Abstract
Objective—the objective of this study was to assess the accuracy of placental growth factor (PlGF), soluble Fms-like Tyrosine Kinase 1 (sFlt-1), and endoglin (sEng) in the diagnosis of suspected preeclampsia (PE) with and without fetal growth restriction (FGR) near delivery. Methods—this is a secondary analysis of a dataset of 125 pregnant women presenting at the high risk pregnancy clinic with suspected PE, FGR or PE + FGR in the University Medical Center of Slovenia. The dataset included 31 PE cases, 16 FGR cases, 42 PE + FGR cases, 15 cases who developed with unrelated complications before 37 weeks (wks) (PTD), and 21 unaffected controls who delivered a healthy baby at term. We also analyzed a sub-group of women who delivered early (<34 wks) including 10 PE, 12 FGR, 28 PE + FGR, and six PTD. Clinical management adhered to hospital guidelines. Marker levels were extracted from the dataset and were used to develop Receiver Operating Characteristic (ROC) curves and to calculate the area under the curve (AUC), the detection rates (DRs), and the false positive rates (FPRs). Previously published marker cutoffs for yes/no admission to hospital wards were extracted from the literature. Negative and positive predictive values (NPVs and PPVs) were evaluated for their value in determining whether hospital admission was required. Non-parametric tests were applied for statistical analysis; p < 0.05 was considered significant. Results—near delivery, all the pro-and anti-angiogenic markers provided diagnostic (ROC = 1.00) accuracy for the early (<34 wks) group of FGR. Diagnostic or near diagnostic (ROC = 0.95) accuracy was achieved by all marker for early PE + FGR but lower accuracy was achieved for early PE. For all cases, all markers, especially PlGF reached diagnostic or near diagnostic accuracy for FGR and PE + FGR. At this accuracy level, they can contribute to the clinical management of FGR, and PE + FGR. All the markers were less accurate for all PE cases. The use of published cutoffs was adequate for clinical management of FGR, whether early or for all cases, using an NPV > 90%. For PE + FGR, the PPV value approached 100%, especially for early cases, and can thus be implemented in clinical management. Neither NPV nor PPV were high enough for managing all cases of PE. There was no added value in measuring the PlGF/(sFlt-1 + sEng) ratio. Conclusion—This is the first study on a Slovenian population. It shows that near-delivery angiogenic biomarkers tests may be useful for confirming the diseases in cases where there is a diagnostic doubt. However, the clinical use of the biomarkers needs to be weighed against resources available and degree of certainty of the diagnosis made with and without them for managing suspected FGR and PE + FGR requiring delivery <34 wks, where they are very accurate, and furthermore in the management of all cases of FGR and FGR+PE. The markers were less accurate for the clinical diagnosis of PE.
1. Introduction
Preeclampsia (PE) is a major pregnancy complication associated with high morbidity and mortality [1,2,3,4,5]. Diagnosis of PE is made if a previously normotensive woman develops hypertension after 20 weeks gestation accompanied by proteinuria [6], and/or thrombocytopenia [7], elevated liver enzymes or hemolysis, along with the occasional involvement of other organs, mainly the cardiovascular system, the eyes, and the brain [1,2,3,4,5,6,7,8,9]. National societies of Maternal and Fetal Medicine and individual medical centers have established protocols to guide proper clinical management for saving the life of the mother and fetus/neonate and reducing morbidity [3,4].
Placental growth factor (PlGF), soluble FMF-like Tyrosine Kinase 1 (sFlt-1), and soluble endoglin (sEng) were suggested as biochemical markers to assist in the diagnosis and clinical management of PE in the second and early third trimesters of pregnancy. Previous studies reported that these biomarkers can reach diagnostic or near-diagnostic accuracy near delivery [10,11,12,13,14,15,16,17]. Some of these studies suggested a shift from estimating marker accuracy using a continuous model to the use of cutoffs of markers level [10,15,16,17]. These cutoffs can help avoid unnecessary hospital admissions for suspected PE.
Interestingly, PlGF, sFlt-1, and sEng have also turned out to be useful in the prediction of fetal growth restriction (FGR), another major pregnancy complication, which develops with or without PE [18,19,20]. FGR may be the consequence of impaired blood supply to the placenta and the fetus and/or a sign of fetal abnormalities. Cases of early (<34 wks) FGR and PE often require emergency delivery. Studies have reported that abnormally low levels of PlGF, and high levels of sFlt-1 and sEng could effectively separate early PE, FGR and PE + FGR cases from other cases of early delivery [15,16,21,22].
This study is the first to assess the accuracy of angiogenic markers in the management of patients with suspected PE, FGR and PE + FGR in Slovenian pregnant women. In a previous paper, we used this dataset to analyze seven biochemical markers (PlGF, sFlt-1, sEng, their ratios, VEGF, Inhibin-A, TNF-alpha, and PP13), evaluated by immunodiagnostic methods, and six biophysical markers (mean arterial blood pressure (MAP), percent augmentation index (Aix (%)) and reactive hyperemia index (RHI) measured with the EndoPAT 2000, and uterine artery pulsatility index (UTPI), uterine artery resistance index (RI), and peak systolic velocity index (PSV) measured by sonography). The semi-quantitative analysis yielded a differential marker profile for each of suspected PE, FGR, PE + FGR and constitutes a possible way to use the different marker profiles to assist in the clinical management [21,23]. In this study, the objective was to conduct accuracy analysis of the angiogenic markers and their ratios to see if they reach diagnostic level in identifying patients with suspected PE, FGR and PE + FGR. We did it using receiver operation characteristic (ROC) curves to calculate the area under the curves (AUCs), and the detection rate (DRs) at fixed false positive rates (FPRs).
We aimed to determine whether marker tests can provide sufficient diagnostic accuracy or whether they should be considered simply as additional tools in clinical management when patients present at the delivery admission clinic with suspected PE with and without FGR, or FGR alone. We used both the continuous and the cutoff models. In the cutoff model, positive and negative predictive values (PPVs and NPVs) were introduced to evaluate who should be admitted and who could be sent home for a later repeated evaluation [15,16,17]. Cutoffs were extracted from previously published papers. We compared the accuracy of the continuous versus the cutoff model in defining diagnostic, near-diagnostic or lower accuracy, and to evaluate these markers as tools in the clinical management of these complications. Although this evaluation has been conducted elsewhere by others [13,14,15,16,17,18,19], including in the neighboring country of Hungary [24], this analysis is the first to be carried out on a cohort from Slovenia.
2. Sample and Methods
2.1. Sample
This secondary analysis was performed on a population described elsewhere [21,23]. The dataset originally covered cases enrolled between 2012 to 2015 after obtaining ethical approval from the National Medical Ethics Committee of the Republic of Slovenia (Approval on 4 December 2011, No. 104/04/12). All the women signed a written informed consent form. Patients presenting with suspected PE, FGR and PE + FGR were invited to enroll in the clinics of the Department of Perinatology of the University Medical Center of Ljubljana, Slovenia. Inclusion criteria, in addition to informed consent, were—(a) admission with suspected complications of PE, FGR and PE + FGR, (b) a maternal age of 18 years and above, and (c) a singleton viable fetus at gestational week > 24 wks for women who were not in labor. We excluded patients with major fetal abnormalities, and those with pre-existing renal, hematological, and autoimmune complications, those who had a twin demise to singleton, and those having mental disorders that undermined their ability to sign the informed consent. Patients presenting with suspected preterm delivery complications unrelated to PE, FGR or PE + FGR were also included as controls except those with major fetal abnormalities and chorioamnionitis. The unaffected controls were patients who delivered a normal baby at term. They were also enrolled in the outpatient clinics along with those with a history of previous pregnancy complications to assure blood tests at a similar period and gestational weeks compared to the cases of complications. All the women were Caucasian. Case management was according to hospital guidelines and the clinical team was blinded to the marker results.
Pregnancy dating was by last menstrual period, verified by first trimester measurements of fetal crown-rump length [24].
2.2. Preeclampsia (PE), Fetal Growth Restriction (FGR) and Preterm Delivery (PTD)
Diagnosis of PE was based on the International Society for the Study of Hypertensive Disorders in Pregnancy (ISSHP) classification [1] in terms of diastolic blood pressure of 90 mm Hg or above or systolic blood pressure of 140 mm Hg or above, and proteinuria of 2+ in dipstick. In the case of no proteinuria, the criteria of thrombocytopenia, hemolysis, renal insufficiency, impaired liver function, or pulmonary edema were applied [1,6,7,8,9].
We used the criteria of International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) to define FGR [18,19,20] that combined the measurements of fetal biometry with measurements of the pulsatility index (PI) of the maternal uterine (UTPI) arteries, the PI of the umbilical arteries and middle cerebral arteries (MCAs), and of the ductus venosus [18].
Our preterm birth group (PTD) included women who delivered <37 weeks [25,26] for reasons unrelated to PE, FGR, and FE+FGR, and not associated with placenta abruption (a complication of PE), or chorioamnionitis (that is known to be associated with a non-specific biomarker response). We also excluded PTD due to major fetal abnormalities. In fact, this group mainly consisted of spontaneous preterm births or early membrane rupture (PPROM).
For each study complication we evaluated the entire group (all cases) and a subgroup of cases who delivered early (<34 wks).
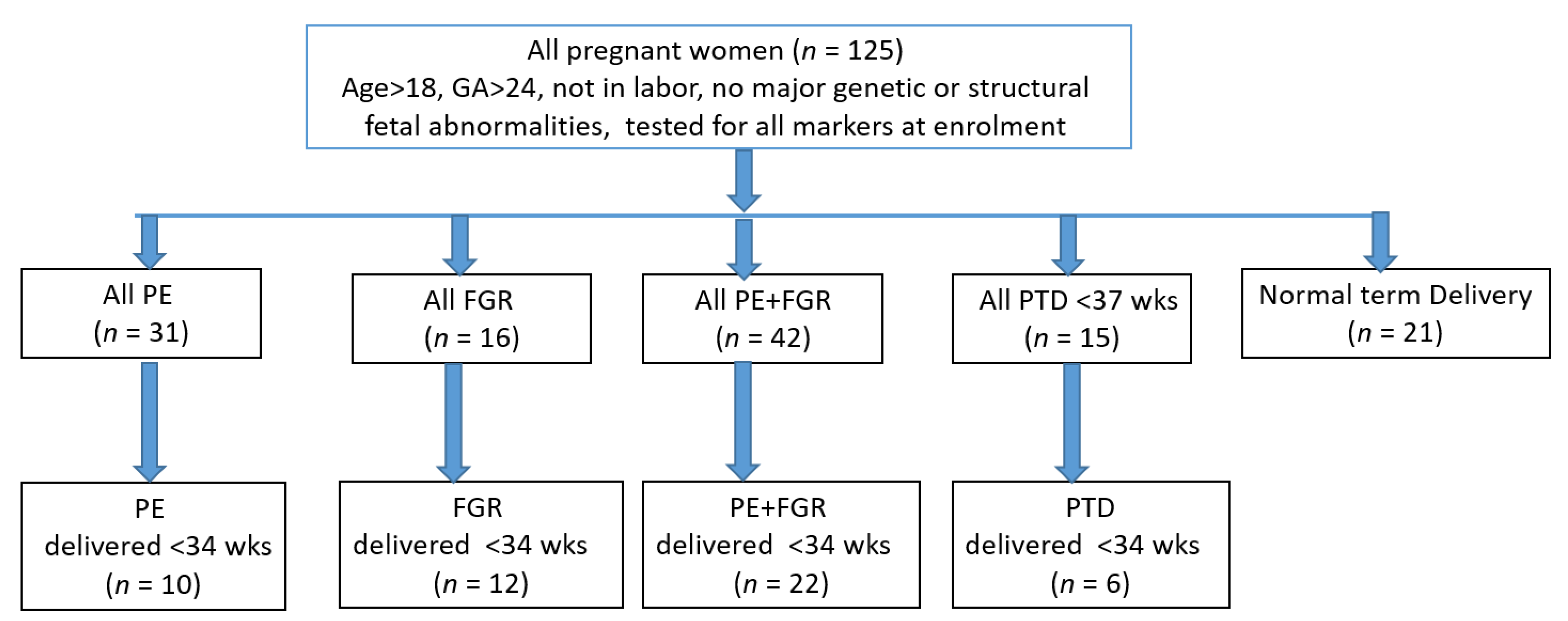
The study design is presented in the flow chart of Figure 1.
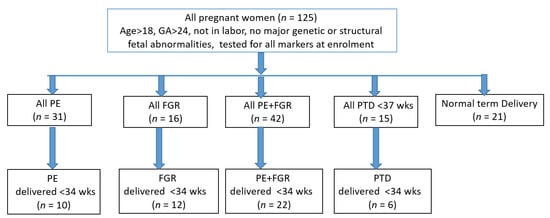
Figure 1.
Study and analysis design.
2.3. Immunodiagnostic Test of Angiogenic Markers
All patients were tested once at the time of enrolment. They provided a 10 mL blood sample for serum testing at admission to the study. Blood was drawn into vacutainer tubes. After 30 min for clotting at room temperature, the samples were centrifuged for 10 min at 1000× g, stored in aliquots of 0.5 mL cryovials at −70 °C, and thawed once for testing.
PlGF and sFlt-1 were determined by the Cobas e411 system (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s specifications. The coefficient of variation (CV%) for both markers was 2%, the inter- and intra-assay CV% were 2.3% to 4.3% (sFlt-1) and 3.6% and 4.1% (PlGF). The sFlt-1/PlGF ratio was used to determine the anti-angiogenic state [10,16]. Personal LAB Microplate Analyzer (Adaltis, Guidonia Montecelio, Italy) was used to determine sEng (Abcam, Cambridge, UK), according to the manufacturer’s instructions. The inter- and intra-CVs (%) were <10% and <12%. PlGF/(sFlt-1 + sEng) ratio [21] reflected the pro-angiogenic state. PlGF/(sFlt-1 + sEng) ratio was calculated from marker values. All marker performances were within the manufacturer’s ranges.
2.4. Biophysical Markers
The reported blood pressure was measured at the time of enrolment with OMRON M6 Comfort devices (Omron Healthcare Co., Ltd., Kyoto, Japan). The devices were regularly calibrated as per protocol. Measurements were made according to the guidelines of the Fetal Medicine Foundation (FMF). We applied arm adjusted cuffs placed on both arms to measure the diastolic and systolic blood pressure twice, 20 min apart, and calculated the mean arterial blood pressure (MAP), according to (systolic + diastolic × 2)/3 [5].
The reported ultrasound results were measured at the time of enrolment. For ultrasound analysis we used a GE Voluson U6 and GE Voluson 8Expert with 2–7 MHz GE RAB6-D probe (GE Healthcare GmbH, Solingen, Germany). All patients underwent fetal biometry at admission including measurements of the fetal head and abdominal circumference and femur length, according to the guidelines established by ISUOG [18]. The estimated fetal weight (EFW) was calculated according to Hadlock’s formula based on four fetal measurements [27].
The uterine artery pulsatility index (UTPI) was measured with a Doppler transducer that was placed on the mother’s abdomen, after a sagittal section of the cervix was obtained. The transducer tilted from side to side to identify the uterine arteries at the level of the internal os. Measurements were made from both maternal vessels and the results were reported as the average of the right and left sides. The A pulsed Doppler sampling gate of 2 mm was used to cover the entire vessel, and an angle of insonation <30 with peak systolic velocity of >60 cm/sec was used to obtain the necessary waveforms before calculating the average of the pulsatility index in the left and right uterine arteries [18].
2.5. Statistical Analyses
We used the statistical SPSS version 24 (SPSS Inc., Chicago, IL, USA). The differences across groups were calculated by Kruskal–Wallis non-parametric tests and corrected by Bonferroni post hoc corrections for multiple comparisons (p < 0.05 considered significant). Mann–Whitney non-parametric test was used to compare marker levels and the AUCs of each complication group compared to term delivery (all cases of the group) or to preterm delivery <34 wks for the respective early subgroup. The symbols * and **, respectively, represent a statistical significance of p < 0.05 and p < 0.001.
Box and Whiskers Plot graphs provided a visualization of the markers’ distribution across quartiles. Cutoffs are marked on the box plots.
The area under the curve (AUC) with a 95% Confidence Interval (95% CI) was calculated from the receiver operating characteristic (ROC) curves according to the marker raw values or from their ratios for the continuous model, or above/below the cutoff. The detection rates (DR%) at 10% fixed false positive rates (FPRs) were extracted from the ROC curves. Published cutoffs were used to calculate the positive predictive value (PPV) according to the true cases at cutoff divided by all cases at cutoff. The negative predictive value (NPV) was calculated as all true negative cases at cutoff divided by all negative cases at cutoff.
3. Results
3.1. Cohort Characteristics
As shown in Figure 1, the dataset was composed of 125 pregnant women divided into the following groups—PE, 31; FGR, 16; PE + FGR, 42; PTD < 37 wks, 15; and 21 who delivered a normal baby at term. Each complication was analyzed as a whole group with a sub-analysis performed for women who delivered <34 wks, which included 10 cases of early PE, 12 cases of early FGR, 28 cases of early PE + FGR and six cases of early PTD. The maternal characteristics, medical history and biomarker levels in the study population are summarized in Table 1.

Table 1.
Characterization of the Study Population pregnancy and delivery features and the biomarker levels.
3.2. Marker Levels
3.2.1. PlGF
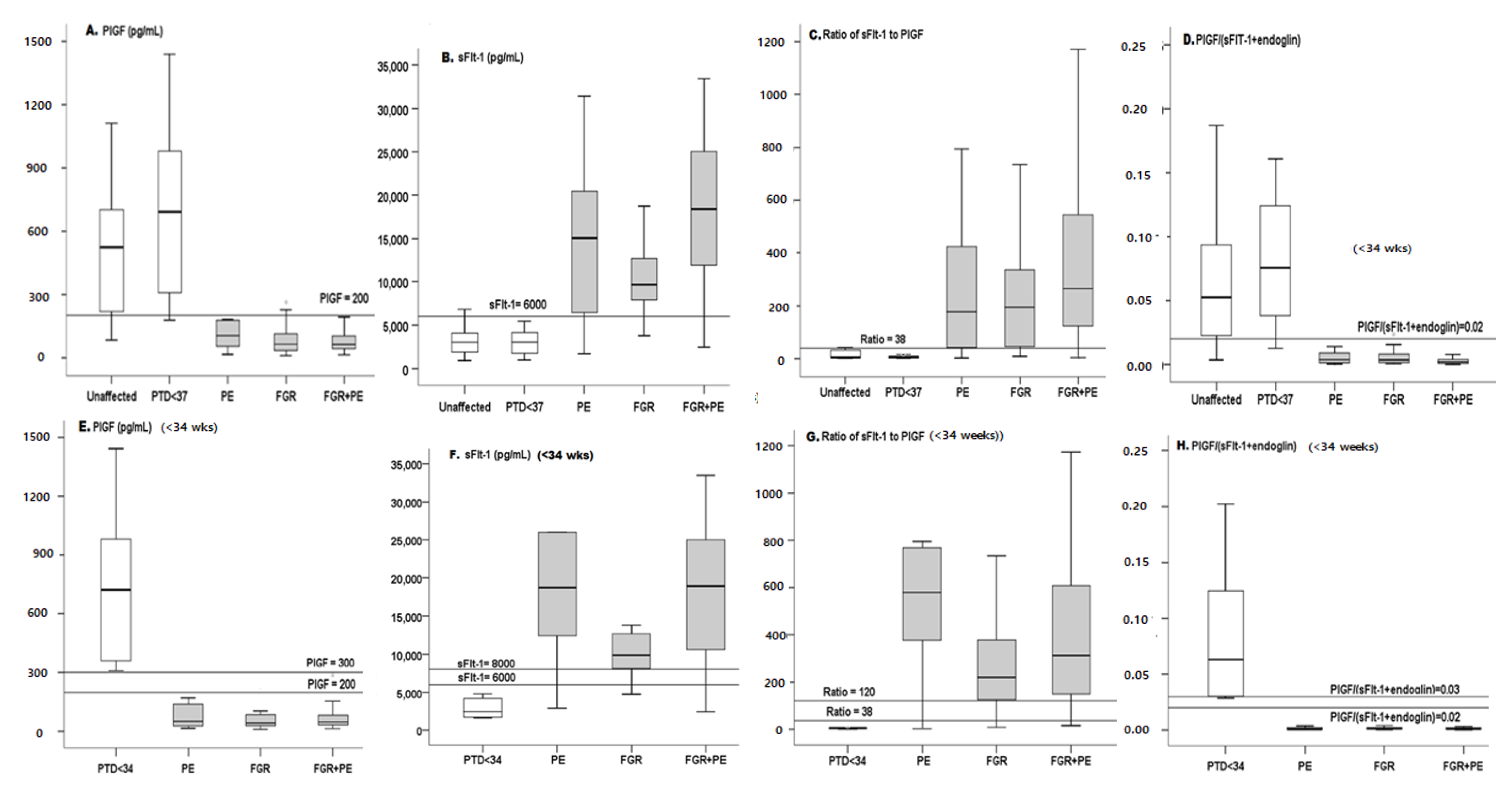
Serum PlGF was lower in the PE, FGR and/or PE + FGR groups compared to the unaffected control or the PTD group in both the entire groups and the sub-groups delivered <34 wks (Table 1, Figure 2). For the continuous model, PlGF yielded an AUC of 0.85 and 0.92 for all cases of PE, and PE + FGR, respectively, with the highest level of AUC = 0.95 for FGR. The respective DRs were 53% for the PE group, 73% for the FGR group and 71% for the PE + FGR group. For the early cases, the accuracy increased to AUC = 0.95 for early PE and reached a diagnostic level of AUC = 1.00 and DR = 100% for early FGR and PE + FGR. Of note—Diagnostic Accuracy (AUC = 1.00 DR = 100%, PPV and NPV+100) met the WHO definition [1,2,3,4].
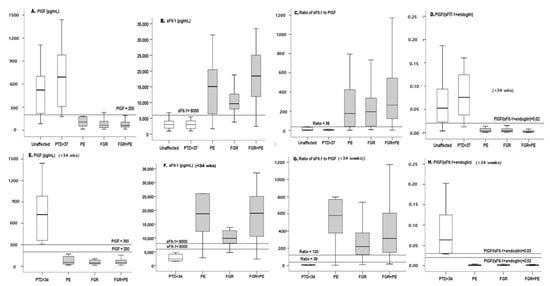
Figure 2.
Box plot of all markers in all cases and in cases delivered <34 wks. Box plots of PlGF (A), sFlt-1 (B), Sflt-1/PlGF ratio (C) and PlGF/(SFlt-1 + sEng) ratio (D) for all cases and for cases before 34 wks (E–H). On each curve, the horizontal lines represent the cutoffs. PE—preeclampsia, FGR—fetal growth restriction, PTD—in addition, we excluded patients from the PTD group who delivered early because of chorioamnionitis (which is known to modify the level of biochemical markers due to the non-specific response) and patients who delivered early as a result of major fetal abnormalities. Note that most cases were spontaneous preterm births or a few cases of PPROM.
The cutoff model yielded somewhat smaller AUCs, PPVs and NPVs in the range of 81–85% for all cases, but for the early cases of FGR and PE + FGR, there was complete diagnostic accuracy with AUC = 1.0, DR = 100% and PPV = 100% (Table 2).

Table 2.
Use of Biomarker for the Prediction of the Complication Groups.
3.2.2. sFlt-1
The sFlt-1 was higher in PE, FGR, PE + FGR, in both the entire groups and the sub-groups delivered <34 wks (Table 1, Figure 2).
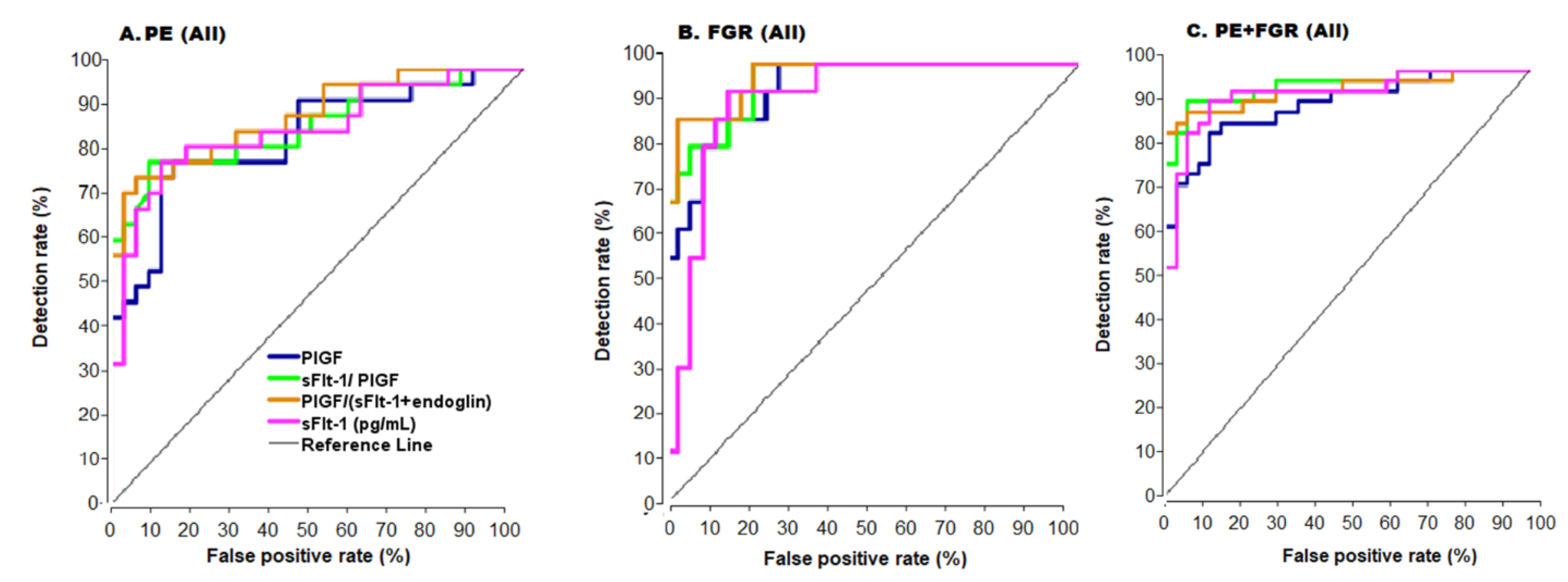
In the AUC analysis, the best results were obtained with PE + FGR (near diagnostic AUC = 0.95 for all cases, Figure 3, Table 2) and AUC = 0.98 for the early cases (Table 2). Performance for all cases of PE or FGR was less accurate, whereas very high accuracy was obtained with the early cases (AUC = 0.95 and 1.0, respectively).
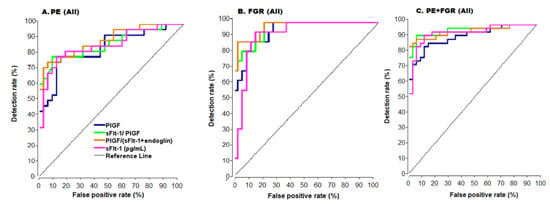
Figure 3.
ROC Curves for all cases. ROC- Receiver operator characteristic curve. (A) Preeclampsia (PE), (B) Fetal growth restriction (FGR), (C) PE + FGR, PlGF—placental growth factor, sFlt-1—soluble Fms-like tyrosinekinase-1. Color code of (A) are the same for (B) and (C).
Using the sFlt-1 cutoff of 6000 pg/mL for all cases yielded adequate NPVs of 81%, 94% and 88%, respectively, for PE, FGR, and PE + FGR with respective PPVs of 85%, 78%, and 90%. Thus, for this marker, the NPV appears useful mainly for directing clinical decisions of FGR whereas the PPV appears useful for the clinical management of cases of PE + FGR (Table 2).
At a cutoff of sFlt-1 = 8000 for the early cases, the perfect PPV of 100% provided diagnostic accuracy for FGR. High PPV was also obtained for PE and PE + FGR, which can thus contribute to the clinical decision to keep patients in the hospital and to optimize the time for delivery (Table 2).
3.2.3. Anti-Angiogenic Ratio of sFlt-1/PlGF
The use of the anti-angiogenic ratio achieved diagnostic accuracy (AUCs = 0.98, 1.0 and 1.0 for the early PE, FGR, and PE + FGR before 34 weeks, respectively (Table 2), in the continuous model. In the cutoff model PPV = 100% for each of the complication groups (Table 2) corresponding to diagnostic accuracy for either the continuous or the cutoff models for the early cases. Lower accuracy was found for all cases of PE, FGR, and PE + FGR (Figure 3, Table 2) indicating that this marker’s ratio can serve as a tool in clinical management with the continuous or the cutoff model (Table 2 and Figure 3).
3.2.4. Pro-Angiogenic Ratio of PlGF/(sFlt-1 + Eng)
The ratio of these markers provided high accuracy for the early cases with both the continuous and the cutoff model (AUC = 0.95, 1.0, and 1.0 for early PE, FGR, and PE + FGR, Figure 3 and Table 2) with a cutoff = 0.03 (Table 2). In comparison, for all cases in each of the complication groups, the ratio of these markers was less accurate and as such it should be seen as a complementary tool in clinical diagnostics.
It should be noted that for all the listed markers, using different cutoffs did not yield better results.
4. Discussion
This study is the first to analyze pro- and anti-angiogenic markers and evaluate whether they can serve in the diagnosis and clinical management of suspected PE, FGR and PE + FGR in the near-delivery period in a Slovenian population. The study shows that the markers in question are effective for diagnosis of FGR PE + FGR and a little less for PE, and that they are very sensible and rather specific. One needs to be cautious before defining their clinical usefulness as it is requires carrying out a different study where the markers would be tested against standard diagnostic techniques, and this is out of the scope of this study.
There are six major findings. First, PlGF achieved diagnostic accuracy, whereas the sFlt-1, sFlt-1/PlGF ratio and PlGF/(sFlt-1 + sEng) ratio provided near diagnostic accuracy in the case of early FGR. Second, for early FGR, both the continuous and the cut-off models generated very adequate results. Third, for all cases of FGR, performance was near diagnostic accuracy and can be considered useful in clinical management. Fourth, for early PE + FGR, all of the PlGF, sFlt-1, sFlt-1/PlGF ratio and PlGF/(sFlt-1 + sEng) ratio resulted in diagnostic accuracy for both the continuous and the cut-off models. Fifth, for all cases of PE + FGR, accuracy was near diagnostic level with either the continuous or the cut-off model. Sixth, all markers and their ratios were less accurate in the case of PE.
PlGF emerged as the best marker in that it achieved diagnostic accuracy for FGR < 34 wks, and a near diagnostic level for all FGR cases. These results are consistent with the literature suggesting that this marker is placental in origin; its decreased level in FGR corresponds to reports that the placenta is smaller in FGR [18,19,20]. Today, FGR diagnosis requires experienced fetal medicine sonographers who are not always available. The PlGF test is simple, can be completed within 2–3 h, and is relatively easy to conduct, and affordable. Thus, analyzers could be installed in community clinics, points of care or hospital admission clinics, at a test cost of 30–50 Euros. This simple immunodiagnostic PlGF test appears suitable for the diagnosis of early FGR and to provide clinical guidance.
PlGF showed good performance for PE + FGR, both for the early cases where it achieved diagnostic accuracy, and in all other cases where it exhibited near diagnostic accuracy. If budgeting is an issue, the use of PlGF as a single marker can be considered adequate. It is worth noting that this marker is useful not only near delivery but also for PE prediction in the 1st and 2nd trimesters. In fact, in ASPRE, SPREE and other studies, PlGF was found to an effective marker of preterm PE and was used to identify women who could benefit from the use of aspirin to prevent preterm PE [28,29,30,31].
sFlt-1 reached diagnostic accuracy for the early cases of FGR and PE + FGR. As mentioned for PlGF, the immunodiagnostic test is simple compared to sophisticated sonography.
The results of our Slovenian cohort, and the extremely high accuracy of marker performance, especially for early cases, is consistent with previous large-scale, high-quality studies pointing to the value of pro- and anti-angiogenic measures near delivery for managing PE, FGR, and PE + FGR [10,11,12,13,14,15,16,17,21,32,33,34,35,36,37]. Thus, our findings constitute additional evidence of the benefits of using PlGF and/or sFlt-1 near delivery for the robust diagnosis of early cases and for guidance in clinical management in all cases.
The anti-angiogenic sFlt-1/PlGF ratio achieved diagnostic accuracy in the early (<34 wks) cases. The pro-angiogenic PlGF/(sFlt-1 + sEng) ratio contributed little to the profile achieved with PlGF or sFlt-1 or their ratio. Hence, pairing PlGF and sFlt-1 appears more useful than the use of triple markers (the above plus sEng). This is important for cost considerations when introducing biomarker tests for patient management when women are admitted with suspected complications.
It is not surprising that marker performance for the cases of PE + FGR exhibited higher accuracy since these are more severe complications with higher risk for both the mother and the fetus. In the current study, the markers appeared to perform better for FGR than for PE. This may be related to differences in the pathological pathways leading to the development of FGR vs. PE [38,39,40,41,42]. Although both PE and FGR have multifactorial origins, the underlying pathways leading to PE appear to be more diverse. Our previous study [21,23] confirmed the value of measuring endothelial dysfunction along with arterial stiffness, which on some occasions were also linked to additional cardiovascular disorders. Immunological and inflammatory components are major underlying pathways leading to the development of PE, and some features of this disorder may become systemic later on [42,43,44]. This might also be the reason why low-dose aspirin is less effective in women with previous FGR compared to women with previous PE and may account for the lower efficacy of aspirin in preventing PE in women with chronic hypertension [28,29,30,31].
The diagnostic accuracy level of FGR identified in this study might be linked to the very strict criteria used for FGR definition, which combined biometry with additional assessments of the blood flow in the umbilical cord, middle cerebral arteries and the ductus venosus [18,19,20,45].
In this cohort, there were 14–16% of cases of superimposed PE who were included in the PE and the PE + FGR groups. Our sub-analysis (not shown) confirmed that the marker levels of cases of superimposed PE were less different from the unaffected controls than the remainder of their PE or PE + FGR. However, the inclusion of superimposed PE in their respective groups appears justified since they require similar care [46].
5. Strengths and Weaknesses
This study has several limitations. The first is the small sample size and the low statistical power of this model.
The second is that the biomarkers were measured when the patients were enrolled in the study rather than at fixed time points. However, this reflects clinical reality. For the women who delivered at <34 wks there was no difference for the GA at enrollment. Gestational week in all cases enrolment was ~3 weeks later for the normal outcome group. However, despite this difference, the marker levels for the normal outcome group and the PTD < 37 weeks were the same, indicating that at this period of pregnancy, these differences do not affect comparisons of marker performance.
Third, the design of the study was such that we did not perform repeated measurements during pregnancy, which could have improved prediction accuracy. Another weakness was its relatively small cohort size. Thus, further studies with larger cohorts are recommended to evaluate the actual benefits for clinical management before introducing these new methods for routine screening.
In the current COVID 19 era, not all women attend prenatal screening, and they often choose to go to the hospital as late as possible for delivery. Some women avoid visiting medical facilities, others face periods of lockdown, and occasionally, when they finally get to a hospital, they may find that some medical facilities are temporarily unavailable [47]. Our study may contribute to reducing unnecessary hospital admissions, which is a benefit, given the high burden on hospital admissions due to the current pandemic, and the need to reduce bed occupancy. In this respect, cheap, fast, simple immunodiagnostic methods are an advantage especially for early cases where the markers have good diagnostic accuracy. In fact, in Germany the sFlt-1/PlGF ratio is already used for identifying which women can be sent home to return for a secondary visit roughly a week later, and for optimizing time of delivery [15,16]. In Israel, one similar study along these lines has been published using PlGF alone [48]. NICE also recommends such use of PlGF [4].
From a different angle, the Fetal Medicine Institute in the UK has conducted large clinical studies for new promising screening markers that may contribute to the use of angiogenic markers. In addition, they used angiogenic markers to identify patients at risk and recruit them for testing novel therapies for PE and PE + FGR [49]. Accordingly, we see this study as an important step toward for improving clinical management in Slovenia and elsewhere using pro- and anti-angiogenic markers.
6. Conclusions
This study shows that the pro- and anti-angiogenic markers, and especially PlGF and the sFlt-1/PlGF, are effective as they are sensible and rather specific for diagnosis of PGR and PE + FGR, especially in early cases delivered <34 wks. Their clinical usefulness needs to be tested with a different study carried out against standard diagnostic techniques, yet this is out of the scope of this study.
Author Contributions
This publication reflects the extended cooperation among all the co-authors. J.O., K.K., T.P.S., and N.T. designed the study protocol, and also applied for and obtaining the ethical approval. T.P.S., V.F.V., and N.T. enrolled the patients in the ObGyn clinics, obtained patient signatures for the informed consent, and conducted all the clinical evaluations and management. K.K., T.F., and J.O. performed the immunodiagnostic tests. Blood pressure, the ultrasound examination and standard blood biochemistry were performed by T.P.S., V.F.V. and N.T., K.K. and A.S.-N. and H.M. constructed the database. The accuracy analysis by Box Plot and ROCs, statistical and mathematical modeling were carried out by K.K., A.S.-N. and H.M. together with K.H.N. and J.O., K.H.N., H.M., J.O. and K.K. obtained study funding. All the authors were involved in writing the manuscript, its editing, and revisions. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the ASPRE project (EU, FP7 # 601852, H.M., K.H.N.), and by the Graduate School of the University Medical Center, Ljubljana, Slovenia (K.K., J.O.).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki for Human subjects involved in clinical research, and was approved b, The National Medical Ethics Committee of the Republic of Slovenia involved in regulating human subjects participation in clinical trials approved the study on 4 December 2012 (Approval No. 104/04/12).
Informed Consent Statement
A written informed consent was obtained from each pregnant woman involved in the study.
Data Availability Statement
Data are available at the websites of Tel Hai College and the University Medical Center, Ljubljana, Slovenia. Due to cyber security issues, links to the websites should be pre-arranged in advanced from A.S.-N. (Tel Hai College) and from K.K. or J.O. (University Medical Center, Ljubljana).
Conflicts of Interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| BMI | Body mass Index |
| BP | Blood pressure |
| dBP | diastolic blood pressure |
| DR | detection rate (sensitivity) |
| FGR | Fetal growth restriction |
| FPR | False Positive rate (1-specificity) |
| ISSHP | International Society for the study of hypertension disorder in pregnancy |
| ISUOG | International society for the ultrasound in obstetrics and gynecology |
| IVF | In-vitro fertilization |
| MAP | Mean arterial blood pressure |
| MCA | Middle Cerebral artery |
| NPV | Negative predictive value |
| PE | Preeclampsia |
| PPV | positive predictive value |
| PSF | Peak systolic flow |
| PlGF | Placenta growth factor |
| PTD | Preterm delivery |
| UTPI | Uterine artery pulsatility index |
| sBP | systolic blood pressure |
| sFlt-1 | soluble Fms like-tyrosine kinase 1 |
| VEGF | Vascular endothelial growth factor |
| 95% CI | 95% Confidence Interval |
References
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- WHO Managing Complications in Pregnancy and Childbirth: A Guide for Midwives and Doctors. 2019. Available online: http://apps.who.int/iris/bitstream/handle/10665/255760/9789241565493-eng.pdf;jsessionid=2BF1F3D8022566FA91FE021D76D50256?sequence=1 (accessed on 1 July 2019).
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef]
- NICE Guidelines, Hypertension in Pregnancy. 2010. Available online: https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-pdf-35109334011877 (accessed on 25 June 2019).
- Poon, L.; Kametas, N.A.; Valencia, C.; Chelemen, T.; Nicolaides, K.H. Hypertensive disorders in pregnancy: Screening by systolic diastolic and mean arterial pressure at 11–13 weeks. Hypertens. Pregnancy 2010, 30, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Jodkowska, A.; Martynowicz, H.; Kaczmarek-Wdowiak, B.; Mazur, G. Thrombocytopenia in pregnancy - pathogenesis and diagnostic approach. Postepy Hig. Med. Dosw. 2015, 12, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Arnaldo Cassia, M.; Daminelli, G.; Zambon, M.; Cardellicchio, M.; Cetin, I.; Gallieni, M. Proteinuria in pregnancy: Clinically driven considerations. Nephrol. Point of Care 2008, 4, 1–5. [Google Scholar]
- Burwick, R.M.; Rincon, M.; Beeraka, S.S.; Gupta, M.; Feinberg, B.B. Evaluation of hemolysis as a severe feature of preeclampsia. Hypertension 2018, 72, 460–465. [Google Scholar] [CrossRef]
- Ekun, O.A.; Olawumi, O.M.; Makwe, C.C.; Ogidi, N.O. Biochemical assessment of renal and liver function among preeclamptics in lagos metropolis. Int. J. Reprod. Med. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Saleh, L.; Vergouwe, Y.; van den Meiracker, A.H.; Verdonk, K.; Russcher, H.; Bremer, H.A.; Versendaal, H.J.; Steegers, E.A.P.; Danser, J.A.H.; Visser, W. Angiogenic markers predict pregnancy complications and prolongation in preeclampsia continuous versus cut-off values. Hypertension 2017, 70, 1–9. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef]
- Engels, T.; Pape, J.; Schoofs, K.; Henrich, W.; Verlohren, S. Automated measurement of sFlt1, PlGF and sFlt1/PlGF ratio in differential diagnosis of hypertensive pregnancy disorders. Hypertens. Pregnancy 2013, 32, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Stubert, J.; Ullmann, S.; Bolz, M.; Külz, T.; Dieterich, M.; Richter, D.-U.; Reimer, T. Prediction of preeclampsia and induced delivery at <34 weeks gestation by sFlt-1 and PlGF in patients with abnormal mid trimester uterine Doppler velocimetry: A prospective cohort analysis. BMC Pregnancy Childbirth 2014, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, J.; Lurba, E.; Chantraine, F.; Vatish, M.; Phil, D.; Staff, A.D.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Schlembach, D.; Hund, M.; Schroer, A.; Wolf, C. Affiliations expand economic assessment of the use of the sFlt-1/PlGF ratio test to predict preeclampsia in Germany. BMC Health Serv. Res. 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- St Pierre, A.; Zaharatos, J.; Goodman, D.; Callaghan, W.M. Challenges and opportunities in identifying, reviewing, and preventing maternal deaths. Obstet. Gynecol. 2018, 131, 138–142. [Google Scholar] [CrossRef]
- Salomon, L.; Alfirevic, Z.; Costa, F.D.S.; Deter, R.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG practice guidelines: Ultrasound assessment of fetal biometry and growth. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef]
- Khalil, A.; Gordijn, S.J.; Beune, I.M.; Wynia, K.; Ganzevoort, W.; Figueras, F.; Kingdom, J.; Marlow, N.; Papageorghiou, A.; Sebire, N.; et al. Essential variables for reporting research studies on fetal growth restriction: A Delphi consensus. Ultrasound Obstet. Gynecol. 2019, 53, 609–614. [Google Scholar] [CrossRef]
- Figueras, F.; Gratacos, E. An integrated approach to fetal growth restriction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 38, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Sharabi-Nov, A.; Kumar, K.; Vodušek, V.F.; Sršen, T.P.; Tul, N.; Fabjan, T.; Meiri, H.; Nicolaides, K.H.; Osredkar, J. Establishing a differential marker profile for pregnancy complications near delivery. Fetal Diagn. Ther. 2019, 47, 471–484. [Google Scholar] [CrossRef]
- Molvarec, A.; Szarka, A.; Walentin, S.; Szűcs, E.; Nagy, B.; Rigó, J. Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with pre-eclampsia. Hypertens. Res. 2010, 33, 892–898. [Google Scholar] [CrossRef]
- Kumer, K.; Premru-Srsen, T.; Fabjan-Vodušek, V.; Tul, N.; Fabjan, T.; Osredkar, J. Peripheral arterial tonometry and angiogenic biomarkers in preeclampsia. Hypertens. Pregnancy 2018, 37, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Shah, Y.P.; Kanon, D.J.; Lindsey, J.V. Fetal crown-rump length: Reevaluation of relation to menstrual age (5–18 weeks) with high-resolution real-time US. Radiology 1992, 182, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Meertens, L.J.; Van Montfort, P.; Scheepers, H.C.; Van Kuijk, S.M.; Aardenburg, R.; Langenveld, J.; Van Dooren, I.M.; Zwaan, I.M.; Spaanderman, M.E.; Smits, L.J. Prediction models for the risk of spontaneous preterm birth based on maternal characteristics: A systematic review and independent external validation. Acta Obstet. Gynecol. Scand. 2018, 97, 907–920. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef]
- Rolnik, D.L.O.D.; Wright, D.; Poon, L.L.; O’Gorman, N.N.; Syngelaki, A.A.; Matallana, C.C.D.P.; Akolekar, R.R.; Cicero, S.S.; Janga, D.D.; Singh, M.M.; et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.Y.; Poon, L.C.; Rolnik, D.L.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; Molina, F.S.; et al. Prediction and prevention of small-for-gestational-age neonates: Evidence from SPREE and ASPRE. Ultrasound Obstet. Gynecol. 2018, 52, 52–59. [Google Scholar] [CrossRef]
- Poon, L.C.; Wright, D.; Rolnik, D.L.; Syngelaki, A.; Delgado, J.L.; Tsokaki, T.; Leipold, G.; Akolekar, R.; Shearing, S.; De Stefani, L.; et al. Aspirin for evidence-based preeclampsia prevention trial: Effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am. J. Obstet. Gynecol. 2017, 217, 585.e1–585.e5. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhu, F.; Ding, Y. Serum screening in first trimester to predict pre-eclampsia, small for gestational age and preterm delivery: Systematic review and meta-analysis. BMC Pregnancy Childbirth 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2020, 20. [Google Scholar] [CrossRef]
- Verlohren, S.; Dröge, L.-A. The diagnostic value of angiogenic and antiangiogenic factors in differential diagnosis of preeclampsia. Am. J. Obstet. Gynecol. 2020, 28. [Google Scholar] [CrossRef]
- Suresh, S.; Mueller, A.; Salahuddin, S.; Naseem, H.; Dhir, R.; Rana, S. Evaluation of angiogenic factors in the decision to admit women with suspected preeclampsia. Pregnancy Hypertens. 2020, 21, 124–131. [Google Scholar] [CrossRef]
- Agrawal, S.; Shinar, S.; Cerdeira, A.S.; Redman, C.; Vatish, M. Predictive performance of PlGF (placental growth factor) for screening preeclampsia in asymptomatic women: A systematic review and meta-analysis. Hypertension 2019, 74, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.R.; Woelkers, D.A.; Newman, R.B.; Combs, C.A.; How, H.Y.; Boggess, K.A.; Martin, J.N.; Kupfer, K.; Sibai, B.M.; PETRA (Preeclampsia Triage by Rapid Assay) Trial. Placental growth factor predicts time to delivery in women with signs or symptoms of early preterm preeclampsia: A prospective multicenter study. Am. J. Obstet. Gynecol. 2020, 222, 259.e1–259.e11. [Google Scholar] [CrossRef] [PubMed]
- Dymara-Konopka, W.; Laskowska, M.; Błażewicz, A. Angiogenic imbalance as a contributor of preeclampsia. Curr. Pharm. Biotechnol. 2018, 19, 797–815. [Google Scholar] [CrossRef]
- Stott, D.; Papastefanou, I.; Paraschiv, D.; Clark, K.; Kametas, N.A. Longitudinal maternal hemodynamics in pregnancies affected by fetal growth restriction. Ultrasound Obstet. Gynecol. 2017, 49, 761–768. [Google Scholar] [CrossRef]
- Stott, D.; Nzelu, O.; Nicolaides, K.H.; Kametas, N.A. Maternal haemodynamics in normal pregnancies and in pregnancies affected by pre-eclampsia. Ultrasound Obstet Gynecol. 2018, 52, 359–364. [Google Scholar] [CrossRef]
- Ferrazzi, E.; Stampalija, T.; Monasta, L.; Di Martino, D.; Vonck, S.; Gyselaers, W. Maternal hemodynamics: A method to classify hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2018, 218, 124.e1–124.e11. [Google Scholar] [CrossRef]
- Benton, S.J.; Leavey, K.; Grynspan, D.; Cox, B.J.; Bainbridge, S.A. The clinical heterogeneity of preeclampsia is related to both placental gene expression and placental histopathology. Am. J. Obstet. Gynecol. 2018, 219, 604.e1–604.e25. [Google Scholar] [CrossRef]
- Baschat, A.A.; Dewberry, D.; Seravalli, V.; Miller, J.L.; Block-Abraham, D.; Blitzer, M.G. Maternal blood-pressure trends throughout pregnancy and development of pre-eclampsia in women receiving first-trimester aspirin prophylaxis. Ultrasound Obstet. Gynecol. 2018, 52, 728–733. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Stefańska, K.; Zieliński, M.; Sakowska, J.; Jankowiak, M.; Trzonkowski, P.; Marek-Trzonkowska, N.; Kwiatkowski, S. Podocytes—The most vulnerable renal cells in preeclampsia. Int. J. Mol. Sci. 2020, 21, 5051. [Google Scholar] [CrossRef] [PubMed]
- Molvarec, A.; Fügedi, G.; Szabó, E.; Stenczer, B.; Walentin, S.; Rigó, J.J. Decreased circulating anandamide levels in preeclampsia. Hypertens. Res. 2015, 38, 413–418. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.; Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG practice guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Guedes-Martins, L. Superimposed preeclampsia. Adv. Exp. Med. Biol. 2016, 956, 409–417. [Google Scholar] [CrossRef]
- Özalp, M.; Demir, Ö.; Akbas, H.; Kaya, E.; Celik, C.; Osmanağaoğlu, M.A. Effect of COVID-19 pandemic process on prenatal diagnostic procedures. J. Matern. Fetal. Neonatal. Med. 2020, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tsviban, A.; Maymon, R.; Meiri, H.; Wiener, Y. Using maternal serum placental growth factor (PlGF) for ruling out preeclampsia near delivery: Preliminary results and economic implications in Israel. Harefuah 2020, 159, 492–497. [Google Scholar]
- Lai, J.; Syngelaki, A.; Nicolaides, K.H.; Von Dadelszen, P.; Magee, L.A. Impact of new definitions of preeclampsia at term on identification of adverse maternal and perinatal outcomes. Am. J. Obstet. Gynecol. 2020. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).