Abstract
The presence in a recipient of antibodies directed against donor-specific antigens represents a major obstacle to transplantation. Removal of these antibodies represents a challenge for physicians dealing with kidney transplantation. Several strategies, techniques, and old and new drugs are currently used for desensitizing these patients. Desensitization may either occur before transplantation, at the time of transplantation, or after transplantation according to whether physicians are dealing with living or deceased donors. Different techniques may be used to reveal the presence of antibodies in the recipients; each technique has different sensitivities and specificities, and different advantages and drawbacks. The targets of the drugs used to desensitize are B cells, plasma cells, the antibodies themselves, and, finally, the complement that is the final actor causing tissue disruption. B cells are relatively easy to target; targeting the plasma cell is more difficult. Indeed, several new drugs are also used in randomized trials to defeat plasma cells. Antibodies may be removed easily, but their removal is often followed by antibody rebound. The complement is not easy to defeat and new drugs are currently used for this aim. Overall, despite difficulties, desensitization is currently possible in many cases, to obtain a safe and successful transplantation.
1. Introduction
Desensitization is among the different strategies that allow kidney transplantation in highly sensitized and incompatible patients [1]. Indeed, humoral alloimmunity against human leukocyte antigens (HLAs) is one of the major barriers for successful transplantation. A number of B cell subsets drive this immune mechanism even if a number of intimately related B and T cell subsets maintain an effective humoral alloimmune response. These subsets are alloreactive memory B cells (mBC) [2], T follicular helper (TFH) cells, and long-lived plasma cells (LLPC) located in different lymphoid organs.
2. Development of Sensitization
After the first alloantigen exposure, events such as pregnancies, transfusions, previous transplants, or any contact with alloimmune antigens may cause a second exposure that generates a memory alloimmunity, both cellular and serological [3,4].
When B cells bind to their cognate antigen, they initiate a migration toward the boundary between the B and T cell zones in lymphoid organs, where they compete for interactions with follicular helper cells. T follicular helper cells provide selection signals required for their differentiation into Germinal Center (GC) cells and into antibody-secreting cells. Additionally, it has been observed that after transplantation, circulating TFH expanded more significantly in patients who developed de novo anti-HLA antibodies than in those who remained not sensitized [5]. An important role in generating an alloimmune response is exerted by the long-lived plasma cells. After the generation of memory B cells, these transform into plasmablasts that are competent for homing into survival niches. Plasmablasts migrating to bone marrow generate long-lived plasma cells. The latter, under the effect of pathogen-associated molecular patterns (PAMPs), migrate to survival niches [6,7]. Most of the plasmablasts migrate to inflamed tissues, under the control of the interferon-gamma-induced expression of CXC-chemokine receptor 3 (CXCR3), which binds CXC-chemokine ligand 9 (CXCL9), CXCL10, and CXCL11 [8,9].
3. Techniques to Identify Sensitization Level and to Stratify the Risk
With different techniques, it is now possible to detect both the alloreactive serological memory and the alloreactive cellular memory in patients waiting for kidney transplantation. Through this approach, it is now possible to stratify the humoral and cellular risk of a candidate to receive a specific solid organ transplantation [10].
The serological memory may be detected using complement-dependent cytotoxicity [11], flow cytometry [12], solid-phase assays such as ELISA [13], and bead-based assays such as Luminex [14]. All these assays are shown in Table 1. On the other hand, assays to evaluate alloreactive cellular memory are, among others, flow cytometry [15], Elispot assay [16], solid-phase assay [17], and flow cytometry [18].

Table 1.
Available assays evaluating alloreactive serological memory.
The immune-pathophysiology of DSA-mediated damages may cause antibody-mediated rejection and graft loss. The pathogenicity of DSAs is routinely evaluated by their titer (MFI or dilution) or their ability to bind donor cells (by flow cytometry crossmatch). Ex vivo complement binding can be evaluated with the C1q and/or C3d assays. Analysis of complement-fixing IgG subclasses or complement genetic variations, the number of innate immune effectors, and the polymorphism of Fcγ receptors could all help achieve a better stratification of the risk for antibody-mediated rejection (AMR). Measurement of DSA affinity and glycosylation profile is not yet available. Finally, the characteristics of the target graft endothelial cell (level of expression of HLA molecules, stress-induced ligands, or expression level of complement regulators or cytoprotective proteins) influence the pathogenicity of the DSA.
All the cited assays for detecting the presence of HLA sensitization have different sensitivities and specificities. CDC has the lowest sensitivity. Sensitivity increases with the use of flow cytometry and a further increase is reached with the use of ELISA. The optimal sensitivity and sensibility are obtained through the use of single beads and complement binding. This fact allows for realizing the ENGAGE’s proposal for categorization of the humoral risk of solid-organ transplant categories. The EuropeaN Guidelines for the mAnagement of Graft rEcipients (ENGAGE) [10] is an initiative from the European Society for Organ Transplantation that stratify the patient’s risk as follows:
- (a)
- If the patient has no DSA and no cellular memory, the transplant is possible with a low risk for AMR;
- (b)
- If at the time of transplantation, there is an absence of DSAs but there is a potential cellular memory against the donor HLA, the transplant is possible with an increased risk for AMR. The cellular memory is possible if there are historical DSAs and/or pregnancy or a previous transplant with repeat antigens. Other possibilities are transfusions with no information on blood donors.
- (c)
- If at the time of transplantation, there are DSAs, but with negative flow, the transplant is possible with a risk for acute AMR and acceptable medium-term graft survival.
- (d)
- If at the time of transplantation, there are DSAs with positive flow and negative CDC, the transplant is possible, but there is a very high risk for acute AMR and accelerated chronic AMR.
- (e)
- If at the time of transplantation, there are DSAs with positive CDC, the transplant is not possible and there is the need of desensitization before proceeding with the transplant.
4. Incidence of Hyperimmune Patients and Graft Survival with Desensitization
The number of hyperimmune patients on the waiting list for kidney transplantation is increasing with time and with the technique used. According to Spanish data of 2020, the proportion of sensitized patients with CDC-PRA > 50% ranged from 10% to 15%, but the same patients evaluated by PRA-SAB (single antigen beads) increased to 40–50% [19].
In the USA, according to data from Montgomery et al. [20], more than 20,000 candidates for kidney transplant are sensitized. The authors conducted a study with desensitization on 211 sensitized patients and found that patient survival rates were 80.6% at 8 years from transplantation, as compared with 30.5% for patients that remained on the waiting list. In a different multicenter study on the risk of incompatible kidney transplantation, Orandi et al. [21] compared the graft survival of patients with positive Luminex and negative flow cross match (PLNC) with positive flow and negative CDC (PFNC) and patients with positive CDC (PCC). All these patients were compared with compatible transplants. The hazard ratio (HR) for graft loss was 1.20 for PLNC, 1.65 for PFNC, and 1.80 for PCC. The graft loss for the last two groups was significant (p < 0.001). In a different study conducted in the UK, Manook et al. [22] compared hyperimmune patients desensitized before transplantation with compatible living donors (CLDs) and compatible deceased donors (CDDs). The 5-year graft survival rates were similar and the authors concluded that desensitization has no detrimental influence on patient survival rates even if it does not offer a survival benefit.
5. Timing of Desensitization
Considering the day of transplantation, different timing and strategies may be applied.
In the case of living donation, an early pre-transplant desensitization is preferred until obtaining CDC or Flow X-match-negative. Clearly different drugs at different dosages may be applied. In case deceased donors do not exist for enough time, two strategies may essentially be applied: the immediate pre-transplant desensitization as used principally in Austria [23] or the post-transplant desensitization as used at the Necker Hospital in Paris [24].
In the case of immediate pre-transplant desensitization, Immunoadsorption (IA) is the preferred method due to its ability to efficiently remove IgG [25,26]. The IA treatment is immediately followed by the administration of anti-thymoglobulin (ATG) and/or anti-CD20 (rituximab). The graft survival rates at 3 years are similar in CDCXM-positive and in CDCXM-negative patients.
The protocol used at the Necker Hospital in the case of deceased donors consists of an induction therapy with ATG, which is started on the day of transplantation, followed by high-dose immunoglobulin (IVIg) that is repeated every 3 weeks for a total of four courses. At the end of plasmapheresis, 1 or 2 rituximab infusions are administered. The graft survival rates 7 years post transplantation are 80% even if lower than the graft survival of the control group represented by patients that do not need desensitization.
6. Desensitization Strategies and Drugs
Summarizing what has been described above, four steps can be seen in the process leading to target tissue destruction. B cells are formed after antigen binding and B cells may act as antigen presentation and are precursors of plasma cells. The second step is represented by plasma cell formation with consequent antibody formation. The antibodies that form immune complexes and activate the complement represent the third step. Finally, complement activation induces chemotaxis and leads to target tissue destruction (Figure 1) [27].
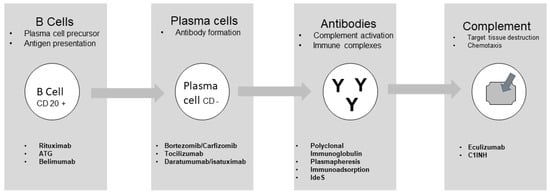
Figure 1.
Targeting different cells or functions.
Different drugs may act on each step described. These drugs are well described by the study of Jordan et al. [28] and their action is summarized in Table 2. In addition to the drugs described, there is an old but efficient pleiotropic drug that may act at any stage. This drug is intravenous immunoglobulin (IVIg), generally given at a dose of 2 g/kg multiple times. The efficacy of IVIg, frequently associated with other drugs, has already been described [24]. Vo et al. [29] conducted an interesting study documenting the efficacy of IVIg in association with rituximab in inducing desensitization.

Table 2.
Summary of pharmacologic options.
7. Drugs Acting on B Cells
Three main drugs act on B cells leading to their reduction and reducing their activity of Rituximab (RTX), ATG, and Belimumab. In a study of Ramos et al. [30] conducted on 25 recipients of living donors needing desensitization, the effectiveness of RTX, IVIg, and ATG in reducing the number of splenic B cells and plasma cells was evaluated. The effect of multiple plasmapheresis plus low-dose IVIg reduced naïve B cells, plasma cells, and memory B cells. Adding RTX to the treatment was effective in a further reduction in naïve B cells, without an effect on memory B cells and plasma cells. Finally, adding ATG to the treatment led to a reduction in memory B cells (CD27+), but again without any effect on plasma cells.
Belimumab inhibits the growth and differentiation of B cells by blocking B lymphocyte stimulator (BAFF or BlyS). Indeed, in normal conditions, BAFF binds to the receptors, BAFF-R, B cell maturation antigen (BCMA), and transmembrane activator and CAML interactor (TACI), leading to immature B cell survival and maturation, plasma cell survival, and B cell survival and proliferation [31,32,33]. Belimumab is a complete human IgG1λ recombinant monoclonal antibody directed against BAFF, initially used for the treatment of systemic lupus erythematosus (SLE) [34].
Belimumab monotherapy was studied as a desensitization agent in kidney transplantation. Nevertheless, the study was terminated early for a reported lack of efficacy (NCT01025193) [35].
Another phase 2 double-blinded randomized placebo-controlled trial of Belimumab plus standard-of-care therapy is being examined for prevention of allograft rejection in renal transplant recipients (NCT01536379) [36]. Finally, the study of Banham et al. [37] in an experimental medicine, randomized, placebo-controlled trial documented the efficacy of Belimumab. In this study, the concentration of activated memory B cells decreased from week 21 to 28 of the treatment and the addition of Belimumab to standard-of-care immunosuppression significantly reduced de novo IgG antibodies.
8. Drugs Acting on Plasma Cells
The second step is represented by plasma cells both short and long living that are principally responsible for antibody production. FcγRIIb, which is an inhibitory receptor for the Fc portion of IgG, controls the persistence and apoptosis of bone marrow plasma cells. It is expressed on B cells [38]. The crosslinking of FcγIIb on naïve B cells may induce apoptosis of B cells [39,40]. Another study documented that FcγIIb controls bone marrow plasma cells and, when crosslinked, induces plasma cells apoptosis [41]. Inside the germinal centers, the balance between survival and apoptosis is related to several factors, such as FcγRIIB. Indeed, the overexpression of FcγRIIB regulates protein phosphorylation and suppresses B cell activation to ameliorate SLE, as documented in mice and in vitro. [42].
A drug efficient in acting against plasma cells is proteasome inhibitors. It is well documented that after proteasome inhibitor administration such as Bortezomib, there is a reduction in the number of antigen-specific plasma cells in candidates of living-donor kidney transplantation [43]. Bortezomib is able to reduce serum levels of DSAs in patients, which were not affected by IVIg or RTX. In addition, Bortezomib reduces the number of antigen-specific plasma cells, without decreasing the total number of plasma cells. In a different study [44], proteasome inhibition caused apoptosis of normal human plasma cells, preventing alloantibody production. Treatment with Bortezomib resulted in a significant increase in the percentage of apoptotic cells, while RTX, ATG, and IVIg had no effect.
However, in different well-conducted studies, Bortezomib did not confirm its efficacy compared to other treatments in reducing DSAs after transplantation in sensitized patients. In particular, Ejaz et al. [45] divided their patients into four groups. One group received ATG alone, a second group received ATG + RTX, a third group received ATG + Bortezomib, and a fourth group received ATG + RTX + Bortezomib. The results in their capacity to reduce DSAs post transplant were similar for all groups, as shown in Table 3. Similarly, Eskandary et al. [46] conducted the study BORTEJECT. The study was a randomized, placebo-controlled trial to investigate the effect of Bortezomib on the course of late ABMR. Bortezomib given as a single agent did not obtain an improvement in the course of late rejection. These studies called for new, more effective agents acting on plasma cells. Carfilzomib is a second-generation irreversible proteasome inhibitor. It is an epoxyketone nonboronated molecule that proved to be effective and with a reduced toxicity in the treatment of patients with multiple myeloma [47]. In a randomized clinical trial (NCT02442648) [48], the B-cell-targeted desensitization with Carfilzomib for preformed anti-HLA antibodies in patients awaiting kidney transplantation was evaluated. A study from Tremblay et al. evaluated the prospective [49], iterative, adaptive trial of carfilzomib-based desensitization. The study documented that HLA antibodies were substantially reduced in the group treated with carfilzomib alone.

Table 3.
DSA monitoring for the first year after transplantation with different treatments [45].
A different strategy for effective desensitization is the use of the Interleukin-6-receptor-specific humanized monoclonal antibody, better known as Tocilizumab. Indeed, IL-6 promotes B cell differentiation to plasma cells and induces Th17 cells. Vo et al. [50] used Tocilizumab in addition to IVIg in patients difficult to desensitize. In their study, Tocilizumab reduced DSA strength and numbers at transplant and 12 months after transplantation. Protocol biopsies showed no evidence of antibody-mediated rejection or transplant glomerulopathy. In the study, after effective desensitization and transplantation, patients subsequently received IVIg once and Tocilizumab monthly for 6 months. The number of patients is low and the authors themselves highlight that larger controlled studies are needed. Later on, Doberer et al. [51] verified the efficacy of a different anti-IL-6 antibody, Clazakizumab, on late ABMR. Clazakizumab is a humanized monoclonal IgG1 antibody. Compared to Tocilizumab, it has a higher affinity for IL-6 and a longer half-life, as documented by studies on psoriatic arthritis [52].
The receptor specific for plasma cells is CD38. Daratumumab is a human immunoglobulin IgGk1 monoclonal antibody that targets the CD38 surface antigen on plasma cells. Daratumumab has been used successfully in treating multiple myeloma and AL amyloidosis. Moreover, unlike Bortezomib, Daratumumab targets nonmalignant plasma cells, hence its efficacy in desensitization and in treatment of ABMR [53,54].
Daratumumab has been successfully used in sensitized kidney transplantation in a nonhuman primate model [55]. Recently [56], four cases of transplant patients desensitized and treated with Daratumumab for AMBR have been reported [55,57,58,59] (Table 4).

Table 4.
Results of 4 patients with ABMR treated by anti CD38 [56].
Another drug acting on DSAs is Belatacept, but its efficacy is only documented when associated with proteasome inhibitors as documented by the studies of Alishetti et al. and of Jain et al. [60,61].
A clinical trial is ongoing (NCT04827979) [62] to verify the efficacy of Belatacept when associated with Daratuzumab.
9. Drugs Acting on Antibodies
Plasma cells produce antibodies that are dangerous by generating immune complexes and by activating the complement cascade.
Removal of DSA antibodies is essential in the different methods of desensitization or in the treatment of ABMR.
The use of IVIg has already been described and represents an important strategy for desensitization as used at the Necker Hospital [24].
A pioneer study suggested that polyclonal Ig could be efficient in decreasing anti-HLA antibodies [63]. Later on, a randomized trial (NIH IG02) compared the pre-transplant administration of polyclonal Ig with placebo in highly sensitized patients [64]. Unfortunately, the NIH IG02 documented that, even if the transplantation rates were higher in the IVIg group, there was only a mild and transient effect on PRA. This fact led to a higher incidence of ABMR in the IVIg treatment group.
IVIg seems to be more efficient when associated with mechanical antibody removal as obtained with plasmapheresis. Montgomery et al. [65] conducted a study in sensitized living-donor kidney transplant recipients. Desensitization was conducted with a combination of plasmapheresis (PE) and administration of IVIg. Post-transplant ABMR occurred as an effect of antibody rebound after contact with allogenic antigens. The ABMRs were easier to control with new cycles of PE and low-dose IVIg. As recommended by the already mentioned desensitization protocol [24], it is essential that the administration of IVIg always follows the PE to avoid the removal of Ig with the PE treatment.
According to the European Guideline for the management of kidney transplant patients with HLA antibodies, both PE and Immunoadsorption are effective [66]. Their efficacy is higher when associated with IVIg and RTX. IA is more selective and is the preferred method by some authors, particularly in the preparation of AB0-incompatible kidney transplantation [67,68,69].
A different method to neutralize antibodies is the use of the IgG-degrading enzyme derived from Streptococcus pyogenes (IdeS) that cleaves intact IgG. Intact human IgG is cleaved by IdeS in two steps. The first step results in a single cleavage of the IgG molecule in which one heavy chain remains intact. The second step generates a fully cleaved molecule that cannot mediate complement-dependent cytotoxicity (CDC) or antibody-dependent cytotoxicity (ADCC) by means of Fcγ receptors [70,71]. A study from Jordan et al. [72] represents the first pilot study that combines two phase 1–2 trials undertaken independently. Overall, 25 highly immunized recipients were treated: 14 in the USA and 11 in Sweden. One shot of IdeS 4 to 6 h was taken before transplantation. IdeS is extremely potent at cleaving circulating IgG, but the effect may be transient. The extent and frequency of DSA rebound after transplantation highly varied between the Swedish and the American arms of the study. Indeed, in the Swedish group, DSAs remained undetectable up to 14 days from transplantation, with a subsequent rebound. In the USA patients, the rebound was very low. This is possibly the effect of the use of IVIg plus RTX before and after transplantation [73,74].
Later on, the safety and efficacy of IdeS were documented by the studies of Lorant [75] and Ge et al. [76].
10. Drugs Acting on Complement
The fourth and final step to be targeted is the complement that favors chemotaxis and leads to tissue destruction. It has been documented that terminal complement inhibition decreases ABMR rates in sensitized renal transplant recipients [77]. In this study, complement inhibition and desensitization were obtained with the use of the anti C5 eculizimab. Eculizumab was given at a dose of 1200 mg immediately prior to transplantation, 600 mg on postoperative day 1, and 600 mg weekly for 4 weeks. Eculizumab was then discontinued if DSAs had significantly decreased or continued until the B flow crossmatch channel shift was <200. Graft survival and ABMR rates were significantly lower in the eculizumab group than the control group. However, considering the outcomes beyond 1 year, the incidence of transplant glomerulopathy did not differ in the two groups [78]. In a more recent study, Marks et al. [79] conducted a randomized trial on the safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization. There were lower rates of ABMR in the eculizumab arm than the arm with standard-of-care therapy. However, at 3 years, there were similar graft survival rates.
A different and new way of targeting the complement is to target the enzymes of the initiating complement cascade [80]. This can be obtained by the use of the serine protease inhibitor (C1INH) [81]. There are two forms of C1INH: the ultra-pure derived C1INH and the full-length recombinant C1INH. These drugs have their advantages and their drawbacks. An advantage is represented by the knowledge of the drugs because they represent the standard of care for hereditary angioedema [82,83]. Additionally, plasma-derived molecules are not immunogenic and they have a broad effect on classical and lectin pathways. The main drawback is the lack of specificity. Indeed, C1INH also controls mannose-binding lectin-associated serine protease (MASP) and proteases in the coagulation and kinin systems. These drugs are new and promising. Indeed, Vo et al. reported that C1-INH resulted in significant elevations in C1-INH levels, C3, and C4, and reduced C1q+HLA antibodies, and that the combination of antibody reduction and C1-INH may prove useful in the prevention and treatment of AMR. Anyway, further controlled studies are warranted [84].
11. Conclusions
The improvement of techniques to detect both serological and cellular memory in recipients waiting for kidney transplantation has allowed us to detect a larger number of sensitized patients than previously achived. As a consequence, the improvement of drugs and strategies to allow desensitization is of outmost importance. In particular, new drugs acting on plasma cells, on antibodies and on complement cascades are promising.
12. Future Directions
New drugs acting on different cells and proteins involved in sensitization and acting on ABMR represent the most important developments for the future. Many trials are ongoing to verify the efficacy and safety of proteosome second-generation and new anti IL-6 monoclonal antibodies. Similarly, new complement inhibitors have been reported as highly promising. Finally, the association of two or more desensitizing drugs, such as belatacept in association with daratuzumab, is an interesting avenue to explore.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed consent Statement
Not applicable.
Data availability Statement
Not applicable.
Conflicts of Interest
The author declare no conflict of interest.
References
- Salvadori, M. Strategies for access to kidney transplantation for highly sensitized and incompatible patients. Transplantology 2023, 4, 85–89. [Google Scholar] [CrossRef]
- Luque, S.; Lúcia, M.; Bestard, O. Refinement of humoral immune monitoring in kidney transplantation: The role of “hidden” alloreactive memory B cells. Transpl. Int. 2017, 30, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Torija, A.; Favà, A.; Meneghini, M.; Crespo, E.; Bestard, O. Novel insights into the pathobiology of humoral alloimmune memory in kidney transplantation. Curr. Opin. Organ. Transplant. 2020, 25, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.S. New insights into the development of B cell responses: Implications for solid organ transplantation. Hum. Immunol. 2019, 80, 378–384. [Google Scholar] [CrossRef]
- Cano-Romero, F.L.; Laguna Goya, R.; Utrero-Rico, A.; Gómez-Massa, E.; Arroyo-Sánchez, D.; Suárez-Fernández, P.; Lora, D.; Andrés, A.; Castro-Panete, M.J.; Paz-Artal, E. Longitudinal profile of circulating T follicular helper lymphocytes parallels anti-HLA sensitization in renal transplant recipients. Am. J. Transplant. 2019, 19, 89–97. [Google Scholar] [CrossRef]
- Radbruch, A.; Muehlinghaus, G.; Luger, E.O.; Inamine, A.; Smith, K.G.; Dörner, T.; Hiepe, F. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006, 6, 741–750. [Google Scholar] [CrossRef]
- Manz, R.A.; Thiel, A.; Radbruch, A. Lifetime of plasma cells in the bone marrow. Nature 1997, 388, 133–134. [Google Scholar] [CrossRef]
- Muehlinghaus, G.; Cigliano, L.; Huehn, S.; Peddinghaus, A.; Leyendeckers, H.; Hauser, A.E.; Hiepe, F.; Radbruch, A.; Arce, S.; Manz, R.A. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood 2005, 105, 3965–3971. [Google Scholar] [CrossRef]
- Baggiolini, M. Chemokines and leukocyte traffic. Nature 1998, 392, 565–568. [Google Scholar] [CrossRef]
- Bestard, O.; Couzi, L.; Crespo, M.; Kessaris, N.; Thaunat, O. Stratifying the humoral risk of candidates to a solid organ transplantation: A proposal of the ENGAGE working group. Transpl. Int. 2021, 34, 1005–1018. [Google Scholar] [CrossRef]
- Patel, R.; Terasaki, P.I. Significance of the positive crossmatch test in kidney transplantation. N. Engl. J. Med. 1969, 280, 735–739. [Google Scholar] [CrossRef]
- Bray, R.A.; Tarsitani, C.; Gebel, H.M.; Lee, J.H. Clinical cytometry and progress in HLA antibody detection. Methods Cell. Biol. 2011, 103, 285–310. [Google Scholar]
- Schlaf, G.; Pollok-Kopp, B.; Manzke, T.; Schurat, O.; Altermann, W. Novel solid phase-based ELISA assays contribute to an improved detection of anti-HLA antibodies and to an increased reliability of pre- and post-transplant crossmatching. NDT Plus 2010, 3, 527–538. [Google Scholar] [CrossRef][Green Version]
- Tait, B.D. Detection of HLA Antibodies in Organ Transplant Recipients—Triumphs and Challenges of the Solid Phase Bead Assay. Front. Immunol. 2016, 7, 570. [Google Scholar] [CrossRef]
- Zachary, A.A.; Kopchaliiska, D.; Montgomery, R.A.; Leffell, M.S. HLA-specific B cells: I. A method for their detection, quantification, and isolation using HLA tetramers. Transplantation 2007, 83, 982–988. [Google Scholar] [CrossRef]
- Lúcia, M.; Luque, S.; Crespo, E.; Melilli, E.; Cruzado, J.M.; Martorell, J.; Jarque, M.; Gil-Vernet, S.; Manonelles, A.; Grinyó, J.M.; et al. Preformed circulating HLA-specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int. 2015, 88, 874–887. [Google Scholar] [CrossRef]
- Karahan, G.E.; Krop, J.; Wehmeier, C.; de Vaal, Y.J.H.; Langerak-Langerak, J.; Roelen, D.L.; Lardy, N.M.; Bemelman, F.J.; Ten Berge, I.J.M.; Reinders, M.E.J.; et al. An Easy and Sensitive Method to Profile the Antibody Specificities of HLA-specific Memory B Cells. Transplantation 2019, 103, 716–723. [Google Scholar] [CrossRef]
- Dahdal, S.; Saison, C.; Valette, M.; Bachy, E.; Pallet, N.; Lina, B.; Koenig, A.; Monneret, G.; Defrance, T.; Morelon, E.; et al. Residual Activatability of Circulating Tfh17 Predicts Humoral Response to Thymodependent Antigens in Patients on Therapeutic Immunosuppression. Front. Immunol. 2019, 9, 3178. [Google Scholar] [CrossRef]
- Gobierno de España, Ministerio de Sanidad Organizatión National de Transplantes Informe 2020. Available online: https://www.sanidad.gob.es (accessed on 15 May 2023).
- Montgomery, R.A.; Lonze, B.E.; King, K.E.; Kraus, E.S.; Kucirka, L.M.; Locke, J.E.; Warren, D.S.; Simpkins, C.E.; Dagher, N.N.; Singer, A.L.; et al. Desensitization in HLA-incompatible kidney recipients and survival. N. Engl. J. Med. 2011, 365, 318–326. [Google Scholar] [CrossRef]
- Orandi, B.J.; Garonzik-Wang, J.M.; Massie, A.B.; Zachary, A.A.; Montgomery, J.R.; Van Arendonk, K.J.; Stegall, M.D.; Jordan, S.C.; Oberholzer, J.; Dunn, T.B.; et al. Quantifying the risk of incompatible kidney transplantation: A multicenter study. Am. J. Transplant. 2014, 14, 1573–1580. [Google Scholar] [CrossRef]
- Manook, M.; Koeser, L.; Ahmed, Z.; Robb, M.; Johnson, R.; Shaw, O.; Kessaris, N.; Dorling, A.; Mamode, N. Post-listing survival for highly sensitised patients on the UK kidney transplant waiting list: A matched cohort analysis. Lancet 2017, 389, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, E.; Eskandary, F.; Kozakowski, N.; Bond, G.; Kikić, Ž.; Yoo, D.; Rasoul-Rockenschaub, S.; Oberbauer, R.; Böhmig, G.A. Deceased donor kidney transplantation across donor-specific antibody barriers: Predictors of antibody-mediated rejection. Nephrol. Dial. Transplant. 2016, 31, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Amrouche, L.; Aubert, O.; Suberbielle, C.; Rabant, M.; Van Huyen, J.D.; Martinez, F.; Sberro-Soussan, R.; Scemla, A.; Tinel, C.; Snanoudj, R.; et al. Long-term Outcomes of Kidney Transplantation in Patients with High Levels of Preformed DSA: The Necker High-Risk Transplant Program. Transplantation 2017, 101, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Regele, H.; Schillinger, M.; Kletzmayr, J.; Haidbauer, B.; Derfler, K.; Druml, W.; Böhmig, G.A. Peritransplant immunoadsorption: A strategy enabling transplantation in highly sensitized crossmatch-positive cadaveric kidney allograft recipients. Transplantation 2005, 79, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Morath, C.; Beimler, J.; Opelz, G.; Scherer, S.; Schmidt, J.; Macher-Goeppinger, S.; Klein, K.; Sommerer, C.; Schwenger, V.; Zeier, M.; et al. Living donor kidney transplantation in crossmatch-positive patients enabled by peritransplant immunoadsorption and anti-CD20 therapy. Transpl. Int. 2012, 25, 506–517. [Google Scholar] [CrossRef]
- Fehr, T.; Gaspert, A. Antibody-mediated kidney allograft rejection: Therapeutic options and their experimental rationale. Transpl. Int. 2012, 25, 623–632. [Google Scholar] [CrossRef]
- Jordan, S.C.; Ammerman, N.; Choi, J.; Huang, E.; Peng, A.; Sethi, S.; Najjar, R.; Toyoda, M.; Lim, K.; Louie, S.; et al. Novel Therapeutic Approaches to Allosensitization and Antibody-mediated Rejection. Transplantation 2019, 103, 262–272. [Google Scholar] [CrossRef]
- Vo, A.A.; Lukovsky, M.; Toyoda, M.; Wang, J.; Reinsmoen, N.L.; Lai, C.-H.; Peng, A.; Villicana, R.; Jordan, S.C. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N. Engl. J. Med. 2008, 359, 242–251. [Google Scholar] [CrossRef]
- Ramos, E.; Pollinger, H.; Stegall, M.D.; Gloor, J.; Dogan, A.; Grande, J. The Effect of Desensitization Protocols on Human Splenic B-Cell Populations In Vivo. Am. J. Transplant. 2007, 7, 402–407. [Google Scholar] [CrossRef]
- Dhilleswara, R.V. Belimumab: Therapeutic mechanism and current status of clinical trials. Biomed. Res. 2018, 29, 3034–3039. [Google Scholar]
- Treml, J.F.; Hao, Y.; Stadanlick, J.E.; Cancro, M.P. The BLyS family: Toward a molecular understanding of B cell homeostasis. Cell Biochem. Biophys. 2009, 53, 1–16. [Google Scholar] [CrossRef]
- Mackay, F.; Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 2009, 9, 491–502. [Google Scholar] [CrossRef]
- Dubey, A.K.; Handu, S.S.; Dubey, S.; Sharma, P.; Sharma, K.K.; Ahmed, Q.M. Belimumab: First targeted biological treatment for systemic lupus erythematosus. J. Pharmacol. Pharmacother. 2011, 2, 317–319. [Google Scholar] [CrossRef]
- NCT01025193 Clinical trial.gov Naji A University of Pennsylvania Desensitization with Belimumab in Sensitized Patients Awaiting Kidney Transplant. Available online: https://www.clinicaltrials.gov (accessed on 19 May 2023).
- NCT01536379 Clinical trial.gov GSK Investigational Site Cambridge UK A Study of Belimumab in the Prevention of Kidney Transplant Rejection. Available online: https://www.clinicaltrials.gov (accessed on 19 May 2023).
- Banham, G.D.; Flint, S.M.; Torpey, N.; Lyons, P.; Shanahan, D.N.; Gibson, A.; Watson, C.; O’Sullivan, A.-M.; Chadwick, J.A.; Foster, K.E.; et al. Belimumab in kidney transplantation: An experimental medicine, randomised, placebo-controlled phase 2 trial. Lancet 2018, 391, 2619–2630. [Google Scholar] [CrossRef]
- Ravetch, J.V.; Bolland, S. IgG Fc receptors. Ann. Rev. Immunol 2001, 19, 275–290. [Google Scholar] [CrossRef]
- Pearse, R.N. SHIP recruitment attenuates FcγIIB-induced apoptosis. Immunity 1999, 10, 753–760. [Google Scholar] [CrossRef]
- Mackay, M.; Stanevsky, A.; Wang, T.; Aranow, C.; Li, M.; Koenig, S.J.; Ravetch, J.V.; Diamond, B. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J. Exp. Med. 2006, 203, 2157–2164. [Google Scholar] [CrossRef]
- Xiang, Z.; Cutler, A.J.; Brownlie, R.J.; Fairfax, K.; Lawlor, K.E.; Severinson, E.; Walker, E.U.; Manz, R.A.; Tarlinton, D.M.; Smith, K.G. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat. Immunol. 2007, 8, 419–429. [Google Scholar] [CrossRef]
- Sheng, L.; Cao, X.; Chi, S.; Wu, J.; Xing, H.; Liu, H.; Yang, Z. Overexpression of FcγRIIB regulates downstream protein phosphorylation and suppresses B cell activation to ameliorate systemic lupus erythematosus. Int. J. Mol. Med. 2020, 46, 1409–1422. [Google Scholar] [CrossRef]
- Diwan, T.S.; Raghavaiah, S.; Burns, J.M.; Kremers, W.K.; Gloor, J.M.; Stegall, M.D. The impact of proteasome inhibition on alloantibody-producing plasma cells in vivo. Transplantation 2011, 9, 536–541. [Google Scholar] [CrossRef]
- Perry, D.K.; Burns, J.M.; Pollinger, H.S.; Amiot, B.P.; Gloor, J.M.; Gores, G.J.; Stegall, M.D. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am. J. Transplant. 2009, 9, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, N.S.; Shields, A.R.; Alloway, R.R.; Sadaka, B.; Girnita, A.L.; Mogilishetty, G.; Cardi, M.; Woodle, E.S. Randomized controlled pilot study of B cell-targeted induction therapy in HLA sensitized kidney transplant recipients. Am. J. Transplant. 2013, 13, 3142–3154. [Google Scholar] [CrossRef] [PubMed]
- Eskandary, F.; Regele, H.; Baumann, L.; Bond, G.; Kozakowski, N.; Wahrmann, M.; Hidalgo, L.G.; Haslacher, H.; Kaltenecker, C.C.; Aretin, M.B.; et al. A Randomized Trial of Bortezomib in Late Antibody-Mediated Kidney Transplant Rejection. J. Am. Soc. Nephrol. 2018, 29, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017, 36, 561–584. [Google Scholar] [CrossRef] [PubMed]
- NCT 02442648 Clinical Trial.gov. Woodle ES B-Cell Targeted Desensitization with Carfilzomib for Preformed Anti-HLA Antibodies in Patients Awaiting Kidney Transplantation. Available online: https://www.clinicaltrials.gov (accessed on 22 May 2023).
- Tremblay, S.; Driscoll, J.J.; Rike-Shields, A.; Hildeman, D.A.; Alloway, R.R.; Girnita, A.L.; Brailey, P.A.; Woodle, E.S. A prospective, iterative, adaptive trial of carfilzomib-based desensitization. Am. J. Transplant. 2020, 20, 411–421. [Google Scholar] [CrossRef]
- Vo, A.A.; Choi, J.; Kim, I.; Louie, S.; Cisneros, K.; Kahwaji, J.; Toyoda, M.; Ge, S.; Haas, M.; Puliyanda, D.; et al. A Phase I/II Trial of the Interleukin-6 Receptor-Specific Humanized Monoclonal (Tocilizumab) + Intravenous Immunoglobulin in Difficult to Desensitize Patients. Transplantation 2015, 99, 2356–2363. [Google Scholar] [CrossRef]
- Doberer, K.; Duerr, M.; Halloran, P.F.; Eskandary, F.; Budde, K.; Regele, H.; Reeve, J.; Borski, A.; Kozakowski, N.; Reindl-Schwaighofer, R.; et al. A Randomized Clinical Trial of Anti-IL-6 Antibody Clazakizumab in Late Antibody-Mediated Kidney Transplant Rejection. J. Am. Soc. Nephrol. 2021, 32, 708–722. [Google Scholar] [CrossRef]
- Mease, P.J.; Gottlieb, A.B.; Berman, A.; Drescher, E.; Xing, J.; Wong, R.; Banerjee, S. The Efficacy and Safety of Clazakizumab, an Anti-Interleukin-6 Monoclonal Antibody, in a Phase IIb Study of Adults with Active Psoriatic Arthritis. Arthritis Rheumatol. 2016, 68, 2163–2173. [Google Scholar] [CrossRef]
- Kaufman, G.P.; Schrier, S.L.; Lafayette, R.A.; Arai, S.; Witteles, R.M.; Liedtke, M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood 2017, 130, 900–902. [Google Scholar] [CrossRef]
- Mateos, M.V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. ALCYONE Trial Investigators. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef]
- Kwun, J.; Matignon, M.; Manook, M.; Guendouz, S.; Audard, V.; Kheav, D.; Poullot, E.; Gautreau, C.; Ezekian, B.; Bodez, D.; et al. Daratumumab in Sensitized Kidney Transplantation: Potentials and Limitations of Experimental and Clinical Use. J. Am. Soc. Nephrol. 2019, 30, 1206–1219. [Google Scholar] [CrossRef]
- Joher, N.; Matignon, M.; Grimbert, P. HLA Desensitization in Solid Organ Transplantation: Anti-CD38 to Across the Immunological Barriers. Front. Immunol. 2021, 12, 688301. [Google Scholar] [CrossRef]
- Doberer, K.; Kläger, J.; Gualdoni, G.A.; Mayer, K.A.; Eskandary, F.; Farkash, E.A.; Agis, H.; Reiter, T.; Reindl-Schwaighofer, R.; Wahrmann, M.; et al. CD38 Antibody Daratumumab for the Treatment of Chronic Active Antibody-mediated Kidney Allograft Rejection. Transplantation 2021, 105, 451–457. [Google Scholar] [CrossRef]
- Spica, D.; Junker, T.; Dickenmann, M.; Schaub, S.; Steiger, J.; Rüfli, T.; Halter, J.; Hopfer, H.; Holbro, A.; Hirt-Minkowski, P. Daratumumab for Treatment of Antibody-Mediated Rejection after ABO-Incompatible Kidney Transplantation. Case Rep. Nephrol. Dial. 2019, 9, 149–157. [Google Scholar] [CrossRef]
- Aguilera Agudo, C.; Gómez Bueno, M.; Krsnik Castello, I. Daratumumab for Antibody-mediated Rejection in Heart Transplant-A Novel Therapy: Successful Treatment of Antibody-mediated Rejection. Transplantation 2021, 105, e30–e31. [Google Scholar] [CrossRef]
- Alishetti, S.; Farr, M.; Jennings, D.; Serban, G.; Uriel, N.; Sayer, G.; Vasilescu, R.; Restaino, S.; Chong, A.S.; Habal, M.V. Desensitizing highly sensitized heart transplant candidates with the combination of belatacept and proteasome inhibition. Am. J. Transplant. 2020, 20, 3620–3630. [Google Scholar] [CrossRef]
- Jain, D.; Rajab, A.; Young, J.S.; Yin, D.; Nadasdy, T.; Chong, A.S.; Pelletier, R.P. Reversing donor-specific antibody responses and antibody-mediated rejection with bortezomib and belatacept in mice and kidney transplant recipients. Am. J. Transplant. 2020, 20, 2675–2685. [Google Scholar] [CrossRef]
- NCT 04827979 Clinical trial. Gov. Shreya Mall. Univerity of California San Francisco, CA Daratumumab and Belatacept for Desensitization. Available online: https://www.clinicaltrials.gov (accessed on 19 May 2023).
- Glotz, D.; Antoine, C.; Julia, P.; Suberbielle-Boissel, C.; Boudjeltia, S.; Fraoui, R.; Hacen, C.; Duboust, A.; Bariety, J. Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg). Am. J. Transplant. 2002, 2, 758–760. [Google Scholar] [CrossRef]
- Jordan, S.C.; Tyan, D.; Stablein, D.; McIntosh, M.; Rose, S.; Vo, A.; Toyoda, M.; Davis, C.; Shapiro, R.; Adey, D.; et al. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: Report of the NIH IG02 trial. J. Am. Soc. Nephrol. 2004, 15, 3256–3562. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Zachary, A.A.; Racusen, L.C.; Leffell, M.S.; King, K.E.; Burdick, J.; Maley, W.R.; Ratner, L.E. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation 2000, 70, 887–895. [Google Scholar] [CrossRef]
- Mamode, N.; Bestard, O.; Claas, F.; Furian, L.; Griffin, S.; Legendre, C.; Pengel, L.; Naesens, M. European Guideline for the Management of Kidney Transplant Patients with HLA Antibodies: By the European Society for Organ Transplantation Working Group. Transpl. Int. 2022, 35, 10511. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Dong, P.; Wang, J.; Yu, X.; Yu, B. Efficacy of Combined Desensitization Therapy Based on Protein A Immunoadsorption on Anti-human Leukocyte Antigen Antibodies in Sensitized Kidney Transplant Recipients: A Retrospective Study. Cureus 2022, 14, e28661. [Google Scholar] [CrossRef] [PubMed]
- Kälble, F.; Süsal, C.; Pego da Silva, L.; Speer, C.; Benning, L.; Nusshag, C.; Pham, L.; Tran, H.; Schaier, M.; Sommerer, C.; et al. Living Donor Kidney Transplantation in Patients with Donor-Specific HLA Antibodies After Desensitization with Immunoadsorption. Front. Med. 2021, 8, 781491. [Google Scholar] [CrossRef] [PubMed]
- Junker, T.; Volken, T.; Stehle, G.; Drexler, B.; Infanti, L.; Buser, A.; Passweg, J.; Schaub, S.; Dickenmann, M.; Halter, J.; et al. Safety and Feasibility of Immunoadsorption with Heparin Anticoagulation in Preparation of ABO-Incompatible Kidney Transplantation: A Retrospective Single-Center Study. Transfus. Med. Hemother. 2023, 50, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Vonpawelrammingen, U.; Björck, L. IdeS and SpeB: Immunoglobulin-degrading cysteine proteinases of Streptococcus pyogenes. Curr. Opin. Microbiol. 2003, 6, 50–55. [Google Scholar] [CrossRef]
- Järnum, S.; Bockermann, R.; Runström, A.; Winstedt, L.; Kjellman, C. The Bacterial Enzyme IdeS Cleaves the IgG-Type of B Cell Receptor (BCR), Abolishes BCR-Mediated Cell Signaling, and Inhibits Memory B Cell Activation. J. Immunol. 2015, 195, 5592–5601. [Google Scholar] [CrossRef]
- Jordan, S.C.; Lorant, T.; Choi, J.; Kjellman, C.; Winstedt, L.; Bengtsson, M.; Zhang, X.; Eich, T.; Toyoda, M.; Eriksson, B.M.; et al. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N. Engl. J. Med. 2017, 377, 442–453. [Google Scholar] [CrossRef]
- Vo, A.A.; Choi, J.; Cisneros, K.; Reinsmoen, N.; Haas, M.; Ge, S.; Toyoda, M.; Kahwaji, J.; Peng, A.; Villicana, R.; et al. Benefits of rituximab combined with intravenous immunoglobulin for desensitization in kidney transplant recipients. Transplantation 2014, 98, 312–319. [Google Scholar] [CrossRef]
- Zachary, A.A.; Lucas, D.P.; Montgomery, R.A.; Leffell, M.S. Rituximab prevents an anamnestic response in patients with cryptic sensitization to HLA. Transplantation 2013, 95, 701–704. [Google Scholar] [CrossRef]
- Lorant, T.; Bengtsson, M.; Eich, T.; Eriksson, B.M.; Winstedt, L.; Järnum, S.; Stenberg, Y.; Robertson, A.K.; Mosén, K.; Björck, L.; et al. Safety, immunogenicity, pharmacokinetics, and efficacy of degradation of anti-HLA antibodies by IdeS (imlifidase) in chronic kidney disease patients. Am. J. Transplant. 2018, 18, 2752–2762. [Google Scholar] [CrossRef]
- Ge, S.; Chu, M.; Choi, J.; Louie, S.; Vo, A.; Jordan, S.C.; Toyoda, M. Imlifidase Inhibits HLA Antibody-mediated NK Cell Activation and Antibody-dependent Cell-mediated Cytotoxicity (ADCC) In Vitro. Transplantation 2020, 104, 1574–1579. [Google Scholar] [CrossRef]
- Stegall, M.D.; Diwan, T.; Raghavaiah, S.; Cornell, L.D.; Burns, J.; Dean, P.G.; Cosio, F.G.; Gandhi, M.J.; Kremers, W.; Gloor, J.M. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am. J. Transplant. 2011, 11, 2405–2413. [Google Scholar] [CrossRef]
- Cornell, L.D.; Schinstock, C.A.; Gandhi, M.J.; Kremers, W.K.; Stegall, M.D. Positive crossmatch kidney transplant recipients treated with eculizumab: Outcomes beyond 1 year. Am. J. Transplant. 2015, 15, 1293–1302. [Google Scholar] [CrossRef]
- Marks, W.H.; Mamode, N.; Montgomery, R.A.; Stegall, M.D.; Ratner, L.E.; Cornell, L.D.; Rowshani, A.T.; Colvin, R.B.; Dain, B.; Boice, J.A.; et al. Safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization therapy: A randomized trial. Am. J. Transplant. 2019, 19, 2876–2888. [Google Scholar] [CrossRef]
- Morgan, B.P.; Harris, C.L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug. Discov. 2015, 14, 857–877. [Google Scholar] [CrossRef]
- Sharp, J.A.; Whitley, P.H.; Cunnion, K.M.; Krishna, N.K. Peptide inhibitor of complement c1, a novel suppressor of classical pathway activation: Mechanistic studies and clinical potential. Front. Immunol. 2014, 5, 406. [Google Scholar] [CrossRef]
- Gadek, J.E.; Hosea, S.W.; Gelfand, J.A.; Santaella, M.; Wickerhauser, M.; Triantaphyllopoulos, D.C.; Frank, M.M. Replacement therapy in hereditary angioedema: Successful treatment of acute episodes of angioedema with partly purified C1 inhibitor. N. Engl. J. Med. 1980, 302, 542–546. [Google Scholar] [CrossRef]
- Zanichelli, A.; Mansi, M.; Periti, G.; Cicardi, M. Therapeutic management of hereditary angioedema due to C1 inhibitor deficiency. Expert Rev. Clin. Immunol. 2013, 9, 477–488. [Google Scholar] [CrossRef]
- Vo, A.A.; Zeevi, A.; Choi, J.; Cisneros, K.; Toyoda, M.; Kahwaji, J.; Peng, A.; Villicana, R.; Puliyanda, D.; Reinsmoen, N.; et al. A phase I/II placebo-controlled trial of C1-inhibitor for prevention of antibody-mediated rejection in HLA sensitized patients. Transplantation 2015, 99, 299–308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).