Strategies for Access to Kidney Transplantation for Highly Sensitized and Incompatible Patients
- Hum oral memory
- Strategies used in immune patients
- Desensitization
- PRA was calculated to evaluate the unacceptable HLA antigens using the “virtual cross-match”.
- A sliding scale score from low but positive cPRA (>20%) to the highest cPRA (100%) was developed.
- Local to regional or national kidney organs allocation were prioritized.
- The best organs were given to the best recipients.
- ABO compatible rather than identical organs were allocated.
- Living-donor kidney-paired exchange programs
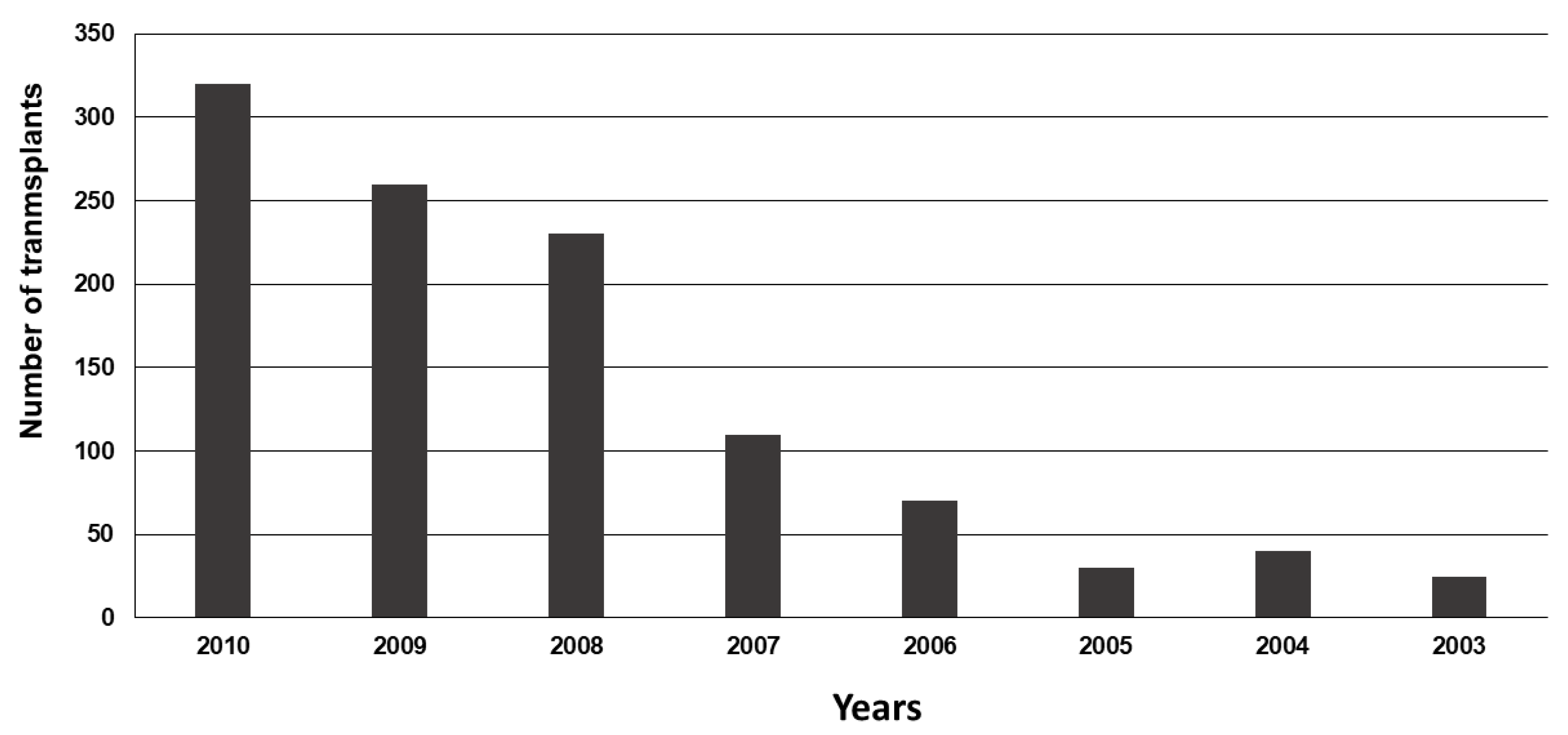
- Acceptable mismatch programs
- Define the humoral risk in kidney transplants; the use of the ENGAGE 5 strata system is recommended;
- Implement strategies to maximize the access to HLA-compatible transplantation:
- ○
- Sliding scale priority score programs for the very HS groups (cPRA > 98%);
- ○
- Expand the living-donor kidney exchange programs (LDKEP) (including low-risk ABOi donors) and develop tools to help assess the probability of an organ match;
- ○
- Expand the AM Program to more European countries;
- Prioritization policies should be linked across countries for equity of access;
- However, a number of HS candidates will never find a compatible donor and would need to undergo desensitization to receive a transplant
Funding
Conflicts of Interest
References
- Torija, A.; Favà, A.; Meneghini, M.; Crespo, E.; Bestard, O. Novel insights into the pathobiology of humoral alloimmune memory in kidney transplantation. Curr. Opin. Organ. Transplant. 2020, 25, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.S. New insights into the development of B cell responses: Implications for solid organ transplantation. Hum. Immunol. 2019, 80, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Bestard, O.; Couzi, L.; Crespo, M.; Kessaris, N.; Thaunat, O. Stratifying the humoral risk of candidates to a solid organ transplantation: A proposal of the ENGAGE working group. Transpl. Int. 2021, 34, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Orandi, B.J.; Garonzik-Wang, J.M.; Massie, A.B.; Zachary, A.A.; Montgomery, J.R.; Van Arendonk, K.J.; Stegall, M.D.; Jordan, S.C.; Oberholzer, J.; Dunn, T.B.; et al. Quantifying the risk of incompatible kidney transplantation: A multicenter study. Am. J. Transplant. 2014, 14, 1573–1580. [Google Scholar] [CrossRef]
- Manook, M.; Koeser, L.; Ahmed, Z.; Robb, M.; Johnson, R.; Shaw, O.; Kessaris, N.; Dorling, A.; Mamode, N. Post-listing survival for highly sensitised patients on the UK kidney transplant waiting list: A matched cohort analysis. Lancet 2017, 389, 727–734. [Google Scholar] [CrossRef]
- Amrouche, L.; Aubert, O.; Suberbielle, C.; Rabant, M.; Van Huyen, J.D.; Martinez, F.; Sberro-Soussan, R.; Scemla, A.; Tinel, C.; Snanoudj, R.; et al. Long-term Outcomes of Kidney Transplantation in Patients with High Levels of Preformed DSA: The Necker High-Risk Transplant Program. Transplantation 2017, 101, 2440–2448. [Google Scholar] [CrossRef]
- Jordan, S.C.; Ammerman, N.; Choi, J.; Huang, E.; Peng, A.; Sethi, S.; Najjar, R.; Toyoda, M.; Lim, K.; Louie, S.; et al. Novel Therapeutic Approaches to Allosensitization and Antibody-mediated Rejection. Transplantation 2019, 103, 262–272. [Google Scholar] [CrossRef]
- Banham, G.D.; Flint, S.M.; Torpey, N.; Lyons, P.A.; Shanahan, D.N.; Gibson, A.; Watson, C.J.E.; O’Sullivan, A.M.; Chadwick, J.A.; Foster, K.E.; et al. Belimumab in kidney transplantation: An experimental medicine, randomised, placebo-controlled phase 2 trial. Lancet 2018, 391, 2619–2630. [Google Scholar] [CrossRef]
- Diwan, T.S.; Raghavaiah, S.; Burns, J.M.; Kremers, W.K.; Gloor, J.M.; Stegall, M.D. The impact of proteasome inhibition on alloantibody-producing plasma cells in vivo. Transplantation 2011, 91, 536–541. [Google Scholar] [CrossRef]
- Eskandary, F.; Regele, H.; Baumann, L.; Bond, G.; Kozakowski, N.; Wahrmann, M.; Hidalgo, L.G.; Haslacher, H.; Kaltenecker, C.C.; Aretin, M.B.; et al. A Randomized Trial of Bortezomib in Late Antibody-Mediated Kidney Transplant Rejection. J. Am. Soc. Nephrol. 2018, 29, 591–605. [Google Scholar] [CrossRef]
- Doberer, K.; Duerr, M.; Halloran, P.F.; Eskandary, F.; Budde, K.; Regele, H.; Reeve, J.; Borski, A.; Kozakowski, N.; Reindl-Schwaighofer, R.; et al. A Randomized Clinical Trial of Anti-IL-6 Antibody Clazakizumab in Late Antibody-Mediated Kidney Transplant Rejection. J. Am. Soc. Nephrol. 2021, 32, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Kwun, J.; Matignon, M.; Manook, M.; Guendouz, S.; Audard, V.; Kheav, D.; Poullot, E.; Gautreau, C.; Ezekian, B.; Bodez, D.; et al. Daratumumab in Sensitized Kidney Transplantation: Potentials and Limitations of Experimental and Clinical Use. J. Am. Soc. Nephrol. 2019, 30, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C.; Lorant, T.; Choi, J.; Kjellman, C.; Winstedt, L.; Bengtsson, M.; Zhang, X.; Eich, T.; Toyoda, M.; Eriksson, B.M.; et al. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N. Engl. J. Med. 2017, 377, 442–453. [Google Scholar] [CrossRef]
- Cornell, L.D.; Schinstock, C.A.; Gandhi, M.J.; Kremers, W.K.; Stegall, M.D. Positive crossmatch kidney transplant recipients treated with eculizumab: Outcomes beyond 1 year. Am. J. Transplant. 2015, 15, 1293–1302. [Google Scholar] [CrossRef]
- Marks, W.H.; Mamode, N.; Montgomery, R.A.; Stegall, M.D.; Ratner, L.E.; Cornell, L.D.; Rowshani, A.T.; Colvin, R.B.; Dain, B.; Boice, J.A.; et al. Safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization therapy: A randomized trial. Am. J. Transplant. 2019, 19, 2876–2888. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.P.; Harris, C.L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug. Discov. 2015, 14, 857–877. [Google Scholar] [CrossRef]
- Stewart, D.E.; Kucheryavaya, A.Y.; Klassen, D.K.; Turgeon, N.A.; Formica, R.N.; Aeder, M.I. Changes in Deceased Donor Kidney Transplantation One Year After KAS Implementation. Am. J. Transplant. 2016, 16, 1834–1847. [Google Scholar] [CrossRef]
- Wallis, C.B.; Samy, K.P.; Roth, A.E.; Rees, M.A. Kidney paired donation. Nephrol. Dial. Transplant. 2011, 26, 2091–2099. [Google Scholar] [CrossRef]
- Claas, F.H.; Witvliet, M.D.; Duquesnoy, R.J.; Persijn, G.G.; Doxiadis, I.I. The acceptable mismatch program as a fast tool for highly sensitized patients awaiting a cadaveric kidney transplantation: Short waiting time and excellent graft outcome. Transplantation 2004, 78, 190–193. [Google Scholar] [CrossRef]
- Heidt, S.; Haasnoot, G.W.; van Rood, J.J.; Witvliet, M.D.; Claas, F.H.J. Kidney allocation based on proven acceptable antigens results in superior graft survival in highly sensitized patients. Kidney Int. 2018, 93, 491–500. [Google Scholar] [CrossRef]
- Heidt, S.; Witvliet, M.D.; Haasnoot, G.W.; Claas, F.H. The 25th anniversary of the Eurotransplant Acceptable Mismatch program for highly sensitized patients. Transpl. Immunol. 2015, 33, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Heidt, S.; Haasnoot, G.W.; van der Linden-van Oevelen, M.J.H.; Claas, F.H.J. Highly Sensitized Patients Are Well Served by Receiving a Compatible Organ Offer Based on Acceptable Mismatches. Front. Immunol. 2021, 12, 687254. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvadori, M. Strategies for Access to Kidney Transplantation for Highly Sensitized and Incompatible Patients. Transplantology 2023, 4, 85-89. https://doi.org/10.3390/transplantology4020009
Salvadori M. Strategies for Access to Kidney Transplantation for Highly Sensitized and Incompatible Patients. Transplantology. 2023; 4(2):85-89. https://doi.org/10.3390/transplantology4020009
Chicago/Turabian StyleSalvadori, Maurizio. 2023. "Strategies for Access to Kidney Transplantation for Highly Sensitized and Incompatible Patients" Transplantology 4, no. 2: 85-89. https://doi.org/10.3390/transplantology4020009
APA StyleSalvadori, M. (2023). Strategies for Access to Kidney Transplantation for Highly Sensitized and Incompatible Patients. Transplantology, 4(2), 85-89. https://doi.org/10.3390/transplantology4020009



