Biochar-Supported Titanium Oxide for the Photocatalytic Treatment of Orange II Sodium Salt
Abstract
1. Introduction
2. Titanium Oxide Modifications for Enhanced Solar Activities
2.1. Metal Ion Doping
2.2. Non-Metal Doping
2.3. Titanium Oxide-Supported Carbon Composites in Photocatalysis
3. Port Jackson Willow (Acacia saligna)
3.1. Chemical Reagents and Materials
3.2. Preparation of Biochar
4. Synthesis of the Titanium Oxide Nanoparticles
Synthesis of Biochar-Supported TiO2 Nanocomposite
5. Energy Bandgap Determination
5.1. Characterization of Biochar Obtained from PJW Leaves
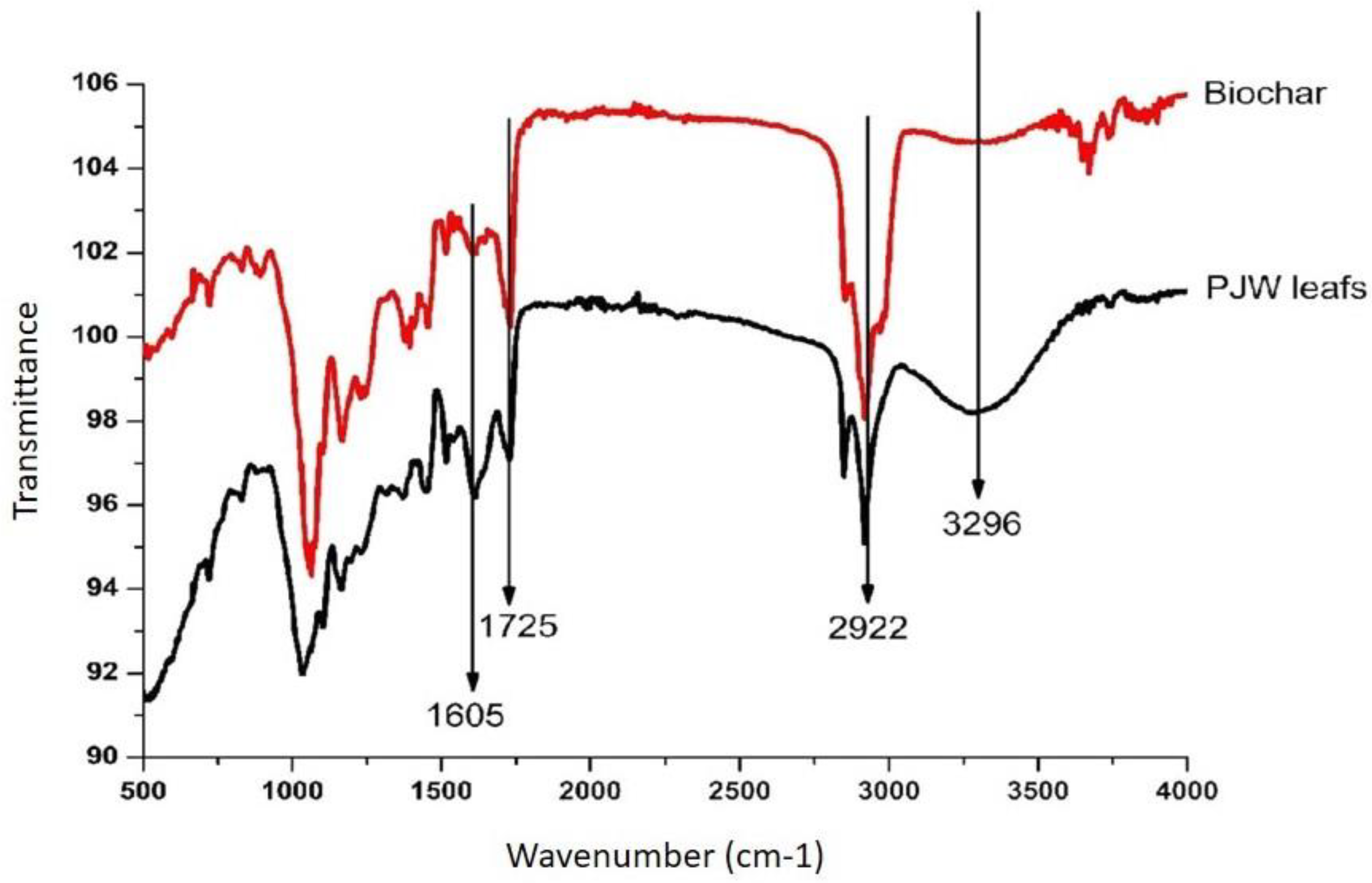
5.2. Functional Group Identification
5.3. Surface Morphology of the Prepared Biochar
5.4. Energy Bandgap of Biochar-Supported Titanium Oxide
5.5. SEM-EDS of the Synthesized Titanium Oxide Nanoparticles
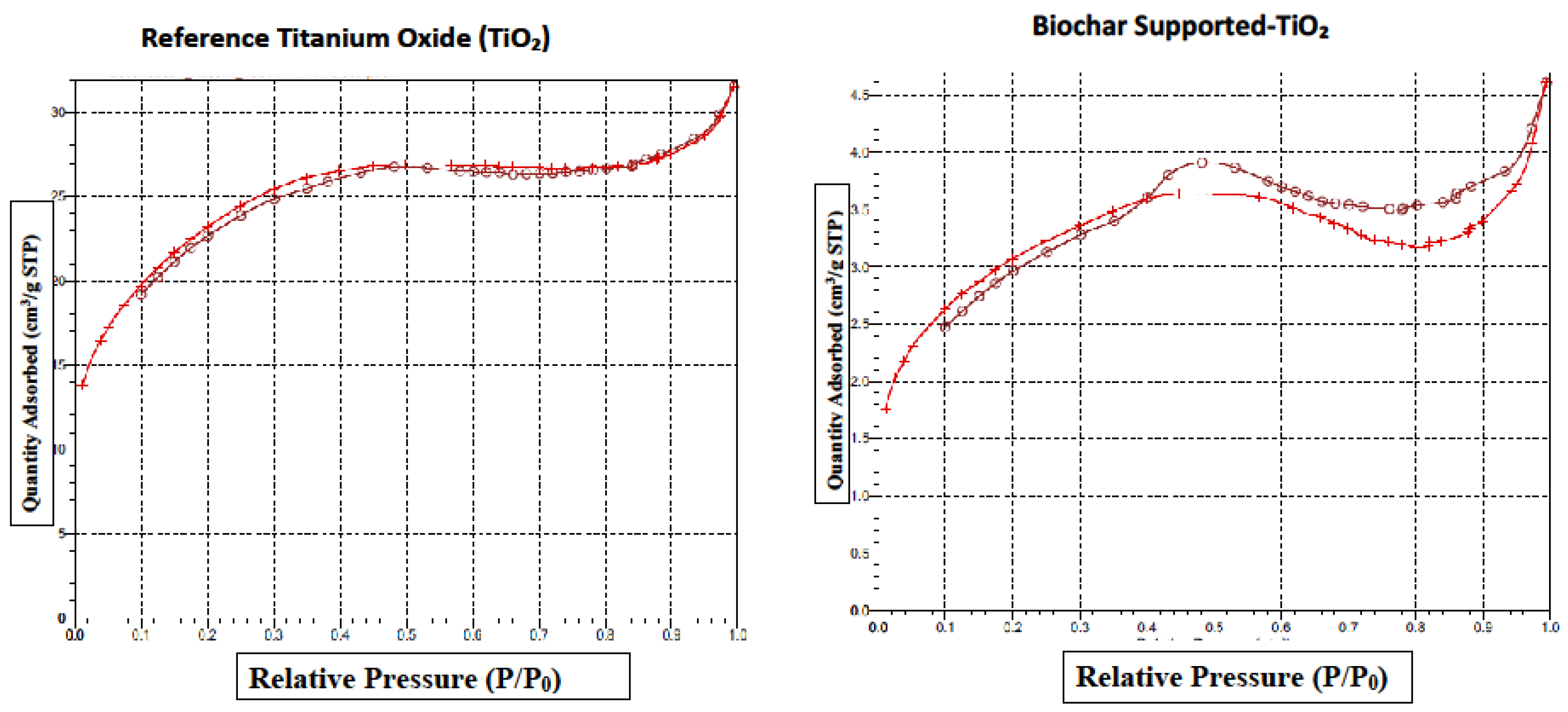
5.6. Brunauer–Emmett–Teller (BET) Surface Area Analysis
5.7. X-ray Diffraction (XRD) Analysis
5.8. Application of the Biochar-Supported Titanium Oxide Nanoparticles for the Degradation of Orange II Sodium Azo-Dye
5.9. Effect of Photocatalyst Loading
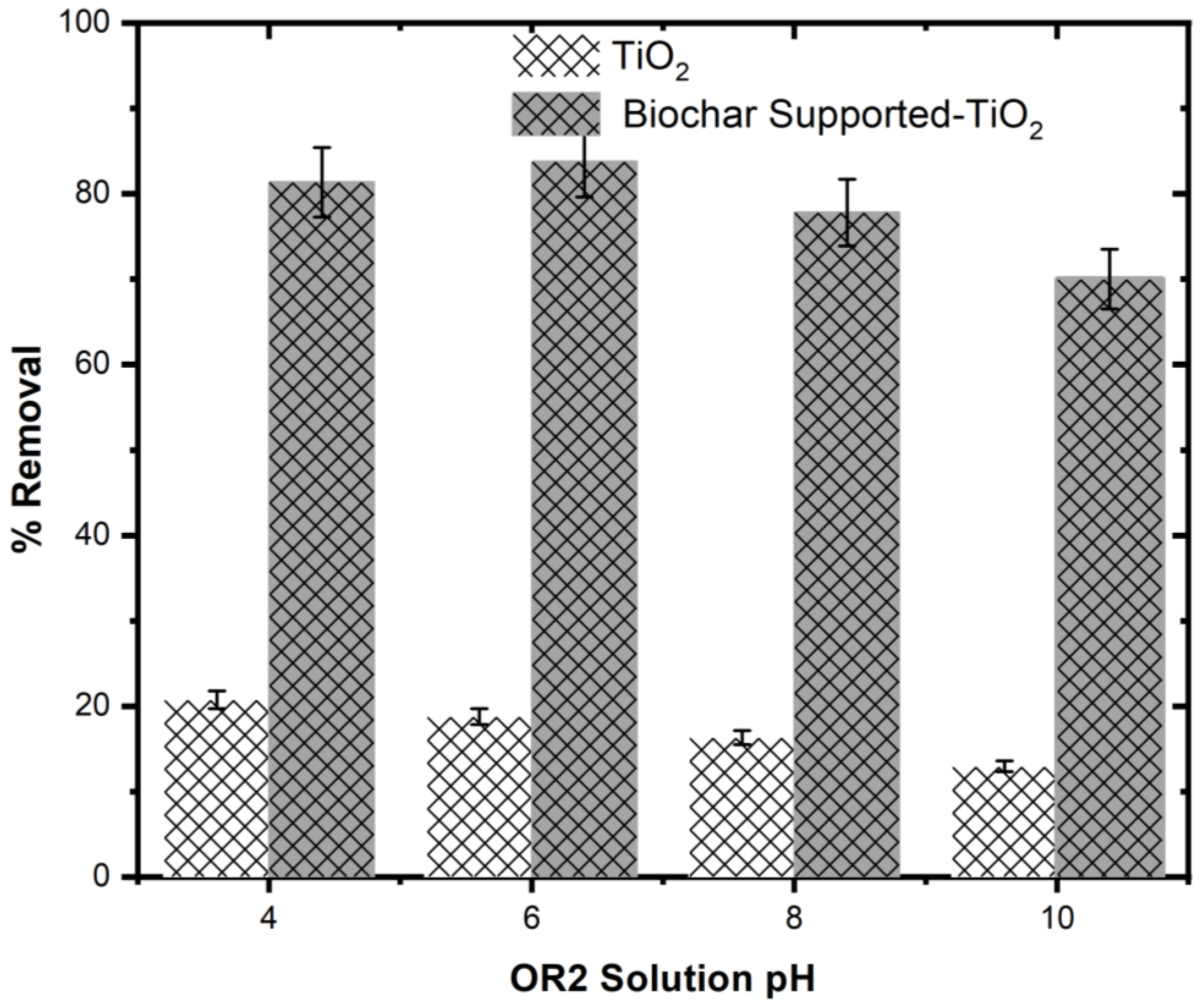
5.10. Impact of Solution pH on Photodegradation of OR2
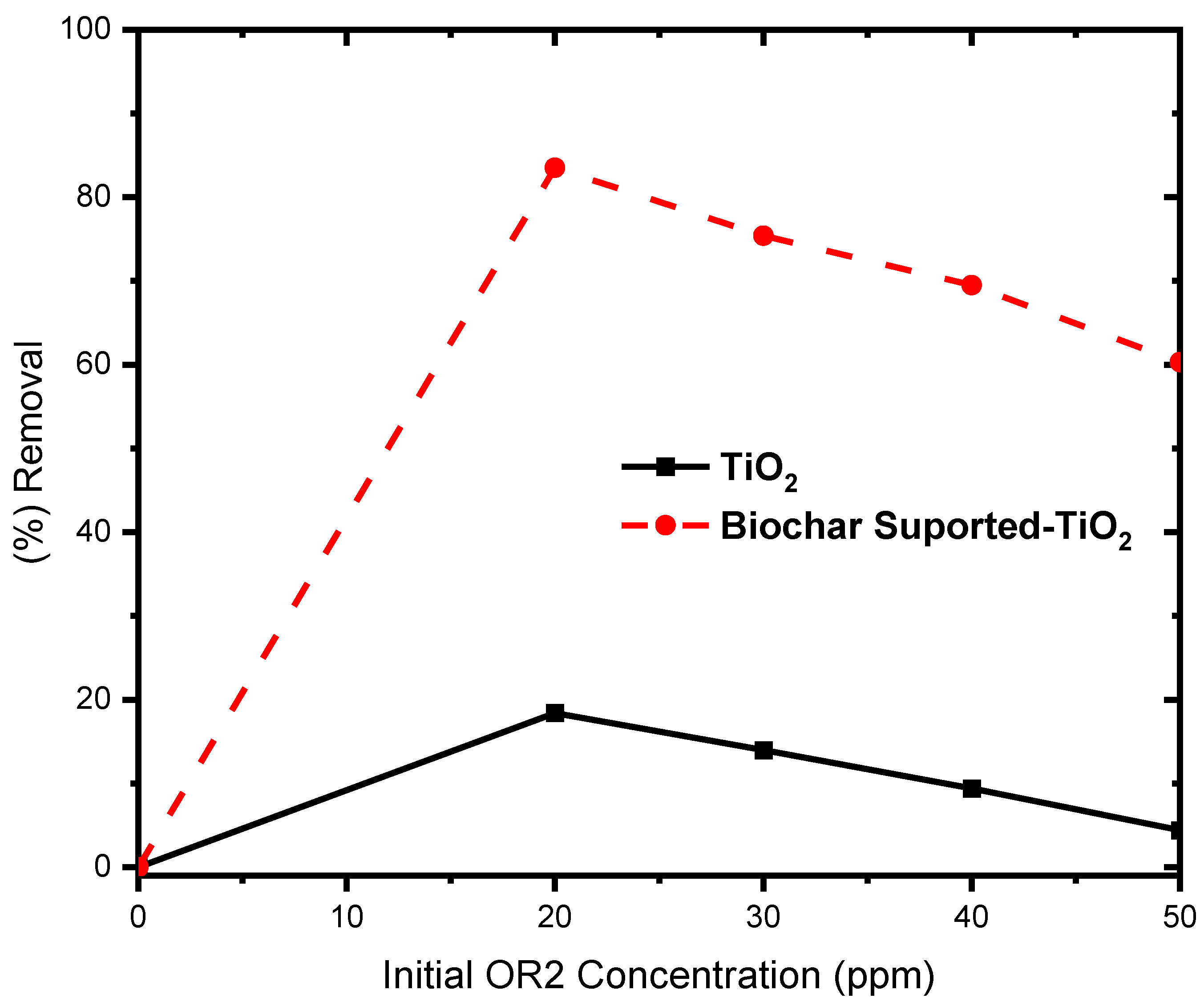
5.11. Effect of Initial Dye Concentration
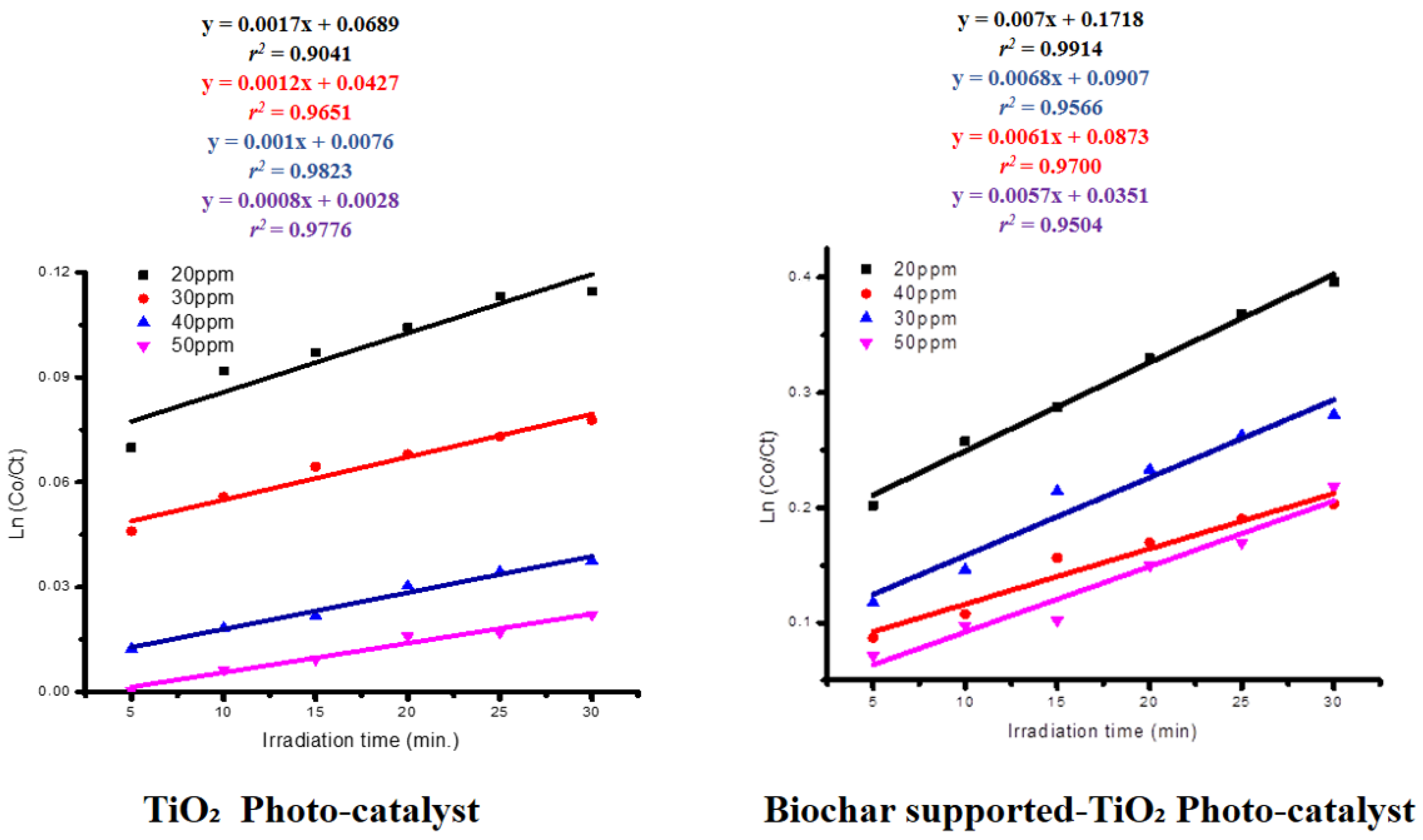
5.12. Kinetics of the Photodegradation of Orange II Sodium Dye
5.13. Electrical Energy Efficiency Per Order (EEo) of the Photocatalytic Degradation of OR2
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeribi, F.; Attaf, A.; Derbali, A.; Saidi, H.; Benmebrouk, L.; Aida, M.S.; Dahnoun, M.; Nouadji, R.; Ezzaouia, H. Dependence of the Physical Properties of Titanium Dioxide (TiO2) Thin Films Grown by Sol-Gel (Spin-Coating) Process on Thickness. ECS J. Solid State Sci. Technol. 2022, 11, 023003. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Hofierka, J.; Kaňuk, J. Assessment of photovoltaic potential in urban areas using open-source solar radiation tools. Renew. Energy 2009, 34, 2206–2214. [Google Scholar] [CrossRef]
- Rashed, M.N.; Eltaher, M.A.; Abdou, A.N.A. Adsorption and photocatalysis for methyl orange and Cd removal from wastewater using TiO2/sewage sludge-based activated carbon nanocomposites. R. Soc. Open Sci. 2017, 4, 170834. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhang, Q.; Liu, Y.; Shi, Y.; Qin, Z. Preparation of Fe3O4/TiO2 magnetic photocatalyst for photocatalytic degradation of phenol. J. Mater. Sci. Mater. Electron. 2018, 29, 8258–8266. [Google Scholar] [CrossRef]
- Hengerer, R.; Bolliger, B.; Erbudak, M.; Grätzel, M. Structure and stability of the anatase TiO2 (101) and (001) surfaces. Surf. Sci. 2000, 460, 162–169. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, S.; Wang, J.; Wang, C.; Wu, M. Characterization and computation of Yb/TiO2 and its photocatalytic degradation with benzohydroxamic acid. Int. J. Environ. Res. Public Health 2017, 14, 1471. [Google Scholar] [CrossRef]
- de Oliveira Pereira, L.; Marques Sales, I.; Pereira Zampiere, L.; Silveira Vieira, S.; do Rosário Guimarães, I.; Magalhães, F. Preparation of magnetic photocatalysts from TiO2, activated carbon and iron nitrate for environmental remediation. J. Photochem. Photobiol. A Chem. 2019, 382, 111907. [Google Scholar] [CrossRef]
- Avilés-García, O.; Espino-Valencia, J.; Romero-Romero, R.; Rico-Cerda, J.L.; Arroyo-Albiter, M.; Solís-Casados, D.A.; Natividad-Rangel, R. Enhanced Photocatalytic Activity of Titania by Co-Doping with Mo and W. Catalysts 2018, 8, 631. [Google Scholar] [CrossRef]
- Wong, T. ScienceDirect Smog induces oxidative stress and microbiota disruption. J. Food Drug Anal. 2017, 477, 1–10. [Google Scholar] [CrossRef]
- Jiang, W.; Pelaez, M.; Dionysiou, D.D.; Entezari, M.H.; Tsoutsou, D.; O’Shea, K. Chromium(VI) removal by maghemite nanoparticles. Chem. Eng. J. 2013, 222, 527–533. [Google Scholar] [CrossRef]
- Mahlambi, M.M.; Ngila, C.J.; Mamba, B.B. Recent Developments in Environmental Photocatalytic Degradation of Organic Pollutants: The Case of Titanium Dioxide Nanoparticles-A Review. J. Nanomater. 2015, 2015, 790173. [Google Scholar] [CrossRef]
- Badmus, K.O.; Wewers, F.; Al-Abri, M.; Shahbaaz, M.; Petrik, L.F. Synthesis of oxygen deficient TiO2 for improved photocatalytic efficiency in solar radiation. Catalysts 2021, 11, 904. [Google Scholar] [CrossRef]
- Bourquard, F.; Bleu, Y.; Loir, A.-S.; Caja-Munoz, B.; Avila, J.; Asensio, M.-C.; Raimondi, G.; Shokouhi, M.; Rassas, I.; Farre, C.; et al. Electroanalytical Performance of Nitrogen-Doped Graphene Films Processed in One Step by Pulsed Laser Deposition Directly Coupled with Thermal Annealing. Materials 2019, 12, 666. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.M.; Ghasemi, N. Influence of temperature and concentration on biosynthesis and characterization of zinc oxide nanoparticles using cherry extract. J. Nanostructure Chem. 2018, 8, 93–102. [Google Scholar] [CrossRef]
- Hejri, Z.; Hejri, M.; Omidvar, M.; Morshedi, S. Synthesis of TiO2/nZVI nanocomposite for nitrate removal from aqueous solution. Int. J. Ind. Chem. 2019, 10, 225–236. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium dioxide: From engineering to applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Yanhui, A.; Wang, K.; Wang, P.; Wang, C.; Hou, J. Synthesis of novel 2D-2D p-n heterojunction BiOBr/La2Ti2O7 composite photocatalyst with enhanced photocatalytic performance under both UV and visible light irradiation. Appl. Catal. B Environ. 2016, 194, 157–168. [Google Scholar] [CrossRef]
- Abel, S.; Jule, L.T.; Belay, F.; Shanmugam, R.; Dwarampudi, L.P.; Nagaprasad, N.; Krishnaraj, R. Application of Titanium Dioxide Nanoparticles Synthesized by Sol-Gel Methods in Wastewater Treatment. J. Nanomater. 2021, 2021, 3039761. [Google Scholar] [CrossRef]
- Castro, A.M.; Lopes, I.; Rocha-santos, T. Evaluation of the Potential Toxicity of Effluents from the Textile Industry before and after Treatment. Appl. Sci. 2019, 9, 3804. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Subramani, A.K.; Byrappa, K.; Ananda, S.; Lokanatha Rai, K.M.; Ranganathaiah, C.; Yoshimura, M. Photocatalytic degradation of indigo carmine dye using TiO2 impregnated activated carbon. Bull. Mater. Sci. 2007, 30, 37–41. [Google Scholar] [CrossRef]
- Singh, P.; Vishnu, M.C.; Sharma, K.K.; Singh, R.; Madhav, S.; Tiwary, D.; Mishra, P.K. Comparative study of dye degradation using TiO2-activated carbon nanocomposites as catalysts in photocatalytic, sonocatalytic, and photosonocatalytic reactor. Desalin. Water Treat. 2016, 57, 20552–20564. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Tran, H.N.; Fu, C.C.; Lu, Y.T.; Juang, R.S. Roles of adsorption and photocatalysis in removing organic pollutants from water by activated carbon–supported titania composites: Kinetic aspects. J. Taiwan Inst. Chem. Eng. 2020, 109, 51–61. [Google Scholar] [CrossRef]
- Mondol, B.; Sarker, A.; Shareque, A.M.; Dey, S.C.; Islam, M.T.; Das, A.K.; Shamsuddin, S.M.; Molla, M.A.I.; Sarker, M. Preparation of Activated Carbon/TiO2 Nanohybrids for Photodegradation of Reactive Red-35 Dye Using Sunlight. Photochem 2021, 1, 54–66. [Google Scholar] [CrossRef]
- Lian, W.; Yang, L.; Joseph, S.; Shi, W.; Bian, R.; Zheng, J.; Li, L.; Shan, S.; Pan, G. Utilization of biochar produced from invasive plant species to efficiently adsorb Cd (II) and Pb (II). Bioresour. Technol. 2020, 317, 124011. [Google Scholar] [CrossRef]
- Xu, J.; Cui, K.; Gong, T.; Zhang, J.; Zhai, Z.; Hou, L.; Zaman, F.U.; Yuan, C. Ultrasonic-Assisted Synthesis of N-Doped, Multicolor Carbon Dots toward Fluorescent Inks, Fluorescence Sensors, and Logic Gate Operations. Nanomaterials 2022, 12, 312. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Nguyen, H.T.; Pham, T.D.; Tran, T.D.; Chu, H.T.; Dang, H.T.; Nguyen, V.H.; Nguyen, K.M.; Pham, T.T.; Van der Bruggen, B. UV–Visible Light Driven Photocatalytic Degradation of Ciprofloxacin by N,S Co-doped TiO2: The Effect of Operational Parameters. Top. Catal. 2020, 63, 985–995. [Google Scholar] [CrossRef]
- Pudza, M.Y.; Abidin, Z.Z.; Rashid, S.A.; Yasin, F.M.; Noor, A.S.M.; Issa, M.A. Eco-friendly sustainable fluorescent carbon dots for the adsorption of heavy metal ions in aqueous environment. Nanomaterials 2020, 10, 315. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Eppa, R.; Durgam, K.; Ramana, M.V.; Reddy, S.R.; Sivakumar, J. Structural, Optical and Photocatalytic Properties of Anatase/Rutile TiO2 Nanoparticles. i-Manag. J. Mater. Sci. 2018, 6, 43. [Google Scholar] [CrossRef]
- Wang, X.; Cai, W.; Lin, Y.; Wang, G.; Liang, C. Mass production of micro/nanostructured porous ZnO plates and their strong structurally enhanced and selective adsorption performance for environmental remediation. J. Mater. Chem. 2010, 20, 8582–8590. [Google Scholar] [CrossRef]
- Zhang, F. Grand Challenges for Nanoscience and Nanotechnology in Energy and Health. Front. Chem. 2017, 5, 80. [Google Scholar] [CrossRef]
- Tong, Y.; Shi, G.; Hu, G.; Hu, X.; Han, L.; Xie, X.; Xu, Y.; Zhang, R.; Sun, J.; Zhong, J. Photo-catalyzed TiO2 inactivates pathogenic viruses by attacking viral genome. Chem. Eng. J. 2021, 414, 128788. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Pittman, C.U.; Mohan, D. Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J. Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Mehmood, M.; Ahmed, J.; Nizam, M.N. Synthesis of graphitic nanofibers and carbon nanotubes by catalytic chemical vapor deposition method on nickel chloride alcogel for high oxygen evolution reaction activity in alkaline media. Nano-Struct. Nano-Objects 2020, 24, 100574. [Google Scholar] [CrossRef]
- Bhati, M.; Rai, R. Nanotechnology and water purification: Indian know-how and challenges. Environ. Sci. Pollut. Res. 2017, 24, 23423–23435. [Google Scholar] [CrossRef]
- Madhvi; Singh, L.; Saroj, S.; Lee, Y.; Singh, S.V. Facile synthesis of nano-crystalline anatase TiO2 and their applications in degradation of Direct blue 199. J. Mater. Sci. Mater. Electron. 2016, 27, 2581–2588. [Google Scholar] [CrossRef]
- Guaraldo, T.T.; Vakili, R.; Wenk, J.; Mattia, D. Highly efficient ZnO photocatalytic foam reactors for micropollutant degradation. Chem. Eng. J. 2023, 455, 140784. [Google Scholar] [CrossRef]



| Weight of TiO2 (mg) | Weight of Biochar (mg) | Ratio | Energy Bandgap (eV) |
|---|---|---|---|
| 200 | 50 | 4:1 | 2.52 |
| 200 | 100 | 2:1 | 2.50 |
| 200 | 200 | 1:1 | 2.49 |
| 200 | 300 | 2:3 | 1.96 |
| 200 | 400 | 1:2 | 2.12 |
| 200 | 500 | 2:5 | 2.33 |
| Pyrolysis | Yield (%) | Carbon (%) | Oxygen (%) | Other Elements (%) |
|---|---|---|---|---|
| Time (h) | ||||
| 1 | 68.90 | 78.62 | 19.05 | 2.33 |
| 2 | 70.90 | 75.11 | 22.26 | 2.63 |
| 3 | 70.58 | 77.57 | 19.30 | 3.14 |
| Elements | TiO2 (Wt %) | Biochar-Supported TiO2 (Wt %) |
|---|---|---|
| Titanium | 43.45 | 24.29 |
| Oxygen | 41.40 | 39.71 |
| Carbon | 15.14 | 34.59 |
| K | - | 0.61 |
| OR2 Concentration (ppm) | Amount of Photocatalyst (mg/L) | Time (min) | OR2 Solution pH |
|---|---|---|---|
| 20 | 50 | 60 | 6.8 |
| 30 | 50 | 60 | 6.8 |
| 40 | 50 | 60 | 6.8 |
| 50 | 50 | 60 | 6.8 |
| 20 | 50 | 60 | 6.8 |
| 20 | 100 | 60 | 6.8 |
| 20 | 150 | 60 | 6.8 |
| 20 | 200 | 60 | 6.8 |
| 20 | 50 | 5 | 6.8 |
| 20 | 50 | 10 | 6.8 |
| 20 | 50 | 15 | 6.8 |
| 20 | 50 | 20 | 6.8 |
| 20 | 50 | 60 | 4.0 |
| 20 | 50 | 60 | 6.0 |
| 20 | 50 | 60 | 8.0 |
| 20 | 50 | 60 | 10.0 |
| Photocatalyst | OR2 Concentration (ppm) | K₁ × 10−3 (s−1) | r2 | 1/K (s) |
|---|---|---|---|---|
| Synthesized Titanium Oxide Nanoparticles | 20 | 10.2 | 0.9041 | 98.3 |
| 30 | 7.5 | 0.9651 | 132.7 | |
| 40 | 4.9 | 0.9823 | 202.2 | |
| 50 | 2.2 | 0.9776 | 442.8 | |
| Biochar-Supported Titanium Oxide Nanoparticles | 20 | 15 | 0.9914 | 66.7 |
| 30 | 13.2 | 0.9566 | 75.8 | |
| 40 | 9.8 | 0.9700 | 102 | |
| 50 | 7.8 | 0.9504 | 128.2 |
| Photocatalyst | pH | Removal (%) | K × 10−3 (min−1) | r2 | EEO (kWh/m3) |
|---|---|---|---|---|---|
| Synthesized Titanium Oxide Nanoparticles | 4 | 20.75 | 1.94 | 0.7170 | 1056.75 |
| 6.8 | 18.77 | 1.73 | 0.7719 | 1182.19 | |
| 8 | 16.32 | 1.49 | 0.8766 | 1379.36 | |
| 10 | 12.96 | 1.16 | 0.8273 | 1770.58 | |
| Biochar-Supported Titanium Oxide Nanoparticles | 4 | 81.73 | 14.17 | 0.9549 | 144.57 |
| 6.8 | 83.48 | 15.01 | 0.9525 | 136.49 | |
| 8 | 77.81 | 12.53 | 0.9677 | 163,39 | |
| 10 | 70.03 | 10.94 | 0.9435 | 187.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanku, L.; Badmus, K.O.; Wewers, F. Biochar-Supported Titanium Oxide for the Photocatalytic Treatment of Orange II Sodium Salt. Appl. Nano 2024, 5, 190-204. https://doi.org/10.3390/applnano5030013
Kanku L, Badmus KO, Wewers F. Biochar-Supported Titanium Oxide for the Photocatalytic Treatment of Orange II Sodium Salt. Applied Nano. 2024; 5(3):190-204. https://doi.org/10.3390/applnano5030013
Chicago/Turabian StyleKanku, Laury, Kassim Olasunkanmi Badmus, and Fracois Wewers. 2024. "Biochar-Supported Titanium Oxide for the Photocatalytic Treatment of Orange II Sodium Salt" Applied Nano 5, no. 3: 190-204. https://doi.org/10.3390/applnano5030013
APA StyleKanku, L., Badmus, K. O., & Wewers, F. (2024). Biochar-Supported Titanium Oxide for the Photocatalytic Treatment of Orange II Sodium Salt. Applied Nano, 5(3), 190-204. https://doi.org/10.3390/applnano5030013







