The Impact of Alloantibodies on Clinical VCA Outcomes and the Need for Immune Tolerance
Abstract
1. Introduction
2. Methods
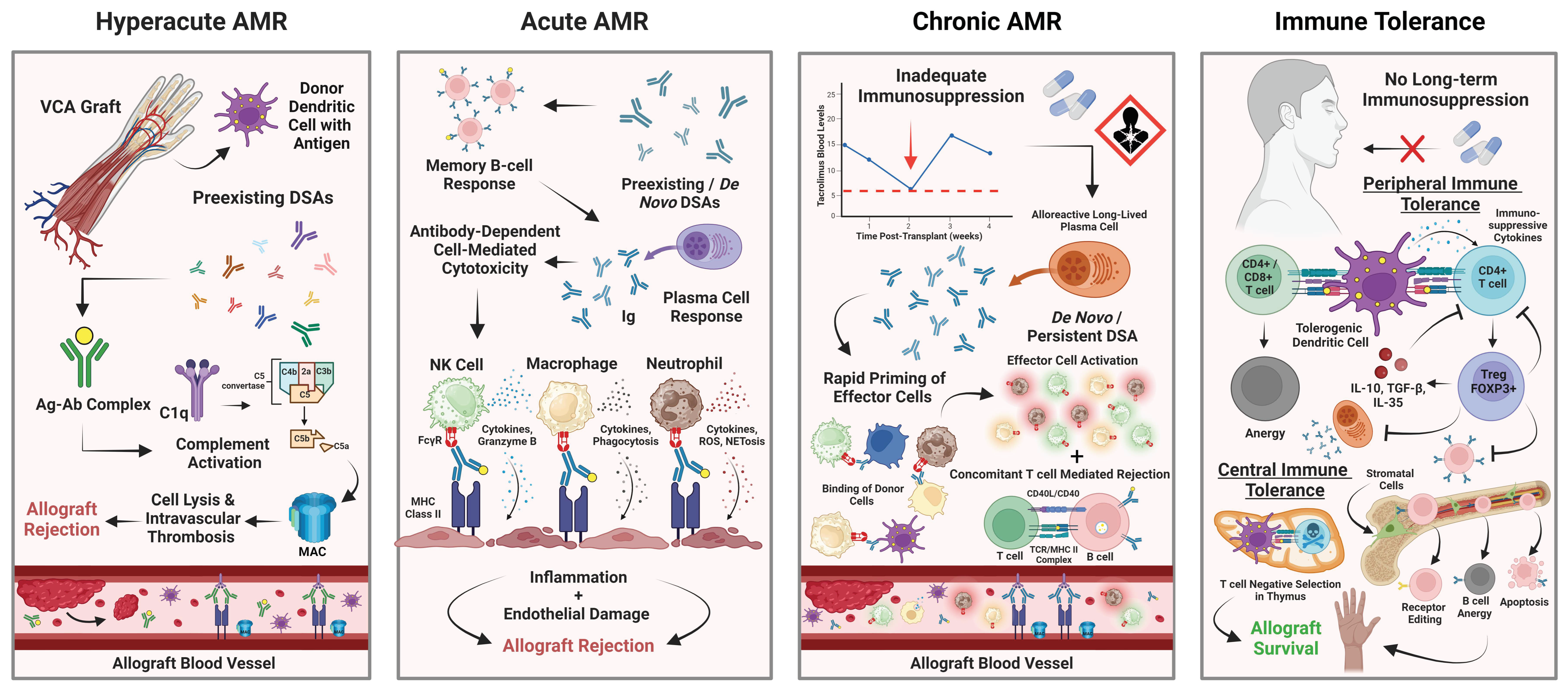
3. AMR in Animal VCA Models
| Animal Model & Study | VCA Model | Immunosuppression | Experimental Groups | Sample Size (n=) | Alloantibody Development | Average Graft Survival (POD) |
|---|---|---|---|---|---|---|
| Non-Human Primates Shockcor et al. [24] | Heterotopic Hemifacial Allograft | Tacrolimus and Mycophenolate Mofetil | Group 1: VCA graft + VBM | 4 | No | 348.3 ± 85.9 |
| Group 2: VCA graft | 3 | Yes | 24.7 ± 16.6 | |||
| Group 3: VCA graft + BMC infusion | 3 | Yes | 75.7 ± 44.1 | |||
| Group 4: Heterotopic myocutaneous graft + VBM | 4 | - | 36.8 ± 20.7 | |||
| Group 5: VCA + VBM + donor T cell depletion with ATG | 3 | Yes | 35.0 ± 26.2 | |||
| Group 6: VCA + VBM + 1.5 Gy donor irradiation | 4 | No | 32.0 ± 21.4 | |||
| Group 7: VCA + 1.5 Gy donor irradiation + BMC infusion | 3 | Yes | 20.7 ± 7.09 | |||
| Group 8: VCA + VBM + recipient T cell depletion with ATG + 21-day steroid taper | 3 | Yes | 28.3 ± 8.02 | |||
| Miniature Swine Elgendy et al. [26] | VRAM Flap Allograft |
| Pig A1 | 1 | Yes | 19.0 ± 2.83 |
| Pig A2 | 1 | No | ||||
| Pig B1 | 1 | Yes | 71.5 ± 3.54 | ||
| Pig B2 | 1 | Yes | ||||
| Pig B3 | 1 | No | 58.0 |
4. AMR in Human Facial VCAs
| Study | Patient # | Facial VCA Model | Alloantibody Development | Time from VCA to CR (Months) | Max Histological Follow-Up (Months) |
|---|---|---|---|---|---|
| Moktefi et al. [29] | 1 | Partial | No | 35 | 153 |
| 2 | Full | No | - | 122 | |
| 4 | Full | Yes | 77 | 124 | |
| 5 | Full | Yes | 48 | 89 ** | |
| 6 | Full | No * | 48 | 94 | |
| 7 | Full | No * | - | 40 | |
| Kiukas et al. [31] | 1 | Partial | Yes | - | 72 |
| 2 | Full | No | - | 48 |
5. AMR in Human Upper Extremity VCAs
| Study | Patient # | Alloantibody Development | Time from VCA to DSA Development (Months) | Maximum Follow-Up (Months) |
|---|---|---|---|---|
| Azoury et al. [40] | 1 | Yes | 26 | 12 |
| Petruzzo et al. [43] | 1 | No | - | 156 |
| 2 | Yes | 84 | 108 | |
| 3 | No | - | 60 | |
| 4 | No | - | 60 | |
| 5 | Yes | 60 | 36 | |
| 6 | No | - | 6 | |
| 7 | No | - | 6 |
6. Therapies for AMR in the Setting of VCA
7. The Need for Immune Tolerance Strategies in VCA
8. Tolerance Induction Using CD3IT T Cell Depletion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siemionow, M.Z.; Kulahci, Y.; Bozkurt, M. Composite tissue allotransplantation. Plast. Reconstr. Surg. 2009, 124, e327–e339. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Hautz, T.; Pratschke, J.; Schneeberger, S. Vascularized composite allografts and solid organ transplants: Similarities and differences. Curr. Opin. Organ Transplant. 2013, 18, 640–644. [Google Scholar] [CrossRef]
- Thuong, M.; Petruzzo, P.; Landin, L.; Mahillo, B.; Kay, S.; Testelin, S.; Jablecki, J.; Laouabdia-Sellami, K.; Lopez-Fraga, M.; Dominguez-Gil, B. Vascularized composite allotransplantation—A Council of Europe position paper. Transpl. Int. 2019, 32, 233–240. [Google Scholar] [CrossRef]
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Cendales, L.; Hardy, M.A. Immunologic considerations in composite tissue transplantation: Overview. Microsurgery 2000, 20, 412–419. [Google Scholar] [CrossRef]
- van Sandwijk, M.S.; Bemelman, F.J.; Ten Berge, I.J. Immunosuppressive drugs after solid organ transplantation. Neth. J. Med. 2013, 71, 281–289. [Google Scholar]
- Enderby, C.; Keller, C.A. An overview of immunosuppression in solid organ transplantation. Am. J. Manag. Care 2015, 21, S12–S23. [Google Scholar]
- Cherikh, W.S.; Kauffman, H.M.; McBride, M.A.; Maghirang, J.; Swinnen, L.J.; Hanto, D.W. Association of the type of induction immunosuppression with posttransplant lymphoproliferative disorder, graft survival, and patient survival after primary kidney transplantation. Transplantation 2003, 76, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Koulmanda, M.; Pomahac, B.; Fan, Z.; Murphy, G.F.; Strom, T.B. Hand transplants and the mandate for tolerance. Curr. Opin. Organ Transplant. 2014, 19, 545–551. [Google Scholar] [CrossRef]
- Vasilic, D.; Alloway, R.R.; Barker, J.H.; Furr, A.; Ashcroft, R.; Banis, J.C., Jr.; Kon, M.; Woodle, E.S. Risk assessment of immunosuppressive therapy in facial transplantation. Plast. Reconstr. Surg. 2007, 120, 657–668. [Google Scholar] [CrossRef]
- Nasir, S.; Bozkurt, M.; Klimczak, A.; Siemionow, M. Large antigenic skin load in total abdominal wall transplants permits chimerism induction. Ann. Plast. Surg. 2008, 61, 572–579. [Google Scholar] [CrossRef]
- Moris, D.; Cendales, L.C. Sensitization and Desensitization in Vascularized Composite Allotransplantation. Front. Immunol. 2021, 12, 682180. [Google Scholar] [CrossRef]
- Schinstock, C.A.; Mannon, R.B.; Budde, K.; Chong, A.S.; Haas, M.; Knechtle, S.; Lefaucheur, C.; Montgomery, R.A.; Nickerson, P.; Tullius, S.G.; et al. Recommended Treatment for Antibody-mediated Rejection After Kidney Transplantation: The 2019 Expert Consensus From the Transplantion Society Working Group. Transplantation 2020, 104, 911–922. [Google Scholar] [CrossRef]
- Moreau, A.; Varey, E.; Anegon, I.; Cuturi, M.C. Effector mechanisms of rejection. Cold Spring Harb. Perspect. Med. 2013, 3, a015461. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.R.; Glotz, D.; Mooney, N. The Role of the Endothelium during Antibody-Mediated Rejection: From Victim to Accomplice. Front. Immunol. 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, C.L.; Cascalho, M.; Ozyurekoglu, T.; Jones, C.M.; Ramirez, A.; Roberts, T.; Tien, H.Y.; Moreno, R.; Galvis, E.; Tsai, T.M.; et al. The role of B cell immunity in VCA graft rejection and acceptance. Hum. Immunol. 2019, 80, 385–392. [Google Scholar] [CrossRef]
- Berger, M.; Baliker, M.; Van Gelder, T.; Bohmig, G.A.; Mannon, R.B.; Kumar, D.; Chadban, S.; Nickerson, P.; Lee, L.A.; Djamali, A. Chronic Active Antibody-mediated Rejection: Opportunity to Determine the Role of Interleukin-6 Blockade. Transplantation 2024, 108, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Ezekian, B.; Schroder, P.M.; Freischlag, K.; Yoon, J.; Kwun, J.; Knechtle, S.J. Contemporary Strategies and Barriers to Transplantation Tolerance. Transplantation 2018, 102, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, M.R. Targeting B cells and antibody in transplantation. Am. J. Transplant. 2011, 11, 1359–1367. [Google Scholar] [CrossRef]
- Sicard, A.; Kanitakis, J.; Dubois, V.; Morelon, E.; Thaunat, O. Humoral Alloreactivity in VCA Recipients: Should We Learn From Our Experience? Transplantation 2020, 104, 2003–2010. [Google Scholar] [CrossRef]
- Hautz, T.; Messner, F.; Weissenbacher, A.; Hackl, H.; Kumnig, M.; Ninkovic, M.; Berchtold, V.; Krapf, J.; Zelger, B.G.; Zelger, B.; et al. Long-term outcome after hand and forearm transplantation—A retrospective study. Transpl. Int. 2020, 33, 1762–1778. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Loupy, A.; Chandraker, A.; Schneeberger, S. Donor-specific antibodies and antibody-mediated rejection in vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2016, 21, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.L.; Loh, C.Y.Y. Reviewing immunosuppressive regimens in animal models for vascularized composite allotransplantation. Plast. Aesthet. Res. 2018, 5, 10. [Google Scholar] [CrossRef]
- Shockcor, N.; Buckingham, E.B.; Hassanein, W.; Dhru, U.; Khalifeh, A.; Uluer, M.; Woodall, J.; Brazio, P.; Drachenberg, C.; Nam, A.J.; et al. Vascularized Bone Marrow Cellular Depletion or Discontinuity Abrogates Protection of Vascularized Composite Allografts in Nonhuman Primates. Transplant. Direct 2021, 7, e659. [Google Scholar] [CrossRef] [PubMed]
- Gassen, R.B.; Borges, T.J.; Perez-Saez, M.J.; Zhang, H.; Al Jurdi, A.; Llinas-Mallol, L.; Aoyama, B.; Lima, M.; Pascual, J.; Sage, P.T.; et al. T cell depletion increases humoral response by favoring T follicular helper cells expansion. Am. J. Transplant. 2022, 22, 1766–1778. [Google Scholar] [CrossRef]
- Elgendy, T.Y.; Waldner, M.; Zhang, W.; Kim, D.Y.; Minervini, M.I.; Komatsu, C.; Kulahci, Y.; Washington, K.M.; Gorantla, V.S.; Ezzelarab, M.B.; et al. Tacrolimus before CTLA4Ig and rapamycin promotes vascularized composite allograft survival in MGH miniature swine. Transpl. Immunol. 2022, 75, 101696. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K.; Bakri, K.; Gibreel, W.; Cotofana, S.; Amer, H.; Mardini, S. Facial Transplantation. Facial Plast. Surg. Clin. N. Am. 2022, 30, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Kauke, M.; Panayi, A.C.; Safi, A.F.; Haug, V.; Perry, B.; Kollar, B.; Nizzi, M.C.; Broyles, J.; Annino, D.J.; Marty, F.M.; et al. Full facial retransplantation in a female patient-Technical, immunologic, and clinical considerations. Am. J. Transplant. 2021, 21, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Moktefi, A.; Hivelin, M.; Grimbert, P.; Carmagnat, M.; Sbidian, E.; Papouin, B.; Suberbielle, C.; Wolkenstein, P.; Bosc, R.; Meningaud, J.P.; et al. Face transplantation: A longitudinal histological study focusing on chronic active and mucosal rejection in a series with long-term follow-up. Am. J. Transplant. 2021, 21, 3088–3100. [Google Scholar] [CrossRef]
- Siemionow, M. The past the present and the future of face transplantation. Curr. Opin. Organ Transplant. 2020, 25, 568–575. [Google Scholar] [CrossRef]
- Kiukas, E.L.; Sipila, M.; Manninen, A.; Yla-Kotola, T.; Lindford, A.; Lassus, P. Comprehensive outcome analysis in two composite face transplants in Helsinki: Have we succeeded? J. Plast. Reconstr. Aesthet. Surg. 2023, 80, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Amat, M.; Duralde, E.; Masutani, R.; Glassman, R.; Shen, C.; Graham, K.L. “Patient Lost to Follow-up”: Opportunities and Challenges in Delivering Primary Care in Academic Medical Centers. J. Gen. Intern. Med. 2022, 37, 2678–2683. [Google Scholar] [CrossRef] [PubMed]
- Office of the Surgeon General (US); Center for Mental Health Services (US); National Institute of Mental Health (US). Mental Health: Culture, Race, and Ethnicity: A Supplement to Mental Health: A Report of the Surgeon General; US Department of Health and Human Services: Rockville, MD, USA, 2001.
- van Ryn, M.; Burke, J. The effect of patient race and socio-economic status on physicians’ perceptions of patients. Soc. Sci. Med. 2000, 50, 813–828. [Google Scholar] [CrossRef] [PubMed]
- McQuinn, M.W.; Kimberly, L.L.; Parent, B.; Diaz-Siso, J.R.; Caplan, A.L.; Blitz, A.G.; Rodriguez, E.D. Self-Inflicted Gunshot Wound as a Consideration in the Patient Selection Process for Facial Transplantation. Camb. Q. Healthc. Ethics 2019, 28, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Kiwanuka, H.; Aycart, M.A.; Gitlin, D.F.; Devine, E.; Perry, B.J.; Win, T.S.; Bueno, E.M.; Alhefzi, M.; Krezdorn, N.; Pomahac, B. The role of face transplantation in the self-inflicted gunshot wound. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Bouquegneau, A.; Loheac, C.; Aubert, O.; Bouatou, Y.; Viglietti, D.; Empana, J.P.; Ulloa, C.; Murad, M.H.; Legendre, C.; Glotz, D.; et al. Correction: Complement-activating donor-specific anti-HLA antibodies and solid organ transplant survival: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002637. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transplant. 2020, 20, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Vionnet, J.; Sempoux, C.; Pascual, M.; Sanchez-Fueyo, A.; Colmenero, J. Donor-specific antibodies in liver transplantation. Gastroenterol. Hepatol. 2020, 43, 34–45. [Google Scholar] [CrossRef]
- Azoury, S.C.; Johnson, F.B.; Levine, M.; Veasey, S.; McAndrew, C.; Shaked, A.; Lantieri, L.; Kamoun, M.; Levin, L.S. Successful transatlantic bilateral hand transplant in a young female highly sensitized to HLA class II antigens. Transpl. Immunol. 2021, 65, 101377. [Google Scholar] [CrossRef]
- Kanitakis, J.; Karayannopoulou, G.; Lanzetta, M.; Petruzzo, P. Graft vasculopathy in the skin of a human hand allograft: Implications for diagnosis of rejection of vascularized composite allografts. Transpl. Int. 2014, 27, e118–e123. [Google Scholar] [CrossRef]
- Valenzuela, N.M.; McNamara, J.T.; Reed, E.F. Antibody-mediated graft injury: Complement-dependent and complement-independent mechanisms. Curr. Opin. Organ Transplant. 2014, 19, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Petruzzo, P.; Luong, S.; Kanitakis, J.; Sardu, C.; Feugier, P.; Danjou, F.; Gazarian, A.; Badet, L.; Morelon, E. Graft vasculopathy in upper extremity allotransplantation: Results of a retrospective high-resolution ultrasonographic study. Clin. Transplant. 2021, 35, e14130. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, C.; Nochy, D.; Andrade, J.; Verine, J.; Gautreau, C.; Charron, D.; Hill, G.S.; Glotz, D.; Suberbielle-Boissel, C. Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am. J. Transplant. 2009, 9, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.A.; Lukovsky, M.; Toyoda, M.; Wang, J.; Reinsmoen, N.L.; Lai, C.H.; Peng, A.; Villicana, R.; Jordan, S.C. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N. Engl. J. Med. 2008, 359, 242–251. [Google Scholar] [CrossRef]
- Garces, J.C.; Giusti, S.; Staffeld-Coit, C.; Bohorquez, H.; Cohen, A.J.; Loss, G.E. Antibody-Mediated Rejection: A Review. Ochsner J. 2017, 17, 46–55. [Google Scholar] [PubMed]
- Weissenbacher, A.; Hautz, T.; Zelger, B.; Zelger, B.G.; Mayr, V.; Brandacher, G.; Pratschke, J.; Schneeberger, S. Antibody-mediated rejection in hand transplantation. Transpl. Int. 2014, 27, e13–e17. [Google Scholar] [CrossRef] [PubMed]
- Kueckelhaus, M.; Fischer, S.; Seyda, M.; Bueno, E.M.; Aycart, M.A.; Alhefzi, M.; ElKhal, A.; Pomahac, B.; Tullius, S.G. Vascularized composite allotransplantation: Current standards and novel approaches to prevent acute rejection and chronic allograft deterioration. Transpl. Int. 2016, 29, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Martin, S.T.; Townsend, K.R.; Gabardi, S. Antibody-mediated rejection in kidney transplantation: A review of pathophysiology, diagnosis, and treatment options. Pharmacotherapy 2014, 34, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.A.; Choi, J.; Cisneros, K.; Reinsmoen, N.; Haas, M.; Ge, S.; Toyoda, M.; Kahwaji, J.; Peng, A.; Villicana, R.; et al. Benefits of rituximab combined with intravenous immunoglobulin for desensitization in kidney transplant recipients. Transplantation 2014, 98, 312–319. [Google Scholar] [CrossRef]
- Kaufman, C.L.; Ouseph, R.; Blair, B.; Kutz, J.E.; Tsai, T.M.; Scheker, L.R.; Tien, H.Y.; Moreno, R.; Ozyurekoglu, T.; Banegas, R.; et al. Graft vasculopathy in clinical hand transplantation. Am. J. Transplant. 2012, 12, 1004–1016. [Google Scholar] [CrossRef]
- Schneeberger, S.; Gorantla, V.S.; Brandacher, G.; Zeevi, A.; Demetris, A.J.; Lunz, J.G.; Metes, D.M.; Donnenberg, A.D.; Shores, J.T.; Dimartini, A.F.; et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann. Surg. 2013, 257, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Dorafshar, A.H.; Bojovic, B.; Christy, M.R.; Borsuk, D.E.; Iliff, N.T.; Brown, E.N.; Shaffer, C.K.; Kelley, T.N.; Kukuruga, D.L.; Barth, R.N.; et al. Total face, double jaw, and tongue transplantation: An evolutionary concept. Plast. Reconstr. Surg. 2013, 131, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.N.; Janus, C.A.; Lillesand, C.A.; Radke, N.A.; Pirsch, J.D.; Becker, B.N.; Fernandez, L.A.; Thomas Chin, L.; Becker, Y.T.; Odorico, J.S.; et al. Outcomes at 3 years of a prospective pilot study of Campath-1H and sirolimus immunosuppression for renal transplantation. Transpl. Int. 2006, 19, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Woodle, E.S.; Walsh, R.C.; Alloway, R.R.; Girnita, A.; Brailey, P. Proteasome inhibitor therapy for antibody-mediated rejection. Pediatr. Transplant. 2011, 15, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Orandi, B.J.; Zachary, A.A.; Dagher, N.N.; Bagnasco, S.M.; Garonzik-Wang, J.M.; Van Arendonk, K.J.; Gupta, N.; Lonze, B.E.; Alachkar, N.; Kraus, E.S.; et al. Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation 2014, 98, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Yelken, B.; Arpali, E.; Gorcin, S.; Kocak, B.; Karatas, C.; Demiralp, E.; Turkmen, A. Eculizumab for Treatment of Refractory Antibody-Mediated Rejection in Kidney Transplant Patients: A Single-Center Experience. Transplant. Proc. 2015, 47, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.H.; Abt, P.L. Treatment options and strategies for antibody mediated rejection after renal transplantation. Semin. Immunol. 2012, 24, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, S.; Gorantla, V.S.; van Riet, R.P.; Lanzetta, M.; Vereecken, P.; van Holder, C.; Rorive, S.; Remmelink, M.; Le Moine, A.; Abramowicz, D.; et al. Atypical acute rejection after hand transplantation. Am. J. Transplant. 2008, 8, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Kauke-Navarro, M.; Knoedler, S.; Panayi, A.C.; Knoedler, L.; Haller, B.; Parikh, N.; Huelsboemer, L.; Stoegner, V.A.; Kiefer, J.; Eisenhardt, S.U.; et al. Correlation between facial vascularized composite allotransplantation rejection and laboratory markers: Insights from a retrospective study of eight patients. J. Plast. Reconstr. Aesthet. Surg. 2023, 83, 155–164. [Google Scholar] [CrossRef]
- Brenner, M.J.; Tung, T.H.; Jensen, J.N.; Mackinnon, S.E. The spectrum of complications of immunosuppression: Is the time right for hand transplantation? J. Bone Jt. Surg. Am. 2002, 84, 1861–1870. [Google Scholar] [CrossRef]
- Sachs, D.H. Transplant tolerance: Bench to bedside--26th annual Samuel Jason Mixter Lecture. Arch. Surg. 2011, 146, 501–505. [Google Scholar] [CrossRef]
- Teshima, T.; Ferrara, J.L. Understanding the alloresponse: New approaches to graft-versus-host disease prevention. Semin. Hematol. 2002, 39, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.; Chandran, S.; Kawai, T.; Levitsky, J.; Mapara, M.; Mathew, J.; Thomson, A.; Yamada, K. Meeting Report: The Fifth International Samuel Strober Workshop on Clinical Immune Tolerance. Transplantation 2023, 107, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.; Chodoff, L.; Leventhal, J.R.; Ho, B.; Richards, M.; Schaumberg, D.A.; Laidlaw, D.; Ildstad, S.T.; Axelrod, D.A. Immune tolerance via FCR001 cell therapy compared with maintenance immunosuppression for kidney transplantation: Real-world evidence analysis of safety and efficacy. Clin. Transplant. 2023, 37, e15074. [Google Scholar] [CrossRef] [PubMed]
- Lowsky, R.; Strober, S. Establishment of Chimerism and Organ Transplant Tolerance in Laboratory Animals: Safety and Efficacy of Adaptation to Humans. Front. Immunol. 2022, 13, 805177. [Google Scholar] [CrossRef]
- Markov, P.V.; Pybus, O.G. Evolution and Diversity of the Human Leukocyte Antigen(HLA). Evol. Med. Public Health 2015, 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Duran-Struuck, R.; Crepeau, R.; Matar, A.; Hanekamp, I.; Srinivasan, S.; Neville, D.M., Jr.; Sachs, D.H.; Huang, C.A. Development of a diphtheria toxin based antiporcine CD3 recombinant immunotoxin. Bioconjug. Chem. 2011, 22, 2014–2020. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wamala, I.; Matar, A.J.; Farkash, E.; Wang, Z.; Huang, C.A.; Sachs, D.H. Recombinant anti-monkey CD3 immunotoxin depletes peripheral lymph node T lymphocytes more effectively than rabbit anti-thymocyte globulin in naive baboons. Transpl. Immunol. 2013, 29, 60–63. [Google Scholar] [CrossRef]
- Fuchimoto, Y.; Huang, C.A.; Gleit, Z.L.; Kitamura, H.; Griesemer, A.; Melendy, E.; Scheier-Dolberg, R.; White-Scharf, M.E.; Sachs, D.H. Mixed chimerism using a nonmyelosuppressive regimen in miniature swine. Transplant. Proc. 2001, 33, 118–119. [Google Scholar] [CrossRef]
- Horner, B.M.; Randolph, M.A.; Duran-Struuck, R.; Hirsh, E.L.; Ferguson, K.K.; Teague, A.G.; Butler, P.E.; Huang, C.A. Induction of tolerance to an allogeneic skin flap transplant in a preclinical large animal model. Transplant. Proc. 2009, 41, 539–541. [Google Scholar] [CrossRef]
- Leonard, D.A.; Kurtz, J.M.; Mallard, C.; Albritton, A.; Duran-Struuck, R.; Farkash, E.A.; Crepeau, R.; Matar, A.; Horner, B.M.; Randolph, M.A.; et al. Vascularized composite allograft tolerance across MHC barriers in a large animal model. Am. J. Transplant. 2014, 14, 343–355. [Google Scholar] [CrossRef]
- Frankel, A.E.; Woo, J.H.; Ahn, C.; Foss, F.M.; Duvic, M.; Neville, P.H.; Neville, D.M. Resimmune, an anti-CD3epsilon recombinant immunotoxin, induces durable remissions in patients with cutaneous T-cell lymphoma. Haematologica 2015, 100, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Barbagiovanni, G.; Jofra, T.; Stabilini, A.; Perol, L.; Baeyens, A.; Anand, S.; Cagnard, N.; Gagliani, N.; Piaggio, E.; et al. Heterogeneous CD3 expression levels in differing T cell subsets correlate with the in vivo anti-CD3-mediated T cell modulation. J. Immunol. 2015, 194, 2117–2127. [Google Scholar] [CrossRef]
- Kim, S.; Shukla, R.K.; Kim, E.; Cressman, S.G.; Yu, H.; Baek, A.; Choi, H.; Kim, A.; Sharma, A.; Wang, Z.; et al. Comparison of CD3e Antibody and CD3e-sZAP Immunotoxin Treatment in Mice Identifies sZAP as the Main Driver of Vascular Leakage. Biomedicines 2022, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Duran-Struuck, R.; Matar, A.; Crepeau, R.; Gusha, A.; Schenk, M.; Hanekamp, I.; Pathiraja, V.; Spitzer, T.R.; Sachs, D.H.; Huang, C.A. Lack of antidonor alloantibody does not indicate lack of immune sensitization: Studies of graft loss in a haploidentical hematopoietic cell transplantation swine model. Biol. Blood Marrow Transplant. 2012, 18, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Suryawanshi, G.W.; Kim, S.; Guan, X.; Bonifacino, A.C.; Metzger, M.E.; Donahue, R.E.; Kim, S.; Chen, I.S.Y. CD3-immunotoxin mediated depletion of T cells in lymphoid tissues of rhesus macaques. Heliyon 2023, 9, e19435. [Google Scholar] [CrossRef]
- Vaccari, M.; Franchini, G. T Cell Subsets in the Germinal Center: Lessons from the Macaque Model. Front. Immunol. 2018, 9, 348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blades, C.M.; Navarro-Alvarez, N.; Huang, C.A.; Mathes, D.W. The Impact of Alloantibodies on Clinical VCA Outcomes and the Need for Immune Tolerance. Transplantology 2024, 5, 148-162. https://doi.org/10.3390/transplantology5030015
Blades CM, Navarro-Alvarez N, Huang CA, Mathes DW. The Impact of Alloantibodies on Clinical VCA Outcomes and the Need for Immune Tolerance. Transplantology. 2024; 5(3):148-162. https://doi.org/10.3390/transplantology5030015
Chicago/Turabian StyleBlades, Caitlin M., Nalu Navarro-Alvarez, Christene A. Huang, and David W. Mathes. 2024. "The Impact of Alloantibodies on Clinical VCA Outcomes and the Need for Immune Tolerance" Transplantology 5, no. 3: 148-162. https://doi.org/10.3390/transplantology5030015
APA StyleBlades, C. M., Navarro-Alvarez, N., Huang, C. A., & Mathes, D. W. (2024). The Impact of Alloantibodies on Clinical VCA Outcomes and the Need for Immune Tolerance. Transplantology, 5(3), 148-162. https://doi.org/10.3390/transplantology5030015






