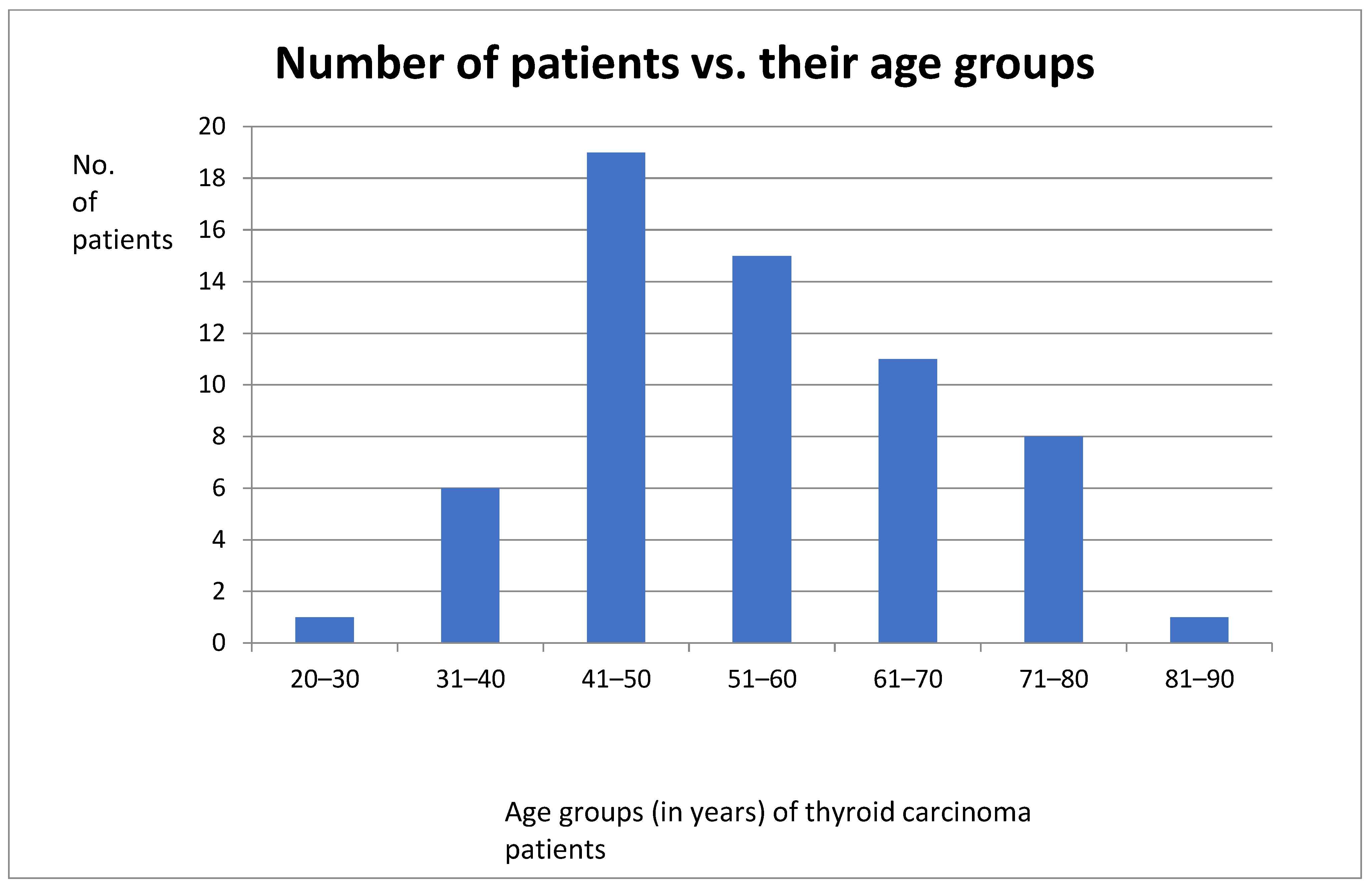Abstract
Diagnosing thyroid carcinoma is not always easy on basic haemtoxylin and eosin staining since nuclear features are inconsistent and controversial. In view of this, studies on the role of immunohistochemical markers in the diagnosis of malignant thyroid carcinoma are necessary. Proposed immunohistochemical markers for papillary thyroid cancer include Hector Battifora mesothelial-1 (HBME-1), and Galectin-3 (Gal-3) which have been studied in this project. Immunohistochemical staining of fifty-eight formalin-fixed paraffin embedded surgically removed thyroid tissue from the years 2008 and 2013 was undertaken to determine the diagnostic accuracy of these two markers. We have concluded that both Gal-3 and HBME-1 are useful markers to aid in the diagnosis of papillary thyroid carcinoma and also in distinguishing between benign and malignant thyroid lesions. The sensitivity and specificity of Gal-3 over the 2years studied was found to be 96.2% and 92.6%, respectively, whilst HBME-1 was found to have sensitivity of 93.6% and specificity of 69.02%.
1. Introduction
Thyroid nodules are a common clinical problem and their diagnosis and management pose a significant challenge to the physician. The ability to properly diagnose, and the knowledge of when and how to treat, makes a difference to the patients’ prognosis and quality of life. On average, 586,202 people were diagnosed with thyroid carcinoma worldwide in 2020, 14.9% of which were European, and the majority, (59.7%) Asian. A total of 2.3% of new thyroid carcinoma cases were registered in Malta in 2020, ranking it the twelfth commonest type of carcinoma locally [1]
Papillary thyroid carcinoma is the commonest type of malignant thyroid cancer [2] An earlier diagnosis will give a better prognosis to the patient, with delayed diagnosis leading to increased mortality [3]. An increase in the rate of thyroid cancer cases has been noted over the years, the cause of which has not yet been fully understood. One contribution is definitely the increase in diagnosis of microcarcinoma and occult disease [4]. In view of the significant numbers of yearly new cases and the importance of early diagnosis for prognosis, it is of importance to study thyroid cancer and its diagnosis in more detail.
The diagnosis of papillary carcinoma relies on nuclear features which include optical clearing, elongation, micronuclei and pseudoinclusions [5]. However, morphological overlaps between follicular adenoma, papillary carcinoma and multinodular goitre showing features of papillary budding cause a diagnostic dilemma. In view of these inconsistencies, several immunohistochemical markers have been studied to assess their use in aiding diagnoses.
Some useful markers for differentiated thyroid cancer which have already been studied, are Galectin-3 (Gal-3), Hector-Battifora mesothelial antigen-1 (HBME-1), cytokeratin-19 (CK-19) and RET/PTC. The latter two each identify a sub-population of papillary carcinoma.
RET/PTC rearrangement is the most common genetic alteration in thyroid carcinoma, recognised to date. It is most commonly found in children and young adults, and also in papillary carcinoma associated with exposure radiation. The prevalence of papillary thyroid carcinoma related to RET/PTC is higher in North America (35%) [6]. The variation in prevalence is also due the tumour heterogeneity [7]. If the distribution of this marker is heterogenous, it will be found in most neoplastic cells and is referred to as clonal RET/PTC whilst if it is present in a small fraction of cells it is known as non-clonal RET/PTC.
It is interesting to note that clonal RET/PTC has only been found in papillary thyroid carcinoma whilst non-clonal RET/PTC has been also found in 10–45% of thyroid adenomas and other non-neoplastic thyroid lesions and Hashimoto’s thyroiditis, as concluded from various studies [8,9].
CK-19 is a type I intermediate filament protein present in simple or glandular epithelial cells. It was shown to be sensitive to papillary thyroid carcinoma, usually with a strong diffuse plasma reactivity [10] however, its specificity for both malignancy and papillary cancer was low (63.1%). CK-19 expression in benign lesions was noted to be high in several studies, with a low specificity and sensitivity when compared to HBME-1 and Gal-3 [11]. In view of the low specificity of CK-19 in the literature, it was not included in our study. RET/PTC was tested; however, it did not stain any cells, both in the control and in the thyroid specimen, and after several trials as described in the method, this marker had to be abandoned.
Therefore, the study focused on the expression of Gal-3 and HBME-1 separately and as a panel on a spectrum of thyroid lesions to determine their diagnostic accuracy.
Gal-3 is a member of the beta-galactoside-binding protein family and has an important role in biological processes such as: cell–cell adhesion and cell–matrix interactions associated with tumour spread [12].Galectins have been extensively investigated regarding their role in cancer especially metastasis [13].A recent meta-analysis by Tang et al., demonstrated that Gal-3 is useful in differentiating between non-papillary thyroid carcinoma and papillary thyroid carcinoma. Moreover, it was also more commonly found in patients with lymph node metastasis [14]. Gal-3 was found to be positive in 100% of papillary carcinomas, 62.5% of follicular carcinomas, 18.8% of follicular adenomas and negative in nodular goiters in a paper by Miskad et al., Galectin’s sensitivity was noted to be 81.25% and at a specificity of 90.62%.The same study showed that sensitivity increased by 10% when both Gal-3 and HBME-1 expression was combined [15].
HBME-1 is amembrane antigen found in the microvilli of mesothelial cells with an unknown function. It has gained popularity in the past decade and its expression in benign and malignant thyroid lesions has been investigated. In normal thyroid tissue, there is no expression of HBME-1; however, it is over-expressed in malignant tumours especially papillary thyroid carcinoma.Several studies reviewed by Rossi et al. show that HBME-1 is over-expressed in 78.8% in thyroid malignancy; 87.3% of papillary thyroid carcinoma and 65.2% of follicular thyroid carcinoma. HBME-1 was shown to detect malignant thyroid tumours with a specificity of 82.1% [16].
The objectives of this study were to look at the different types of thyroid cancer in the Maltese population and to study the use of Gal-3 and HBME-1 as immunohistochemical markers in thyroid cancer.
2. Materials and Method
Formalin-fixed, paraffin embedded tissue from fifty-eight surgically resected thyroid tissue were obtained from the pathology archives of Mater Dei Hospital for the years of 2008 and 2013. (Formalin used: 10% Nuetral Buffered Formalin, CellPath Ltd., Newton, Powys, UK. Fixation was performed for 24–48 h). Reagent obtained from Leica BioSystems, Newcastle Upon Tyne, UK).
The study was approved by the University Ethics Research Committee of the University of Malta on 25th September 2018, (FREC unique ID: FRECMDS_1718_048), and the Mater Dei Hospital Data Protection Office. All patients who underwent surgery due to a thyroid carcinoma during the years 2008 and 2013 were included. Use of tissue and patient clinical information was subject to written informed consent, apart from those who were deceased, following which the information was anonymised and de-identified prior to analyses. Patients who did not give informed consent to participate were excluded from this study, together with those who had inadequate samples which could not undergo immunohistochemical staining in view of their state. The tissue sections representing the tumour were chosen by a senior pathologist, blinded to the type of thyroid cancer present, to avoid bias.
2.1. Tissue Preparation and Staining
3 μm sections of paraffin embedded thyroid tumour tissue were placed on glass slides and dried at 37 °C overnight. Sections were de-waxed through a sequential treatment in xylene for 7 min, xylene-alcohol for 2 min, graded alcohol (100%, 70% and 30%, for 4, 2 and 2 min, respectively) and then rinsed in water. Heat-induced antigen retrieval was carried out for both markers using citrate buffer at pH 6 (3.15 g of citric acid in 1.5 L of distilled water). The slides were then boiled in a pressure cooker for one hour in the antigen retrieval solution and afterwards they were cooled down to room temperature. This method was chosen after several runs of water bath and pressure cooker with citrate buffer at different concentrations. The polymer detection method was chosen over ABC detection method, since the former produced better results after staining.
Sections were then placed in 0.3% hydrogen peroxide solution for 20 min at room temperature. Subsequently, the slides were rinsed in tap water and mounted in Sequenza humid chambers, washed with 1× PBS (1 g KCl, 40 g NaCl, 7.2 g Na2HPO4, 1.2 g KH4PO4, 5 L of distilled H2O; pH 7.4 and then incubated with 100 μL of UltraCruz® blocking reagent (sc-516214 from Santa Cruz) for 10 min at room temperature to block non-specific binding.
2.2. Immunostaining
Primary antibodies were diluted in antibody diluent (1 g sodium azide, 1 g bovine albumin and 100 mL PBS). A total of 100 μL of the diluted antibodies was added to each slide and left at 4 °C overnight. After the primary antibodies, the sections were processed using the polymer detection method. A total of 100 μL of post-primary reagent (secondary antibody) was applied for 15 min at room temperature, and then 100 μL of polymer reagent was added for another 15 min and then washed sequentially 3 times with PBS. The sections were then stained using DAB (3,3-Diaminobenzidine), and counterstained with Harris haematoxylin. Sections were then dehydrated in graded ethanol, xylene and finally mounted in DPX. Refer to Table 1 for primary antibodies details.

Table 1.
Details of primary antibodies used for immunohistochemical analysis.
RET (monoclonal C-3 Santa Cruz biotechnology Inc., Santa Cruz, CA, USA) was the only marker which did not demonstrate satisfactory and consistent staining after immunohistochemical processing in our study. A new batch of the same antibody was obtained and re-evaluated; however, it did not stain any cells, both in the controls and the actual thyroid specimen. Different concentrations were also tested to no avail, and due to time constraints in view of a degree-related deadline for completion of research study, immunohistochemistry of RET/PTC was abandoned.
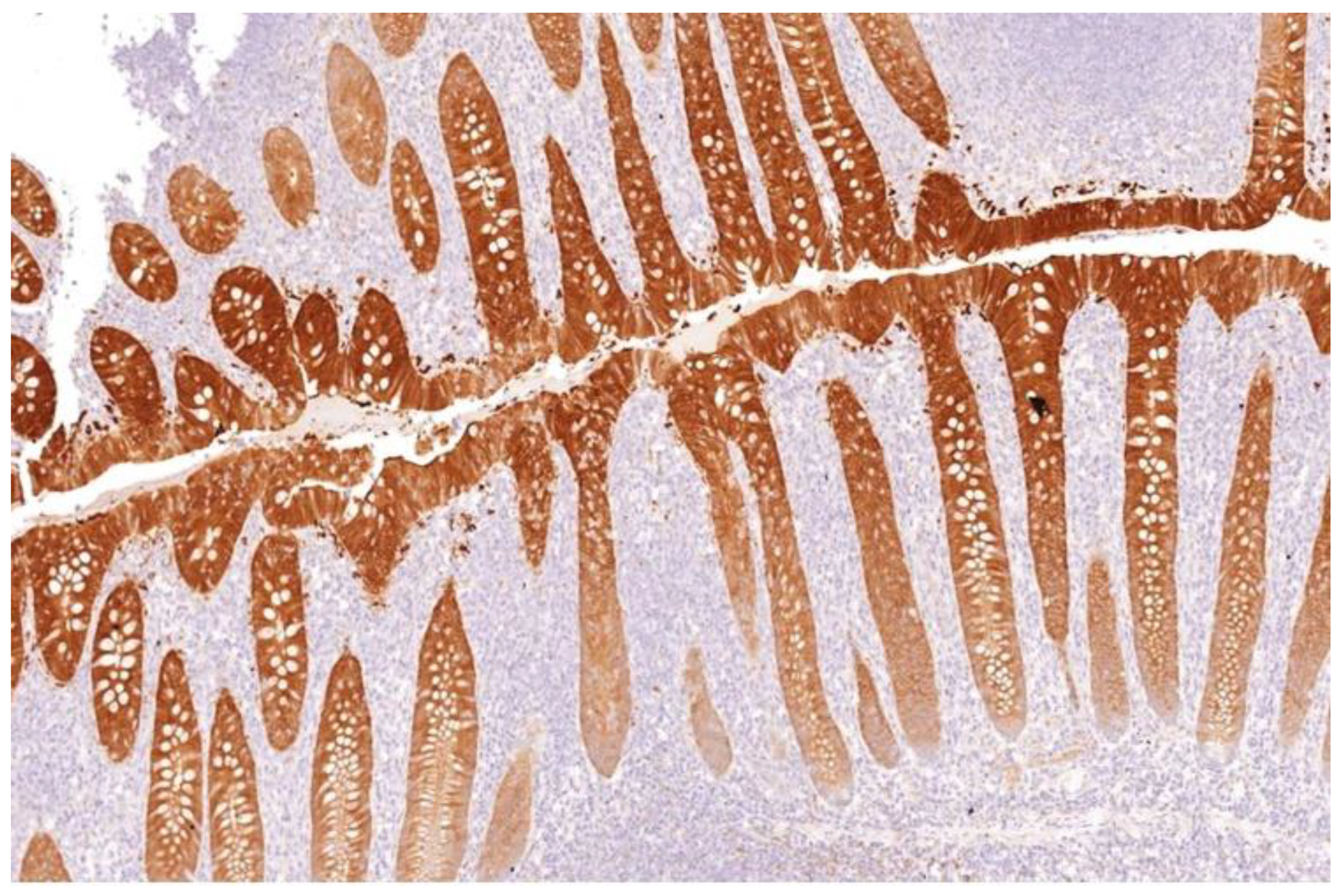
Appendix and skin tissues were used as positive controls for Gal-3 (Figure 1), while tonsil and mesothelioma tissues were used for HBME-1. Patient-matched normal thyroid tissue was included as negative control where available.
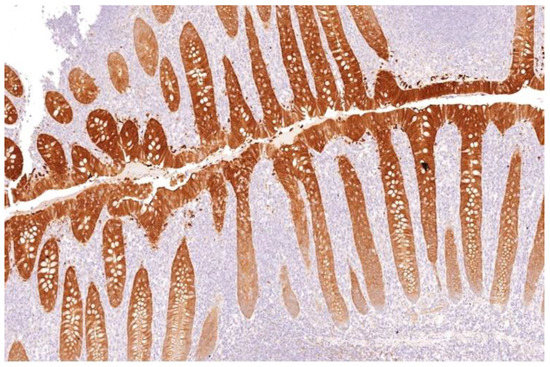
Figure 1.
Galectin-3 staining strongly as brown stain after IHC in appendix crypts of Lierberkuhn (control) (×100).
2.3. Statistical Analysis
The analysis involved the evaluation of HBME-1 and Gal-3 and their relation to benign and malignant thyroid lesions with the aim of identifying any significant difference between the two. Their sensitivity, specificity and positive predictive values were also calculated using MedCalc Software version 19.4.1 (https://www.medcalc.org/calc/diagnostic_test.php, accessed on 20 March 2019). The immunohistochemical markers were analysed according to their percentage positivity index, the intensity of the staining and the H-score. From these values, the true positives, true negatives, false positives and false negatives were identified, and the sensitivity, specificity, positive and negative predictive values were calculated. True positive was taken as a papillary thyroid carcinoma which tested positive for the marker in question (>15% staining for Gal-3 and >30% staining for HBME). True negatives were thyroid lesions other than papillary thyroid cancer which did not stain significantly.
The Kruskal–Wallis test was used to compare the mean Gal-3 percentage staining scores between the different types of carcinoma, since Gal-3 score distribution did not satisfy the normality assumption (p-value = 0) and a non-parametric test had to be used for this analysis. On the other hand, the one-way ANOVA test, which is a parametric test, was used to compare mean HBME-1 percentage staining scores between the different types of thyroid cancer since HBME-1 score distribution satisfied the normality assumption (p-value = 0.2).For both tests, the null hypothesis specifies that the mean scores vary marginally between the cancer types and is accepted if the p-value exceeds the 0.05 level of significance. The alternative hypothesis specifies that the mean scores vary significantly between the different types of cancer and is accepted if the p-value is less than 0.05.
Receiver operating characteristics curves (ROC) were plotted using SPSS. Each point on the ROC plot represents a sensitivity/specificity pair corresponding to a particular cut-off. Quantitative measures of accuracy including the area under the curve (AUC), was also calculated using this software.
3. Interpretation and Analysis
A number of the slides were reviewed with two independent senior pathologists, to aid in identifying benign and malignant areas of thyroid tissue. All sections were scanned at 20× objective magnification using the Pannoramic MIDI viewer (3DHISTECH, Budapest, Hungary). Positive staining was quantified using CellQuant Quantification software (3DHISTECH, Budapest, Hungary), as an intensity (0–3) and a percentage staining to calculate the H-score. Staining was assessed in both the area of tumour and also normal tissue.
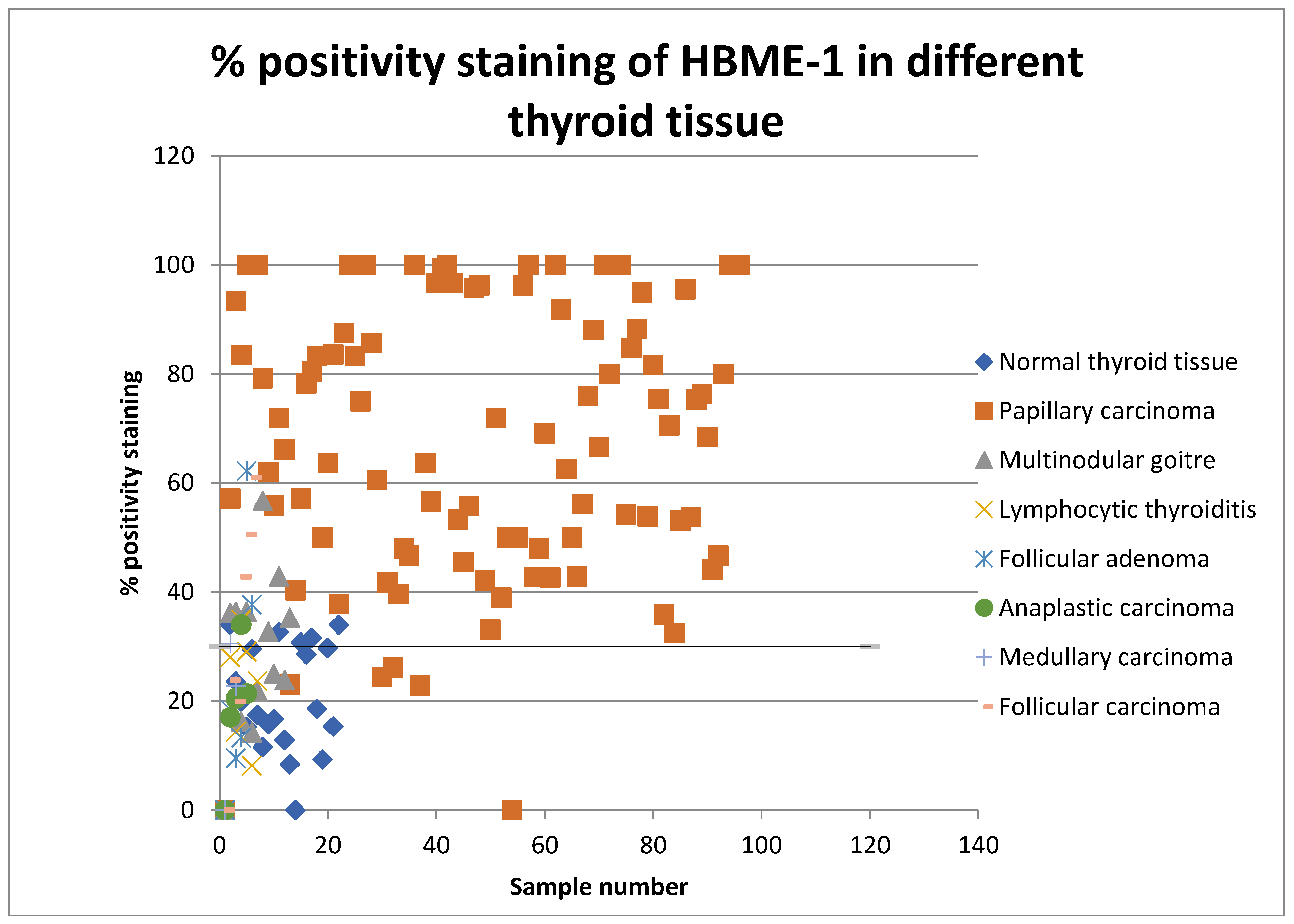
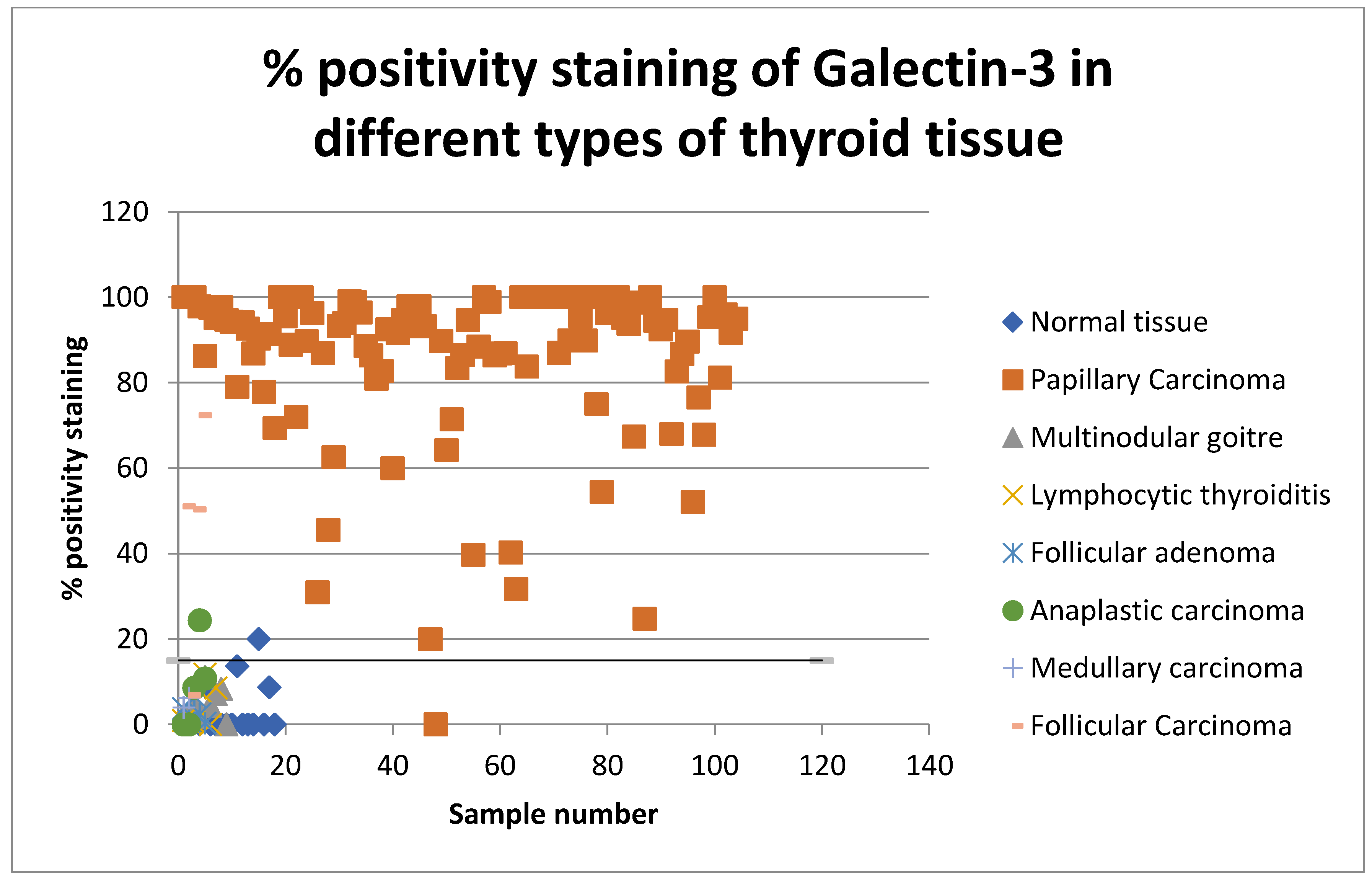
The percentages of positive cell values for all thyroid lesions were then plotted on a scatter graph and the sensitivity and specificity for detecting papillary thyroid carcinoma was assessed (shown in Figure 2 and Figure 3). Percentage positivity is the percentage of positively stained cells with the marker in question. Scatter graphs were plotted and their average values for positive cells in benign and malignant thyroid samples suggested a threshold.
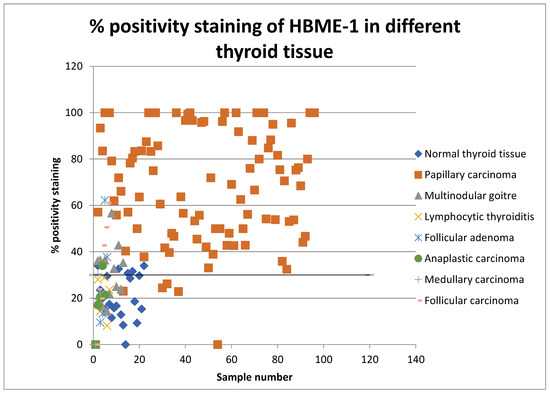
Figure 2.
Scatter graph showing percentage positivity staining rates of HBME-1 in all types of thyroid tissue in 2008 and 2013.
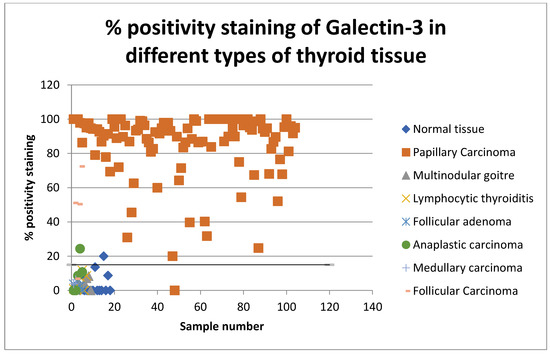
Figure 3.
Scatter graph showing percentage positivity staining rates of Gal-3 in all types of thyroid tissue in 2008 and 2013.
The positivity index represents the percentage of cells staining positive for the examined marker. The H-score is a summation of the percentage of area stained at each intensity level multiplied by the weighted intensity. The score is calculated by using the following formula: 3× percentage of strongly stained nuclei + 2× percentage of moderately stained nuclei + percentage of weakly staining nuclei. The range of the H-score is between 0 and 300 [17].
The staining results were then correlated with the original histological diagnosis and results tabulated. HBME-1 expression was cytoplasmic with luminal attenuation whilst Galectin-3 was expressed in both the nuclear and cytoplasmic compartments. Controls stained strongly positive for both markers, respectively.
4. Results
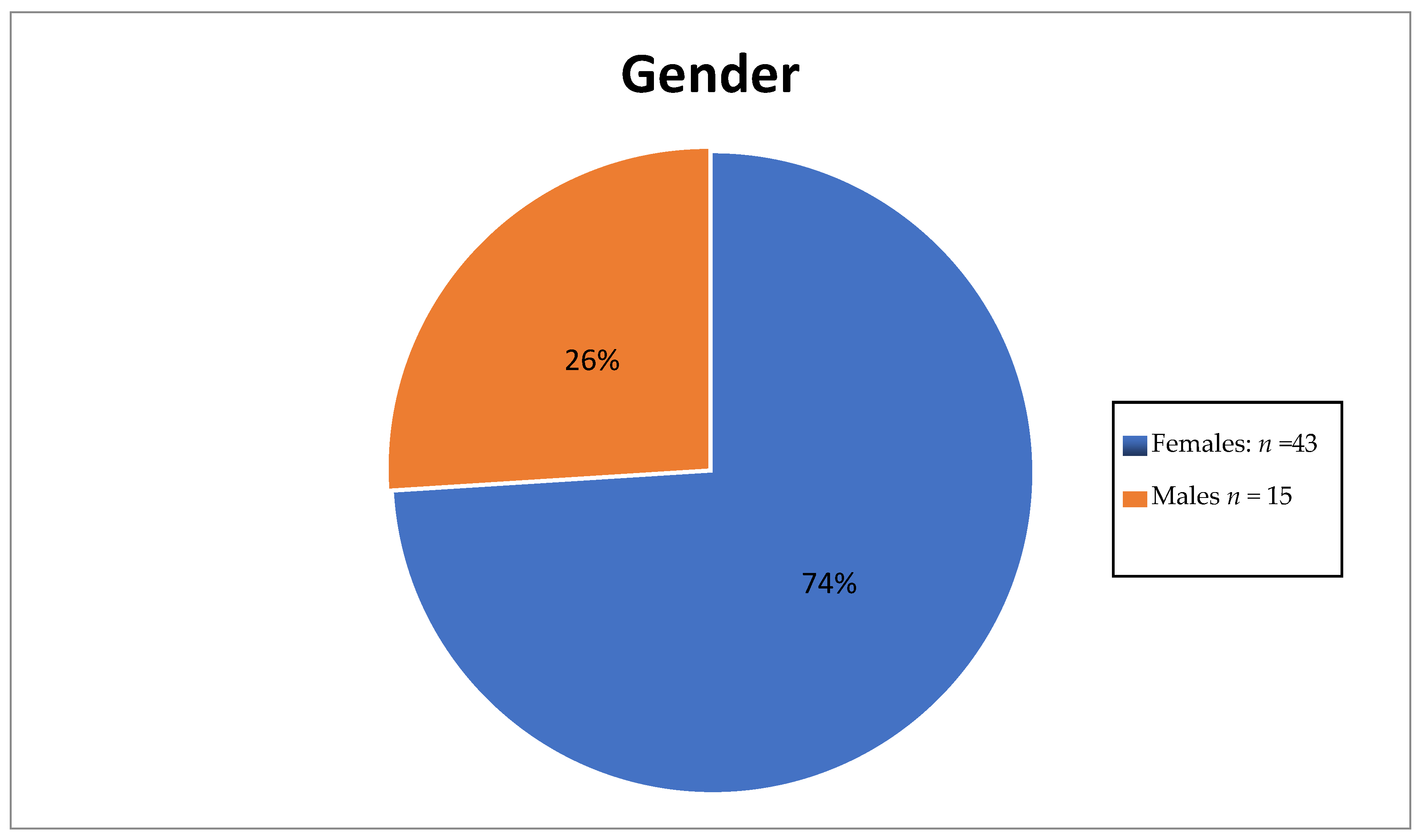
Table 2 shows the numbers of different subtypes of thyroid carcinoma diagnosed in 2008 and 2013, whilst gender and age distribution results are shown in Figure 4 and Figure 5, respectively.

Table 2.
Distribution of thyroid carcinoma subtypes diagnosed in 2008 and 2013.
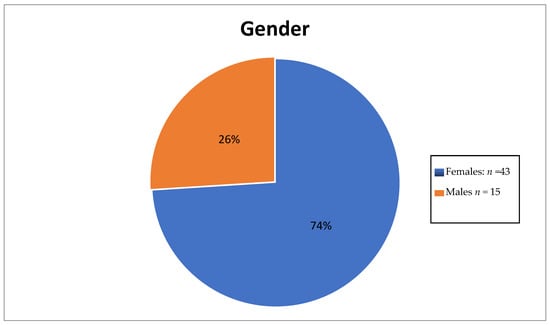
Figure 4.
Pie chart showing gender disparity.
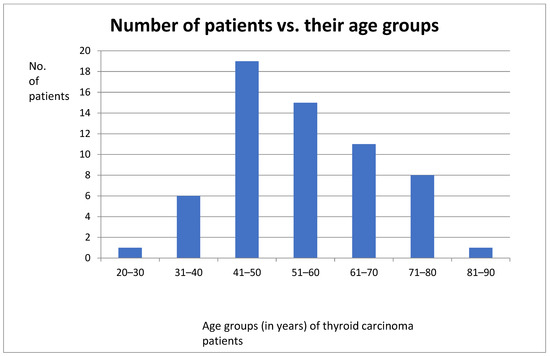
Figure 5.
Bar graph showing distribution of patients and their age groups.
The number of female thyroid cancer patients was three times that of male patients. The ages of these patients ranged between the third and eight decades with the highest number of patients being in between the 41st and 50th year of age.
4.1. Immunohistochemical Results
The mean Gal-3 staining score for papillary carcinoma (80.49) was found to be significantly higher (p-value = 0.012) than that for other malignant thyroid diseases. This was also the case for HBME-1 where the staining score was highest for papillary carcinoma (68.28, p-value = 0.002). This data supports the relevance of Gal-3 and HBME-1 for use in the diagnosis of papillary thyroid carcinoma.
On the other hand, the staining of both markers was not significantly different between benign lesions, meaning that both markers are not specific to a particular benign lesion. These results are shown in Table 3.

Table 3.
Mean staining scores of Gal-3 and HBME-1 across subtypes of thyroid carcinoma and subtypes of benign thyroid lesions.
Each marker’s specificity and sensitivity to detect papillary thyroid carcinoma were evaluated further. A value of >15% staining was taken as positive for Gal-3 whilst >30% staining was taken as positive for HBME-1. This chosen cut-off value was based on the histology sections as described by Zhang et al. [18]). A total of 2/4 cases of follicular carcinoma and 1/1 case of high-grade anaplastic carcinoma were found to express high levels of Gal-3 protein, whilst 2/41 cases of follicular carcinoma were considered to be false negative showing a low expression to Gal-3. The cases which were documented as true negative included anaplastic thyroid carcinoma, multinodular goitre and lymphocytic thyroiditis. All normal thyroid tissue did not stain with Galectin-3.
The sensitivity and specificity of both markers were calculated and shown in Table 4, Table 5 and Table 6.

Table 4.
Sensitivity, specificity, positive and negative predictive values of combined Gal-3 and HBME-1 with a 95% confidence interval (CI).

Table 5.
Sensitivity and specificity of Gal-3.

Table 6.
Sensitivity and specificity values for HBME-1.
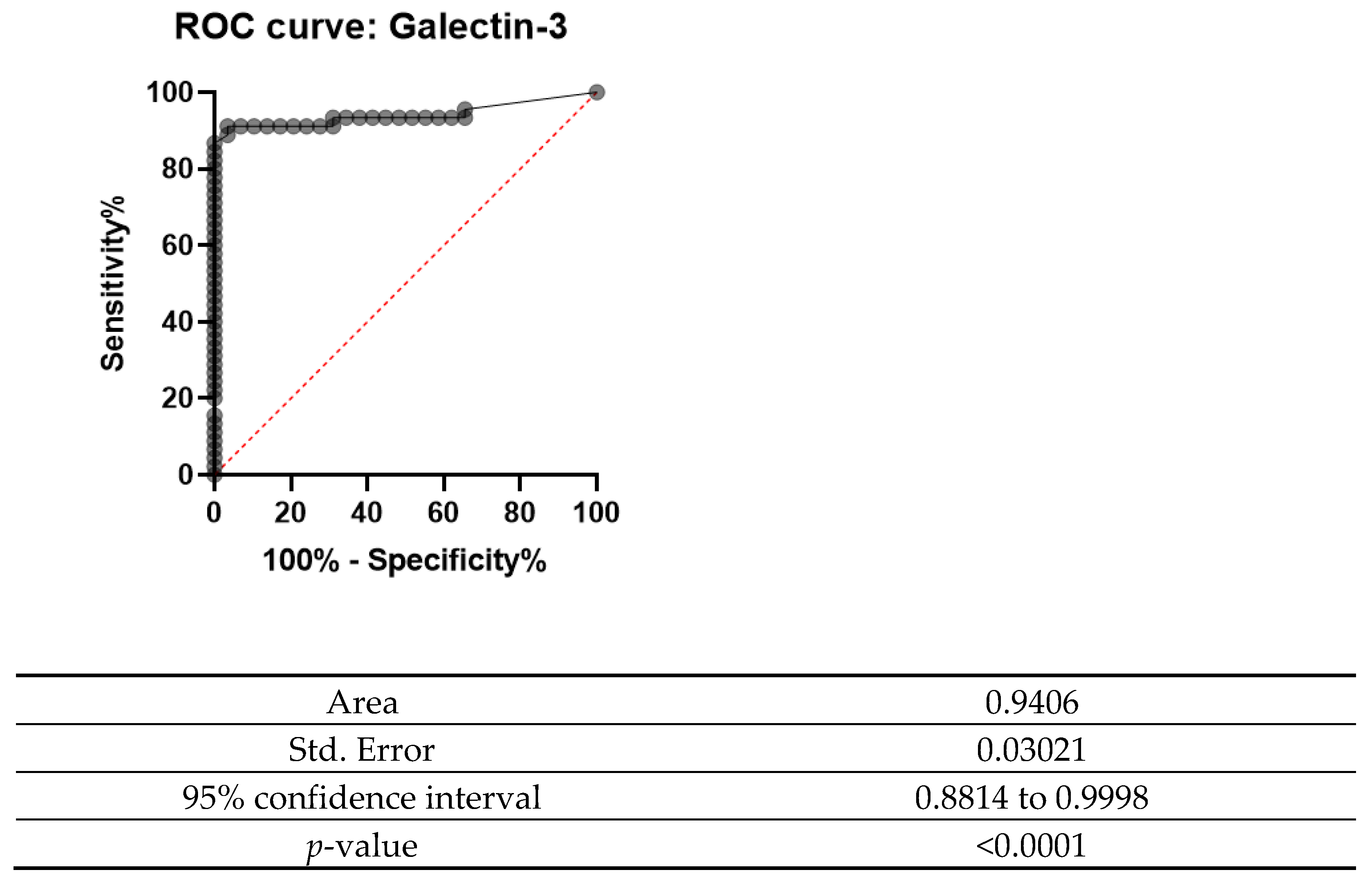
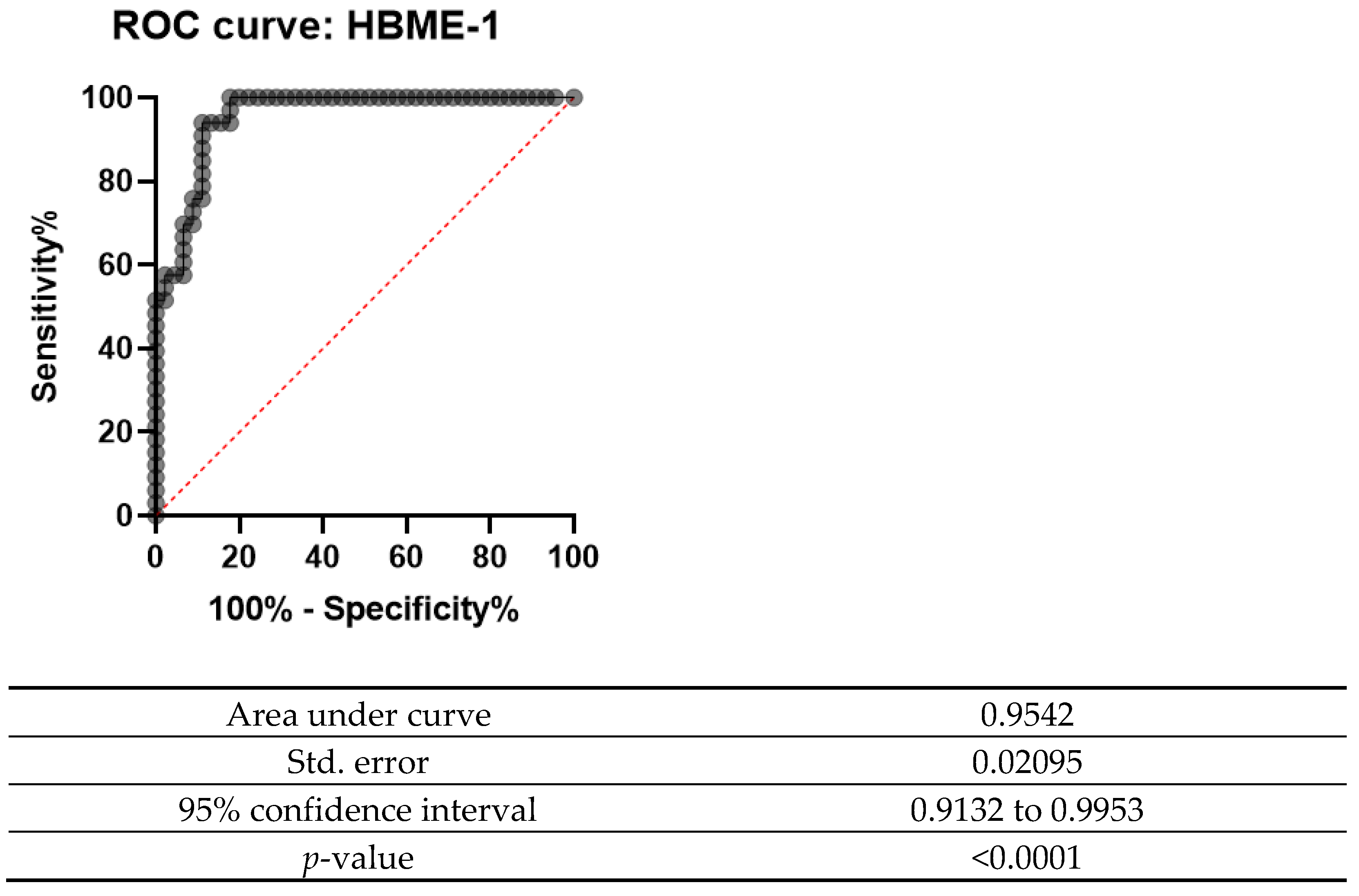
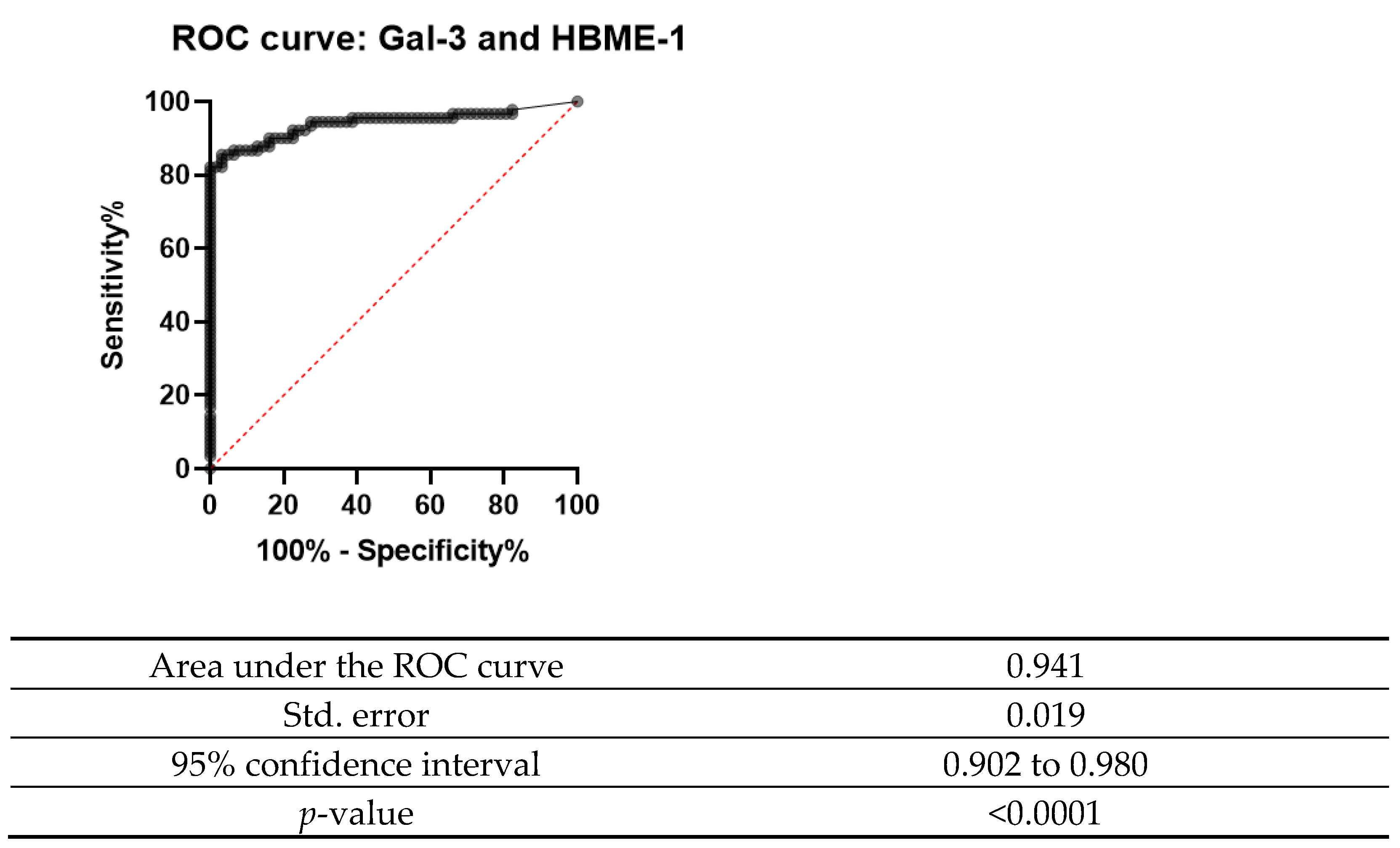
Galectin-3 had the highest value for both sensitivity and specificity (95% and 92.5%, respectively). Moreover, it also showed a high percentage of positive and negative predictive values (92.68% and 94.87%, respectively). These values show that this marker is valuable in the diagnosis of thyroid carcinoma, since it is present in the true positive cases and negative in the true negative cases. On the other hand, HBME-1 had a sensitivity of 90.91% and a specificity of 67.35%. Its positive predictive value and negative predictive value were also noted to be less than for Gal-3 (71.43% and 89.19%); however, when the two markers were combined, they had a sensitivity of 92.86% and specificity of 78.65% and a positive predictive value of 80.41% and a negative predictive value of 92.11%. These values show that both these markers can be useful in the diagnosis of thyroid carcinoma.
4.2. How Beneficial Are These Markers in the Clinical Setting?
A receiver operating characteristic curve (ROC curve) is plotted with the true positive rate against the false positive rate. This curve measures the diagnostic performance of biomarkers under study. The area under the curve (AUC) is equal to the probability that a classifier will rank a randomly chosen positive instance higher than a randomly chosen negative one.
The ROC curve was designed for both Gal-3 and HBME-1 as a combination of markers as shown in Figure 6, Figure 7 and Figure 8. In all the below curves, the p-value was always found to be less than the 0.05 level of significance, thus it can be deduced that the areas under the graphs are larger than 0.5 and so, the markers have a better significance than if they were to be attributed to chance.
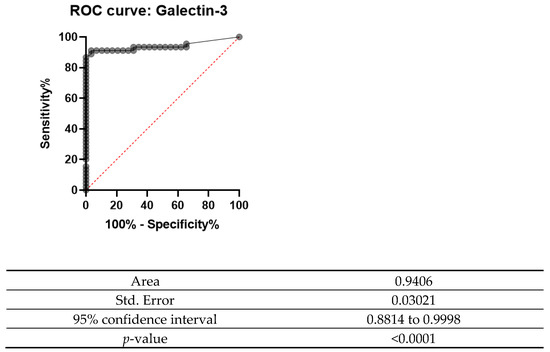
Figure 6.
ROC curve for Gal-3 (2008 and 2013).
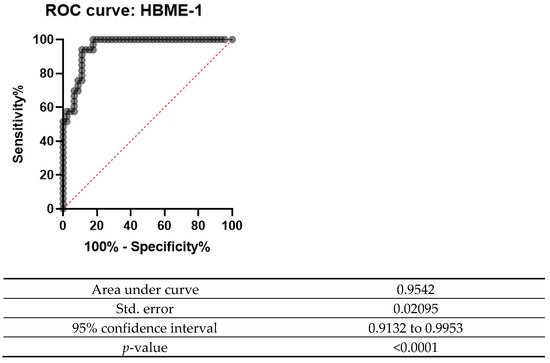
Figure 7.
ROC curve for HBME-1 (2008 and 2013).
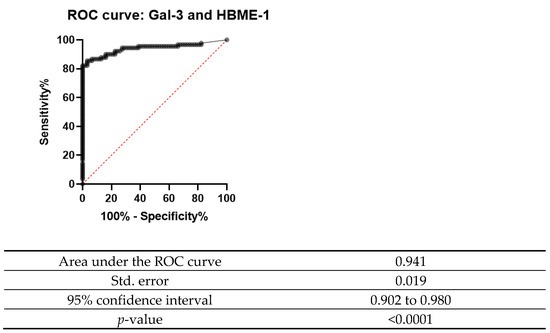
Figure 8.
ROC curve for Gal-3 and HBME-1 combined.
5. Discussion
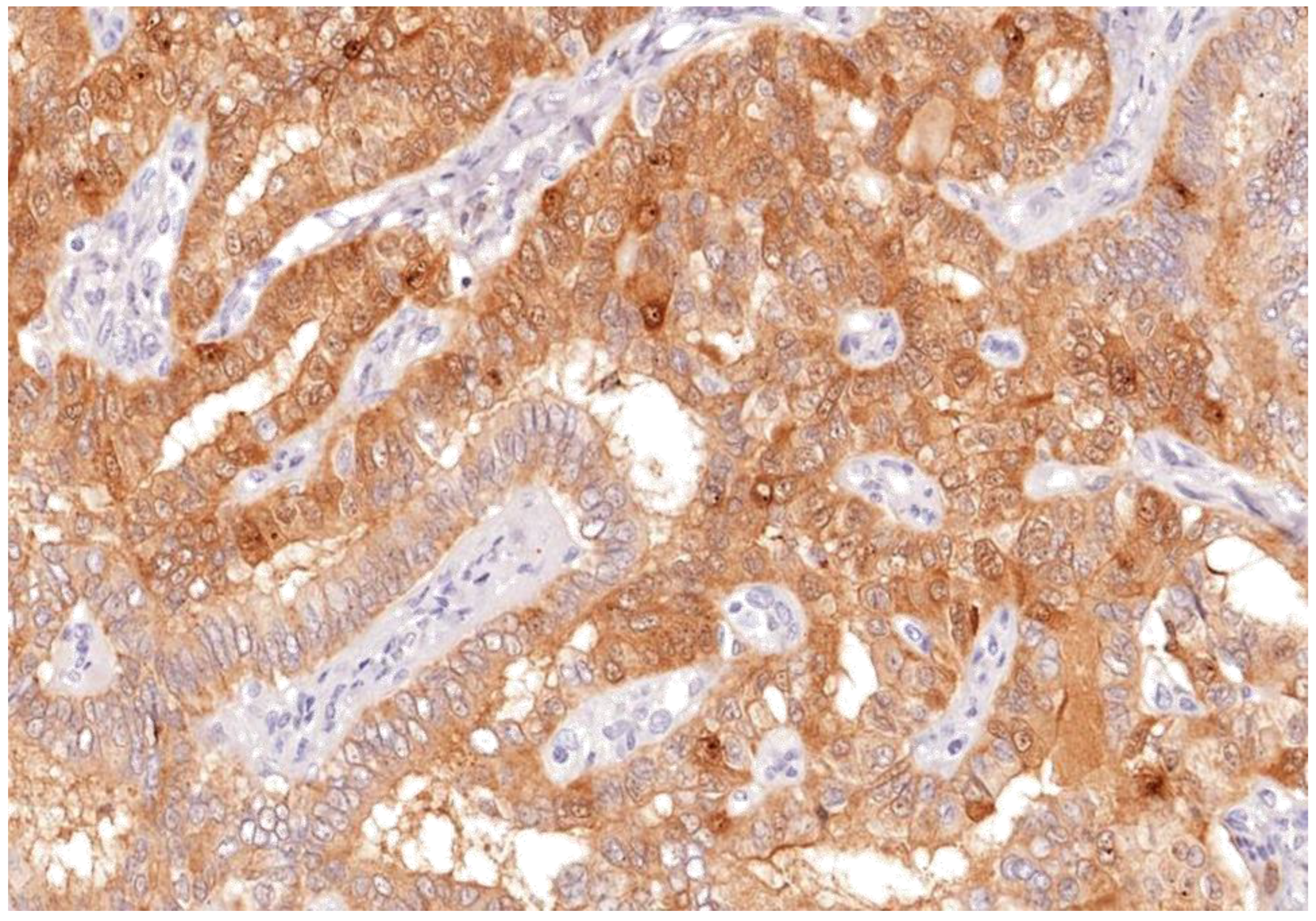
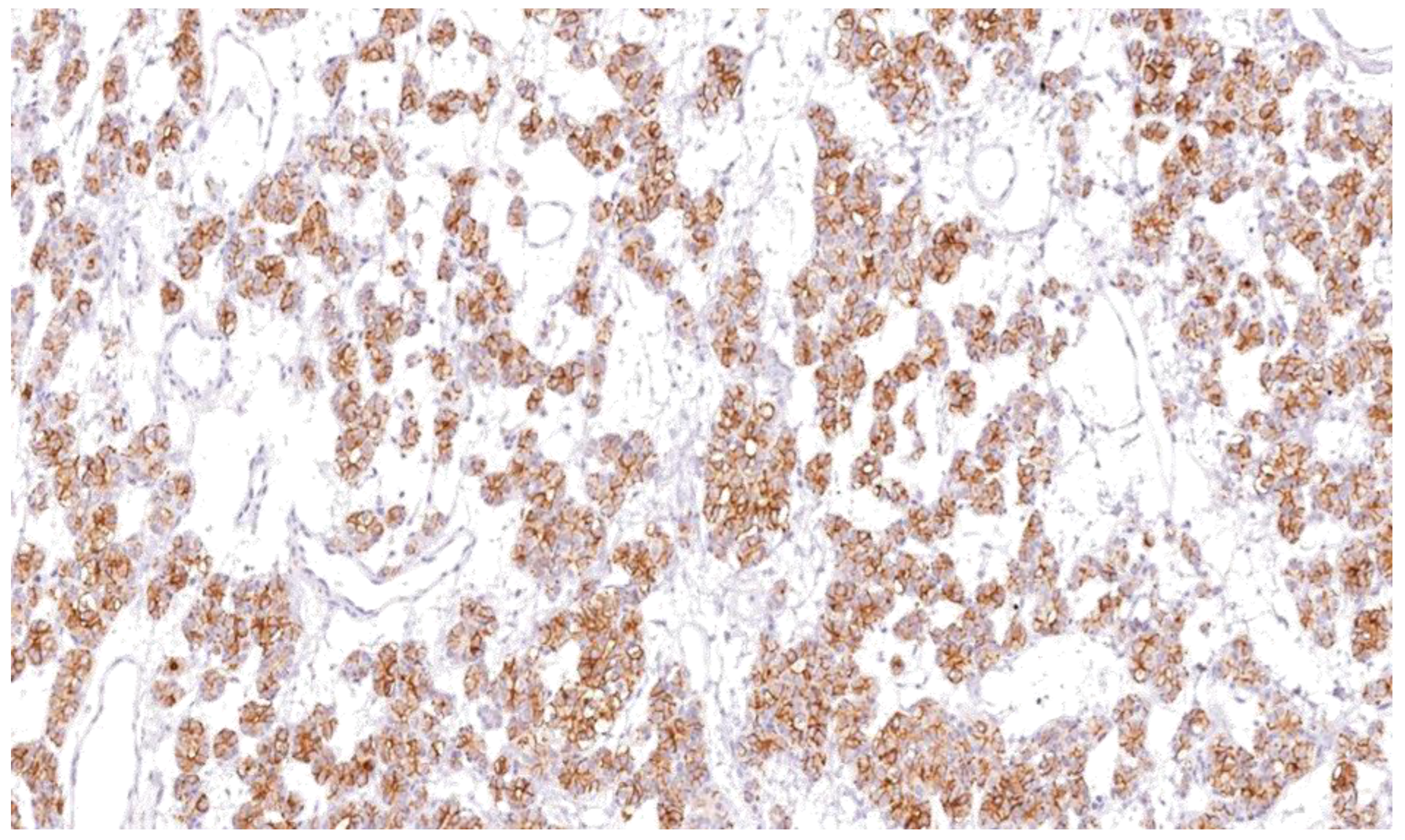
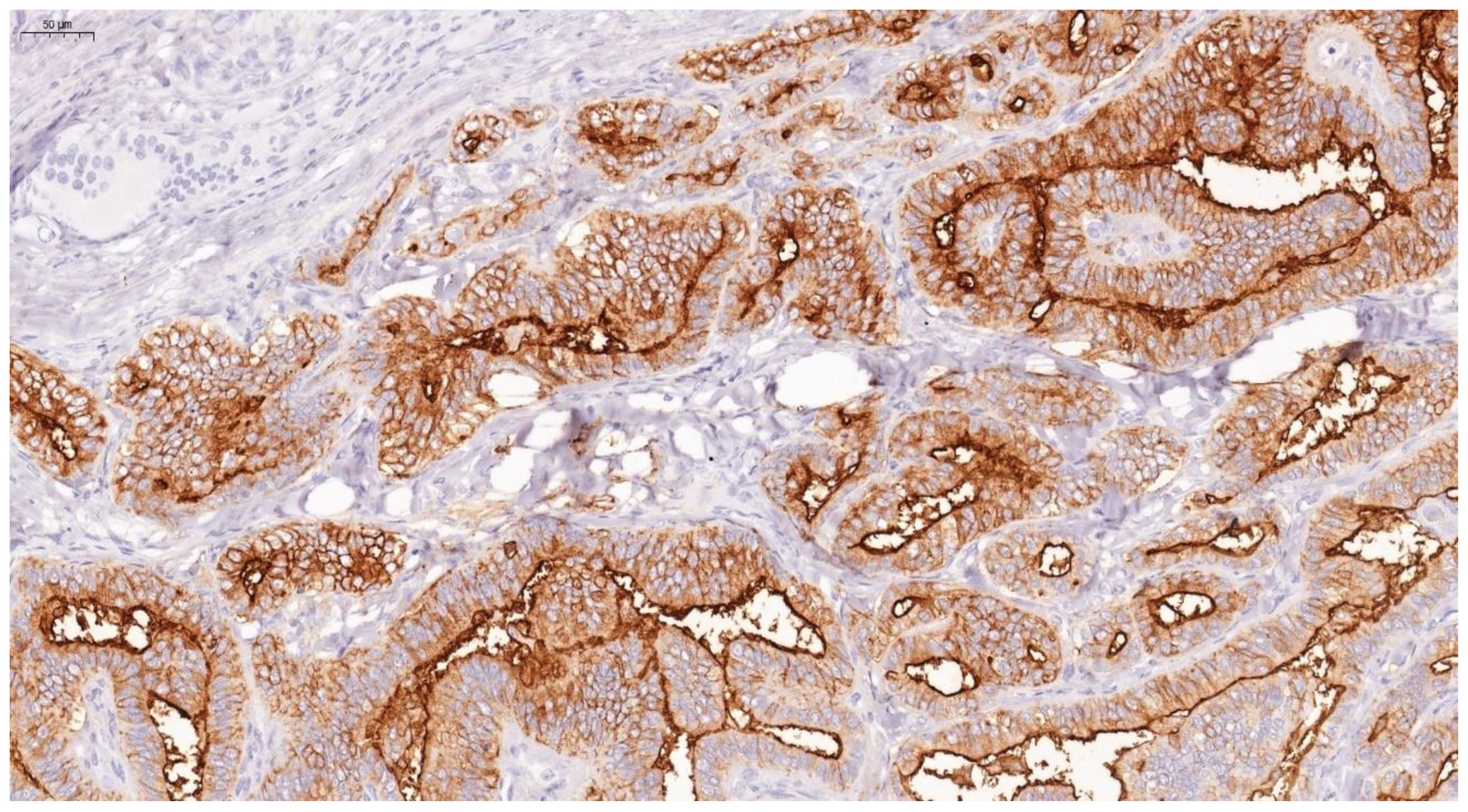
Thyroid lesions are common clinical findings especially in women and between the ages of 30 and 60 years, and thyroid cancer has been documented to be the commonest endocrine malignancy [19]. Fortunately, the majority of clinically diagnosed thyroid lesions are benign tumours. However, it is important to identify cancerous lesions with immediate optimum management for maximum patient benefit [20]. Incorrect diagnosis and interpretation of the cytology can lead to significant social and psychological problems, causing an unnecessary increase in healthcare costs [21]. Additionally, FNAB is not the most reliable investigation to differentiate between benign and malignant tumours, especially in tumours which have a follicular growth pattern, with difficulty to assess capsular invasion at times, in fact 30% of FNA examinations yield indeterminate or non-diagnostic results, complicating patient management [22]. In view of these diagnostic dilemmas, studies on immunohistochemical markers have increased in an attempt to help in distinguishing between difficult cases [23]. Our study confirms the usefulness of Gal-3 and HBME-1 and shows their sensitivity and specificity as a combination in papillary thyroid carcinoma. In this study, Gal-3 scores were significantly higher for papillary thyroid carcinoma than for other types of thyroid carcinoma. Similar results were noted with HBME-1, making both markers useful in distinguishing papillary from other types of thyroid carcinoma (Figure 9, Figure 10 and Figure 11). These results provide further evidence to the literature, in favour of these markers’ diagnostic use in papillary thyroid carcinoma [24].
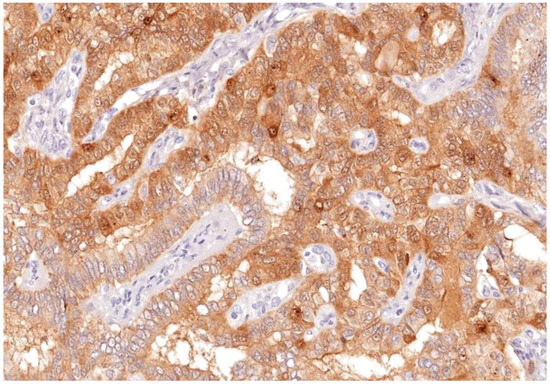
Figure 9.
Galectin-3 staining shown nuclear and membranous staining as brown stain after IHC in apillary thyroid carcinoma; (labelled) (Magnification ×100).
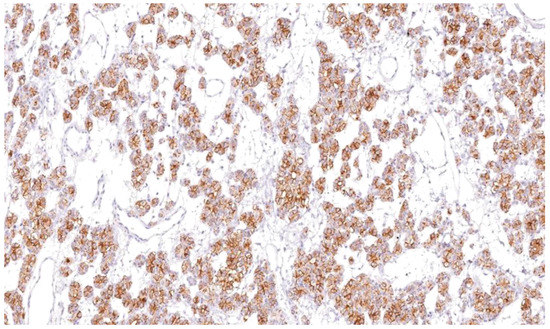
Figure 10.
HBME-1 showing cytoplasmic and membranous staining as brown after IHC in papillary thyroidcarcinoma (×100).
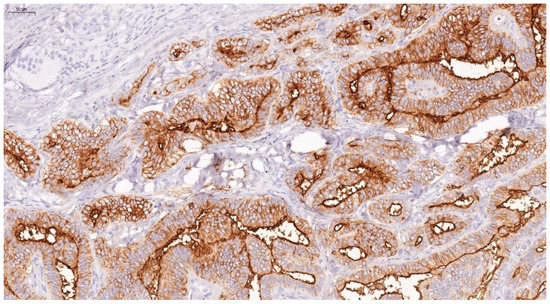
Figure 11.
HBME-1 showing strong membranous and cytoplasmic staining as brown, after IHC in papillary thyroid carcinoma (×100).
Both markers were also more commonly found in cancerous tissue and absent or stained weakly in benign lesions. In the case of lymphocytic thyroiditis where Gal-3 showed weak staining, the literature had already debatedthe relationship between Gal-3 and lymphocytic thyroiditis, and it was concluded that in Hashimoto’s thyroiditis, features of papillary thyroid carcinoma, including follicular epithelial dysplasia are present, and thus immunomarkers may stain and overlap [25]. Several studies have also found Galectin-3 to be weakly expressed in the follicular epithelium and in entrapped follicles in lymphocytic thyroiditis. [26].Likewise, Gal-3 staining in advanced medullary thyroid carcinoma has been documented and it has been suggested that this marker might play a role in its pathogenesis [27]. Follicular adenomas which stain positively with Gal-3 are considered an indication of early or incipient carcinoma where capsular or vascular invasion has not yet been noted on histology [28].In the case of HBME-1 where it showed weak positivity in some cases of lymphocytic thyroiditis and one case of follicular adenoma, it was noted that these cells were adjacent to the cancerous tissue itself. HBME-1 has already been linked to follicular carcinoma, multinodular goitre and follicular adenoma [29]. Moreover, lymphocytic thyroiditis has been linked to malignancy in a number of studies [30]. and HBME-1 was shown to be positive in foci of follicular epithelial dysplasia in lymphocytic thyroiditis [31].
6. Conclusions
The current challenge is to find immunohistochemical markers that are mostly or exclusively positive in cancer tissue. This study has shed more light on the use of Galectin-3 and HBME-1 as immunohistochemical markers as a supplementary test in the diagnosis of thyroid neoplasms. Both Gal-3 and HBME-1 showed high values of sensitivity and specificity both separately and combined and thus offer reliability in distinguishing benign from malignant. A panel of two markers combined helps in avoiding technical problems or processing issues. This low cost immunohistochemical test helps to reduce the number of unnecessary surgical resections of benign nodules, leading to a better quality of life for the patients and less burden on the healthcare services.
Limitations of This Study
It would have been useful to study Gal-3 and HBME-1 expression both in histopathology and also in cytological specimens, where in the latter IHC stains to determine malignancy can be very useful in the absence of morphology. Secondly, some subtypes were underrepresented in this study. A study using larger numbers is needed to confirm Gal-3 and HBME-1 across distinct thyroid lesions.
Thirdly, a standardised protocol for the application of Gal-3 and HBME-1 in the differentiation between benign and malignant thyroid nodules is not available at present. A standard staining protocol and method of scoring is crucial to facilitate implementation into diagnostic lab.
Author Contributions
Conceptualization, C.V. and J.V.; methodology, S.B., R.F. and J.V.; software, S.B. and C.V.; validation, R.F. and S.B.; formal analysis, C.V. and S.B.; investigation, C.V. and S.B.; resources, S.B.; data curation, C.V. and J.V.; writing-original draft preparation, C.V.; writing-review and editing, J.V. and S.B.; visualisation, C.V. and S.B.; supervision, J.V., S.B. and R.F.; project administration, J.V.; funding acquisition, C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Faculty of Medicine and Surgery, University of Malta (Research Fund Vote number MDSBM 20-53).
Institutional Review Board Statement
The study was conducted according to the guidelines of Declaration of Helsinki, and approved by the Research Ethics Committee of University of Malta (Reference No. FRECMDS_1718_048 on 25 September 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not Applicable.
Acknowledgments
We would like thank all the staff of the labs used, namely the Immunohistochemistry lab at Mater Dei Hospital and the Molecular Genetics lab in the Biomedical Sciences building at the University of Malta. Mostly, I sincerely thank Godfrey Grech and James DeGaetano for allowing me to use their labs and facilities, and for going out of their way to share their knowledge and advice when needed. Particular thanks goes to Sharon Cassar who has helpedwith the tissue cutting and slide preparation.
Conflicts of Interest
The authors declare that there was no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Longo, D.L.; Kasper, D.L.; Jameson, J.L.; Fauci, A.S.; Hauser, S.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 16th ed.; McGraw-Hill Publishers: New York, NY, USA, 2012. [Google Scholar]
- Xu, D.; Wang, L.; Long, B.; Ye, X.; Ge, M.; Wang, K.; Guo, L.; Li, L. Radiofrequency ablation for postsurgical thyroid removal of differentiated thyroid carcinoma. Am. J. Transl. 2016, 8, 1876–1885. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21190442 (accessed on 14 June 2019).
- Chen, A.Y.; Jemal, A.; Ward, E.M. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 2009, 115, 3801–3807. [Google Scholar] [CrossRef]
- Xu, B. Papillary Thyroid Carcinoma Overview. 2020. Available online: https://www.pathologyoutlines.com/topic/thyroidpapillary.html (accessed on 25 April 2022).
- Nikiforov, Y. RET/PTC re-arrangement in thyroid tumours. EndocrPathol. 2002, 13, 3–16. [Google Scholar] [CrossRef]
- Puxeddu, E.; Moretti, S.; Elisei, R.; Romei, C.; Pascucci, R.; Martinelli, M.; Marino, C.; Avenia, N.; Rossi, E.D.; Fadda, G.; et al. BRAF(V599E) Mutation is the Leading Genetic Event in Adult Sporadic Papillary Thyroid Carcinomas. J. Clin. Endocrinol. Metab. 2004, 89, 2414–2420. [Google Scholar] [CrossRef] [Green Version]
- Saad, A.; Falciglia, M.; Steward, D.L.; Nikiforov, Y.E. Amiodarone-induced Thyrotoxicosis and Thyroid Cancer: Clinical, Immunohistochemical, and Molecular Genetic Studies of a Case and Review of the Literature. Arch. Pathol. Lab. Med. 2004, 128, 807–810. [Google Scholar] [CrossRef]
- Mochizuki, K.; Kondo, T.; Nakazawa, T.; Iwashina, M.; Kawasaki, T.; Nakamura, N.; Yamane, T.; Murata, S.; Ito, K.; Kameyama, K.; et al. RET Rearrangements and BRAF Mutation in Undifferentiated Thyroid Carcinomas Having Papillary Carcinoma Components. Histopathology 2010, 57, 444–450. [Google Scholar] [CrossRef]
- Song, Q.; Wang, D.; Lou, Y.; Li, C.; Fang, C.; He, X.; Li, J. Diagnostic significance of CK19, TG, Ki 67 and galectin-3 expression for papillary thyroid carcinoma in the northeastern region of China. Diagn. Pathol. 2011, 6, 126. [Google Scholar] [CrossRef] [Green Version]
- Barroeta, J.E.; Baloch, Z.W.; Lal, P.; Pasha, T.L.; Zhang, P.J.; LiVolsi, V.A. Diagnostic Value of Differential Expression of CK19, Galectin-3, HBME-1, ERK, RET, and p16 in Benign and Malignant Follicular-derived Lesions of the Thyroid: An Immunohistochemical Tissue Microarray Analysis. Endocr. Pathol. 2006, 17, 225–234. Available online: https://www.ncbi.nlm.nih.gov/pubmed/17308359 (accessed on 5 May 2019). [CrossRef]
- Husain, A.S.; Jining, F.; Farah, T.; Al-Zohaili, O.; Husain, M.; Giorgadze, T. Differential expression of galectin-3, CK19, HBME1 and Ret iciprotein in the diagnosis of thyroid neoplasms by fine-needle aspiration biopsy. Cytojournal 2009, 6, 18. [Google Scholar]
- Newlaczyl, A.U.; Yu, L.G. Galectin-3—A Jack-of-All-Trades in Cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef]
- Tang, W.; Huang, C.; Tang, C.; Xu, J.; Wang, H. Galectin-3 may serve as a potential marker for diagnosis and prognosis in papillary thyroid carcinoma: A meta-analysis. Onco Targets Ther. 2016, 9, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Miskad, U.A.; Leiwakabessy, W.N.; Durry, M.F.; Rahawarin, H.; Cangara, M.H.; Djimahit, T. The Utility of Galectin-3 and HBME-1 in Differentiating Thyroid Lesions. Eur. J. Mol. Clin. Med. 2020, 7, 1177–1182. Available online: https://ejmcm.com/article_4599_36ffe00127a377c814d530ec7b8f5383.pdf (accessed on 5 May 2019).
- Rossi, E.D.; Straccia, P.; Palumbo, M.; Stigliano, E.; Revelli, L.; Lombardi, C.P.; Santeusanio, G.; Pontecorvi, A.; Fadda, G. Diagnostic and Prognostic Role of HBME-1, Galectin-3, and β-catenin in Poorly Differentiated and Anaplastic Thyroid Carcinomas. Appl. Immunohistochem. Mol. Morphol. 2013, 21, 237–241. [Google Scholar] [CrossRef]
- Ishibashi, H.; Suzuki, T.; Suzuki, S.; Moriya, T.; Kaneko, C.; Takizawa, T.; Sunamori, M.; Handa, M.; Kondo, T.; Sasano, H. Sex steroid hormone receptors in human thymoma. J. Clin. Endocrinol. Metab. 2003, 88, 2309–2317. [Google Scholar] [CrossRef]
- Zhang, L.; Krausz, T.; DeMay, R. A Pilot Study of Galectin03, HBME-1 and p27 Triple Immunostaining Pattern for Diagnosis of Indeterminate Thyroid Nodules in Cytology with Correlation to Histology. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Griebeler, M.; Gharib, H. Thyroid Nodules and Cancer. In Endocrinology and Diabetes; Bandiera, F., Gharib, H., Griz, L., Faria, M., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Nasr, M.R.; Mukhopadhyay, S.; Zhang, S.; Katzenstein, A.L. Immunohistochemical markers in diagnosis of papillary thyroid carcinoma: Utility of HBME1 combined with CK19 immunostaining. Mod. Pathol. 2006, 19, 1631–1637. [Google Scholar] [CrossRef]
- Collet, J.F.; Fajac, A. Galectin-3 immunodetection in fine-needle aspirates: Technical procedure and results. Ann. Pathol. 2006, 26, 347–351. [Google Scholar] [CrossRef]
- Giuliano, S.; Mirabelli, M.; Chiefari, E.; Vergine, M.; Gervasi, R.; Brunetti, F.; Innaro, N.; Donato, N.; Aversa, A.; Brunetti, A. Malignancy analysis of thyroid nodules in patients subjected to surgery with cytologcal and ultrasound based risk stratification. Endocrines 2020, 1, 102–118. [Google Scholar] [CrossRef]
- Artus, O.; Zenons, N.; Ilze, G.; Volanska, G.; Gardovskis, J. Immunohistochemical expression of HBME-1, E-cadherin and CD56 in the differential diagnosis of thyroid nodules. Medicina 2012, 48, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Papotti, M.; Rodriguez, J.; Pompa, R.; Bartolazzi, A.; Rosai, J. Galectin-3 and HBME-1 Expression in Well-differentiated Thyroid Tumors with Follicular Architecture of Uncertain Malignant Potential. Mod. Pathol. 2005, 18, 541–546. [Google Scholar] [CrossRef]
- Mehdi, M.A.; JAsim, A.M.; Al-Ganber, M.F. The expression of Gal-3 and CK-19 in Hashimoto’s thyroiditis compared to Papillary thyroid carcinoma. Iraq. Med. J. 2018, 2, 86–90. [Google Scholar] [CrossRef]
- Herrmann, M.E.; LiVolsi, V.A.; Pasha, T.L.; Roberts, S.A.; Wojcik, E.M.; Baloch, Z.W. Immunohistochemical Expression of Galectin-3 in Benign and Malignant Thyroid Lesions. Arch. Pathol. Lab. Med. 2002, 126, 710–713. Available online: https://www.ncbi.nlm.nih.gov/pubmed/12033961 (accessed on 12 June 2019). [CrossRef]
- Cvejic, D.; Savin, S.; Golubovic, S.; Paunovic, I.; Tatic, S.; Havelka, M. Galectin-3 and Carcinoembryonic Antigen Expression in Medullary Thyroid Carcinoma: Possible Relation to Tumour Progression. Histopathology 2002, 37, 530–535. [Google Scholar] [CrossRef]
- Kovacs, R.B.; Foldes, J.; Winkler, G.; Bodo, M.; Sapi, Z. The investigation of galectin-3 in diseases of the thyroid gland. Eur. J. Endocrinol. 2003, 149, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Alshenawy, H.A. Utility of Immunohistochemical Markers in Differential Diagnosis of Follicular-cell Derived Thyroid Lesions. J. Microsc. Ultrastruct. 2014, 2, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Pusztaszeri, M.P.; Faquin, W.C.; Sadow, P.M. Tumor-Associated Inflammatory Cells in Thyroid Carcinomas. Surg. Pathol. Clin. 2014, 7, 501–514. [Google Scholar] [CrossRef]
- Kholova, I.; Kalfert, D.; Lintusaari, J.; Rajakorpi, E.; Ludvikova, M. Follicular epithelial Dysplasia as Hashimoto Thyroiditis- related Atypia: A Series of 91 specimen. Endocr. Pathol. 2021, 32, 368–374. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).