Developmental Origin and Functional Diversity of Foxp3+ Regulatory T Cells in Diabetes and Obesity
Abstract
1. Introduction
2. Thymic and Peripheral Pathways of Treg Cell Development
3. Treg Cells in β-Cell Autoimmunity and T1D
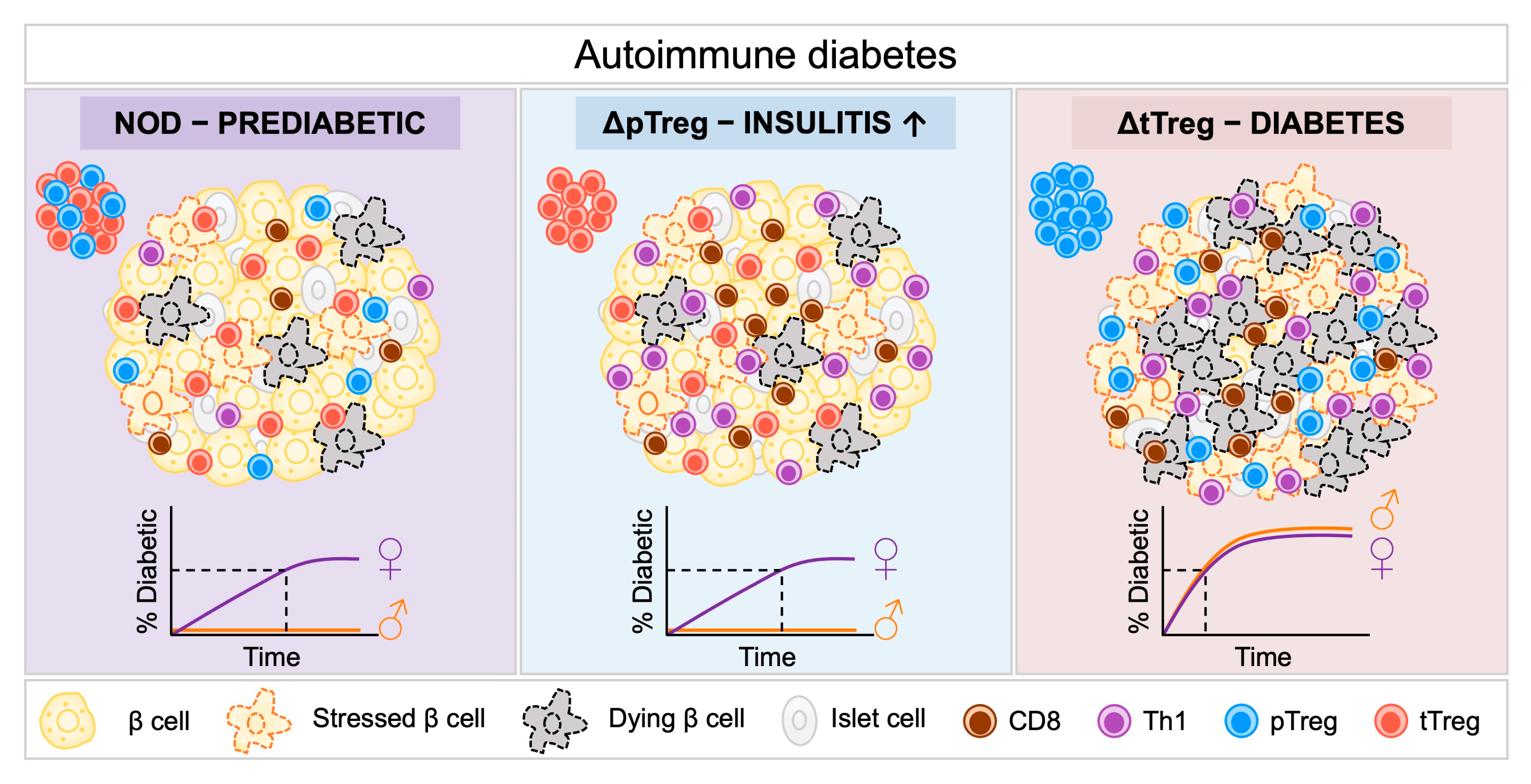
Genetic Ablation of pTreg and tTreg Subsets in the NOD Model
4. Treg Cells in Adipose Tissue Inflammation, Obesity, and T2D
4.1. Phenotypic Adaptation of VAT Treg Cells
4.2. Function of VAT Treg Cells
4.3. pTreg Cells—Central Regulators of Adipose Tissue Function and Systemic Metabolism
4.4. Roles of VAT pTreg and tTreg Cells in Obesity
4.5. Non-Immune Functions of VAT pTreg Cells
5. Treg Cell Plasticity: Pathogenic Threat in β Cell Autoimmunity and Obesity?
Transdifferentiation of Treg Cells into Pathogenic T Effector Cells
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gale, E.A. The Discovery of Type 1 Diabetes. Diabetes 2001, 50, 217–226. [Google Scholar] [CrossRef]
- Bottazzo, G.F.; Florin-Christensen, A.; Doniach, D. Islet-Cell Antibodies in Diabetes Mellitus with Autoimmune Polyendocrine Deficiencies. Lancet 1974, 2, 1279–1283. [Google Scholar] [CrossRef]
- MacCuish, A.C.; Irvine, W.J.; Barnes, E.W.; Duncan, L.J. Antibodies to Pancreatic Islet Cells in Insulin-Dependent Diabetics with Coexistent Autoimmune Disease. Lancet 1974, 2, 1529–1531. [Google Scholar] [CrossRef] [PubMed]
- Cudworth, A.G.; Woodrow, J.C. Genetic Susceptibility in Diabetes Mellitus: Analysis of the HLA Association. Br. Med. J. 1976, 2, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C.; Crook, M.A. Is Type II Diabetes Mellitus a Disease of the Innate Immune System? Diabetologia 1998, 41, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C.; Mattock, M.B.; Chusney, G.D.; Burt, D. NIDDM as a Disease of the Innate Immune System: Association of Acute-Phase Reactants and Interleukin-6 with Metabolic Syndrome X. Diabetologia 1997, 40, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Lio, C.-W.J.; Hsieh, C.-S. A Two-Step Process for Thymic Regulatory T Cell Development. Immunity 2008, 28, 100–111. [Google Scholar] [CrossRef]
- Schallenberg, S.; Tsai, P.-Y.; Riewaldt, J.; Kretschmer, K. Identification of an Immediate Foxp3− Precursor to Foxp3+ Regulatory T Cells in Peripheral Lymphoid Organs of Nonmanipulated Mice. J. Exp. Med. 2010, 207, 1393–1407. [Google Scholar] [CrossRef]
- Haribhai, D.; Williams, J.B.; Jia, S.; Nickerson, D.; Schmitt, E.G.; Edwards, B.; Ziegelbauer, J.; Yassai, M.; Li, S.-H.; Relland, L.M.; et al. A Requisite Role for Induced Regulatory T Cells in Tolerance Based on Expanding Antigen Receptor Diversity. Immunity 2011, 35, 109–122. [Google Scholar] [CrossRef]
- Apostolou, I.; Sarukhan, A.; Klein, L.; von Boehmer, H. Origin of Regulatory T Cells with Known Specificity for Antigen. Nat. Immunol. 2002, 3, 756–763. [Google Scholar] [CrossRef]
- Apostolou, I.; von Boehmer, H. In Vivo Instruction of Suppressor Commitment in Naive T Cells. J. Exp. Med. 2004, 199, 1401–1408. [Google Scholar] [CrossRef]
- Hawiger, D.; Inaba, K.; Dorsett, Y.; Guo, M.; Mahnke, K.; Rivera, M.; Ravetch, J.V.; Steinman, R.M.; Nussenzweig, M.C. Dendritic Cells Induce Peripheral T Cell Unresponsiveness under Steady State Conditions in Vivo. J. Exp. Med. 2001, 194, 769–779. [Google Scholar] [CrossRef]
- Hawiger, D.; Masilamani, R.F.; Bettelli, E.; Kuchroo, V.K.; Nussenzweig, M.C. Immunological Unresponsiveness Characterized by Increased Expression of CD5 on Peripheral T Cells Induced by Dendritic Cells in Vivo. Immunity 2004, 20, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, K.; Apostolou, I.; Hawiger, D.; Khazaie, K.; Nussenzweig, M.C.; von Boehmer, H. Inducing and Expanding Regulatory T Cell Populations by Foreign Antigen. Nat. Immunol. 2005, 6, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, K.; Heng, T.S.P.; von Boehmer, H. De Novo Production of Antigen-Specific Suppressor Cells in Vivo. Nat. Protoc. 2006, 1, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Jaeckel, E.; Kretschmer, K.; Apostolou, I.; von Boehmer, H. Instruction of Treg Commitment in Peripheral T Cells Is Suited to Reverse Autoimmunity. Semin. Immunol. 2006, 18, 89–92. [Google Scholar] [CrossRef]
- Kretschmer, K.; Apostolou, I.; Jaeckel, E.; Khazaie, K.; von Boehmer, H. Making Regulatory T Cells with Defined Antigen Specificity: Role in Autoimmunity and Cancer. Immunol. Rev. 2006, 212, 163–169. [Google Scholar] [CrossRef]
- Hsieh, C.-S.; Lee, H.-M.; Lio, C.-W.J. Selection of Regulatory T Cells in the Thymus. Nat. Rev. Immunol. 2012, 12, 157–167. [Google Scholar] [CrossRef]
- Pohar, J.; Simon, Q.; Fillatreau, S. Antigen-Specificity in the Thymic Development and Peripheral Activity of CD4+FOXP3+ T Regulatory Cells. Front. Immunol. 2018, 9, 1701. [Google Scholar] [CrossRef]
- Owen, D.L.; Mahmud, S.A.; Sjaastad, L.E.; Williams, J.B.; Spanier, J.A.; Simeonov, D.R.; Ruscher, R.; Huang, W.; Proekt, I.; Miller, C.N.; et al. Thymic Regulatory T Cells Arise via Two Distinct Developmental Programs. Nat. Immunol. 2019, 20, 195–205. [Google Scholar] [CrossRef]
- Josefowicz, S.Z.; Niec, R.E.; Kim, H.Y.; Treuting, P.; Chinen, T.; Zheng, Y.; Umetsu, D.T.; Rudensky, A.Y. Extrathymically Generated Regulatory T Cells Control Mucosal TH2 Inflammation. Nature 2012, 482, 395–399. [Google Scholar] [CrossRef]
- Campbell, C.; Dikiy, S.; Bhattarai, S.K.; Chinen, T.; Matheis, F.; Calafiore, M.; Hoyos, B.; Hanash, A.; Mucida, D.; Bucci, V.; et al. Extrathymically Generated Regulatory T Cells Establish a Niche for Intestinal Border-Dwelling Bacteria and Affect Physiologic Metabolite Balance. Immunity 2018, 48, 1245–1257.e9. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Josefowicz, S.Z.; Arvey, A.; Treuting, P.M.; Rudensky, A.Y. Extrathymic Generation of Regulatory T Cells in Placental Mammals Mitigates Maternal-Fetal Conflict. Cell 2012, 150, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Hanna, B.S.; Wang, G.; Galván-Peña, S.; Mann, A.O.; Ramirez, R.N.; Muñoz-Rojas, A.R.; Smith, K.; Wan, M.; Benoist, C.; Mathis, D. The Gut Microbiota Promotes Distal Tissue Regeneration via RORγ+ Regulatory T Cell Emissaries. Immunity 2023, 56, 829–846.e8. [Google Scholar] [CrossRef] [PubMed]
- Petzold, C.; Steinbronn, N.; Gereke, M.; Strasser, R.H.; Sparwasser, T.; Bruder, D.; Geffers, R.; Schallenberg, S.; Kretschmer, K. Fluorochrome-Based Definition of Naturally Occurring Foxp3+ Regulatory T Cells of Intra- and Extrathymic Origin. Eur. J. Immunol. 2014, 44, 3632–3645. [Google Scholar] [CrossRef]
- Simonetti, M.; Yilmazer, A.; Kretschmer, K. Genetic Tools for Analyzing Foxp3+ Treg Cells: Fluorochrome-Based Transcriptional Reporters and Genetic Fate-Mapping. In Methods in Molecular Biology; Humana: New York, NY, USA, 2023; Volume 2559, pp. 95–114. ISBN 978-1-0716-2647-4. [Google Scholar]
- Yilmazer, A.; Zevla, D.M.; Malmkvist, R.; Rodríguez, C.A.B.; Undurraga, P.; Kirgin, E.; Boernert, M.; Voehringer, D.; Kershaw, O.; Schlenner, S.; et al. Selective Ablation of Thymic and Peripheral Foxp3+ Regulatory T Cell Development. Front. Immunol. 2023, 14, 1298938. [Google Scholar] [CrossRef]
- Chatila, T.A.; Blaeser, F.; Ho, N.; Lederman, H.M.; Voulgaropoulos, C.; Helms, C.; Bowcock, A.M. JM2, Encoding a Fork Head-Related Protein, Is Mutated in X-Linked Autoimmunity-Allergic Disregulation Syndrome. J. Clin. Investig. 2000, 106, R75–R81. [Google Scholar] [CrossRef]
- Brunkow, M.E.; Jeffery, E.W.; Hjerrild, K.A.; Paeper, B.; Clark, L.B.; Yasayko, S.-A.; Wilkinson, J.E.; Galas, D.; Ziegler, S.F.; Ramsdell, F. Disruption of a New Forkhead/Winged-Helix Protein, Scurfin, Results in the Fatal Lymphoproliferative Disorder of the Scurfy Mouse. Nat. Genet. 2001, 27, 68–73. [Google Scholar] [CrossRef]
- Bennett, C.L.; Christie, J.; Ramsdell, F.; Brunkow, M.E.; Ferguson, P.J.; Whitesell, L.; Kelly, T.E.; Saulsbury, F.T.; Chance, P.F.; Ochs, H.D. The Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked Syndrome (IPEX) Is Caused by Mutations of FOXP3. Nat. Genet. 2001, 27, 20–21. [Google Scholar] [CrossRef]
- Wildin, R.S.; Ramsdell, F.; Peake, J.; Faravelli, F.; Casanova, J.L.; Buist, N.; Levy-Lahad, E.; Mazzella, M.; Goulet, O.; Perroni, L.; et al. X-Linked Neonatal Diabetes Mellitus, Enteropathy and Endocrinopathy Syndrome Is the Human Equivalent of Mouse Scurfy. Nat. Genet. 2001, 27, 18–20. [Google Scholar] [CrossRef]
- Bacchetta, R.; Barzaghi, F.; Roncarolo, M.-G. From IPEX Syndrome to FOXP3 Mutation: A Lesson on Immune Dysregulation. Ann. N. Y. Acad. Sci. 2018, 1417, 5–22. [Google Scholar] [CrossRef]
- Johnson, M.B.; Cerosaletti, K.; Flanagan, S.E.; Buckner, J.H. Genetic Mechanisms Highlight Shared Pathways for the Pathogenesis of Polygenic Type 1 Diabetes and Monogenic Autoimmune Diabetes. Curr. Diab. Rep. 2019, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.M.; Peakman, M.; Tree, T.I.M. Regulatory T Cell Dysfunction in Type 1 Diabetes: What’s Broken and How Can We Fix It? Diabetologia 2017, 60, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Lindley, S.; Dayan, C.M.; Bishop, A.; Roep, B.O.; Peakman, M.; Tree, T.I.M. Defective Suppressor Function in CD4+CD25+ T-Cells from Patients with Type 1 Diabetes. Diabetes 2005, 54, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Long, S.A.; Cerosaletti, K.; Wan, J.Y.; Ho, J.-C.; Tatum, M.; Wei, S.; Shilling, H.G.; Buckner, J.H. An Autoimmune-Associated Variant in PTPN2 Reveals an Impairment of IL-2R Signaling in CD4+ T Cells. Genes Immun. 2011, 12, 116–125. [Google Scholar] [CrossRef]
- Ferraro, A.; D’Alise, A.M.; Raj, T.; Asinovski, N.; Phillips, R.; Ergun, A.; Replogle, J.M.; Bernier, A.; Laffel, L.; Stranger, B.E.; et al. Interindividual Variation in Human T Regulatory Cells. Proc. Natl. Acad. Sci. USA 2014, 111, E1111–E1120. [Google Scholar] [CrossRef]
- Brusko, T.M.; Wasserfall, C.H.; Clare-Salzler, M.J.; Schatz, D.A.; Atkinson, M.A. Functional Defects and the Influence of Age on the Frequency of CD4+ CD25+ T-Cells in Type 1 Diabetes. Diabetes 2005, 54, 1407–1414. [Google Scholar] [CrossRef]
- Long, S.A.; Cerosaletti, K.; Bollyky, P.L.; Tatum, M.; Shilling, H.; Zhang, S.; Zhang, Z.-Y.; Pihoker, C.; Sanda, S.; Greenbaum, C.; et al. Defects in IL-2R Signaling Contribute to Diminished Maintenance of FOXP3 Expression in CD4+CD25+ Regulatory T-Cells of Type 1 Diabetic Subjects. Diabetes 2010, 59, 407–415. [Google Scholar] [CrossRef]
- Aghili, B.; Amirzargar, A.A.; Rajab, A.; Rabbani, A.; Sotoudeh, A.; Assadiasl, S.; Larijani, B.; Massoud, A. Altered Suppressor Function of Regulatory T Cells in Type 1 Diabetes. Iran. J. Immunol. 2015, 12, 240–251. [Google Scholar]
- Brusko, T.; Wasserfall, C.; McGrail, K.; Schatz, R.; Viener, H.L.; Schatz, D.; Haller, M.; Rockell, J.; Gottlieb, P.; Clare-Salzler, M.; et al. No Alterations in the Frequency of FOXP3+ Regulatory T- Cells in Type 1 Diabetes. Diabetes 2007, 56, 604–612. [Google Scholar] [CrossRef]
- McClymont, S.A.; Putnam, A.L.; Lee, M.R.; Esensten, J.H.; Liu, W.; Hulme, M.A.; Hoffmüller, U.; Baron, U.; Olek, S.; Bluestone, J.A.; et al. Plasticity of Human Regulatory T Cells in Healthy Subjects and Patients with Type 1 Diabetes. J. Immunol. 2011, 186, 3918–3926. [Google Scholar] [CrossRef] [PubMed]
- Putnam, A.L.; Vendrame, F.; Dotta, F.; Gottlieb, P.A. CD4+CD25high Regulatory T Cells in Human Autoimmune Diabetes. J. Autoimmun. 2005, 24, 55–62. [Google Scholar] [CrossRef]
- Watts, D.; Janßen, M.; Jaykar, M.; Palmucci, F.; Weigelt, M.; Petzold, C.; Hommel, A.; Sparwasser, T.; Bonifacio, E.; Kretschmer, K. Transient Depletion of Foxp3(+) Regulatory T Cells Selectively Promotes Aggressive β Cell Autoimmunity in Genetically Susceptible DEREG Mice. Front. Immunol. 2021, 12, 720133. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Kuchroo, J.R.; Sage, P.T.; Liang, D.; Francisco, L.M.; Buck, J.; Thaker, Y.R.; Zhang, Q.; McArdel, S.L.; Juneja, V.R.; et al. PD-1 Restraint of Regulatory T Cell Suppressive Activity Is Critical for Immune Tolerance. J. Exp. Med. 2021, 218, e20182232. [Google Scholar] [CrossRef] [PubMed]
- Holohan, D.R.; Van Gool, F.; Bluestone, J.A. Thymically-Derived Foxp3+ Regulatory T Cells Are the Primary Regulators of Type 1 Diabetes in the Non-Obese Diabetic Mouse Model. PLoS ONE 2019, 14, e0217728. [Google Scholar] [CrossRef]
- Schuster, C.; Jonas, F.; Zhao, F.; Kissler, S. Peripherally Induced Regulatory T Cells Contribute to the Control of Autoimmune Diabetes in the NOD Mouse Model. Eur. J. Immunol. 2018, 48, 1211–1216. [Google Scholar] [CrossRef]
- Zheng, Y.; Josefowicz, S.; Chaudhry, A.; Peng, X.P.; Forbush, K.; Rudensky, A.Y. Role of Conserved Non-Coding DNA Elements in the Foxp3 Gene in Regulatory T-Cell Fate. Nature 2010, 463, 808–812. [Google Scholar] [CrossRef]
- Lathrop, S.K.; Bloom, S.M.; Rao, S.M.; Nutsch, K.; Lio, C.-W.; Santacruz, N.; Peterson, D.A.; Stappenbeck, T.S.; Hsieh, C.-S. Peripheral Education of the Immune System by Colonic Commensal Microbiota. Nature 2011, 478, 250–254. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Sefik, E.; Geva-Zatorsky, N.; Oh, S.; Konnikova, L.; Zemmour, D.; McGuire, A.M.; Burzyn, D.; Ortiz-Lopez, A.; Lobera, M.; Yang, J.; et al. MUCOSAL IMMUNOLOGY. Individual Intestinal Symbionts Induce a Distinct Population of RORγ+ Regulatory T Cells. Science 2015, 349, 993–997. [Google Scholar] [CrossRef]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but Not Obese, Fat Is Enriched for a Unique Population of Regulatory T Cells That Affect Metabolic Parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef]
- Eller, K.; Kirsch, A.; Wolf, A.M.; Sopper, S.; Tagwerker, A.; Stanzl, U.; Wolf, D.; Patsch, W.; Rosenkranz, A.R.; Eller, P. Potential Role of Regulatory T Cells in Reversing Obesity-Linked Insulin Resistance and Diabetic Nephropathy. Diabetes 2011, 60, 2954–2962. [Google Scholar] [CrossRef]
- Franczyk, M.P.; He, M.; Yoshino, J. Removal of Epididymal Visceral Adipose Tissue Prevents Obesity-Induced Multi-Organ Insulin Resistance in Male Mice. J. Endocr. Soc. 2021, 5, bvab024. [Google Scholar] [CrossRef]
- Kälin, S.; Becker, M.; Ott, V.B.; Serr, I.; Hosp, F.; Mollah, M.M.H.; Keipert, S.; Lamp, D.; Rohner- Jeanrenaud, F.; Flynn, V.K.; et al. A Stat6/Pten Axis Links Regulatory T Cells with Adipose Tissue Function. Cell Metab. 2017, 26, 475–492.e7. [Google Scholar] [CrossRef]
- Muñoz-Rojas, A.R.; Mathis, D. Tissue Regulatory T Cells: Regulatory Chameleons. Nat. Rev. Immunol. 2021, 21, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Joseph, S.S.; Garcia-Carrizo, F.; Tom, R.Z.; Opaleva, D.; Serr, I.; Tschöp, M.H.; Schulz, T.J.; Hofmann, S.M.; Daniel, C. Regulatory T Cells Require IL6 Receptor Alpha Signaling to Control Skeletal Muscle Function and Regeneration. Cell Metab. 2023, 35, 1736–1751.e7. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Kälin, S.; Neubig, A.H.; Lauber, M.; Opaleva, D.; Hipp, H.; Salb, V.K.; Ott, V.B.; Legutko, B.; Kälin, R.E.; et al. Regulatory T Cells in the Mouse Hypothalamus Control Immune Activation and Ameliorate Metabolic Impairments in High-Calorie Environments. Nat. Commun. 2025, 16, 2744. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Spallanzani, R.G.; Mathis, D. Visceral Adipose Tissue Tregs and the Cells That Nurture Them. Immunol. Rev. 2020, 295, 114–125. [Google Scholar] [CrossRef]
- Elkins, C.; Li, C. Deciphering Visceral Adipose Tissue Regulatory T Cells: Key Contributors to Metabolic Health. Immunol. Rev. 2024, 324, 52–67. [Google Scholar] [CrossRef]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ Is a Major Driver of the Accumulation and Phenotype of Adipose Tissue Treg Cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Moro, K.; Xin, A.; Liao, Y.; Gloury, R.; Kawamoto, S.; Fagarasan, S.; Mielke, L.A.; Afshar-Sterle, S.; Masters, S.L.; et al. The Transcriptional Regulators IRF4, BATF and IL-33 Orchestrate Development and Maintenance of Adipose Tissue-Resident Regulatory T Cells. Nat. Immunol. 2015, 16, 276–285. [Google Scholar] [CrossRef]
- Delacher, M.; Imbusch, C.D.; Hotz-Wagenblatt, A.; Mallm, J.-P.; Bauer, K.; Simon, M.; Riegel, D.; Rendeiro, A.F.; Bittner, S.; Sanderink, L.; et al. Precursors for Nonlymphoid-Tissue Treg Cells Reside in Secondary Lymphoid Organs and Are Programmed by the Transcription Factor BATF. Immunity 2020, 52, 295–312.e11. [Google Scholar] [CrossRef] [PubMed]
- Delacher, M.; Imbusch, C.D.; Weichenhan, D.; Breiling, A.; Hotz-Wagenblatt, A.; Träger, U.; Hofer, A.-C.; Kägebein, D.; Wang, Q.; Frauhammer, F.; et al. Genome-Wide DNA-Methylation Landscape Defines Specialization of Regulatory T Cells in Tissues. Nat. Immunol. 2017, 18, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, D.; Cohen, P.; Spiegelman, B.M.; Benoist, C.; Mathis, D. Appearance and Disappearance of the MRNA Signature Characteristic of Treg Cells in Visceral Adipose Tissue: Age, Diet, and PPARγ Effects. Proc. Natl. Acad. Sci. USA 2015, 112, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Kolodin, D.; van Panhuys, N.; Li, C.; Magnuson, A.M.; Cipolletta, D.; Miller, C.M.; Wagers, A.; Germain, R.N.; Benoist, C.; Mathis, D. Antigen- and Cytokine-Driven Accumulation of Regulatory T Cells in Visceral Adipose Tissue of Lean Mice. Cell Metab. 2015, 21, 543–557. [Google Scholar] [CrossRef]
- DiSpirito, J.R.; Zemmour, D.; Ramanan, D.; Cho, J.; Zilionis, R.; Klein, A.M.; Benoist, C.; Mathis, D. Molecular Diversification of Regulatory T Cells in Nonlymphoid Tissues. Sci. Immunol. 2018, 3, eaat5861. [Google Scholar] [CrossRef]
- Frias, A.B.J.; Hyzny, E.J.; Buechel, H.M.; Beppu, L.Y.; Xie, B.; Jurczak, M.J.; D’Cruz, L.M. The Transcriptional Regulator Id2 Is Critical for Adipose-Resident Regulatory T Cell Differentiation, Survival, and Function. J. Immunol. 2019, 203, 658–664. [Google Scholar] [CrossRef]
- Han, J.M.; Wu, D.; Denroche, H.C.; Yao, Y.; Verchere, C.B.; Levings, M.K. IL-33 Reverses an Obesity-Induced Deficit in Visceral Adipose Tissue ST2+ T Regulatory Cells and Ameliorates Adipose Tissue Inflammation and Insulin Resistance. J. Immunol. 2015, 194, 4777–4783. [Google Scholar] [CrossRef]
- Sullivan, J.M.; Höllbacher, B.; Campbell, D.J. Cutting Edge: Dynamic Expression of Id3 Defines the Stepwise Differentiation of Tissue-Resident Regulatory T Cells. J. Immunol. 2019, 202, 31–36. [Google Scholar] [CrossRef]
- Li, C.; DiSpirito, J.R.; Zemmour, D.; Spallanzani, R.G.; Kuswanto, W.; Benoist, C.; Mathis, D. TCR Transgenic Mice Reveal Stepwise, Multi-Site Acquisition of the Distinctive Fat-Treg Phenotype. Cell 2018, 174, 285–299.e12. [Google Scholar] [CrossRef]
- Ilan, Y.; Maron, R.; Tukpah, A.-M.; Maioli, T.U.; Murugaiyan, G.; Yang, K.; Wu, H.Y.; Weiner, H.L. Induction of Regulatory T Cells Decreases Adipose Inflammation and Alleviates Insulin Resistance in Ob/Ob Mice. Proc. Natl. Acad. Sci. USA 2010, 107, 9765–9770. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.V.; Man, K.; Elmzzahi, T.; Malko, D.; Chisanga, D.; Liao, Y.; Prout, M.; Abbott, C.A.; Tang, A.; Wu, J.; et al. Two Regulatory T Cell Populations in the Visceral Adipose Tissue Shape Systemic Metabolism. Nat. Immunol. 2024, 25, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Schmidleithner, L.; Thabet, Y.; Schönfeld, E.; Köhne, M.; Sommer, D.; Abdullah, Z.; Sadlon, T.; Osei-Sarpong, C.; Subbaramaiah, K.; Copperi, F.; et al. Enzymatic Activity of HPGD in Treg Cells Suppresses Tconv Cells to Maintain Adipose Tissue Homeostasis and Prevent Metabolic Dysfunction. Immunity 2019, 50, 1232–1248.e14. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Muñoz-Rojas, A.R.; Spallanzani, R.G.; Franklin, R.A.; Benoist, C.; Mathis, D. Adipose- Tissue Treg Cells Restrain Differentiation of Stromal Adipocyte Precursors to Promote Insulin Sensitivity and Metabolic Homeostasis. Immunity 2024, 57, 1345–1359.e5. [Google Scholar] [CrossRef]
- Bettini, M.L.; Pan, F.; Bettini, M.; Finkelstein, D.; Rehg, J.E.; Floess, S.; Bell, B.D.; Ziegler, S.F.; Huehn, J.; Pardoll, D.M.; et al. Loss of Epigenetic Modification Driven by the Foxp3 Transcription Factor Leads to Regulatory T Cell Insufficiency. Immunity 2012, 36, 717–730. [Google Scholar] [CrossRef]
- Darce, J.; Rudra, D.; Li, L.; Nishio, J.; Cipolletta, D.; Rudensky, A.Y.; Mathis, D.; Benoist, C. An N- Terminal Mutation of the Foxp3 Transcription Factor Alleviates Arthritis but Exacerbates Diabetes. Immunity 2012, 36, 731–741. [Google Scholar] [CrossRef]
- Yilmazer, A.; Eugster, A.; Zevla, D.M.; Helbich, S.S.; Boernert, M.; Torun, B.; Marsela, E.; Kirgin, E.; Dahl, A.; Petzold, A.; et al. Functional Dichotomy of Developmental Foxp3+ Treg Cell Subsets in the Visceral Adipose Tissue of Lean and Obese Mice. bioRxiv 2025. [Google Scholar] [CrossRef]
- Miragaia, R.J.; Gomes, T.; Chomka, A.; Jardine, L.; Riedel, A.; Hegazy, A.N.; Whibley, N.; Tucci, A.; Chen, X.; Lindeman, I.; et al. Single-Cell Transcriptomics of Regulatory T Cells Reveals Trajectories of Tissue Adaptation. Immunity 2019, 50, 493–504.e7. [Google Scholar] [CrossRef]
- Martins, T.; Castro-Ribeiro, C.; Lemos, S.; Ferreira, T.; Nascimento-Gonçalves, E.; Rosa, E.; Oliveira, P.A.; Antunes, L.M. Murine Models of Obesity. Obesities 2022, 2, 127–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Suleiman, J.B.; Mohamed, M.; Bakar, A.B.A. A Systematic Review on Different Models of Inducing Obesity in Animals: Advantages and Limitations. J. Adv. Vet. Anim. Res. 2019, 7, 103–114. [Google Scholar] [CrossRef]
- Collins, S.; Martin, T.L.; Surwit, R.S.; Robidoux, J. Genetic Vulnerability to Diet-Induced Obesity in the C57BL/6J Mouse: Physiological and Molecular Characteristics. Physiol. Behav. 2004, 81, 243–248. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Sherman, A.; Tsao, P.; Gonzalez, O.; Yee, G.; Lamendola, C.; Reaven, G.M.; Cushman, S.W. Enhanced Proportion of Small Adipose Cells in Insulin-Resistant vs Insulin- Sensitive Obese Individuals Implicates Impaired Adipogenesis. Diabetologia 2007, 50, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Guo, F.; Zhou, L.; Stahl, R.; Grams, J. The Cell Size and Distribution of Adipocytes from Subcutaneous and Visceral Fat Is Associated with Type 2 Diabetes Mellitus in Humans. Adipocyte 2015, 4, 273–279. [Google Scholar] [CrossRef]
- Krautbauer, S.; Neumeier, M.; Rein-Fischboeck, L.; Haberl, E.M.; Tilg, H.; Eisinger, K.; Buechler, C. Adipocyte Hypertrophy and Improved Postprandial Lipid Response in Beta 2 Syntrophin Deficient Mice. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2019, 52, 1151–1165. [Google Scholar] [CrossRef]
- Floess, S.; Freyer, J.; Siewert, C.; Baron, U.; Olek, S.; Polansky, J.; Schlawe, K.; Chang, H.-D.; Bopp, T.; Schmitt, E.; et al. Epigenetic Control of the Foxp3 Locus in Regulatory T Cells. PLoS Biol. 2007, 5, e38. [Google Scholar] [CrossRef]
- Polansky, J.K.; Kretschmer, K.; Freyer, J.; Floess, S.; Garbe, A.; Baron, U.; Olek, S.; Hamann, A.; von Boehmer, H.; Huehn, J. DNA Methylation Controls Foxp3 Gene Expression. Eur. J. Immunol. 2008, 38, 1654–1663. [Google Scholar] [CrossRef]
- Alvarez, F.; Istomine, R.; Shourian, M.; Pavey, N.; Al-Aubodah, T.A.-F.; Qureshi, S.; Fritz, J.H.; Piccirillo, C.A. The Alarmins IL-1 and IL-33 Differentially Regulate the Functional Specialisation of Foxp3(+) Regulatory T Cells during Mucosal Inflammation. Mucosal Immunol. 2019, 12, 746–760. [Google Scholar] [CrossRef]
- Hua, J.; Inomata, T.; Chen, Y.; Foulsham, W.; Stevenson, W.; Shiang, T.; Bluestone, J.A.; Dana, R. Pathological Conversion of Regulatory T Cells Is Associated with Loss of Allotolerance. Sci. Rep. 2018, 8, 7059. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic Conversion of Foxp3+ T Cells into TH17 Cells in Autoimmune Arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Fu, Z.; Ye, J.; Dean, J.W.; Bostick, J.W.; Weinberg, S.E.; Xiong, L.; Oliff, K.N.; Chen, Z.E.; Avram, D.; Chandel, N.S.; et al. Requirement of Mitochondrial Transcription Factor A in Tissue-Resident Regulatory T Cell Maintenance and Function. Cell Rep. 2019, 28, 159–171.e4. [Google Scholar] [CrossRef]
- Huynh, A.; DuPage, M.; Priyadharshini, B.; Sage, P.T.; Quiros, J.; Borges, C.M.; Townamchai, N.; Gerriets, V.A.; Rathmell, J.C.; Sharpe, A.H.; et al. Control of PI(3) Kinase in Treg Cells Maintains Homeostasis and Lineage Stability. Nat. Immunol. 2015, 16, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Yang, K.; Guy, C.; Vogel, P.; Neale, G.; Chi, H. Treg Cells Require the Phosphatase PTEN to Restrain TH1 and TFH Cell Responses. Nat. Immunol. 2015, 16, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chi, H. Metabolic Control of Treg Cell Stability, Plasticity, and Tissue-Specific Heterogeneity. Front. Immunol. 2019, 10, 2716. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Bailey-Bucktrout, S.L.; Jeker, L.T.; Penaranda, C.; Martínez-Llordella, M.; Ashby, M.; Nakayama, M.; Rosenthal, W.; Bluestone, J.A. Instability of the Transcription Factor Foxp3 Leads to the Generation of Pathogenic Memory T Cells in Vivo. Nat. Immunol. 2009, 10, 1000–1007. [Google Scholar] [CrossRef]
- Bailey-Bucktrout, S.L.; Martinez-Llordella, M.; Zhou, X.; Anthony, B.; Rosenthal, W.; Luche, H.; Fehling, H.J.; Bluestone, J.A. Self-Antigen-Driven Activation Induces Instability of Regulatory T Cells during an Inflammatory Autoimmune Response. Immunity 2013, 39, 949–962. [Google Scholar] [CrossRef]
- Lal, G.; Zhang, N.; van der Touw, W.; Ding, Y.; Ju, W.; Bottinger, E.P.; Reid, S.P.; Levy, D.E.; Bromberg, J.S. Epigenetic Regulation of Foxp3 Expression in Regulatory T Cells by DNA Methylation. J. Immunol. 2009, 182, 259–273. [Google Scholar] [CrossRef]
- Ohkura, N.; Hamaguchi, M.; Morikawa, H.; Sugimura, K.; Tanaka, A.; Ito, Y.; Osaki, M.; Tanaka, Y.; Yamashita, R.; Nakano, N.; et al. T Cell Receptor Stimulation-Induced Epigenetic Changes and Foxp3 Expression Are Independent and Complementary Events Required for Treg Cell Development. Immunity 2012, 37, 785–799. [Google Scholar] [CrossRef]
- Bovenschen, H.J.; van de Kerkhof, P.C.; van Erp, P.E.; Woestenenk, R.; Joosten, I.; Koenen, H.J.P.M. Foxp3+ Regulatory T Cells of Psoriasis Patients Easily Differentiate into IL-17A- Producing Cells and Are Found in Lesional Skin. J. Investig. Dermatol. 2011, 131, 1853–1860. [Google Scholar] [CrossRef]
- Setoguchi, R.; Hori, S.; Takahashi, T.; Sakaguchi, S. Homeostatic Maintenance of Natural Foxp3+ CD25+ CD4+ Regulatory T Cells by Interleukin (IL)-2 and Induction of Autoimmune Disease by IL-2 Neutralization. J. Exp. Med. 2005, 201, 723–735. [Google Scholar] [CrossRef]
- Miyao, T.; Floess, S.; Setoguchi, R.; Luche, H.; Fehling, H.J.; Waldmann, H.; Huehn, J.; Hori, S. Plasticity of Foxp3+ T Cells Reflects Promiscuous Foxp3 Expression in Conventional T Cells but Not Reprogramming of Regulatory T Cells. Immunity 2012, 36, 262–275. [Google Scholar] [CrossRef]
- Rubtsov, Y.P.; Niec, R.E.; Josefowicz, S.; Li, L.; Darce, J.; Mathis, D.; Benoist, C.; Rudensky, A.Y. Stability of the Regulatory T Cell Lineage in Vivo. Science 2010, 329, 1667–1671. [Google Scholar] [CrossRef]
- Junius, S.; Mavrogiannis, A.V.; Lemaitre, P.; Gerbaux, M.; Staels, F.; Malviya, V.; Burton, O.; Gergelits, V.; Singh, K.; Tito Tadeo, R.Y.; et al. Unstable Regulatory T Cells, Enriched for Naïve and Nrp1(Neg) Cells, Are Purged after Fate Challenge. Sci. Immunol. 2021, 6, eabe4723. [Google Scholar] [CrossRef]
- Thirawatananond, P.; Brown, M.E.; Sachs, L.K.; Arnoletti, J.M.; Yeh, W.-I.; Posgai, A.L.; Shapiro, M.R.; Chen, Y.-G.; Brusko, T.M. Treg-Specific CD226 Deletion Reduces Diabetes Incidence in NOD Mice by Improving Regulatory T-Cell Stability. Diabetes 2023, 72, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, G.; Sivasami, P.; Ramirez, R.N.; Zhang, Y.; Benoist, C.; Mathis, D. Interferon-α-Producing Plasmacytoid Dendritic Cells Drive the Loss of Adipose Tissue Regulatory T Cells during Obesity. Cell Metab. 2021, 33, 1610–1623.e5. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Rojas, A.R.; Wang, G.; Benoist, C.; Mathis, D. Adipose-Tissue Regulatory T Cells Are a Consortium of Subtypes That Evolves with Age and Diet. Proc. Natl. Acad. Sci. USA 2024, 121, e2320602121. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Banks, A.S.; Estall, J.L.; Kajimura, S.; Boström, P.; Laznik, D.; Ruas, J.L.; Chalmers, M.J.; Kamenecka, T.M.; Blüher, M.; et al. Anti-Diabetic Drugs Inhibit Obesity-Linked Phosphorylation of PPARgamma by Cdk5. Nature 2010, 466, 451–456. [Google Scholar] [CrossRef]
- Bradley, D.; Smith, A.J.; Blaszczak, A.; Shantaram, D.; Bergin, S.M.; Jalilvand, A.; Wright, V.; Wyne, K.L.; Dewal, R.S.; Baer, L.A.; et al. Interferon Gamma Mediates the Reduction of Adipose Tissue Regulatory T Cells in Human Obesity. Nat. Commun. 2022, 13, 5606. [Google Scholar] [CrossRef]
- Goudy, K.S.; Johnson, M.C.; Garland, A.; Li, C.; Samulski, R.J.; Wang, B.; Tisch, R. Reduced IL-2 Expression in NOD Mice Leads to a Temporal Increase in CD62Llo FoxP3+ CD4+ T Cells with Limited Suppressor Activity. Eur. J. Immunol. 2011, 41, 1480–1490. [Google Scholar] [CrossRef]
- Yamanouchi, J.; Rainbow, D.; Serra, P.; Howlett, S.; Hunter, K.; Garner, V.E.S.; Gonzalez-Munoz, A.; Clark, J.; Veijola, R.; Cubbon, R.; et al. Interleukin-2 Gene Variation Impairs Regulatory T Cell Function and Causes Autoimmunity. Nat. Genet. 2007, 39, 329–337. [Google Scholar] [CrossRef]
- Grinberg-Bleyer, Y.; Baeyens, A.; You, S.; Elhage, R.; Fourcade, G.; Gregoire, S.; Cagnard, N.; Carpentier, W.; Tang, Q.; Bluestone, J.; et al. IL-2 Reverses Established Type 1 Diabetes in NOD Mice by a Local Effect on Pancreatic Regulatory T Cells. J. Exp. Med. 2010, 207, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Adams, J.Y.; Penaranda, C.; Melli, K.; Piaggio, E.; Sgouroudis, E.; Piccirillo, C.A.; Salomon, B.L.; Bluestone, J.A. Central Role of Defective Interleukin-2 Production in the Triggering of Islet Autoimmune Destruction. Immunity 2008, 28, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Burchill, M.A.; Yang, J.; Vogtenhuber, C.; Blazar, B.R.; Farrar, M.A. IL-2 Receptor Beta- Dependent STAT5 Activation Is Required for the Development of Foxp3+ Regulatory T Cells. J. Immunol. 2007, 178, 280–290. [Google Scholar] [CrossRef]
- Li, X.; Liang, Y.; LeBlanc, M.; Benner, C.; Zheng, Y. Function of a Foxp3 Cis-Element in Protecting Regulatory T Cell Identity. Cell 2014, 158, 734–748. [Google Scholar] [CrossRef]
- Feng, Y.; Arvey, A.; Chinen, T.; van der Veeken, J.; Gasteiger, G.; Rudensky, A.Y. Control of the Inheritance of Regulatory T Cell Identity by a Cis Element in the Foxp3 Locus. Cell 2014, 158, 749–763. [Google Scholar] [CrossRef]
- Passerini, L.; Allan, S.E.; Battaglia, M.; Di Nunzio, S.; Alstad, A.N.; Levings, M.K.; Roncarolo, M.G.; Bacchetta, R. STAT5-Signaling Cytokines Regulate the Expression of FOXP3 in CD4+CD25+ Regulatory T Cells and CD4+CD25− Effector T Cells. Int. Immunol. 2008, 20, 421–431. [Google Scholar] [CrossRef]
- Dwyer, C.J.; Ward, N.C.; Pugliese, A.; Malek, T.R. Promoting Immune Regulation in Type 1 Diabetes Using Low-Dose Interleukin-2. Curr. Diab. Rep. 2016, 16, 46. [Google Scholar] [CrossRef]
- Aylward, A.; Chiou, J.; Okino, M.-L.; Kadakia, N.; Gaulton, K.J. Shared Genetic Risk Contributes to Type 1 and Type 2 Diabetes Etiology. Hum. Mol. Genet. 2018. Epub ahead of printing. [Google Scholar] [CrossRef]
- Nyaga, D.M.; Vickers, M.H.; Jefferies, C.; Fadason, T.; O’Sullivan, J.M. Untangling the Genetic Link between Type 1 and Type 2 Diabetes Using Functional Genomics. Sci. Rep. 2021, 11, 13871. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmazer, A.; Zevla, D.M.; Kretschmer, K. Developmental Origin and Functional Diversity of Foxp3+ Regulatory T Cells in Diabetes and Obesity. Endocrines 2025, 6, 41. https://doi.org/10.3390/endocrines6030041
Yilmazer A, Zevla DM, Kretschmer K. Developmental Origin and Functional Diversity of Foxp3+ Regulatory T Cells in Diabetes and Obesity. Endocrines. 2025; 6(3):41. https://doi.org/10.3390/endocrines6030041
Chicago/Turabian StyleYilmazer, Acelya, Dimitra Maria Zevla, and Karsten Kretschmer. 2025. "Developmental Origin and Functional Diversity of Foxp3+ Regulatory T Cells in Diabetes and Obesity" Endocrines 6, no. 3: 41. https://doi.org/10.3390/endocrines6030041
APA StyleYilmazer, A., Zevla, D. M., & Kretschmer, K. (2025). Developmental Origin and Functional Diversity of Foxp3+ Regulatory T Cells in Diabetes and Obesity. Endocrines, 6(3), 41. https://doi.org/10.3390/endocrines6030041






