The Evolution of the Cryopreservation Techniques in Reproductive Medicine—Exploring the Character of the Vitrified State Intra- and Extracellularly to Better Understand Cell Survival after Cryopreservation
Abstract
1. Cryopreservation: A Field of Emerging Interest
1.1. In the Animal Field
1.2. In the Human Field
2. Evolution of the Cryopreservation Techniques in ART
2.1. Cryopreservation in the 1980s–1990s: Applied for a Limited Number of Indications, Why?
2.2. Slow Freezing and the Undesirable Events Associated with Ice Crystal Formation
2.3. One Century Ago: The Rise of the Vitrification Concept
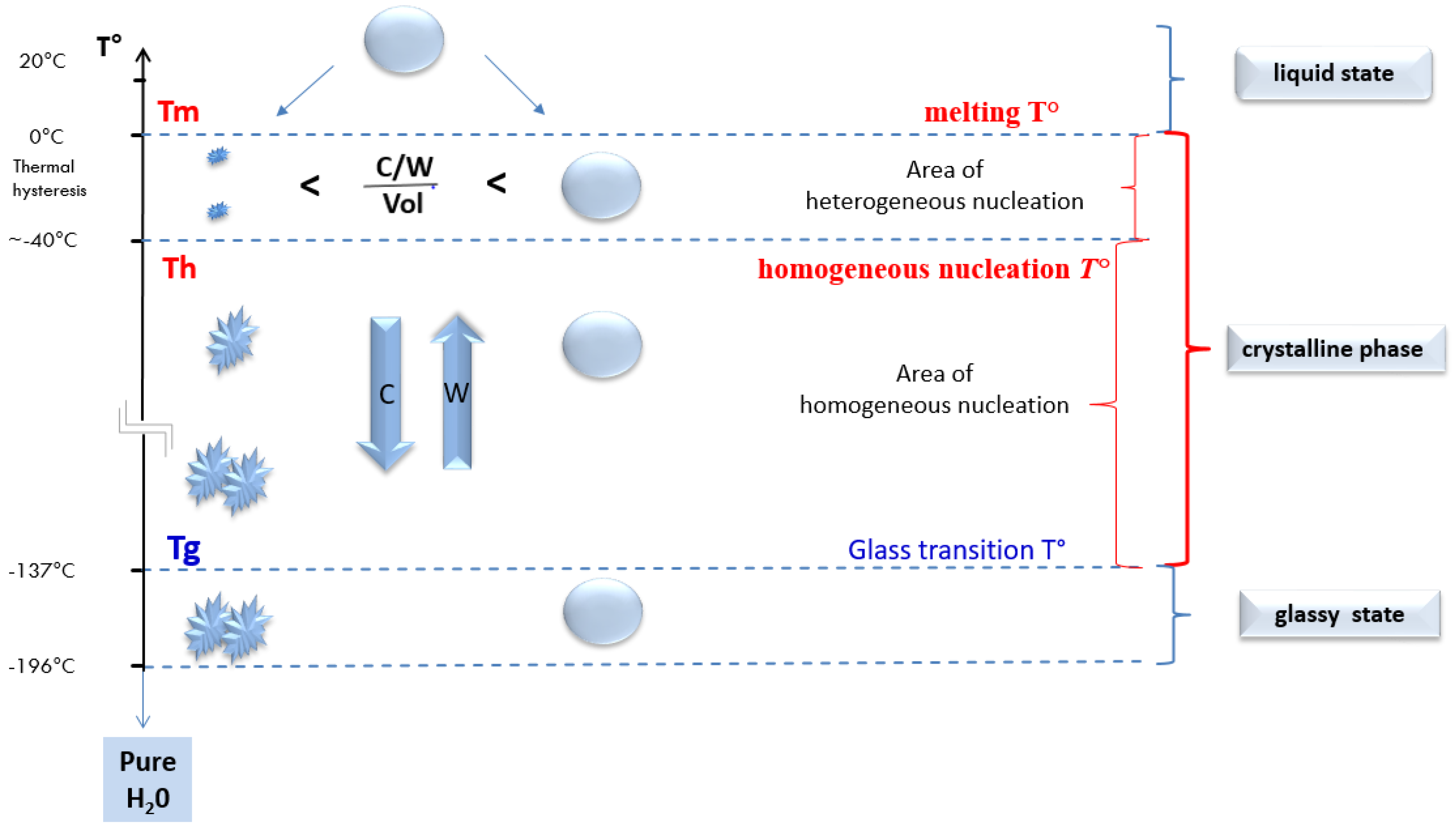
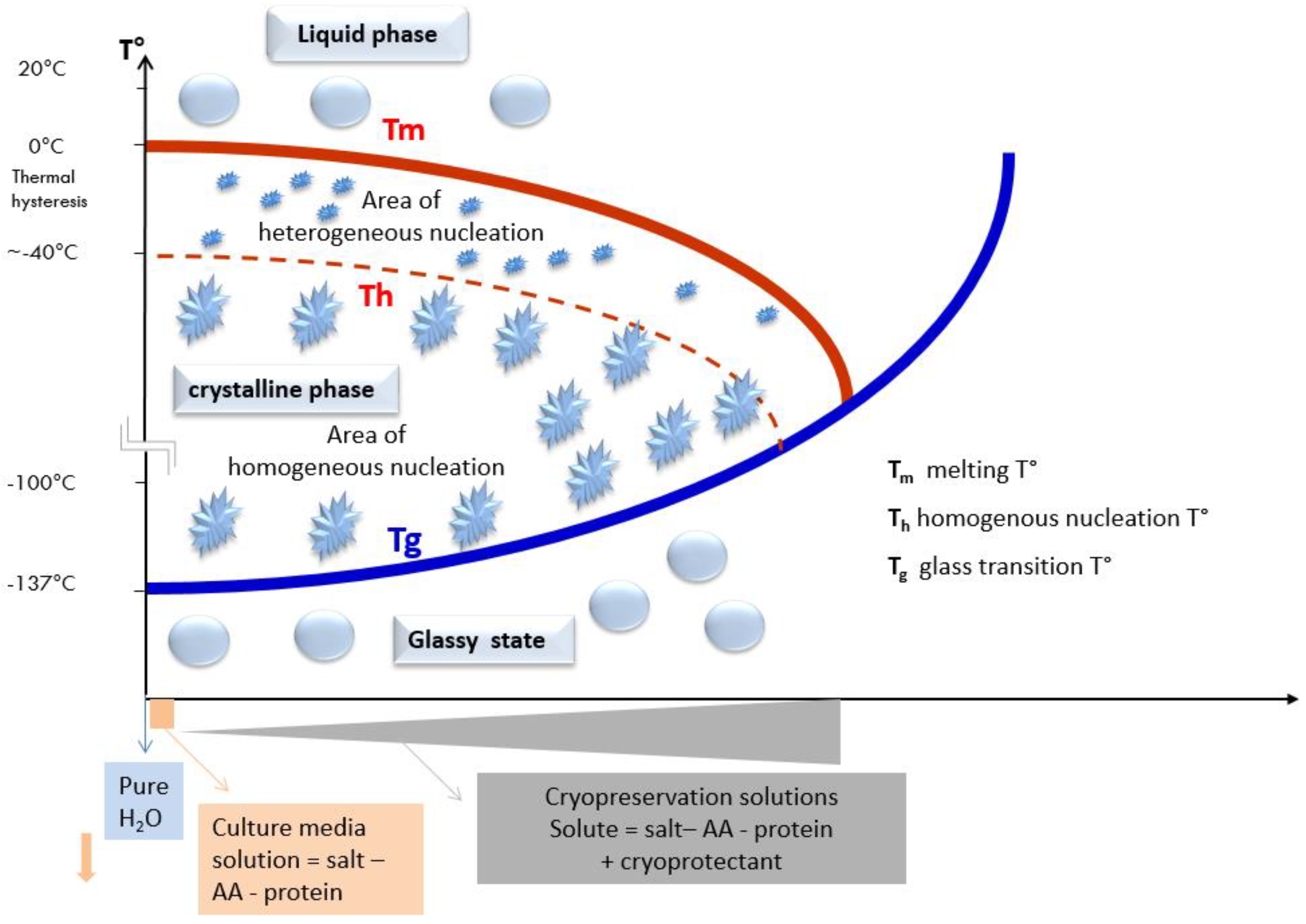
2.4. At Which Temperature Do We Obtain a Glass-Like Amorphous Solid State?
2.4.1. Temperature and the Change of States of Pure Water during a Cooling Process
2.4.2. Shifting of the Glass Transition Temperature (Tg) in Cryoprotectant Solutions
2.5. The General Principle of the Vitrification Technique
2.5.1. The Concentration of CPA (Viscosity), Cooling/Warming Rates and Volume: Three Parameters to Control Achieving a Vitrified State
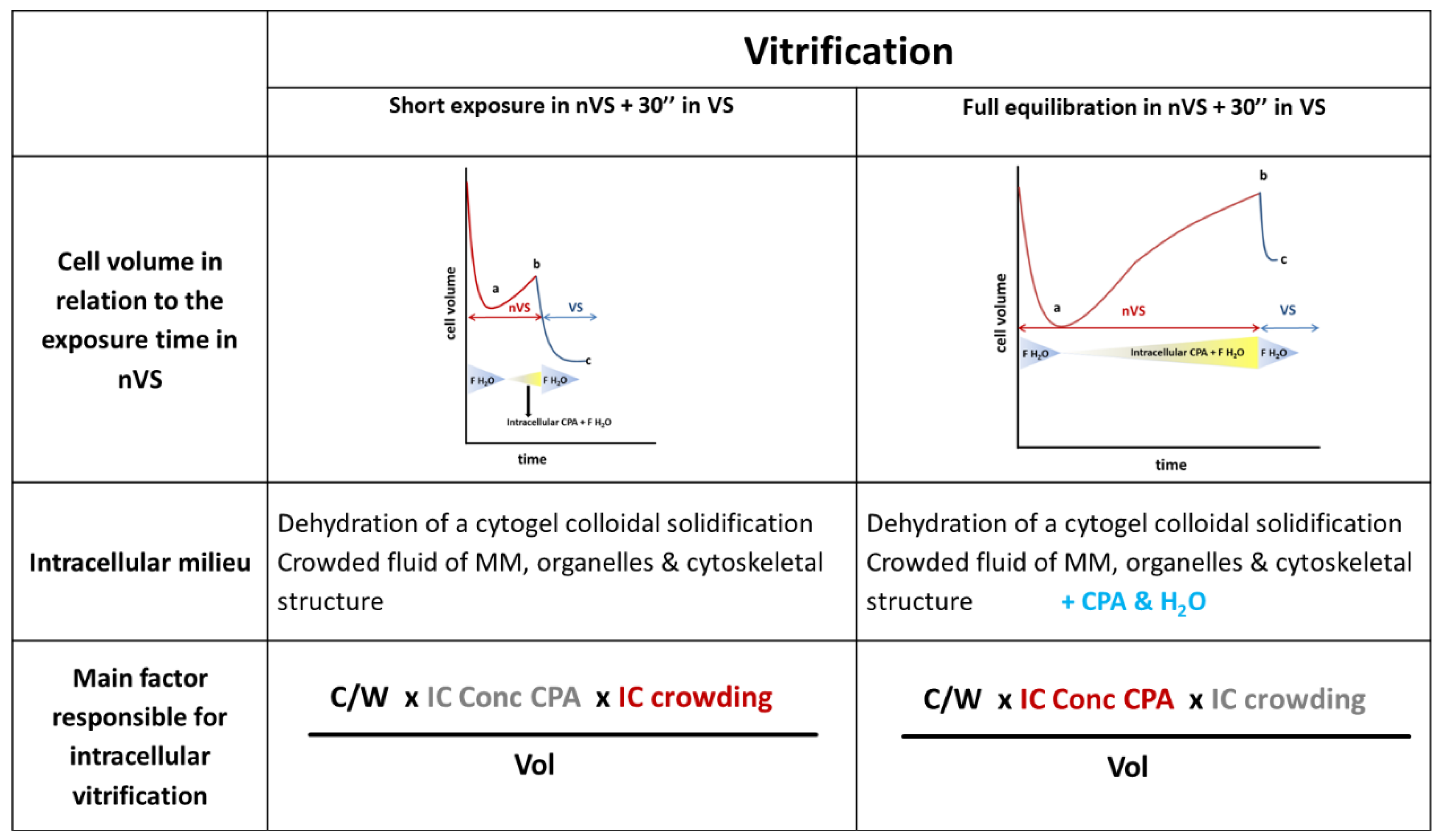
2.5.2. How to Prepare Oocytes and Embryos before Plunging Them into LN2?
2.6. Exploration of the Intracellular Compartment
2.6.1. Mode of Action of the High Concentrated VS
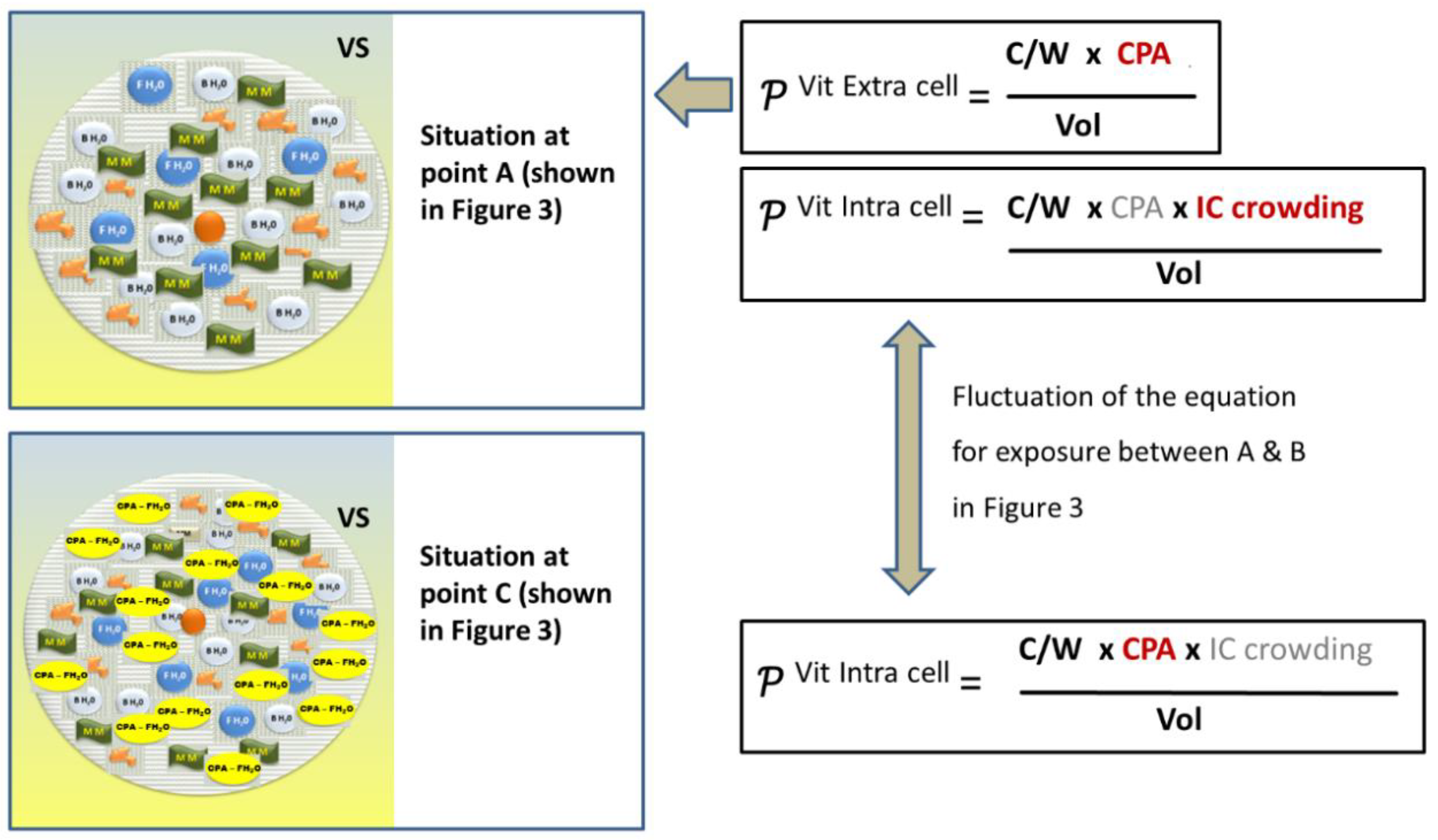
2.6.2. The Intracellular Concentration of Cryoprotectant Is Far below the Number of CPA in the Vitrifying Solution
2.6.3. Two Physical Arguments to Explain the Absence of Intracellular Crystallization
Intracellular Crowding and the Colloidal Vitrification
Revision of the Classical Equation on the Probability of Achieving a Vitrifying State
The Difference in the Intra- and Extracellular Glass Transition Temperature
2.6.4. What Can Be Deducted from the Technique of Vitrification?
2.7. Intracellular Vitrification: A Common Denominator for All Successful Cryopreservation Procedures
3. Conclusions
Funding
Conflicts of Interest
References
- Whittingham, D.G.; Leibo, S.P.; Mazur, P. Survival of mouse embryos frozen to −96 degrees and −269 degrees C. Science 1972, 178, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Vatja, G. Vitrification in animal reproduction: Vitrification of embryos using open pulled straws. In Vitrification in Assisted Reproduction. A User´s Manual and Trouble-Shooting Guide; Tucker, M.J., Liebermann, J., Eds.; Informa Healthcare: London, UK, 2007; pp. 65–73. [Google Scholar]
- Mandawala, A.A.; Harvey, S.C.; Roy, T.K.; Fowler, K.E. Cryopreservation of animal oocytes and embryos: Current progress and future prospects. Theriogenology 2016, 86, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I. In vitro embryo production. In Reproductive Technologies in Farm Animals, 2nd ed.; Gordon, I., Ed.; CABI Pub: Cambridge, MA, USA, 2017; pp. 100–101. [Google Scholar]
- Mochida, K.; Ogura, A. Cryopreservation of embryos in laboratory species. J. Mamm. Ova Res. 2010, 27, 87–92. [Google Scholar] [CrossRef]
- Tsang, W.H.; Chow, K. Cryopreservation of embryos from model animals and human. In Current Frontiers in Cryobiology; Katkov, I., Ed.; InTech: Houston, TX, USA, 2012; pp. 259–290. Available online: https://www.intechopen.com/books/current-frontiers-in-cryobiology/cryopreservation-of-embryos-of-model-animals-and-human (accessed on 28 August 2020).
- Neubauer, J.C.; Beier, A.F.; Stracke, F.; Zimmermann, H. Vitrification in pluripotent stem cell banking: Requirements and technical solutions for large-scale biobanks. In Vitrification in Assisted Reproduction, 2nd ed.; Tucker, M.J., Liebermann, J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 203–224. [Google Scholar]
- Connan, D. Contribution à L’étude de la Cryopréservation des Cellules Souches Embryonnaires Humaines à Visée Thérapeutique. Thése Doctorat, Université de Liège, Liège, Belgique, 2013. Available online: https://orbi.uliege.be/handle/2268/157842 (accessed on 28 July 2020).
- Courbiere, B.; Decanter, C.; Bringer-Deutsch, S.; Rives, N.; Mirallié, S.; Pech, J.C.; De Ziegler, D.; Carré-Pigeon, F.; May-Panloup, P.; Sifer, C.; et al. Emergency IVF for embryo freezing to preserve female fertility: A French multicentre cohort study. Hum. Reprod. 2013, 28, 2381–2382. [Google Scholar] [CrossRef]
- Peccatori, F.A.; Mangili, G.; Bergamini, A.; Filippi, F.; Martinelli, F.; Ferrari, F.; Noli, S.; Rabaiotti, E.; Candiani, M.; Somigliana, E. Fertility preservation in women harboring deleterious BRCA mutations: Ready for prime time? Hum. Reprod. 2018, 33, 181–187. [Google Scholar] [CrossRef]
- Zeilmaker, G.H.; Alberda, A.T.; Van Gent, I.; Rijkmans, C.M.; Drogendijk, A.C. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil. Steril. 1984, 42, 293–296. [Google Scholar] [CrossRef]
- Mazur, P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977, 14, 251–272. [Google Scholar] [CrossRef]
- Leibo, S.P. Fundamental cryobiology of mouse ova and embryos. In The Freezing of Mammalian Embryos; Elliot, K., Whelan, J., Eds.; Elsevier/Excerpta Medica/North-Holland: Amsterdam, The Netherlands, 1977; Volume 52, pp. 69–92. [Google Scholar]
- Mazur, P.; Cole, K.W. Influence of cell concentration on the contribution of unfrozen fraction and salt concentration to the survival of slowly frozen human erythrocytes. Cryobiology 1985, 22, 509–536. [Google Scholar] [CrossRef]
- Pegg, D.E. Principles of Cryopreservation. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F., Oldenhof, H., Eds.; Methods in Molecular Biology; Springer Science + Business Media: New York, NY, USA, 2015; Volume 1257, pp. 3–19. [Google Scholar]
- Luyet, B. The vitrification of organic colloids and of protoplasm. Biodynamica 1937, 1, 1–14. [Google Scholar]
- Luyet, B.; Rapatz, G. Patterns of ice formation in some aqueous solutions. Biodynamica 1958, 8, 1–68. [Google Scholar]
- Rall, W.F.; Fahy, G.M. Ice-free cryopreservation of mouse embryos at 196 C by vitrification. Nature 1985, 313, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Scheffen, B.; Vanderzwalmen, P.; Massip, A. A simple and efficient procedure for preservation of mouse embryos by vitrification. Cryo Lett. 1986, 7, 260–269. [Google Scholar]
- Vanderzwalmen, P.; Gaurois, B.; Ectors, F.J.; Massip, A.; Ectors, F. Some factors affecting successful vitrification of mouse blastocysts. Theriogenology 1988, 30, 1177–1183. [Google Scholar] [CrossRef][Green Version]
- Massip, A.; Vanderzwalmen, P.; Scheffen, B.; Ectors, F. Pregnancies following transfer of cattle embryos preserved by vitrification. Cryo Lett. 1986, 7, 270–273. [Google Scholar]
- Vanderzwalmen, P.; Delval, A.; Chatziparasidou, A.; Bertin, G.; Ectors, F.; Lejeune, B.; Nijs, M.; Prapas, N.; Prapas, Y.; Van Damme, B.I.; et al. Pregnancies after vitrification of human day 5 embryos. Hum. Reprod. 1997, 12, 98. [Google Scholar] [CrossRef]
- Benson, E.E. Cryopreservation Theory. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 15–32. [Google Scholar]
- Chian, R.C. Cryobiology: An overview. In Fertility Cryopreservation; Chian, R.C., Quinn, P., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 1–9. [Google Scholar]
- Brüggeller, P.; Mayer, E. Complete vitrification in pure liquid water and dilute aqueous solutions. Nature 1980, 288, 569–571. [Google Scholar] [CrossRef]
- Mayer, E.; Brüggeller, P. Vitrification of pure liquid water by high pressure jet freezing. Nature 1982, 298, 715–718. [Google Scholar] [CrossRef]
- Dubochet, J.; McDowall, A.W. Vitrification of pure water for electron microscopy. J. Microsc. 1981, 124, RP3–RP4. [Google Scholar] [CrossRef]
- Fahy, G.M.; Rall, W.F. Vitrification: An overview. In Assisted Reproduction: A User’s Manual and Troubleshooting Guide; Tucker, M.J., Liebermann, J., Eds.; Informa Healthcare: London, UK, 2007; pp. 1–20. [Google Scholar]
- Fahy, G.M.; MacFarlane, D.R.; Angell, C.A.; Meryman, H.T. Vitrification as an approach to cryopreservation. Cryobiology 1984, 21, 407–426. [Google Scholar] [CrossRef]
- Van der Elsken, J.; Dings, J.; Michielsen, C.F. The freezing of supercooled water. J. Mol. Struct. 1991, 250, 245–251. [Google Scholar] [CrossRef]
- Muldrew, K.; Acker, J.P.; Elliott, J.A.W.; McGann, L.E. The Water to Ice transition: Implications for living cells. In Life in the Frozen State; Fuller, B.J., Lane, N., Benson, E.E., Eds.; CRC Press: Boca Raton, FL, USA; New York, NY, USA; Washington, DC, USA, 2004; pp. 67–107. [Google Scholar]
- Franks, F. Metastable water at subzero temperatures. J. Microsc. 1986, 141, 243–249. [Google Scholar] [CrossRef]
- Starr, F.W.; Angell, C.A.; Stanley, H.E. Prediction of entropic and dynamic properties of water below the homogeneous nucleation temperature. Phys. A Stat. Mech. Appl. 2003, 323, 51–66. [Google Scholar] [CrossRef]
- Angell, C.A. Liquid fragility and the glass transition in water and aqueous solutions. Chem. Rev. 2002, 102, 2627–2650. [Google Scholar] [CrossRef] [PubMed]
- Mullen, S.F.; Critser, J.K. The Science of Cryobiology. In Oncofertility: Fertility Preservation for Cancer Survivors; Woodruff, T.K., Snyder, K.A., Eds.; Springer: New York, NY, USA, 2007; pp. 83–109. [Google Scholar]
- Cocks, F.; Brower, W. Phase diagram relationships in cryobiology. Cryobiology 1974, 11, 340–358. [Google Scholar] [CrossRef]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryobiology Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Forsyth, M. Recent insights on the role of cryoprotective agents in vitrification. Cryobiology 1990, 27, 345–358. [Google Scholar] [CrossRef]
- Fuller, B.J. Cryoprotectants: The essential antifreezes to protect life in the frozen state. Cryo Let. 2004, 25, 375–388. [Google Scholar]
- Yavin, S.; Arav, A. Measurement of essential physical properties of vitrification solutions. Theriogenology 2007, 67, 81–89. [Google Scholar] [CrossRef]
- Fahy, G.M.; Levy, D.I.; Ali, S.E. Some emerging principles underlying the physical properties, biological actions, and utility of vitrification solutions. Cryobiology 1987, 24, 196–213. [Google Scholar] [CrossRef]
- Vanderzwalmen, P.; Ectors, F.; Grobet, L.; Prapas, Y.; Panagiotidis, Y.; Vanderzwalmen, S.; Stecher, A.; Frias, P.; Liebermann, J.; Zech, N.H. Aseptic vitrification of blastocysts from infertile patients, egg donors and after IVM. Reprod. BioMed. Online 2009, 5, 700–7007. [Google Scholar] [CrossRef]
- Vanderzwalmen, P.; Zech, N.H.; Ectors, F.; Stecher, A.; Lejeune, B.; Vanderzwalmen, S.; Wirleitner, B. Blastocyst transfer after aseptic vitrification of zygotes: An approach to overcome an impaired uterine environment. Reprod. BioMed. Online 2012, 6, 591–599. [Google Scholar] [CrossRef][Green Version]
- Vanderzwalmen, P.; Connan, D.; Grobet, L.; Wirleitner, B.; Remy, B.; Vanderzwalmen, S.; Zech, N.; Ectors, F.J. Lower intracellular concentration of cryoprotectants after vitrification than after slow freezing despite exposure to higher concentration of cryoprotectant solutions. Hum. Reprod. 2013, 28, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Luyet, B. The problem of structural instability and molecular mobility in aqueous solutions “solidified” at low temperatures. Biodynamica 1966, 10, 1–32. [Google Scholar] [PubMed]
- Galashev, A.E. Vitrification and structural differences between metal glass, quasicrystal and Frank-Kasper phases. J. Struct. Chem. 1996, 37, 120–136. [Google Scholar] [CrossRef]
- Mochida, K.; Hasegawa, A.; Li, M.W.; Fray, M.D.; Kito, S.; Vallelunga, J.M.; Lloyd, K.C.; Yoshiki, A.; Obata, Y.; Ogura, A. High Osmolality Vitrification: A New Method for the Simple and Temperature-Permissive Cryopreservation of Mouse Embryos. PLoS ONE 2013, 8, e49316. [Google Scholar] [CrossRef]
- Zhou, E.H.; Trepat, X.; Park, C.Y.; Lenormand, G.; Oliver, M.N.; Mijailovich, S.M.; Hardin, C.; Weitz, D.A.; Butler, J.P.; Fredberg, J.J. Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition: Version 2. Proc. Natl. Acad. Sci. USA 2009, 106, 10632–10637. [Google Scholar] [CrossRef]
- Parry, B.R.; Surovtsev, I.V.; Cabeen, M.T.; O’Hern, C.S.; Dufresne, E.R.; Jacobs-Wagner, C. The Bacterial Cytoplasm Has Glass-like Properties and Is Fluidized by Metabolic Activity. Cell 2014, 156, 183–194. [Google Scholar] [CrossRef]
- Nishizawa, K.; Fujiwara, K.; Ikenaga, M.; Nakajo, N.; Yanagisawa, M.; Mizuno, D. Universal glass-forming behavior of in vitro and living cytoplasm. Sci. Rep. 2017, 7, 15143. [Google Scholar] [CrossRef]
- Hunter, G.L.; Weeks, E.R. The physics of the colloidal glass transition. Rep. Prog. Phys. 2012, 75, 066501. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.; Meneghel, J.; Cenard, S.; Passot, S.; Morris, G.J. Determination of Intracellular Vitrification Temperatures for Unicellular Micro Organisms under Conditions Relevant for Cryopreservation. PLoS ONE 2016, 11, e0152939. [Google Scholar] [CrossRef]
- Mourão, M.A.; Hakim, J.B.; Schnell, S. Connecting the Dots: The Effects of Macromolecular Crowding on Cell Physiology. Biophys. J. 2014, 107, 2761–2766. [Google Scholar] [CrossRef]
- Tao, Y.; Sanger, E.; Saewu, A.; Leveille, M.C. Human sperm vitrification: The state of the art. Reprod. Biol. Endocrinol. 2020, 18, 17. [Google Scholar] [CrossRef]
- Álvarez, C.; González, N.; Luño, V.; Gil, L. Ejaculated compared with epididymal stallion sperm vitrification. Anim. Reprod. Sci. 2019, 211, 106205. [Google Scholar] [CrossRef]
- Morris, J.G.; Acton, E.; Murray, B.J.; Fonseca, F. Freezing injury: The special case of the sperm cell. Cryobiology 2012, 64, 71–80. [Google Scholar] [CrossRef]

| Zygote or Embryo Cryopreservation: |
|
| Indications for Oocyte Cryopreservation: |
|
| Vitrification | Crystallization | |
|---|---|---|
| No Molecular Organization Infinite Increase of Viscosity | Molecular Organization of Water | |
| Of a solution: Vitreous amorphous state | Of a colloid: Colloidal amorphous state | 13 types of crystal structures (hexagonal or cubic) |
| Tg = −130 °C | Tg = <−100 °C | Tm = −5 °C |
| = solidification in a state typical of a liquid | = solidification is related to dehydration of a colloid | Solution effect -> amorphous vitrification between ice crystals |
| Extracellular only | Intra- or extracellular | Extracellular only |
| Cryopreservation Technics | Vitrification | Slow Freezing | Ultra-Rapid Freezing | Sperm Vitrification | |
|---|---|---|---|---|---|
| Short time in nVS | Long time in nVS | ||||
| Extracellular crystallization | No | No | Yes | Yes | Yes |
| Intracellular vitrification | Yes | Yes | Yes | Yes | Yes |
| Intracellular CPA concentration | Low | High | High | Low | Very low |
| Intracellular crowding of MM colloid | +++++ | ++ | ++++ | ++ | +++++ |
| Extracellular Tg | Low | Low | low | ? | Low |
| Intracellular Tg | higher | higher | higher | higher | higher |
| Solution effect during cooling | No | No | Yes | No | No |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanderzwalmen, P.; Ectors, F.; Panagiotidis, Y.; Schuff, M.; Murtinger, M.; Wirleitner, B. The Evolution of the Cryopreservation Techniques in Reproductive Medicine—Exploring the Character of the Vitrified State Intra- and Extracellularly to Better Understand Cell Survival after Cryopreservation. Reprod. Med. 2020, 1, 142-157. https://doi.org/10.3390/reprodmed1020011
Vanderzwalmen P, Ectors F, Panagiotidis Y, Schuff M, Murtinger M, Wirleitner B. The Evolution of the Cryopreservation Techniques in Reproductive Medicine—Exploring the Character of the Vitrified State Intra- and Extracellularly to Better Understand Cell Survival after Cryopreservation. Reproductive Medicine. 2020; 1(2):142-157. https://doi.org/10.3390/reprodmed1020011
Chicago/Turabian StyleVanderzwalmen, Pierre, Fabien Ectors, Yannis Panagiotidis, Maximilian Schuff, Maximilian Murtinger, and Barbara Wirleitner. 2020. "The Evolution of the Cryopreservation Techniques in Reproductive Medicine—Exploring the Character of the Vitrified State Intra- and Extracellularly to Better Understand Cell Survival after Cryopreservation" Reproductive Medicine 1, no. 2: 142-157. https://doi.org/10.3390/reprodmed1020011
APA StyleVanderzwalmen, P., Ectors, F., Panagiotidis, Y., Schuff, M., Murtinger, M., & Wirleitner, B. (2020). The Evolution of the Cryopreservation Techniques in Reproductive Medicine—Exploring the Character of the Vitrified State Intra- and Extracellularly to Better Understand Cell Survival after Cryopreservation. Reproductive Medicine, 1(2), 142-157. https://doi.org/10.3390/reprodmed1020011





