Production and Properties of Quercetin-Loaded Liposomes and Their Influence on the Properties of Galactomannan-Based Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Development of the Liposomes
| Sample | Quercetin Diluted into the Ethanolic Solution of Lipoid S45 [µg/mL] |
| QT-LL 0 | 0 |
| QT-LL 25 | 25 |
| QT-LL 50 | 50 |
| QT-LL 75 | 75 |
| QT-LL 100 | 100 |
| QT-LL 125 | 125 |
| QT-LL 150 | 150 |
2.3. Quercetin Encapsulation Efficiency and Loading
2.4. Physicochemical Characterization of the Liposomes
2.5. Antioxidant Activity Assays
2.6. Film Characterization
2.6.1. Film Preparation
2.6.2. Film Thickness
2.6.3. Scanning Electron Microscopy
2.6.4. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6.5. X-ray Patterns
2.6.6. Color and Opacity
2.6.7. Moisture Content
2.6.8. Solubility
2.6.9. Water Vapor Permeability (WVP)
2.6.10. Contact Angle
2.7. Statistical Analyses
3. Results and Discussion
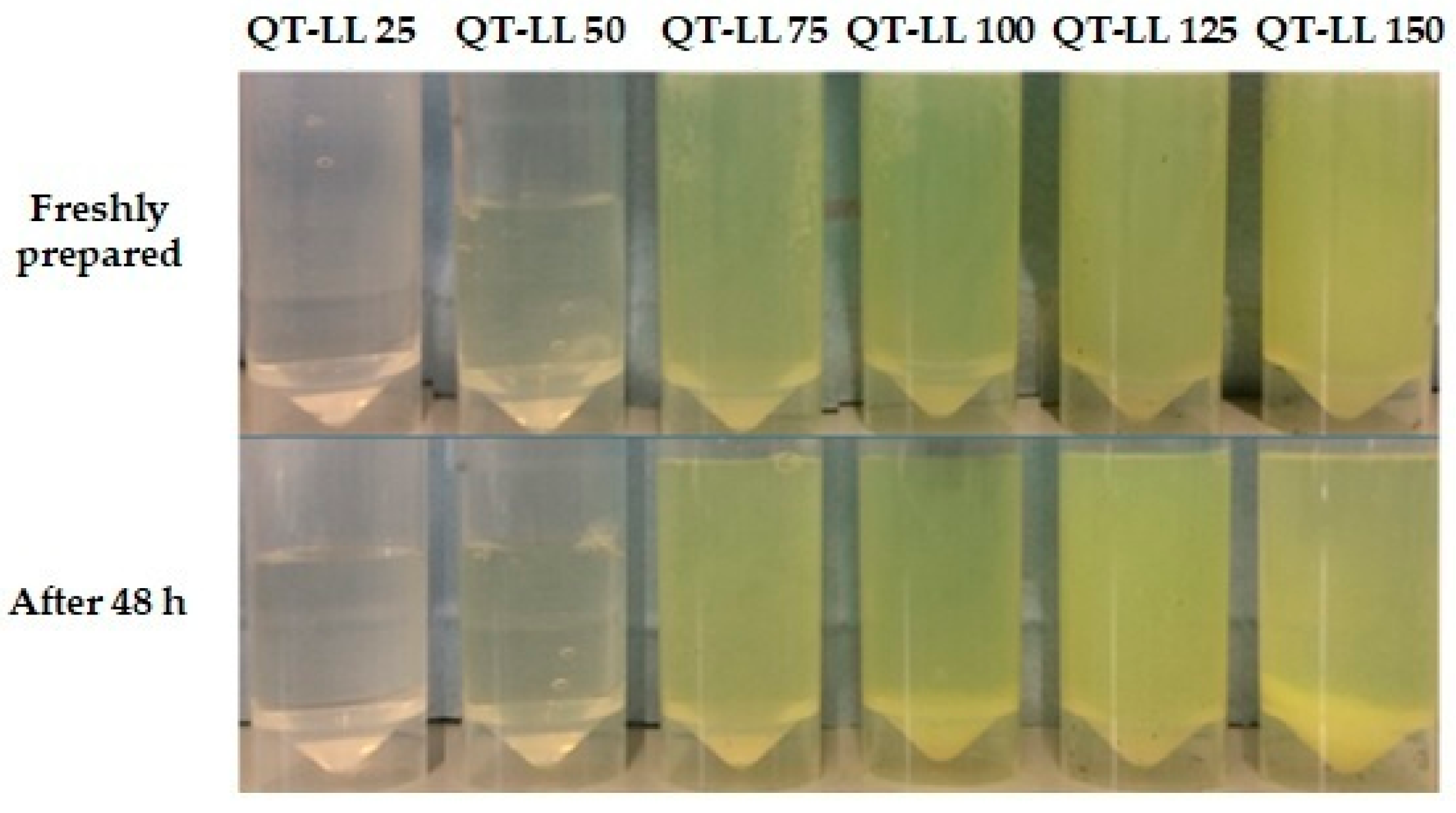
3.1. Liposomes’ Physicochemical Characterization
3.2. Antioxidant Activity Assays
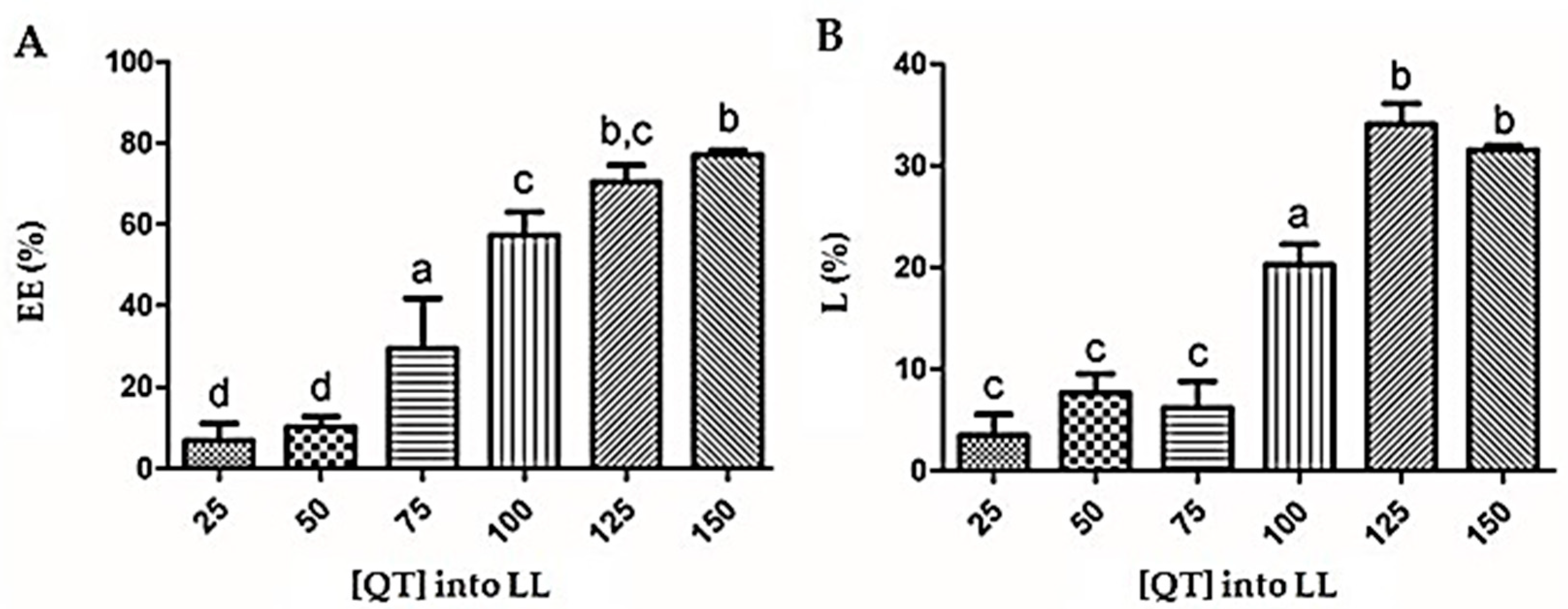
3.3. Quercetin Encapsulation Efficiency (EE %) and Loading Content (LC %)
3.4. Films’ Characterization
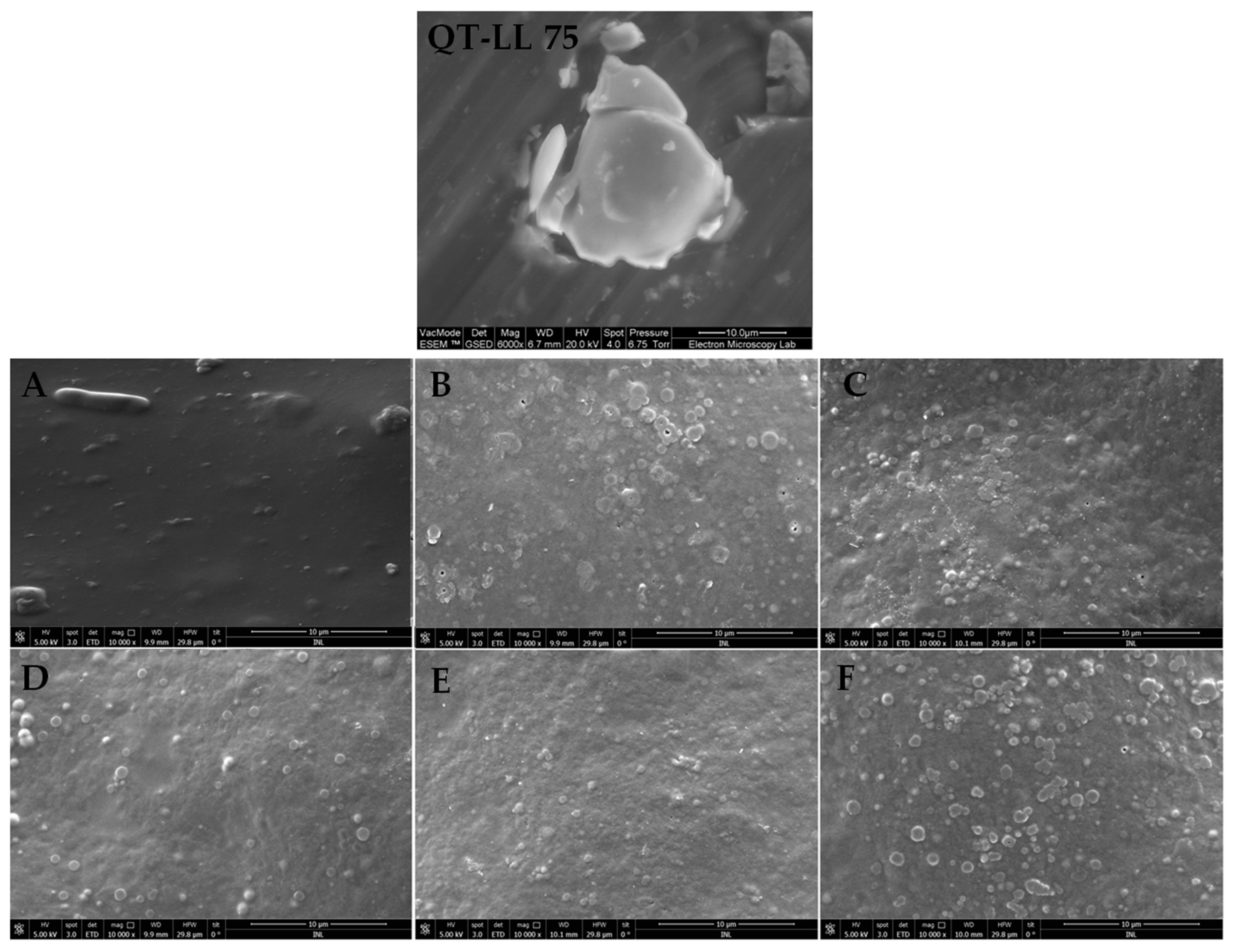
3.4.1. Scanning Electron Microscopy (SEM)
3.4.2. Fourier-Transform Infrared (FTIR) Spectroscopy
3.4.3. X-ray Patterns
3.4.4. Color and Opacity
3.5. Film Thickness, Moisture Content (MC), Solubility (S %), Water Vapor Permeability (WVP), and Contact Angle (CA)
- (1)
- Higher concentration of phospholipids, for example, the 60 mg/mL of Lecinova® or Lipoid S75 used for the development of liposomes by Gil et al. [18];
- (2)
- Other reagents and sophisticated equipment, as mentioned by AbouSamra, Elgohary, and Mansy [19] on the development of pirfenidone-loaded lecithin core nanocapsules, which included Span 60, Tween 80, and chloroform, in addition to a homogenizer that reached more than 20,000 rpm;
- (3)
- Longer experimental period, for example the stirring overnight of the suspensions containing whey lecithin, maltodextrin, eugenol, oleic acid, and chitosan; these suspensions also required a high-pressure homogenizer for complete homogenization [21].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albuquerque, P.B.S.; de Oliveira, W.F.; Silva, P.M.D.S.; Correia, M.T.D.S.; Kennedy, J.F.; Coelho, L.C.B.B. Epiphanies of well-known and newly discovered macromolecular carbohydrates—A review. Int. J. Biol. Macromol. 2020, 156, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.B.; Barros, W.; Santos, G.R.; Correia, M.T.; Mourão, P.A.; Teixeira, J.A.; Carneiro-Da-Cunha, M.G. Characterization and rheological study of the galactomannan extracted from seeds of Cassia grandis. Carbohydr. Polym. 2014, 104, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.B.; Cerqueira, M.A.; Vicente, A.A.; Teixeira, J.A.; Carneiro-Da-Cunha, M.G. Immobilization of bioactive compounds in Cassia grandis galactomannan-based films: Influence on physicochemical properties. Int. J. Biol. Macromol. 2017, 96, 727–735. [Google Scholar] [CrossRef]
- Albuquerque, P.B.; Soares, P.A.; Aragão-Neto, A.C.; Albuquerque, G.S.; Silva, L.C.; Lima-Ribeiro, M.H.; Neto, J.C.S.; Coelho, L.C.; Correia, M.T.; Teixeira, J.A.; et al. Healing activity evaluation of the galactomannan film obtained from Cassia grandis seeds with immobilized Cratylia mollis seed lectin. Int. J. Biol. Macromol. 2017, 102, 749–757. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0141813017304683 (accessed on 1 February 2023). [CrossRef] [PubMed]
- Gouda, A.; Sakr, O.S.; Nasr, M.; Sammour, O. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102174. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Xiao, Q.; Fan, L.; Huang, D.; Chen, W.; He, W. Liposomes and liposome-like nanoparticles: From anti-fungal infection to the COVID-19 pandemic treatment. Asian J. Pharm. Sci. 2022, 17, 817–837. [Google Scholar] [CrossRef]
- Imura, T.; Otake, K.; Hashimoto, S.; Gotoh, T.; Yuasa, M.; Yokoyama, S.; Sakai, H.; Rathman, J.F.; Abe, M. Preparation and physicochemical properties of various soybean lecithin liposomes using supercritical reverse phase evaporation method. Colloids Surf. B Biointerfaces 2003, 27, 133–140. [Google Scholar] [CrossRef]
- Zahednezhad, F.; Zakeri-Milani, P.; Mojarrad, J.S.; Sarfraz, M.; Mahmoudian, M.; Baradaran, B.; Valizadeh, H. Acetyl carnitine modified liposomes elevate cisplatin uptake in macrophage and cancer cells. J. Drug Deliv. Sci. Technol. 2023, 81, 104198. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Z.; Chen, Y.; Gao, D.; Wang, P.; Lin, Y.; Wang, Y.; Wang, F.; Han, Y.; Yuan, H. Co-delivery of Docetaxel and Resveratrol by liposomes synergistically boosts antitumor efficiency against prostate cancer. Eur. J. Pharm. Sci. 2022, 174, 106199. [Google Scholar] [CrossRef]
- Yu, S.; Li, D.; Shi, A.; Long, Y.; Deng, J.; Ma, Y.; Li, X.; Wen, J.; Hu, Y.; He, X.; et al. Multidrug-loaded liposomes prevent ischemic stroke through intranasal administration. Biomed. Pharmacother. 2023, 162, 114542. [Google Scholar] [CrossRef]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M. Self-assembled tacrolimus-loaded lecithin-chitosan hybrid nanoparticles for in vivo management of psoriasis. Int. J. Pharm. 2021, 608, 121114. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.-M.; Müller, R.; Bégu, S. Liposomes, lipid nanocapsules and smartCrystals®: A comparative study for an effective quercetin delivery to the skin. Int. J. Pharm. 2018, 542, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Katsube, T.; Wahyudiono; Goto, M. Preparation of Liposomes from Soy Lecithin Using Liquefied Dimethyl Ether. Foods 2021, 10, 1789. [Google Scholar] [CrossRef] [PubMed]
- Płaczek, M.; Wątróbska-Świetlikowska, D.; Stefanowicz-Hajduk, J.; Drechsler, M.; Ochocka, J.R.; Sznitowska, M. Comparison of the in vitro cytotoxicity among phospholipid-based parenteral drug delivery systems: Emulsions, liposomes and aqueous lecithin dispersions (WLDs). Eur. J. Pharm. Sci. 2018, 127, 92–101. [Google Scholar] [CrossRef]
- Savić, V.; Ilić, T.; Nikolić, I.; Marković, B.; Čalija, B.; Cekic, N.; Savic, S. Tacrolimus-loaded lecithin-based nanostructured lipid carrier and nanoemulsion with propylene glycol monocaprylate as a liquid lipid: Formulation characterization and assessment of dermal delivery compared to referent ointment. Int. J. Pharm. 2019, 569, 118624. [Google Scholar] [CrossRef]
- Vater, C.; Hlawaty, V.; Werdenits, P.; Cichoń, M.A.; Klang, V.; Elbe-Bürger, A.; Wirth, M.; Valenta, C. Effects of lecithin-based nanoemulsions on skin: Short-time cytotoxicity MTT and BrdU studies, skin penetration of surfactants and additives and the delivery of curcumin. Int. J. Pharm. 2020, 580, 119209. [Google Scholar] [CrossRef]
- Gil, K.A.; Jerković, I.; Marijanović, Z.; Manca, M.L.; Caddeo, C.; Tuberoso, C.I.G. Evaluation of an innovative sheep cheese with antioxidant activity enriched with different thyme essential oil lecithin liposomes. LWT 2021, 154, 112808. [Google Scholar] [CrossRef]
- AbouSamra, M.M.; Elgohary, R.; Mansy, S.S. Innovated pirfenidone loaded lecithin nanocapsules for targeting liver fibrosis: Formulation, characterization and in vivo study. Int. J. Pharm. 2023, 631, 122539. [Google Scholar] [CrossRef]
- Correa, V.L.R.; Martins, J.A.; de Souza, T.R.; Rincon, G.D.C.N.; Miguel, M.P.; de Menezes, L.B.; Amaral, A.C. Melatonin loaded lecithin-chitosan nanoparticles improved the wound healing in diabetic rats. Int. J. Biol. Macromol. 2020, 162, 1465–1475. [Google Scholar] [CrossRef]
- Talón, E.; Lampi, A.-M.; Vargas, M.; Chiralt, A.; Jouppila, K.; González-Martínez, C. Encapsulation of eugenol by spray-drying using whey protein isolate or lecithin: Release kinetics, antioxidant and antimicrobial properties. Food Chem. 2019, 295, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.P.; Vaz, A.F.M.; Correia, M.T.S.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Quercetin-Loaded Lecithin/Chitosan Nanoparticles for Functional Food Applications. Food Bioprocess Technol. 2014, 7, 1149–1159. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0003269796902924 (accessed on 1 February 2023). [CrossRef] [PubMed]
- Torres, E.; Marín, V.; Aburto, J.; Beltran, H.I.; Shirai, K.; Villanueva, S.; Sandoval, G. Enzymatic modification of chitosan with quercetin and its application as antioxidant edible films. Appl. Biochem. Microbiol. 2012, 48, 151–158. Available online: http://link.springer.com/10.1134/S0003683812020123 (accessed on 1 February 2023). [CrossRef]
- Mahaling, B.; Katti, D.S. Understanding the influence of surface properties of nanoparticles and penetration enhancers for improving bioavailability in eye tissues in vivo. Int. J. Pharm. 2016, 501, 1–9. [Google Scholar] [CrossRef]
- Gibis, M.; Vogt, E.; Weiss, J. Encapsulation of polyphenolic grape seed extract in polymer-coated liposomes. Food Funct. 2012, 3, 246–254. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Carbone, C.; Ennas, G.; Puglisi, G.; Fadda, A.M.; Manconi, M. Cross-linked chitosan/liposome hybrid system for the intestinal delivery of quercetin. J. Colloid Interface Sci. 2016, 461, 69–78. [Google Scholar] [CrossRef]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Igarzabal, C.I. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll. 2018, 89, 758–764. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Du, Y.; Wang, L.; Tong, C.; Hu, Y.; Pang, J.; Yan, Z. Preparation and characterization of konjac glucomannan-based bionanocomposite film for active food packaging. Food Hydrocoll. 2018, 89, 682–690. [Google Scholar] [CrossRef]
- Rodriguez-Canto, W.; Cerqueira, M.A.; Chel-Guerrero, L.; Pastrana, L.M.; Aguilar-Vega, M. Delonix regia galactomannan-based edible films: Effect of molecular weight and k-carrageenan on physicochemical properties. Food Hydrocoll. 2020, 103, 105632. [Google Scholar] [CrossRef]
- De Sousa, F.D.; Vasconselos, P.D.; da Silva, A.F.B.; Mota, E.F.; Tomé, A.D.R.; Mendes, F.R.D.S.; Gomes, A.M.M.; Abraham, D.J.; Shiwen, X.; Owen, J.S.; et al. Hydrogel and membrane scaffold formulations of Frutalin (breadfruit lectin) within a polysaccharide galactomannan matrix have potential for wound healing. Int. J. Biol. Macromol. 2019, 121, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.; Campia, P.; Merlini, L.; Brasca, M.; Pastori, N.; Farris, S.; Melone, L.; Punta, C.; Galante, Y.M. An aerogel obtained from chemo-enzymatically oxidized fenugreek galactomannans as a versatile delivery system. Carbohydr. Polym. 2016, 144, 353–361. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0144861716300479 (accessed on 1 February 2023). [CrossRef] [PubMed]
- Chouaibi, M.; Rezig, L.; Lakoud, A.; Boussaid, A.; Hassouna, M.; Ferrari, G.; Hamdi, S. Exploring potential new galactomannan source of Retama reatam seeds for food, cosmetic and pharmaceuticals: Characterization and physical, emulsifying and antidiabetic properties. Int. J. Biol. Macromol. 2019, 124, 1167–1176. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B. X-ray diffraction, IR spectroscopy and thermal characterization of partially hydrolyzed guar gum. Int. J. Biol. Macromol. 2012, 50, 1035–1039. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.; Li, R.; Mao, L.; Liu, F.; Gao, Y. Structural characterization, formation mechanism and stability of curcumin in zein-lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chem. 2017, 237, 1163–1171. [Google Scholar] [CrossRef]
- Ben Jeddou, K.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 2016, 205, 97–105. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0308814616302783 (accessed on 1 February 2023). [CrossRef]
- Ghribi, A.M.; Sila, A.; Gafsi, I.M.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Structural, functional, and ACE inhibitory properties of water-soluble polysaccharides from chickpea flours. Int. J. Biol. Macromol. 2015, 75, 276–282. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Z.; Xiao, Z. Preparation, characterization, and thermal stability of β-cyclodextrin/soybean lecithin inclusion complex. Carbohydr. Polym. 2014, 101, 1027–1032. [Google Scholar] [CrossRef]
- Xie, H.; Liu, C.; Gao, J.; Shi, J.; Ni, F.; Luo, X.; He, Y.; Ren, G.; Luo, Z. Fabrication of Zein-Lecithin-EGCG complex nanoparticles: Characterization, controlled release in simulated gastrointestinal digestion. Food Chem. 2021, 365, 130542. [Google Scholar] [CrossRef]
- Pool, H.; Quintanar, D.; Figueroa, J.D.D.; Mano, C.M.; Bechara, J.E.H.; Godínez, L.A.; Mendoza, S. Antioxidant Effects of Quercetin and Catechin Encapsulated into PLGA Nanoparticles. J. Nanomater. 2012, 2012, 145380. [Google Scholar] [CrossRef]
- González, A.; Barrera, G.N.; Galimberti, P.I.; Ribotta, P.D.; Igarzabal, C.I.A. Development of edible films prepared by soy protein and the galactomannan fraction extracted from Gleditsia triacanthos (Fabaceae) seed. Food Hydrocoll. 2019, 97, 105227. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.M.; Silva, H.D.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-Da-Cunha, M.G. Development and Characterization of an Active Chitosan-Based Film Containing Quercetin. Food Bioprocess Technol. 2015, 8, 2183–2191. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Zhong, F. Characterization of tara gum edible films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Food Hydrocoll. 2015, 44, 309–319. Available online: http://www.sciencedirect.com/science/article/pii/S0268005X14003294 (accessed on 1 February 2023). [CrossRef]
- Libio, I.C.; Demori, R.; Ferrão, M.F.; Lionzo, M.I.; da Silveira, N.P. Films based on neutralized chitosan citrate as innovative composition for cosmetic application. Mater. Sci. Eng. C 2016, 67, 115–124. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0928493116304337 (accessed on 1 February 2023). [CrossRef] [PubMed]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—A comparative study. Food Hydrocoll. 2012, 27, 175–184. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0268005X11002086 (accessed on 1 February 2023). [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2018, 89, 56–66. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Qazi, H.J.; Zhong, F. Physicochemical and thermomechanical characterization of tara gum edible films: Effect of polyols as plasticizers. Carbohydr. Polym. 2014, 111, 359–365. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, D.; Wang, L. Preparation and physical properties of tara gum film reinforced with cellulose nanocrystals. Int. J. Biol. Macromol. 2016, 86, 606–612. Available online: http://linkinghub.elsevier.com/retrieve/pii/S0141813016301052 (accessed on 1 February 2023). [CrossRef] [PubMed]


| Sample | Average Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| QT-LL 0 | 94.26 ± 0.823 c | 0.27 ± 0.004 b,d | −44.98 ± 0.98 a |
| QT-LL 25 | 93.85 ± 4.343 c | 0.28 ± 0.012 c,d | −46.85 ± 10.92 a |
| QT-LL 50 | 100.2 ± 0.567 d | 0.23 ± 0.009 a | −50.83 ± 10.85 a |
| QT-LL 75 | 100.5 ± 4.569 d | 0.27 ± 0.0124 c,d | −48.14 ± 5.64 a |
| QT-LL 100 | 104.1 ± 4.214 d | 0.28 ± 0.007 c | −46.76 ± 1.85 a |
| QT-LL 125 | 114.0 ± 1.045 b | 0.27 ± 0.006 d | −47.10 ± 2.59 a |
| QT-LL 150 | 131.8 ± 3.469 a | 0.26 ± 0.005 b | −45.30 ± 0.59 a |
| Concentration of Quercetin | ABTS (%) | FRAP (AA/μL) | ||
|---|---|---|---|---|
| QT-LL | Free Quercetin | QT-LL | Free Quercetin | |
| 25 | −4.20 ± 5.09 | 10.76 ± 0.33 | 41.61 ± 11.93 | 165.71 ± 12.23 |
| 50 | 6.53 ± 11.31 | 25.97 ± 3.63 | 100.90 ± 12.06 | 502.58 ± 6.82 |
| 75 | 28.05 ± 0.18 | 36.03 ± 0.18 | 312.59 ± 13.67 | 1023.47 ± 12.04 |
| 100 | 35.88 ± 2.75 | 42.34 ± 1.18 | 476.37 ± 24.88 | 2281.29 ± 15.56 |
| 125 | 42.53 ± 2.99 | 47.68 ± 0.84 | 731.81 ± 38.18 | 2579.89 ± 9.78 |
| 150 | 55.49 ± 3.07 | 52.87 ± 1.09 | 812.70 ± 49.80 | 2933.27 ± 35.19 |
| Film | QT-LL 75 (v/v) | L* | a* | b* | Y |
|---|---|---|---|---|---|
| A | 0 | 95.62 ± 0.40 a | 0.35 ± 0.06 b,c | 7.93 ± 0.61 a | 12.30 ± 0.32 a |
| B | 0.1% | 95.66 ± 0.22 a | 0.35 ± 0.01 b,c | 7.46 ± 0.09 a | 12.59 ± 0.26 a |
| C | 0.2% | 95.77 ± 0.22 a | 0.30 ± 0.01 a,b,c | 7.24 ± 0.33 a | 12.49 ± 0.24 a |
| D | 0.3% | 95.01 ± 1.09 a | 0.39 ± 0.04 b | 8.17 ± 2.08 a | 12.11 ± 0.36 a |
| E | 0.4% | 96.05 ± 0.31 a | 0.27 ± 0.03 a,c | 7.46 ± 0.99 a | 12.27 ± 0.29 a |
| F | 0.5% | 95.84 ± 0.42 a | 0.25 ± 0.04 a | 7.25 ± 0.36 a | 12.62 ± 0.14 a |
| Film | QT-LL 75 (v/v) | Thickness (mm) | S (%) | MC (%) | WVP × 10−7 (g·h−1·m−1·Pa−1) |
|---|---|---|---|---|---|
| A | 0 | 0.046 ± 0.004 a | 77.0 ± 8.0 a | 17.0 ± 1.0 a | 7.27 ± 0.80 a |
| B | 0.1% | 0.051 ± 0.001 a | 68.0 ± 10.0 a | 19.0 ± 2.0 a | 8.07 ± 0.63 a,c |
| C | 0.2% | 0.058 ± 0.002 a | 77.0 ± 4.0 a | 22.0 ± 3.0 a | 10.44 ± 0.48 b,c |
| D | 0.3% | 0.056 ± 0.008 a | 75.0 ± 2.0 a | 27.0 ± 5.0 a | 11.41 ± 1.05 b |
| E | 0.4% | 0.051 ± 0.002 a | 54.0 ± 22.0 a | 28.0 ± 9.0 a | 9.72 ± 0.57 a,b,c |
| F | 0.5% | 0.054 ± 0.008 a | 73.0 ± 13.0 a | 23.0 ± 2.0 a | 9.94 ± 1.46 b,c |
| Sample | CA at 5 s | CA at 30 s |
|---|---|---|
| A | 113.5 ± 9.57 a | 111.5 ± 2.61 a |
| B | 114.2 ± 5.93 a | 119.1 ± 3.47 a |
| C | 108.3 ± 4.36 a | 112.2 ± 3.04 a |
| D | 108.2 ± 3.98 a | 114.2 ± 6.02 a |
| E | 112.8 ± 7.03 a | 115.6 ± 6.22 a |
| F | 113.8 ± 4.81 a | 117.1 ± 3.78 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Albuquerque, P.B.S.; de Souza, M.P.; Bourbon, A.I.; Cerqueira, M.A.; Pastrana, L.; Jauregi, P.; Teixeira, J.A.; das Graças Carneiro-da-Cunha, M. Production and Properties of Quercetin-Loaded Liposomes and Their Influence on the Properties of Galactomannan-Based Films. Appl. Nano 2023, 4, 159-177. https://doi.org/10.3390/applnano4020009
de Albuquerque PBS, de Souza MP, Bourbon AI, Cerqueira MA, Pastrana L, Jauregi P, Teixeira JA, das Graças Carneiro-da-Cunha M. Production and Properties of Quercetin-Loaded Liposomes and Their Influence on the Properties of Galactomannan-Based Films. Applied Nano. 2023; 4(2):159-177. https://doi.org/10.3390/applnano4020009
Chicago/Turabian Stylede Albuquerque, Priscilla Barbosa Sales, Marthyna Pessoa de Souza, Ana Isabel Bourbon, Miguel A. Cerqueira, Lorenzo Pastrana, Paula Jauregi, José A. Teixeira, and Maria das Graças Carneiro-da-Cunha. 2023. "Production and Properties of Quercetin-Loaded Liposomes and Their Influence on the Properties of Galactomannan-Based Films" Applied Nano 4, no. 2: 159-177. https://doi.org/10.3390/applnano4020009
APA Stylede Albuquerque, P. B. S., de Souza, M. P., Bourbon, A. I., Cerqueira, M. A., Pastrana, L., Jauregi, P., Teixeira, J. A., & das Graças Carneiro-da-Cunha, M. (2023). Production and Properties of Quercetin-Loaded Liposomes and Their Influence on the Properties of Galactomannan-Based Films. Applied Nano, 4(2), 159-177. https://doi.org/10.3390/applnano4020009















