Abstract
Turner syndrome (TS) affects approximately 1 out of every 1500–2500 live female births, with clinical features including short stature, premature ovarian failure, dysmorphic features and other endocrine, skeletal, cardiovascular, renal, gastrointestinal and neurodevelopmental organ system involvement. TS, a common genetic syndrome, is caused by sex chromosome aneuploidy, mosaicism or abnormalities with complete or partial loss of function of the second X chromosome. Advances in genetic and genomic testing have further elucidated other possible mechanisms that contribute to pathogenic variability in phenotypic expression that are not necessarily explained by monosomy or haploinsufficiency of the X chromosome alone. The role of epigenetics in variations of gene expression and how this knowledge can contribute to more individualized therapy is currently being explored. TS is established as a multisystemic condition, with several endocrine manifestations of TS affecting growth, puberty and fertility having significant impact on quality of life. Treatment guidelines are in place for the management of these conditions; however, further data on optimal management is needed.
1. Introduction
Turner syndrome (TS) is a complex multisystem genetic condition, first described in 1938 by an endocrinologist noting growth and congenital hypergonadotropic hypogonadism, with a reported prevalence of 1:2000 to 1:4000 among females [1,2]. It is a heterogenous genetic disorder with 40–50% of patients having classic genotype 45,X and the rest having a variety of mosaicisms [2,3]. There is a diverse range of phenotypic characteristics, influenced by an individual’s karyotype. However, despite extensive research showing the effects of haploinsufficiency of the X chromosome, exact mechanisms are still not fully understood. More recent data support the role of altered gene expression as a result of epigenetic mechanisms as contributory to the varied clinical manifestations of TS [4], and is further discussed in this review. Furthermore, TS is associated with an increased risk for a variety of health complications, and the particular interest of this review are the endocrine-related manifestations and their subsequent management.
2. Etiology
2.1. Chromosomal Aberrations in TS
Turner Syndrome is considered to be the most common aneuploidy, although 99% of congenital occurrences result in spontaneous abortion [4]. As with other aneuploidies, the primary mechanism believed to contribute to TS is meiotic non-disjunction, a process which leads to an unequal distribution of genetic material amongst daughter cells, such that some cells may have additional chromosomes, whereas others will have missing chromosomes (Figure 1) [5].
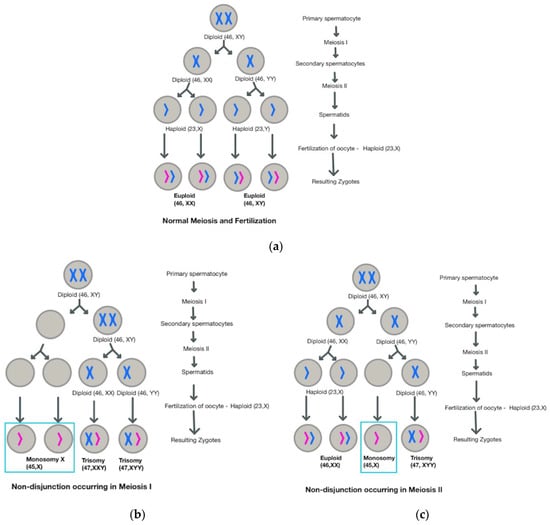
Figure 1.
Comparison of (a) normal meiosis with (b) non-disjunction occurring in Meiosis I and (c) non-disjunction occurring in Meiosis II.
Approximately 40–50% of individuals with TS have a monosomy (45,X) karyotype [2], meaning that all cells only have one X chromosome; 15–25% have 45,X/46,XX mosaicism and another 10–12% have 45,X/46,XY mosaicism, meaning that some cells only have one X chromosome, whereas others have two X chromosomes or X and Y chromosomes [2]. Interestingly, in mosaic individuals, the distribution of cells with only one X chromosome and cells with two sex chromosomes does not need to be split evenly [6].
Other structural abnormalities of the X chromosome (Table 1), such as deletions, translocations, ring formation and isochromes contribute to mosaic forms [7]. Large, inverted repeats within the short arm (p) of X chromosome at the Xp11.2 locus have been found to create a complex repetitive architecture that predisposes it to rearrangements that correlate to the isodicentric X chromosome formation that is the second most common chromosomal abnormality associated with TS [8]. Additionally, studies have shown that the parental origin for the single complete X chromosome is maternal (Xm) in 90% of cases [9]. The origin of the abnormal or missing chromosome is inconclusive at this point, with some studies demonstrating increased likelihood of maternal origin (Xm) for pseudo dicentric and deletions of long arm (q) of X chromosome (Xq) [10], paternal in origin (Xp) for deletions of short arm (p) of X chromosome, and the extremely rare cases of abnormal Y chromosomes, but equally likely to originate in either parent in the case of either isochromosome Xq or Ring X abnormalities. Other studies, however, postulate that the majority of Turner syndrome karyotypes are caused by paternal meiotic or mitotic errors, leading to dominance of the maternal X chromosome [11].

Table 1.
TS karyotypes and description of associated structural abnormalities.
The X chromosome is important not just for sex determination, but also contains several genes responsible for growth and development during the embryonic period and any aberration or abnormal defects can ultimately lead to pathogenesis of TS disease conditions. The ‘Gene Dosage Effect’ hypothesis also postulates that TS features can be mapped to particular regions of the X-chromosome that are gene dosage sensitive [12]. However, phenotypic variability in TS cannot be explained by genomic imbalance alone. It is postulated that other processes such as X-chromosome inactivation and altered gene expression as a result of epigenetic factors are also contributory [4,13].
2.2. Contributions of X-Inactivation to the TS Phenotype
The genes on the short arm of X chromosome which escape X-inactivation are implicated in the TS phenotype [14,15]. X-inactivation is the process by which one of the X chromosomes is silenced to equalize the gene dosages between male and female mammals, resulting in one functional copy of the X chromosome on all somatic cells [16]. Under normal conditions, an X inactivation epigenetic process randomly methylates one of the two X chromosomes present in each cell, so that the genes from only one X chromosome are actively expressed. However, certain genes in the pseudoautosomal region 1 (PAR1) and pseudoautosomal region 2 (PAR2) of the X chromosome may escape X-inactivation and still be expressed, even if these genes are on the silenced X chromosome [17]. The Y chromosome in males also contains genes found on PARs; hence, both 46,XX and 46,XY individuals express two copies of the pseudoautosomal genes that escape X-inactivation in females [17]. In contrast, for TS, cells with only one X chromosome lack the second copy of these pseudoautosomal genes or other genes that would typically escape X-inactivation due to not having a second sex chromosome present. This results in decreased expression of those genes, which is termed haploinsufficiency [6]. The degree of haploinsufficiency involved in TS depends on the karyotype; the 45,X karyotype involves greater haploinsufficiency than mosaic karyotypes.
An example of the contribution of haploinsufficiency associated with the skewed inactivation pattern resulting from Turner syndrome karyotype is with respect to the previously identified short stature homeobox, or SHOX gene, which is located on Xp22.23, and thus far is the only gene that has been compellingly associated with TS attributes. SHOX is expressed in bone marrow fibroblasts, which can give rise to osteogenic genes that can contribute to bone growth [18]. This gene’s function is dosage-dependent, with decreased gene expression or haploinsufficiency leading to short stature and other features such as Madelung deformity, high arched palate, scoliosis and micrognathia [19,20]. Another gene located in PAR1 is the CSF2RA gene [17], which could be implicated in the high rates of in utero deaths associated with TS [21]. One study has shown that expression of various genes that are active in the placenta, including CSF2RA, was higher in wild-type human embryonic stem cells than in cells that had spontaneously lost an X chromosome (mimicking the 45,X karyotype) [21]. Although haploinsufficiency of the SHOX gene could interfere with growth in TS patients, haploinsufficiency of CSF2RA could interfere with proper placental function.
Other genes on the X chromosome besides those found in PARs can also escape X-inactivation. For instance, TIMP1 usually escapes X-inactivation, so TS would involve haploinsufficiency of TIMP1, potentially promoting the formation of a bicuspid aortic valve (rather than a normal tricuspid aortic valve) [22]. Of note, certain variants of TIMP3, a gene located on chromosome 22, are also associated with bicuspid aortic valve, and decreased expression of TIMP1 can heighten risk for heart defects if the individual has these TIMP3 variants [22]. The relationship between TIMP1 and TIMP3 indicates that the inadequate expression of X chromosome genes combined with the presence of certain autosomal genes can bring about the clinical manifestations seen in TS.
2.3. Role of Epigenetics in TS
Epigenetic differences that influence gene expression without altering base sequences exist between 45,X and 46,XX individuals, with extensive hypomethylation throughout 45,X genome compared to 46,XX individuals, apart from differences in hypermethylation [23,24] Epigenetic modification and resulting altered gene expression can contribute to various pathogenesis seen in TS. For example, differentially methylated genes such as STAT4 and KDM6A can affect T helper 1 (TH1) cell development and function [13], and T follicular helper (TFH) cells development [25], respectively, and contribute to resultant inadequate immune responses and autoimmunity in affected TS individuals. KDM6A, implicated in ovarian dysfunction in TS [22,26], is also differentially expressed in Klinefelter syndrome [23,27].
Levels of microRNA (miRNA), which can bind to mRNA to block translation into protein, can also differ between TS and 46,XX patients. For example, blood samples from TS patients had lower amounts of miR-126-3p compared to controls [28]. Furthermore, miR-126-3p levels were higher in TS patients who had abnormal aortic valves compared to TS patients who did not; miR-126-3p can limit Bcl-2 expression and lead to dysregulated apoptosis in the heart [28]. Fetal gonadal tissues with a 45,X karyotype have decreased amounts of KITLG protein compared to tissues with a 46,XX karyotype, and the difference was found to be mediated by levels of miR-320a. In 46,XX individuals, KDM5C blocks expression of miR-320a, allowing the expression of KITLG, which supports ovarian development. However, since KDM5C escapes X-inactivation, haploinsufficiency of KDM5C allows for increased levels of miR-320a to be present in 45,X individuals, thus leading to deficient KITLG expression and possibly impaired ovarian development [29]. Thus, this demonstrates that differences in miRNA abundance can influence the clinical presentation of TS. Another example is how PPARGC1A promoter DNA methylation status affects Homeostasis Model Assessment for Insulin Resistance (HOMA-IR) independently of BMI or age in TS subjects [30].
RPS4X and JPX are other escape genes that are also differentially expressed in TS; however, their precise roles in TS pathogenesis are still to be elucidated [31,32,33]. The gene IL3RA, which encodes for a subunit of the IL-3 receptor, is noted to be differentially methylated in TS women. This gene has been linked to increased risk for autoimmune disorders among these patients [34,35]. One study compared the transcriptomes (the collection of mRNA expressed) of 45,X and 46,XX fibroblasts and revealed that the BMP2 and BMPER genes were dysregulated in 45,X cells such that normal bone mineralization could be impaired [28]. Other interesting findings in 45,X cells from this transcriptome analysis include downregulation of STC1 that could lead to dysregulated follicle development, downregulation of IGF2 and ENPP1 that could lead to metabolic pathologies, and upregulation of CLDN11, which is involved in spermatogenesis [32]. Notably, these six genes are all located on autosomes, indicating that the genetic etiologies of TS manifestations extend beyond the X chromosome.
Another role of epigenetics that has been explored is its involvement in X chromosome monosomy. It is postulated that differential methylation of sex chromosomes could cause errors in chromosome alignment and spindle formation during meiosis or mitosis [4]. Herrera et al. studied hypomethylated cells as caused by mutations in DNA methyltransferase 3b gene (DNMT3B), and found delayed centromere separation leading to aneuploidy [36]. Underlying pathogenesis mechanism in various TS phenotypic presentation is evolving with improved understanding of notable difference in RNA expression, autosomal DNA methylation and X chromosome methylation in TS patients.
3. Clinical Presentation
As alluded to earlier, the karyotype a TS individual possesses can influence which TS features appear and how severely these features present. A study of 656 individuals with TS in London found that 45,X patients were diagnosed at a younger age than patients with other TS karyotypes [6]. This data suggests that the 45,X karyotype gives rise to more serious or noticeable complications to warrant an earlier diagnosis. In the same study, a significantly higher percentage of 45,X patients had primary amenorrhea compared to 45,X/46,XX, ring X, and isochromosome Xq patients, and a significantly lower percentage of patients with 45,X/46,XX had hearing loss, hypertension, and obesity compared with 45,X patients [6]. Another study involving adults from the UK Biobank revealed that 45,X/46,XX and 46,XX individuals had a similar age of menarche, rate of pregnancy, and number of pregnancies [37]. Furthermore, 45,X/46,XX individuals had a smaller height deficit than 45,X [37], and a different study found that 45,X patients had more congenital heart malformations than other TS patients [38]. The difference in phenotype between 45,X and 45,X/46,XX individuals could be explained by genes that escape X-inactivation. Monosomy X is the most extreme form of haploinsufficiency, whereas 45,X/46,XX individuals have some cell lines with normal levels of gene expression intact. These normal cell lines in 45,X/46,XX individuals can be protective.
Table 2 lists some of the more common clinical features of TS. Clinical presentation can be very varied, ranging from having all or most classical features to minimal or no apparent symptoms or signs. Because of heterogeneity of clinical manifestations, TS may be diagnosed at any age. Prenatal diagnosis has increased throughout the years because of prenatal testing, particularly for at-risk mothers. Findings suggestive of TS include chromosome abnormalities detected with chorionic villous sampling or amniocentesis; increased nuchal translucency, presence of cystic hygroma, coarctation or evidence of left sided cardiac defects, brachycephaly, renal anomalies, polyhydramnios, oligohydramnios and growth retardation on ultrasound studies; and abnormal maternal triple or quadruple testing [2]. The presence of lymphedema of the extremities, dysmorphic features, failure to thrive and developmental delay in a female infant or young child should alert the medical provider to proceed with further evaluation and testing for TS. However, the diagnosis can be delayed until the patient presents with short stature later in childhood or delayed pubertal development during the adolescent period.

Table 2.
Clinical features of TS.
4. Endocrine-Related Manifestations of Turner Syndrome
4.1. Growth and Short Stature
The short stature in TS is attributed to haploinsufficiency of SHOX gene which regulates differentiation of chondrocytes. This also explains other features noted in TS including high arched palate, obstructive sleep apnea, prominent ears, and chronic otitis media [39]. Although individuals with TS do not have true growth hormone deficiency, they respond to growth hormone as seen in those with isolated SHOX gene deficiency [40], and can have a mean height gain of about 7 cm; however, this is still dependent on age when related to age of initiation and duration of treatment [41]. Results of a French observational multicenter study published in 2016 demonstrated association between karyotype subgroup and phenotypic variation in spontaneous intrauterine and postnatal growth and adult height after GH therapy [42]. The authors concluded that haploinsufficiency of unknown Xp gene increases the risk of deficit in prenatal and postnatal growth and short adult height after GH treatment [42].
4.2. Ovarian Insufficiency
Ovarian insufficiency resulting from rapid and progressive loss of oocytes is another feature of the syndrome in almost all TS individuals, presenting as absent or delayed pubertal development in adolescent girls, or infertility in women of childbearing age. This accelerated degeneration of oocytes is attributed to failure of chromosome pairing in the early stage of meiotic prophase [12]. Spontaneous pubertal development is more common in mosaics, occurring in about a third of this population; however, only a smaller percentage of this group will continue to progress to the occurrence of menarche [43]. Consistent with the variability of clinical presentation in TS, there is also significant differences in the size primordial follicle pool. This explains how mosaics with a large enough pool may undergo spontaneous puberty and menarche [43]; however, they will still have a faster rate of follicle apoptosis than women with normal 46,XX [44]. Anti-Mullerian hormone (AMH) is a good marker of ovarian reserve of growing follicles and Visser et al., found that AMH levels corelate with karyotype of TS with measurable levels found in 77% of TS patients with 45,X/4XX mosaicism and in only 10% with classic 45,X karyotype [45].
4.3. Autoimmune Thyroiditis
Individuals with TS are at increased risk of developing autoimmune diseases, particularly the autoimmune thyroid diseases (ATD). As per the recent meta-analysis report the overall prevalence of ATD in TS is 38.6% [46]. About 41–45% of individuals with TS are found to have thyroid peroxidase antibodies [47,48], with prevalence of Hashimoto’s disease (HD) is 30–50% [48,49]. Graves’ disease (GD), which is rarer than HD, still occurs more frequently in TS compared with general population with an estimated prevalence of 1.7–3% [50,51] Although ATD is observed in all TS karyotypes, several studies have shown that autoimmune disorders including ATD are more likely to occur in those with isolated Xp deletion and isochromosome Xq [47,52,53,54]. Both these chromosomal anomalies lack the short arm of X chromosome and the haploinsufficiency of immune regulatory genes located in the Xter-p11.2 region is the most likely explanation for increased predisposition to autoimmune disorders [54]. Other proposed mechanisms for the cause of increased frequency of autoimmune disorders in TS include: maternal origin of X chromosome, hypogonadism and cytokine imbalance with more pro-inflammatory cytokines and less anti-inflammatory cytokines [55].
4.4. Diabetes and Metabolic Syndrome
The patients with TS are more likely to develop either type 1 or type 2 diabetes mellitus (DM) with a relative risk of 11.56 and 4.38, respectively [56]. The increased risk for Type 1 diabetes is explained by the overall higher risk for development of autoimmune conditions in TS, as previously discussed.
The associated risk for developing metabolic syndrome including Type 2 diabetes in TS involves a more complicated pathophysiologic process, with several contributory factors. The features of metabolic syndrome observed in patients with TS include Increased abdominal adiposity, impaired vascular function and hypertension, dyslipidemia and insulin resistance [57]. Lebenthal et al. looked at the evolution of metabolic comorbidities in TS, with the longitudinal and cross-sectional retrospective study that showed that increasing age and weight gain increase metabolic risks in this cohort of 98 TS patients [58]. Similar to previous studies, these cardiometabolic risk factors are already apparent during childhood, and in a good number of patients who have normal BMI at this age [58,59,60]. X chromosome gene dosage is thought to influence the occurrence of metabolic disorders in TS, with reports of clustering of risk factors in 45,X monosomy [3,61].
Individuals with TS were noted to have higher body mass index (BMI) and higher waist circumference when compared with BMI matched women without TS [62]. As many as 20–40% of youth and 60% of adult individuals with TS have systemic hypertension, which may be due to renal anomalies, or idiopathic [2]. An atherogenic lipid profile of high triglycerides, high low-density lipoprotein (LDL) and low high-density lipoprotein (HDL) is also observed in some studies, with hypercholesterolemia being reported in 37–50% of patients with TS [2,61,63]. Altered glucose and insulin metabolism in TS is most likely due to decreased beta-cell responsiveness with diminished first-phase insulin release [64]. Haploid gene deficiency of PAR1 is believed to result into altered expression of molecules that the gene encodes for, which include certain types of receptors, transcription factors, phospholipase and protein lipases involved in appropriate insulin response [65]. Another genetic mechanism explored is the association of the long arm of the X chromosome (iXq) with higher incidence of DM, with additional copies of Xq triggering overexpression of diabetes-related genes such as islet cell antigen (ICA) and insulin-like growth factor II [66]. This overexpression is then linked to immune-mediated injury to beta-cells, leading to decreased responsiveness.
5. Diagnosis—Karyotyping and Beyond
A standard 20-cell karyotype using the peripheral blood sample is recommended for all girls and women suspected with TS. Sampling of another tissue such as buccal mucosa cells or skin fibroblasts may be needed if the standard karyotype is reported normal and there is strong clinical suspicion of TS. [2,67]. If the diagnosis was made prenatally with chromosomal analysis using chronic villous sampling or amniocentesis, it is recommended to repeat the karyotyping postnatally [2].
Presence of Y chromosome material suggests increased risk of gonadoblastoma and germ cell tumors [68]. In patients with virilizing features, fluorescent in situ hybridization (FISH) analysis of at least two to three different tissues using X and Y probes is recommended to detect cryptic Y material [2,67]. Additional testing has also been recommended for patients with 45,X karyotype, as true sex chromosome monosomy is incompatible with life and these patients may have cryptic mosaicism potentially with Y chromosome material [69]. FISH using a probe to DYZ3 locus at Y-centromere is suggested to detect cryptic mosaicism for Y chromosome as this region is associated with increased susceptibility to gonadoblastoma [69].
The polymerase chain reaction test using multiple Y-chromosome-specific DNA probes is more sensitive than FISH [70,71]; however, it may be susceptible to contamination [72]. Non-invasive prenatal screening tests such as cell-free fetal DNA screening in maternal plasma by microarray or whole genome sequencing helps to detect aneuploidy, but both methods have limitations such as failure to identify balanced translocations and triploidies, or missing X-chromosome structural abnormalities and mosaicism, respectively [73,74]. Single nucleotide polymorphism (SNP) array genotyping has been compared with karyotyping in patients with TS and it was found that SNP array can better detect cryptic Y chromosome; however, this may cause misinterpretation of rare cell lines and cannot detect fully balanced translocations of the X chromosome [75].
Further advancements in genetic testing during the past decade have allowed for understanding genomic mechanisms of TS, elucidating further on what affects growth, puberty, neurocognitive development and occurrence of associated conditions in these patients. Advances in epigenetic research has seen the use of assays that started with DNA methylation and histone acetylation studies that were site-specific, to genome-wide assessments [76]. More recently, bioinformatics analysis has been used to identify differentially expressed genes that may further increase knowledge on the pathogenesis of TS [77,78]. While newer gene editing technological advances such as Crispr/Cas9 can offer potential therapeutic options, their use in human disease can elicit multitude of ethical issues and such discussion is beyond the scope of this review.
6. Treatment and Management
6.1. Growth Hormone Therapy
Growth hormone (GH) treatment started at an early age of 4–6 years in patients with TS helps to achieve normal growth pattern similar to that for peers of the same age [2]. The mean average height of TS patients untreated with GH is cited to be around 142–144 cm, which is approximately 20 cm less than the mean height of general population [79]. When treated with GH, the annual height gain of TS patients increased by 1–2 cm for every year of GH therapy [2,79]. Besides promoting growth, GH augments bone mass, regulates lipid and glucose metabolism and increases amino acid transport in the muscle [79]. Long-term therapy is also associated with positive effects on craniofacial development in TS, mostly affecting mandibular ramus and posterior facial height [80]. A recent systematic review looking at effects of GH on the cardiovascular system showed positive effect on lipid profile, reducing the risk of cardiovascular disease, particularly if with concomitant estradiol therapy [81].
Several factors affect success of growth hormone therapy. Dose, duration, as well as adherence and compliance to therapy all influence final adult height [79]. The “Toddler Turner” study, which looked at effects of early initiation of GH therapy beginning at 9 months to 4 years of age, revealed that the early treated group were taller all through out, but with no significant difference in near-adult height when compared with early untreated group. This was attributed to the catch down growth noted in the early treated group during lapses of GH therapy, demonstrating the significance of uninterrupted treatment to promote better adult height [82]. Later initiation not only limits adult height predictions but may delay growth associated with puberty as well. The presence of other health conditions such as congenital heart disease, hypothyroidism or celiac disease may contribute to growth deficits, independent of growth hormone effects [79]. Other non-modifiable factors include height of parents and height of the patient at the beginning of GH therapy. Specific genetic markers have also been linked with response to GH therapy in TS, such as estrogen receptor alpha (ESR1) and tyrosine-protein phosphatase nonreceptor type1 (PTPN1) both noted to influence height velocity in TS [83], whereas homozygosity for SOCS-2, GHR exon3 full length and IGFBP3-202 C alleles was associated with poor response to GH therapy [84].
The initial dose of GH is 0.35–0.375 mg/kg/week and patients with poor height prognosis may be started on higher doses after careful consideration [2]. Concomitant treatment with Oxandrolone is an option to improve final height if there is delay in initiation of GH therapy due to delayed diagnosis. GH therapy along with Oxandrolone at a dose of 0.03–0.05 mg/kg/day in TS patients 10 years and older results in gain of final height by 2–5 cm as compared to those treated with GH alone [2]. Although Oxandrolone can have synergistic effect on growth acceleration, it is associated with undesirable effects including virilization and delayed pubertal development [85]. These side effects are modest when treated with doses less than 0.06 mg/kg/day [86].
6.2. Estrogen Replacement
The goal of estrogen replacement therapy in TS is to induce and maintain normal pubertal development with secondary sexual characteristics including normal breasts and uterine size and shape. The other goals include achievement of physiological effects of endogenous estrogens such as bone mineralization and maintenance of cardiovascular health. The negative effects of estrogen deficiency in TS include poor intrauterine growth, decreased cognitive and motor reaction time, reduced bone mass, poor cardiovascular outcomes, low self-esteem and poor quality of life [87,88]. In a study by Viuff et al., hormone replace therapy is found to reduce endocrine and cardiovascular morbidity in TS adults with decreased use of antidiabetics, thyroid hormone replacement and antihypertensives and reduced hospitalizations due to osteoporotic fractures and stroke [89]. Other studies show that earlier induction of puberty and start of estrogen replacement may be beneficial for adult bone density [90].
Current recommendations call for starting estrogen replacement at around age 11–12 years, with transdermal 17-β estradiol being the preferred treatment [2,88]. Monitoring gonadotropins, particularly follicle stimulating hormone (FSH), starting at about 11 years of age, aid in detecting and confirming hypergonadotropic hypogonadism before puberty induction [2]. Low levels of anti-Mullerian hormone (AMH) can also suggest ovarian failure [45,89,91]. To mimic the normal physiologic milieu during the peripubertal period, initiation with low doses of estrogen (3–7 µg/kg/day) is recommended, gradually increasing every 6 months, eventually reaching adult doses of up to 100 mg/day in 2–3 years [2]. Ultra-low dose estrogen therapy using oral ethinyl estradiol during the prepubertal period has been suggested, with studies showing normalization of onset and tempo of puberty development, as well as improvements in cognition and memory [92,93,94]. However, routine use of this therapy is not recommended due to lack of long-term safety data [2].
As mentioned, transdermal Estradiol (E2) is the more widely used preparation that theoretically provides a more physiologic, systemic route of delivery. Orally administered estrogen, on the other hand, reaches the systemic circulation after undergoing metabolism in the liver. A randomized clinical trial by Torres-Santiago et al. demonstrated no significant differences between the TS patients treated with oral and transdermal E2 regarding their body composition, bone mineral density and lipid profile when their estradiol levels were titrated to those of normal menstruating adolescents [95]. However, the increased amounts of conjugated estrogen precursors and metabolites associated with oral estrogen preparations pose a higher risk for thromboembolic events as seen in the post-menopausal women [96]. To date, there are no data available to suggest the same risk in TS population, as long-term studies assessing the optimal dose, route and duration of hormone replacement treatment are still to be published.
TS individuals usually have normal uterus, and it is recommended to add progestin therapy 2 years after induction of puberty or once spotting or menstrual bleeding has commenced [2]. Progestins minimize risk of endometrial hyperplasia due to unopposed estrogen therapy and thereby prevent endometrial cancer [97]. There are several proposed progestin and estrogen/progestin replacement options after puberty induction [88]. After establishing adult dosing for hormone replacement therapy, it needs to be continued until the usual age of menopause. These patients need to be monitored for risks associated with estrogen therapy on an annual basis.
6.3. Fertility Preservation
Premature ovarian failure results in infertility in majority of patients with TS with spontaneous pregnancy reported only in 2–10% [98]. In those with mosaicism (45X/46XX) salvage of existing oocytes using assisted reproductive technologies was proposed [99]. Oocyte retrieval and cryopreservation after ovarian stimulation in adults and post pubertal adolescents with Turner mosaicism has been reported [100,101,102]. Ovarian stimulation was initiated with FSH (recombinant or highly purified) along with LH supplementation in the form of human menopausal gonadotropins or recombinant LH due to concerns of hypothalamic immaturity in post pubertal adolescents with TS [101].
Ovarian tissue cryopreservation which is still experimental fertility preservation technique may be an option in prepubertal patients with TS who are at increased risk of accelerated ovarian failure based declining AMH levels (<2 ng/mL) and cannot wait until sufficient maturity to undergo oocyte cryopreservation [99]. Though fertility preservation is no longer consider experimental in adult patients, ovarian stimulation for oocyte cryopreservation and ovarian tissue cryopreservation in young patients needs consent from parents and patients above nine years of age and also approval of institutional review board (IRB) is strongly recommended [99]. More recently, another approach for fertility preservation is suggested, primarily based on the patient’s genotype (monosomy vs. mosaic), then subsequently based on AMH concentrations over time [103]. This approach also takes into strong consideration the expected maternal risks that may vary significantly from person to person. Prenatal genetic counseling plays an important role in prenatal diagnostic procedures in all pregnant women and in future reproductive options such as in vitro fertilization for TS adults.
7. Conclusions
Scientific advancements have led to improved diagnostic and management options in the care of patients with TS. Still there is more to discover how the impact of more detailed genetic and genomic testing could affect health outcomes, help decrease health burdens, and ultimately improve quality of life. Although clinical manifestations could be highly variable, more often the condition affects multiple organ systems, thus needing multidisciplinary team approach to ensure optimal outcomes.
Author Contributions
Conceptualization: E.G.C. and S.K.; Administrative support: E.G.C. and S.K.; Provision of study materials or patients: E.G.C. and S.K.; Manuscript writing: E.G.C., S.K.P., T.D., B.S. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kansra, A.; Donohue, P. Hypofunction of the Ovaries. In Nelson Textbook of Pediatrics, 21st ed.; Kliegman, R.M., St. Geme, J., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2020; pp. 3001–3007. [Google Scholar]
- Gravholt, C.H.; Andersen, N.H.; Conway, G.S.; Dekkers, O.M.; Geffner, M.E.; Klein, K.O.; Lin, A.E.; Mauras, N.; Quigley, C.A.; Rugin, K.; et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: Proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur. J. Endocrinol. 2017, 177, G1–G70. [Google Scholar] [CrossRef]
- El-Mansoury, M.; Barrenäs, M.L.; Bryman, I.; Hanson, C.; Larsson, C.; Wilhelmsen, L.; Landin-Wilhelmsen, K. Chromosomal mosaicism mitigates stigmata and cardiovascular risk factors in Turner syndrome. Clin. Endocrinol. 2007, 66, 744–751. [Google Scholar] [CrossRef]
- Álvarez-Nava, F.; Lanes, R. Epigenetics in Turner syndrome. Clin. Epigenetics 2018, 10, 45. [Google Scholar] [CrossRef]
- Scott, S.A.; Cohen, N.; Brandt, T.; Warburton, P.E.; Edelmann, L. Large inverted repeats within Xp11.2 are present at the breakpoints of isodicentric X chromosomes in Turner syndrome. Hum. Mol. Genet. 2010, 19, 3383–3393. [Google Scholar] [CrossRef]
- Cameron-Pimblett, A.; La Rosa, C.; King, T.F.J.; Davies, M.C.; Conway, G.S. The Turner syndrome life course project: Karyotype-phenotype analyses across the lifespan. Clin. Endocrinol. 2017, 87, 532–538. [Google Scholar] [CrossRef]
- Catović, A. Cytogenetics findings at Turner Syndrome and their correlation with clinical findings. Bosn. J. Basic Med. Sci. 2005, 5, 54–58. [Google Scholar] [CrossRef][Green Version]
- Monroy, N.; López, M.; Cervantes, A.; Garcia-Cruz, D.; Zafra, G.; Canun, S.; Zenteno, J.C.; Kofman-Alfaro, S. Microsatellite analysis in Turner syndrome: Parental origin of X chromosomes and possible mechanism of formation of abnormal chromosomes. Am. J. Med. Genet. 2002, 107, 181–189. [Google Scholar] [CrossRef]
- Gottlieb, S.F.; Tupper, C.; Kerndt, C.C.; Tegay, D.H. Genetics, Nondisjunction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Jacobs, P.; Dalton, P.; James, R.; Mosse, K.; Power, M.; Robinson, D.; Skuse, D. Turner syndrome: A cytogenetic and molecular study. Ann. Hum. Genet. 1997, 61 Pt 6, 471–483. [Google Scholar] [CrossRef]
- Uematsu, A.; Yorifuji, T.; Muroi, J.; Kawai, M.; Mamada, M.; Kaji, M.; Yamanaka, C.; Momoi, T.; Nakahata, T. Parental origin of normal X chromosomes in Turner syndrome patients with various karyotypes: Implications for the mechanism leading to generation of a 45,X karyotype. Am. J. Med. Genet. 2002, 111, 134–139. [Google Scholar] [CrossRef]
- Ogata, T.; Matsuo, N. Turner syndrome and female sex chromosome aberrations: Deduction of the principal factors involved in the development of clinical features. Hum. Genet. 1995, 95, 607–629. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Trolle, C.; Vang, S.; Hornshøj, H.; Skakkebæk, A.; Hedegaard, J.; Nordentoft, I.; Pederson, J.S.; Gravholt, C.H. Epigenetic and transcriptomic consequences of excess X-chromosome material in 47,XXX syndrome-A comparison with Turner syndrome and 46,XX females. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 279. Available online: https://pubmed.ncbi.nlm.nih.gov/32489015/ (accessed on 2 April 2022). [CrossRef]
- Bondy, C.A.; Cheng, C. Monosomy for the X chromosome. Chromosome Res. 2009, 17, 649. Available online: https://pubmed.ncbi.nlm.nih.gov/19802705/ (accessed on 2 April 2022). [CrossRef]
- Gravholt, C.H.; Viuff, M.H.; Brun, S.; Stochholm, K.; Andersen, N.H. Turner syndrome: Mechanisms and management. Nat. Rev. Endocrinol. 2019, 15, 601–614. [Google Scholar] [CrossRef]
- Disteche, C.; Berletch, J. X-chromosome inactivation and escape. J. Genet. 2015, 94, 591. Available online: https://pubmed.ncbi.nlm.nih.gov/26690513/ (accessed on 2 April 2022). [CrossRef]
- Ross, M.T.; Grafham, D.V.; Coffey, A.J.; Scherer, S.; McLay, K.; Muzny, D.; Platzer, M.; Howell, G.R.; Burrows, C.; Bird, C.P.; et al. The DNA sequence of the human X chromosome. Nature 2005, 434, 325–337. [Google Scholar] [CrossRef]
- Ellison, J.W.; Wardak, Z.; Young, M.F.; Robey, P.G.; Laig-Webster, M.; Chiong, W. PHOG, a Candidate Gene for Involvement in the Short Stature of Turner Syndrome. Hum. Mol. Genet. 1997, 6, 1341–1347. [Google Scholar] [CrossRef]
- Clement-Jones, M.; Schiller, S.; Rao, E.; Blaschke, R.J.; Zuniga, A.; Zeller, R.; Robson, S.C.; Binder, G.; Glass, I.; Strachan, T.; et al. The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Hum. Mol. Genet. 2000, 9, 695–702. [Google Scholar] [CrossRef]
- Fukami, M.; Seki, A.; Ogata, T. SHOX Haploinsufficiency as a Cause of Syndromic and Nonsyndromic Short Stature. Mol. Syndromol. 2016, 7, 3–11. [Google Scholar] [CrossRef]
- Urbach, A.; Benvenisty, N. Studying Early Lethality of 45,XO (Turner’s Syndrome) Embryos Using Human Embryonic Stem Cells. PLoS ONE 2009, 4, e4175. [Google Scholar] [CrossRef]
- Corbitt, H.; Gutierrez, J.; Silberbach, M.; Maslen, C.L. The genetic basis of Turner syndrome aortopathy. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 117–125. [Google Scholar] [CrossRef]
- Trolle, C.; Nielsen, M.M.; Skakkebæk, A.; Lamy, P.; Vang, S.; Hedegaard, J.; Nordentoft, I.; Orntoft, T.F.; Pedersen, J.S.; Gravholt, C.H. Widespread DNA hypomethylation and differential gene expression in Turner syndrome. Sci. Rep. 2016, 6, 34220. Available online: https://pubmed.ncbi.nlm.nih.gov/27687697/ (accessed on 2 April 2022). [CrossRef]
- Sharma, A.; Jamil, M.A.; Nuesgen, N.; Schreiner, F.; Priebe, L.; Hoffmann, P.; Herns, P.; Nothen, M.M.; Frohlich, H.; Oldenburg, J.; et al. DNA methylation signature in peripheral blood reveals distinct characteristics of human X chromosome numerical aberrations. Clin. Epigenetics 2015, 7, 76. Available online: https://pubmed.ncbi.nlm.nih.gov/26221191/ (accessed on 2 April 2022). [CrossRef]
- Cook, K.D.; Shpargel, K.B.; Starmer, J.; Whitfield-Larry, F.; Conley, B.; Allard, D.E.; Rager, J.E.; Fry, R.C.; Davvenport, M.; Magnuson, T.; et al. T Follicular Helper Cell-Dependent Clearance of a Persistent Virus Infection Requires T Cell Expression of the Histone Demethylase UTX. Immunity 2015, 43, 703–714. [Google Scholar] [CrossRef]
- Berletch, J.B.; Deng, X.; Nguyen, D.K.; Disteche, C.M. Female bias in Rhox6 and 9 regulation by the histone demethylase KDM6A. PLoS Genet. 2013, 9, e1003489. [Google Scholar] [CrossRef]
- Skakkebæk, A.; Nielsen, M.M.; Trolle, C.; Vang, S.; Hornshoj, H.; Hedegaard, J.; Wallentin, M.; Bojesen, A.; Hertz, J.M.; Fedder, J.; et al. DNA hypermethylation and differential gene expression associated with Klinefelter syndrome. Sci Rep. 2018, 8, 13740. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Oberhoffer, F.S.; El Rahman, M.A.; Jung, A.M.; Zemlin, M.; Rohrer, T.R.; Kahraman, M.; Keller, A.; Meese, E.; Abdul-Khaliq, H. Insights from circulating microRNAs in cardiovascular entities in turner syndrome patients. PLoS ONE 2020, 15, e0231402. Available online: https://pubmed.ncbi.nlm.nih.gov/32271829/ (accessed on 2 April 2022). [CrossRef]
- Sun, Y.X.; Zhang, Y.X.; Zhang, D.; Xu, C.M.; Chen, S.C.; Zhang, J.Y.; Ruan, Y.C.; Chen, F.; Zhang, R.J.; Qian, Y.Q.; et al. XCI-escaping gene KDM5C contributes to ovarian development via downregulating miR-320a. Hum. Genet. 2017, 136, 227–239. [Google Scholar] [CrossRef]
- Álvarez-Nava, F.; Salinas, M.; Bastidas, D.; Vicuña, Y.; Racines-Orbe, M. PPARGC1A promoter DNA-methylation level and glucose metabolism in Ecuadorian women with Turner syndrome. Horm. Mol. Biol. Clin. Investig. 2020, 42, 159–165. [Google Scholar] [CrossRef]
- Rajpathak, S.N.; Vellarikkal, S.K.; Patowary, A.; Scaria, V.; Sivasubbu, S.; Deobagkar, D.D. Human 45,X fibroblast transcriptome reveals distinct differentially expressed genes including long noncoding RNAs potentially associated with the pathophysiology of Turner syndrome. PLoS ONE 2014, 9, e100076. [Google Scholar] [CrossRef]
- Zhang, R.; Hao, L.; Wang, L.; Chen, M.; Li, W.; Li, R.; Yu, J.; Xiao, J.; Wu, J. Gene expression analysis of induced pluripotent stem cells from aneuploid chromosomal syndromes. BMC Genom. 2013, 14 (Suppl. 5), S8. [Google Scholar] [CrossRef]
- Bellott, D.W.; Hughes, J.F.; Skaletsky, H.; Brown, L.G.; Pyntikova, T.; Cho, T.J.; Koutseva, N.; Zaghul, S.; Graves, T.; Rock, S.; et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature 2014, 508, 494–499. [Google Scholar] [CrossRef]
- Lleo, A.; Moroni, L.; Caliari, L.; Invernizzi, P. Autoimmunity and Turner’s syndrome. Autoimmun. Rev. 2012, 11, A538–A543. [Google Scholar] [CrossRef]
- Berglund, A.; Cleemann, L.; Oftedal, B.E.; Holm, K.; Husebye, E.S.; Gravholt, C.H. 21-hydroxylase autoantibodies are more prevalent in Turner syndrome but without an association to the autoimmune polyendocrine syndrome type I. Clin. Exp. Immunol. 2019, 195, 364–368. [Google Scholar] [CrossRef]
- Herrera, L.A.; Prada, D.; Andonegui, M.A.; Dueñas-González, A. The epigenetic origin of aneuploidy. Curr. Genom. 2008, 9, 43–50. [Google Scholar] [CrossRef]
- Tuke, M.A.; Ruth, K.S.; Wood, A.R.; Beaumont, R.N.; Tyrrell, J.; Jones, S.E.; Yaghootkar, H.; Turner, C.L.S.; Donohoe, M.E.; Brooke, A.M.; et al. Mosaic Turner syndrome shows reduced penetrance in an adult population study. Genet. Med. 2019, 21, 877–886. [Google Scholar] [CrossRef]
- Fiot, E.; Zénaty, D.; Boizeau, P.; Haignere, J.; Dos Santos, S.; Leger, J.; French Turner Syndrome Study Group. X chromosome gene dosage as a determinant of congenital malformations and of age-related comorbidity risk in patients with Turner syndrome, from childhood to early adulthood. Eur. J. Endocrinol. 2019, 180, 397–406. [Google Scholar] [CrossRef]
- Davenport, M.L. Approach to the patient with Turner syndrome. J. Clin. Endocrinol. Metab. 2010, 95, 1487–1495. [Google Scholar] [CrossRef]
- Blum, W.F.; Ross, J.L.; Zimmermann, A.G.; Quigley, C.A.; Child, C.J.; Kalifa, G.; Deal, C.; Drop, S.L.S.; Rappold, G.; Butler, G.B., Jr. GH treatment to final height produces similar height gains in patients with SHOX deficiency and turner syndrome: Results of a multicenter trial. J. Clin. Endocrinol. Metab. 2013, 98, 1383–1392. [Google Scholar] [CrossRef]
- Soriano-Guillen, L.; Coste, J.; Ecosse, E.; Leger, J.; Tauber, M.; Cabrol, S.; Nicolino, M.; Chaussain, J.L.; Carel, J.C. Adult height and pubertal growth in Turner syndrome after treatment with recombinant growth hormone. J. Clin. Endocrinol. Metab. 2005, 90, 5197–5204. [Google Scholar] [CrossRef][Green Version]
- Fiot, E.; Zenaty, D.; Boizeau, P.; Haignere, J.; Dos Santos, S.; Leger, J.; French Turner Syndrome Study Group. X-chromosome gene dosage as a determinant of impaired pre and postnatal growth and adult height in Turner syndrome. Eur. J. Endocrinol. 2016, 174, 281–288. [Google Scholar] [CrossRef]
- Pasquino, A.M.; Passeri, F.; Pucarelli, I.; Segni, M.; Municchi, G. Spontaneous pubertal development in Turner’s syndrome. J. Clin. Endocrinol. Metab. 1997, 82, 1810–1813. [Google Scholar] [CrossRef]
- Reindollar, R.H. Turner syndrome: Contemporary thoughts and reproductive issues. Semin. Reprod. Med. 2011, 29, 342–352. [Google Scholar] [CrossRef]
- Visser, J.A.; Hokken-Koelega, A.C.S.; Zandwijken, G.R.J.; Limacher, A.; Ranke, M.B.; Flück, C.E. Anti-Müllerian hormone levels in girls and adolescents with Turner syndrome are related to karyotype, pubertal development and growth hormone treatment. Hum. Reprod. 2013, 28, 1899–1907. [Google Scholar] [CrossRef]
- Mohamed, S.O.O.; Elkhidir, I.H.E.; Abuzied, A.I.H.; Noureddin, A.A.M.H.; Ibrahim, G.A.A.; Mohmoud, A.A.A. Prevalence of autoimmune thyroid diseases among the Turner Syndrome patients: Meta-analysis of cross-sectional studies. BMC Res. Notes 2018, 11, 842. [Google Scholar] [CrossRef]
- Elsheikh, M.; Wass, J.A.H.; Conway, G.S. Autoimmune thyroid syndrome in women with Turner’s syndrome—The association with karyotype. Clin. Endocrinol. 2001, 55, 223–226. [Google Scholar] [CrossRef]
- Mortensen, K.H.; Cleemann, L.; Hjerrild, B.E.; Nexo, E.; Locht, H.; Jeppesen, E.M.; Gravholt, C.H. Increased prevalence of autoimmunity in Turner syndrome—Influence of age. Clin. Exp. Immunol. 2009, 156, 205–210. [Google Scholar] [CrossRef]
- El-Mansoury, M.; Bryman, I.; Berntorp, K.; Hanson, C.; Wilhelmsen, L.; Landin-Wilhelmsen, K. Hypothyroidism is common in turner syndrome: Results of a five-year follow-up. J. Clin. Endocrinol. Metab. 2005, 90, 2131–2135. [Google Scholar] [CrossRef]
- Livadas, S.; Xekouki, P.; Fouka, F.; Kanaka-Gantenbein, C.; Kaloumenou, I.; Mavrou, A.; Constantinidou, N.; Dacou-Voutetakis, C. Prevalence of thyroid dysfunction in Turner’s syndrome: A long-term follow-up study and brief literature review. Thyroid 2005, 15, 1061–1066. [Google Scholar] [CrossRef]
- Valenzise, M.; Aversa, T.; Corrias, A.; Mazzanti, L.; Cappa, M.; Ubertini, G.; Scarano, E.; Mussa, A.; Messina, M.F.; De Luca, F.; et al. Epidemiology, presentation and long-term evolution of Graves’ disease in children, adolescents and young adults with Turner syndrome. Horm. Res. Paediatr. 2014, 81, 245–250. [Google Scholar] [CrossRef]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef]
- Mondal, S.; Bhattacharjee, R.; Chowdhury, S.; Mukhopadhyah, S. Karyotype-Phenotype Correlation in Turner Syndrome at a Single Center in Eastern India. Indian Pediatr. 2021, 58, 34–37. [Google Scholar] [CrossRef]
- Stoklasova, J.; Zapletalova, J.; Frysak, Z.; Hana, V.; Cap, J.; Pavlikova, M.; Soucek, O.; Lebl, J. An isolated Xp deletion is linked to autoimmune diseases in Turner syndrome. J. Pediatr. Endocrinol. Metab. 2019, 32, 479–488. [Google Scholar] [CrossRef]
- De Sanctis, V.; Khater, D. Autoimmune diseases in Turner syndrome: An overview. Acta Biomed. 2019, 90, 341–344. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Juul, S.; Naeraa, R.W.; Hansen, J. Morbidity in Turner syndrome. J. Clin. Epidemiol. 1998, 51, 147–158. [Google Scholar] [CrossRef]
- Davis, S.M.; Geffner, M.E. Cardiometabolic health in Turner syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 52–58. [Google Scholar] [CrossRef]
- Lebenthal, Y.; Levy, S.; Sofrin-Drucker, E.; Nagelberg, N.; Weintrob, N.; Shalitin, S.; de Vries, L.; Tenenbaum, A.; Phillip, M.; Lazar, L. The Natural History of Metabolic Comorbidities in Turner Syndrome from Childhood to Early Adulthood: Comparison between 45,X Monosomy and Other Karyotypes. Front. Endocrinol. 2018, 9, 27. [Google Scholar] [CrossRef]
- Pirgon, Ö.; Atabek, M.E.; Oran, B.; Güçlü, R. Atherogenic lipid profile and systolic blood pressure are associated with carotid artery intima-media thickness in children with Turner syndrome. J. Clin. Res. Pediatr. Endocrinol. 2008, 1, 62–71. [Google Scholar] [CrossRef]
- O’Gorman, C.S.; Syme, C.; Lang, J.; Bradley, T.J.; Wells, G.D.; Hamilton, J.K. An evaluation of early cardiometabolic risk factors in children and adolescents with Turner syndrome. Clin. Endocrinol. 2013, 78, 907–913. [Google Scholar] [CrossRef]
- Van, P.L.; Bakalov, V.K.; Bondy, C.A. Monosomy for the X-chromosome is associated with an atherogenic lipid profile. J. Clin. Endocrinol. Metab. 2006, 91, 2867–2870. [Google Scholar] [CrossRef]
- Calcaterra, V.; Brambilla, P.; Maffè, G.C.; Klersy, C.; Albertini, R.; Introzzi, F.; Bozzola, E.; Bozzola, M.; Larizza, D. Metabolic syndrome in Turner syndrome and relation between body composition and clinical, genetic, and ultrasonographic characteristics. Metab. Syndr. Relat. Disord. 2014, 12, 159–164. [Google Scholar] [CrossRef]
- Mavinkurve, M.; O’Gorman, C.S. Cardiometabolic and vascular risks in young and adolescent girls with Turner syndrome. BBA Clin. 2015, 3, 304–309. [Google Scholar] [CrossRef]
- Hjerrild, B.E.; Holst, J.J.; Juhl, C.B.; Christiansen, J.S.; Schmitz, O.; Gravholt, C.H. Delayed β-cell response and glucose intolerance in young women with Turner syndrome. BMC Endocr. Disord. 2011, 11, 6. [Google Scholar] [CrossRef]
- Helena Mangs, A.; Morris, B.J. The Human Pseudoautosomal Region (PAR): Origin, Function and Future. Curr. Genom. 2007, 8, 129–136. [Google Scholar] [CrossRef]
- Bakalov, V.K.; Cheng, C.; Zhou, J.; Bondy, C.A. X-chromosome gene dosage and the risk of diabetes in Turner syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 3289–3296. [Google Scholar] [CrossRef]
- Wolff, D.J.; Van Dyke, D.L.; Powell, C.M. Laboratory guideline for turner syndrome. Genet. Med. 2010, 12, 52–55. [Google Scholar] [CrossRef]
- de Oliveira, R.M.R.; do Nascimento Verreschi, I.T.; Lipay, M.V.N.; Eça, L.P.; Guedes, A.D.; Bianco, B. Y chromosome in Turner syndrome: Review of the literature. Sao Paulo Med. J. 2009, 127, 373–378. [Google Scholar] [CrossRef]
- Ackermann, A.; Bamba, V. Current controversies in turner syndrome: Genetic testing, assisted reproduction, and cardiovascular risks. J. Clin. Transl. Endocrinol. 2014, 1, 61–65. [Google Scholar] [CrossRef]
- Knauer-Fischer, S.; Besikoglu, B.; Inta, I.; Kneppo, C.; Vogt, P.H.; Bettendorf, M. Analyses of Gonadoblastoma Y (GBY)-locus and of Y centromere in Turner syndrome patients. Exp. Clin. Endocrinol. Diabetes 2015, 123, 61–65. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Fedder, J.; Naeraa, R.W.; Müller, J. Occurrence of gonadoblastoma in females with Turner syndrome and Y chromosome material: A population study. J. Clin. Endocrinol. Metab. 2000, 85, 3199–3202. [Google Scholar]
- Nishi, M.Y.; Domenice, S.; Medeiros, M.A.; Mendonca, B.B.; Billerbeck, A.E.C. Detection of Y-specific sequences in 122 patients with Turner syndrome: Nested PCR is not a reliable method. Am. J. Med. Genet. 2002, 107, 299–305. [Google Scholar] [CrossRef]
- Wapner, R.J.; Martin, C.L.; Levy, B.; Ballif, B.C.; Eng, C.M.; Zachary, J.M.; Savage, M.; Platt, L.D.; Saltzman, D.; Grobman, W.A.; et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N. Engl. J. Med. 2012, 367, 2175–2184. [Google Scholar] [CrossRef]
- Mazloom, A.R.; Džakula, Ž.; Oeth, P.; Wang, H.; Jnsen, T.; Tynan, J.; McCullough, R.; Saldivar, J.S.; Ehrich, M.; van den Boom, D.; et al. Noninvasive prenatal detection of sex chromosomal aneuploidies by sequencing circulating cell-free DNA from maternal plasma. Prenat. Diagn. 2013, 33, 591–597. [Google Scholar] [CrossRef]
- Prakash, S.; Guo, D.; Maslen, C.L.; Silberbach, M.; Milewicz, D.; Bondy, C.A.; GenTAC Investigators. Single-nucleotide polymorphism array genotyping is equivalent to metaphase cytogenetics for diagnosis of Turner syndrome. Genet. Med. 2014, 16, 53–59. [Google Scholar] [CrossRef]
- Viuff, M.; Skakkebaek, A.; Nielsen, M.M.; Chang, S.; Gravholt, C.H. Epigenetics and genomics in Turner syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2019, 181, 125–132. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, H.; Zhu, W.; Xu, Y.; Wang, N.; Han, B.; Song, H.; Qiao, J. Bioinformatic Analysis Identifies Potential Key Genes in the Pathogenesis of Turner Syndrome. Front. Endocrinol. 2020, 11, 104. [Google Scholar] [CrossRef]
- Manotas, M.C.; Calderón, J.C.; López-Kleine, L.; Suárez-Obando, F.; Moreno, O.M.; Rojas, A. Identification of common differentially expressed genes in Turner (45,X) and Klinefelter (47,XXY) syndromes using bioinformatics analysis. Mol. Genet. Genom. Med. 2020, 8, e1503. [Google Scholar] [CrossRef]
- Los, E.; Rosenfeld, R.G. Growth and growth hormone in Turner syndrome: Looking back, looking ahead. Am. J. Med. Genet. Part. C Semin. Med. Genet. 2019, 181, 86–90. [Google Scholar] [CrossRef]
- Juloski, J.; Dumančić, J.; Šćepan, I.; Lauc, T.; Milašin, J.; Kaic, Z.; Dumic, M.; Babic, M. Growth hormone positive effects on craniofacial complex in Turner syndrome. Arch. Oral Biol. 2016, 71, 10–15. [Google Scholar] [CrossRef]
- Dyrka, K.; Rozkiewicz, N.; Obara-Moszynska, M.; Niedziela, M. The influence of growth hormone therapy on the cardiovascular system in Turner syndrome. J. Pediatr. Endocrinol. Metab. 2020, 33, 1363–1372. [Google Scholar] [CrossRef]
- Quigley, C.A.; Fechner, P.Y.; Geffner, M.E.; Eugster, E.A.; Ross, J.L.; Habiby, R.L.; Ugrasbul, F.; Rubin, K.; Travers, S.; Antalis, C.J.; et al. Prevention of Growth Failure in Turner Syndrome: Long-Term Results of Early Growth Hormone Treatment in the “Toddler Turner” Cohort. Horm. Res. Paediatr. 2021, 94, 18–35. [Google Scholar] [CrossRef]
- Stevens, A.; Murray, P.; Wojcik, J.; Raelson, J.; Koledova, E.; Chatelain, P.; Clayton, P.; PREDICT Investigator Group. Validating genetic markers of response to recombinant human growth hormone in children with growth hormone deficiency and Turner syndrome: The PREDICT validation study. Eur. J. Endocrinol. 2016, 175, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Braz, A.F.; Costalonga, E.F.; Trarbach, E.B.; Scalco, R.C.; Malaquias, A.C.; Guerra-Junior, G.; Antonini, S.R.R.; Mendonca, B.B.; Arnhold, I.J.P.; Jorge, A.A.L. Genetic predictors of long-term response to growth hormone (GH) therapy in children with GH deficiency and Turner syndrome: The influence of a SOCS2 polymorphism. J. Clin. Endocrinol. Metab. 2014, 99, E1808–E1813. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cheng, F.; Xiu, L. Height outcome of the recombinant human growth hormone treatment in Turner syndrome: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, E1808–E1813. [Google Scholar] [CrossRef] [PubMed]
- Sas, T.C.J.; Gault, E.J.; Zeger Bardsley, M.; Menke, L.A.; Freriks, K.; Perry, R.J.; Otten, N.J.; Keizer-Schrama, S.M.; Timmers, H.; Wit, J.M.; et al. Safety and efficacy of oxandrolone in growth hormone-treated girls with Turner syndrome: Evidence from recent studies and recommendations for use. Horm. Res. Paediatr. 2014, 81, 289–297. [Google Scholar] [CrossRef]
- Cintron, D.; Rodriguez-Gutierrez, R.; Serrano, V.; Latortue-Albino, P.; Erwin, P.J.; Murad, M.H. Effect of estrogen replacement therapy on bone and cardiovascular outcomes in women with turner syndrome: A systematic review and meta-analysis. Endocrine 2016, 55, 366–375. [Google Scholar] [CrossRef]
- Klein, K.O.; Rosenfield, R.L.; Santen, R.J.; Gawlik, A.M.; Backeljauw, P.F.; Gravholt, C.H.; Sas, T.C.J.; Mauras, N. Estrogen Replacement in Turner Syndrome: Literature Review and Practical Considerations. J. Clin. Endocrinol. Metab. 2018, 103, 1790–1803. [Google Scholar] [CrossRef]
- Viuff, M.H.; Berglund, A.; Juul, S.; Andersen, N.H.; Stochholm, K.; Gravholt, C.H. Sex Hormone Replacement Therapy in Turner Syndrome: Impact on Morbidity and Mortality. J. Clin. Endocrinol. Metab. 2020, 105, 468–478. [Google Scholar] [CrossRef]
- Cameron-Pimblett, A.; Davies, M.C.; Burt, E.T.; Talaulikar, V.S.; La Rosa, C.; King, T.F.J.; Conway, G.S. Effects of Estrogen Therapies on Outcomes in Turner Syndrome: Assessment of Induction of Puberty and Adult Estrogen Use. J. Clin. Endocrinol. Metab. 2019, 104, 2820–2826. [Google Scholar] [CrossRef]
- Lunding, S.A.; Aksglaede, L.; Anderson, R.A.; Main, K.M.; Juul, A.; Hagen, C.P.; Pedersen, A.T. AMH as Predictor of Premature Ovarian Insufficiency: A Longitudinal Study of 120 Turner Syndrome Patients. J. Clin. Endocrinol. Metab. 2015, 100, E1030–E1038. [Google Scholar] [CrossRef]
- Quigley, C.A.; Wan, X.; Garg, S.; Kowal, K.; Cutler, G.B., Jr.; Ross, J.L. Effects of low-dose estrogen replacement during childhood on pubertal development and gonadotropin concentrations in patients with Turner syndrome: Results of a randomized, double-blind, placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2014, 99, E1754–E1764. [Google Scholar] [CrossRef]
- Ross, J.L.; Roeltgen, D.; Feuillan, P.; Kushner, H.; Cutler, G.B., Jr. Effects of estrogen on nonverbal processing speed and motor function in girls with Turner’s syndrome. J. Clin. Endocrinol. Metab. 1998, 83, 3198–3204. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.L.; Roeltgen, D.; Feuillan, P.; Kushner, H.; Cutler, G.B., Jr. Use of estrogen in young girls with Turner syndrome: Effects on memory. Neurology 2000, 54, 164. [Google Scholar] [CrossRef] [PubMed]
- Torres-Santiago, L.; Mericq, V.; Taboada, M.; Unanue, N.; Klein, K.O.; Singh, R.; Hossain, J.; Santen, R.J.; Ross, J.L.; Mauras, N. Metabolic effects of oral versus transdermal 17β-estradiol (E₂): A randomized clinical trial in girls with Turner syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, K.; Dabrh, A.M.A.; Benkhadra, K.; Al Nofal, A.; Carranza Leon, B.G.; Prokop, L.J.; Montori, V.M.; Faubion, S.S.; Hurad, M.H. Oral vs Transdermal Estrogen Therapy and Vascular Events: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2015, 100, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Shifren, J.L.; Gass, M.L.S.; NAMS Recommendations for Clinical Care of Midlife Women Working Group. The North American Menopause Society recommendations for clinical care of midlife women. Menopause 2014, 21, 1038–1062. [Google Scholar] [CrossRef] [PubMed]
- Hovatta, O. Ovarian function and in vitro fertilization (IVF) in Turner syndrome. Pediatr. Endocrinol. Rev. 2012, 9 (Suppl. 2), 713–717. [Google Scholar] [PubMed]
- Oktay, K.; Bedoschi, G.; Berkowitz, K.; Bronson, R.; Kashani, B.; McGovern, P.; Pal, L.; Quinn, G.; Rubin, K. Fertility Preservation in Women with Turner Syndrome: A Comprehensive Review and Practical Guidelines. J. Pediatr. Adolesc. Gynecol. 2015, 29, 409–416. [Google Scholar] [CrossRef]
- Kavoussi, S.K.; Fisseha, S.; Smith, Y.R.; Smith, G.D.; Christman, G.M.; Gago, L.A. Oocyte cryopreservation in a woman with mosaic Turner syndrome: A case report. J. Reprod. Med. 2008, 53, 223–226. [Google Scholar]
- Oktay, K.; Bedoschi, G. Oocyte cryopreservation for fertility preservation in postpubertal female children at risk for premature ovarian failure due to accelerated follicle loss in Turner syndrome or cancer treatments. J. Pediatr. Adolesc. Gynecol. 2014, 27, 342–346. [Google Scholar] [CrossRef]
- Oktay, K.; Rodriguez-Wallberg, K.A.; Sahin, G. Fertility preservation by ovarian stimulation and oocyte cryopreservation in a 14-year-old adolescent with Turner syndrome mosaicism and impending premature ovarian failure. Fertil. Steril. 2010, 94, 753.e15–753.e19. [Google Scholar] [CrossRef]
- Nawroth, F.; Schüring, A.N.; von Wolff, M. The indication for fertility preservation in women with Turner syndrome should not only be based on the ovarian reserve but also on the genotype and expected future health status. Acta Obstet. Gynecol. Scand. 2020, 99, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).