Rapid Degradation of Organic Dyes by Nanostructured Gd2O3 Microspheres
Abstract
1. Introduction
2. Materials and Methods
3. Results
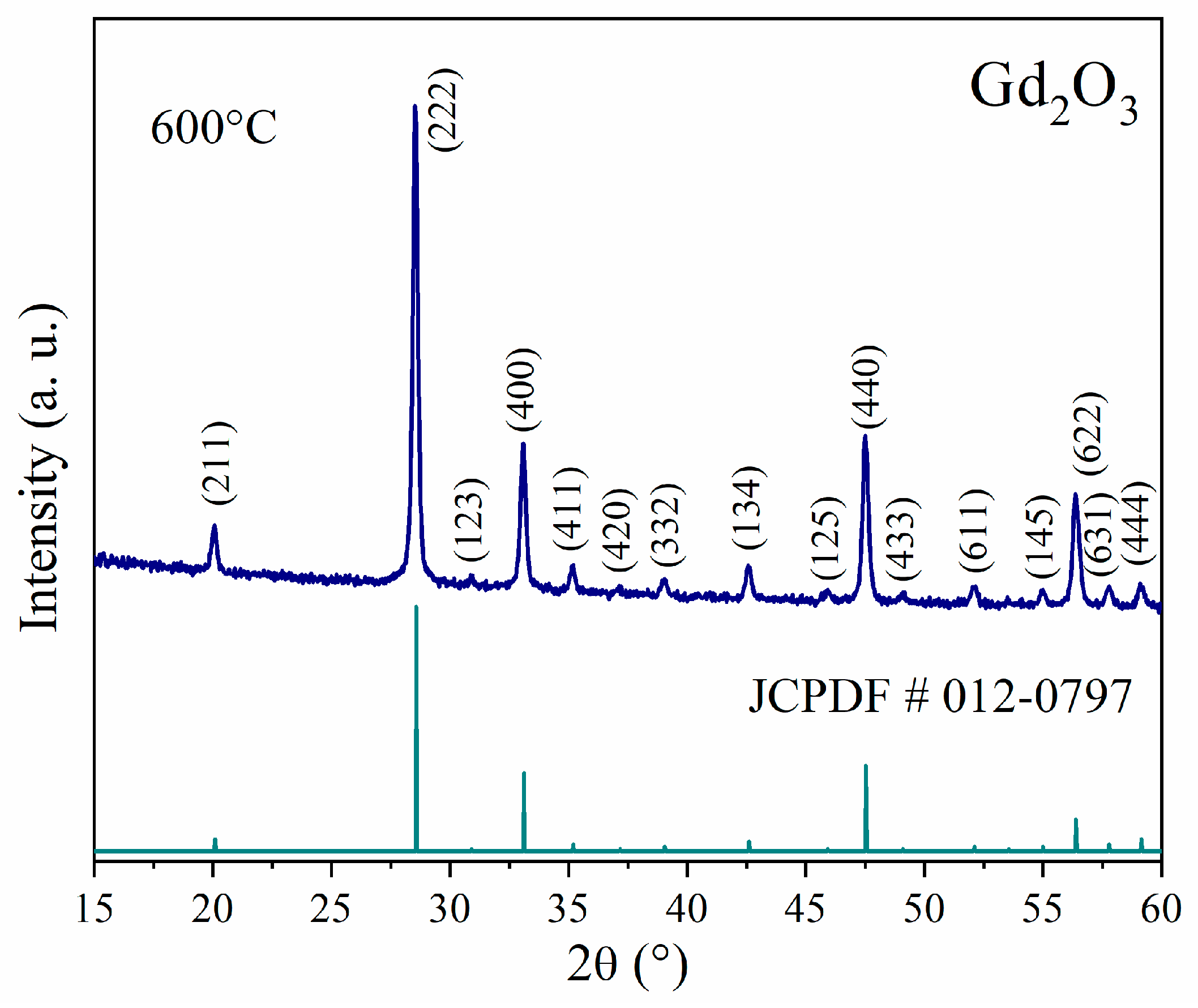
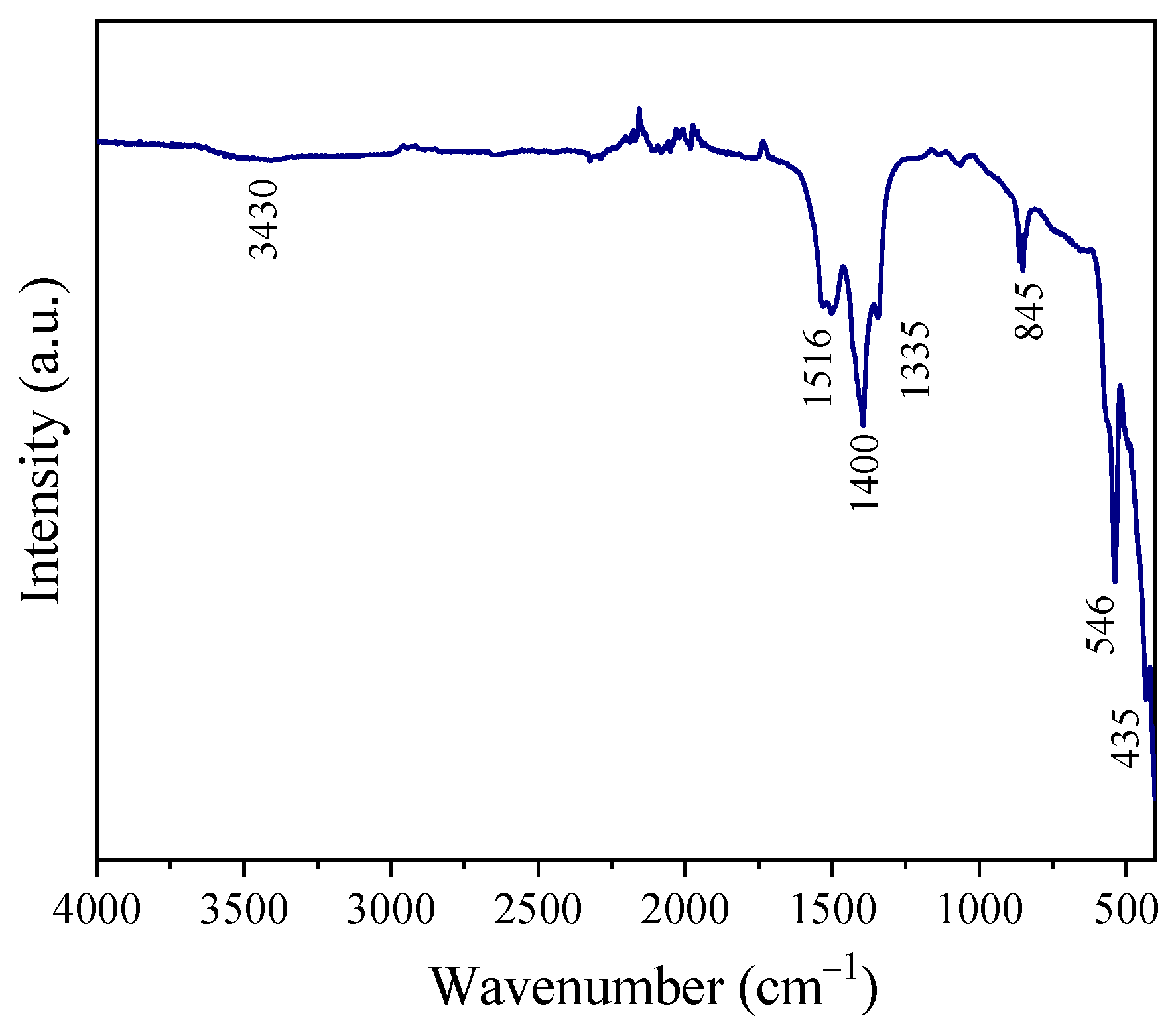
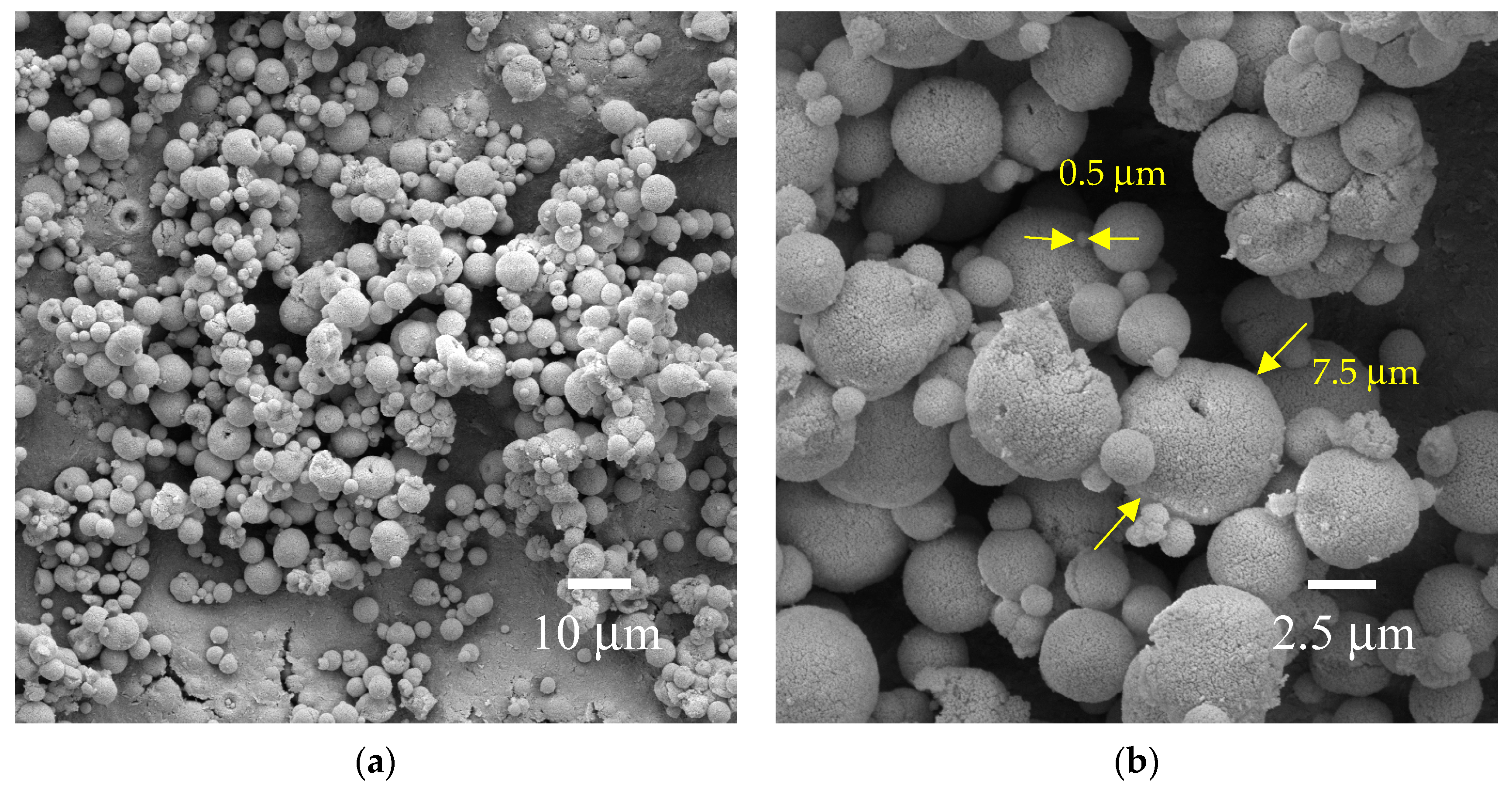
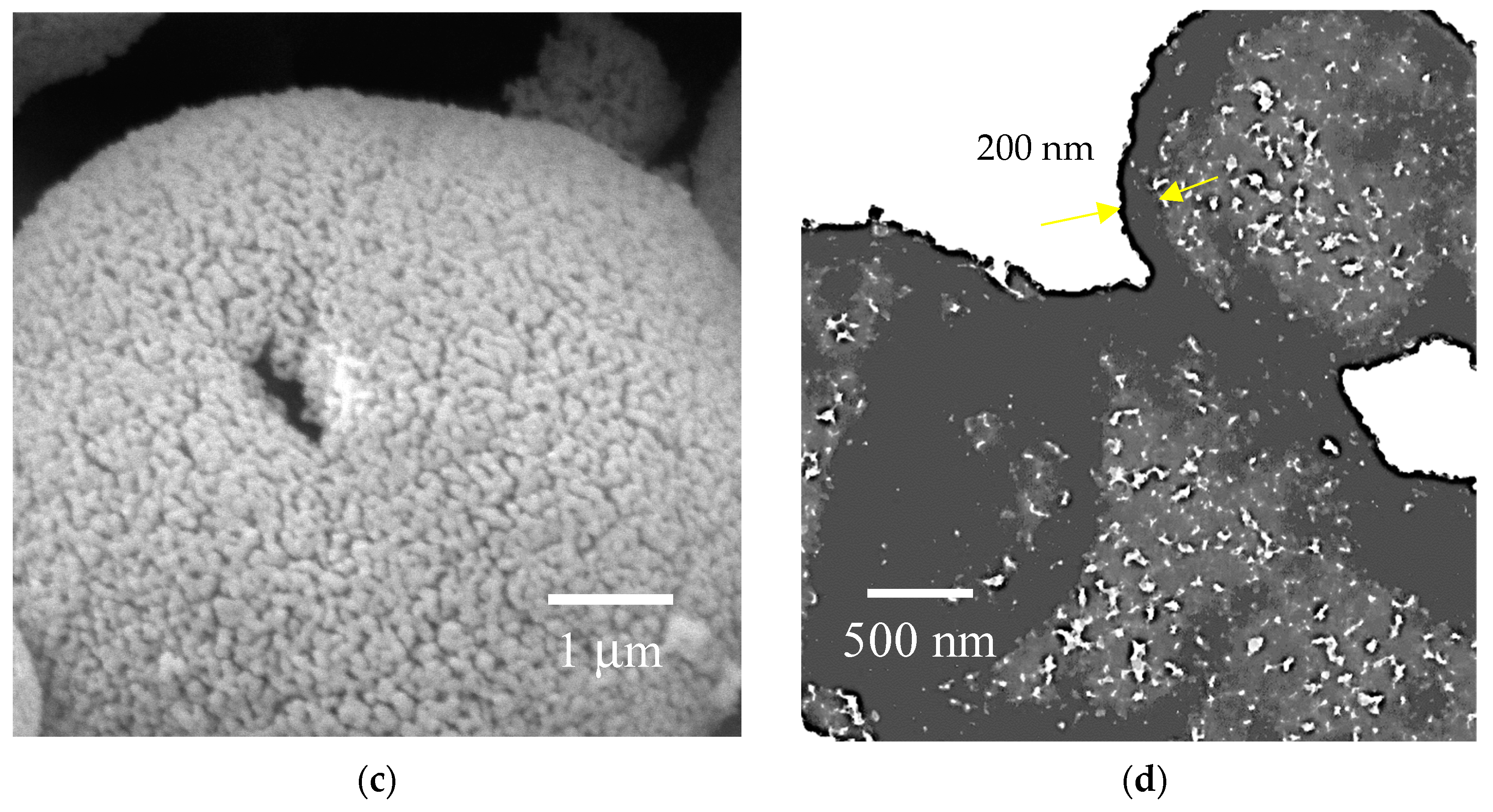
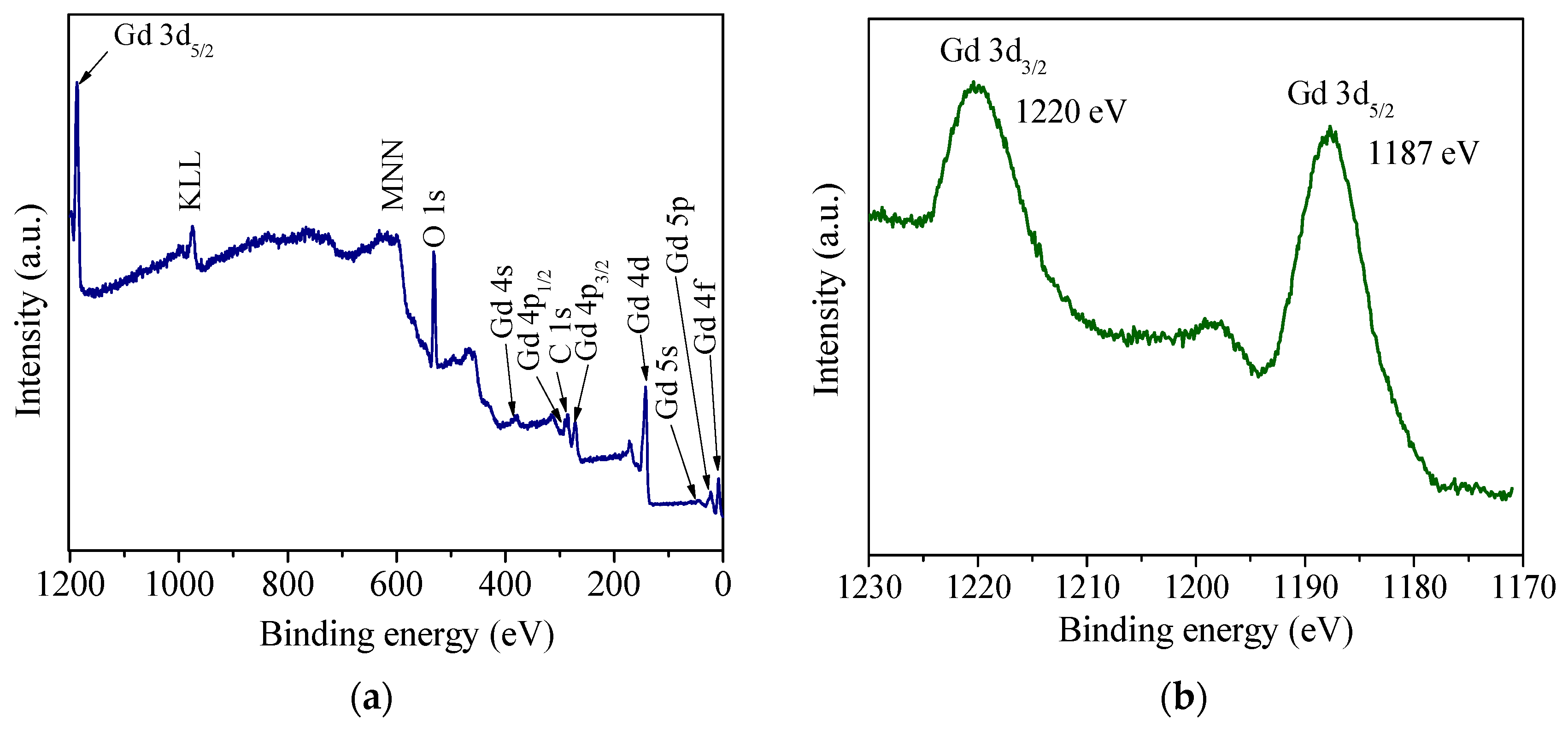
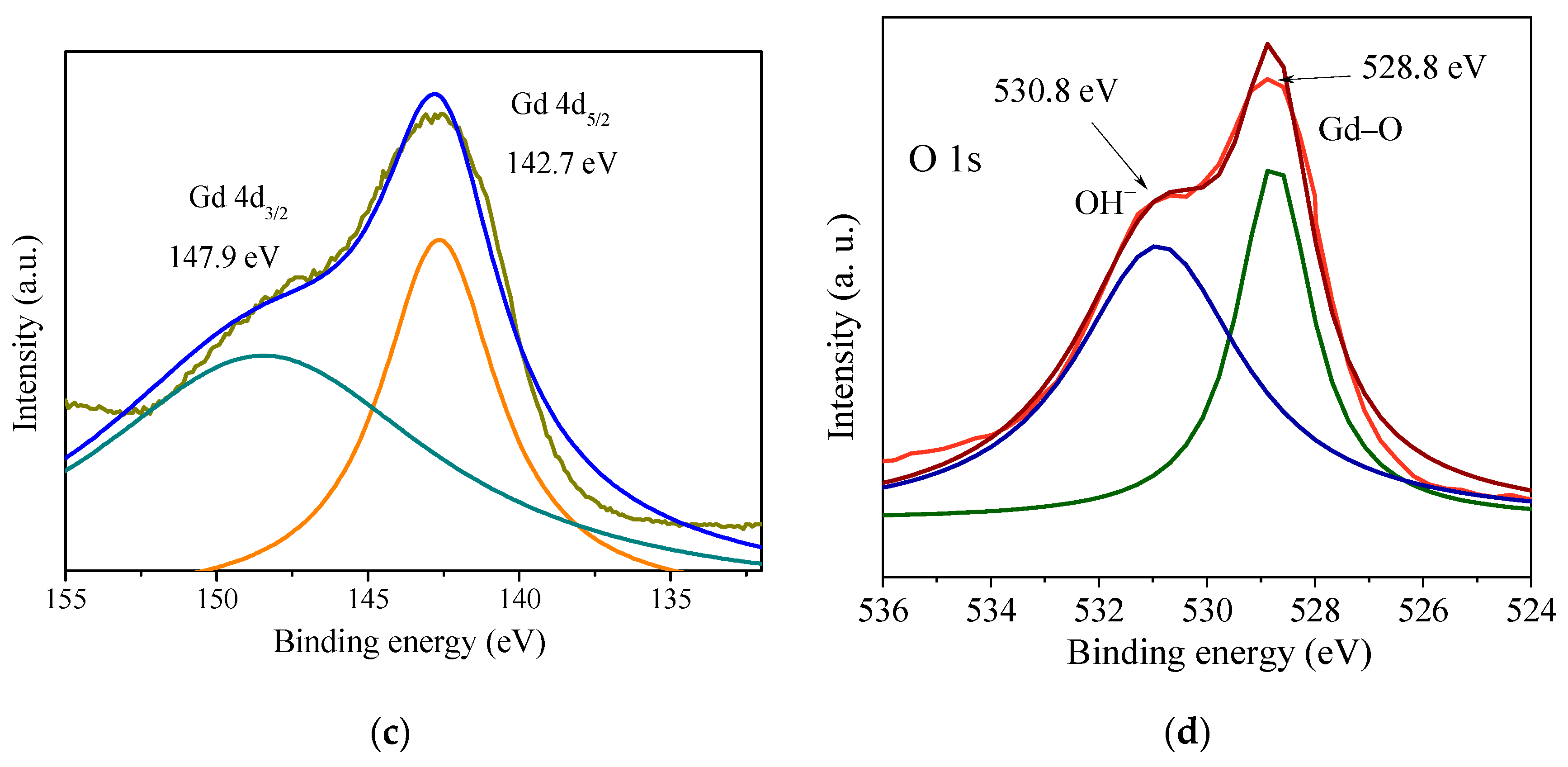
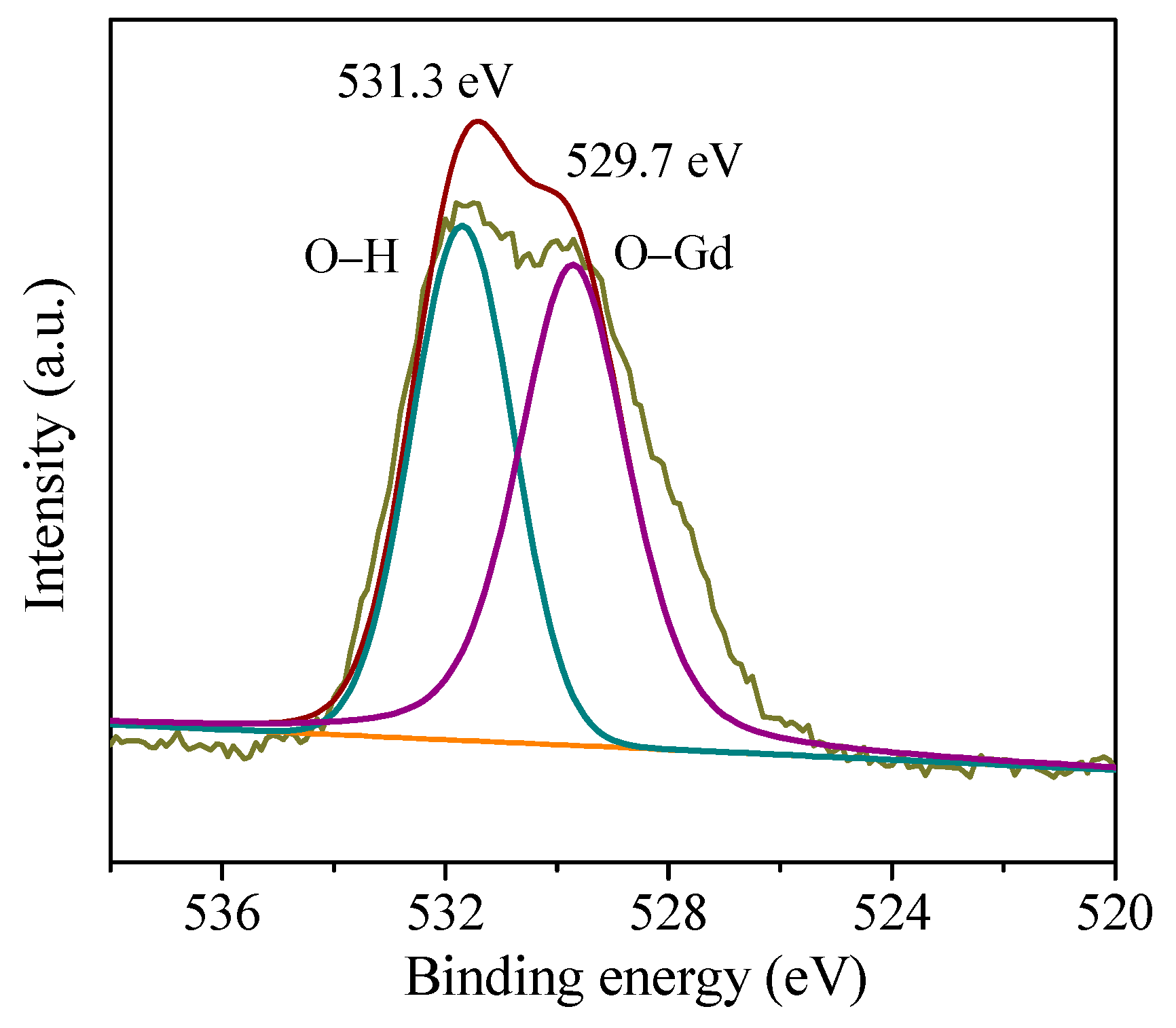
3.1. Crystal Structure, Morphology, and Surface Chemical Composition
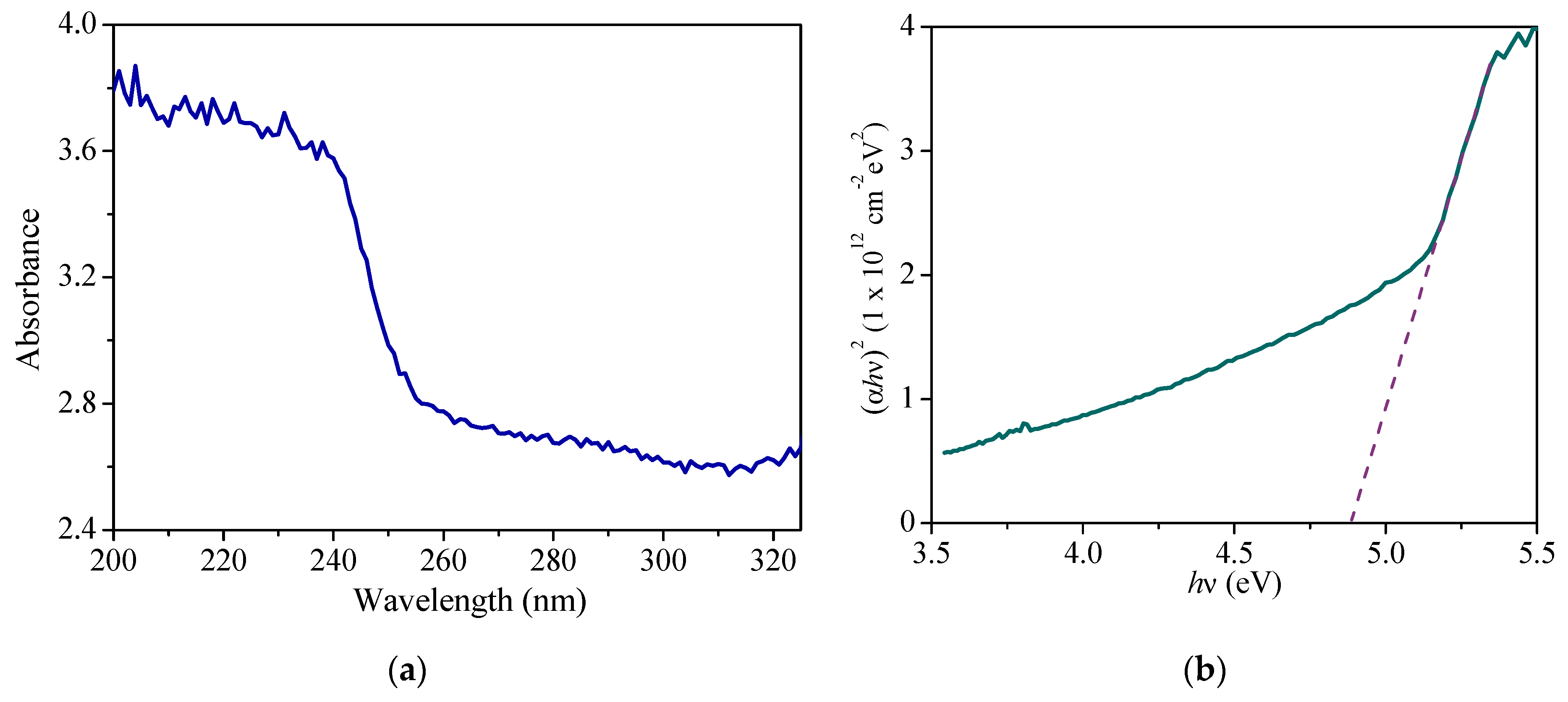
3.2. Determination of the Bandgap Energy
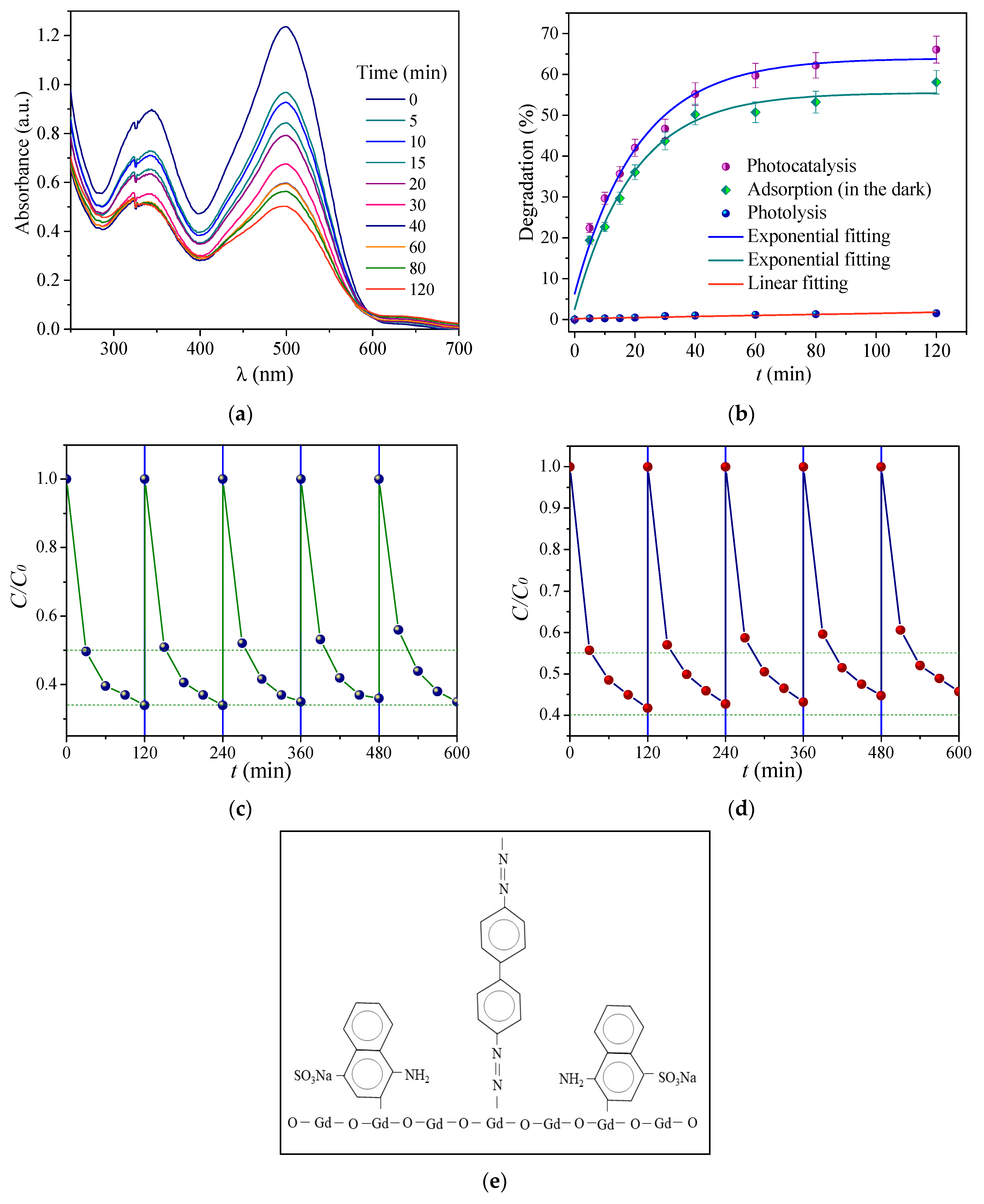
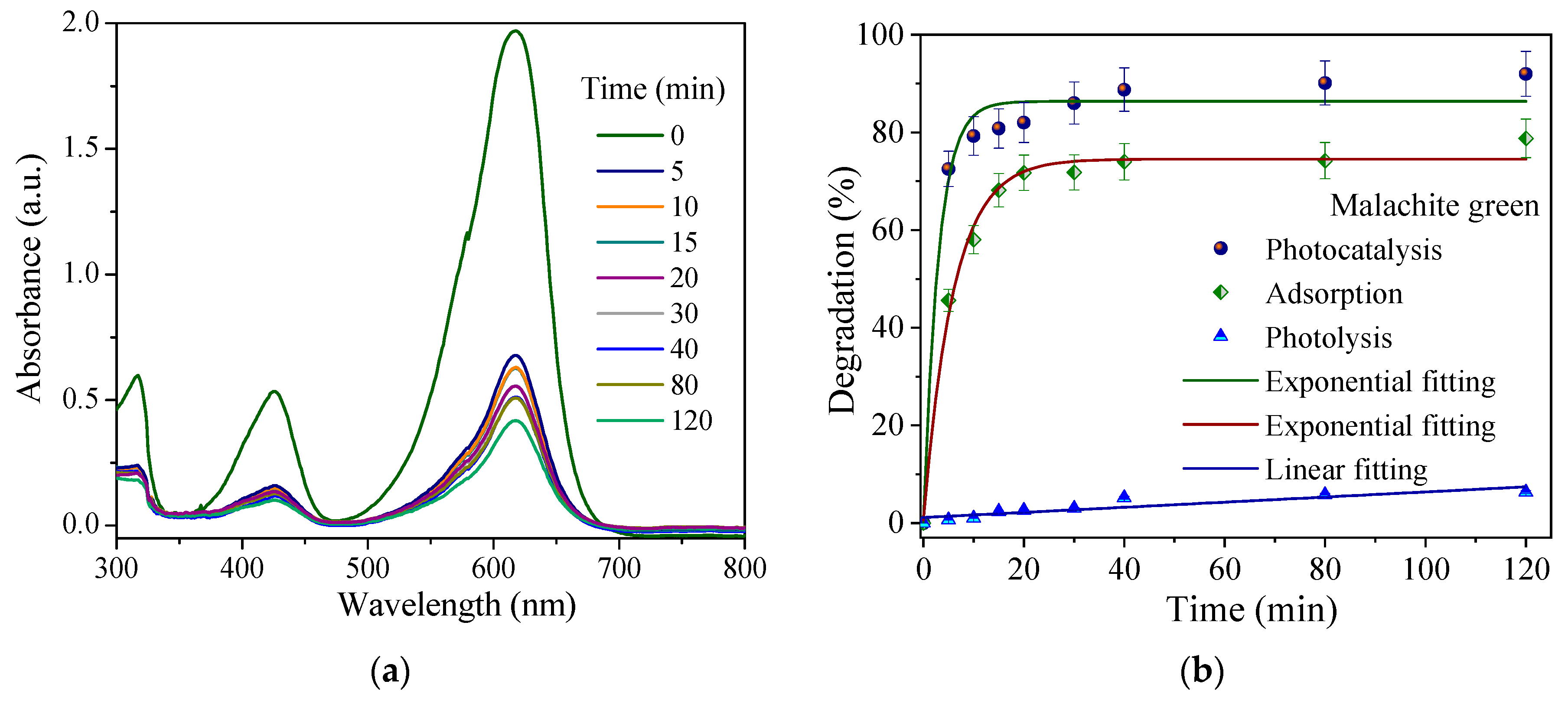
3.3. Degradation of Congo Red and Malachite Green
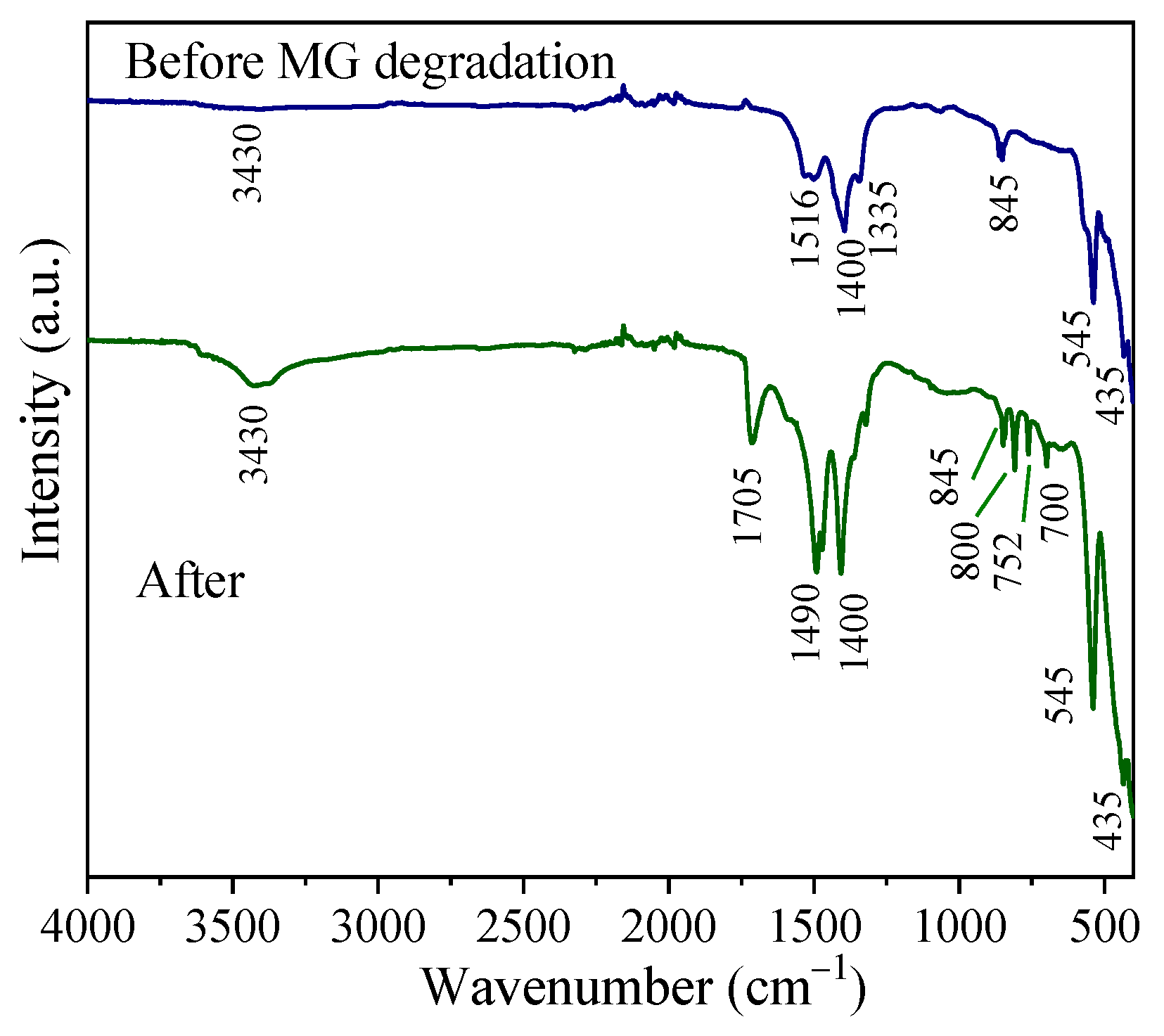
3.4. XPS and FTIR Spectra of Samples After Degradation
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scarel, G.; Svane, A.; Fanciulli, M. Scientific and technological issues related to rare earth oxides: An introduction. In Rare Earth Oxide Thin Films; Fanciulli, M., Scarel, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–14. [Google Scholar] [CrossRef]
- Saha, A.; Devi, P.S. Surface functionalized multifunctional Gd2O3—Fluorescein composite nanorods for redox responsive drug delivery and imaging applications. ACS Appl. Nano Mater. 2018, 1, 2898–2911. [Google Scholar] [CrossRef]
- Tamrakar, R.K.; Bisen, D.P.; Brahme, N. Comparasion of photoluminescence properties of Gd2O3 phosphor synthesized by combustion and solid state reaction method. J. Radiat. Res. Appl. Sci. 2014, 7, 550–559. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Sharma, S.; Nath, M.; Karmakar, A.; Kumari, K.; Sutradhar, S. Dielectric study and magnetic property analysis of Gd2O3 nanorods/nanowire in combination with Monte Carlo simulation. J. Alloys Compd. 2021, 882, 160720. [Google Scholar] [CrossRef]
- Michel, C.R.; López, N.L.; Martínez-Preciado, A.H. Gas sensing properties of Gd2O3 microspheres prepared in aqueous media containing pectin. Sens. Actuatuators B. 2013, 177, 390–396. [Google Scholar] [CrossRef]
- Ning, L.; Liao, S.; Dong, C.; Zhang, M.; Gu, W.; Liu, X. Rare earth oxide anchored platinum catalytic site coated zeolitic imidazolate frameworks toward enhancing selective hydrogenation. ACS Appl. Mater. Interfaces 2020, 12, 7198–7205. [Google Scholar] [CrossRef]
- Druon, F.; Velázquez, M.; Veber, P.; Janicot, S.; Viraphong, O.; Buşe, G.; Ahmed, M.A.; Graf, T.; Rytz, D.; Georges, P. Laser demonstration with highly doped Yb:Gd2O3 and Yb:Y2O3 crystals grown by an original flux method. Opt. Lett. 2013, 38, 4146–4149. [Google Scholar] [CrossRef]
- Kuznetsova, Y.A.; Zatsepin, A.F.; Gavrilov, N.V. The high refractive index of Gd2O3 thin films obtained by magnetron sputtering. Opt. Mater. 2021, 120, 111382. [Google Scholar] [CrossRef]
- Külah, E.; Marot, L.; Steiner, R.; Romanyuk, A.; Jung, T.A.; Wäckerlin, A.; Meyer, E. Surface chemistry of rare-earth oxide surfaces at ambient conditions: Reactions with water and hydrocarbons. Sci. Rep. 2017, 7, 43369. [Google Scholar] [CrossRef]
- Jia, G.; Liu, K.; Zheng, Y.; Song, Y.; Yang, M.; You, H. Highly uniform Gd(OH)3 and Gd2O3:Eu3+ nanotubes: Facile synthesis and luminescence properties. J. Phys. Chem. C 2009, 113, 6050–6055. [Google Scholar] [CrossRef]
- Jegadeesan, P.; Sen, S.; Padmaprabu, C.; Srivastava, S.K.; Das, A.; Amirthapandian, S. Morphological and optical investigations on Gd2O3 nanostructures. Inorg. Chem. Commun. 2023, 150, 110493. [Google Scholar] [CrossRef]
- Kang, J.G.; Min, B.K.; Sohn, Y. Synthesis and characterization of Gd(OH)3 and Gd2O3 nanorods. Ceram. Int. 2015, 41, 1243–1248. [Google Scholar] [CrossRef]
- Xiao, H.; Li, P.; Jia, F.; Zhang, L. General nonaqueous sol-gel synthesis of nanostructured Sm2O3, Gd2O3, Dy2O3, and Gd2O3:Eu3+ phosphor. J. Phys. Chem. C 2009, 113, 21034–21041. [Google Scholar] [CrossRef]
- Huang, C.C.; Liu, T.Y.; Su, C.H.; Lo, Y.W.; Chen, J.H.; Yeh, C.S. Superparamagnetic hollow and paramagnetic porous Gd2O3 particles. Chem. Mater. 2008, 20, 3840–3848. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Y.; Li, H.; Duosiken, D.; Tang, N.; Sun, K.; Tao, K. Revisiting the factors influencing the magnetic resonance contrast of Gd2O3 nanoparticles. Nanoscale Adv. 2022, 4, 95–101. [Google Scholar] [CrossRef]
- Ortega-Berlanga, B.; Betancourt-Mendiola, L.; Del Angel-Olarte, C.; Hernández-Adame, L.; Rosales-Mendoza, S.; Palestino, G. An overview of gadolinium-based oxide and oxysulfide particles: Synthesis, properties, and biomedical applications. Crystals 2021, 11, 1094. [Google Scholar] [CrossRef]
- Jenks, W.S. Photocatalytic reaction pathways. In Photocatalysis and Water Purification; Pichat, P., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 25–52. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, L.; Yao, C.; Zhang, F.; Zhang, J.; Li, C. Synthesis and characterization of Gd2O3 hollow microspheres using a template-directed method. Materials 2016, 9, 323. [Google Scholar] [CrossRef]
- Jeon, S.; Ko, J.-W.; Ko, W.-B. Synthesis of Gd2O3 nanoparticles and their photocatalytic activity for degradation of azo dyes. Catalysts 2021, 11, 742. [Google Scholar] [CrossRef]
- Dhanalakshmi, S.; Kumar, P.S.; Karuthapandian, S.; Muthuraj, V.; Prithivikumaran, N. Design of Gd2O3 nanorods: A challenging photocatalyst for the degradation of neurotoxicity chloramphenicol drug. J. Mater. Sci. Mater. Electron. 2019, 4, 3744–3752. [Google Scholar] [CrossRef]
- Eprasatya, A.; Yulizar, Y.; Yunarti, R.T.; Apriandanu, D.O.B. Fabrication of Gd2O3 nanoparticles in hexane-water system using Myristica fragrans Houtt leaves extract and their photodegradation activity of malachite green. IOP Conf. Ser. Mater. Sci. Eng. 2020, 902, 012004. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Zhang, D.; Yang, S. Structural, luminescence and magnetic properties of Yb3+-Er3+ codoped Gd2O3 hierarchical architectures. CrystEngComm 2015, 17, 1106–1114. [Google Scholar] [CrossRef]
- Guo, H.; Yang, X.; Xiao, T.; Zhang, W.; Lou, L.; Mugnier, J. Structure and optical properties of sol–gel derived Gd2O3 waveguide films. Appl. Surf. Sci. 2004, 230, 215–221. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Jeon, S.; Hwang, H. Effect of hygroscopic nature on the electrical characteristics of lanthanide oxides (Pr2O3, Sm2O3, Gd2O3 and Dy2O3). J. Appl. Phys. 2003, 93, 6393. [Google Scholar] [CrossRef]
- Lal, H.B.; Gaur, K.A. Electrical conduction in non-metallic rare-earth solids. J. Mater. Sci. 1988, 23, 919–923. [Google Scholar] [CrossRef]
- Prokofiev, A.M.; Shelykh, A.I.; Melekh, B.T. Periodicity in the band gap variation of Ln2X3 (X = O, S, Se) in the lanthanide series. J. Alloys Compd. 1996, 242, 41–44. [Google Scholar] [CrossRef]
- Gillen, R.; Clark, S.J.; Robertson, J. Nature of the electronic band gap in lanthanide oxides. Phys. Rev. B 2013, 87, 125116. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductors; Plenum Press: New York, NY, USA, 1974; pp. 171–186. [Google Scholar] [CrossRef]
- Yaneva, Z.L.; Georgieva, N.V. Insights into Congo red adsorption on agro-industrial materials-spectral, equilibrium, kinetic, thermodynamic, dynamic and desorption studies. A review. Int. Rev. Chem. Eng. 2012, 4, 127–146. [Google Scholar]
- Bernal, S.; Blanco, G.; Gatica, J.M.; Pérez-Omil, J.A.; Pintado, J.M.; Vidal, H. Chemical Reactivity of Binary Rare Earth Oxides. In Binary Rare Earth Oxides; Adachi, G., Imanaka, N., Kang, Z.C., Eds.; Kluwer: New York, NY, USA, 2004; pp. 9–15. [Google Scholar] [CrossRef]
- Do, C.; Ford, B.; Lee, D.Y.; Tan, C.; Escobar, P.; Wagner, B. Gadolinium-based contrast agents: Stimulators of myelo-idinduced renal fibrosis and major metabolic disruptors. Toxicol. Appl. Pharm. 2019, 375, 32–35. [Google Scholar] [CrossRef]
- Malhotra, N.; Hsu, H.S.; Liang, S.T.; Jmelou, M.; Roldan, M.; Lee, J.S.; Ger, T.R.; Hsiao, C.D. An updated review of toxicity effect of the rare earth elements (REEs) on aquatic organisms. Animals 2020, 10, 1663. [Google Scholar] [CrossRef]
- Cottrell, T.L. The Strengths of Chemical Bonds; Butterworth: London, UK, 1958; pp. 178–238. [Google Scholar]
- Kerr, J.A. Bond dissociation energies by kinetic methods. Chem. Rev. 1966, 66, 465–500. [Google Scholar] [CrossRef]
- Liu, S.; Cai, Y.; Cai, X.; Li, H.; Zhang, F.; Mua, Q.; Liu, Y.; Wang, Y. Catalytic photodegradation of Congo red in aqueous solution by Ln(OH)3(Ln = Nd, Sm, Eu, Gd, Tb, and Dy) nanorods. Appl. Catal. A Gen. 2013, 453, 45–53. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef] [PubMed]
- La, L.B.T.; Leong, Y.K.; Watts, H.P.; Au, P.-I.; Hayward, K.J.; Zhang, L.C. A novel approach for the preparation of nanosized Gd2O3 structure: The influence of surface force on the morphology of ball milled particles. Colloids Surface. A 2016, 506, 13–19. [Google Scholar] [CrossRef]
- Parks, G.A. The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr)oxides: 50 years after Parks’ review. Adv. Colloid Interface Sci. 2016, 238, 1–61. [Google Scholar] [CrossRef]
- Kosmulski, M. Chemical Properties of Material Surfaces; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Giacomo Fais, G.; Desogus, F.; Loy, F.; Licheri, R.; Lai, N.; Cao, G.; Concas, A. Eco-friendly photocatalytic treatment of dyes with Ag nanoparticles obtained through sustainable process involving Spirulina platensis. Sustainability 2024, 16, 8758. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding hydroxyl radical (•OH) generation processes in photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
| Method | Reagents | Morphology and Size | T (°C) | Ref. |
|---|---|---|---|---|
| Magnetron sputtering | Metallic Gd | Thin films 370–800 nm thick | 200 | [5] |
| Magnetron sputtering | Gd2O3 | Thin films 8–90 nm thick | 600 | [6] |
| Coprecipitation | Gd(NO3)3, HNO3, NaOH | Nanotubes of length (l) 200–300 nm, diameter (Ø) ∼40 nm | 800 | [7] |
| Hydrothermal | Gd(OH)3, NaOH | Microrods with Ø = 50–300 nm | 600 | [8] |
| Sol–gel | Gd(C5H7O2)3 | Microspheres, Ø = 50–300 nm | 500 | [9] |
| Sol–gel | GdCl3, Gd(C3H7O)3 | Hollow spheres, Ø ∼20 nm | 580 | [10] |
| Sol–gel | GdCl3, NH4OH | Nanoparticles, l = 50–200 nm | 1100 | [11] |
| Thermolysis | GdCl3, C18H34O2 | Nanospheres, Ø < 20 nm | 320 | [12] |
| Coprecipitation | Gd(NO3)3, CH2O2 | Microspheres, Ø = 0.5–7.5 μm | 600 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, C.R. Rapid Degradation of Organic Dyes by Nanostructured Gd2O3 Microspheres. Appl. Nano 2025, 6, 1. https://doi.org/10.3390/applnano6010001
Michel CR. Rapid Degradation of Organic Dyes by Nanostructured Gd2O3 Microspheres. Applied Nano. 2025; 6(1):1. https://doi.org/10.3390/applnano6010001
Chicago/Turabian StyleMichel, Carlos R. 2025. "Rapid Degradation of Organic Dyes by Nanostructured Gd2O3 Microspheres" Applied Nano 6, no. 1: 1. https://doi.org/10.3390/applnano6010001
APA StyleMichel, C. R. (2025). Rapid Degradation of Organic Dyes by Nanostructured Gd2O3 Microspheres. Applied Nano, 6(1), 1. https://doi.org/10.3390/applnano6010001







