1. Introduction
Engineered nanoparticles (ENPs) are of particular interest to industry, medicine and research due to their chemical, optical, electronic and magnetic properties [
1]. Increasing industrial, commercial, medical and household applications lead to a permanent release in aquatic environment with ecological and health implications [
2,
3]. The release of ENPs via wastewater treatment plants and drain-off into surface waters, which are also indirectly used for drinking water production, makes it necessary to know the stability and behavior of ENPs in natural waters and their elimination during the water treatment process [
4,
5,
6,
7,
8].
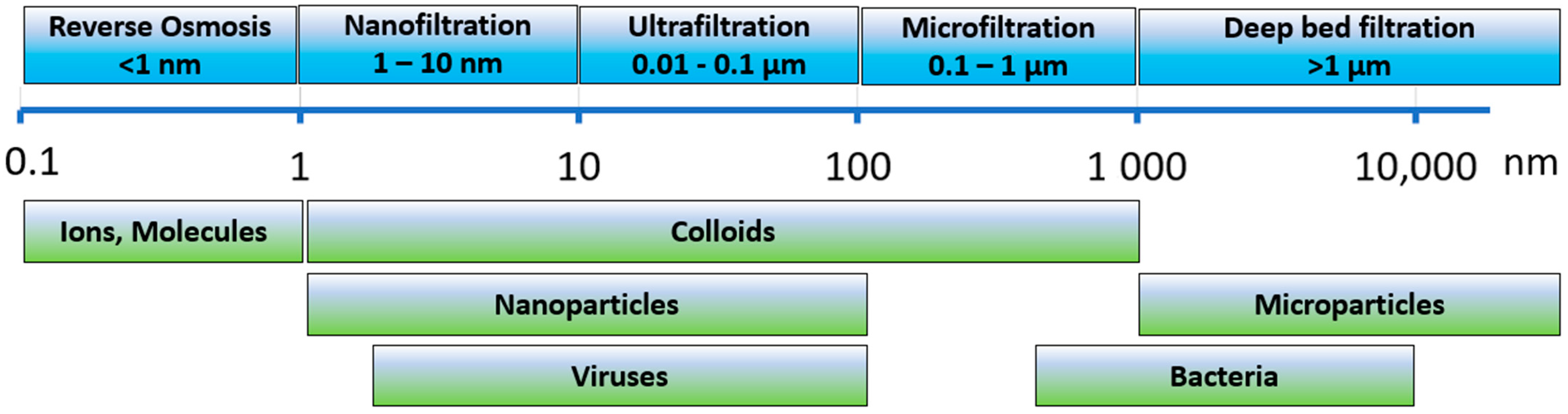
Nanoparticles (NPs) are defined as nanocrystals or aggregates ranging in size from 1 to 100 nm (
Figure 1). As such, they are part of the colloid group, which includes dispersed, molecular, and associative colloids defined by their physical behavior [
9,
10]. Due to their small size, NPs have a very high surface area-to-mass ratio, and a high surface energy, which accelerate aggregation and reactions, and are also responsible for quantum effects such as localized surface plasmon resonance (LSPR), the absorption of light at specific wavelengths [
11]. Because NPs try to reduce their high surface energy by aggregation, free nanoparticles are usually not stable. This can be prevented in aqueous media by a protective shell (anions, surfactants, polymers). Anions like citrate form a negatively charged coordination sphere around NPs, which prevents aggregation by electrical repulsion, while neutral polymers separate the NPs by steric hindrance [
12]. Since NPs in an aqueous solution are mostly negatively charged, the addition of positively charged ions can cause coagulation and result in precipitation, which can be used as a separation technique in water treatment [
13,
14]. Ligand stabilization is especially important when using ENPs under destabilizing conditions, e.g., low pH or high salt concentration [
15,
16]. NPs are formed naturally by the large-scale weathering of minerals, degradation of natural organic matter (NOM), and anthropogenic materials such as plastics. In contrast, ENPs are manufactured for technical, medical or other applications. They have a defined composition, size and shape, and are synthesized using top–down or bottom–up methods [
1]. Metallic nanoparticles are of increasing interest due to their widespread use in chemical catalysis [
17], sensing [
18], electronics [
19] and health applications [
3,
20]. For example, the silver NP market was valued at
$1.5 billion in 2020 and is projected to reach
$6.6 billion by 2030, growing at a CAGR of 15.6% from 2021 to 2030 [
21]. The use of gold NPs in electronic goods such as electronic chips, printing inks, and medical assays is widespread, and the market value in the United States was
$4.7 billion in 2022 and will increase at a CAGR of 10.6% from 2023 to 2031 to
$11.8 billion in 2031 [
22].
The environmental release modeling of silver ENPs in all relevant matrices predicted a silver concentration of 1.51 ng/L in surface water in the EU [
2]. Antunes et al. reviewed the synthesis, application, environmental behavior and mitigation strategies of AgNPs, and found published values ranging from 0.1 to 3.2 µg/L, which is in good agreement with the predicted value [
23]. After release into surface water, ENPs can directly impact the aquatic environment as NPs or transformed material. The degradation of metallic ENPs often results in the release of their metal and ligands, so their transformation products must also be considered in the toxicological assessment. For example, Louie et al. found that the PVP coating of PVP/AuNPs were rapidly oxidized upon UV irradiation, which reduced the colloidal stability of the AuNPs [
24]. While dissolved organic matter often has a stabilizing effect on ENPs, the presence of multivalent ions can lead to bridging and flocculation [
25]. Silver NPs are known to readily oxidize to elemental silver covered with an oxide layer, which has a long-lasting negative potential for ecosystems. Metallic silver released from AgNPs is more toxic to microorganisms than bulk silver due to the increased surface area [
26].
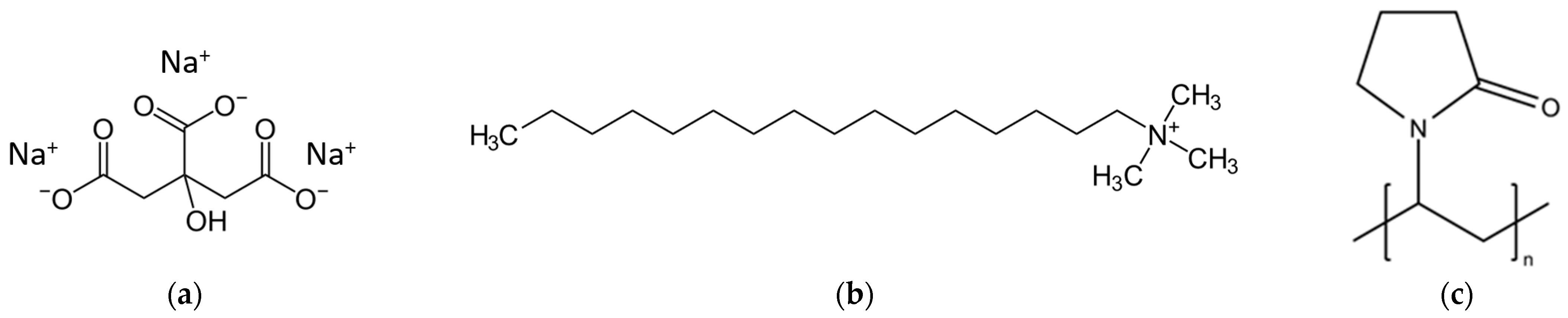
Important stabilizers for ENPs are citrate (CA), cetyltrimethylammonium bromide (CTAB) and polyvinylpyrrolidone (PVP). CA (
Figure 2a) is the anion of citric acid with a molecular weight of 189 g/mol. The stability of CA-capped NPs mainly depends on the pH due to CA protonation, which reduces the repulsive forces and stability, and to the presence of multivalent ions, which can link the nanoparticles into chains and further into aggregates and coagulates [
27]. CA/AuNPs are, therefore, subject to aging [
28]. Citrate is non-toxic and a metabolite of the citric acid cycle. In contrast, CTAB (
Figure 2b), a cationic surfactant, protects NPs by forming a bilayer around the NP core, resulting in a positive-charged NPs in water. The hydrophilic amine groups of the inner layer bind to the gold surface, while the amine groups of the outer layer interact with the aqueous phase [
29]. Two groups have recently elucidated the self-assembly of CTAB around the metallic core [
30,
31]. CTAB is toxic and has been shown to affect biological systems by promoting cell lysis, and is used as an apoptosis-promoting anticancer agent [
32,
33].
PVP (
Figure 2c) is a nonionic, linear and atactic polymer with molecular weights ranging from 2.5 to 2500 kDa. It is non-toxic and is used in pharmaceutical formulations [
34]. In NP synthesis, PVP can serve as a surface stabilizer, growth modifier, dispersant, and reducing agent [
35]. Studies have shown that both oxygen and nitrogen atoms of the pyrrolidone ring are responsible for the adsorption of PVP on the surface of silver NPs [
36]. Silver nanoparticles (AgNPs) have a significant impact on the aquatic environment, as demonstrated in numerous studies [
23,
37,
38,
39,
40]. They can be absorbed through the respiratory tract, digestive system, and skin, and have recently been reviewed [
41]. PVP/AgNPs cause cytotoxic and inflammatory responses in the lungs of healthy adult female rats after intratracheal instillation [
42]. Although gold is known to be an inert metal, CTAB/AuNPs have been shown to be taken up by cells and bound to serum proteins [
43,
44]. CTAB/AuNPs bind strongly to enzymes, which is used for analysis [
29]. A recent review has highlighted the toxicological aspects of AuNPs [
45].
The detection and quantification of ENPs in the environment is still challenging due to the low concentration, structural heterogeneity and transformation [
5,
7]. However, there are several methods currently available for the determination of ENPs in fresh water [
8,
46,
47,
48]. In this study, the size- and shape-dependent localized surface plasmon resonance (LSPR) of gold and silver nanoparticles was used for analysis [
11,
49]. LSPR can be used to determine both the concentration and the change in size of the metal core in ENPs due to aggregation. As the concentration decreases, the adsorption decreases, and as the particle size increases, a red shift of the absorption maximum is observed [
50,
51]. The Lambert–Beer law (Equation (1)) describes the attenuation of light by an appropriate absorption band of a dissolved substance. The logarithm of the quotient,
lg(
I0/
I), is called the absorbance (
A) and is proportional to the concentration (
c) of the substance, which allows for its quantitative determination (
ℓ: optical path length,
ε: molar attenuation coefficient).
Due to the low concentration, small size and wide variety of stabilized ENPs, the options for removing ENPs from natural waters are limited. There are many studies in the laboratory, but few on removal at a pilot or technical DWTP [
4,
5,
8]. Lawler and coworkers used flocculation and filtration to remove CA/AgNPs, and identified calcium ion concentration and NOM as important factors [
52]. Hofman-Caris et al. spiked various water qualities with CA/AuNPs and CA/AgNPs, and found ENP reductions of 57 to 77% with coagulation/flocculation/sedimentation (CFS). Preloaded sand provided good retention up to 82% for AuNPs and 85% for AgNPs, while purified sand was ineffective. Activated carbon (AC) had a low retention rate of about 30% [
53]. In a laboratory-scale study, Chalew et al. spiked various water samples with unstabilized ENPs and tested CFS, microfiltration (MF), and ultrafiltration (UF) as treatment methods [
54]. CFS resulted in partial reductions of 2–20% for Ag in finished water. Breakthrough after MF was 1–45%, and after UF was 0–2%. Although the majority of aggregated or stable NPs were removed, the authors concluded that “NPs should be regarded as emerging drinking water contaminants and that drinking water treatment processes should be enhanced to fully remove NPs in order to reduce their potential harmful health outcomes”. In their review, Westerhoff et al. estimated a low risk of ENPs for drinking water based on the fact that conventional DWTPs remove >80% [
55]. In contrast, Sousa and Teixera stated that data on the contamination of raw water and drinking water are insufficient, and that the detection of ENPs in drinking water poses a risk to human health because their properties make their removal difficult [
5]. Park et al. concluded that ENPs pose a serious threat to the environment and human health if water treatment is inadequate. An important question is the extent to which ENP removal processes are efficient [
7]. Reverse osmosis is probably the only single process that can completely remove NPs from raw water (
Figure 1). This paper considers the treatment processes commonly used by many waterworks: ozonation, advanced oxidation process (AOP), flocculation, anthracite sand filtration and adsorption on activated carbon.
Ozonation is used for oxidation or disinfection in larger DWTPs. Ozone (O
3) has a high standard oxidation potential (+2.07 V) and is commonly used in water treatment for disinfection and oxidation of iron(II), manganese(II), and NOM. In addition, O
3 can micro-flocculate the colloidal components of natural water by destabilizing them [
56]. The oxidation reactions of O
3 (aq) cover a wide range of second-order rate constants from <0.1 M
−1s
−1 to 10
−8 M
−1s
−1, while the hydroxyl radical reaction is diffusion controlled with k(∙OH) from 10
9 M
−1s
−1 to 10
10 M
−1s
−1 [
57]. The radical process often leads to a reaction even with compounds that are difficult to oxidize. It is known that CA/AuNPs react reversibly with O
3 [
58], while polyphosphate/AgNPs were partially oxidized [
59].
The radical process can become the dominant reaction channel, for example, through the addition of hydrogen peroxide, in which case it is referred to as an Advanced Oxidation Process (AOP) [
60]. The process must be optimized to achieve maximum OH radical yield and is mainly dependent on the concentrations of ozone, hydrogen peroxide and pH. Due to parallel radical scavenging processes, the yield of hydroxyl radicals is only ~50% [
61]. Partial aggregation of PVP/AgNPs was observed during laboratory ozonation, which increased with reaction time and pH [
62].
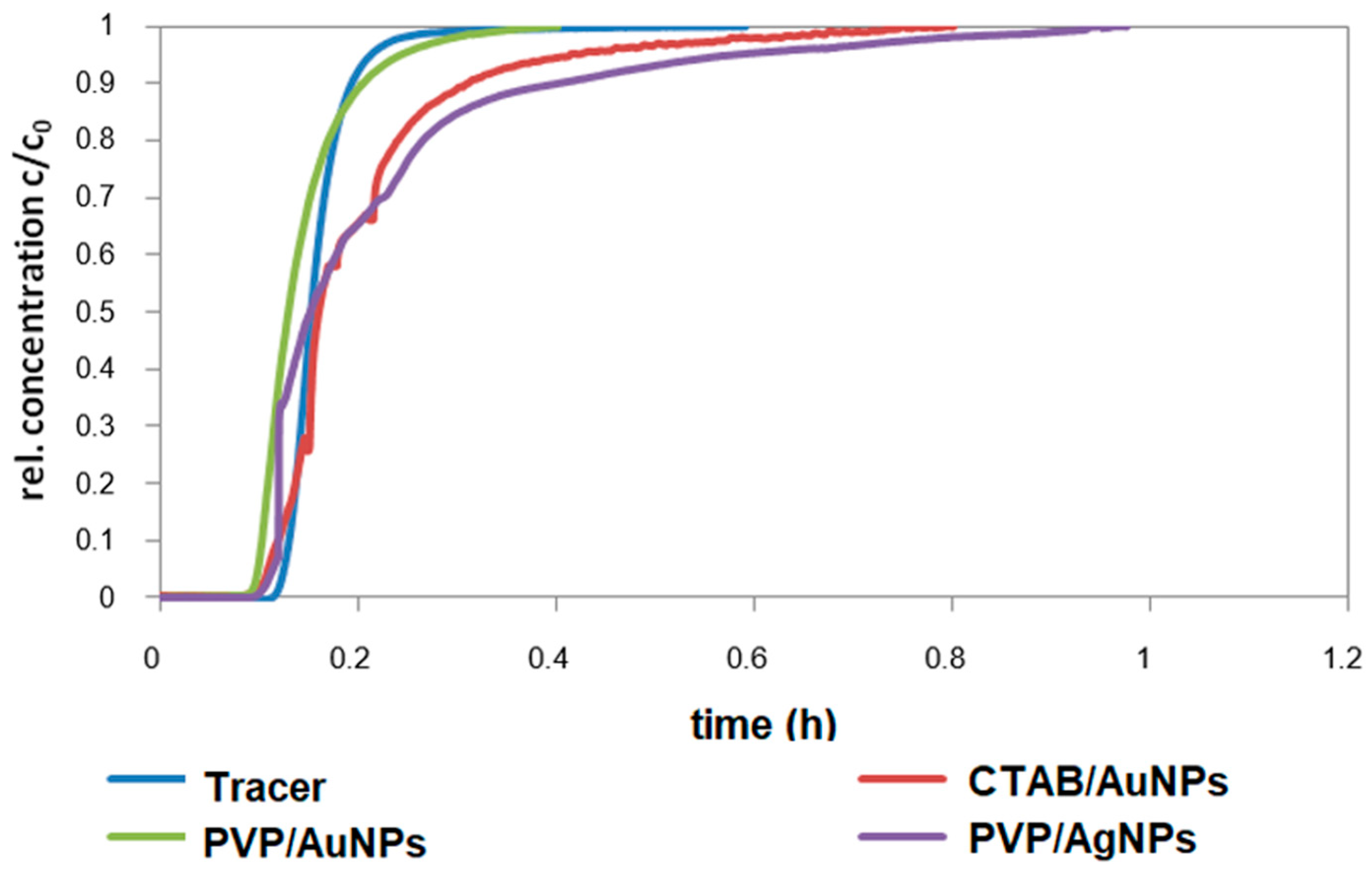
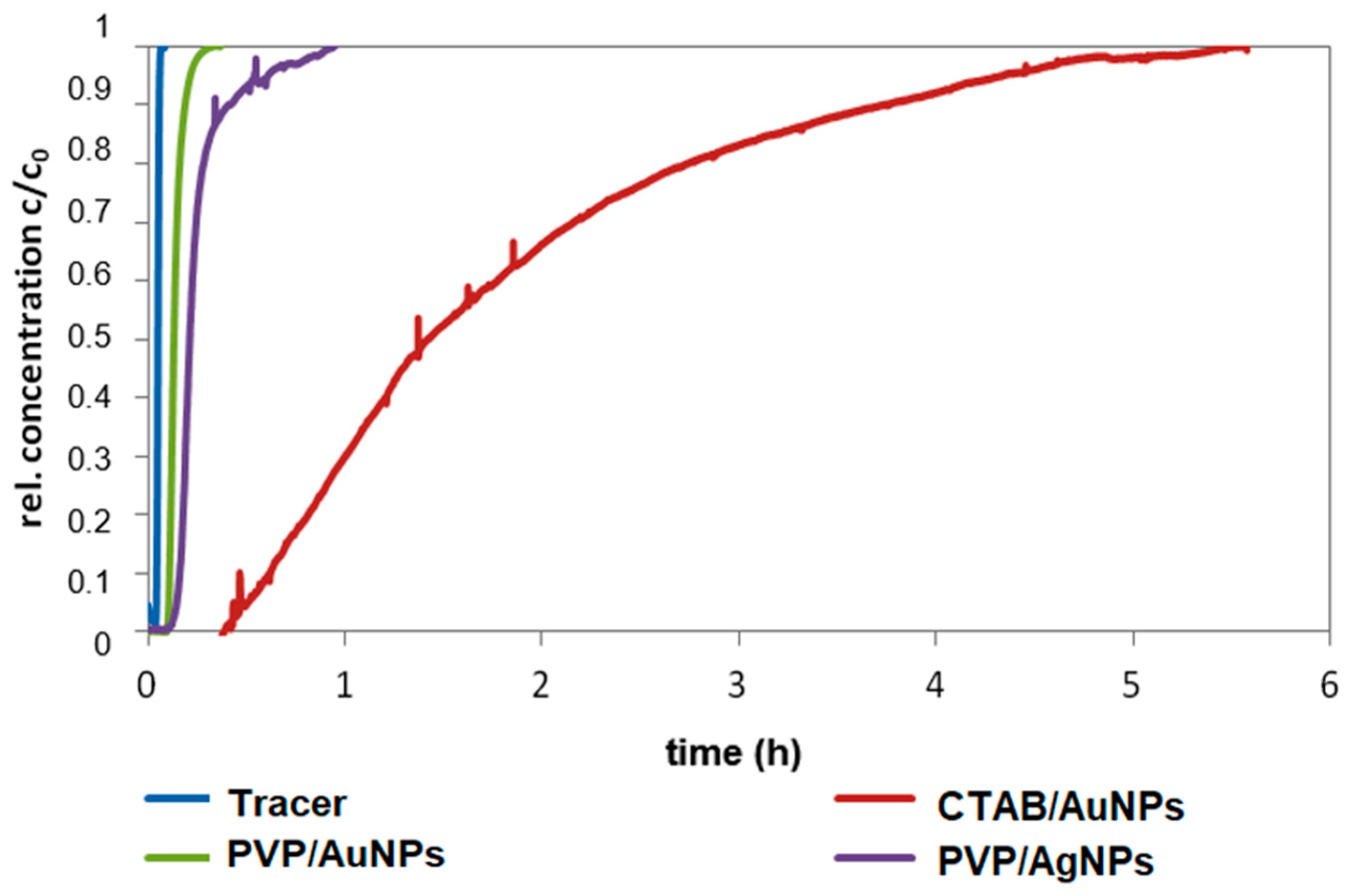
Filtration is the retention of suspended (>1 mm) and dispersed (<1 mm) particles on a filter material. In deep-bed filtration, particles are removed during the flow of a suspension through a deep granular bed [
63]. Various mechanisms are responsible for retention, including inertia, interception, sedimentation, and electrostatic forces, while the forced fluid flow is directed toward the outlet [
64]. The NP retention on porous material, e.g., riverbank filtration, retention on filter materials and adsorbents can be described with the colloid filtration theory (CFT). The relevant mechanisms are filtration, adsorption, and partitioning of colloids in water-saturated, porous media [
65].
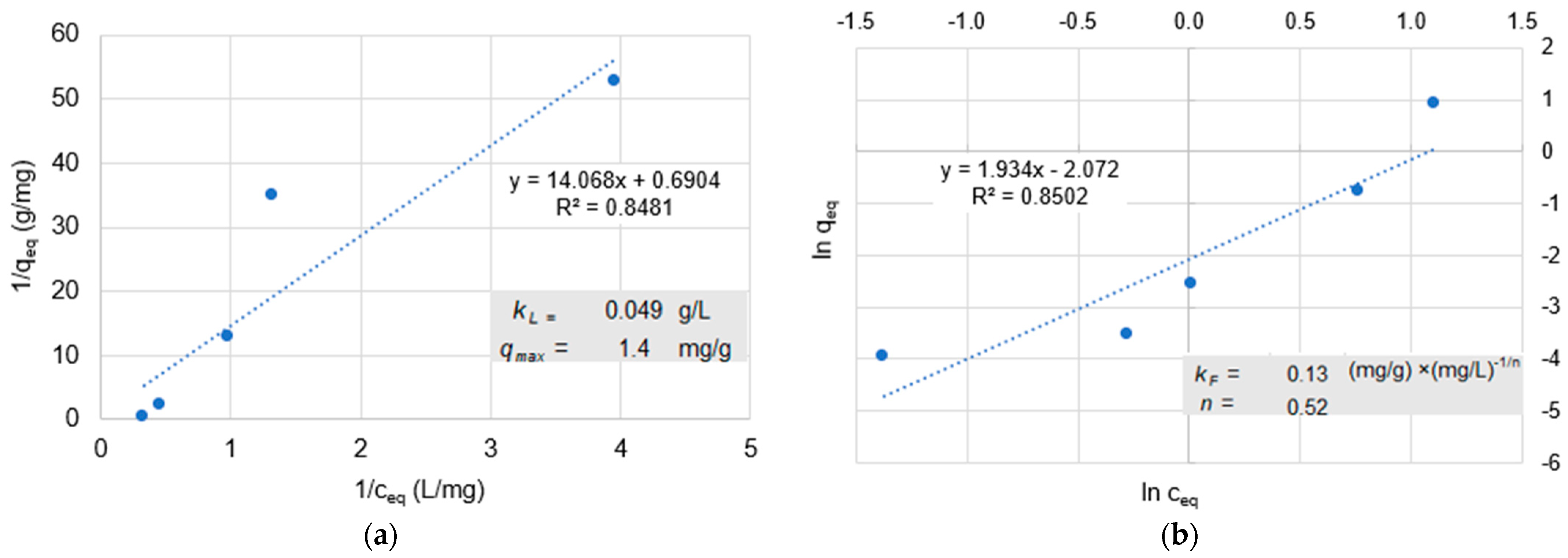
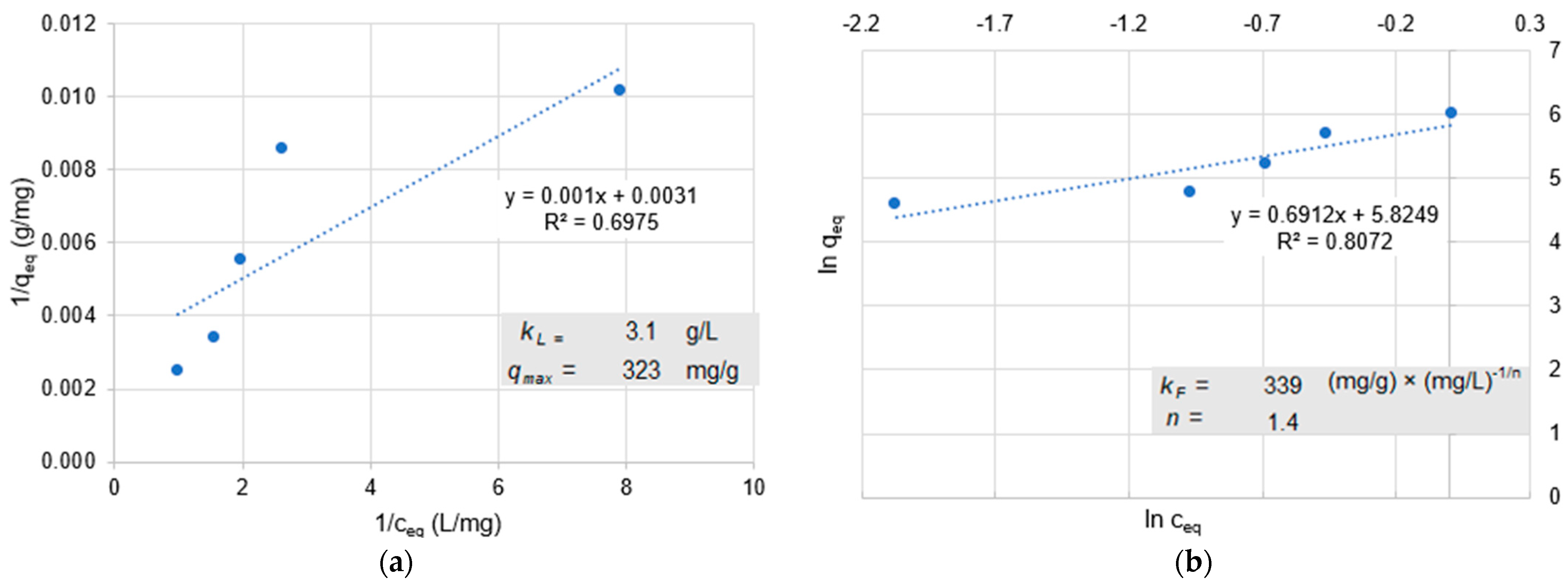
Adsorption is the process of attaching a compound to the outer or inner surface of an adsorbent [
66]. Adsorption depends on the physical and chemical properties of the adsorptive, the solvent, and the adsorbent, such as the surface structure and the process conditions. In batch tests, the equilibrium loading capacity is determined as a function of the equilibrium concentration at a constant temperature. An adsorption isotherm is obtained by plotting the two values. Closer to technical implementation than batch tests are column tests, in which adsorption occurs as the solution or dispersion of the adsorptive passes through the adsorbent and the residual concentration in the effluent is measured as a function of the eluted pore volume or time [
67]. Activated carbon is often used in DWTPs as an adsorbent because it contains many pores of different sizes and diameters. Macropores have a diameter of more than 30 nm, followed by mesopores with a diameter of 30 to 2 nm, and micropores in the range of 2 to 0.4 nm. The macropores and mesopores, in particular, may be important for the adsorption of NPs [
23]. The Langmuir or Freundlich isotherms are mainly used to describe the adsorption of solutes in water.
Flocculation can be used to remove turbidity, color, bacteria, biological matter or other substances from water. Flocculation always involves the use of flocculants to overcome the electrostatic repulsion forces of suspended particles (coagulation), followed by flocculation and sedimentation (CFS) [
68]. Multivalent ions, which have a greater potential-changing effect than monovalent ions, are preferred. The most common flocculants are ferric chloride (FeCl
3) and aluminum chloride/sulfate (AlCl
3/Al
2(SO
4)
3), which are used as solutions and mixed with the water to be treated [
69]. By adding polyvalent metal salts which hydrolyze to hydroxy species in water, dense and easily separable flakes can be formed. When particles are captured and bound in one process, it is called sweep flocculation [
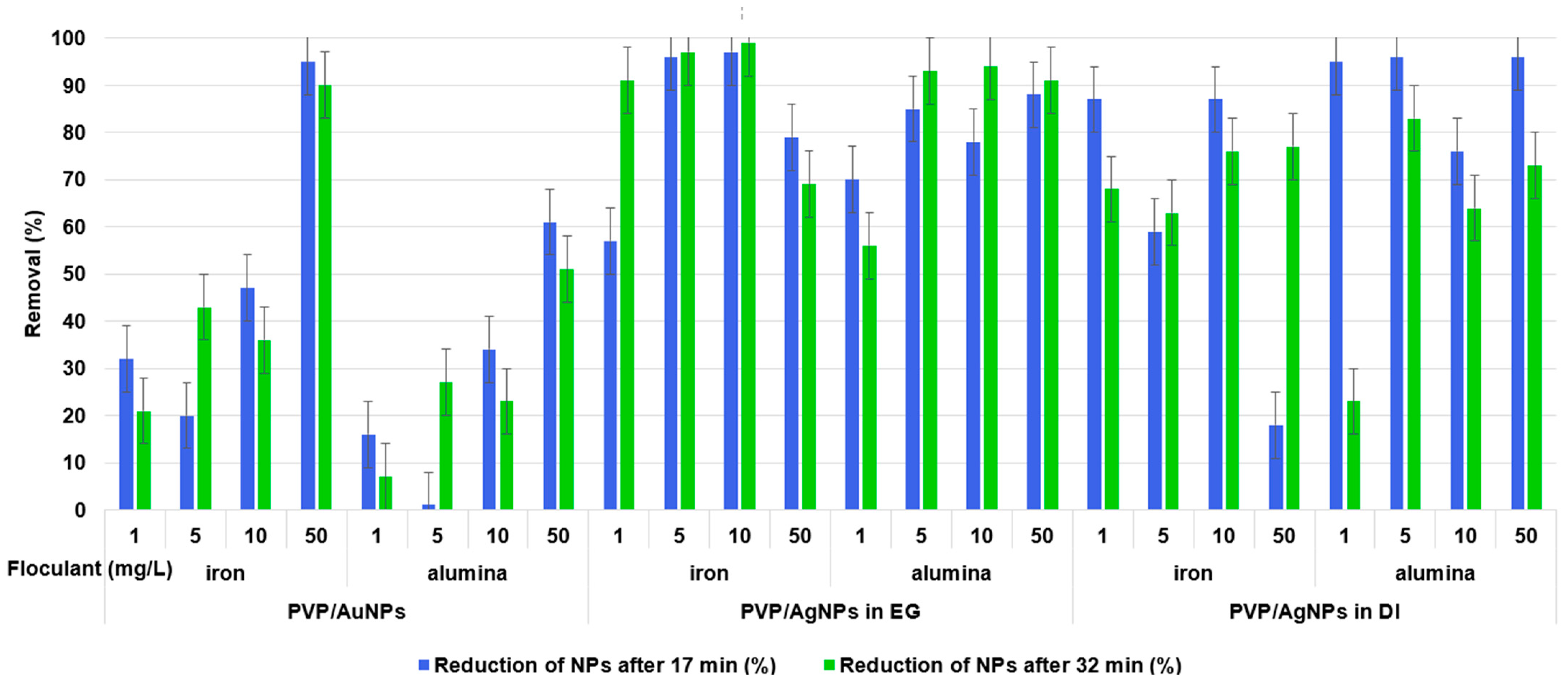
70]. Polyvinyl alcohol (PVA)-stabilized AgNPs with a core size of 5 to 20 nm and a concentration of 1 mg/L in natural water can only be flocculated with high flocculant concentrations of 30 to 485 mg/L, while the presence of NOM improves the removal efficiency, and a pH of about 7.5 was found to be optimal [
14]. Hofman-Caris et al. used batch CFS with low doses of 0.5 to 1.5 mg/L iron(III) and observed a reduction in CA/AuNPs by 8 to 77% depending on the water quality [
53].
The waterworks considered here are located near the river Rhine and the raw water treatment is carried out by bank filtration, ozonation, anthracite and activated carbon filtration. The underground passage of the river water through the collimated riverbed and quaternary sands cleans it on its way to the extraction wells. Particles and microorganisms are retained, organic matter is microbiologically degraded, and peak concentrations of salts in the river are smoothed and reduced [
71,
72,
73]. At the DWTP, raw water is treated with ozone to disinfect and oxidize dissolved iron(II) and manganese(II). Filtration through anthracite removes the particles generated during ozonation. Trace organic compounds (TrOCs) and their transformation products, NOM, and heavy metals are adsorbed on granular activated carbon. In order to gain further insight into the treatment processes and identify the risks and improvement potentials of a conventional water treatment plant, e.g., the removal of TrOCs and chromium(VI), a modular pilot plant was built and operated [
74,
75].
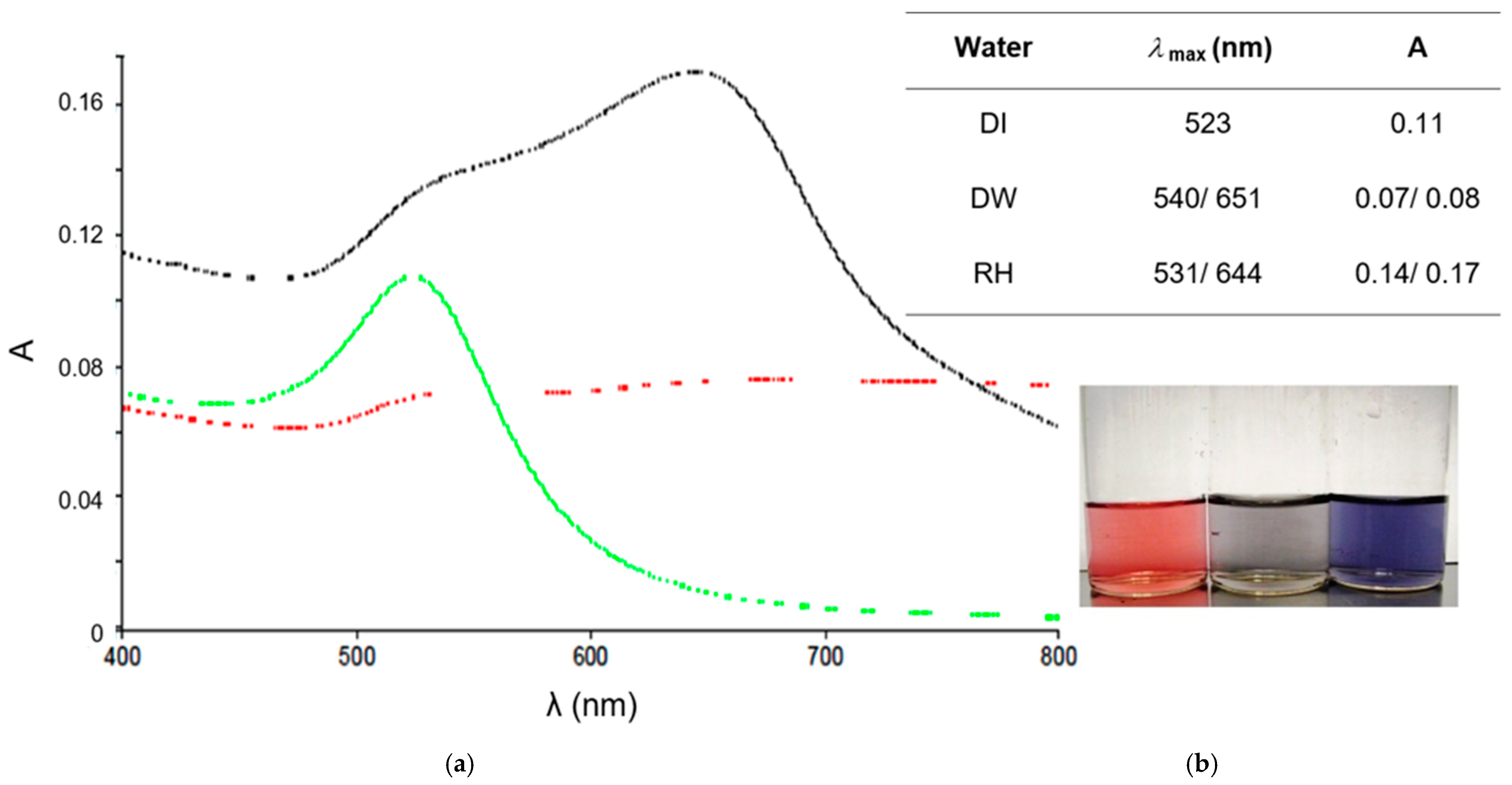
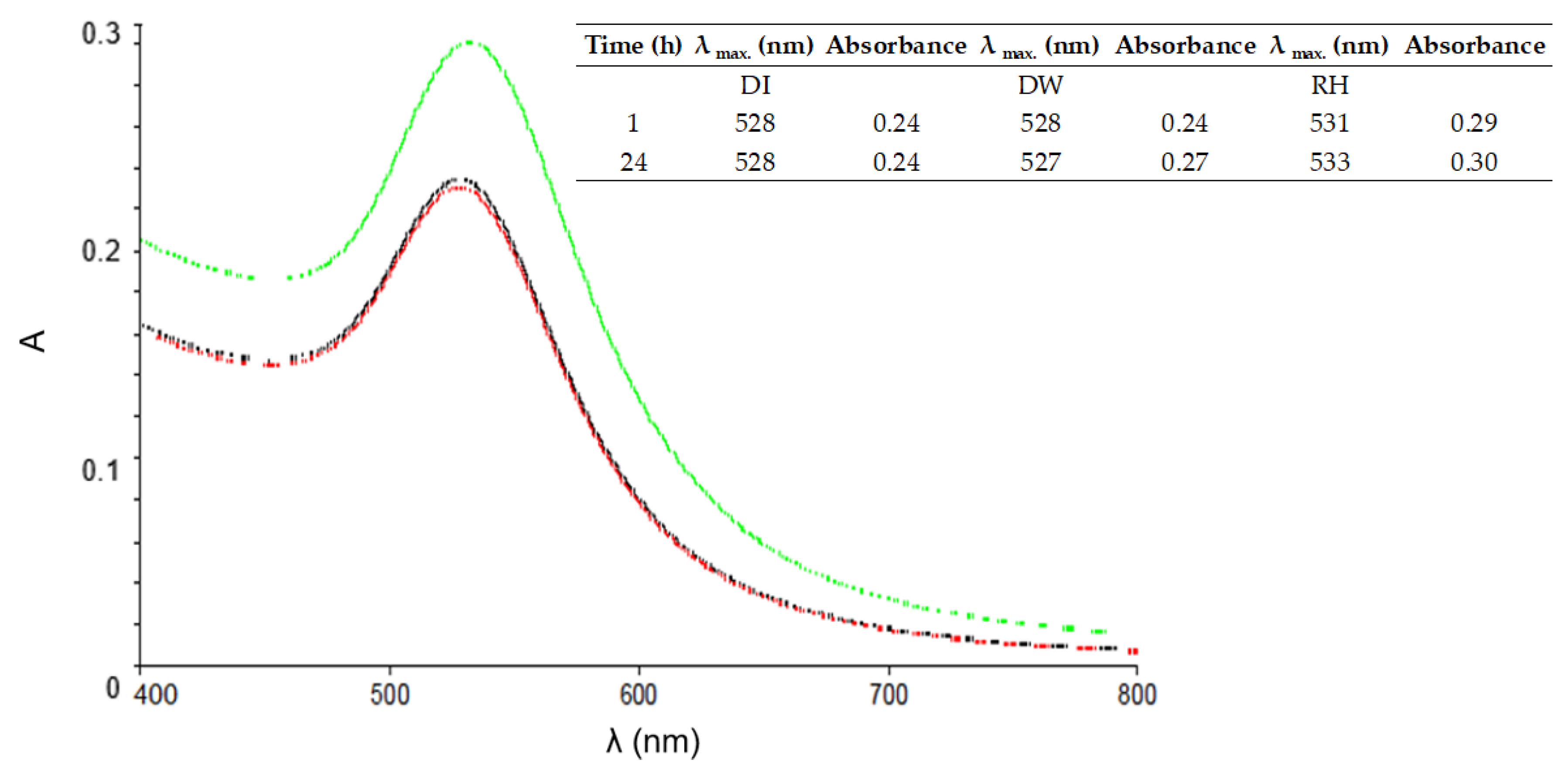
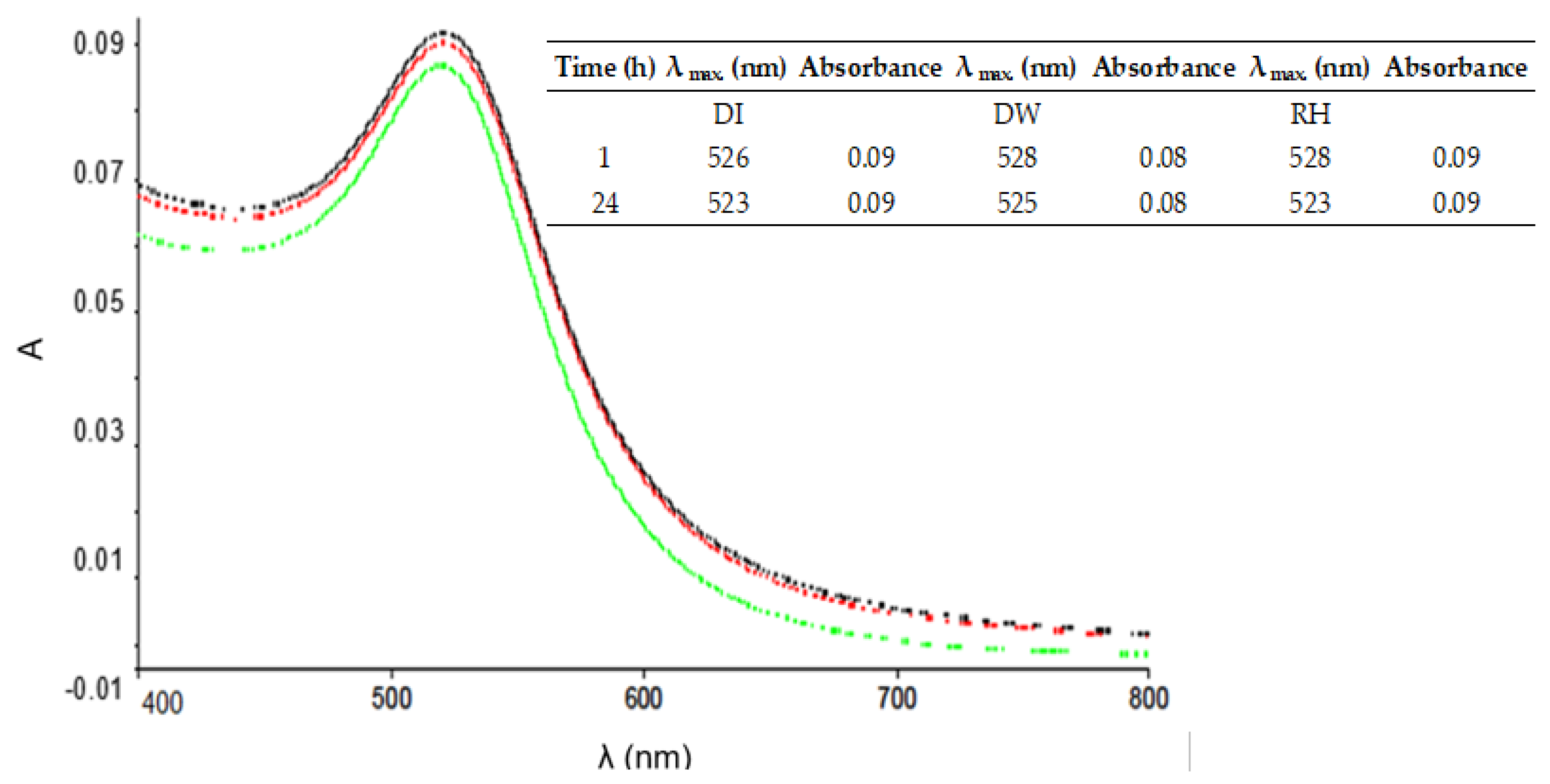
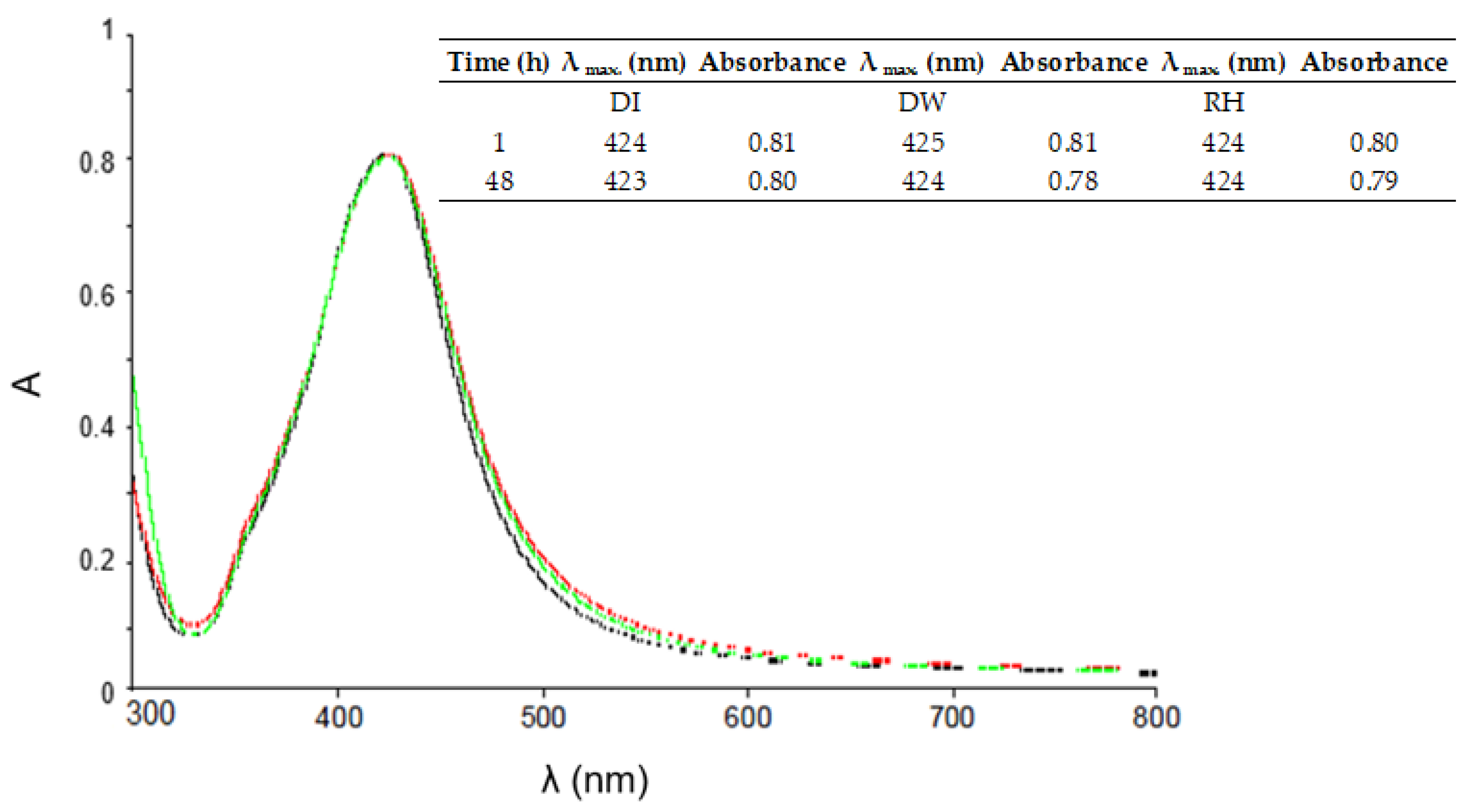
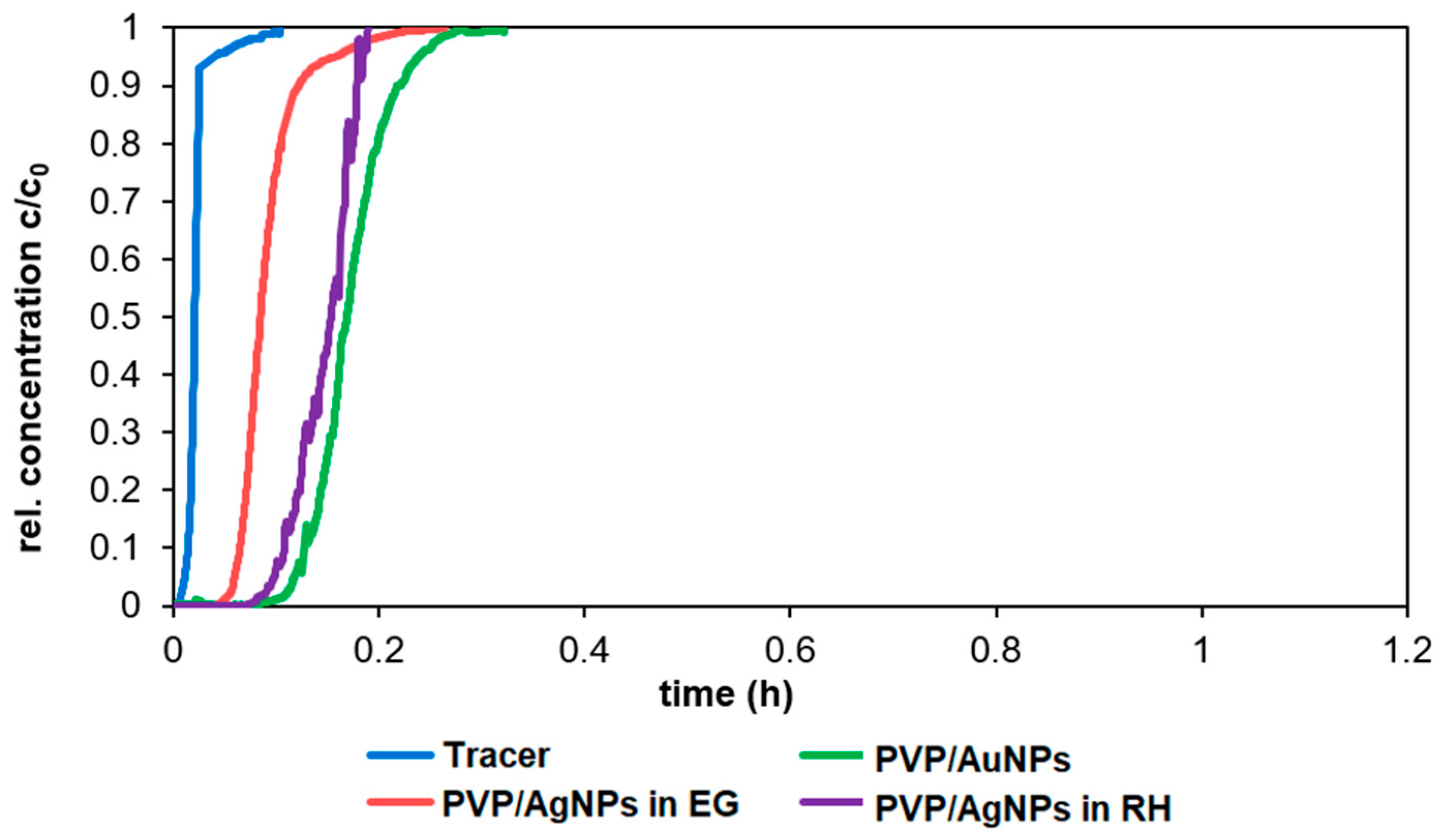
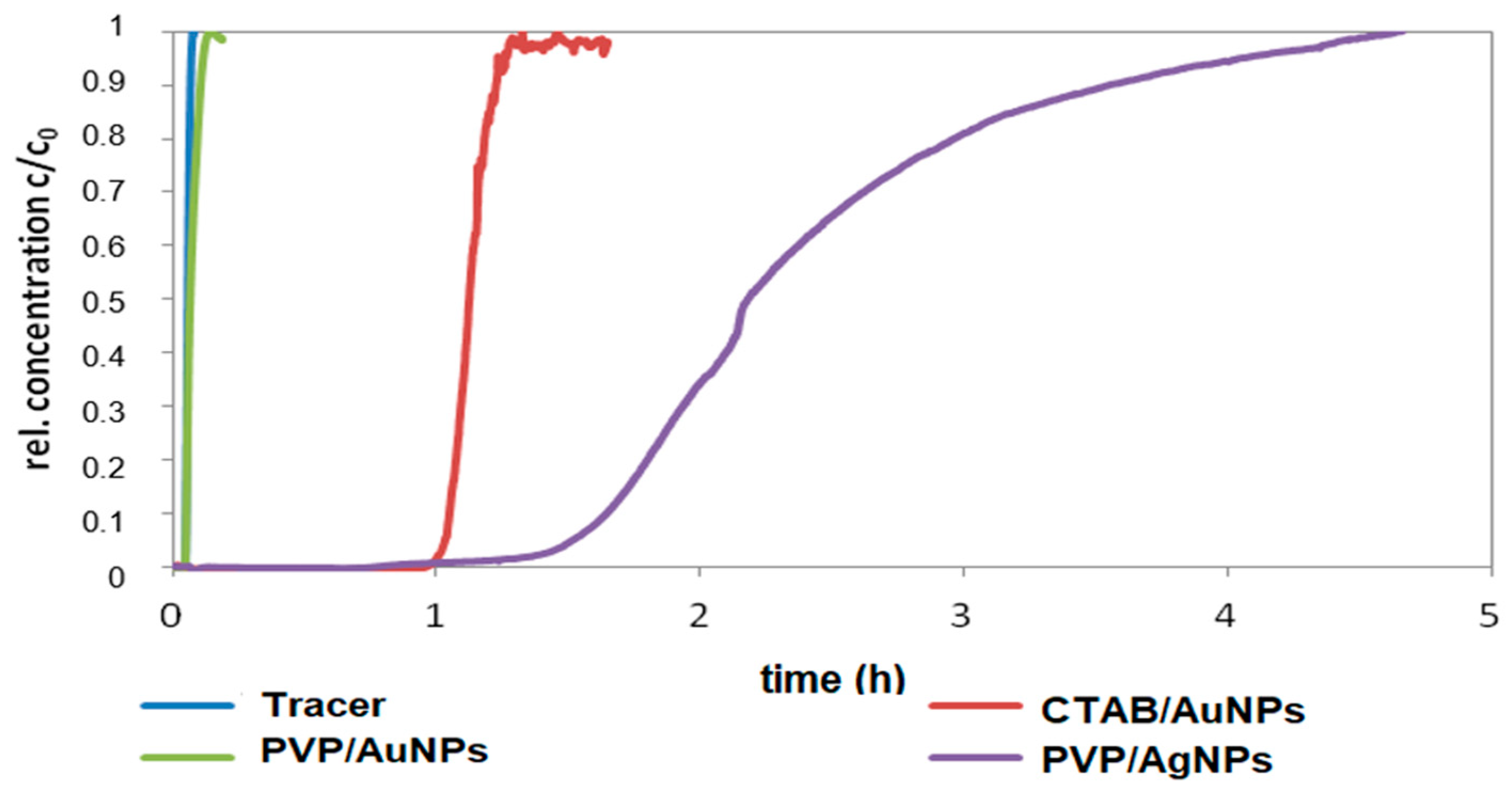
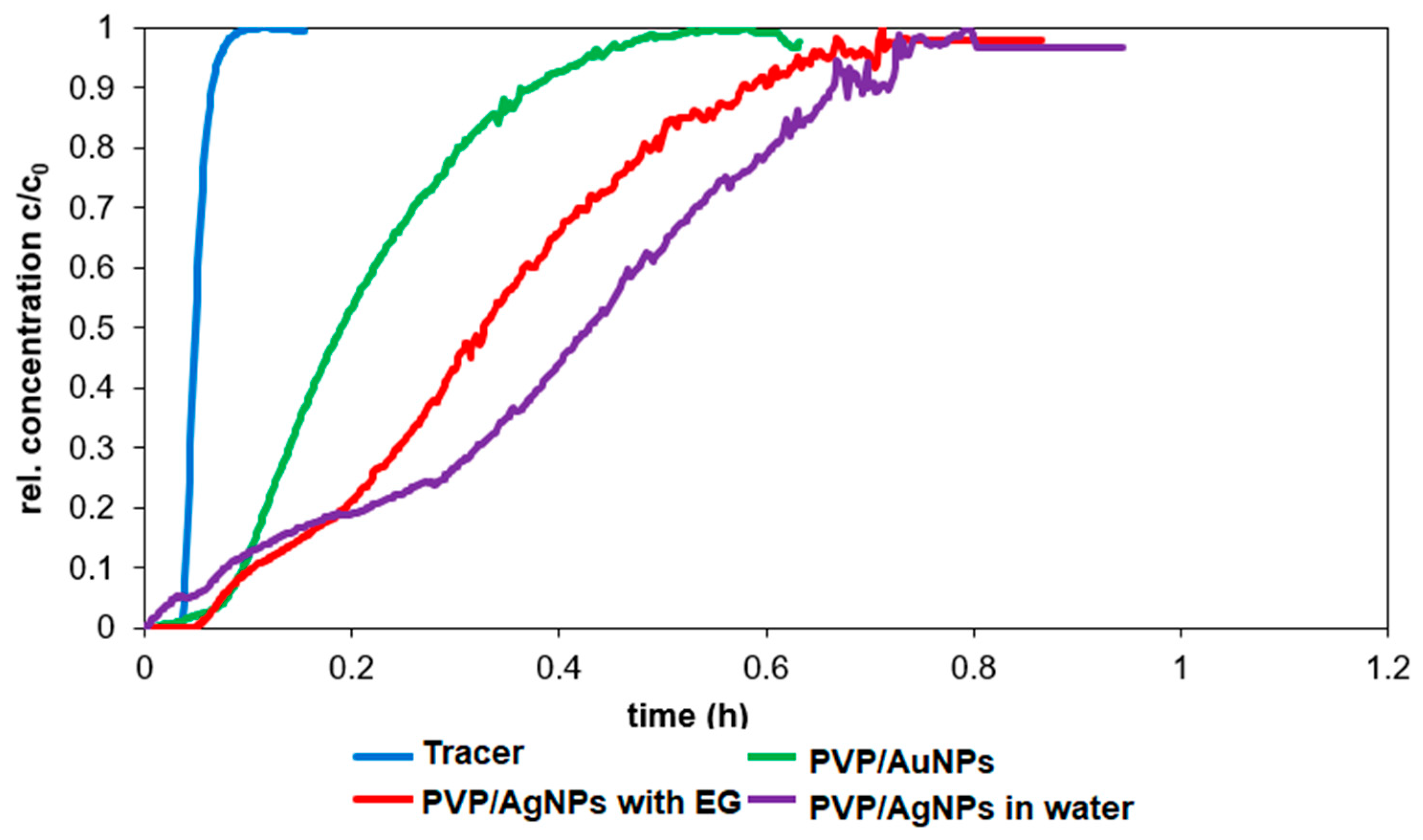
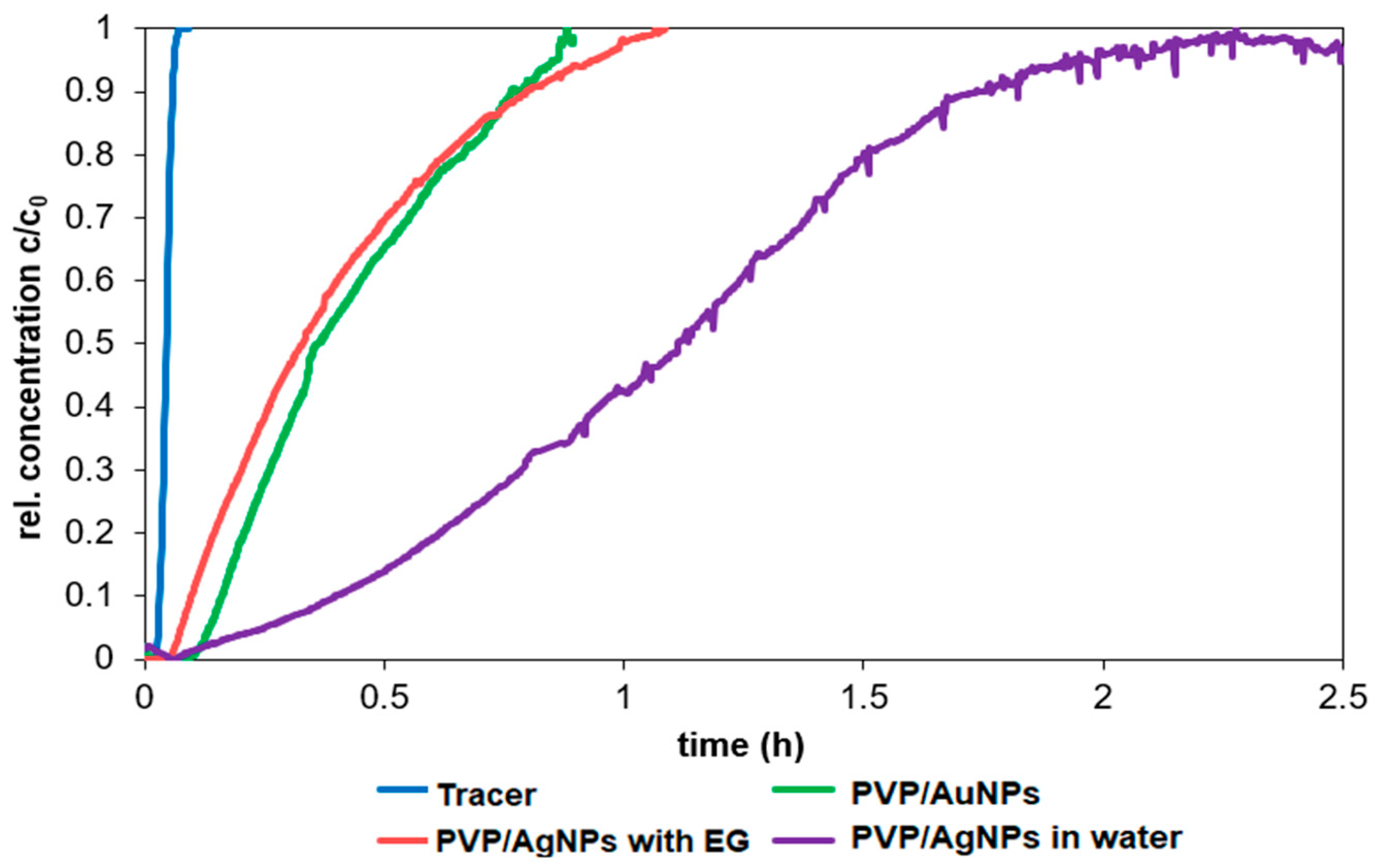
The objective of this study was to demonstrate the behavior of stabilized ENPs at a DWTP for risk assessment. Although there are many laboratory tests for the removal of ENPs, their behavior in a working pilot plant or technical DWTP has rarely been described [
52,
53,
54]. The results of the study can be used as a basis for risk assessment by authorities and water suppliers. First, several ENPs were synthesized and tested for their stability in natural water (surface and drinking water). The stable ENPs were then tested for retention in riverbank filtration on a laboratory scale using Rhine sand as a model, while purified quartz sand and silica gel were studied as references. The ENPs not retained during bank filtration were then investigated for retention during filtration on fresh and used anthracite and for adsorption on activated carbon. CFS was studied as an upgrade in DWTPs for ENP removal. The most stable ENPs were injected into the pilot plant for a short period of time and their behavior in ozonation, AOP and retention on anthracite sand filter was determined. This work is the first to consider the retention of stabilized ENPs throughout the process chain from surface water to drinking water, which is essential for a comprehensive risk assessment.