Novel PITX2 Homeodomain-Contained Mutations from ATRIAL Fibrillation Patients Deteriorate Calcium Homeostasis
Abstract
:1. Introduction
2. Methods
2.1. Patient Recruitment and Sample Collection
2.2. DNA Sequencing
2.3. Generation of Reporter and Mutated PITX2c Constructs
2.4. Cell Culture and Luciferase Transactivation Assays
2.5. qRT-PCR Analyses
2.6. Intracellular Calcium Recordings in HL-1 Myocytes
2.7. Statistical Analysis
3. Results
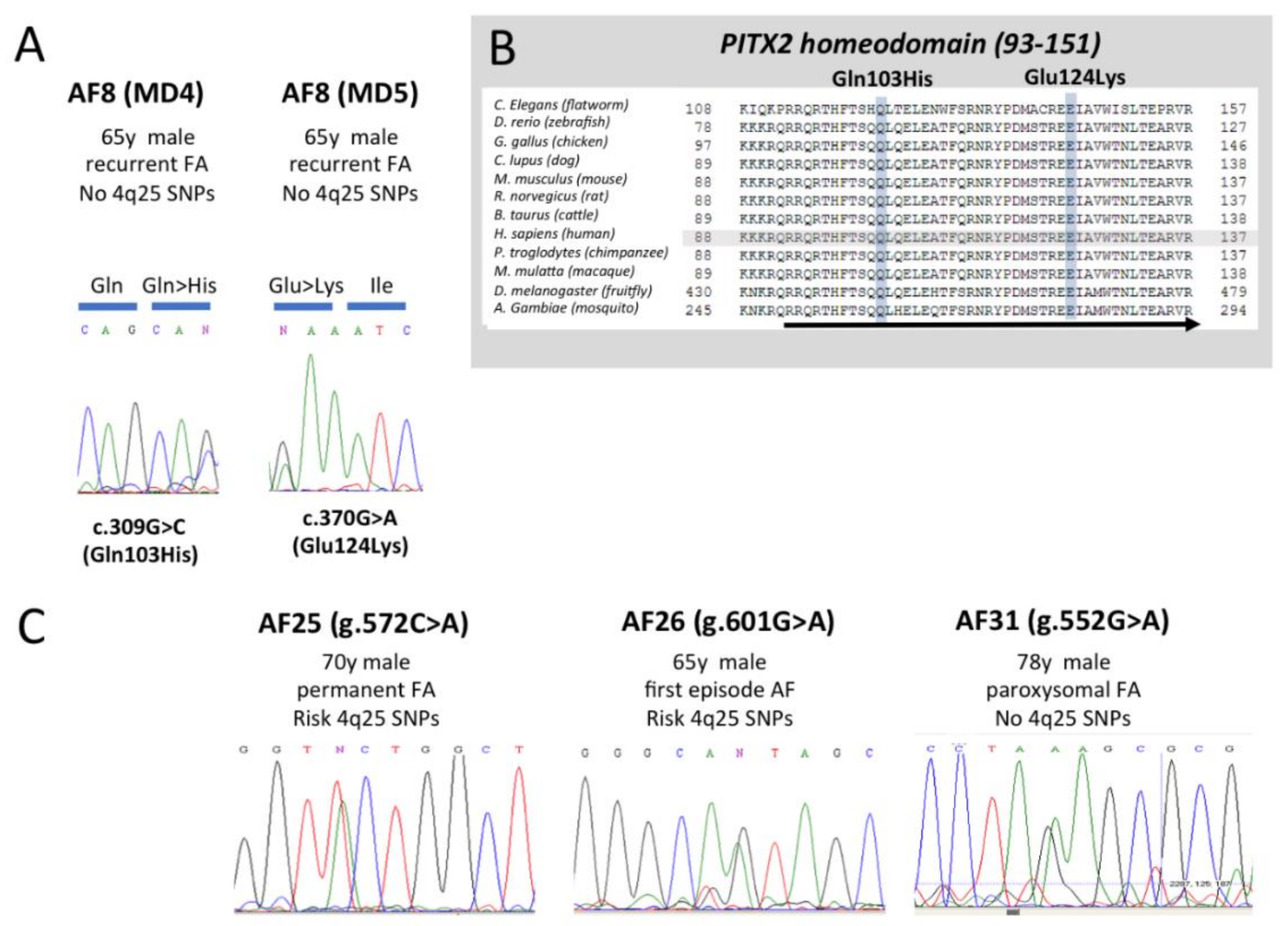
3.1. Mutation Screening of PITX2c Reveals in AF Patients Revealed Two Novel HD Mutations
3.2. Gln103His and Glu124Lys Mutations Impairs PITX2c Transactivation Activity
3.3. Gln103His and Glu124Lys PITX2 Mutations Alter Calcium Homeostasis
3.4. Impact of Gln103His and Glu124Lys PITX2c Mutations on the Beat-to-Beat Response
4. Discussion
4.1. Impact of AF Associated PITX2 Homeodomain Mutants on PITX2c Function
4.2. Impact of the PITX2 Mutants on Intracellular Calcium Homeostasis
4.3. Impact of the PITX2 Mutants on the Rate-Dependency of the Beat-To-Beat Response
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camm, A.J.; Lip, G.Y.; De Caterina, R.; Savelieva, I.; Atar, D.; Hohnloser, S.H.; Hindricks, G.; Kirchhof, P.; Bax, J.J.; Baumgartner, H.; et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur. Hear. J. 2012, 33, 2719–2747. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Arnar, D.O.; Helgadottir, A.; Gretarsdottir, S.; Holm, H.; Sigurdsson, A.; Jonasdottir, A.; Baker, A.; Thorleifsson, G.; Kristjansson, K.; et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nat. Cell Biol. 2007, 448, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, S.A.; Sinner, M.F.; Lunetta, K.L.; Makino, S.; Pfeufer, A.; Rahman, R.; Veltman, C.E.; Barnard, J.; Bis, J.C.; Danik, S.P.; et al. Independent Susceptibility Markers for Atrial Fibrillation on Chromosome 4q25. Circulation 2010, 122, 976–984. [Google Scholar] [CrossRef] [Green Version]
- Lubitz, S.A.; Lunetta, K.L.; Lin, H.; Arking, D.E.; Trompet, S.; Li, G.; Krijthe, B.P.; Chasman, D.I.; Barnard, J.; Kleber, M.E.; et al. Novel Genetic Markers Associate With Atrial Fibrillation Risk in Europeans and Japanese. J. Am. Coll. Cardiol. 2014, 63, 1200–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, J.B.; Thorolfsdottir, R.B.; Fritsche, L.G.; Zhou, W.; Skov, M.W.; Graham, S.E.; Herron, T.J.; McCarthy, S.; Schmidt, E.M.; Sveinbjornsson, G.; et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat. Genet. 2018, 50, 1234–1239. [Google Scholar] [CrossRef]
- Franco, D. The Role of Pitx2 during Cardiac Development Linking Left–Right Signaling and Congenital Heart Diseases. Trends Cardiovasc. Med. 2003, 13, 157–163. [Google Scholar] [CrossRef]
- Campione, M.; Ros, M.A.; Icardo, J.M.; Piedra, E.; Christoffels, V.M.; Schweickert, A.; Blum, M.; Franco, D.; Moorman, A.F. Pitx2 Expression Defines a Left Cardiac Lineage of Cells: Evidence for Atrial and Ventricular Molecular Isomerism in the iv/iv Mice. Dev. Biol. 2001, 231, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Mommersteeg, M.T.M.; Brown, N.A.; Prall, O.W.J.; Vries, C.D.G.-D.; Harvey, R.P.; Moorman, A.F.M.; Christoffels, V.M. Pitx2c and Nkx2-5 Are Required for the Formation and Identity of the Pulmonary Myocardium. Circ. Res. 2007, 101, 902–909. [Google Scholar] [CrossRef] [Green Version]
- Chinchilla, A.; Daimi, H.; Lozano-Velasco, E.; Dominguez, J.N.; Caballero, R.; Delpón, E.; Tamargo, J.; Cinca, J.; Hove-Madsen, L.; Aranega, A.E.; et al. PITX2 Insufficiency Leads to Atrial Electrical and Structural Remodeling Linked to Arrhythmogenesis. Circ. Cardiovasc. Genet. 2011, 4, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Kirchhof, P.; Kahr, P.C.; Kaese, S.; Piccini, I.; Vokshi, I.; Scheld, H.-H.; Rotering, H.; Fortmueller, L.; Laakmann, S.; Verheule, S.; et al. PITX2c Is Expressed in the Adult Left Atrium, and Reducing Pitx2c Expression Promotes Atrial Fibrillation Inducibility and Complex Changes in Gene Expression. Circ. Cardiovasc. Genet. 2011, 4, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Klysik, E.; Sood, S.; Johnson, R.L.; Wehrens, X.H.T.; Martin, J.F. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc. Natl. Acad. Sci. USA 2010, 107, 9753–9758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, M.M.; Ahlberg, G.; Hansen, C.V.; Guenther, S.; Marín-Juez, R.; Sokol, A.M.; El-Sammak, H.; Piesker, J.; Hellsten, Y.; Olesen, M.S.; et al. Early sarcomere and metabolic defects in a zebrafish pitx2c cardiac arrhythmia model. Proc. Natl. Acad. Sci. USA 2019, 116, 24115–24121. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Velasco, E.; Hernández-Torres, F.; Daimi, H.; Serra, S.A.; Herraiz, A.; Hove-Madsen, L.; Aránega, A.; Franco, D. Pitx2 impairs calcium handling in a dose-dependent manner by modulating Wnt signalling. Cardiovasc. Res. 2016, 109, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herraiz-Martínez, A.; Llach, A.; Tarifa, C.; Gandía, J.; Jiménez-Sabado, V.; Lozano-Velasco, E.; Serra, S.A.; Vallmitjana, A.; De Castroviejo, E.V.R.; Benítez, R.; et al. The 4q25 variant rs13143308T links risk of atrial fibrillation to defective calcium homoeostasis. Cardiovasc. Res. 2019, 115, 578–589. [Google Scholar] [CrossRef] [Green Version]
- Semina, E.V.; Reiter, R.; Leysens, N.J.; Alward, W.L.M.; Small, K.W.; Datson, N.A.; Siegel-Bartelt, J.; Bierke-Nelson, D.; Bitoun, P.; Zabel, B.U.; et al. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat. Genet. 1996, 14, 392–399. [Google Scholar] [CrossRef]
- Priston, M.; Kozlowski, K.; Gill, D.; Letwin, K.; Buys, Y.; Levin, A.V.; Walter, M.A.; Héon, E. Functional analyses of two newly identified PITX2 mutants reveal a novel molecular mechanism for Axenfeld-Rieger syndrome. Hum. Mol. Genet. 2001, 10, 1631–1638. [Google Scholar] [CrossRef] [Green Version]
- Reis, L.M.; Tyler, R.C.; Kloss, B.A.V.; Schilter, K.F.; Levin, A.V.; Lowry, R.B.; Zwijnenburg, P.J.G.; Stroh, E.; Broeckel, U.; Murray, J.C.; et al. PITX2 and FOXC1 spectrum of mutations in ocular syndromes. Eur. J. Hum. Genet. 2012, 20, 1224–1233. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.W.; Cho, H.-K.; Oh, S.-Y.; Ki, C.-S.; Kee, C. Novel c.300_301delinsT Mutation in PITX2 in a Korean Family with Axenfeld-Rieger Syndrome. Ann. Lab. Med. 2013, 33, 360–363. [Google Scholar] [CrossRef]
- Yin, H.-F.; Fang, X.-Y.; Jin, C.-F.; Yin, J.-F.; Li, J.-Y.; Zhao, S.-J.; Miao, Q.; Song, F.-W. Identification of a novel frameshift mutation in PITX2 gene in a Chinese family with Axenfeld-Rieger syndrome. J. Zhejiang Univ. Sci. B 2014, 15, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Alward, W.L.; Semina, E.V.; Kalenak, J.W.; Héon, E.; Sheth, B.P.; Stone, E.M.; Murray, J.C. Autosomal dominant iris hypoplasia is caused by a mutation in the Rieger syndrome (RIEG/PITX2) gene. Am. J. Ophthalmol. 1998, 125, 98–100. [Google Scholar] [CrossRef]
- Doward, W.; Perveen, R.; Lloyd, I.C.; Ridgway, A.; Wilson, L.; Black, G.C. A mutation in the RIEG1 gene associated with Peters’ anomaly. J. Med. Genet. 1999, 36, 152–155. [Google Scholar]
- Xia, K. Mutation in PITX2 is associated with ring dermoid of the cornea. J. Med. Genet. 2004, 41, e129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xin, Y.-F.; Xu, W.-J.; Liu, Z.-M.; Qiu, X.-B.; Qu, X.-K.; Xu, L.; Li, X.; Yang, Y.-Q. Prevalence and Spectrum of PITX2c Mutations Associated with Congenital Heart Disease. DNA Cell Biol. 2013, 32, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Probst, F.J.; Fox, S.E.; Schimmenti, L.A.; Semina, E.V.; Hefner, M.A.; Belmont, J.W.; Camper, S.A. Exclusion ofPITX2 mutations as a major cause of CHARGE association. Am. J. Med. Genet. 2002, 111, 27–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechakra, A.; Footz, T.; Walter, M.; Aránega, A.; Hernández-Torres, F.; Morel, E.; Millat, G.; Yang, Y.-Q.; Chahine, M.; Chevalier, P.; et al. A Novel PITX2c Gain-of-Function Mutation, p.Met207Val, in Patients With Familial Atrial Fibrillation. Am. J. Cardiol. 2019, 123, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Serzanti, M.; Giacomelli, A.; Beltramone, S.; Marchina, E.; Bertini, V.; Piovani, G.; Refsgaard, L.; Olesen, M.S.; Cortellini, V.; et al. Generation of induced pluripotent stem cells (iPSC) from an atrial fibrillation patient carrying a PITX2 p.M200V mutation. Stem. Cell Res. 2017, 24, 8–11. [Google Scholar] [CrossRef]
- Tsai, C.-T.; Hsieh, C.-S.; Chang, S.-N.; Chuang, E.Y.; Juang, J.-M.J.; Lin, L.-Y.; Lai, L.-P.; Hwang, J.-J.; Chiang, F.-T.; Lin, J.-L. Next-generation sequencing of nine atrial fibrillation candidate genes identified novel de novo mutations in patients with extreme trait of atrial fibrillation. J. Med. Genet. 2015, 52, 28–36. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, P.; Yang, Y.; Ge, Z.; Kang, W. A novel PITX2c loss-of-function mutation underlies lone atrial fibrillation. Int. J. Mol. Med. 2013, 32, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Boldt, L.-H.; Posch, M.G.; Perrot, A.; Polotzki, M.; Rolf, S.; Parwani, A.S.; Huemer, M.; Wutzler, A.; Özcelik, C.; Haverkamp, W. Mutational analysis of the PITX2 and NKX2-5 genes in patients with idiopathic atrial fibrillation. Int. J. Cardiol. 2010, 145, 316–317. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Xu, Y.-J.; Li, R.-G.; Qu, X.-K.; Fang, W.-Y.; Liu, X. Prevalence and spectrum of PITX2c mutations associated with familial atrial fibrillation. Int. J. Cardiol. 2013, 168, 2873–2876. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.-F.; Sun, Y.-M.; Yang, Y.-Q. A novel PITX2c loss-of-function mutation associated with familial atrial fibrillation. Eur. J. Med. Genet. 2014, 57, 25–31. [Google Scholar] [CrossRef]
- Toro, R.; Saadi, I.; Kuburas, A.; Nemer, M.; Russo, A.F. Cell-specific Activation of the Atrial Natriuretic Factor Promoter by PITX2 and MEF2A. J. Biol. Chem. 2004, 279, 52087–52094. [Google Scholar] [CrossRef] [Green Version]
- Ganga, M.; Espinoza, H.M.; Cox, C.J.; Morton, L.; Hjalt, T.A.; Lee, Y.; Amendt, B.A. PITX2 Isoform-specific Regulation of Atrial Natriuretic Factor Expression. J. Biol. Chem. 2003, 278, 22437–22445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunnen, J.T.D.; Antonarakis, S.E. Nomenclature for the description of human sequence variations. Hum. Genet. 2001, 109, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, K.U.; Baracchini, E.; Ross, R.S.; Harris, A.N.; Henderson, S.A.; Evans, S.M.; Glembotski, C.C.; Chien, K.R. Co-regulation of the atrial natriuretic factor and cardiac myosin light chain-2 genes during alpha-adrenergic stimulation of neonatal rat ventricular cells. Identification of cis sequences within an embryonic and a constitutive contractile protein gene which mediate inducible expression. J Biol Chem. 1991, 266, 7759–7768. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shioi, T.; Kasahara, H.; Jobe, S.M.; Wiese, R.J.; Markham, B.E.; Izumo, S. The cardiac tissue-restricted homeobox protein Csx/Nkx2.5 physically associates with the zinc finger protein GATA4 and cooperatively activates atrial natriuretic factor gene expression. Mol. Cell Biol. 1998, 18, 3120–3129. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Torres, F.; Rastrojo, A.; Aguado, B. Intron retention and transcript chimerism conserved across mammals: Ly6g5b and Csnk2b-Ly6g5b as examples. BMC Genom. 2013, 14, 199. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Torres, F.; Aránega, A.E.; Franco, D. Identification of regulatory elements directing miR-23a–miR-27a–miR-24-2 transcriptional regulation in response to muscle hypertrophic stimuli. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1839, 885–897. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Molina, C.E.; Llach, A.; Herraiz-Martínez, A.; Tarifa, C.; Barriga, M.; Wiegerinck, R.F.; Fernandes, J.; Cabello, N.; Vallmitjana, A.; Benitez, R.; et al. Prevention of adenosine A2A receptor activation diminishes beat-to-beat alternation in human atrial myocytes. Basic Res. Cardiol. 2015, 111, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Syeda, F.; Holmes, A.P.; Yu, T.Y.; Tull, S.; Kuhlmann, S.M.; Pavlovic, D.; Betney, D.; Riley, G.; Kucera, J.P.; Jousset, F.; et al. PITX2 Modulates Atrial Membrane Potential and the Antiarrhythmic Effects of Sodium-Channel Blockers. J. Am. Coll. Cardiol. 2016, 68, 1881–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Tucker, N.R.; Weng, L.-C.; Clauss, S.; Lubitz, S.A.; Ellinor, P.T. A Functional Variant Associated with Atrial Fibrillation Regulates PITX2c Expression through TFAP2a. Am. J. Hum. Genet. 2016, 99, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- Gore-Panter, S.R.; Hsu, J.; Hanna, P.; Gillinov, A.M.; Pettersson, G.; Newton, D.W.; Moravec, C.S.; Van Wagoner, D.R.; Chung, M.K.; Barnard, J.; et al. Atrial Fibrillation Associated Chromosome 4q25 Variants Are Not Associated with PITX2c Expression in Human Adult Left Atrial Appendages. PLoS ONE 2014, 9, e86245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karck, M.; Wehrens, X.H.; Nattel, S.; Dobrev, D. Cellular and Molecular Mechanisms of Atrial Arrhythmogenesis in Patients With Paroxysmal Atrial Fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Yao, J.; Ni, M.; Wei, J.; Zhong, X.; Guo, W.; Zhang, L.; Wang, R.; Belke, D.; Chen, Y.-X.; et al. Cardiac ryanodine receptor calcium release deficiency syndrome. Sci. Transl. Med. 2021, 13, eaba7287. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yao, J.; Belke, D.; Guo, W.; Zhong, X.; Sun, B.; Wang, R.; Estillore, J.P.; Vallmitjana, A.; Benitez, R.; et al. Ca 2+ -CaM Dependent Inactivation of RyR2 Underlies Ca 2+ Alternans in Intact Heart. Circ. Res. 2021, 128. [Google Scholar] [CrossRef] [PubMed]
- Lalani, G.G.; Schricker, A.A.; Clopton, P.; Krummen, D.E.; Narayan, S.M. Frequency Analysis of Atrial Action Potential Alternans. Circ. Arrhythmia Electrophysiol. 2013, 6, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Xiong, F.; Qi, X.-Y.; Xiao, J.; Villeneuve, L.; Abu-Taha, I.; Dobrev, D.; Huang, C.; Nattel, S. Altered calcium handling produces reentry-promoting action potential alternans in atrial fibrillation–remodeled hearts. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [Green Version]
- Llach, A.; Molina, C.E.; Fernandes, J.; Padro, J.; Cinca, J.; Hove-Madsen, L. Sarcoplasmic reticulum and L-type Ca2+channel activity regulate the beat-to-beat stability of calcium handling in human atrial myocytes. J. Physiol. 2011, 589, 3247–3262. [Google Scholar] [CrossRef]






| Sequencing | Forward Primers | Reverse Primers |
|---|---|---|
| 1 | 5′ AGATTTCAGAGGGCCCAGAT 3′ | 5′ CGACCTCCTTCCTTTTGCTT 3′ |
| 2 | 5′ GGCACACTTTTCGAGTGAGA 3′ | 5′ CCAAGAGACGGAACAAAGGA 3′ |
| 3 | 5′ CTCACACCCACACTCCACAC 3′ | 5′ ATTCCACCAAACTCCACTGC 3′ |
| 4 | 5′ CTCCCCTGCCTCGTTTCT 3′ | 5′ GGGATAGATGGCCTCCACTT 3′ |
| 5 | 5′ CGAACAGACATCTCAATCAGG 3′ | 5′ GGTGGGAGCAAGCGTTGT 3′ |
| 6 | 5′ AGTCTCATCTGAGCCCTGCT 3′ | 5′ CTGGCGATTTGGTTCTGATT 3′ |
| 7 | 5′ TGGGTCTTTGCTCTTTGTCC 3′ | 5′ CTTCCCTCCCGGCCTTAC 3′ |
| 8 | 5′ CCCGCCTCTGGTTTTAAGAT 3′ | 5′ AAAGTCCGGAGACGGAAAGT 3′ |
| 9 | 5′ CTTGTTTCGCTTTGGAGCTT 3′ | 5′ AGGGGCTGACTTCCTTGG 3′ |
| 10 | 5′ ATGCTGACGGGAAAGTGTGT 3′ | 5′ GGCCTGTACCTCCACAACAT 3′ |
| 11 | 5′ GCATCTGTTTGCTCCCTTG 3′ | 5′ GACGGGCTACTCAGGTTGTT 3′ |
| 12 | 5′ ATGAGCATGTCGTCCAGCAT 3′ | 5′ TGAAAGATGTCAGACACTGAGGA 3′ |
| 13 | 5′ GAAAAGGAAACCACTGAATCAAA 3′ | 5′ TGTTAGAAACATACAGTGTGGCATT 3′ |
| 14 | 5′ CAACAGTGTTTTTAAAGGTTAGGC 3′ | 5′ AGGAGGGGAGAAAGAATCCA 3′ |
| Mutagenesis | Forward Primers | Reverse Primers |
| G947C (Gln103His) | 5′actttaccagccagcaCctccaggagctggagg 3′ | 5′cctccagctcctggagGtgctggctggtaaagt 3′ |
| G1008C (Glu124Lys) | 5′catgtccacacgcgaaCaaatcgctgtgtggac 3′ | 5′gtccacacagcgatttGttcgcgtgtggacatg 3′ |
| Gene | Forward Primers | Reverse Primers |
|---|---|---|
| Mm Gapdh | 5′ TCTTGCTCAGTGTCCTTGCTGG 3′ | 5′ TCCTGGTATGACAATGAATACGC 3′ |
| Mm Gusb | 5′ ACGCATCAGAAGCCGATTAT 3′ | 5′ ACTCTCAGCGGTGACTGGTT 3′ |
| Mm Atp2a2 | 5′ TGGGAGAATATCTGGCTCGT 3′ | 5′ AGGCAAGGAGATTTTCAGCA 3′ |
| Mm Pln | 5′ ATGCTCTGCACTGTGACGAT 3′ | 5′ TTTCCATTATGCCAGGAAGG 3′ |
| Mm Casq1 | 5′ CCCGTACTGGGAGAAGACCT 3′ | 5′ CAGGTCCTCCTCGTTATCCA 3′ |
| Mm Nkx2.5 | 5′ AGGTACCGCTGTTGCTTGAA 3′ | 5′ CAAGTGCTCTCCTGCTTTCC 3′ |
| Mm Gata4 | 5′ GCAGCAGCAGTGAAGAGATG 3′ | 5′ GCGATGTCTGAGTGACAGGA 3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herraiz-Martínez, A.; Tarifa, C.; Lozano-Velasco, E.; Jiménez-Sábado, V.; Casabella, S.; Hernández-Torres, F.; Daimi, H.; Vázquez Ruiz de Castroviejo, E.; Delpón, E.; Caballero, R.; et al. Novel PITX2 Homeodomain-Contained Mutations from ATRIAL Fibrillation Patients Deteriorate Calcium Homeostasis. Hearts 2021, 2, 251-269. https://doi.org/10.3390/hearts2020020
Herraiz-Martínez A, Tarifa C, Lozano-Velasco E, Jiménez-Sábado V, Casabella S, Hernández-Torres F, Daimi H, Vázquez Ruiz de Castroviejo E, Delpón E, Caballero R, et al. Novel PITX2 Homeodomain-Contained Mutations from ATRIAL Fibrillation Patients Deteriorate Calcium Homeostasis. Hearts. 2021; 2(2):251-269. https://doi.org/10.3390/hearts2020020
Chicago/Turabian StyleHerraiz-Martínez, Adela, Carmen Tarifa, Estefanía Lozano-Velasco, Verónica Jiménez-Sábado, Sergi Casabella, Francisco Hernández-Torres, Houria Daimi, Eduardo Vázquez Ruiz de Castroviejo, Eva Delpón, Ricardo Caballero, and et al. 2021. "Novel PITX2 Homeodomain-Contained Mutations from ATRIAL Fibrillation Patients Deteriorate Calcium Homeostasis" Hearts 2, no. 2: 251-269. https://doi.org/10.3390/hearts2020020
APA StyleHerraiz-Martínez, A., Tarifa, C., Lozano-Velasco, E., Jiménez-Sábado, V., Casabella, S., Hernández-Torres, F., Daimi, H., Vázquez Ruiz de Castroviejo, E., Delpón, E., Caballero, R., Aránega, A., Franco, D., & Hove-Madsen, L. (2021). Novel PITX2 Homeodomain-Contained Mutations from ATRIAL Fibrillation Patients Deteriorate Calcium Homeostasis. Hearts, 2(2), 251-269. https://doi.org/10.3390/hearts2020020








