Molecular Basis of Atrial Fibrillation Initiation and Maintenance
Abstract
1. Introduction
2. Electrophysiological Mechanisms Associated with AF
2.1. Ectopic Activity
2.2. Conduction Abnormalities
2.3. Enhanced Automaticity
3. Molecular Regulatory Networks of Proarrhythmic Mechanisms in the Atria
3.1. Genetic Regulation
3.2. Autonomic Remodeling and Upregulation of Sympathetic Signaling
3.3. Oxidative Stress and Inflammation
4. Therapeutic Implications and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colilla, S.; Crow, A.; Petkun, W.; Singer, D.E.; Simon, T.; Liu, X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am. J. Cardiol. 2013, 112, 1142–1147. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial Remodeling and Atrial Fibrillation. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef]
- Nattel, S.; Guasch, E.; Savelieva, I.; Cosio, F.G.; Valverde, I.; Halperin, J.L.; Conroy, J.M.; Al-Khatib, S.M.; Hess, P.L.; Kirchhof, P.; et al. Early management of atrial fibrillation to prevent cardiovascular complications. Eur. Heart J. 2014, 35, 1448–1456. [Google Scholar] [CrossRef]
- Sanchez-Quintana, D.; Ramon Lopez-Mínguez, J.; Pizarro, G.; Murillo, M.; Cabrera, J.A. Triggers and Anatomical Substrates in the Genesis and Perpetuation of Atrial Fibrillation. Curr. Cardiol. Rev. 2012, 8, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.R.; Cha, T.J.; Zhang, L.; Chartier, D.; Melnyk, P.; Hohnloser, S.H.; Nattel, S. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: Action potential and ionic current properties. J. Physiol. 2003, 551, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Kholova, I.; Kautzner, J. Anatomic Characteristics of Extensions of Atrial Myocardium into the Pulmonary Veins in Subjects With and Without Atrial Fibrillation. Pacing Clin. Electrophysiol. 2003, 26, 1348–1355. [Google Scholar] [CrossRef]
- Li, N.; Csepe, T.A.; Hansen, B.J.; Sul, L.V.; Kalyanasundaram, A.; Zakharkin, S.O.; Zhao, J.; Guha, A.; Van Wagoner, D.R.; Kilic, A.; et al. Adenosine-Induced Atrial Fibrillation. Circulation 2016, 134, 486–498. [Google Scholar] [CrossRef]
- Hocini, M.; Nault, I.; Wright, M.; Veenhuyzen, G.; Narayan, S.M.; Jaïs, P.; Lim, K.T.; Knecht, S.; Matsuo, S.; Forclaz, A.; et al. Disparate Evolution of Right and Left Atrial Rate During Ablation of Long-Lasting Persistent Atrial Fibrillation. J. Am. Coll. Cardiol. 2010, 55, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.J. Cardiac adrenergic control and atrial fibrillation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010, 381, 235–249. [Google Scholar] [CrossRef]
- Hiraoka, M.; Sunami, A.; Fan, Z.; Sawanobori, T. Multiple Ionic Mechanisms of Early Afterdepolarizations in Isolated Ventricular Myocytes from Guinea-pig Hearts. Ann. N. Y. Acad. Sci. 1992, 644, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.M.; Alekseev, A.E.; Liu, X.K.; Park, S.; Zingman, L.V.; Bienengraeber, M.; Sattiraju, S.; Ballew, J.D.; Jahangir, A.; Terzic, A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum. Mol. Genet. 2006, 15, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Volders, P. Similarities between early and delayed afterdepolarizations induced by isoproterenol in canine ventricular myocytes. Cardiovasc. Res. 1997, 34, 348–359. [Google Scholar] [CrossRef]
- Nattel, S.; Maguy, A.; le Bouter, S.; Yeh, Y.H. Arrhythmogenic Ion-Channel Remodeling in the Heart: Heart Failure, Myocardial Infarction, and Atrial Fibrillation. Physiol. Rev. 2007, 87, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Hüser, J.; Blatter, L.A.; Lipsius, S.L. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J. Physiol. 2000, 524, 415–422. [Google Scholar] [CrossRef]
- Kockskämper, J.; Sheehan, K.A.; Bare, D.J.; Lipsius, S.L.; Mignery, G.A.; Blatter, L.A. Activation and Propagation of Ca2+ Release during Excitation-Contraction Coupling in Atrial Myocytes. Biophys. J. 2001, 81, 2590–2605. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Llach, A.; Bayes-Genís, A.; Roura, S.; Rodriguez Font, E.; Arís, A.; Cinca, J. Atrial Fibrillation Is Associated With Increased Spontaneous Calcium Release From the Sarcoplasmic Reticulum in Human Atrial Myocytes. Circulation 2004, 110, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Bögeholz, N.; Pauls, P.; Dechering, D.G.; Frommeyer, G.; Goldhaber, J.I.; Pott, C.; Eckhardt, L.; Müller, F.U.; Schulte, J.S. Distinct Occurrence of Proarrhythmic Afterdepolarizations in Atrial Versus Ventricular Cardiomyocytes: Implications for Translational Research on Atrial Arrhythmia. Front. Pharmacol. 2018, 9, 933. [Google Scholar] [CrossRef]
- Lehnart, S.E.; Wehrens, X.H.T.; Marks, A.R. Calstabin deficiency, ryanodine receptors, and sudden cardiac death. Biochem. Biophys. Res. Commun. 2004, 322, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Kobayashi, S.; Kohno, M.; Doi, M.; Tokuhisa, T.; Okuda, S.; Suetsugu, M.; Hisaoka, T.; Obayashi, M.; Ohkusa, T.; et al. FKBP12.6-Mediated Stabilization of Calcium-Release Channel (Ryanodine Receptor) as a Novel Therapeutic Strategy Against Heart Failure. Circulation 2003, 107, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xiao, J.; Jiang, D.; Wang, R.; Vembaiyan, K.; Wang, A.; Smith, C.D.; Xie, C.; Chen, W.; Zhang, J.; et al. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat. Med. 2011, 17, 1003–1009. [Google Scholar] [CrossRef]
- Gupta, R.C.; Mishra, S.; Rastogi, S.; Imai, M.; Habib, O.; Sabbah, H.N. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2373–H2381. [Google Scholar] [CrossRef]
- Shanmugam, M.; Molina, C.E.; Gao, S.; Severac-Bastide, R.; Fischmeister, R.; Babu, G.J. Decreased sarcolipin protein expression and enhanced sarco(endo)plasmic reticulum Ca2+ uptake in human atrial fibrillation. Biochem. Biophys. Res. Commun. 2011, 410, 97–101. [Google Scholar] [CrossRef]
- Ziolo, M.T.; Martin, J.L.; Bossuyt, J.; Bers, D.M.; Pogwizd, S.M. Adenoviral Gene Transfer of Mutant Phospholamban Rescues Contractile Dysfunction in Failing Rabbit Myocytes With Relatively Preserved SERCA Function. Circ. Res. 2005, 96, 815–817. [Google Scholar] [CrossRef]
- Llach, A.; Molina, C.E.; Fernandes, J.; Padró, J.; Cinca, J.; Hove-Madsen, L. Sarcoplasmic reticulum and L-type Ca2+ channel activity regulate the beat-to-beat stability of calcium handling in human atrial myocytes. J. Physiol. 2011, 589, 3247–3262. [Google Scholar] [CrossRef] [PubMed]
- Tzeis, S.; Asvestas, D.; Vardas, P. Atrial Fibrosis: Translational Considerations for the Management of AF Patients. Arrhythmia Electrophysiol. Rev. 2019, 8, 37–41. [Google Scholar] [CrossRef]
- Gaudesius, G.; Miragoli, M.; Thomas, S.P.; Rohr, S. Coupling of Cardiac Electrical Activity Over Extended Distances by Fibroblasts of Cardiac Origin. Circ. Res. 2003, 93, 421–428. [Google Scholar] [CrossRef] [PubMed]
- McArthur, L.; Riddell, A.; Chilton, L.; Smith, G.L.; Nicklin, S.A. Regulation of connexin 43 by interleukin 1β in adult rat cardiac fibroblasts and effects in an adult rat cardiac myocyte: Fibroblast co-culture model. Heliyon 2020, 6, e03031. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, M.; Gaudesius, G.; Rohr, S. Electrotonic Modulation of Cardiac Impulse Conduction by Myofibroblasts. Circ. Res. 2006, 98, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, M.; Salvarani, N.; Rohr, S. Myofibroblasts Induce Ectopic Activity in Cardiac Tissue. Circ. Res. 2007, 101, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.A.; Camelliti, P.; Rog-Zielinska, E.A.; Siedlecka, U.; Poggioli, T.; O’Toole, E.T.; Knöpfel, T.; Kohl, P. Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proc. Natl. Acad. Sci. USA 2016, 113, 14852–14857. [Google Scholar] [CrossRef]
- De Bakker, J.M.T.; Stein, M.; van Rijen, H.V.M. Three-dimensional anatomic structure as substrate for ventricular tachycardia/ventricular fibrillation. Heart Rhythm 2005, 2, 777–779. [Google Scholar] [CrossRef]
- Delaunay, M.; Osman, H.; Kaiser, S.; Diviani, D. The Role of Cyclic AMP Signaling in Cardiac Fibrosis. Cells 2019, 9, 69. [Google Scholar] [CrossRef]
- Moreira, L.M.; Takawale, A.; Hulsurkar, M.; Menassa, D.A.; Antanaviciute, A.; Lahiri, S.K.; Mehta, N.; Evans, N.; Psarros, C.; Robinson, P.; et al. Paracrine signalling by cardiac calcitonin controls atrial fibrogenesis and arrhythmia. Nature 2020, 587, 460–465. [Google Scholar] [CrossRef]
- Kostin, S. Structural correlate of atrial fibrillation in human patients. Cardiovasc. Res. 2002, 54, 361–379. [Google Scholar] [CrossRef]
- Gollob, M.H.; Jones, D.L.; Krahn, A.D.; Danis, L.; Gong, X.Q.; Shao, Q.; Liu, X.; Veinot, J.P.; Tang, A.S.; Stewart, A.F.; et al. Somatic Mutations in the Connexin 40 Gene (GJA5) in Atrial Fibrillation. N. Engl. J. Med. 2006, 354, 2677–2688. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Y.; Lakatta, E.G.; Maltsev, V.A. From two competing oscillators to one coupled-clock pacemaker cell system. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Joung, B.; Chen, P.-S.; Lin, S.-F. The Role of the Calcium and the Voltage Clocks in Sinoatrial Node Dysfunction. Yonsei Med. J. 2011, 52, 211. [Google Scholar] [CrossRef]
- Stillitano, F.; Lonardo, G.; Zicha, S.; Varro, A.; Cerbai, E.; Mugelli, A.; Nattel, S. Molecular basis of funny current (If) in normal and failing human heart. J. Mol. Cell. Cardiol. 2008, 45, 289–299. [Google Scholar] [CrossRef]
- Darbar, D.; Herron, K.J.; Ballew, J.D.; Jahangir, A.; Gersh, B.J.; Shen, W.K.; Hammill, S.C.; Packer, D.L.; Olson, T.M. Familial atrial fibrillation is a genetically heterogeneous disorder. J. Am. Coll. Cardiol. 2003, 41, 2185–2192. [Google Scholar] [CrossRef]
- Ellinor, P.T.; Lunetta, K.L.; Glazer, N.L.; Pfeufer, A.; Alonso, A.; Chung, M.K.; Sinner, M.F.; de Bakker, P.I.; Mueller, M.; Lubitz, S.A.; et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat. Genet. 2010, 42, 240–244. [Google Scholar] [CrossRef]
- Husser, D.; Büttner, P.; Ueberham, L.; Dinov, B.; Sommer, P.; Arya, A.; Hindricks, G.; Bollmann, A. Association of atrial fibrillation susceptibility genes, atrial fibrillation phenotypes and response to catheter ablation: A gene-based analysis of GWAS data. J. Transl. Med. 2017, 15, 71. [Google Scholar] [CrossRef]
- Husser, D.; Büttner, P.; Stübner, D.; Ueberham, L.; Platonov, P.G.; Dinov, B.; Arya, A.; Hindricks, G.; Bollmann, A. PR Interval Associated Genes, Atrial Remodeling and Rhythm Outcome of Catheter Ablation of Atrial Fibrillation—A Gene-Based Analysis of GWAS Data. Front. Genet. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Thorolfsdottir, R.B.; Fritsche, L.G.; Zhou, W.; Skov, M.W.; Graham, S.E.; Herron, T.J.; McCarthy, S.; Schmidt, E.M.; Sveinbjornsson, G.; et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat. Genet. 2018, 50, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Van Ouwerkerk, A.F.; Hall, A.W.; Kadow, Z.A.; Lazarevic, S.; Reyat, J.S.; Tucker, N.R.; Nadadur, R.D.; Bosada, F.M.; Bianchi, V.; Ellinor, P.T.; et al. Epigenetic and Transcriptional Networks Underlying Atrial Fibrillation. Circ. Res. 2020, 127, 34–50. [Google Scholar] [CrossRef]
- Chinchilla, A.; Daimi, H.; Lozano-Velasco, E.; Dominguez, J.N.; Caballero, R.; Delpón, E.; Tamargo, J.; Cinca, J.; Hove-Madsen, L.; Aranega, A.E.; et al. PITX2 Insufficiency Leads to Atrial Electrical and Structural Remodeling Linked to Arrhythmogenesis. Circ. Cardiovasc. Genet. 2011, 4, 269–279. [Google Scholar] [CrossRef]
- Kirchhof, P.; Kahr, P.C.; Kaese, S.; Piccini, I.; Vokshi, I.; Scheld, H.H.; Rotering, H.; Fortmueller, L.; Laakmann, S.; Verheule, S.; et al. PITX2c Is Expressed in the Adult Left Atrium, and Reducing Pitx2c Expression Promotes Atrial Fibrillation Inducibility and Complex Changes in Gene Expression. Circ. Cardiovasc. Genet. 2011, 4, 123–133. [Google Scholar] [CrossRef]
- Syeda, F.; Holmes, A.P.; Yu, T.Y.; Tull, S.; Kuhlmann, S.M.; Pavlovic, D.; Betney, D.; Riley, G.; Kucera, J.P.; Jousset, F.; et al. PITX2 Modulates Atrial Membrane Potential and the Antiarrhythmic Effects of Sodium-Channel Blockers. J. Am. Coll. Cardiol. 2016, 68, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Syeda, F.; Kirchhof, P.; Fabritz, L. PITX2 -dependent gene regulation in atrial fibrillation and rhythm control. J. Physiol. 2017, 595, 4019–4026. [Google Scholar] [CrossRef]
- Bai, J.; Lo, A.; Gladding, P.A.; Stiles, M.K.; Federov, V.V.; Zhao, J. In silico investigation of the mechanisms underlying atrial fibrillation due to impaired Pitx2. PLoS Comput. Biol. 2020, 16, e1007678. [Google Scholar] [CrossRef]
- Lozano-Velasco, E.; Hernández-Torres, F.; Daimi, H.; Serra, S.A.; Herraiz, A.; Hove-Madsen, L.; Aránega, A.; Franco, D. Pitx2 impairs calcium handling in a dose-dependent manner by modulating Wnt signalling. Cardiovasc. Res. 2016, 109, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Gore-Panter, S.R.; Hsu, J.; Hanna, P.; Gillinov, A.M.; Pettersson, G.; Newton, D.W.; Moravec, C.S.; Van Wagoner, D.R.; Chung, M.K.; Barnard, J.; et al. Atrial Fibrillation Associated Chromosome 4q25 Variants Are Not Associated with PITX2c Expression in Human Adult Left Atrial Appendages. PLoS ONE 2014, 9, e86245. [Google Scholar] [CrossRef]
- Pérez-Hernández, M.; Matamoros, M.; Barana, A.; Amorós, I.; Gómez, R.; Núñez, M.; Sacristán, S.; Pinto, Á.; Fernández-Avilés, F.; Tamargo, J.; et al. Pitx2c increases in atrial myocytes from chronic atrial fibrillation patients enhancing I Ks and decreasing I Ca,L. Cardiovasc. Res. 2016, 109, 431–441. [Google Scholar] [CrossRef]
- Herraiz-Martínez, A.; Llach, A.; Tarifa, C.; Gandía, J.; Jiménez-Sabado, V.; Lozano-Velasco, E.; Serra, S.A.; Vallmitjana, A.; Vázquez Ruiz de Castroviejo, E.; Benítez, R.; et al. The 4q25 variant rs13143308T links risk of atrial fibrillation to defective calcium homoeostasis. Cardiovasc. Res. 2019, 115, 578–589. [Google Scholar] [CrossRef]
- Guo, D.-F.; Li, R.-G.; Yuan, F.; Shi, H.Y.; Hou, X.M.; Qu, X.K.; Xu, Y.J.; Zhang, M.; Liu, X.; Jiang, J.Q.; et al. TBX5 loss-of-function mutation contributes to atrial fibrillation and atypical Holt-Oram syndrome. Mol. Med. Rep. 2016, 13, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Yi Li, Q.; Newbury-Ecob, R.A.; Terrett, J.A.; Wilson, D.I.; Curtis, A.R.; Yi, C.H.; Gebuhr, T.; Bullen, P.J.; Robson, S.C.; Strachan, T.; et al. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat. Genet. 1997, 15, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Nadadur, R.D.; Broman, M.T.; Boukens, B.; Maturek, S.R.; Yang, X.; van den Boogaard, M.; Bekeny, J.; Gadek, M.; Ward, T.; Zhang, M.; et al. Pitx2 modulates a Tbx5 -dependent gene regulatory network to maintain atrial rhythm. Sci. Transl. Med. 2016, 8, 354ra115. [Google Scholar] [CrossRef]
- Laforest, B.; Dai, W.; Tyan, L.; Lazarevic, S.; Shen, K.M.; Gadek, M.; Broman, M.T.; Weber, C.R.; Moskowitz, I.P. Atrial fibrillation risk loci interact to modulate Ca2+-dependent atrial rhythm homeostasis. J. Clin. Investig. 2019, 129, 4937–4950. [Google Scholar] [CrossRef]
- Olesen, M.S.; Jabbari, J.; Holst, A.G.; Nielsen, J.B.; Steinbrüchel, D.A.; Jespersen, T.; Haunsø, S.; Svendsen, J.H. Screening of KCNN3 in patients with early-onset lone atrial fibrillation. Europace 2011, 13, 963–967. [Google Scholar] [CrossRef]
- Rahm, A.-K.; Wieder, T.; Gramlich, D.; Müller, M.E.; Wunsch, M.N.; El Tahry, F.A.; Heimberger, T.; Weis, T.; Most, P.; Katus, H.A.; et al. HDAC2-dependent remodeling of KCa2.2 (KCNN2) and KCa2.3 (KCNN3) K+ channels in atrial fibrillation with concomitant heart failure. Life Sci. 2021, 266, 118892. [Google Scholar] [CrossRef]
- Heijman, J.; Voigt, N.; Nattel, S.; Dobrev, D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 2014, 114, 1483–1499. [Google Scholar] [CrossRef]
- Lipovsky, C.E.; Jimenez, J.; Guo, Q.; Li, G.; Yin, T.; Hicks, S.C.; Bhatnagar, S.; Takahashi, K.; Zhang, D.M.; Brumback, B.D.; et al. Chamber-specific transcriptional responses in atrial fibrillation. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Garnier, A.; Bork, N.I.; Jacquet, E.; Zipfel, S.; Muñoz-Guijosa, C.; Baczkó, I.; Reichenspurner, H.; Donzeau-Gouge, P.; Maier, L.S.; Dobrev, D.; et al. Mapping genetic changes in the cAMP-signaling cascade in human atria. J. Mol. Cell. Cardiol. 2021, 155, 10–20. [Google Scholar] [CrossRef]
- Darbar, D.; Kannankeril, P.J.; Donahue, B.S.; Kucera, G.; Stubblefield, T.; Haines, J.L.; George, A.L., Jr.; Roden, D.M. Cardiac Sodium Channel (SCN5A) Variants Associated with Atrial Fibrillation. Circulation 2008, 117, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.M. Sodium Channel Mutations and Susceptibility to Heart Failure and Atrial Fibrillation. JAMA 2005, 293, 447. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Amin, A.S.; Remme, C.A. Disease Modifiers of Inherited SCN5A Channelopathy. Front. Cardiovasc. Med. 2018, 5. [Google Scholar] [CrossRef]
- Grammer, J.B.; Zeng, X.; Bosch, R.-F.; Kühlkamp, V. Atrial L-type Ca2+ -channel, β-adrenoreceptor, and 5-hydroxytryptamine type 4 receptor mRNAs in human atrial fibrillation. Basic Res. Cardiol. 2001, 96, 82–90. [Google Scholar] [CrossRef]
- Arora, R. Recent Insights into the Role of the Autonomic Nervous System in the Creation of Substrate for Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2012, 5, 850–859. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyauchi, Y.; Zhou, S.; Okuyama, Y.; Miyauchi, M.; Hayashi, H.; Hamabe, A.; Fishbein, M.C.; Mandel, W.J.; Chen, L.S.; Chen, P.S.; et al. Altered atrial electrical restitution and heterogeneous sympathetic hyperinnervation in hearts with chronic left ventricular myocardial infarction: Implications for atrial fibrillation. Circulation 2003, 108, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.L.; Fishbein, M.C.; Chen, L.S.; Chen, P.S.; Masroor, S. Histopathological substrate for chronic atrial fibrillation in humans. Heart Rhythm 2009, 6, 454–460. [Google Scholar] [CrossRef]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef]
- Amar, D.; Zhang, H.; Miodownik, S.; Kadish, A.H. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J. Am. Coll. Cardiol. 2003, 42, 1262–1268. [Google Scholar] [CrossRef]
- Patterson, E.; Po, S.S.; Scherlag, B.J.; Lazzara, R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005, 2, 624–631. [Google Scholar] [CrossRef]
- Lehnart, S.E.; Wehrens, X.H.T.; Reiken, S.; Warrier, S.; Belevych, A.E.; Harvey, R.D.; Richter, W.; Jin, S.L.; Conti, M.; Marks, A.R. Phosphodiesterase 4D Deficiency in the Ryanodine-Receptor Complex Promotes Heart Failure and Arrhythmias. Cell 2005, 123, 25–35. [Google Scholar] [CrossRef]
- Vest, J.A.; Wehrens, X.H.T.; Reiken, S.R.; Lehnart, S.E.; Dobrev, D.; Chandra, P.; Danilo, P.; Ravens, U.; Rosen, M.R.; Marks, A.R. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 2005, 111, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Neef, S.; Dybkova, N.; Sossalla, S.; Ort, K.R.; Fluschnik, N.; Neumann, K.; Seipelt, R.; Schöndube, F.A.; Hasenfuss, G.; Maier, L.S. CaMKII-Dependent Diastolic SR Ca2+ Leak and Elevated Diastolic Ca2+ Levels in Right Atrial Myocardium of Patients With Atrial Fibrillation. Circ. Res. 2010, 106, 1134–1144. [Google Scholar] [CrossRef]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karack, M.; Wehrens, X.H.T.; Nattel, S.; Dobrev, D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef]
- Christ, T.; Boknik, P.; Wöhrl, S.; Wettwer, E.; Graf, E.M.; Bosch, R.F.; Knaut, M.; Schmitz, W.; Ravens, U.; Dobrev, D. L-Type Ca2+ Current Downregulation in Chronic Human Atrial Fibrillation Is Associated With Increased Activity of Protein Phosphatases. Circulation 2004, 110, 2651–2657. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.E.; Leroy, J.; Richter, W.; Xie, M.; Scheitrum, C.; Lee, I.O.; Maack, C.; Rucker-Martin, C.; Donzeau-Gouge, P.; Verde, I.; et al. Cyclic Adenosine Monophosphate Phosphodiesterase Type 4 Protects Against Atrial Arrhythmias. J. Am. Coll. Cardiol. 2012, 59, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Rozmaritsa, N.; Christ, T.; van Wagoner, D.R.; Haase, H.; Stasch, J.P.; Matschke, K.; Ravens, U. Attenuated response of L-type calcium current to nitric oxide in atrial fibrillation. Cardiovasc. Res. 2014, 101, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Tovar, A.; Vargas, M.L.; Escudero, E.; Kaumann, A.J. Ontogenic changes of the control by phosphodiesterase-3 and -4 of 5-HT responses in porcine heart and relevance to human atrial 5-HT 4 receptors. Br. J. Pharmacol. 2009, 156, 237–249. [Google Scholar] [CrossRef]
- Pabel, S.; Mustroph, J.; Stehle, T.; Lebek, S.; Dybkova, N.; Keyser, A.; Rupprecht, L.; Wagner, S.; Neef, S.; Maier, L.S.; et al. Dantrolene reduces CaMKIIδC-mediated atrial arrhythmias. Europace 2020, 22, 1111–1118. [Google Scholar] [CrossRef]
- Xiao, R.-P.; Zhu, W.; Zheng, M.; Cao, C.; Zhang, Y.; Lakatta, E.G.; Han, Q. Subtype-specific α1- and β-adrenoceptor signaling in the heart. Trends Pharmacol. Sci. 2006, 27, 330–337. [Google Scholar] [CrossRef]
- Maupoil, V.; Bronquard, C.; Freslon, J.L.; Cosnay, P.; Findlay, I. Ectopic activity in the rat pulmonary vein can arise from simultaneous activation of α 1- and β 1-adrenoceptors. Br. J. Pharmacol. 2007, 150, 899–905. [Google Scholar] [CrossRef]
- Daaka, Y.; Luttrell, L.M.; Lefkowitz, R.J. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 1997, 390, 88–91. [Google Scholar] [CrossRef]
- Kilts, J.D.; Gerhardt, M.A.; Richardson, M.D.; Sreeram, G.; Machensen, G.B.; Grocott, H.P.; White, W.D.; Davis, R.D.; Newman, M.F.; Reves, J.G.; et al. β 2 -Adrenergic and Several Other G Protein-Coupled Receptors in Human Atrial Membranes Activate Both G s and G i. Circ. Res. 2000, 87, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.-H.; Leblais, V.; Wang, P.H.; Crow, M.T.; Xiao, R.P. Phosphatidylinositol 3-Kinase Functionally Compartmentalizes the Concurrent G s Signaling During β 2 -Adrenergic Stimulation. Circ. Res. 2002, 91, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Naro, F.; Zoudilova, M.; Jin, S.L.; Conti, M.; Kobilka, B. Phosphodiesterase 4D is required for 2 adrenoceptor subtype-specific signaling in cardiac myocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cheng, C.; Huang, J.; Chen, Y.A.; Lu, Y.Y.; Chen, Y.C.; Chen, S.A.; Chen, Y.J. Various subtypes of phosphodiesterase inhibitors differentially regulate pulmonary vein and sinoatrial node electrical activities. Exp. Ther. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yu, Y.; Lan, H.; Ou, X.; Yang, L.; Li, T.; Cao, J.; Zeng, X.; Li, M. Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) Increases Small-Conductance Ca2+-Activated K+ Current in Patients with Chronic Atrial Fibrillation. Med. Sci. Monit. 2018, 24, 3011–3023. [Google Scholar] [CrossRef]
- Christ, T.; Rozmaritsa, N.; Engel, A.; Berk, E.; Knaut, M.; Metzner, K.; Canteras, M.; Ravens, U.; Kaumann, A. Arrhythmias, elicited by catecholamines and serotonin, vanish in human chronic atrial fibrillation. Proc. Natl. Acad. Sci. USA 2014, 111, 11193–11198. [Google Scholar] [CrossRef] [PubMed]
- Dolce, B.; Christ, T.; Grammatika Pavlidou, N.; Yildirim, Y.; Reichenspurner, H.; Eschenhagen, T.; Nikolaev, V.O.; Kaumann, A.J.; Molina, C.E. Impact of phosphodiesterases PDE3 and PDE4 on 5-hydroxytryptamine receptor4-mediated increase of cAMP in human atrial fibrillation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Berk, E.; Christ, T.; Schwarz, S.; Ravens, U.; Knaut, M.; Kaumann, A.J. In permanent atrial fibrillation, PDE3 reduces force responses to 5-HT, but PDE3 and PDE4 do not cause the blunting of atrial arrhythmias. Br. J. Pharmacol. 2016, 173, 2478–2489. [Google Scholar] [CrossRef]
- Pino, R.; Cerbai, E.; Calamai, G.; Alajmo, F.; Borgioli, A.; Braconi, L.; Cassai, M.; Montesi, G.F.; Mugelli, A. Effect of 5-HT4 receptor stimulation on the pacemaker current If in human isolated atrial myocytes. Cardiovasc. Res. 1998, 40, 516–522. [Google Scholar] [CrossRef]
- Scala, E.D.; Findlay, I.; Rose, S.; Aupart, M.; Argibay, J.; Cosnay, P.; Bozon, V. High Efficiency Activation of L-Type Ca2+ Current by 5-HT in Human Atrial Myocytes. Recept. Channels 2004, 10, 159–165. [Google Scholar] [CrossRef]
- Pfaffinger, P.J.; Martin, J.M.; Hunter, D.D.; Nathanson, N.M.; Hille, B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature 1985, 317, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Hisatome, I.; Wasserstrom, J.A.; Arentzen, C.E.; Singer, D.H. Acetylcholine-sensitive potassium channels in human atrial myocytes. Am. J. Physiol. Heart Circ. Physiol. 1990, 259, H1730–H1735. [Google Scholar] [CrossRef]
- Christ, T.; Wettwer, E.; Voigt, N.; Hála, O.; Radicke, S.; Matschke, K.; Várro, A.; Dobrev, D.; Ravens, U. Pathology-specific effects of the I Kur/I to/I K,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br. J. Pharmacol. 2008, 154, 1619–1630. [Google Scholar] [CrossRef]
- Dobrev, D.; Friedrich, A.; Voigt, N.; Jost, N.; Wettwer, E.; Christ, T.; Knaut, M.; Ravens, U. The G Protein–Gated Potassium Current I K,ACh Is Constitutively Active in Patients With Chronic Atrial Fibrillation. Circulation 2005, 112, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Friedrich, A.; Bock, M.; Wettwer, E.; Christ, T.; Knaut, M.; Strasser, R.H.; Ravens, U.; Dobrev, D. Differential phosphorylation-dependent regulation of constitutively active and muscarinic receptor-activated IK,ACh channels in patients with chronic atrial fibrillation. Cardiovasc. Res. 2007, 74, 426–437. [Google Scholar] [CrossRef]
- Soattin, L.; Lubberding, A.F.; Bentzen, B.H.; Christ, T.; Jespersen, T. Inhibition of Adenosine Pathway Alters Atrial Electrophysiology and Prevents Atrial Fibrillation. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef]
- Belardinelli, L.; Shryock, J.C.; Song, Y.; Wang, D.; Srinivas, M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995, 9, 359–365. [Google Scholar] [CrossRef]
- Pelleg, A.; Mitsuoka, T.; Michelson, E.L.; Menduke, H. Adenosine mediates the negative chronotropic action of adenosine 5′-triphosphate in the canine sinus node. J. Pharmacol. Exp. Ther. 1987, 242, 791–795. [Google Scholar]
- George, E. Adenosine and acetylcholine reduce isoproterenol-induced protein phosphorylation of rat myocytes. J. Mol. Cell. Cardiol. 1991, 23, 749–764. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Llach, A.; Molina, C.E.; Prat-Vidal, C.; Farré, J.; Roura, S.; Cinca, J. The proarrhythmic antihistaminic drug terfenadine increases spontaneous calcium release in human atrial myocytes. Eur. J. Pharmacol. 2006, 553, 215–221. [Google Scholar] [CrossRef]
- Molina, C.E.; Llach, A.; Herraiz-Martínez, A.; Tarifa, C.; Barriga, M.; Wiegerinck, R.F.; Fernandes, J.; Cabello, N.; Vallmitjana, A.; Benitéz, R.; et al. Prevention of adenosine A2A receptor activation diminishes beat-to-beat alternation in human atrial myocytes. Basic Res. Cardiol. 2016, 111, 5. [Google Scholar] [CrossRef]
- Furukawa, S.; Satoh, K.; Taira, N. Opening of ATP-sensitive K+ channels responsible for adenosine A2 receptor-mediated vasodepression does not involve a pertussis toxin-sensitive G protein. Eur. J. Pharmacol. 1993, 236, 255–262. [Google Scholar] [CrossRef]
- Llach, A.; Molina, C.E.; Prat-Vidal, C.; Fernandes, J.; Casadó, V.; Ciruela, F.; Lluís, C.; Francro, R.; Cianca, J.; Hove-Madsen, L. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur. Heart J. 2011, 32, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Tikh, E.I.; Fenton, R.A.; Dobson, J.G. Contractile effects of adenosine A 1 and A 2A receptors in isolated murine hearts. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H348–H356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Headrick, J.P.; Peart, J.N.; Reichelt, M.E.; Haseler, L.J. Adenosine and its receptors in the heart: Regulation, retaliation and adaptation. Biochim. Biophys. Acta (BBA) Biomembr. 2011, 1808, 1413–1428. [Google Scholar] [CrossRef]
- Maggirwar, S.B.; Dhanraj, D.N.; Somani, S.M.; Ramkumar, V. Adenosine Acts as an Endogenous Activator of the Cellular Antioxidant Defense System. Biochem. Biophys. Res. Commun. 1994, 201, 508–515. [Google Scholar] [CrossRef]
- Phosri, S.; Arieyawong, A.; Bunrukchai, K.; Parichatikanond, W.; Nishimura, A.; Nishida, M.; Mangmool, S. Stimulation of Adenosine A2B Receptor Inhibits Endothelin-1-Induced Cardiac Fibroblast Proliferation and α-Smooth Muscle Actin Synthesis Through the cAMP/Epac/PI3K/Akt-Signaling Pathway. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Phosri, S.; Bunrukchai, K.; Parichatikanond, W.; Sato, V.H.; Mangmool, S. Epac is required for exogenous and endogenous stimulation of adenosine A2B receptor for inhibition of angiotensin II-induced collagen synthesis and myofibroblast differentiation. Purinergic Signal. 2018, 14, 141–156. [Google Scholar] [CrossRef]
- Villarreal, F.; Epperson, S.A.; Ramirez-Sanchez, I.; Yamazaki, K.G.; Brunton, L.L. Regulation of cardiac fibroblast collagen synthesis by adenosine: Roles for Epac and PI3K. Am. J. Physiol. Cell Physiol. 2009, 296, C1178–C1184. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Zhong, H.; Mezzaroma, E.; Van Tassell, B.W.; Kannan, H.; Zeng, D.; Belardinelli, L.; Voelkel, N.F.; Abbate, A. GS-6201, a Selective Blocker of the A 2B Adenosine Receptor, Attenuates Cardiac Remodeling after Acute Myocardial Infarction in the Mouse. J. Pharmacol. Exp. Ther. 2012, 343, 587–595. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, H.; Everett, T.H.; Wilson, E.; Chang, R.; Zeng, D.; Belardinelli, L.; Olgin, J.E. Blockade of A2B adenosine receptor reduces left ventricular dysfunction and ventricular arrhythmias 1 week after myocardial infarction in the rat model. Heart Rhythm 2014, 11, 101–109. [Google Scholar] [CrossRef]
- Asakura, M.; Asanuma, H.; Kim, J.; Liao, Y.; Nakamaru, K.; Fujita, M.; Komamura, K.; Isomura, T.; Furukawa, H.; Tomoike, H.; et al. Impact of Adenosine Receptor Signaling and Metabolism on Pathophysiology in Patients with Chronic Heart Failure. Hypertens. Res. 2007, 30, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-T.; Chiang, F.-T.; Tseng, C.-D.; Hwang, J.J.; Kuo, K.T.; Wu, C.K.; Yu, C.; Wang, Y.C.; Lai, L.P.; Lin, J.L. Increased Expression of Mineralocorticoid Receptor in Human Atrial Fibrillation and a Cellular Model of Atrial Fibrillation. J. Am. Coll. Cardiol. 2010, 55, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, F.; Alzoubi, K.H.; van Wagoner, D.R. Impact of aldosterone antagonists on the substrate for atrial fibrillation: Aldosterone promotes oxidative stress and atrial structural/electrical remodeling. Int. J. Cardiol. 2013, 168, 5135–5142. [Google Scholar] [CrossRef]
- Neefs, J.; van den Berg, N.W.E.; Limpens, J.; Berger, W.R.; Boekholdt, S.M.; Sanders, P.; de Groot, J.R. Aldosterone Pathway Blockade to Prevent Atrial Fibrillation: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017, 231, 155–161. [Google Scholar] [CrossRef]
- Van Wagoner, D.R. Oxidative Stress and Inflammation in Atrial Fibrillation: Role in Pathogenesis and Potential as a Therapeutic Target. J. Cardiovasc. Pharmacol. 2008, 52, 306–313. [Google Scholar] [CrossRef]
- Ishii, Y.; Schuessler, R.B.; Gaynor, S.L.; Hames, K.; Damiano, R.J. Postoperative atrial fibrillation: The role of the inflammatory response. J. Thorac. Cardiovasc. Surg. 2017, 153, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Scott, L.; Veleva, T.; Müller, F.U.; Nattel, S.; Dobrev, D.; Wehrens, X.H.T.; Li, N. Enhanced Activation Inflammasome Promotes Atrial Fibrillation. J. Mol. Cell. Cardiol. 2017, 112, 147. [Google Scholar] [CrossRef]
- Shiroshitatakeshita, A.; Brundel, B.; Lavoie, J.; Nattel, S. Prednisone prevents atrial fibrillation promotion by atrial tachycardia remodeling in dogs. Cardiovasc. Res. 2006, 69, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Karabacak, K.; Kubat, E.; Akyol, F.B.; Kadan, M.; Erol, G.; Doğanci, S.; Yildirim, V.; Bolcal, C. The C-reactive protein/albumin ratio as a new predictor for postoperative atrial fibrillation after coronary artery bypass graft surgery. J. Card. Surg. 2020, 35, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Martin, D.O.; Sprecher, D.; Wazni, O.; Kanderian, A.; Carnes, C.A.; Bauer, J.A.; Tchou, P.J.; Niebauer, M.J.; Natale, A.; et al. C-Reactive Protein Elevation in Patients With Atrial Arrhythmias. Circulation 2001, 104, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.H.; Kang, J.G.; Lee, H.J.; Kim, N.H.; Sung, J.W.; Cheong, E.; Sung, K.C. C-reactive protein and risk of atrial fibrillation in East Asians. Europace 2017, 19, 1643–1649. [Google Scholar] [CrossRef]
- Minutoli, L.; Puzzolo, D.; Rinaldi, M.; Irrera, N.; Marini, H.; Arcoraci, V.; Bitto, A.; Crea, G.; Pisani, A.; Squadrito, F.; et al. ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Yao, C.; Veleva, T.; Scott, L.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Alsina, K.M.; Abu-Taha, I.; Ghezelbash, S. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Chen, J.; Zhao, S.; Li, H. Pirfenidone Attenuates Cardiac Fibrosis in a Mouse Model of TAC-Induced Left Ventricular Remodeling by Suppressing NLRP3 Inflammasome Formation. Cardiology 2013, 126, 1–11. [Google Scholar] [CrossRef]
- Pinho-Gomes, A.C.; Reilly, S.; Brandes, R.P.; Casadei, B. Targeting Inflammation and Oxidative Stress in Atrial Fibrillation: Role of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Inhibition with Statins. Antioxid. Redox Signal. 2014, 20, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, V.; Andrié, R.P.; Rudolph, T.K.; Friedrichs, K.; Klinke, A.; Hirsch-Hoffmann, B.; Schwoerer, A.P.; Lau, D.; Fu, X.; Klingel, K.; et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat. Med. 2010, 16, 470–474. [Google Scholar] [CrossRef]
- Simon, J.N.; Vrellaku, B.; Monterisi, S.; Chu, S.M.; Rawlings, N.; Lomas, O.; Marchal, G.A.; Waithe, D.; Syeda, F.; Gajendragadkar, P.R.; et al. Oxidation of Protein Kinase A Regulatory Subunit PKARIα Protects Against Myocardial Ischemia-Reperfusion Injury by Inhibiting Lysosomal-Triggered Calcium Release. Circulation 2020. [Google Scholar] [CrossRef]
- Camelliti, P. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc. Res. 2004, 62, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Schultz, F.; Swiatlowska, P.; Alvarez-Laviada, A.; Sanchez-Alonso, J.L.; Song, Q.; de Vries, A.A.F.; Pijnappels, D.A.; Ongstad, E.; Braga, V.M.M.; Entcheva, E.; et al. Cardiomyocyte–myofibroblast contact dynamism is modulated by connexin-43. FASEB J. 2019, 33, 10453–10468. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Yan, Y.; Zeng, Z.; Zhang, Z.; Liu, H.; Liu, H.; Li, J.; Huang, W.; Wu, J.; He, Y. Connexin 43 reduces susceptibility to sympathetic atrial fibrillation. Int. J. Mol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Laing, J.G.; Kanter, E.M.; Berthoud, V.M.; Bao, M.; Rohrs, H.W.; Townsend, R.R.; Yamada, K.A. Identification of CaMKII Phosphorylation Sites in Connexin43 by High-Resolution Mass Spectrometry. J. Proteome Res. 2011, 10, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Kameritsch, P.; Kiemer, F.; Mannell, H.; Beck, H.; Pohl, U.; Pogoda, K. PKA negatively modulates the migration enhancing effect of Connexin 43. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2019, 1866, 828–838. [Google Scholar] [CrossRef]
- Lampe, P.D.; TenBroek, E.M.; Burt, J.M.; Kurata, W.E.; Johnson, R.G.; Lauf, A.F. Phosphorylation of Connexin43 on Serine368 by Protein Kinase C Regulates Gap Junctional Communication. J. Cell Biol. 2000, 149, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Xie, W.; Betzenhauser, M.; Reiken, S.; Chen, B.X.; Wronska, A.; Marks, A.R. Calcium Leak Through Ryanodine Receptors Leads to Atrial Fibrillation in 3 Mouse Models of Catecholaminergic Polymorphic Ventricular Tachycardia. Circ. Res. 2012, 111, 708–717. [Google Scholar] [CrossRef]
- Greer-Short, A.; Musa, H.; Alsina, K.M.; Ni, L.; Word, T.A.; Reynolds, J.O.; Gratz, D.; Lane, C.; El-Refaey, M.; Unudurthi, S.; et al. Calmodulin kinase II regulates atrial myocyte late sodium current, calcium handling, and atrial arrhythmia. Heart Rhythm 2020, 17, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Toischer, K.; Hartmann, N.; Wagner, S.; Fischer, T.H.; Herting, J.; Danner, B.C.; Sag, C.M.; Hund, T.J.; Mohler, P.J.; Belardinelli, L.; et al. Role of late sodium current as a potential arrhythmogenic mechanism in the progression of pressure-induced heart disease. J. Mol. Cell. Cardiol. 2013, 61, 111–122. [Google Scholar] [CrossRef]
- Van der Velden, H. Gap junctional remodeling in relation to stabilization of atrial fibrillation in the goat. Cardiovasc. Res. 2000, 46, 476–486. [Google Scholar] [CrossRef]
- Bonilla, I.M.; Sridhar, A.; Györke, S.; Cardounel, A.J.; Carnes, C.A. Nitric Oxide Synthases and Atrial Fibrillation. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Guzik, T.J.; Zhang, Y.H.; Zhang, M.H.; Kattach, H.; Ratnatunga, C.; Pillai, R.; Channon, K.M.; Casadei, B. A Myocardial Nox2 Containing NAD(P)H Oxidase Contributes to Oxidative Stress in Human Atrial Fibrillation. Circ. Res. 2005, 97, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.N.; Jayaram, R.; Nahar, K.; Antoniades, C.; Verheule, S.; Channon, K.M.; Alp, N.J.; Schotten, U.; Casadei, B. Atrial Sources of Reactive Oxygen Species Vary With the Duration and Substrate of Atrial Fibrillation. Circulation 2011, 124, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Eager, K.R.; Dulhunty, A.F. Activation of the Cardiac Ryanodine Receptor by Sulfhydryl Oxidation is Modified by Mg 2+ and ATP. J. Membr. Biol. 1998, 163, 9–18. [Google Scholar] [CrossRef]
- Marengo, J.J.; Hidalgo, C.; Bull, R. Sulfhydryl Oxidation Modifies the Calcium Dependence of Ryanodine-Sensitive Calcium Channels of Excitable Cells. Biophys. J. 1998, 74, 1263–1277. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; Marbán, E.; O’Rourke, B. Synchronized Whole Cell Oscillations in Mitochondrial Metabolism Triggered by a Local Release of Reactive Oxygen Species in Cardiac Myocytes. J. Biol. Chem. 2003, 278, 44735–44744. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-β Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6. [Google Scholar] [CrossRef]
- Burger, D.E.; Lu, X.; Lei, M.; Xiang, F.L.; Hammoud, L.; Jiang, M.; Wang, H.; Jones, D.L.; Sims, S.M.; Feng, Q. Neuronal Nitric Oxide Synthase Protects Against Myocardial Infarction-Induced Ventricular Arrhythmia and Mortality in Mice. Circulation 2009, 120, 1345–1354. [Google Scholar] [CrossRef]
- Sears, C.E.; Bryant, S.M.; Ashley, E.A.; Lygate, C.A.; Rakovic, S.; Wallis, H.L.; Neubauer, S.; Terrar, D.A.; Casadei, B. Cardiac Neuronal Nitric Oxide Synthase Isoform Regulates Myocardial Contraction and Calcium Handling. Circ. Res. 2003, 92. [Google Scholar] [CrossRef] [PubMed]
- Layland, J.; Li, J.; Shah, A.M. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J. Physiol. 2002, 540, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, B.D.; Sagach, V.F.; Shah, A.M. Basal Release of Nitric Oxide Augments the Frank-Starling Response in the Isolated Heart. Circulation 1997, 96, 1320–1329. [Google Scholar] [CrossRef]
- Wang, H.; Kohr, M.J.; Wheeler, D.G.; Ziolo, M.T. Endothelial nitric oxide synthase decreases β-adrenergic responsiveness via inhibition of the L-type Ca2+ current. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1473–H1480. [Google Scholar] [CrossRef] [PubMed]
- Loke, K.E.; McConnell, P.I.; Tuzman, J.M.; Shesely, E.G.; Smith, C.J.; Stackpole, C.J.; Thompson, C.I.; Kaley, G.; Wolin, M.S.; Hintze, T.H. Endogenous Endothelial Nitric Oxide Synthase–Derived Nitric Oxide Is a Physiological Regulator of Myocardial Oxygen Consumption. Circ. Res. 1999, 84, 840–845. [Google Scholar] [CrossRef]
- Petroff, M.G.V.; Kim, S.H.; Pepe, S.; Dessy, C.; Marbán, E.; Balligand, J.L.; Sollott, S.J. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat. Cell Biol. 2001, 3, 867–873. [Google Scholar] [CrossRef]
- Cai, H.; Li, Z.; Goette, A.; Mera, F.; Honeycutt, C.; Feterik, K.; Wilcox, J.N.; Dudley, S.C.; Harrison, D.G.; Langberg, J.J. Downregulation of Endocardial Nitric Oxide Synthase Expression and Nitric Oxide Production in Atrial Fibrillation. Circulation 2002, 106, 2854–2858. [Google Scholar] [CrossRef]
- Gomez, R.; Núñez, L.; Vaquero, M.; Amorós, I.; Barana, A.; de Prada, T.; Macaya, C.; Moroto, L.; Rodrígues, E.; Caballero, R.; et al. Nitric oxide inhibits Kv4.3 and human cardiac transient outward potassium current (Ito1). Cardiovasc. Res. 2008, 80, 375–384. [Google Scholar] [CrossRef]
- Richards, M.A.; Simon, J.N.; Ma, R.; Loonat, A.A.; Crabtree, M.J.; Paterson, D.J.; Fahlman, R.P.; Casadei, B.; Fliegerl, L.; Swietach, P. Nitric oxide modulates cardiomyocyte pH control through a biphasic effect on sodium/hydrogen exchanger-1. Cardiovasc. Res. 2020, 116, 1958–1971. [Google Scholar] [CrossRef]
- Tamura, N.; Ogawa, Y.; Chusho, H.; Nakamura, K.; Nakao, K.; Suda, M.; Kasahara, M.; Hashimoto, R.; Katsuura, G.; Mukoyama, M.; et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc. Natl. Acad. Sci. USA 2000, 97, 4239–4244. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, M.; Rivet-Bastide, M.; Hatem, S.; Bénardeau, A.; Mercadier, J.J.; Fischmeister, R. Nitric oxide regulates the calcium current in isolated human atrial myocytes. J. Clin. Investig. 1995, 95, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Mizuno, K. Increased Concentration of Plasma Cyclic GMP from Aconitine-Induced Atrial Fibrillation in Dogs and Patients with Paroxysmal Atrial Fibrillation. Advances in Myocardiology; Springer US: Boston, MA, USA, 1982; pp. 177–183. [Google Scholar] [CrossRef]
- Mizuno, K.; Ogawa, K. Increased Concentration of Plasma Cyclic GMP During Aconitine-Induced Atrial Fibrillation in Dogs and Paroxysmal Atrial Fibrillation in Patients. J. Cardiovasc. Pharmacol. 1981, 3, 1211–1220. [Google Scholar] [CrossRef]
- Suffee, N.; Moore-Morris, T.; Farahmand, P.; Rücker-Martin, C.; Dilanian, G.; Fradet, M.; Sawaki, D.; Derumeaux, G.; LePrince, P.; Clément, K.; et al. Atrial natriuretic peptide regulates adipose tissue accumulation in adult atria. Proc. Natl. Acad. Sci. USA 2017, 114, E771–E780. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.R.; Joiner, M.A.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef]
- Rahmutula, D.; Zhang, H.; Wilson, E.E.; Olgin, J.E. Absence of natriuretic peptide clearance receptor attenuates TGF-β1-induced selective atrial fibrosis and atrial fibrillation. Cardiovasc. Res. 2019, 115, 357–372. [Google Scholar] [CrossRef]
- Yoo, S.; Aistrup, G.; Shiferaw, Y.; Ng, J.; Mohler, P.J.; Hund, T.J.; Waugh, T.; Browne, S.; Gussak, G.; Gilani, M.; et al. Oxidative stress creates a unique, CaMKII-mediated substrate for atrial fibrillation in heart failure. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, C.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Lafuente, C.; Valembois, L.; Bergmann, J.-F.; Belmin, J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Reimold, F.R.; Reynolds, M.R. Proarrhythmia and death with antiarrhythmic drugs for atrial fibrillation, and the unfulfilled promise of comparative effectiveness research. Am. Heart J. 2018, 205, 128–130. [Google Scholar] [CrossRef] [PubMed]
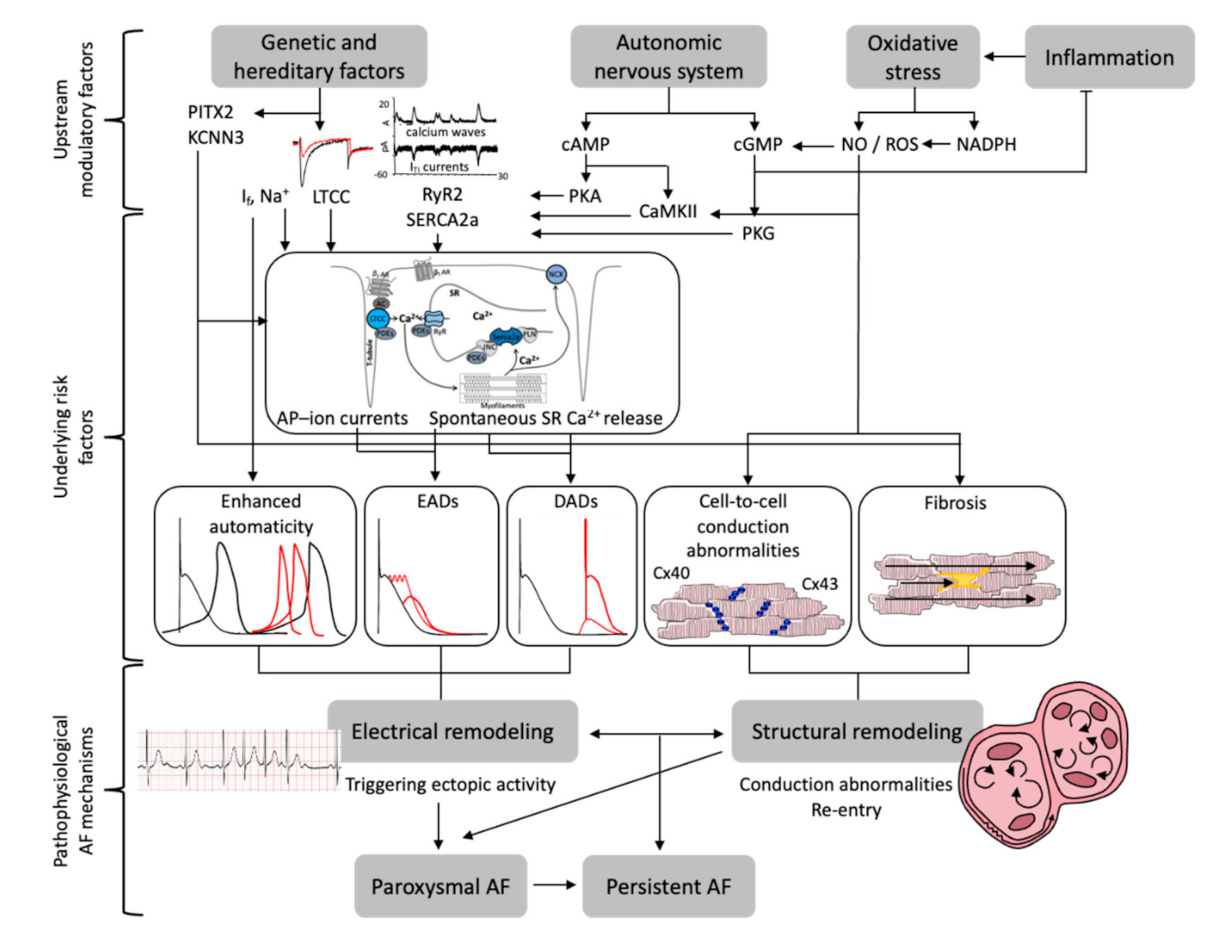
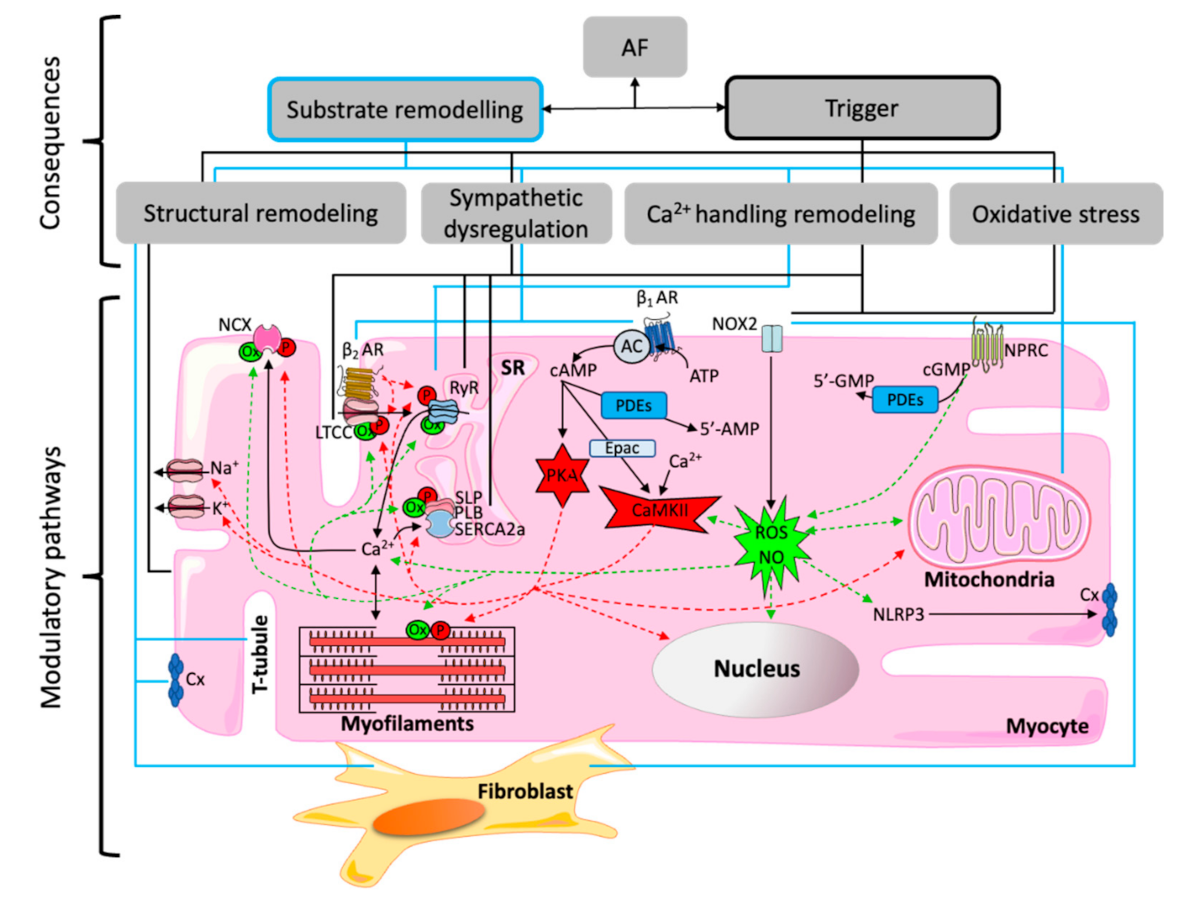
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beneke, K.; Molina, C.E. Molecular Basis of Atrial Fibrillation Initiation and Maintenance. Hearts 2021, 2, 170-187. https://doi.org/10.3390/hearts2010014
Beneke K, Molina CE. Molecular Basis of Atrial Fibrillation Initiation and Maintenance. Hearts. 2021; 2(1):170-187. https://doi.org/10.3390/hearts2010014
Chicago/Turabian StyleBeneke, Kira, and Cristina E. Molina. 2021. "Molecular Basis of Atrial Fibrillation Initiation and Maintenance" Hearts 2, no. 1: 170-187. https://doi.org/10.3390/hearts2010014
APA StyleBeneke, K., & Molina, C. E. (2021). Molecular Basis of Atrial Fibrillation Initiation and Maintenance. Hearts, 2(1), 170-187. https://doi.org/10.3390/hearts2010014





