Overview of Nutraceuticals and Cardiometabolic Diseases following Socio-Economic Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Burden of Disease
3.1.1. Burden of CVD
3.1.2. Burden of MetS
3.1.3. Burden of T2DM
3.1.4. Burden of NAFLD
3.2. Nutraceuticals
3.2.1. Vitamin D
- RCTs regarding the relationship between vitamin D and type II diabetes: five out of 18 trials (Nilas et al. [52], Pittas et al. [51], von Hurts et al. [53], Hsia et al. [54], de Boer et al. [55]) found that vitamin D had no effect on FBG (−0.002 mmol/L vitamin D vs. placebo). In a subgroup analysis of participants with impaired FBG at baseline, a combined administration of vitamin D and calcium carbonate (700 iu/day and 500 mg/day) resulted in attenuated FBG (Pittas et al. [51]).
- RCTs that analyzed outcomes concerning hypertension: two studies reported how supplementation with vitamin D brought about a positive lowering of SBP of −7 mmHg and −14 mmHg, respectively (Pfeifer et al. [56], and Sugden et al. [57]). In a study (Margolis et al. [58]) with 7 years of follow up, supplementation of vitamin D and calcium carbonate (400 iu/day and 1000 mg/day) had no effect on the improvement of parameters related to hypertension. In all other studies, there was no significant improvement in SBP and DBP, either with vitamin D supplement alone or in combination with calcium.
- RCTs analyzing the relationship between vitamin D and cardiovascular disease: five studies (Hsia et al. [54], Trivedi et al. [59], Brazier et al. [60], LaCroix et al. [61], Price et al. [62]) analyzed the effect of vit-amin D with or without calcium on various cardiovascular outcomes including myocardial infarction, stroke, and other cardiac and cerebrovascular outcomes. In patients’ follow-up period (from 1 year to 5–7 years), no study showed any statistically significant effect. Another systematic review, the one conducted by Dolinsky et al. [63], aimed to investigate the effects of vitamin D on young children in health or affected by type II diabetes. The 35 studies analyzed in the study evaluated the relationship between vitamin D and some cardiometabolic biomarkers. We therefore found relationships between:
- -
- vitamin D and arterial stiffness: A study (Dong et al. [64]) that involved the randomized administration of 400 IU/day (control group) or 2000 IU/day (experimental group) of vitamin D for 16 weeks to black adolescents and the subsequent measurement of the femoral carotid value pulse found an increase in the latter in the first group (from 5.38 to 5.71 m/s), thus indicating a worsening of the arterial stiffness, while in the second group the value decreased (5.41 to 5.33 m/s).
- -
- Vitamin D and endothelial dysfunction: This relationship was evaluated only in one study, which however did not find any correlation worthy of note.
- -
- Vitamin D and BP: Out of a total of 16 studies evaluating this parameter, 3 (Dong et al. [65], Ashraf et al. [66], Pirgon et al. [67]) found no significant changes in SBP or DBP. 10 (Ganji et al. [68], Ashraf et al. [66], Williams et al. [69], Kumar et al. [70], Reis et al. [71], Dong et al. [65], Pacifico et al. [72], Al-Daghri et al. [73], Nsiah-Kumi et al. [74], Zhou et al. [75]) found an inverse correlation between SBP and 25(OH)D, while four (Kumar et al. [70], Al-Daghri et al. [73], Nsiah-Kumi et al. [74], Sharma et al. [76]) found an inverse correlation between DBP and 25(OH)D.
- -
- Vitamin D and lipid levels: Of 22 studies evaluating this correlation, one (Ashraf et al. [66]) found a positive correlation between vitamin D and a decrease in LDL cholesterol, while a second study (Boucher-Berry et al. [77]) found a negative relationship. Between vitamin D and HDL, out of six studies, five (Ganji et al. [68], Williams et al. [69], Kumar et al. [70], Smotkin-Tangorra et al. [78], Johnson et al. [79]) demonstrated an increase in HDL concomitant with the administration of vitamin D. Of eight studies evaluating the correlation with the level of triglycerides, six found an inverse correlation between levels of vitamin D and TG. Finally, of 18 studies evaluating the relationship between vitamin D and total cholesterol, only two studies (Kumar et al. [70], Delvin et al. [80]) actually found a positive correlation.
- -
- Vitamin D, glucose, and insulin metabolism: This association was evaluated in 30 studies. Nunle Bland et al. [81], study found a correlation between HOMA and 25(OH)D, while Olson et al. [82], an inverse correlation. Another study (Pirgon et al. [67]) proposed to hire 87 obese children with or without non-alcoholic Fatty Liver Disease (NAFLD) for the evaluation. An association was found between HOMA and 25(OH)D in the group of obese children with concomitant manifestation of NAFLD, but not in the group not affected by NAFLD. Two studies (Reis et al. [71], Johnson et al. [79]) showed that as the concentration of endogenous vitamin D increased, FBG decreased (of which one of 0.09% for 2.5 noml/L). Out of 17 studies, eight found an inverse relationship between 25(OH)D and FBG and one a positive relationship. Of eight studies evaluating the relationship between 25(OH)D and HbA1c, only one (Williams et al. [69]) resulted in a positive finding. Finally, among the 12 studies evaluating the correspondence between HOMA and 25(OH)D, just over half (7) noted an inverse relationship. The 16 studies analyzed by Kunutsor et al. [83] provided for a variable administration of vitamin D2 ergocalciferol or D3 cholecalciferol with a range of doses between 800 and 8571 IU/day, with an average of 600 IU/day, involving a minimum of 34 subjects up to a maximum of 438, including both healthy individuals and obese, with type II diabetes or hypertension. The main evaluated outcome was BP, calculated in the levels of SBP and DBP. The duration of the studies ranged from 5 weeks to 12 months. No significant results were found for the desired outcomes of SBP or DBP, but a subgroup of analyses, including only patients with a history of previous cardiometabolic disorders, showed a significant and positive lowering of DBP (−0.34 mmHg). Alkharfy et al. [84] conducted a study with a total duration of 12 months involving 499 Saudi women and men, with or without T2DM, divided into eight groups: 151 subjects without type 2 diabetes mellitus T2DM, called “control group”; 49 diabetic subjects treated with oral hypoglycemic agent rosiglitazone; 15 subjects with diabetes subjected to a particular diet; 55 diabetic subjects who were only given insulin; 12 diabetic subjects who were given insulin in combination with other oral drugs; 121 diabetic patients who received metformin; 37 diabetics undergoing a combined administration of oral hypoglycemic agents; and 59 diabetic subjects taking sulfonylureas. All these subjects were also given 2000 IU/day vitamin D, except for non-T2DM, who, however, were encouraged to expose themselves to sunlight, known to be a beneficial factor for increasing endogenous vitamin D levels via termoisomerization of provitamin D3. The results of this study were as follows: the levels of 25(OH)D naturally increased, except for the dietary group and the group with a combination of oral agents; BMI levels did not show noteworthy changes; SBP levels decreased only in males who were given insulin in combination with oral agents and in the group of women and men who were combined with oral agents; DBP was increased in the rosiglitazone-taking group and in males treated with insulin plus oral agents and in the group undergoing treatment with a combination of oral agents; as far as lipid levels are concerned, the average cholesterol levels improved both in males and females receiving insulin plus oral agents and in females with single insulin administration. A lowering of TG levels was noted in the rosiglitazone-taking group, as well as in the groups receiving insulin plus oral agents and in males receiving a combination of oral agents only. Finally, the non-T2DM control group saw its HDL levels increase.
3.2.2. Vitamin K
3.2.3. Omega-3 Polyunsaturated Acids
- -
- omega-3 (OI3) levels: At the sixth week, the level of omega-3 index rose in the group of patients taking Almega PL by 5.51 ± 1.05%, while after 12 weeks the values were found to be 5.75 ± 0.90%; moreover, the levels of EPA and DPA also saw an increase.
- -
- Cardiometabolic markers: Total cholesterol (TC) levels decreased by 3% and VLDL by 25%. No difference was found in the levels of HDL, LDL and triglycerides.
- -
- Anthropometric measures: The administration of Almega PL for 12 weeks led to a significant reduction in BW and hip circumference.
- -
- Inflammation markers: No difference between the intervention group and patients taking placebo was found.
3.2.4. Polyphenols
3.2.5. Bergamot Flavonoids
3.2.6. Probiotics
- -
- FBG: of the six studies that evaluated it, five found a decrease in FBG, while one (Asemi et al. [142]) did not, but in general there were no significant results.
- -
- -
- -
- -
- -
- -
- LDL: of four studies evaluating this parameter, only 1 (Asemi et al. [142]) found a lowering of LDL levels.
- -
- -
- Levels of C reactive protein (CRP): only two out of four studies showed a significant decrease in this value after taking probiotics; however, the overall effect was found to be insignificant (SMD: −1.73).
3.2.7. Prebiotics
- -
- BW: Of five trials evaluating this parameter, two (Genta et al. [150], Parnell et al. [151]) demonstrated a significant reduction, while the other three (Dehghan et al. [152], De Luis et al. [153], Seidel et al. [154]) showed no effect after administration of prebiotic products. In general, however, it can be stated that, in this study, no significant general values of BW reduction (SMD: −0.48) were found; glucose homeostasis: of four studies measuring the effect of prebiotics on postprandial glucose levels, only two found a significant reduction in blood glucose levels in obese and overweight patients (Cani et al. [155], Dewulf et al. [156]). After the meta-analysis, the SMD turned out to be −0.76, which indicates a significant effect of supplementation with prebiotics. Two of three studies found a reduction in postprandial insulin levels in overweight and hypercholesterolemic individuals. The SMD index for this value turned out to be −0.77. A reduction in HbA1c was found in healthy patients after 5 weeks of supplementation (Russo et al. [157]), and in women with type II diabetes after 8 weeks (Dehghan et al. [152]), while no reduction in HbA1c in obese women was found in a third study after prebiotic supplementation for 3 months (Dewulf et al. [156]).
- -
- Cardiovascular and hepatic outcomes: No significant evidence of a reduction in LDL cholesterol or lipid levels.
- -
- Outcomes evaluating the degree of inflammation: Out of four studies evaluating C reactive protein (biomarker inflammation), three (Dehghan et al. [152], De Luis et al. [153], Vulevic et al. [158]) noted a significant reduction in this parameter in obese and overweight adults, and women with type II diabetes. The results were considered non-significant when a -0.85 SMD was found.
3.2.8. Nutraceuticals Main Findings
4. Discussion
- -
- Antidiabetic properties: Phloridzin has been shown to have properties capable of improving dyslipidemia and decreasing the level of glucose in the blood [200], as well as a reduction in beta cells that usually lead to insulin resistance, and an improvement in hyperglycemia [201]. In addition, phloridzin, in a study by Chai et al. [202], was shown to lower BW and decrease FBG and TG; it also improved the levels of the enzyme glucokinase in the liver [203]. In general, the phenols derived from apples inhibit the sodium/glucose co-transporter in the intestine and kidney and consequently decrease the renal reabsorption of glucose.
- -
- Cardioprotective effects: Phenols present in apples have been found to have a lowering effect on TC levels, which naturally leads to a lowering of the risk of developing CVD. They also decrease LDL levels by limiting oxidation processes [204,205]. Chai et al. [202] found lower total and LDL cholesterol levels in postmenopausal women who consumed apples daily. In particular, the flavonoid phloretin limits the expression of TNF alpha in a dose-dependent manner (1–100 micromol/L) [206]. The systematic review by Hausenblas et al. [99] saw the polyphenol resveratrol as the subject. The action mechanism of resveratrol, a polyphenol found in red wine, tea, berries, blueberries, pomegranates and nuts is as follows: it activates the SIRT1 receptor (sirtuin family of transcription factors), whose main function is to regulate the energy metabolism and homeostasis of mitochondria, and the AMP-dependent protein kinase (AMPK), that present with a lipid-lowering effect, essential in the regulation of diabetes [207,208,209,210] and for a contrasting effect on obesity, promoting lipolysis, and inhibiting lipogenesis [211]. These biochemical signals are also activated by exercise and a decrease in caloric intake, which, in their turn, are associated with a decrease in the risk of developing type II diabetes, NAFLD and cardiometabolic risks and are also the target of antidiabetic drugs, such as metformin [212]. Therefore, resveratrol could lead to improvement in antidiabetic therapy, and may be able, in the future, to replace metformin use or improve adherence to this therapy, representing a new therapeutic choice able to reduce the costs of direct drugs spent on antidiabetic treatment. Resveratrol has also shown to improve blood flow and vascular endothelial function [213,214]. Concretely, in this study, the most significant results to be found were: an increase in HDL levels, and reduction in SBP and in the levels of glycated hemoglobin HbA1c (high in diabetic subjects, symptom of an imbalance in glycemic levels) and creatinine, the high rates of which are indicative of nephropathy. In two studies analyzed by Bocellino et al. [105], resveratrol was instead shown to be effective in reducing TG levels, BFM, and WC indices. Resveratrol can also be useful in reducing TC, LDL, and HOMA-IR levels in NAFLD patients. Flavonols are a subtype of flavonoids that can be found in onions, spinach, asparagus, and some berries. A very important type of flavonols is quercetin [215,216], found mainly in vegetables such as onions, garlic and ginger, apples, and wine. Its mechanism of action has been hypothesized to be the following: it acts by decreasing BP through an improvement in endothelial function, an action on the renin angiotensin aldosterone system, and a down regulation of sodium channels in the kidneys [217,218]. Quercetin was also the object of evaluation of two studies analyzed by Boccellino et al. [105], in which the administration of 150 mg/day–162 mg/day for 8–6 weeks was found to be effective in lowering TG and WC levels in overweight or obese subjects; moreover, in a study carried out by Amiot et al. [116], quercetin was found to be effective in decreasing the levels of SBP, DBP, and TNF-alpha. A meta-analysis conducted by Menezes et al. [104] analyzed the effects of flavonols relative to cardiometabolic biomarkers: TG, TC, SBP, DBP, FBG, and LDL levels decreased, while HDL levels increased; the most interesting real finding was found in a higher incidence of results, that turned out to be more significant when they included an Asian population and patients with a diagnosis of disease rather than healthy ones. Curcumin is the most abundant polyphenol present in Curcuma longa [105]. It has been shown to have properties that in five studies analyzed led to a lowering of the levels of TG, pro-inflammatory interleukin 4 (IL-4) and interleukin 1-beta (IL-1-beta) in individuals suffering from obesity; it also led to a decrease in WC levels and an increase in HDL lipoproteins. Additionally, in NAFLD patients, supplementation with curcumin was associated with reduction in BMI, WC, TG, LDL, FBG, HOMA-IR Grape polyphenols have shown to be effective in reducing the levels of HOMA-IR, TC, and LDL, but, above all, of insulin in subjects at high risk of developing cardiometabolic disease [115]. One of the possible mechanisms proposed could be the interaction of grape pomace polyphenols with the insulin receptor, thus decreasing the levels of phosphorylated serine, preventing the inactivation of glycogen synthase kinase, and increasing the levels of the receptor for the proliferation of peroxisomes (gamma PPAR). Finally, a high number of polyphenols of different derivations were analyzed in relation to some changes that they implemented in cardiometabolic biomarkers related to obesity, SBP, DBP, dyslipidemia, glycemic levels, insulin resistance, oxidative stress, inflammation, and cardiovascular dysfunctions. Regarding obesity, the use of green tea, rich in catechins, in particular in epigallocatechin 3-O gallate and 5-O-galloylquinic acid, is noteworthy [219]. Catechin-polyphenols act by inhibiting the degradation of cAMP via a phosphodiesterase, as well as inhibiting catechol-O-methyltransferase, which would normally degrade noradrenaline. Furthermore, these green tea polyphenols could stimulate the catabolic process of energy consumption by the cells, which would lead to a decrease in body weight intake and expression of fatty acid synthesis. They have been shown to be effective in decreasing TG and HDL levels, and BW, BMI, and WC. Improvement in these anthropometric biomarkers could be useful in the prevention of obesity manifestation and metabolic disfunction, eventually protecting the subjects at high risk of developing MetS complications and leading, consequently, to a reduction in economic burden of metabolic syndrome. Regarding blood glucose and insulin resistance, this study by Amiot et al. [116] proposed a mechanism of action of polyphenols, which is expressed through: inhibition of glucose uptake via SGLT1 (intestinal sodium glucose transporter), protection of pancreatic beta cells from glucotoxicity, suppression of glucose release from liver, and also through the improvement of glucose uptake via the GLUT4 transporter [220]. Their relationship with oxidative stress and vascular dysfunctions is also being evaluated: polyphenols have an antioxidant effect since they reduce, as previously mentioned, the formation of reactive oxygen species produced in the mitochondria, by NADPH oxidase and NO synthase [221] and increase the production of vasodilator substances such as NO and endothelium-derived hyperpolarizing factor by stimulating AMP kinase and preventing ROS degradation of NO by reducing NADPH oxidase gene expression [221]. Bergamot, or citrus bergamia has a high content of flavonoids (neoeriocitrin, neohesperidin, naringin, rutin, neodesmin, rhoifolin, and poncirin) [194,222]. In particular, three flavonoids extracted from bergamot peel, with the acronym HMGF 3-hydroxy-3-methylglutaryl falavanones (brutieridin, melitidin and neoeriocitrin), have been shown to own activities that mimic the effects of a statin, simvastatin. In fact, both simvastatin and HMGF lower the total level of circulating cholesterol, LDL cholesterol, and raise HDL [223]. HMGFs act by inhibiting HMG-CoA reductase, which leads to a reduction in the formation of cholesterol esters. There is therefore an alternative therapy for the treatment of dyslipidemia and CVD’s complications in those patients who experience adverse effects after taking statins, such as myalgia, myopathy, rhabdomyolysis or liver damage, i.e., products of nutraceutical derivation containing bergamot extracts (object of three clinical studies covered by the paper by Giglio et al. [134]). This is a very important aspect, because the use of bergamot flavonoids in subjects not undergoing statin-lowering cholesterol therapy could lead to an improvement in terms of therapy adherence and, consequently, to a reduction in CVD treatment’s annual costs. These HMGF flavonoids can lower blood glucose and lipid levels [137] through an increase in their consumption by an activation of mitochondrial oxidation, a decrease in VLDL, and an increase in the transcription of the LDL receptor via PKC and gamma PPAR, thus reducing the risk of CVD development [224]. A product based on flavonoids extracted from bergamot (Bergavit) has been shown to be effective in reducing TC and TG levels, but also in anthropometric outcomes through some proposed action mechanisms [138]:
- -
- -
- Activation of protein kinase C (PKC), which, through some gene transcription pathways, leads to the sequestration of circulating LDL.
- -
- Activation of the receptor activating the proliferation of peroxisomes (PPAR) which also sequesters LDL. Another flavanone-based product (subgroup of flavonoids) of bergamot hesperidin and eriocitrin, called CitraVes was analyzed by Raimondo et al. [139] to understand the effects on TC levels and WC. The aglycones of hesperidin, naringenin and hesperetin have been shown to be effective in inhibiting the enzyme acylCoA cholesterol acyltransferase (ACAT) and the microsomal transfer protein, known to be responsible for the synthesis of cholesterol and its esterification in the liver. Consequently, they bring about a reduction in the levels of VLDL and LDL lipoproteins [231]. In addition, hesperidin also has antioxidant and anti-inflammatory activities, while erythrocin can lead to the reduction in LDL and protection from metabolic disorders and an increase in adipose tissue [232,233]. Probiotics are defined as live and vital microorganisms that confer health benefits to the host when consumed, able to reach the intestine, multiply there, and exert a physiological balance action on the bacterial microflora. They must be safe for use in humans and provide at least 109 live cells per day. The intestinal bacterial flora performs functions aimed at maintaining the wellbeing of the host organism:
- -
- stimulates the development of the immune system;
- -
- forms a barrier that protects us from attack by pathogens or viruses (through mechanisms of antagonism for the competition of nutrients and for the attachment sites to the intestinal epithelium);
- -
- intervenes in the digestion processes;
- -
- participates in the synthesis of vitamins;
- -
- promotes the absorption of calcium, magnesium, and iron. The complex of microbes, bacteria, viruses, and archaea, called “Intestinal microbiota”, is very important for our health; if altered, as for example in the case of endotoxemia, it can cause the spread of Gram-bacteria through the intestinal mucosa and throughout circulation causing inflammation [234], leading to conditions like obesity, diabetes, non-alcoholic fatty liver disease, and arteriosclerosis [234,235,236]. The intestinal microbiota can also be altered by drugs such as antibiotics but also by other factors such as advancing age, incorrect diet, and genetic predisposition of the host [237,238]. Therefore, to restore the correct intestinal flora, probiotic products can be used, as they have been shown to be effective in improving the barrier function of the intestinal epithelium, thus preventing the microbiota from passing into the circulation [239,240]. Probiotic supplementation had a beneficial effect on the lipid profile in obese post-menopausal women, who were given two different doses of Ecologic barrier, a multi-species probiotic product. The administration of the higher dose (1 × 1010 CFU per day) resulted in a greater reduction in glucose, insulin, and HOMA-IR levels when compared with administration of the lower dose. The benefits were therefore dose dependent. In general, however, both doses had significant effects in reducing TC and LDL lipoprotein levels. It was also noted that individuals with type II diabetes have fewer bacteria producing butyric acid, a short-chain fatty acid [241,242,243] which serves as a substrate in gluconeogenesis, lipogenesis, and modulation of expression of some genes [244]. Butyric acid binds to a G protein coupled receptor and brings some beneficial effects, such as the regulation of glucagon-like peptide 1, as also happens for probiotics, associated with an improvement in insulin excretion and therefore a lowering glucose level [245]. It has also a trophic action on the mucous membranes of the intestine, stabilizing their turnover and thus exerting a protective effect against the onset of colon cancer. Probiotics, like this acid, have the task of preventing the influx of pro-inflammatory cytokines from the intestine to the bloodstream [245,246]. Therefore, a correct balance of the intestinal microbiota obtained by administering probiotics is of fundamental importance for a regular maintenance of glucose, lipid, and protein metabolism. In this meta-analysis [141], probiotics were found to be effective in having a lowering effect on HbA1c, HOMA-IR and lowering glucose levels in patients with type 2 diabetes mellitus. According to Einarson et al. [8], for the year 2040 the number of people with diabetes would increase to 642 million; to face this enormous number, indicating a future announced global disease, it will be very important to witness the ability of probiotics in the prevention of diabetes type II. Additionally, probiotics could be used alongside classic diabetic therapy (thanks to their ability of mimic butyric acid action) such as metformin, sulfonylureas, glitazones, in order to assist their action. When Gram-bacteria, normally found in our intestinal microbiota, end up in the bloodstream, they cause, as stated above, metabolic endotoxemia [247]. In fact, they stimulate the production of pro-inflammatory cytokines by macrophages and reactive oxygen species, which can cause systemic inflammation, insulin resistance, and weight gain [248,249]. It is possible to stimulate the growth of microorganisms that bring benefits to the patient, preventing the passage of bacteria such as the aforementioned Gram- from our intestinal bacterial flora to the systemic circulation, through the intake of prebiotics, defined as non-digestible but fermentable carbohydrates deriving from plants that act as a fermentation substrate in the colon for those intestinal microbes that confer benefits to the patient’s health [250]. They therefore help to develop the intestinal bacterial flora already present in our body. In the case of concomitant or previous intake of probiotics, prebiotics assist in their growth, development, and action. Some carbohydrates with a prebiotic effect are insulin-type fructans (inulin, oligofructose and fructo-oligosaccharides) and galactans (galactooligosaccharides), which stimulate the production of bifidobacteria and lactic acid-producing lactobacilli [251]. The intestinal microbiota plays a fundamental role in the development of the host’s immune system, modulation of inflammatory processes, in the regulation of glucose and lipid metabolism, in the production of vitamins and in the regulation of intestinal permeability [247,252,253]. For these reasons, therefore, a prebiotic supplement that is capable of favoring the intestinal bacterial flora could represent a therapeutic strategy for the prevention and treatment of metabolic diseases. The fermentation of prebiotic bacteria in the colon also leads to the production of short-chain fatty acids (SCFAs): acetate, propionate, and butyrate (already addressed previously). SCFAs have an important role in maintaining intestinal health and in modulating metabolic and immune processes. In fact, they have two G proteins in the intestine, stimulating the secretion of energy peptides, that is the YY and GLP-1 peptides, hormones that reduce the level of appetite, increase insulin sensitivity, and eliminate gastric emptying. SCFA propionate appears positive in the liver to inhibit cholesterol synthesis by altering a key enzyme [254]. However, in the systematic review conducted by Kellow et al. [149], contradictory and insignificant results were found on the reduction in TC, LDL, and HDL.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Waard, A.-K.M.; Wändell, P.; Holzmann, M.J.; Korevaar, J.C.; Hollander, M.; Gornitzki, C.; De Wit, N.J.; Schellevis, F.G.; Lionis, C.; Søndergaard, J.; et al. Barriers and facilitators to participation in a health check for cardiometabolic diseases in primary care: A systematic review. Eur. J. Prev. Cardiol. 2018, 25, 1326–1340. [Google Scholar] [CrossRef] [PubMed]
- Mutowo, M.P.; Owen, A.J.; Billah, B.; Lorgelly, P.K.; Gumbie, K.E.; Mangwiro, J.C.; Renzaho, A.M. Burden at-tributable to Cardiometabolic Diseases in Zimbabwe: A retrospective cross-sectional study of national mortality data. BMC Public Health 2015, 15, 1213. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Li, B.-L.; Yang, L.; Zhou, D.; Ding, K.-X.; Yan, J.; Gao, Y.-J.; Huang, X.-R.; Zheng, X.-P. Cardiometabolic diseases, frailty, and healthcare utilization and expenditure in community-dwelling Chinese older adults. Sci. Rep. 2021, 11, 7776. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85, Erratum in Eur. Heart J. 2020, 41, 4507. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 2020, 139, e56–e528, Erratum in Circulation 2020, 141, e33. [Google Scholar] [CrossRef]
- Rao, G.H. Prevention or reversal of cardiometabolic diseases. J. Clin. Prev. Cardiol. 2018, 7, 22–28. [Google Scholar] [CrossRef]
- Rao, G.H. Cardiometabolic Diseases, Risk Stratification, and Management. EC Endocrinol. Metab. Res. 2020, 5, 1–8. [Google Scholar]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Economic Burden of Cardiovascular Disease in Type 2 Diabetes: A Systematic Review. Value Health 2018, 21, 881–890. [Google Scholar] [CrossRef]
- Wong, N.D. Metabolic Syndrome. Am. J. Cardiovasc. Drugs 2007, 7, 259–272. [Google Scholar] [CrossRef]
- Boudreau, D.; Malone, D.; Raebel, M.; Fishman, P.; Nichols, G.; Feldstein, A.; Boscoe, A.; Ben-Joseph, R.; Magid, D.; Okamoto, L. Health Care Utilization and Costs by Metabolic Syndrome Risk Factors. Metab. Syndr. Relat. Disord. 2009, 7, 305–314. [Google Scholar] [CrossRef]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ novel formulations: The good, the bad, the unknown and patents involved. In Recent Patents on Drug Delivery & Formulation; Bentham Science Publishers: Sharjah, United Arab Emirates, 2019; Volume 13, pp. 105–156. [Google Scholar]
- Jardim, T.V.; Mozaffarian, D.; Abrahams-Gessel, S.; Sy, S.; Lee, Y.; Liu, J.; Huang, Y.; Rehm, C.; Wilde, P.; Micha, R.; et al. Cardiometabolic disease costs associated with suboptimal diet in the United States: A cost analysis based on a microsimulation model. PLOS Med. 2019, 16, e1002981. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Nichols, M.; Townsend, N.; Luengo-Fernandez, R.; Leal, J.; Gray, A.; Scarborough, P.; Rayner, M. European Cardiovascular Disease Statistics 2012; European Heart Network, Brussels and European Society of Cardiology, Sophia Antipolis: Brussels, Belgium, 2012. [Google Scholar]
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2017, 39, 508–579. [Google Scholar] [CrossRef]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Franco, O.H.; Massaro, J.M.; Civil, J.; Cobain, M.R.; O’Malley, B.; D’AgostinoSr, R.B. Trajectories of Entering the Metabolic Syndrome. Circulation 2009, 120, 1943–1950. [Google Scholar] [CrossRef]
- Ju, S.-Y.; Lee, J.-Y.; Kim, D.-H. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly. Medicine 2017, 96, e8491. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Lin, H.; Fu, X.; Lin, W.; Li, M.; Zeng, X.; Gao, Q. Metabolic syndrome and stroke: A meta-analysis of prospective cohort studies. J. Clin. Neurosci. 2017, 40, 34–38. [Google Scholar] [CrossRef]
- Li, J.; Flammer, A.; Lennon, R.J.; Nelson, R.E.; Gulati, R.; Friedman, P.A.; Thomas, R.; Sandhu, N.P.; Hua, Q.; Lerman, L.O.; et al. Comparison of the Effect of the Metabolic Syndrome and Multiple Traditional Cardiovascular Risk Factors on Vascular Function. Mayo Clin. Proc. 2012, 87, 968–975. [Google Scholar] [CrossRef]
- Smith, J.P.; Haddad, E.V.; Taylor, M.B.; Oram, D.; Blakemore, D.; Chen, Q.; Boutaud, O.; Oates, J.A. Suboptimal Inhibition of Platelet Cyclooxygenase-1 by Aspirin in Metabolic Syndrome. Hypertension 2012, 59, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Sala, C.; Tadic, M.; Gherbesi, E.; Grassi, G.; Mancia, G. Association of metabolic syndrome with carotid thickening and plaque in the general population: A meta-analysis. J. Clin. Hypertens. 2017, 20, 4–10. [Google Scholar] [CrossRef] [PubMed]
- van Vliet-Ostaptchouk, J.V.; Nuotio, M.-L.; Slagter, S.N.; Doiron, D.; Fischer, K.; Foco, L.; Gaye, A.; Gögele, M.; Heier, M.; Hiekkalinna, T.; et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 2014, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Scholze, J.; Alegria, E.; Ferri, C.; Langham, S.; Stevens, W.; Jeffries, D.; Uhl-Hochgraeber, K. Epidemiological and economic burden of metabolic syndrome and its consequences in patients with hypertension in Germany, Spain and Italy; a prevalence-based model. BMC Public Health 2010, 10, 529. [Google Scholar] [CrossRef]
- Sullivan, P.W.; Ghushchyan, V.; Wyatt, H.R.; Hill, J.O. The Medical Cost of Cardiometabolic Risk Factor Clusters in the United States**. Obesity 2007, 15, 3150–3158. [Google Scholar] [CrossRef]
- Nichols, G.A.; Moler, E.J. Metabolic Syndrome Components Are Associated with Future Medical Costs Independent of Cardiovascular Hospitalization and Incident Diabetes. Metab. Syndr. Relat. Disord. 2011, 9, 127–133. [Google Scholar] [CrossRef]
- Seuring, T.; Archangelidi, O.; Suhrcke, M. The Economic Costs of Type 2 Diabetes: A Global Systematic Review. Pharmacoeconomics 2015, 33, 811–831. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Solomon, C.G.; Liu, S.; Willett, W.C.; Speizer, F.E.; Manson, J.E. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch. Intern. Med. 2001, 161, 1717–1723. [Google Scholar] [CrossRef]
- Rao, G.H. The tsunami of cardiometabolic diseases: An overview. J. Diabetes Obes. Metab. Syndr. 2019, 1, 1–9. [Google Scholar]
- Tarantino, G.; Balsano, C.; Santini, S.J.; Brienza, G.; Clemente, I.; Cosimini, B.; Sinatti, G. It Is High Time Physicians Thought of Natural Products for Alleviating NAFLD. Is There Sufficient Evidence to Use Them? Int. J. Mol. Sci. 2021, 22, 13424. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. A changing paradigm for prevention of cardiovascular disease: Emergence of the metabolic syndrome as a multiplex risk factor. Eur. Heart J. Suppl. 2008, 10, B16–B23. [Google Scholar] [CrossRef][Green Version]
- Hyppönen, E.; Power, C. Vitamin D Status and Glucose Homeostasis in the 1958 British Birth Cohort. Diabetes Care 2006, 29, 2244–2246. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, A.; Shidfar, F.; Hosseinpanah, F.; Vafa, M.; Razaghi, M.; Amiri, F. Does vitamin D3 supplementation improve glucose homeostasis in overweight or obese women? A double-blind, randomized, placebo-controlled clinical trial. Diabet. Med. 2013, 30, 1477–1481. [Google Scholar] [CrossRef]
- Grethen, E.; McClintock, R.; Gupta, C.E.; Jones, R.; Cacucci, B.M.; Diaz, D.; Fulford, A.D.; Perkins, S.M.; Considine, R.V.; Peacock, M. Vitamin D and Hyperparathyroidism in Obesity. J. Clin. Endocrinol. Metab. 2011, 96, 1320–1326. [Google Scholar] [CrossRef]
- Chacko, S.A.; Song, Y.; Manson, J.E.; Van Horn, L.; Eaton, C.; Martin, L.W.; McTiernan, A.; Curb, J.D.; Wylie-Rosett, J.; Phillips, L.S.; et al. Serum 25-hydroxyvitamin D concentrations in relation to cardiometabolic risk factors and metabolic syndrome in postmenopausal women. Am. J. Clin. Nutr. 2011, 94, 209–217. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Menon, R.K.; Sharp, S.J.; Mannan, N.; Timms, P.M.; Martineau, A.R.; Rickard, A.P.; Boucher, B.J.; Chowdhury, T.A.; Griffiths, C.J.; et al. Effects of vitamin D2 or D3 supplementation on glycemic control and cardiometabolic risk among people at risk of type 2 diabetes: Results of a randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2016, 18, 392–400. [Google Scholar] [CrossRef]
- Manousopoulou, A.; Garbis, S.D.; Chrousos, G.P.; Al-Daghri, N.M. Vitamin D and cardiovascular risk among adults with obesity: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2015, 45, 1113–1126. [Google Scholar] [CrossRef]
- Wamberg, L.; Kampmann, U.; Stødkilde-Jørgensen, H.; Rejnmark, L.; Pedersen, S.B.; Richelsen, B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels—Results from a randomized trial. Eur. J. Intern. Med. 2013, 24, 644–649. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alkharfy, K.M.; Al-Saleh, Y.; Al-Attas, O.S.; Alokail, M.S.; Al-Othman, A.; Moharram, O.; El-Kholie, E.; Sabico, S.; Kumar, S.; et al. Modest reversal of metabolic syndrome manifestations with vitamin D status correction: A 12-month prospective study. Metabolism 2012, 61, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Sneve, M.; Torjesen, P.; Figenschau, Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3for 1 year. J. Intern. Med. 2010, 267, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Palacios, O.M.; Bell, M.; Toth, P.P. Use of supplemental long-chain omega-3 fatty acids and risk for cardiac death: An updated meta-analysis and review of research gaps. J. Clin. Lipidol. 2017, 11, 1152–1160.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, J.; Pande, J.N.; Bhartia, A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet. Med. 2009, 26, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Sneve, M.; Figenschau, Y.; Jorde, R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur. J. Endocrinol. 2008, 159, 675–684. [Google Scholar] [CrossRef]
- Zittermann, A.; Frisch, S.; Berthold, H.K.; Götting, C.; Kuhn, J.; Kleesiek, K.; Stehle, P.; Koertke, H.; Koerfer, R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am. J. Clin. Nutr. 2009, 89, 1321–1327. [Google Scholar] [CrossRef]
- Kamycheva, E.; Berg, V.; Jorde, R. Insulin-like growth factor I, growth hormone, and insulin sensitivity: The effects of a one-year cholecalciferol supplementation in middle-aged overweight and obese subjects. Endocrine 2012, 43, 412–418. [Google Scholar] [CrossRef]
- Pittas, A.G.; Chung, M.; Trikalinos, T.; Mitri, J.; Brendel, M.; Patel, K.; Lichtenstein, A.H.; Lau, J.; Balk, E.M. Systematic review: Vitamind D and cardiometabolic outcomes. Ann. Intern. Med. 2010, 152, 307–314. [Google Scholar] [CrossRef]
- Nilas, L.; Christiansen, C. Treatment with vitamin D or its analogues does not change body weight or blood glucose level in postmenopausal women. Int. J. Obes. 1984, 8, 407–411. [Google Scholar]
- Von Hurst, P.R.; Stonehouse, W.; Coad, J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient–a randomised, placebo-controlled trial. Br. J. Nutr. 2010, 103, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Hsia, J.; Heiss, G.; Ren, H.; Allison, M.; Dolan, N.C.; Greenland, P.; Heckbert, S.R.; Johnson, K.C.; Manson, J.E.; Sidney, S.; et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007, 115, 846–854. [Google Scholar] [CrossRef] [PubMed]
- De Boer, I.H.; Tinker, L.F.; Connelly, S.; Curb, J.D.; Howard, B.V.; Kestenbaum, B.; Larson, J.C.; Manson, J.E.; Margolis, K.L.; Siscovick, D.S.; et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care 2008, 31, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Nachtigall, D.; Hansen, C. Effects of a Short-Term Vitamin D3 and Calcium Supplementation on Blood Pressure and Parathyroid Hormone Levels in Elderly Women. J. Clin. Endocrinol. Metab. 2001, 86, 1633–1637. [Google Scholar] [CrossRef]
- Sugden, J.A.; Davies, J.I.; Witham, M.D.; Morris, A.D.; Struthers, A.D. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet. Med. 2008, 25, 320–325. [Google Scholar] [CrossRef]
- Margolis, K.L.; Ray, R.M.; Van Horn, L.; Manson, J.E.; Allison, M.A.; Black, H.R.; Beresford, S.A.A.; Connelly, S.A.; Curb, J.D.; Grimm, R.H., Jr.; et al. Effect of calcium and vitamin D supplementation on blood pressure: The Women’s Health Initiative Randomized Trial. Hypertension 2008, 52, 847–855. [Google Scholar] [CrossRef]
- Trivedi, D.P.; Doll, R.; Khaw, K.T. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: Randomised double blind controlled trial. BMJ 2003, 326, 469. [Google Scholar] [CrossRef]
- Brazier, M.; Grados, F.; Kamel, S.; Mathieu, M.; Morel, A.; Maamer, M.; Sebert, J.-L.; Fardellone, P. Clinical and laboratory safety of one year’s use of acombination calcium+ vitamin D tablet in ambulatory elderly women with vitamin D insufficiency: Results of a mlticenter, rndomized, double-blind, placebo-controlled study. Clin. Ther. 2005, 27, 1885–1893. [Google Scholar] [CrossRef]
- LaCroix, A.Z.; Kotchen, J.; Anderson, G.; Brzyski, R.; Cauley, J.A.; Cummings, S.R.; Wactawski-Wende, J. Calcium plus vitamin D supplementation and mortality in postmenopausal women: The Women’s Health Initiative calcium–vitamin D randomized controlled trial. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64, 559–567. [Google Scholar] [CrossRef]
- Prince, R.L.; Austin, N.; Devine, A.; Dick, I.M.; Bruce, D.; Zhu, K. Effects of Ergocalciferol Added to Calcium on the Risk of Falls in Elderly High-Risk Women. Arch. Intern. Med. 2008, 168, 103–108. [Google Scholar] [CrossRef]
- Dolinsky, D.H.; Armstrong, S.; Mangarelli, C.; Kemper, A.R. The Association Between Vitamin D and Cardiometabolic Risk Factors in Children. Clin. Pediatr. 2013, 52, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Stallmann-Jorgensen, I.S.; Pollock, N.K.; Harris, R.A.; Keeton, D.; Huang, Y.; Li, K.; Bassali, R.; Guo, D.-h.; Thomas, J.; et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J. Clin. Endocrinol. Metab. 2010, 95, 4584–4591. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pollock, N.; Stallmann-Jorgensen, I.S.; Gutin, B.; Lan, L.; Chen, T.C.; Keeton, D.; Petty, K.; Holick, M.F.; Zhu, H. Low 25-Hydroxyvitamin D Levels in Adolescents: Race, Season, Adiposity, Physical Activity, and Fitness. Pediatrics 2010, 125, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.P.; Alvarez, J.A.; Gower, B.A.; Saenz, K.H.; McCormick, K.L. Associations of Serum 25-Hydroxyvitamin D and Components of the Metabolic Syndrome in Obese Adolescent Females. Obesity 2011, 19, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Pirgon, O.; Cekmez, F.; Bilgin, H.; Eren, E.; Dundar, B. Low 25-hydroxyvitamin D level is associated with insulin sensitivity in obese adolescents with non-alcoholic fatty liver disease. Obes. Res. Clin. Pr. 2013, 7, e275–e283. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Zhang, X.; Shaikh, N.; Tangpricha, V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001–2006. Am. J. Clin. Nutr. 2011, 94, 225–233. [Google Scholar] [CrossRef]
- Williams, D.M.; Fraser, A.; Lawlor, D.A. Associations of vitamin D, parathyroid hormone and calcium with cardiovascular risk factors in US adolescents. Heart 2011, 97, 315–320. [Google Scholar] [CrossRef]
- Kumar, J.; Muntner, P.; Kaskel, F.J.; Hailpern, S.M.; Melamed, M.L. Prevalence and Associations of 25-Hydroxyvitamin D Deficiency in US Children: NHANES 2001–2004. Pediatrics 2009, 124, e362–e370. [Google Scholar] [CrossRef]
- Reis, J.P.; von Mühlen, D.; Miller, E.R.; Michos, E.D.; Appel, L.J. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics 2009, 124, e371–e379. [Google Scholar] [CrossRef]
- Pacifico, L.; Anania, C.; Osborn, J.F.; Ferraro, F.; Bonci, E.; Olivero, E.; Chiesa, C. Low 25 (OH) D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur. J. Endocrinol. 2011, 165, 603. [Google Scholar] [CrossRef]
- Al-Daghri, N.; Al-Attas, O.; Alokail, M.; Alkharfy, K.; Yousef, M.; Nadhrah, H.; Al-Othman, A.; Al-Saleh, Y.; Sabico, S.; Chrousos, G. Hypovitaminosis D and cardiometabolic risk factors among non-obese youth. Open Med. 2010, 5, 752–757. [Google Scholar] [CrossRef]
- Nsiah-Kumi, P.A.; Erickson, J.M.; Beals, J.L.; Ogle, E.A.; Whiting, M.; Brushbreaker, C.; Borgeson, C.D.; Qiu, F.; Yu, F.; Larsen, J.L. Vitamin D Insufficiency Is Associated With Diabetes Risk in Native American Children. Clin. Pediatr. 2011, 51, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Schechter, C.; Cai, Z.; Markowitz, M. Determinants of 25(OH)D Sufficiency in Obese Minority Children: Selecting Outcome Measures and Analytic Approaches. J. Pediatr. 2011, 158, 930–934.e1. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sharma, V.K.; Jain, R.K.; Jain, P. Vitamin D and the metabolic syndrome in Indian sub-population. J. Clin. Diag. Res. 2012, 6, 89–91. [Google Scholar]
- Boucher-Berry, C.; Speiser, P.W.; Carey, D.E.; Shelov, S.P.; Accacha, S.; Fennoy, I.; Rapaport, R.; Espinal, Y.; Rosenbaum, M.; Altshuler, L.; et al. Vitamin D, osteocalcin, and risk for adiposity as comorbidities in middle school children. J. Bone Miner. Res. 2011, 27, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Smotkin-Tangorra, M.; Purushothaman, R.; Gupta, A.; Nejati, G.; Anhalt, H.; Ten, S. Prevalence of vitamin D insufficiency in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2007, 20, 817–824. [Google Scholar] [CrossRef]
- Johnson, M.D.; Nader, N.S.; Weaver, A.L.; Singh, R.; Kumar, S. Relationships between 25-hydroxyvitamin D levels and plasma glucose and lipid levels in pediatric outpatients. J. Pediatrics 2010, 156, 444–449. [Google Scholar] [CrossRef]
- Delvin, E.E.; Lambert, M.; Levy, E.; O’Loughlin, J.; Mark, S.; Gray-Donald, K.; Paradis, G. Vitamin D status is modestly associated with glycemia and indicators of lipid metabolism in French-Canadian children and adolescents. J. Nutr. 2010, 140, 987–991. [Google Scholar] [CrossRef]
- Nunlee-Bland, G.; Gambhir, K.; Abrams, C.; Abdul, M.; Vahedi, M.; Odonkor, W. Vitamin D deficiency and insulin resistance in obese African- American adolescents. J. Pediatr. Endocrinol. Metab. 2011, 24, 29–33. [Google Scholar] [CrossRef]
- Olson, M.; Maalouf, N.M.; Oden, J.D.; White, P.C.; Hutchison, M.R. Vitamin D Deficiency in Obese Children and Its Relationship to Glucose Homeostasis. J. Clin. Endocrinol. Metab. 2012, 97, 279–285. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Burgess, S.; Munroe, P.B.; Khan, H. Vitamin D and high blood pressure: Causal association or epiphenomenon? Eur. J. Epidemiol. 2013, 29, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Alkharfy, K.M.; Al-Daghri, N.M.; Sabico, S.B.; Al-Othman, A.; Moharram, O.; Alokail, M.S.; Al-Saleh, Y.; Kumar, S.; Chrousos, G.P. Vitamin D supplementation in patients with diabetes mellitus type 2 on different therapeutic regimens: A one-year prospective study. Cardiovasc. Diabetol. 2013, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Vermeer, C. Determination of phylloquinone and menaquinones in food: Effect of food matrix on circulating vitamin K concentrations. Haemostasis 2000, 30, 298–307. [Google Scholar]
- Bolton-Smith, C.; Price, R.J.G.; Fenton, S.T.; Harrington, D.J.; Shearer, M.J. Compilation of a provisional UK database for the phylloquinone (vitamin K1) content of foods. Br. J. Nutr. 2000, 83, 389–399. [Google Scholar]
- Thane, C.W.; Bolton-Smith, C.; Coward, W.A. Comparative dietary intake and sources of phylloquinone (vitamin K1) among British adults in 1986–7 and 2000–1. Br. J. Nutr. 2006, 96, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Guraewal, S.; Wong, Y.L.; Majanbu, D.L.; Mavrodaris, A.; Stranges, S.; Kandala, N.-B.; Clarke, A.; Franco, O.H. Is vitamin K consumption associated with cardio-metabolic disorders? A systematic review. Maturitas 2010, 67, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, A.T.; Booth, S.L.; Hu, F.B.; Jacques, P.F.; Manson, J.E.; Rexrode, K.M.; Stampfer, M.J.; Lichtenstein, A.H. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur. J. Clin. Nutr. 2005, 59, 196–204. [Google Scholar] [CrossRef]
- Erkkilä, A.T.; Booth, S.L.; Hu, F.B.; Jacques, P.F.; Lichtenstein, A.H. Phylloquinone intake and risk of cardiovascular diseases in men. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 58–62. [Google Scholar] [CrossRef]
- Gast, G.-C.M.; De Roos, N.M.; Sluijs, I.; Bots, M.L.; Beulens, J.W.J.; Geleijnse, J.M.; Witteman, J.C.; Grobbee, D.E.; Peeters, P.H.M.; van der Schouw, Y.T.; et al. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 504–510. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Vermeer, C.; Grobbee, D.E.; Schurgers, L.J.; Knapen, M.H.J.; Van Der Meer, I.M.; Hofman, A.; Witteman, J.C.M. Dietary Intake of Menaquinone Is Associated with a Reduced Risk of Coronary Heart Disease: The Rotterdam Study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [CrossRef]
- Shea, M.K.; O’Donnell, C.J.; Hoffmann, U.; Dallal, G.E.; Dawson-Hughes, B.; Ordovas, J.M.; Price, P.A.; Williamson, M.K.; Booth, S.L. Vitamin K supplementation and progression of coronary artery calcium in older men and women. AmericanJ. Clin. Nutr. 2009, 89, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- O’Mahoney, L.; Dunseath, G.; Churm, R.; Holmes, M.; Boesch, C.; Stavropoulos-Kalinoglou, A.; Ajjan, R.A.; Birch, K.M.; Orsi, N.M.; Mappa, G.; et al. Omega-3 polyunsaturated fatty acid supplementation versus placebo on vascular health, glycemic control, and metabolic parameters in people with type 1 diabetes: A randomised controlled preliminary trial. Cardiovasc. Diabetol. 2020, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Briskey, D.; Nalley, J.O.; Ganuza, E. Omega-3 Eicosapentaenoic Acid (EPA) Rich Extract from the Microalga Nannochloropsis Decreases Cholesterol in Healthy Individuals: A Double-Blind, Randomized, Placebo-Controlled, Three-Month Supplementation Study. Nutrients 2020, 12, 1869. [Google Scholar] [CrossRef] [PubMed]
- Oscarsson, J.; Hurt-Camejo, E. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein B-containing lipoproteins in humans: A review. Lipids Health Dis. 2017, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Bagherniya, M.; Atkin, S.L.; Askari, G.; Orafai, H.M.; Sahebkar, A. The beneficial effects of nutraceuticals and natural products on small dense LDL levels, LDL particle number and LDL particle size: A clinical review. Lipids Health Dis. 2020, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus-systematic review and meta-analysis. Mol. Nutr. Food Res. 2014, 59, 147–159. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Kumar, B.J.; Joghee, N.M. Resveratrol supplementation in patients with type 2 diabetes mellitus: A prospective, open label, randomized controlled trial. Int. Res. J. Pharm. 2013, 4, 246–249. [Google Scholar]
- Movahed, A.; Nabipour, I.; Lieben Louis, X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid.-Based Complement. Altern. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.R.; Dumont, J.; et al. Impact of Flavonols on Cardiometabolic Biomarkers: A Meta-Analysis of Randomized Controlled Human Trials to Explore the Role of Inter-Individual Variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Sahebkar, A.; Iranshahi, M.; Amini, M.; Khojasteh, R.; Ghayour-Mobarhan, M.; Ferns, G.A. Effects of supplementation with curcuminoids on dyslipidemia in obese patients: A randomized crossover trial. Phytother. Res. 2013, 27, 374–379. [Google Scholar] [CrossRef]
- Ganjali, S.; Sahebkar, A.; Mahdipour, E.; Jamialahmadi, K.; Torabi, S.; Akhlaghi, S.; Ferns, G.; Reza Parizadeh, S.M.; Ghayour-Mobarhan, M. Investigation of the effects of curcumin on serum cytokines in obese individuals: A randomized controlled trial. Sci. World J. 2014, 2014, 898361. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bressan, A.; Ranaldi, D.; Rapacioli, G.; Giacomelli, L.; Bertuccioli, A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: Preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4195–4202. [Google Scholar]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin on cardiovascular risk factors in obese and overweight adolescent girls: A randomized clinical trial. Sao Paulo Med. J. 2019, 137, 414–422. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Auinger, A.; Bley, U.; Kraus-Stojanowic, I.; Laue, C.; Winkler, P.; Rüfer, C.; Frank, J.; Bösch-Saadatmandi, C.; Rimbach, G.; et al. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 403–409. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Konings, E.; Timmers, S.; Boekschoten, M.V.; Goossens, G.H.; Jocken, J.W.; Afman, L.A.; Müller, M.; Schrauwen, P.; Mariman, E.C.; Blaak, E.E. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int. J. Obes. 2014, 38, 470–473. [Google Scholar] [CrossRef]
- Arzola-Paniagua, M.A.; García-Salgado López, E.R.; Calvo-Vargas, C.G.; Guevara-Cruz, M. Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: A randomized controlled trial. Obesity 2016, 24, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stødkilde-Jørgensen, H.; Møller, S.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O.L. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Maqueda, D.; Zapatera, B.; Gallego-Narbón, A.; Vaquero, M.P.; Saura-Calixto, F.; Pérez-Jiménez, J. A 6-week supplementation with grape pomace to subjects at cardiometabolic risk ameliorates insulin sensitivity, without affecting other metabolic syndrome markers. Food Funct. 2018, 9, 6010–6019. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green Tea Supplementation Affects Body Weight, Lipids, and Lipid Peroxidation in Obese Subjects with Metabolic Syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol. Trace Elem. Res. 2012, 149, 315–322. [Google Scholar] [CrossRef]
- Allison, D.B.; Gadbury, G.; Schwartz, L.G.; Murugesan, R.; Kraker, J.L.; Heshka, S.; Fontaine, K.R.; Heymsfield, S.B. A novel soy-based meal replacement formula for weight loss among obese individuals: A randomized controlled clinical trial. Eur. J. Clin. Nutr. 2003, 57, 514–522. [Google Scholar] [CrossRef]
- Méndez-del Villar, M.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Lizárraga-Valdez, R. Effect of Resveratrol Administration on Metabolic Syndrome, Insulin Sensitivity, and Insulin Secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef]
- Basu, A.; Fu, D.X.; Wilkinson, M.; Simmons, B.; Wu, M.; Betts, N.M.; Du, M.; Lyons, T.J. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr. Res. 2010, 30, 462–469. [Google Scholar] [CrossRef]
- Chu, S.L.; Fu, H.; Yang, J.X.; Liu, G.X.; Dou, P.; Zhang, L.; Tu, P.F.; Wang, X.M. A randomized double-blind placebo-controlled study of Pu’er tea (普洱茶) extract on the regulation of metabolic syndrome. Chin. J. Integr. Med. 2011, 17, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Ledda, A.; Hu, S.; Cesarone, M.R.; Feragalli, B.; Dugall, M. Greenselect phytosome for borderline metabolic syndrome. Evid.-Based Complement. Altern. Med. 2013, 2013, 869061. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Rizzo, M.; Sarlo, F.; Colica, C.; Iacopino, L.; Domino, E.; Sergi, D.; De Lorenzo, A. Effects of dark chocolate in a population of normal weight obese women: A pilot study. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2257–2266. [Google Scholar] [PubMed]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, CR28–CR34. [Google Scholar] [PubMed]
- Davison, G.; Callister, R.; Williamson, G.; Cooper, K.A.; Gleeson, M. The effect of acute pre-exercise dark chocolate consumption on plasma antioxidant status, oxidative stress and immunoendocrine responses to prolonged exercise. Eur. J. Nutr. 2011, 51, 69–79. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Hofheins, J.E.; Mendel, R.W.; Landis, J.; Anderson, R.A. Effects of a Water-Soluble Cinnamon Extract on Body Composition and Features of the Metabolic Syndrome in Pre-Diabetic Men and Women. J. Int. Soc. Sports Nutr. 2006, 3, 45. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Müller, M.J. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Lehtonen, H.-M.; Suomela, J.-P.; Tahvonen, R.; Yang, B.; Venojärvi, M.; Viikari, J.; Kallio, H. Different berries and berry fractions have various but slightly positive effects on the associated variables of metabolic diseases on overweight and obese women. Eur. J. Clin. Nutr. 2011, 65, 394–401. [Google Scholar] [CrossRef]
- Barona, J.; Blesso, C.N.; Andersen, C.J.; Park, Y.; Lee, J.; Fernandez, M.L. Grape consumption increases anti-inflammatory markers and upregulates peripheral nitric oxide synthase in the absence of dyslipidemias in men with metabolic syndrome. Nutrients 2012, 4, 1945–1957. [Google Scholar] [CrossRef]
- Fujitaka, K.; Otani, H.; Jo, F.; Jo, H.; Nomura, E.; Iwasaki, M.; Nishikawa, M.; Iwasaka, T.; Das, D.K. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr. Res. 2011, 31, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Giglio, R.V.; Patti, A.M.; Nikolic, D.; Volti, G.L.; Al-Rasadi, K.; Katsiki, N.; Mikhailidis, D.P.; Montalto, G.; Ivanova, E.; Orekhov, A.N.; et al. The effect of bergamot on dyslipidemia. Phytomedicine 2015, 23, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolinoa, V.; Russoa, V.; Jandaa, E.; Ragusa, S. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Carresi, C.; Musolino, V.; Palma, E.; Muscoli, C.; Vitale, C.; Gratteri, S.; Muscianisi, G.; Janda, E.; Muscoli, S.; et al. The Effect of Bergamot-Derived Polyphenolic Fraction on LDL Small Dense Particles and Non Alcoholic Fatty Liver Disease in Patients with Metabolic Syndrome. Adv. Biol. Chem. 2014, 04, 129–137. [Google Scholar] [CrossRef]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Patti, A.M.; Nikolic, D.; Giglio, R.V.; Castellino, G.; Biancucci, T.; Geraci, F.; David, S.; Montalto, G.; Rizvi, A.; et al. Bergamot Reduces Plasma Lipids, Atherogenic Small Dense LDL, and Subclinical Atherosclerosis in Subjects with Moderate Hypercholesterolemia: A 6 Months Prospective Study. Front. Pharmacol. 2016, 6, 299. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Nikolic, D.; Conigliaro, A.; Giavaresi, G.; Sasso, B.L.; Giglio, R.; Chianetta, R.; Manno, M.; Raccosta, S.; Corleone, V.; et al. Preliminary Results of CitraVes™ Effects on Low Density Lipoprotein Cholesterol and Waist Circumference in Healthy Subjects after 12 Weeks: A Pilot Open-Label Study. Metabolites 2021, 11, 276. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; Van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef]
- Kasińska, M.A.; Drzewoski, J. Effectiveness of probiotics in type 2 diabetes: A meta-analysis. Pol. Arch. Intern. Med. 2015, 125, 803–813. [Google Scholar] [CrossRef]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.; Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolicprofiles, hs-CRP, and oxidati vestress in patients with type 2 diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Asemi, Z.; Khorrami-Rad, A.; Alizadeh, S.; Shakeri, H.; Esmaillzadeh, A. Effects of synbiotic food consumption on metabolic status of diabetic patients: A double-blind ran- domized cross-over controlledclinical trial. Clin. Nutr. 2014, 33, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Jafarabadi, M.A.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Judiono, J.; Hadisaputro, S.; Indranilla, K.S. Effects of clear kefir on bio- molecular aspects of glycemic status of type 2 diabetes mellitus (T2DM) patients in Bandung, West Java [Study on human blood glucose, c pep- tide and insulin]. J. Funct. Foods Health Dis. 2014, 4, 340–348. [Google Scholar] [CrossRef]
- Mazloom, Z.; Yousefinejad, A.; Dabbaghmanesh, M.H. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflam- matory markers in patients with type 2 diabetes: A clinical trial. Iran J. Med. Sci. 2013, 38, 38–43. [Google Scholar] [PubMed]
- Moroti, C.; Souza Magri, L.F.; de Rezende Costa, M.; Cavallini, D.C.U.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol lev- els in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mahboobi, S.; Iraj, B.; Maghsoudi, Z.; Feizi, A.; Ghiasvand, R.; Askari, G.; Maayeshi, N. The Effects of Probiotic Supplementation on Markers of Blood Lipids, and Blood Pressure in Patients with Prediabetes: A Randomized Clinical Trial. Int. J. Prev. Med. 2014, 5, 1239–1246. [Google Scholar] [PubMed]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Metabolic benefits of dietary prebiotics in human subjects: A systematic review of randomised controlled trials. Br. J. Nutr. 2014, 111, 1147–1161. [Google Scholar] [CrossRef]
- Genta, S.B.; Cabrera, W.M.; Habib, N.C.; Pons, J.; Carillo, I.M.; Grau, A.; Sánchez, S. Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin. Nutr. 2009, 28, 182–187. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef]
- Dehghan, P.; Gargari, B.P.; Jafarabadi, M.A.; Aliasgharzadeh, A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized-controlled clinical trial. Int. J. Food Sci. Nutr. 2013, 65, 117–123. [Google Scholar] [CrossRef]
- De Luis, D.A.; De la Fuente, B.; Izaola, O.; Conde, R.; Gutiérrez, S.; Morillo, M.; Torres, C.T. Double blind randomized clinical trial controlled by placebo with an alpha linoleic acid and prebiotic enriched cookie on risk cardiovascular factor in obese patients. Nutr. Hosp. 2011, 26, 827–833. [Google Scholar] [PubMed]
- Seidel, C.; Boehm, V.; Vogelsang, H.; Wagner, A.; Persin, C.; Glei, M.; Pool-Zobel, B.L.; Jahreis, G. Influence of prebiotics and antioxidants in bread on the immune system, antioxidative status and antioxidative capacity in male smokers and non-smokers. Br. J. Nutr. 2007, 97, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Lecourt, E.; Dewulf, E.M.; Sohet, F.M.; Pachikian, B.D.; Naslain, D.; De Backer, F.; Neyrinck, A.M.; Delzenne, N.M. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 2009, 90, 1236–1243. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.-P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Riezzo, G.; Chiloiro, M.; De Michele, G.; Chimienti, G.; Marconi, E.; D’Attoma, B.; Linsalata, M.; Clemente, C. Metabolic Effects of a Diet with Inulin-Enriched Pasta in Healthy Young Volunteers. Curr. Pharm. Des. 2010, 16, 825–831. [Google Scholar] [CrossRef]
- Vulevic, J.; Juric, A.; Tzortzis, G.; Gibson, G.R. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J. Nutr. 2013, 143, 324–331. [Google Scholar] [CrossRef]
- Solomon, S.; Mulugeta, W. Disease burden and associated risk factors for metabolic syndrome among adults in Ethiopia. BMC Cardiovasc. Disord. 2019, 19, 84–89. [Google Scholar] [CrossRef]
- Liu, L.; Miura, K.; Fujiyoshi, A.; Kadota, A.; Miyagawa, N.; Nakamura, Y.; Ohkubo, T.; Okayama, A.; Okamura, T.; Ueshima, H. Impact of metabolic syndrome on the risk of cardiovascular disease mortality in the United States and in Japan. Am. J. Cardiol. 2014, 113, 84–89. [Google Scholar] [CrossRef]
- Xi, B.; He, D.; Hu, Y.; Zhou, D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: The China Health and Nutrition Survey in 2009. Prev. Med. 2013, 57, 867–871. [Google Scholar] [CrossRef]
- Vidigal, F.D.C.; Bressan, J.; Babio, N.; Salas-Salvadó, J. Prevalence of metabolic syndrome in Brazilian adults: A systematic review. BMC Public Health 2013, 13, 1198. [Google Scholar] [CrossRef]
- Salas, R.; Bibiloni, M.D.M.; Ramos, E.; Villarreal, J.Z.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Prevalence among Northern Mexican Adult Population. PLoS ONE 2014, 9, e105581. [Google Scholar] [CrossRef]
- Palomer, X.; González-Clemente, J.M.; Blanco-Vaca, F.; Mauricio, D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes. Metab. 2007, 10, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P.A. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, g2035. [Google Scholar] [CrossRef] [PubMed]
- Burgaz, A.; Orsini, N.; Larsson, S.; Wolk, A. Blood 25-hydroxyvitamin D concentration and hypertension: A meta-analysis. J. Hypertens. 2011, 29, 636–645. [Google Scholar] [CrossRef]
- Geleijnse, J.M. Vitamin D and the Prevention of Hypertension and Cardiovascular Diseases: A Review of the Current Evidence. Am. J. Hypertens. 2011, 24, 253–262. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Ginde, A.A.; Scragg, R.; Schwartz, R.S.; Camargo, C.A., Jr. Prospective Study of Serum 25-Hydroxyvitamin D Level, Cardiovascular Disease Mortality, and All-Cause Mortality in Older U.S. Adults. J. Am. Geriatr. Soc. 2009, 57, 1595–1603. [Google Scholar] [CrossRef]
- Perna, L.; Schöttker, B.; Holleczek, B.; Brenner, H. Serum 25-Hydroxyvitamin D and Incidence of Fatal and Nonfatal Cardiovascular Events: A Prospective Study with Repeated Measurements. J. Clin. Endocrinol. Metab. 2013, 98, 4908–4915. [Google Scholar] [CrossRef]
- Ogata, T.; Miyauchi, T.; Sakai, S.; Irukayama-Tomobe, Y.; Goto, K.; Yamaguchi, I. Stimulation of peroxisome-proliferator-activated receptor α (PPAR α) attenuates cardiac fibrosis and endothelin-1 production in pressure-overloaded rat hearts. Clin. Sci. 2002, 103, 284S–288S. [Google Scholar] [CrossRef]
- Kazlauskaite, R.; Powell, L.H.; Mandapakala, C.; Cursio, J.; Avery, E.F.; Calvin, J. Vitamin D is associated with atheroprotective high-density lipoprotein profile in postmenopausal women. J. Clin. Lipidol. 2010, 4, 113–119. [Google Scholar] [CrossRef][Green Version]
- Richart, T.; Li, Y.; Staessen, J.A. Renal Versus Extrarenal Activation of Vitamin D in Relation to Atherosclerosis, Arterial Stiffening, and Hypertension. Am. J. Hypertens. 2007, 20, 1007–1015. [Google Scholar] [CrossRef]
- Kutlay, S.; Atli, T.; Aydogan, I.; Tutkak, H.; Nergizoglu, G. The association of serum vitamin D levels with several cardiometabolic risk and aortic pulse wave velocity in elderly persons. Eur. Geriatr. Med. 2014, 5, 238–241. [Google Scholar] [CrossRef]
- Pilz, S.; Tomaschitz, A.; Ritz, E.; Pieber, T.R. Vitamin D status and arterial hypertension: A systematic review. Nat. Rev. Cardiol. 2009, 6, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.M.; Schurgers, L.J.; Vermeer, C. Vitamin K: The coagulation vitamin that became omnipotent. Thromb. Haemost. 2007, 98, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Nishiike, T.; Iguchi, H.; Sakamoto, K. Relationship between acute insulin response and vitamin K intake in healthy young male volunteers. Diabetes Nutr. Metab. 1999, 12, 37–41. [Google Scholar] [PubMed]
- Sakamoto, N.; Nishiike, T.; Iguchi, H. Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels. Clin. Nutr. 2000, 19, 259–263. [Google Scholar] [CrossRef]
- Yoshida, M.; Booth, S.L.; Meigs, J.B.; Saltzman, E.; Jacques, P.F. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am. J. Clin. Nutr. 2008, 88, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Jacques, P.F.; Meigs, J.B.; Saltzman, E.; Shea, M.K.; Gundberg, C.; Dawson-Hughes, B.; Dallal, G.; Booth, S.L. Effect of Vitamin K Supplementation on Insulin Resistance in Older Men and Women. Diabetes Care 2008, 31, 2092–2096. [Google Scholar] [CrossRef]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-Chain Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid and Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef]
- Harris, W.S. The omega-3 index as a risk factor for coronary heart disease. Am. J. Clin. Nutr. 2008, 87, 1997S–2002S. [Google Scholar] [CrossRef]
- Murphy, R.A.; Yu, E.A.; Ciappio, E.D.; Mehta, S.; McBurney, M.I. Suboptimal Plasma Long Chain n-3 Concentrations are Common among Adults in the United States, NHANES 2003–2004. Nutrients 2015, 7, 10282–10289. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.M.; Johnson, N.A.; Burdon, C.A.; Cohn, J.S.; O’Connor, H.T.; George, J. Omega-3 supplementation and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 56, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, X.; Shao, S. Effects of Omega-3 Fatty Acid Supplementation on Glucose Control and Lipid Levels in Type 2 Diabetes: A Meta-Analysis. PLoS ONE 2015, 10, e0139565. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.-K.; Kwak, S.M.; Lee, Y.J.; Seo, H.G.; Korean Meta-analysis Study Group. Efficacy of Omega-3 Fatty Acid Supplements (Eicosapentaenoic Acid and Docosahexaenoic Acid) in the Secondary Prevention of Cardiovascular Disease. Arch. Intern. Med. 2012, 172, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Tsubata, H.; Hashimoto, N.; Takabe, M.; Miyata, T.; Aoki, K.; Yamashita, S.; Oishi, S.; Osue, T.; Yokoi, K.; et al. Effects of 6-month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly-diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc. Diabetol. 2016, 15, 121. [Google Scholar] [CrossRef]
- Jacobo-Cejudo, M.G.; Valdés-Ramos, R.; Guadarrama-López, A.L.; Pardo-Morales, R.-V.; Martínez-Carrillo, B.E.; Harbige, L.S. Effect of n-3 Polyunsaturated Fatty Acid Supplementation on Metabolic and Inflammatory Biomarkers in Type 2 Diabetes Mellitus Patients. Nutrients 2017, 9, 573. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- He, X.; Liu, R.H. Phytochemicals of Apple Peels: Isolation, Structure Elucidation, and Their Antiproliferative and Antioxidant Activities. J. Agric. Food Chem. 2008, 56, 9905–9910. [Google Scholar] [CrossRef]
- Di Meo, S.; Iossa, S.; Venditti, P. Skeletal muscle insulin resistance: Role of mitochondria and other ROS sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.G.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed]
- Dugo, P.; Presti, M.L.; Öhman, M.; Fazio, A.; Dugo, G.; Mondello, L. Determination of flavonoids in citrus juices by micro-HPLC-ESI/MS. J. Sep. Sci. 2005, 28, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.F.-F.; Macdonald, R.; Lovegrove, J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. Br. J. Nutr. 2010, 104, S28–S39. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Sowers, J.R.; Clark, S.E.; Li, W.; Ferrario, C.M.; Stump, C.S. Stump Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-κB activation via NADPH oxidase. Am. J. Physiol.-Endocrinol. Metab. 2008, 294, E345–E351. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Aguilera, C.M.; Gil, A. A Systematic Review of the Efficacy of Bioactive Compounds in Cardiovascular Disease: Phenolic Compounds. Nutrients 2015, 7, 5177–5216. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann.-Ist. Super. Di Sanita 2007, 43, 348–361. [Google Scholar]
- Walia, M.; Kumar, S.; Agnihotri, V.K. UPLC-PDA quantification of chemical constituents of two different varieties (golden and royal) of apple leaves and their antioxidant activity. J. Sci. Food Agric. 2015, 96, 1440–1450. [Google Scholar] [CrossRef]
- Najafian, M.; Jahromi, M.Z.; Nowroznejhad, M.J.; Khajeaian, P.; Kargar, M.M.; Sadeghi, M.; Arasteh, A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2011, 39, 5299–5306. [Google Scholar] [CrossRef]
- Rossetti, L.; Smith, D.; Shulman, G.I.; Papachristou, D.; DeFronzo, R.A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J. Clin. Investig. 1987, 79, 1510–1515. [Google Scholar] [CrossRef]
- Chai, S.C.; Hooshmand, S.; Saadat, R.L.; Payton, M.E.; Brummel-Smith, K.; Arjmandi, B.H. Daily Apple versus Dried Plum: Impact on Cardiovascular Disease Risk Factors in Postmenopausal Women. J. Acad. Nutr. Diet. 2012, 112, 1158–1168. [Google Scholar] [CrossRef]
- Ohta, T.; Morinaga, H.; Yamamoto, T.; Yamada, T. Effect of phlorizin on metabolic abnormalities in spontaneously diabetic Torii (SDT) rats. Open J. Anim. Sci. 2012, 2, 113–118. [Google Scholar] [CrossRef][Green Version]
- Chu, Y.F.; Liu, R.H. Cardioprotective potentials of apple phytochemicals in LDL oxidation and LDL receptor expression. In Proceedings of the Cornell Institute of Food Science Symposium, Ithaca, NY, USA, 22–24 May 2005; pp. 22–24. [Google Scholar]
- Liu, R.H.; Sun, J. Antiproliferative Activity of Apples Is Not Due to Phenolic-Induced Hydrogen Peroxide Formation. J. Agric. Food Chem. 2003, 51, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Stangl, V.; Lorenz, M.; Ludwig, A.; Grimbo, N.; Guether, C.; Sanad, W.; Ziemer, S.; Martus, P.; Baumann, G.; Stangl, K. The Flavonoid Phloretin Suppresses Stimulated Expression of Endothelial Adhesion Molecules and Reduces Activation of Human Platelets. J. Nutr. 2005, 135, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting cAMP Phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Kume, S.; Thomas, M.C.; Koya, D. Nutrient Sensing, Autophagy, and Diabetic Nephropathy. Diabetes 2011, 61, 23–29. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; Yáñez-Gascón, M.J.; Davalos, A.; Gil Zamorano, J.; Gonzálvez, M.; García-Almagro, F.J.; Ros, J.A.R.; Tomás-Barberán, F.A.; Espín, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef]
- Mercken, E.M.; Carboneau, B.A.; Krzysik-Walker, S.M.; de Cabo, R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 2011, 11, 390–398. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Wong, R.; Howe, P.; Buckley, J.; Coates, A.; Kunz, I.; Berry, N. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.J.; Symons, J.D.; Jalili, T. Therapeutic Potential of Quercetin to Decrease Blood Pressure: Review of Efficacy and Mechanisms. Adv. Nutr. Int. Rev. J. 2012, 3, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Duarte, J.; Jimenez, R.; Santos-Buelga, C.; Osuna, A. Antihypertensive effects of the flavonoid quercetin. Pharm. Rep. 2009, 61, 67–75. [Google Scholar] [CrossRef]
- Larsson, S.C. Coffee, Tea, and Cocoa and Risk of Stroke. Stroke 2014, 45, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Étienne-Selloum, N.; Li, H.; Martínez, M.C.; Schini-Kerth, V.B.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef]
- Pinto, P.; Santos, C.N. Worldwide (poly)phenol intake: Assessment methods and identified gaps. Eur. J. Nutr. 2017, 56, 1393–1408. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2015, 55, 1359–1375. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef]
- Di Donna, L.; Iacopetta, D.; Cappello, A.R.; Gallucci, G.; Martello, E.; Fiorillo, M.; Dolce, V.; Sindona, G. Hypocholesterolaemic activity of 3-hydroxy-3-methyl-glutaryl flavanones enriched fraction from bergamot fruit (Citrus bergamia): “In vivo” studies. J. Funct. Foods 2014, 7, 558–568. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-inflammatory Activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Salamone, F.; Galvano, F.; Cappello, F.; Mangiameli, A.; Barbagallo, I.; Volti, G.L. Silibinin modulates lipid homeostasis and inhibits nuclear factor kappa B activation in experimental nonalcoholic steatohepatitis. Transl. Res. 2012, 159, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Bruckbauer, A.; Zemel, M. Synergistic Effects of Polyphenols and Methylxanthines with Leucine on AMPK/Sirtuin-Mediated Metabolism in Muscle Cells and Adipocytes. PLoS ONE 2014, 9, e89166. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, R.; Yoshida, T.; Nii, Y.; Okahisa, N.; Iwata, S.; Tsukayama, M.; Hashimoto, R.; Taniguchi, Y.; Sakaue, H.; Hosaka, T.; et al. Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr. Metab. 2014, 11, 32. [Google Scholar] [CrossRef]
- Chang, J.-J.; Hsu, M.-J.; Huang, H.-P.; Chung, D.-J.; Chang, Y.-C.; Wang, C.-J. Mulberry Anthocyanins Inhibit Oleic Acid Induced Lipid Accumulation by Reduction of Lipogenesis and Promotion of Hepatic Lipid Clearance. J. Agric. Food Chem. 2013, 61, 6069–6076. [Google Scholar] [CrossRef]
- Reddy, S.; Yang, W.; Taylor, D.G.; Shen, X.-Q.; Oxender, D.; Kust, G.; Leff, T. Mitogen-activated Protein Kinase Regulates Transcription of the ApoCIII Gene. J. Biol. Chem. 1999, 274, 33050–33056. [Google Scholar] [CrossRef]
- Quesada, H.; del Bas, J.M.; Pajuelo, D.; Díaz, S.; Fernandez-Larrea, J.; Pinent, M.; Arola, L.; Salvadó, M.J.; Bladé, C. Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int. J. Obes. 2009, 33, 1007–1012. [Google Scholar] [CrossRef]
- Wilcox, L.J.; Borradaile, N.M.; De Dreu, L.E.; Huff, M.W. Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. J. Lipid Res. 2001, 42, 725–734. [Google Scholar] [CrossRef]
- Bellavia, D.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Caradonna, F.; Giavaresi, G. Flavonoids in Bone Erosive Diseases: Perspectives in Osteoporosis Treatment. Trends Endocrinol. Metab. 2020, 32, 76–94. [Google Scholar] [CrossRef]
- Kwon, E.-Y.; Choi, M.-S. Eriocitrin Improves Adiposity and Related Metabolic Disorders in High-Fat Diet-Induced Obese Mice. J. Med. Food 2020, 23, 233–241. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, W.H.W.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Hear. J. 2014, 35, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef]
- Muegge, B.D.; Kuczynski, J.; Knights, D.; Clemente, J.C.; González, A.; Fontana, L.; Henrissat, B.; Knight, R.; Gordon, J.I. Diet Drives Convergence in Gut Microbiome Functions Across Mammalian Phylogeny and Within Humans. Science 2011, 332, 970–974. [Google Scholar] [CrossRef]
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef]
- van Hemert, S.; Verwer, J.; Schütz, B. Clinical Studies Evaluation Effects of Probiotics on Parameters of Intestinal Barrier Function. Adv. Microbiol. 2013, 3, 221. [Google Scholar]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Bäckhed, F. Gut metagenome in european women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.K.; Omaye, S.T. Metabolic diseases and pro- and prebiotics: Mechanistic insights. Nutr. Metab. 2012, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Hussey, S.E.; Sanchez-Avila, A.; Tantiwong, P.; Musi, N. Effect of Lipopolysaccharide on Inflammation and Insulin Action in Human Muscle. PLoS ONE 2013, 8, e63983. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2009, 101, 541–550. [Google Scholar] [CrossRef]
- Chung, H.; Kasper, D.L. Microbiota-stimulated immune mechanisms to maintain gut homeostasis. Curr. Opin. Immunol. 2010, 22, 455–460. [Google Scholar] [CrossRef]
- Hill, M.J. Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 1997, 6, S43–S45. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. S2), S1–S63. [Google Scholar] [CrossRef] [PubMed]

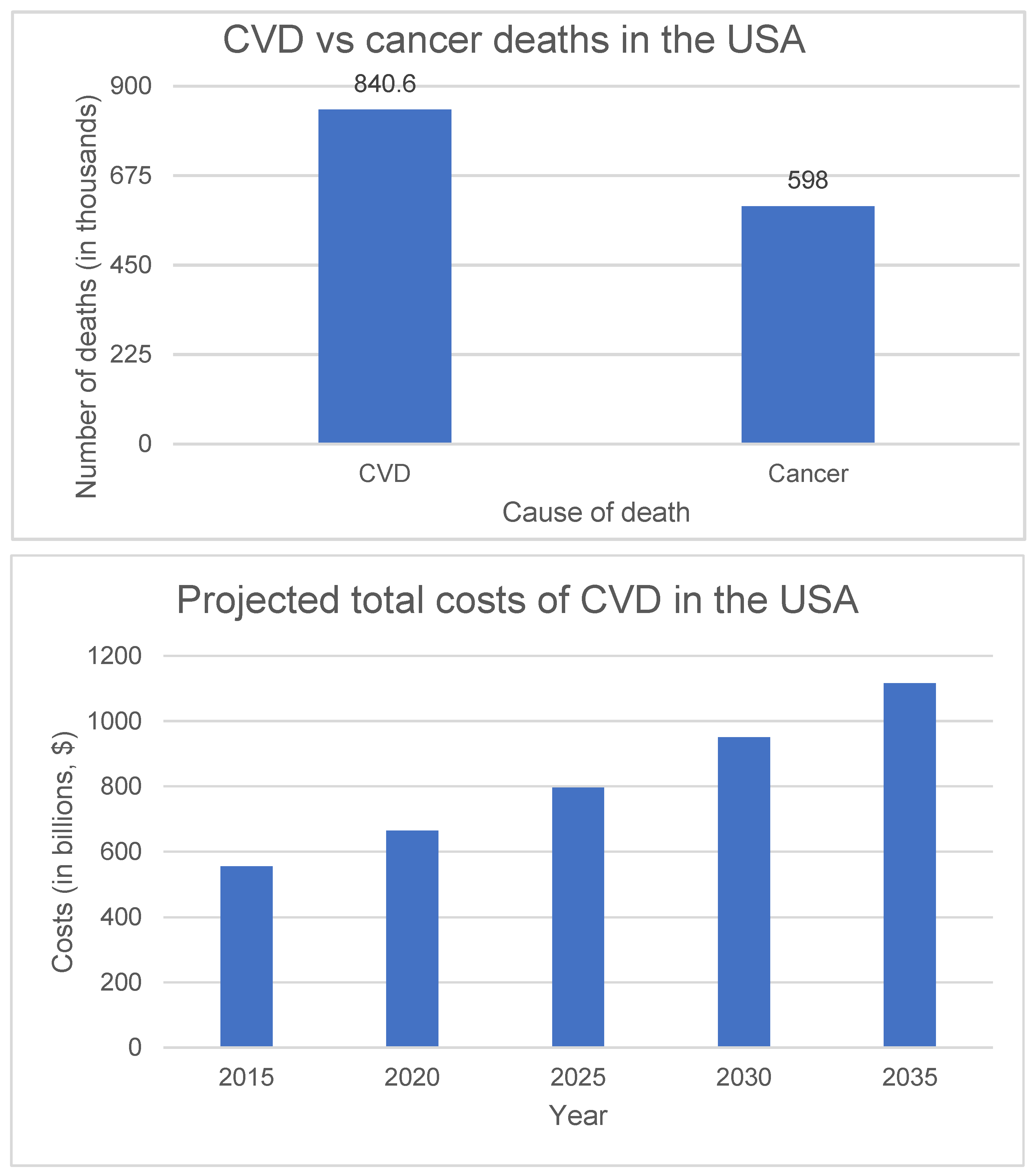
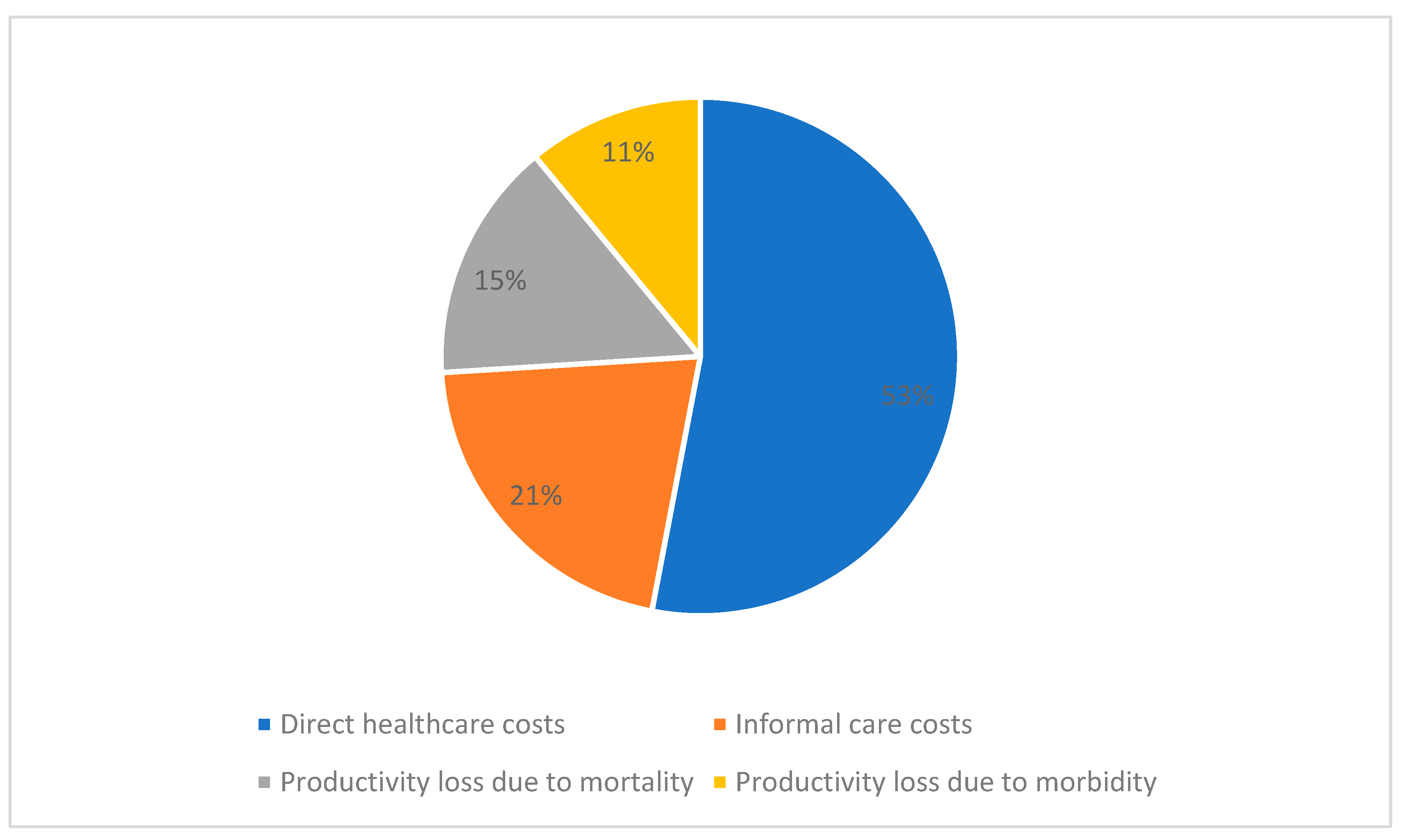
| Heart Disease | Stroke | Hypertensive Disease | Other Circulatory Conditions | Total CVD | |
|---|---|---|---|---|---|
| DIRECT COSTS | 109.4 | 28.0 | 51.3 | 25.1 | 213.8 |
| INDIRECT COSTS | 109.3 | 17.5 | 4.6 | 6.1 | 137.4 |
| GRAND TOTAL | 218.7 | 45.5 | 55.9 | 31.2 | 351.2 |
| Germany | Spain | Italy | ||
|---|---|---|---|---|
| Event Rate per 1000 | Event Rate per 1000 | Event Rate per 1000 | ||
| Annual incidence of CVD events | MetS | 27 | 25 | 24 |
| Without MetS | 14 | 13 | 13 | |
| Annual mortality | MetS | 3 | 2.74 | 2.64 |
| Without MetS | 1.49 | 1.45 | 1.40 | |
| Annual prevalence of type II diabetes | MetS | 248 | 281 | 308 |
| Without MetS | 45 | 51 | 52 |
| Drug Annual Costs | Physician Costs | CVD Costs | T2DM Costs | Total Costs | ||
|---|---|---|---|---|---|---|
| Germany | MetS | 628 | 1952 | 5265 | 16,582 | 24,472 |
| Germany | Without MetS | 407 | 1264 | 1703 | 1967 | 5341 |
| Spain | MetS | 116 | 126 | 699 | 968 | 1909 |
| Spain | Without MetS | 397 | 432 | 1256 | 597 | 2682 |
| Italy | MetS | 258 | 330 | 817 | 3472 | 4877 |
| Italy | Without MetS | 958 | 1222 | 1599 | 2178 | 5957 |
| Total Annual Cost Increase per Patient (in USD, $) | |
|---|---|
| Impaired FBG | 161 |
| Impaired BMI | 408 |
| Impaired blood pressure (BP) | 657 |
| Impaired HDL | 481 |
| Impaired TG | 423 |
| Number of Studies | Study Design | Year of Publication | Country | Authors | Main Findings | References | |
|---|---|---|---|---|---|---|---|
| Vitamin D | 7 | Double blind, placebo-controlled RCT; systematic review and meta-analysis of RCTs; prospective review | 2010–2015 | Iran, UK, USA, Saudi Arabia, Greece | Salehpour A et al., Forouhi NG et al., Manousopoulou A et al., Pittas GA et al., Dolinsky DH et al., Kunutsor SK et al., Alkharfy KM et al. | Non-significant results or ↓DBP ↓↑SBP ↓FBG ↓TC ↓TG ↓↑LDL ↑HDL ↓HOMA-IR | [36,40,41,51,63,83,84] |
| Vitamin K | 1 | Systematic review | 2010 | UK | Rees K et al. | Non-significant results | [88] |
| Omega-3 | 2 | Meta-analysis of RCTs; double blind, randomized, placebo-controlled study | 2018 2020 | UK, USA, Australia | O’Mahoney LL et al., Rao A et al. | ↓BW ↓TC ↓TG ↓LDL ↑HDL ↓VLDL ↓HbA1c | [94,95] |
| Polyphenols | 5 | Systematic review, meta-analysis of RCTs, double blind placebo-controlled RCT, double-blind crossover study | 2015–2020 | USA, Italy, Spain, France | Hausenblas HA et al., Menezes R et al., Boccellino M et al., Martinez-Maqueda D et al., Amiot MJ et al. | ↓HbA1c ↓creatinine ↓HOMA-IR ↓TAG ↓TG ↓FG ↓TC ↓LDL ↑↓HDL ↓SBP ↓DBP ↓BMI ↓WC ↓BW ↓BFM | [99,104,105,115,116] |
| Polyphenols (bergamot flavonoids) | 3 | RCT, prospective study, open label study | 2015 2016 2021 | Italy, USA | Giglio RV et al., Toth PP et al., Raimondo S et al. | ↓HbA1c ↑glucose ↓TC ↓LDL ↑HDL ↓↑TG ↓BG ↓WC | [134,138,139] |
| Probiotics | 2 | RCT, meta-analysis | 2015 2018 | Poland | Szulinska M et al., Kasinska MA et al. | ↓LPS ↓WC ↓BFM ↓TC ↓TG ↓LDL ↓FBG ↓INS ↓HOMA-IR ↓HbA1c | [140,141] |
| Prebiotics | 1 | Systematic-review | 2013 | Australia | Kellow NJ et al. | ↓INS ↓BG | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, G.M.; Dovera, F.; Ciccarone, A.; Colombo, G.L. Overview of Nutraceuticals and Cardiometabolic Diseases following Socio-Economic Analysis. Endocrines 2022, 3, 255-295. https://doi.org/10.3390/endocrines3020023
Bruno GM, Dovera F, Ciccarone A, Colombo GL. Overview of Nutraceuticals and Cardiometabolic Diseases following Socio-Economic Analysis. Endocrines. 2022; 3(2):255-295. https://doi.org/10.3390/endocrines3020023
Chicago/Turabian StyleBruno, Giacomo Matteo, Federico Dovera, Antonio Ciccarone, and Giorgio Lorenzo Colombo. 2022. "Overview of Nutraceuticals and Cardiometabolic Diseases following Socio-Economic Analysis" Endocrines 3, no. 2: 255-295. https://doi.org/10.3390/endocrines3020023
APA StyleBruno, G. M., Dovera, F., Ciccarone, A., & Colombo, G. L. (2022). Overview of Nutraceuticals and Cardiometabolic Diseases following Socio-Economic Analysis. Endocrines, 3(2), 255-295. https://doi.org/10.3390/endocrines3020023






