order-disorder transition in C Cu2ZnSnS4 thin films. Appl. Phys. Lett. 2014, 104, 041911. [Google Scholar] [CrossRef]
- Weber, A.; Mainz, R.; Schock, H.W. On the Sn loss from thin films of the material system Cu–Zn–Sn–S in high vacuum. Appl. Phys. Lett. 2010, 107, 013516. [Google Scholar] [CrossRef]
- Orletskyi, I.G.; Solovana, M.M.; Brus, V.V.; Pinna, F.; Cicero, G.; Maryanchuk, P.D.; Maistruk, E.V.; Ilashchuk, M.I.; Boichuk, T.I.; Tressob, E. Structural, optical and electrical properties of Cu2ZnSnS4 films prepared from a non-toxic DMSO-based sol-gel and synthesized in low vacuum. J. Phys. Chem. Solids 2017, 100, 154. [Google Scholar] [CrossRef]
- Kim, C.; Hong, S. Optical and electrical properties of Cu2ZnSnS4 thin films grown using spray pyrolysis technique and annealing in air. Mol. Cryst. Liq. 2017, 465, 217. [Google Scholar] [CrossRef]
- Swami, S.K.; Chaturvedi, S.N.; Kumar, A.; Dutta, V. Effect of deposition temperature on the structural and electrical properties of spray deposited kesterite (Cu2ZnSnS4) films. Sol. Energy 2015, 122, 508. [Google Scholar] [CrossRef]
- Behera, N.; Mohan, D.B. The phase optimization, optical and electrical properties of kesterite Cu2ZnSnS4 thin film prepared by single target RF magnetron sputtering technique for solar cell application. Mater. Res. Express 2019, 6, 126457. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Oliva, F.; Guc, M.; Giraldo, S.; Saucedo, E.; Pérez-Rodríguez, A.; Izquierdo-Roca, V. Defect Characterisation in Cu2ZnSnSe4 Kesterites via Resonance Raman Spectroscopy and the Impact on Optoelectronic Solar Cell Properties. J. Mater. Chem. A 2019, 7, 13293. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Giraldo, S.; Pistor, P.; Saucedo, E.; Pérez-Rodríguez, A.; Izquierdo-Roca, V. Raman Scattering Analysis of the Surface Chemistry of Kesterites: Impact of Post-Deposition Annealing and Cu / Zn Reordering on Solar Cell Performance. Sol. Energy Mater. Sol. Cells 2016, 157, 462. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Fairbrother, A.; Fontané, X.; Jawhari, T.; Izquierdo-Roca, V.; Saucedo, E.; Pérez-Rodríguez, A. Multiwavelength Excitation Raman Scattering Study of Polycrystalline Kesterite Cu2ZnSnS4 Thin Films. Appl. Phys. Lett. 2014, 104, 021901. [Google Scholar] [CrossRef]
- Havryliuk, Y.; Valakh, M.Y.; Dzhagan, V.; Greshchuk, O.; Yukhymchuk, V.; Raevskaya, A.; Stroyuk, O.; Selyshchev, O.; Gaponik, N.; Zahn, D.R.T. Raman Characterization of Cu2ZnSnS4 Nanocrystals: Phonon Confinement Effect and Formation of CuxS Phases. RSC Adv. 2018, 8, 30736. [Google Scholar] [CrossRef]
- Guc, M.; Litvinchuk, A.P.; Levcenko, S.; Valakh, M.Y.; Bodnar, I.V.; Dzhagan, V.M.; Izquierdo-Roca, V.; Arushanov, E.; Pérez-Rodríguez, A. Optical Phonons in the Wurtzstannite Cu2ZnGeS4 Semiconductor: Polarized Raman Spectroscopy and First-Principle Calculations. RSC Adv. 2016, 6, 13278. [Google Scholar] [CrossRef]
- Lox, J.F.L.; Dang, Z.; Dzhagan, V.M.; Spittel, D.; Martín-García, B.; Moreels, I.; Zahn, D.R.T.; Lesnyak, V. Near-Infrared Cu-In-Se-Based Colloidal Nanocrystals via Cation Exchange. Chem. Mater. 2018, 30, 2607. [Google Scholar] [CrossRef]
- Kempken, J.; Li, B.; Dzhagan, V.; Zahn, D.R.T.; Grzelak, J.; Mackowski, S.; Parisi, J.; Kolny-Olesiak, J. Alloyed CuInS2–ZnS Nanorods: Synthesis, Structure and Optical Properties. CrystEngComm 2015, 17, 5634. [Google Scholar]
- Valle, R.L.E.; Neldner, K.; Gurieva, G.; Schorr, S. Existence of Off-Stoichiometric Single Phase Kesterite. J. Alloys Compd. 2016, 657, 408. [Google Scholar] [CrossRef]
- Dzhagan, V.M.; Azhniuk, Y.M.; Milekhin, A.G.; Zahn, D.R.T. Vibrational Spectroscopy of Compound Semiconductor Nanocrystals. J. Phys. D Appl. Phys. 2018, 51, 503001. [Google Scholar] [CrossRef]
- Selyshchev, O.; Havryliuk, Y.; Valakh, M.Y.; Yukhymchuk, V.O.; Raievska, O.; Stroyuk, O.L.; Dzhagan, V.; Zahn, D.R.T. Raman and X-ray Photoemission Identification of Colloidal Metal Sulfides as Potential Secondary Phases in Nanocrystalline Cu2ZnSnS4 Photovoltaic Absorbers. ACS Appl. Nano Mater. 2020, 3, 5706. [Google Scholar] [CrossRef]
- Chen, G.; Wang, W.; Zhang, J.; Huang, Z. Formation Mechanism of Secondary Phases in Cu2ZnSnS4 Growth under Different Copper Content. Mater. Lett. 2016, 186, 98. [Google Scholar] [CrossRef]
- Guc, M.; Oliva, F.; Fairbrother, A.; Jawhari, T.; Alcobe, X.; Placidi, M.; Pérez, R.A.; Saucedo, E.; Izquierdo-Roca, V. Scripta Materialia Cu-Sn-S System: Vibrational Properties and Coexistence of the Cu2SnS3, Cu3SnS4 and Cu4SnS4 Compounds. Scr. Mater. 2020, 186, 180. [Google Scholar] [CrossRef]
- Naujokaitis, A.; Stalnionis, G.; Giraitis, R.; Kalinauskas, P.; Mockus, Z. Photoelectrochemical, Raman Spectroscopy, XRD and Photoluminescence Study of Disorder in Electrochemically Deposited Kesterite Thin Film. J. Alloys Compd. 2020, 824, 153853. [Google Scholar]
- Pascual-Winter, M.F.; Bruchhausen, A.; Schreiner, W.; Vázquez, M. Cross-Section Analysis of the Composition of Sprayed Cu2ZnSnS4 Thin Films by XPS, EDS, and Multi-Wavelength Raman Spectroscopy. Phys. Status Solidi 2018, 215, 1800639. [Google Scholar]
- Phys, A.; Rudisch, K.; Ren, Y.; Platzer-Björkman, C.; Scragg, J. Order-Disorder Transition in B-Type Cu2ZnSnS4 and Limitations of Ordering through Thermal Treatments. Appl. Phys. Lett. 2018, 108, 231902. [Google Scholar]
- Lafond, A.; Guillot-Deudon, C. Solid-State NMR and Raman Spectroscopy To Address the Local Structure of Defects and the Tricky Issue of the Cu/Zn Disorder in Cu- Poor, Zn-Rich CZTS Materials. Inorg. Chem. 2014, 53, 8646–8653. [Google Scholar]
- Stroyuk, O.; Raevskaya, A.; Selyshchev, O.; Dzhagan, V.; Gaponik, N.; Zahn, D.R.T.; Eychmüller, A. “Green” Aqueous Synthesis and Optical Characterization of Colloidal Cu2ZnSnS4 Nanocrystal Inks. Sci. Rep. 2018, 8, 13677. [Google Scholar] [CrossRef] [PubMed]
- Datenbank CHEMSAFE, Version 2016.0; DECHEMA-PTB-BAM (der Gesellschaft für Chemische Technik und Biotechnologie e.V., der Physikalisch-Technischen Bundesanstalt, der Bundesanstalt für Materialforschung und -prüfung): Berlin, Germany, 2016.
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Briggs, M.P.; Seah, D. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 1990. [Google Scholar]
- Whittles, T.J.; Burton, L.A.; Skelton, J.M.; Walsh, A.; Veal, T.D.; Dhanak, V.R. Band Alignments, Valence Bands and Core Levels in the Tin Sulfides SnS, SnS2 and Sn2S3: Experiment and Theory. Chem. Mater. 2016, 28, 3718–3726. [Google Scholar] [CrossRef]
- Whittles, T.J. Electronic Characterisation of Earth-Abundant Sulphides for Solar Photovoltaics; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Perry, D.L.; Taylor, J.A. X-ray Photoelectron and Auger Spectroscopic Studies of Cu2S and CuS. J. Mater. Sci. Lett. 1986, 5, 384. [Google Scholar] [CrossRef]
- Yeryukov, N.A.; Milekhin, A.G.; Sveshnikova, L.L.; Duda, T.A.; Pokrovsky, L.D.; Gutakovskii, A.K.; Batsanov, S.A.; Rodyakina, E.E.; Latyshev, A.V.; Zahn, D.R.T. Synthesis and Characterization of CuxS (x = 1–2) Nanocrystals Formed by the Langmuir–Blodgett Technique. J. Phys. Chem. C 2014, 118, 23409. [Google Scholar] [CrossRef]
- Milekhin, A.G.; Sveshnikova, L.L.; Duda, T.A.; Yeryukov, N.A.; Rodyakina, E.E.; Gutakovskii, A.K.; Batsanov, S.A.; Latyshev, A.V.; Zahn, D.R.T. Surface-Enhanced Raman Spectroscopy of Semiconductor Nanostructures. Phys. E 2016, 75, 210. [Google Scholar] [CrossRef]
- Kashida, S.; Shimosaka, W.; Mori, M.; Yoshimura, D. Valence Band Photoemission Study of the Copper Chalcogenide Compounds, Cu2S, Cu2Se and Cu2Te. J. Phys. Chem. Solids 2003, 64, 2357. [Google Scholar] [CrossRef]
- Okamoto, K.; Kawai, S. Electrical Conduction and Phase Transition of Copper Sulfides. Jpn. J. Appl. Phys. 1973, 12, 1130. [Google Scholar] [CrossRef]
- Tirado, J.; Roldán-Carmona, C.; Muñoz-Guerrero, F.A.; Bonilla-Arboleda, G.; Ralaiarisoa, M.; Grancini, G.; Queloz, V.I.E.; Koch, N.; Nazeeruddin, M.K.; Jaramillo, F. Copper Sulfide Nanoparticles as Hole-Transporting-Material in a Fully-Inorganic Blocking Layers n-i-p Perovskite Solar Cells: Application and Working Insights. Appl. Surf. Sci. 2019, 478, 607. [Google Scholar] [CrossRef]
- Bosson, C.J.; Birch, M.T.; Halliday, D.P.; Knight, K.S.; Gibbs, A.S.; Hatton, P.D. Cation Disorder and Phase Transitions in the Structurally Complex Solar Cell Material Cu2ZnSnS4. J. Mater. Chem. A 2017, 5, 16672. [Google Scholar] [CrossRef]
- Rein, C.; Engberg, S.; Andreasen, J.W.S. Carbon-Free Inks of Cu2ZnSnS4 Nanoparticles Synthesized at Room Temperature Designed for Roll-to-Roll Fabrication of Solar Cell Absorber Layers. J. Alloys Compd. 2019, 787, 6. [Google Scholar] [CrossRef]
- Khare, A.; Wills, A.W.; Ammerman, L.M.; Norris, D.J.; Aydil, E.S. Size Control and Quantum Confinement in Cu2ZnSnS4 Nanocrystals. Chem. Commun. 2011, 47, 11721. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Guo, B.L.; Wu, X.S.; Zhang, F.M.; Mak, C.L.; Wong, K.H. Facile Hydrothermal Synthesis of Hydrotropic Cu2ZnSnS4 Nanocrystal Quantum Dots: Band-Gap Engineering and Phonon Confinement Effect. J. Mater. Chem. A 2013, 1, 3182. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z.; Shao, C.; Boye, D.M.; Zhao, J. A Facile and General Approach to Polynary Semiconductor Nanocrystals via a Modified Two-Phase Method. Nanotechnology 2011, 22, 245605. [Google Scholar] [CrossRef]
- Flynn, B.; Wang, W.; Chang, C.H.; Herman, G.S. Microwave Assisted Synthesis of Cu2ZnSnS4 Colloidal Nanoparticle Inks. Phys. Status Solidi 2012, 209, 2186. [Google Scholar] [CrossRef]
- Xie, H.; Dimitrievska, M.; Fontané, X.; Sánchez, Y.; López-Marino, S.; Izquierdo-Roca, V.; Bermúdez, V.; Pérez-Rodríguez, A.; Saucedo, E. Formation and Impact of Secondary Phases in Cu-Poor Cu2ZnSn(S1-ySey) (0 < y < 1) Based Solar Cells. Sol. Energy Mater. Sol. Cells 2015, 140, 289. [Google Scholar]
- Dimitrievska, M.; Boero, F.; Litvinchuk, A.P.; Delsante, S.; Borzone, G.; Pérez-Rodríguez, A.; Izquierdo-Roca, V. Structural Polymorphism in “Kesterite” Cu2ZnSnS4: Raman Spectroscopy and First-Principles Calculations Analysis. Inorg. Chem. 2017, 56, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Gurieva, G.; Többens, D.M.; Valakh, M.Y.; Schorr, S. Cu-Zn Disorder in Cu2ZnGeSe4: A Complementary Neutron Diffraction and Raman Spectroscopy Study. J. Phys. Chem. Solids 2016, 99, 100. [Google Scholar] [CrossRef]
- Kandare, S.P.; Dahiwale, S.S.; Dhole, S.D.; Rao, M.N.; Rao, R. Materials Science in Semiconductor Processing Order-Disorder Transition in Nano-Cu2ZnSnS4: A Raman Spectroscopic Study. Mater. Sci. Semicond. Process. 2019, 102, 104594. [Google Scholar] [CrossRef]
- Valakh, M.Y.; Dzhagan, V.M.; Babichuk, I.S.; Fontane, X.; Perez-Rodriquez, A.; Schorr, S. Optically Induced Structural Transformation in Disordered Kesterite Cu2ZnSnS4. JETP Lett. 2013, 98, 255. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordrred and Amorphous Carbon. Phys. Rev. B 2000, 61, 14295. [Google Scholar] [CrossRef]
- Vasin, A.V.; Matveeva, L.A.; Yukhimchuk, V.A. The Photoluminescence of Amorphous Carbon in A-C: C60 Films Obtained by Fullerene C60 Deposition. Tech. Phys. Lett. 2002, 28, 592. [Google Scholar] [CrossRef]
- Vasin, A.; Kysil, D.; Rusavsky, A.; Isaieva, O.; Zaderko, A.; Nazarov, A.; Lysenko, V. Synthesis and Luminescent Properties of Carbon Nanodots Dispersed in Nanostructured Silicas. Nanomaterials 2021, 11, 3267. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ito, K.; Bernstein, H.J. The Vibrational Spectra of the Formate, Acetate, and Oxalate Ions. Can. J. Chem. 1956, 34, 170. [Google Scholar] [CrossRef]
- Marakushev, A.A.; Bezmen, N.I. Chemical Affinity of Metals for Oxygen and Sulfur. Int. Geol. Rev. 1971, 13, 1781. [Google Scholar] [CrossRef]
- Kosyak, V.; Karmarkar, M.A.; Scarpulla, M.A. Temperature Dependent Conductivity of Polycrystalline Cu2ZnSnS4 Thin Films. Appl. Phys. Lett. 2012, 100, 263903. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, W.; Bertoni, G.; Kriegel, I.; Xiong, M.M.; Prato, N.L.; Riedinger, A.; Sathya, A.; Manna, L. Tuning and Locking the Localized Surface Plasmon Resonances of CuS (Covellite) Nanocrystals by an Amorphous CuPdxS Shell. Chem. Mater. 2017, 29, 1716. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.M.; Jain, P.K.; Ewers, T.; Alivisatos, A.P. Localized Surface Plasmon Resonances Arising from Free Carriers in Doped Quantum Dots. Nat. Mater. 2011, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Llorente, V.B.; Dzhagan, V.M.; Gaponik, N.; Iglesias, R.A.; Zahn, D.R.T.; Lesnyak, V. Electrochemical Tuning of Localized Surface Plasmon Resonance in Copper Chalcogenide Nanocrystals. J. Phys. Chem. C 2017, 121, 18244. [Google Scholar] [CrossRef]
- Gaponenko, S.V. Optical Properties of Semiconductor Nanocrystals; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Dzhagan, V.M.; Lokteva, I.; Himcinschi, C.; Kolny-Olesiak, J.; Valakh, M.Y.; Schulze, S.; Zahn, D.R.T. The Influence of Pyridine Ligand onto the Structure and Phonon Spectra of CdSe Nanocrystals. J. Appl. Phys. 2011, 109, 084334. [Google Scholar] [CrossRef]
- Raevskaya, A.E.; Stroyuk, A.L.; Kuchmiy, S.Y.; Dzhagan, V.M.; Valakh, M.Y.; Zahn, D.R.T. Optical Study of CdS- and ZnS-Passivated CdSe Nanocrystals in Gelatin Films. J. Phys. Condens. Matter. 2007, 19, 386237. [Google Scholar] [CrossRef]
- Dzhagan, V.; Milekhin, A.G.; Valakh, M.Y.; Pedetti, S.; Tessier, M.; Dubertret, B.; Zahn, D.R.T. Morphology-Induced Phonon Spectra of CdSe/CdS Nanoplatelets: Core/Shell vs. Core-Crown. Nanoscale 2016, 8, 17204. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Dmytruk, A.; Dmitruk, I.; Yasuto, N.; Kasuya, A.; Takeda, M.; Ohuchi, N. Aqueous-Phase Synthesis of Ultra-Stable Small CdSe Nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 3750. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raievska, O.; Stroyuk, O.; Dzhagan, V.; Solonenko, D.; Zahn, D.R.T. Ultra-Small Aqueous Glutathione-Capped Ag–In–Se Quantum Dots: Luminescence and Vibrational Properties. RSC Adv. 2020, 10, 42178. [Google Scholar] [CrossRef]
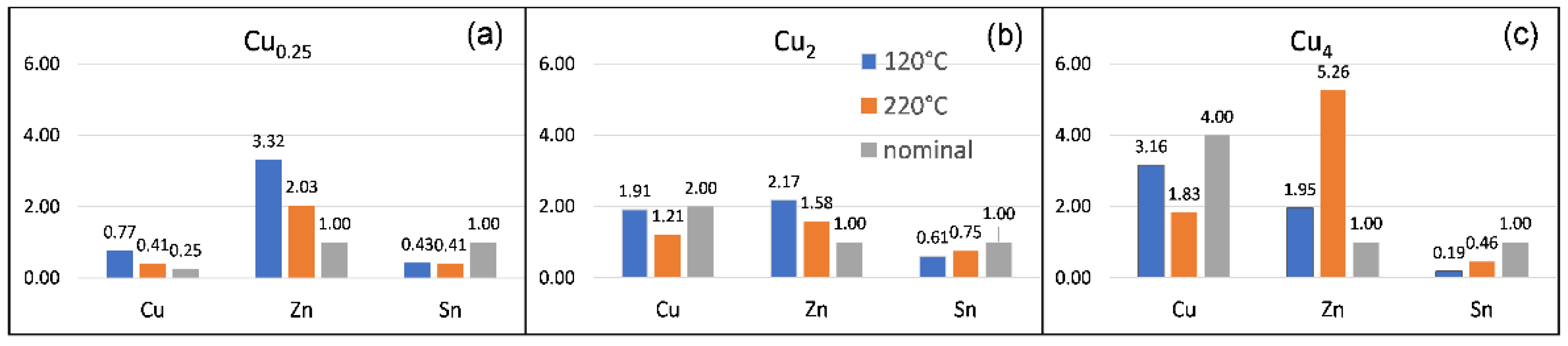
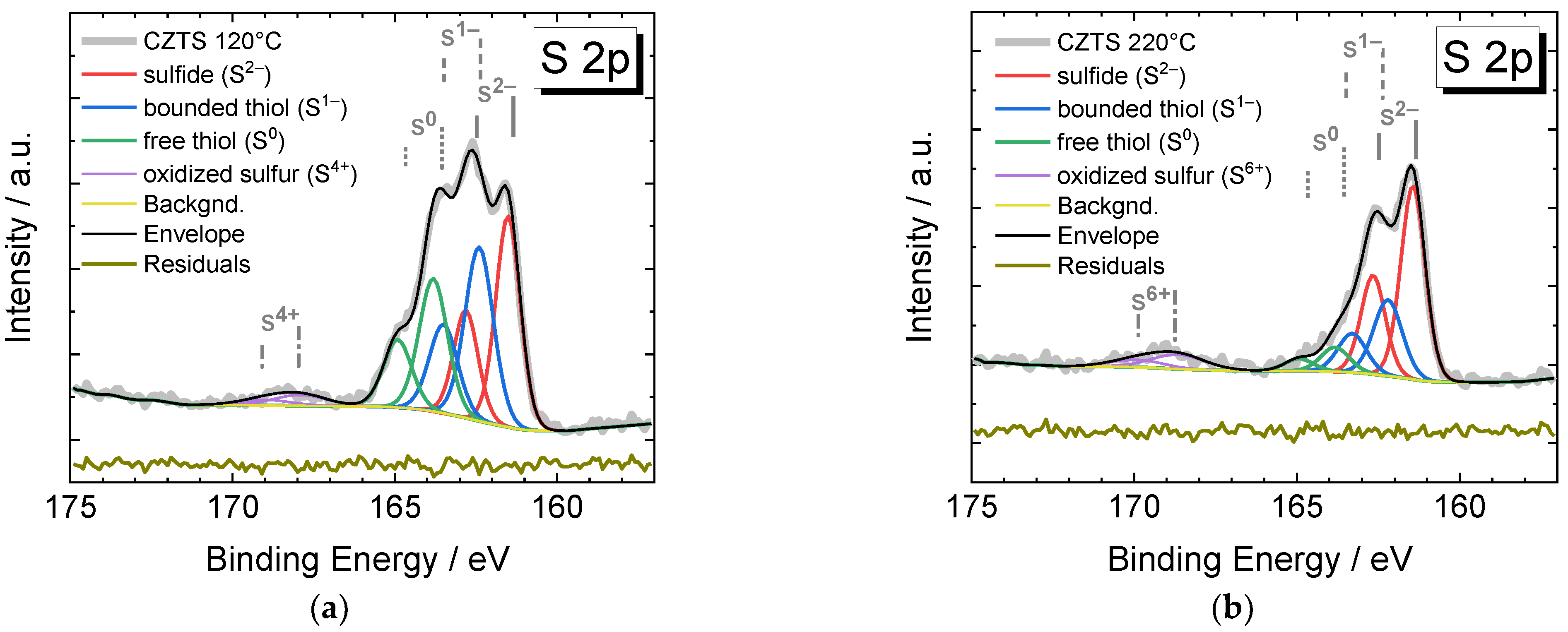


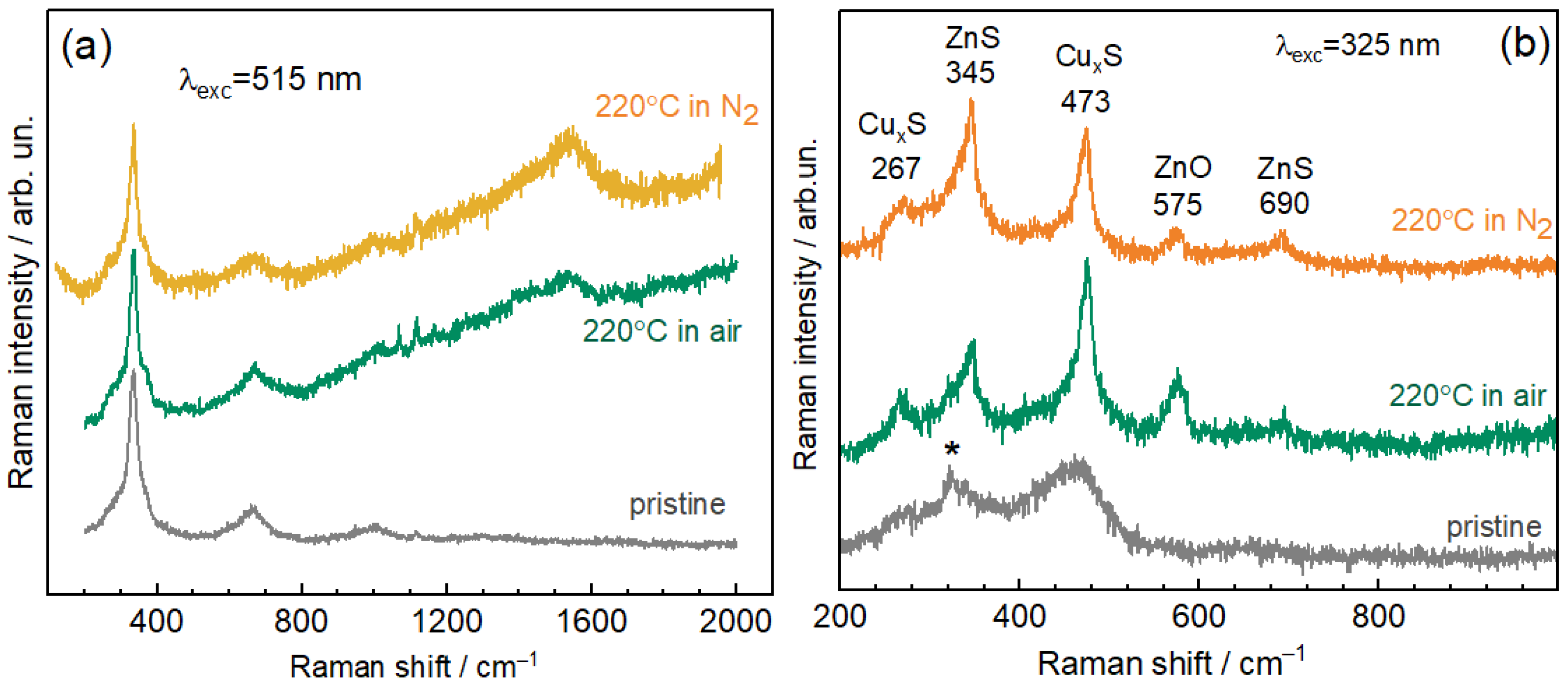
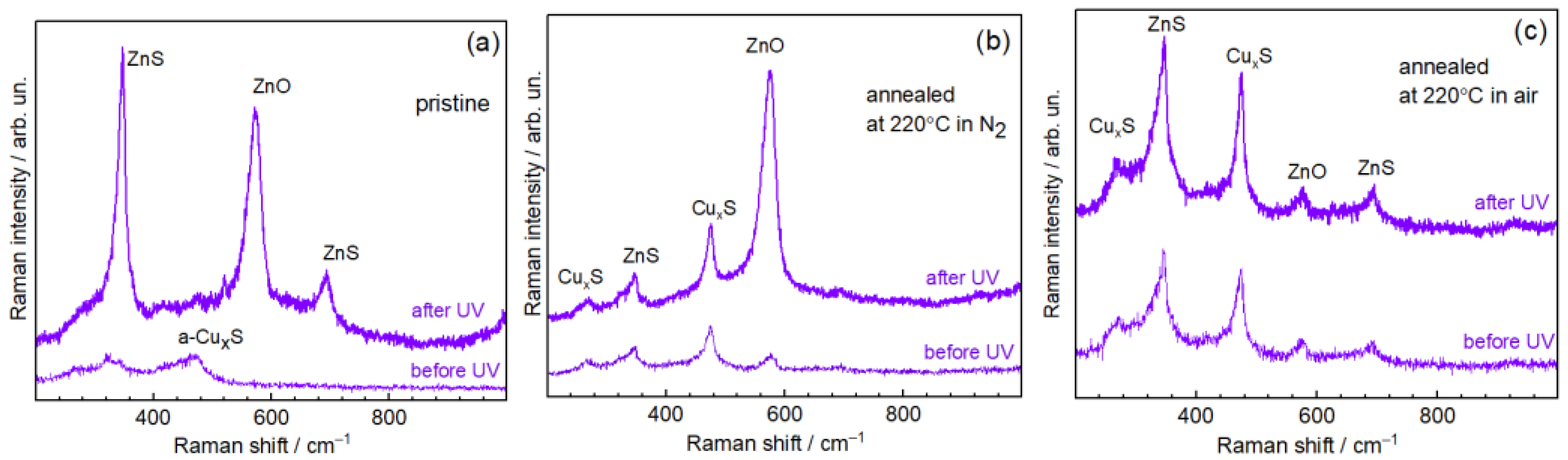

| Cu2p3/2/ ±0.1 eV | Cu LMM (1G4)/±0.1 eV | α’Cu = Cu2p3/2 + CuLMM (1G4)/±0.2 eV | Assignment | Literature Data, α’Cu/eV | |
|---|---|---|---|---|---|
| Cu0.25 (initial, 120 °C) | 931.7 | 917.0 | 1848.7 | CZTS | 1848.6–1849.2, CZTS [30,42] |
| Cu2 (initial, 120 °C) | 932.5 | 917.0 | 1849.5 | Cu2S + CZTS | - |
| Cu4 (initial, 120 °C) | 932.4 | 917.5 | 1849.9 | Cu2S | 1849.8–1849.9, Cu2S [40,42,43] |
| Cu0.25 (annealed, 220 °C) | 932.4 | 917.1 | 1849.5 | Cu2S + CZTS | - |
| Cu2 (annealed, 220 °C) | 932.2 | 917.5 | 1849.7 | Cu2S + CZTS | - |
| Cu4 (annealed, 220 °C) | 932.2 | 917.9 | 1850.1 | CuS | 1850.0–1850.4, CuS [40,42,43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzhagan, V.; Selyshchev, O.; Kondratenko, S.; Mazur, N.; Havryliuk, Y.; Raievska, O.; Stroyuk, O.; Zahn, D.R.T. Copper-Content Dependent Structural and Electrical Properties of CZTS Films Formed by “Green” Colloidal Nanocrystals. Electron. Mater. 2022, 3, 136-153. https://doi.org/10.3390/electronicmat3010013
Dzhagan V, Selyshchev O, Kondratenko S, Mazur N, Havryliuk Y, Raievska O, Stroyuk O, Zahn DRT. Copper-Content Dependent Structural and Electrical Properties of CZTS Films Formed by “Green” Colloidal Nanocrystals. Electronic Materials. 2022; 3(1):136-153. https://doi.org/10.3390/electronicmat3010013
Chicago/Turabian StyleDzhagan, Volodymyr, Oleksandr Selyshchev, Serhiy Kondratenko, Nazar Mazur, Yevhenii Havryliuk, Oleksandra Raievska, Oleksandr Stroyuk, and Dietrich R. T. Zahn. 2022. "Copper-Content Dependent Structural and Electrical Properties of CZTS Films Formed by “Green” Colloidal Nanocrystals" Electronic Materials 3, no. 1: 136-153. https://doi.org/10.3390/electronicmat3010013
APA StyleDzhagan, V., Selyshchev, O., Kondratenko, S., Mazur, N., Havryliuk, Y., Raievska, O., Stroyuk, O., & Zahn, D. R. T. (2022). Copper-Content Dependent Structural and Electrical Properties of CZTS Films Formed by “Green” Colloidal Nanocrystals. Electronic Materials, 3(1), 136-153. https://doi.org/10.3390/electronicmat3010013






