Abstract
The design and necessity of corrosion-resisting nanocarbon nanocomposites have been investigated for cutting-edge aerospace applications. In this regard, nanocarbon nanofillers, especially carbon nanotubes, graphene, nanodiamond, etc. have been used to fill in various polymeric matrices (thermosets, thermoplastics, and conducting polymers) to develop anti-rusting space-related nanocomposites. This review fundamentally emphases the design, anti-corrosion properties, and application of polymer/nanocarbon nanocomposites for the space sector. An electron-conducting network is created in the polymers with nanocarbon dispersion to assist in charge transportation, and thus in the polymers’ corrosion resistance features. The corrosion resistance mechanism depends upon the formation of tortuous diffusion pathways due to nanofiller arrangement in the matrices. Moreover, matrix–nanofiller interactions and interface formation play an important role in enhancing the corrosion protection properties. The anticorrosion nanocomposites were tested for their adhesion, contact angle, and impedance properties, and NaCl tests and scratch tests were carried out. Among the polymers, epoxy was found to be superior corrosion-resisting polymer, relative to the thermoplastic polymers in these nanocomposites. Among the carbon nanotubes, graphene, and nanodiamond, the carbon nanotube with a loading of up to 7 wt.% in the epoxy matrix was desirable for corrosion resistance. On the other hand, graphene contents of up to 1 wt.% and nanodiamond contents of 0.2–0.4 wt.% were desirable to enhance the corrosion resistance of the epoxy matrix. The impedance, anticorrosion, and adhesion properties of epoxy nanocomposites were found to be better than those of the thermoplastic materials. Despite the success of nanocarbon nanocomposites in aerospace applications, thorough research efforts are still needed to design high-performance anti-rusting materials to completely replace the use of metal components in the aerospace industry.
1. Introduction
In advanced technical applications, metal-based industries face corrosion problems [1]. To prevent these corrosion issues, protective coatings, additives, and other strategies have been exploited. In this regard, nanocarbon nanoparticles such as carbon nanotubes, graphene, nanodiamond, etc. have been considered for corrosion prevention [2,3,4]. Nanocarbon nanoparticles have been reinforced in polymers to form corrosion-resisting nanocomposites [5]. Thermoplastics, thermosets, and conjugated/non-conjugated polymers have been employed in anticorrosion nanocomposite coatings [6]. As compared with pristine polymers, polymer nanocomposites have revealed superior anticorrosion properties [7]. Incidentally, homogeneous nanoparticle dispersion and matrix–nanofiller interactions have been found to facilitate the electron conductivity and corrosion-resistant properties of polymers [8,9]. Moreover, nanofiller nanoparticles in anticorrosion coatings may form tortuous pathways to delay the diffusion of corrosive species towards metals [10]. Particularly, anticorrosion coatings based on the polymer/nanocarbon nanocomposites have been found to be effective in preventing the corrosive species from reaching the metal surface [11]. Numerous facile strategies have been adopted to form anticorrosion polymer/nanocarbon nanocomposite coatings on the metal surface, such as solution techniques, spin coating, spray coating, and other approaches [12]. Consequently, anticorrosion and barrier properties have been found to be dependent on uniform nanocarbon nanofiller dispersion and interface formation in the nanocomposites.
This state-of-the-art review describes the remarkable potential of polymer/nanocarbon nanocomposites in corrosion prevention. The potential of these nanocomposites has been explored particularly for the aerospace industry. Space-related polymer/nanocarbon nanomaterials have been scrutinized for their design, physical properties, and anticorrosion characteristics. The review proceeds as follows: Section 1, is the introduction, Section 2 covers polymeric nanocomposites for corrosion resistance, Section 3 covers anticorrosion polymer/nanocarbon nanocomposites, Section 4 is dedicated to corrosion-resisting polymer/nanocarbon nanomaterials for the aerospace industry, and finally, Section 5 provides future prospects and our conclusions. All the sections thoroughly and comprehensively describe the outlined contents. In this cutting-edge review, various notable future prospects of corrosion-resisting polymer/nanocarbon nanocomposites have been highlighted, particularly the design versatility, essential features, and significance of anti-corrosion polymer/nanocarbon nanocomposites for the aerospace industry. In this regard, the indispensable features of various anticorrosion systems prepared using polymers and carbon nanoparticles have been considered. To the best of our knowledge, such a specific and recent review on aerospace-related anti-corrosion polymer/nanocarbon nanocomposites is not currently present in the literature. Some previous literature reports on this particular review topic have been observed; however, the reported literature has not been sufficiently updated to portray the current state of anti-corrosion polymer/nanocarbon nanocomposites in the aerospace industry. Furthermore, future developments in the field of anti-corrosion polymer/nanocarbon nanocomposites are not possible for scientists/researchers before prior knowledge of the recently compiled literature has been attained. Therefore, this innovative review has been designed to assemble and discuss the significant recent literature, and the advancements of anti-corrosion polymer/nanocarbon nanocomposites for application in aerospace.
2. Polymeric Nanocomposites for Corrosion Resistance
Inclusion of nanoparticles in polymers has been found to enhance various physical properties of the matrices, such as heat stability, mechanical robustness, electron transportation, thermal conductivity, and corrosion resistance [13]. Anticorrosion polymer nanocomposites have been applied in a wide range of electronics, energy devices, automobiles/spacecraft, and construction applications [14,15,16]. In this context, numerous polymers and nanoparticle combinations have been used [17]. Most importantly, conducting polymers have fine electron transport properties, leading to superior corrosion-resisting properties [18]. Polyaniline [19], polypyrrole [20], polythiophene [21], and their derived polymers have been commonly applied for their anticorrosion properties. Non-conducting polymers such as epoxies have also been effectively used for corrosion prevention [22]. Furthermore, thermoplastic polymers have been utilized for the purpose of corrosion protection [23].
In anticorrosion nanocomposites, organic and inorganic nanoparticles (nanocarbons, metal oxides, metal nanoparticles, etc.) have been exploited [24,25]. Applying polymeric nanocomposite coatings to metal surfaces may reveal corrosion, scratch, and wear resistance features. The type of polymer as well as the nanofiller selection affect the anticorrosion and tribological characteristics of nanocomposites [26]. The amount of nanofiller also influenced the corrosion resistance of the nanomaterial coated on the metal substrate [27]. Guo et al. [28] produced epoxy resin, poly(dimethyl siloxane), and silica nanoparticle-based corrosion-resisting coatings. These anticorrosion nanomaterials possess fine superhydrophobic properties and a water contact angle of 163°. Guo et al. [29] also formed corrosion-resisting coatings based on epoxy and polypyrrole/Fe3O4 nanoparticles. Polypyrrole functional magnetite nanoparticles have the ability to develop interactions with the epoxy matrix. Inclusion of nanoparticles enhanced the volume resistivity of the nanocomposites up to 1.6 × 1013 Ωcm, therefore enhancing the anticorrosion properties. He et al. [30] developed polypyrrole- and zeolite nanoparticle-based nanocomposite coatings for preventing the corrosion of metals. The nanomaterial was tested in 3.5 wt.% NaCl solution, and revealed a high impedance modulus of 3.7 × 108 Ω cm2. For corrosion prevention on metal surfaces, several mechanisms have been proposed in the literature (Figure 1) [31].
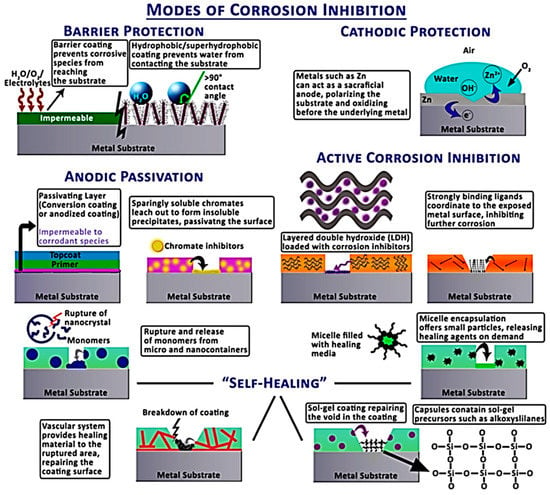
Figure 1.
Schematic of various modes of corrosion inhibition such as barrier protection, cathodic protection, anodic passivation, active corrosion inhibition, and self-healing [31]. Reproduced with permission from IOP publishing.
The major phenomena involved are anodic passivation, cathodic protection, electrolytic prevention, and active corrosion inhibition. Previously, the cathodic protection of steel and aluminum alloys has been achieved using more electropositive metals such as zinc and magnesium. In anodic passivation, anodization has been used to inhibit ion transport to prevent the corrosion process. Electrolytic protection has also been used to prevent ionic transportation between the anode and cathode, therefore producing the diffusion barrier. Active corrosion protection has been achieved using coatings with additives, which can be released upon any damage to reform the protective layer on the metal surface. Similarly, the self-healing phenomenon comprises the use of monomers and catalysts in nanocomposites for corrosion-related damage recovery through reformation of coatings.
3. Anticorrosion Polymer/Nanocarbon Nanocomposites
Among thermosetting polymers, epoxy resins have been widely used in the engineering and aerospace industries [32,33,34]. Epoxies and epoxy-based nanocomposites have superior physical properties, including adhesion, electron conduction, heat stability, thermal conductivity, and flame resistance [35]. Epoxies and derived nanomaterials also exhibit a corrosion resistance phenomenon [36,37]. Subsequently, epoxy/nanocarbon nanocomposites have been effectively applied to improve the anticorrosion properties of metal substrates [38]. Nanocarbon nanoparticles such as carbon nanotubes [39], graphene [40], graphene oxide [41], nanodiamond [42], and other nanoparticles [43] have been utilized to fabricate corrosion-resisting epoxy nanocomposites. The resulting coatings demonstrated fine hydrophobicity, moisture resistance, and a high water contact angle for corrosion prevention [44]. The matrix–nanofiller interactions in polymer/nanocarbon nanoparticles may develop interconnecting pathways in polymers to prevent the corrosive species permeating the metal surface [45]. Consequently, the nanofiller contents, uniform dispersion, and less aggregation enhance the anticorrosion properties of polymers [46].
A carbon nanotube is a one-dimensional tube-like nanocarbon made up of sp2 hybridized carbon atoms [47]. Carbon nanotubes have been considered rolled nanosheets of graphene. They are made up of sp2 hybridized carbon atoms, i.e., a two-dimensional hexagonal lattice nanostructure. Depending upon their overlapping cylindrical structures, carbon nanotubes can be categorized as a single walled carbon nanotube, a double-walled carbon nanotube, or a multi-walled carbon nanotube. The rolled cylinders in carbon nanotubes are hollow. The length of these cylinders is several times the diameter. Carbon nanotubes have unique structural, electronic, magnetic, thermal, and mechanical properties. Technical applications of carbon nanotubes have been observed in the nanoelectronics, and energy devices, and in the biomedical and various nanocomposite-related fields [48]. Yang et al. [49] used tannic acid-modified carbon nanotubes to reinforce epoxy resin for corrosion prevention. The uniform distribution of nanotubes in the epoxy resin enhanced its corrosion resistance properties. In 3.5 wt.% NaCl solution, the nanocomposite coatings revealed a corrosion potential and corrosion current of −0.207 V and 5.281 × 10−11 Acm−2, respectively. Lorwanishpaisarn et al. [50] prepared an epoxy vitrimer/carbon nanotube nanocomposite for corrosion protection. The inclusion of 5 wt.% nanofiller decreased the corrosion resistance to 3.12 × 10−5 MPY, in 3.5 wt.% NaCl solution. A corrosion protection efficiency of 99.99% was attained.
Nanodiamond is a type of nanocarbon with a size of ~4 to 5 nm [51]. Nanodiamonds have gained an important position among carbon nanoparticles. Nanodiamond has frequently been prepared through detonation techniques involving explosion or meteorite effects. It is a low-cost nanocarbon material that can be easily prepared using large-scale synthesis methods. Nanodiamond has also garnered attention due to its easy surface functionalization. Moreover, the superior structural, biocompatibility, strength, and thermal properties of nanodiamonds have been observed. Nanodiamonds have wide-ranging applications in the technical fields of electronics, engineering, and biomedicine. Nanodiamond nanoparticles possess fine surface functionalization, structural, and biocompatibility properties. Rahmani et al. [52] fabricated dodecylamine functional nanodiamond- and epoxy-based anticorrosion nanocomposites. A 1 wt.% nanodiamond-loaded epoxy coating was formed on the mild steel surface. The nanocomposite coating showed fine corrosion resistance and barrier properties in 3.5 wt.% NaCl solution. Mohammadkhani et al. [53] fabricated a polyaniline functional nanodiamond as a nanofiller for epoxy coatings. The synergistic effects of the polyaniline functional nanodiamond and the epoxy resin enhanced the anodic protection and barrier properties of the studied anticorrosion epoxy coatings.
Graphene is a one-atom-thick two-dimensional nanocarbon [54]. Graphene nanosheets are made up of sp2 hybridized carbon atoms. They have a honeycomb lattice consisting of sp2 hybridized carbon atoms [55]. Graphene can be considered a derivation of graphite, given it has stacking graphene layers [56]. Graphene has van der Waals interactions which may cause the wrinkling and restacking of the nanosheets [57]. Moreover, graphene has a high surface area, electron transport, thermal conductivity, Young’s modulus, and strength properties [58]. Due to its unique structure and properties, graphene has attracted considerable research interest [59]. Graphene has been further applied to fabricate various nanomaterials [60]. Owing to its remarkable optical, electrical, mechanical, and thermal properties, graphene has applications in a wide range of technical fields such as energy, electronics, biomedical, aerospace, automotive, composites, etc. [61]. Chen et al. [62] fabricated epoxy/poly(2-butylaniline)/graphene-derived nanocomposites. Poly(2-butylaniline) was used to better disperse the graphene nanoparticles in the epoxy resin. Consequently, fine interactions were developed between the epoxy and graphene [63]. The epoxy/poly(2-butylaniline) nanocomposite with 0.5 wt.% graphene contents revealed improved anticorrosion properties. Yu et al. [64] fabricated a epoxy/reduced graphene oxide nanocomposite via in situ polymerization. The reduced graphene oxide was modified using diamino diphenyl methane to develop covalent interactions with the epoxy resin. Addition of 1 wt.% nanofiller enhanced the corrosion resistance of the coating. Table 1 demonstrates the corrosion potential and corrosion current density of the nanocomposites. The maximum corrosion resistance was observed at a 0.5 wt.% graphene content. At a higher nanofiller loading, the corrosion resistance was decreased due to nanoparticle aggregation [65].

Table 1.
Potentiodynamic polarization of polymer/graphene-coated metal substrate [62]. P2BA = poly(2-butylaniline); Ecorr = corrosion potential; Icorr = corrosion current. Reproduced with permission from Elsevier.
4. Corrosion-Resisting Polymer/Nanocarbon Nanomaterials for Aerospace
The use of metallic structures in industries faces corrosion as major challenge [66,67,68]. Corrosion is the chemical degradation of metal-based materials under the influence of environmental factors such as chemicals, moisture, etc. [69]. Figure 2 illustrates the metal-based structures and engineering properties of an aircraft structure. Space-related structures (outer body, stabilizers, wings, pressure cabins, fuselage, etc.) must have high durability, strength, toughness, fatigue, wear, and corrosion-resisting properties [70,71,72]. Figure 3 demonstrates metallic structures widely applied in aerospace manufacturing. Corrosion processes cause damage to metal parts, leading to poor aerospace performance [73].
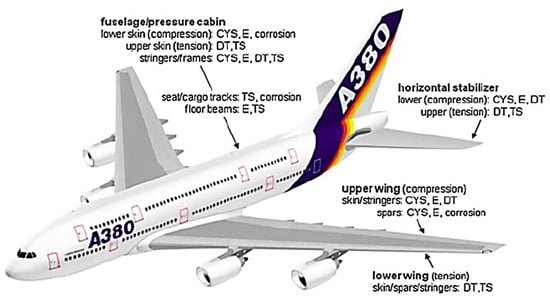
Figure 2.
Engineering property requirements for the main structural areas of a transport aircraft, i.e., corrosion, fatigue, fatigue crack growth, fracture toughness. CYS = compressive yield strength; E = elastic modulus; TS = tensile strength; DT = damage tolerance properties [66]. Reproduced with permission from Springer.
Advanced materials have been focused on for aerospace structures because they have enhanced engineering and anticorrosion properties [74]. Moreover, various corrosion protection coatings have been developed to shield aerospace-related metallic structures. Corrosion damage causes major economic expenses in the aerospace industry [75]. Conventional anticorrosion strategies have been found to be inefficient for corrosion prevention in aerospace parts [76,77,78]. Due to their high strength/weight ratio, ceramic-based anticorrosion materials have not been found useful for space structures [79]. Chromium-based coatings have also been used in aerospace to prevent the corrosion of parts [80]. Lately, metal nanoparticles [81] and carbon nanoparticles [82] have been studied for corrosion prevention in metals.
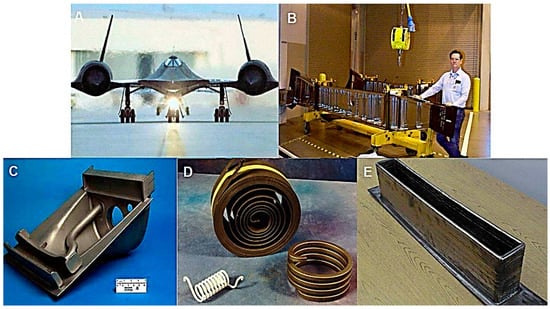
Figure 3.
(A) Front view of the all-titanium SR-71 Blackbird; (B) Photo of the Ti alloy landing gear beam for the Boeing 747; (C) Ti alloy casting for large military transport aircraft; (D) alloy springs used in the Boeing aircraft; and (E) Ti alloy shape with a deep pocket formed by laser additive manufacturing [83]. Reproduced with permission from Elsevier.
Due to their ability to withstand high temperatures, shocks, and corrosion, epoxy-based materials have been employed in the space sector [84]. Consequently, epoxy and other polymer-based nanocomposites have been considered for aerospace [85,86]. Epoxy nanocomposites depicted better corrosion resistance compared with neat epoxy coatings [87]. Ebrahimzad et al. [88] developed carbon nanotube-based coatings for improving the mechanical stability and corrosion resistance of aluminum for aerospace structures. Such coatings have been applied to enhance the life span of space-related metal alloys [89]. Asmatulu et al. [90] developed epoxy/multi-walled carbon nanotube nanocomposite coatings. The nanocomposite (1 mm thick) was coated on the aluminum surface for corrosion prevention. Epoxy/multi-walled carbon nanotube nanocomposites were tested in UV and salt fog chambers for moisture and chemical resistance. The contact angles of the nanocomposites with 0.25–2 wt.% nanofiller were investigated. A contact angle of 84–91° was observed for the coatings, indicating the hydrophobicity and anticorrosion properties of the nanocomposite material made for aerospace [91]. Jakubinek et al. [92] fabricated aerospace-grade epoxy and single-walled carbon nanotube-derived nanocomposites. Nanofiller contents up to ~1 wt.% were reinforced in the matrix. Figure 4 shows the fabrication of aerospace-grade epoxy/single-walled carbon nanotube nanocomposite adhesives. The nanomaterial was de-gassed prior to spreading on the panels. Lap shear and peel tests were performed on the nanocomposite adhesive samples. Figure 5 depicts the performance of adhesive joints with 0.5 wt.% nanofiller contents. With the reinforcement of 1 wt.% nanotube contents in the adhesive, the peel strength was found to be 30% higher than the neat polymer. The fine lap shear performance of the nanocomposites with 0.5–1 wt.% nanofiller loading was observed. The properties the revealed excellent application potential of this nanocomposite adhesive for the aerospace sector.
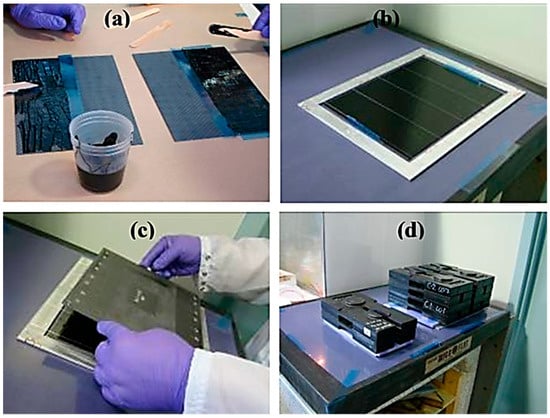
Figure 4.
Formation of adhesive bonded panels using an adhesive composite with single-walled nanotubes, showing (a) spreading of adhesive onto peel test panels (covering half a panel), and (b–d) assembly of lap-shear test panels [92]. Reproduced with permission from Elsevier.
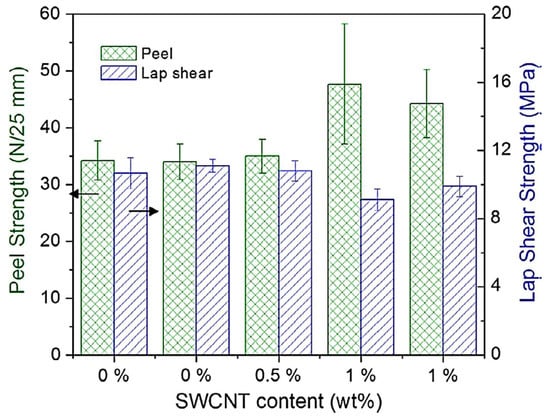
Figure 5.
Joint performance in peel and lap-shear tests with composite adherends [92]. Reproduced with permission from Elsevier.
For the aerospace industry, nickel-carbon nanotube-based anticorrosion coatings have been designed [93]. Jyotheender and co-researchers [94] fabricated nickel–carbon nanotube-filled epoxy nanocomposite coatings for the corrosion resistance of space parts. Nyquist plots indicated the impedance measurements of the nanocomposite coatings (Figure 6). Semicircle capacitive loops were observed in the impedance curves. Inclusion of 7 wt.% nanofiller contents revealed a larger capacitive loop and impedance value, indicating effective corrosion prevention. It was suggested that the uniform nickel–carbon nanotube dispersion and matrix–nanofiller interface formation led to enhanced anticorrosion properties.
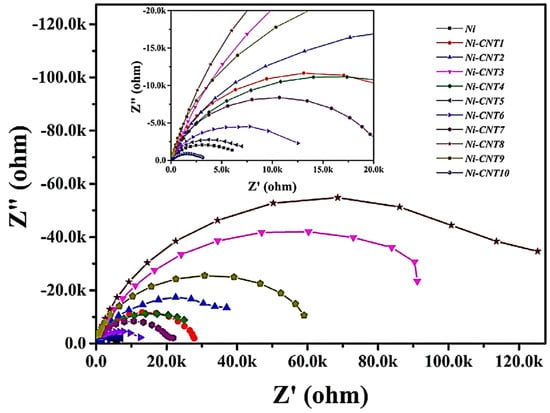
Figure 6.
Nyquist plots of Ni and Ni-CNT composite coatings [94]. Ni = nickle; Ni-CNT = nickle–carbon nanotube. Reproduced with permission from Elsevier.
Zheng et al. [95] designed and investigated neat polyamides, polyamide/purified multi-walled carbon nanotubes, and polyamide/modified multi-walled carbon nanotube nanocomposites for aerospace. Table 2 shows the tensile strength, Young’s modulus, and elongation at break of the materials. The inclusion of modified multi-walled carbon nanotubes enhanced the tensile strength and Young’s modulus of the nanocomposites by 82% and 119%, respectively, relative to the neat polymer. This enhancement was observed due to better nanofiller dispersion and interface formation with the inclusion of modified nanofiller. Figure 7 illustrates the results of the nanoindentation test. Due to the presence of the interlinked modified multi-walled carbon nanotubes, high elastic modulus areas alternating with low elastic modulus areas were observed. Consequently, a higher interfacial transition area of 4.6 GPa was observed for the polyamide/modified multi-walled carbon nanotube nanocomposite, compared with the neat polyamide (1.46 GPa). The results demonstrated the better load transfer properties of the matrix and the modified nanofiller. The nanocomposite depicted 16% improvement in the electrical conductivity of the nanocomposites, indicating superior corrosion prevention.

Table 2.
Tensile properties of neat polyamide and nanocomposites [95]. Reproduced with permission from ACS.
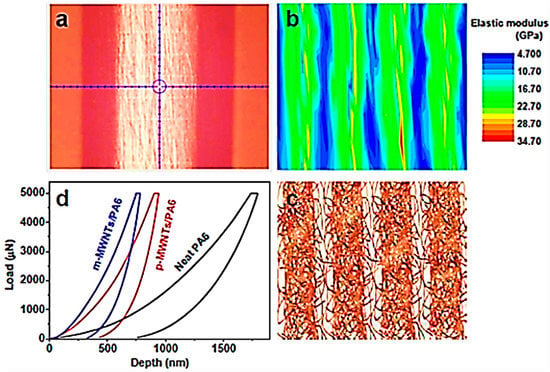
Figure 7.
(a) The image of the sample after nanoindentation test; (b) elastic modulus contour map of cross section of polyamide/modified multi-walled carbon nanotube nanocomposite; (c) internal sandwich structure sketch of polyamide/modified multi-walled carbon nanotube; (d) loading–unloading curves of neat polyamide 6 (PA6), polyamide/purified multi-walled carbon nanotubes (p-MWNTs/PA6), and polyamide/modified multi-walled carbon nanotubes (m-MWNTs/PA6) [95]. Reproduced with permission from ACS.
Verma et al. [96] developed nanocomposite coatings based on poly(vinyl alcohol) and multi-walled carbon nanotubes. The anticorrosion coatings were prepared using a dip-coating technique on aerospace-grade anodized alloys of magnesium, aluminum, and titanium [97]. The nanocomposite layers were heat-treated (200 °C) after the dip-coating process. A hatch tape test was performed to study the adhesion properties of the coatings, and these were found to be significant. Inclusion of 0.5 wt.% nanofiller loading also enhanced the anticorrosion properties of the alloys. The poly(vinyl alcohol)/multi-walled carbon nanotube coatings were found to be effective for spacecraft structural parts. Other research groups have also investigated the effectiveness of poly(vinyl alcohol)/multi-walled carbon nanotube nanocomposites for the corrosion resistance of aerospace structural components [98,99].
Epoxy/nanodiamond nanocomposite coatings have also been used for aerospace structures [100,101], and epoxy and nanodiamond nanomaterials have been applied in aerospace structures [102]. Particularly, functional nanodiamonds have been used to reinforce epoxy matrices to enhance their mechanical and anticorrosion characteristics. Carbon fiber modified with nanodiamond has also been used to reinforce epoxy nanocomposites for aerospace structures [103]. Recently, inclusion of 0.2–0.4 wt.% nanofiller loading has been found to enhance the corrosion resistance of epoxy/carbon fiber composites by 12.6%, relative to unfilled epoxy/carbon fiber materials [104]. Singh et al. [105] investigated the increase in the mechanical and anticorrosion properties of epoxy/glass fiber/carbon fiber composites with nanodiamond loading. However, relatively few studies on aerospace-related polymer/nanodiamond nanocomposites have been found, and more comprehensive efforts are needed in this field.
Additionally, carbon nanotubes, nanodiamond and graphene have been applied as corrosion-resisting materials [106]. Monetta and co-workers [107] considered epoxy/graphene nanocomposites for the corrosion protection of aerospace assemblages. Both a pristine epoxy coating and epoxy/graphene nanocomposite layers have been applied to aluminum alloy using a bar applicator [108,109,110]. The addition of 1 wt.% graphene nanofiller enhanced the adhesion and coating thickness on the metal surface. The water contact angle values were measured to analyze the moisture resistance of the epoxy/graphene nanocomposite (Table 3). As compared to pristine polymers, epoxy/graphene nanocomposites revealed higher contact angles. Consequently, the properties of hydrophobicity and corrosion resistance were attained.

Table 3.
Water contact angle values of neat epoxy and graphene-filled epoxy resin [107]. Reproduced with permission from MDPI.
Accordingly, epoxy resin has been filled with few-layer graphene for enhanced anticorrosion performance in aerospace [111]. Daradmare and co-workers [112] reinforced the epoxy matrix with few-layer graphene for use in aerospace. The few-layer graphene was synthesized using the electro-exfoliation technique [113]. Here, 0.1–1 wt.% graphene nanofiller was included in the epoxy matrix. The coatings were applied to the mild steel substrate. Relative to the neat epoxy coating, the corrosion resistance of the few-layer graphene dispersed polymer was improved by >20 times. The reason was suggested to be the uniform graphene dispersion and formation of twisting paths for restricted diffusion of the corrosion molecules [114]. Electrochemical impedance spectroscopy was used to obtain the Nyquist and Bode plots of the epoxy/few-layer graphene nanocomposite coating (Figure 8). The impedance of the nanocomposite was found to be higher (~47 kΩ cm2), relative to the neat polymer (5.9 kΩ cm2), revealing superior corrosion resistance. Improvement in the anticorrosion properties was also depicted through an increase in the polarization resistance and protection efficiency of the nanocomposite coating. The nanocomposite coating corrosion efficiency was found to be up to 90%, i.e., that required for aerospace applications.
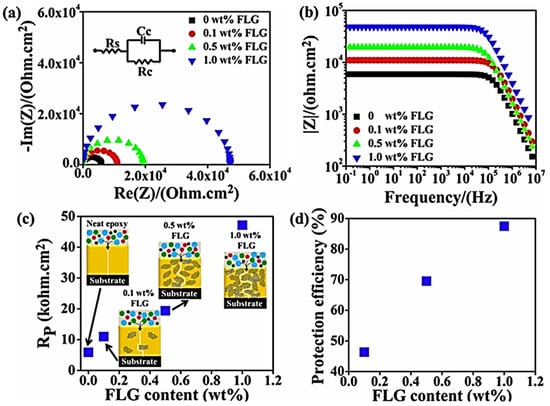
Figure 8.
(a) Nyquist plot; (b) Bode plot; (c) change in polarization resistance (Rp); and (d) variation in protection efficiency of FLG-based nanocomposite coatings of different nanofiller contents [112]. FLG = few-layer graphene. Reproduced with permission from Elsevier.
Nazir and co-researchers [115] designed epoxy and nickel-graphene based nanocomposite coatings on steel substrate. Their corrosion resistance features were studied using a molecular dynamics simulation. The anticorrosion properties were found to be adequate for aerospace applications. Chang and co-workers [116] designed a diglycidyl ether of bisphenol A epoxy resin and graphene-based nanocomposites. Hydrophobic epoxy and hydrophobic epoxy/graphene nanocomposites have been tested for their corrosion resistance performance. Cold-rolled steel and poly(dimethyl siloxane) was used as a substrate. A nanocasting technique was employed to develop the hydrophobic epoxy and epoxy/graphene nanocomposite coatings (Figure 9). Figure 10 illustrates the Nyquist plots of bare metal, epoxy, and nanocomposite coatings. The high impedance of the epoxy/graphene nanocomposite was attained from the large semicircle arc, relative to the neat epoxy and metal substrate. Figure 11 demonstrates the mechanism of the diffusion of O2 and corrosive molecules through the neat epoxy and the epoxy/graphene nanocomposite coating deposited on metal substrate. Fine graphene distribution in polymer matrix formed tortuous pathways for the permeation of corrosion molecules, and so the corrosion phenomenon was hindered [117]. In this way, several polymer/carbon nanotubes, polymer/nanodiamonds, and polymer/graphene-based anticorrosion coatings have been effectively developed for the aerospace sector.
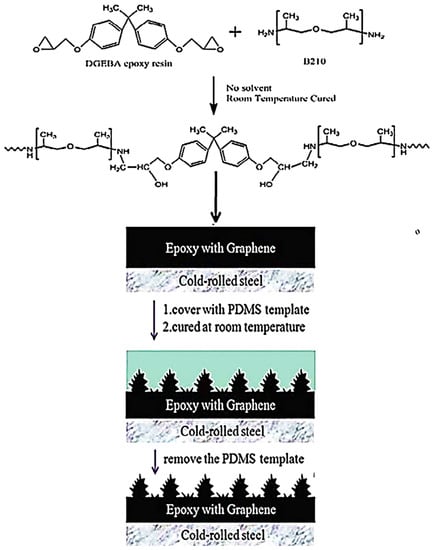
Figure 9.
Fabrication of hydrophobic nanocomposite surfaces through the nanocasting technique [116]. DGEBA = diglycidyl ether of bisphenol A; PDMS = poly(dimethyl siloxane). Reproduced with permission from Elsevier.
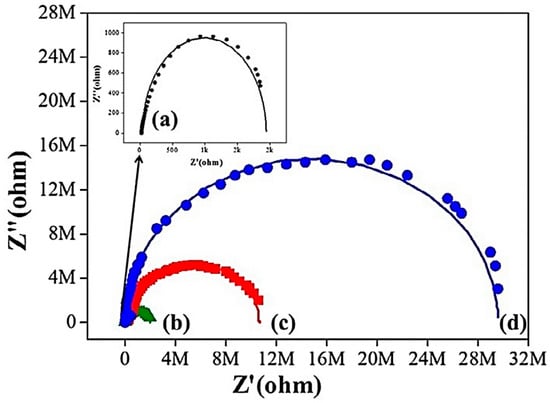
Figure 10.
Nyquist plots for (a) bare metal; (b) epoxy-coated; (c) hydrophobic epoxy-coated; and (d) hydrophobic epoxy/graphene nanocomposite-coated cold-rolled steel [116]. Reproduced with permission from Elsevier.
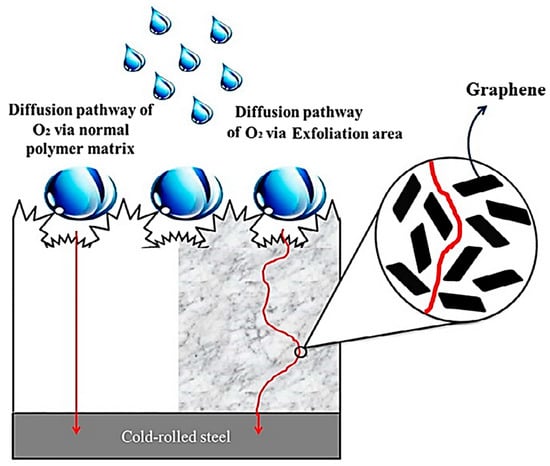
Figure 11.
Schematic of hydrophobic surface and oxygen following a tortuous path through neat epoxy and hydrophobic epoxy/graphene nanocomposite [116]. Reproduced with permission from Elsevier.
In this regard, our research group has reported novel nanocomposite systems for aerospace applications. We have reported on diglycidyl ether of bisphenol-A/tetrabromobisphenol-A/multi-walled carbon nanotube nanocomposites [118]. The electromagnetic interference shielding effectiveness of diglycidyl ether of bisphenol-A/tetrabromobisphenol-A/multi-walled carbon nanotube nanocomposites was found to be ~12.1 dB for aerospace application. The materials had a high thermal stability of 369–569 °C. Moreover, the nonflammability and anticorrosion properties of the nanomaterials were observed. In another attempt, we prepared a diglycidyl ether of bisphenol-A/polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene nanocomposite with a purified and acid-functional graphene nanoplatelet [119]. The ultimate tensile strength and toughness of the nanomaterials were in the range of 60.4–64.5 MPa and 422.6–725.5 MPa, respectively. Thermal stability was observed within ~547–568 °C, and the glass transition temperature was ~229–258 °C. The EMI shielding effectiveness of nanocomposite was sufficiently high, in the range of ~17.9–20.07 dB, for aerospace applications. In another attempt [120], we fabricated diglycidyl ether of bisphenol-A/polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene nanocomposites, with amine-functional graphene nanoplatelets, for aerospace. A high maximum decomposition temperature was observed around 545 °C. The glass transition temperature of the nanocomposite was also observed to be high, at ~230 °C. The EMI shielding effectiveness of the nanocomposite was ~18.87–20.15 dB. Moreover, the nanocomposite had fine anticorrosion properties for space application. The novel epoxy blend and nanocarbon-based systems have fine potential for space application. Moreover, we have developed polyimide/polybenzimidazole and carbon nano-onion-based system for corrosion protection [121]. The electrical conductivity of the nanocomposites was observed to be in the range of 1.6–3.3 S cm−1, with varying nanofiller loading. Inclusion of 5 wt.% nanofiller enhanced the corrosion protection effect up to 70%.
5. Prospects of Corrosion-Resisting Coatings
Corrosion-resisting polymer/nanocarbon nanomaterials have been successfully designed and investigated in the literature [122,123,124]. These nanocomposites possess light weight, high electrical conductivity, and impendence features supporting the corrosion protection characteristics [125]. Anticorrosion polymer/nanocarbon nanocomposites have been found to be effective in protecting metals and metallic alloy substrates [126]. Their corrosion inhibition mechanism was developed through the cathodic behavior of the nanocomposite and the anodic action of the metallic substrates. The anode–cathode behavior in turn prevented the corrosion process. Carbon nanotubes, graphene, and nanodiamonds have been widely used as nanocarbon nanofillers in anticorrosion polymer coatings [127]. The derived nanocomposite coatings restricted the passage of corrosive molecules to the metal surface. In the space sector, thermoplastics as well as thermosetting matrices have been used to develop corrosion-resisting coatings [128]. Epoxy resins and conducting polymer-based nanocomposite coatings have been widely employed for preventing the corrosion of aerospace structures [129]. Polymer/nanocarbon nanomaterials have been found to hinder corrosion-causing factors such as chemicals/moisture, and to prevent gases reaching the metal surface [130]. Several mechanisms have been proposed for the corrosion prevention phenomenon of polymer/nanocarbon nanocomposites [131]. Despite their superior design and properties, these materials face the limitations of porosity, defects, and fragility. Therefore, 100% corrosion resistance cannot be achieved for the aerospace sector. In this regard, future research attempts must focus on the functionalization of carbon nanotubes, graphene, and nanodiamonds, and on polymer modification to enhance the structural stability, coating durability, and corrosion resistance properties of aerospace materials. Moreover, several nanocarbon nanofillers have yet not been explored for corrosion-resisting aerospace materials. Thus, new polymer/nanocarbon designs must be produced to fulfil the demands of future aerospace structures.
6. Conclusions
In a nutshell, this review has portrayed the design and properties of corrosion-resisting polymer/nanocarbon nanocomposites, especially focusing on the aerospace industry. Combinations of various polymers and carbon nanoparticles have been explored for the development and use of anticorrosion nanomaterials in the space industry. Moreover, the factors and mechanisms behind the anticorrosion of these materials have been investigated. Nanocarbon-based corrosion-resisting nanomaterials have been found effective for advanced aerospace applications. Carbon nanotube coatings were tested with carbon nanotube contents in the range of 0.25–2 wt.%. Higher nanotube loadings were found effective for corrosion prevention. Further, higher loading of 7 wt.% nickel–carbon nanotubes in epoxy coatings was needed for anticorrosion properties. Contrarily, graphene loading of below 1 wt.% was needed to enhance the corrosion resistance of the epoxy coatings. Epoxy/carbon nanotube nanocomposites have been tested for their contact angle, which was found to be high, at ~91°. On the other hand, the contact angle of epoxy/graphene nanocomposites was found to be lower, i.e., ~75.3°. Polyamide/carbon nanotube and poly(vinyl alcohol)/carbon nanotube coatings revealed lower corrosion-resisting efficiency than the epoxy/carbon nanotubes. The results revealed that epoxy is a better matrix for the corrosion protection and adhesion properties of aerospace structures, relative to thermoplastic matrices. Moreover, low nanodiamond contents of 0.2–0.4 wt.% were needed for the corrosion protection of the epoxy matrices.
Author Contributions
Conceptualization, A.K.; data curation, A.K.; writing of original draft preparation, A.K.; Review and editing, A.K., I.A. and T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thakur, A.; Kaya, S.; Kumar, A. Recent Trends in the Characterization and Application Progress of Nano-Modified Coatings in Corrosion Mitigation of Metals and Alloys. Appl. Sci. 2023, 13, 730. [Google Scholar] [CrossRef]
- Bikiaris, D.N. Nanocomposites with Different Types of Nanofillers and Advanced Properties for Several Applications. Appl. Nano 2022, 3, 160–162. [Google Scholar] [CrossRef]
- Milana, E.; Santaniello, T.; Azzini, P.; Migliorini, L.; Milani, P. Fabrication of High-Aspect-Ratio Cylindrical Micro-Structures Based on Electroactive Ionogel/Gold Nanocomposite. Appl. Nano 2020, 1, 59–69. [Google Scholar] [CrossRef]
- Kausar, A. Poly (methyl methacrylate) nanocomposite reinforced with graphene, graphene oxide, and graphite: A review. Polym.-Plast. Technol. Mater. 2019, 58, 821–842. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Kontaxis, L.C.; Portan, D.V.; Petropoulos, G.N.; Valeriou, E.; Alexandropoulos, D. Mechanical Performance Enhancement of Aluminum Single-Lap Adhesive Joints Due to Organized Alumina Nanotubes Layer Formation on the Aluminum Adherends. Appl. Nano 2021, 2, 206–221. [Google Scholar] [CrossRef]
- Dagdag, O.; Berisha, A.; Mehmeti, V.; Haldhar, R.; Berdimurodov, E.; Hamed, O.; Jodeh, S.; Lgaz, H.; Sherif, E.-S.M.; Ebenso, E.E. Epoxy coating as effective anti-corrosive polymeric material for aluminum alloys: Formulation, electrochemical and computational approaches. J. Mol. Liq. 2022, 346, 117886. [Google Scholar] [CrossRef]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Pourhashem, S.; Saba, F.; Duan, J.; Rashidi, A.; Guan, F.; Nezhad, E.G.; Hou, B. Polymer/Inorganic nanocomposite coatings with superior corrosion protection performance: A review. J. Ind. Eng. Chem. 2020, 88, 29–57. [Google Scholar] [CrossRef]
- Bakshi, M.I.; Ahmad, S. In-situ synthesis of synergistically active ceria doped polypyrrole oleo-polyesteramide hybrid nanocomposite coatings: Corrosion protection and flame retardancy behaviour. Prog. Org. Coat. 2020, 147, 105778. [Google Scholar] [CrossRef]
- Jeong, N.; Jwa, E.; Kim, C.; Choi, J.Y.; Nam, J.-Y.; Hwang, K.S.; Han, J.-H.; Kim, H.-K.; Park, S.-C.; Seo, Y.S. One-pot large-area synthesis of graphitic filamentous nanocarbon-aligned carbon thin layer/carbon nanotube forest hybrid thin films and their corrosion behaviors in simulated seawater condition. Chem. Eng. J. 2017, 314, 69–79. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2015, 98, 313–333. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Tareq, S. Fabrication and Characterisation of Polymeric Nano-Composites. Master’s Thesis, Western Sydney University, Sydney NSW, Australia, 2019. [Google Scholar]
- Balakrishnan, P.; John, M.J.; Pothen, L.; Sreekala, M.; Thomas, S. Natural fibre and polymer matrix composites and their applications in aerospace engineering. In Advanced Composite Materials for Aerospace Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 365–383. [Google Scholar]
- Kausar, A. Applications of polymer/graphene nanocomposite membranes: A review. Mater. Res. Innov. 2019, 23, 276–287. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, J.; Yan, J.; Liu, X. Advanced Fiber Materials for Wearable Electronics. Adv. Fiber Mater. 2022, 5, 12–35. [Google Scholar] [CrossRef]
- Habibpour, S.; Um, J.G.; Jun, Y.-S.; Bhargava, P.; Park, C.B.; Yu, A. Structural Impact of Graphene Nanoribbon on Mechanical Properties and Anti-corrosion Performance of Polyurethane Nanocomposites. Chem. Eng. J. 2021, 405, 126858. [Google Scholar] [CrossRef]
- Sánchez-Romate, X.F. Fundamentals of Electrical Conductivity in Polymers. In Multifunctional Epoxy Resins; Springer: Berlin/Heidelberg, Germany, 2023; pp. 327–364. [Google Scholar]
- Yu, J.; Liu, Y.; Wang, H.; Yan, Q.; Luo, J. Insight into the corrosion inhibition of the iron anode with electro-deposited polyaniline during the electrocoagulation treatment process of electroplating wastewater. Environ. Sci. Water Res. Technol. 2022, 9, 406–418. [Google Scholar] [CrossRef]
- Cho, L.Y.; Guiotti, L.G.; Liu, A.S. Corrosion performance of polypyrrole-bilayer coating on carbon steel. J. Mater. Sci. 2023, 58, 1436–1451. [Google Scholar] [CrossRef]
- Hassan, A.; Ismail, M.; Reshak, A.H.; Zada, Z.; Khan, A.A.; Arif, M.; Siraj, K.; Zada, S.; Murtaza, G.; Ramli, M.M. Effect of heteroatoms on structural, electronic and spectroscopic properties of polyfuran, polythiophene and polypyrrole: A hybrid DFT approach. J. Mol. Struct. 2023, 1274, 134484. [Google Scholar] [CrossRef]
- Ai, Y.-F.; Xia, L.; Pang, F.-Q.; Xu, Y.-L.; Zhao, H.-B.; Jian, R.-K. Mechanically strong and flame-retardant epoxy resins with anti-corrosion performance. Compos. Part B Eng. 2020, 193, 108019. [Google Scholar] [CrossRef]
- Nurhamiyah, Y.; Amir, A.; Finnegan, M.; Themistou, E.; Edirisinghe, M.; Chen, B. Wholly biobased, highly stretchable, hydrophobic, and self-healing thermoplastic elastomer. ACS Appl. Mater. Interfaces 2021, 13, 6720–6730. [Google Scholar] [CrossRef]
- Kausar, A. Thermally conducting polymer/nanocarbon and polymer/inorganic nanoparticle nanocomposite: A review. Polym. Technol. Mater. 2020, 59, 895–909. [Google Scholar] [CrossRef]
- Anjana, P.; Bindhu, M.; Rakhi, R. Green synthesized gold nanoparticle dispersed porous carbon composites for electrochemical energy storage. Mater. Sci. Energy Technol. 2019, 2, 389–395. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Yang, W.; Feng, W.; Liao, Z.; Yang, Y.; Miao, G.; Yu, B.; Pei, X. Protection of mild steel with molecular engineered epoxy nanocomposite coatings containing corrosion inhibitor functionalized nanoparticles. Surf. Coat. Technol. 2020, 406, 126639. [Google Scholar] [CrossRef]
- Guo, X.-J.; Zhang, D.; Xue, C.-H.; Liu, B.-Y.; Huang, M.-C.; Wang, H.-D.; Wang, X.; Deng, F.-Q.; Pu, Y.-P.; An, Q.-F. Scalable and Mechanically Durable Superhydrophobic Coating of SiO2/Polydimethylsiloxane/Epoxy Nanocomposite. ACS Appl. Mater. Interfaces 2023, 15, 4612–4622. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Gu, H.; Wang, Y.; Yan, X.; Ding, D.; Long, J.; Tadakamalla, S.; Wang, Q.; Khan, M.A. Reinforced magnetic epoxy nanocomposites with conductive polypyrrole nanocoating on nanomagnetite as a coupling agent. RSC Adv. 2014, 4, 36560–36572. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.; Zhang, X.; Chen, Y.; Bai, W.; Lin, Y.; Jian, R.; Xu, Y. Self-healing epoxy composite coating based on polypyrrole@MOF nanoparticles for the long-efficiency corrosion protection on steels. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 657, 130601. [Google Scholar] [CrossRef]
- Dennis, R.V.; Patil, V.; Andrews, J.L.; Aldinger, J.P.; Yadav, G.D.; Banerjee, S. Hybrid nanostructured coatings for corrosion protection of base metals: A sustainability perspective. Mater. Res. Express 2015, 2, 032001. [Google Scholar] [CrossRef]
- Ramon, E.; Sguazzo, C.; Moreira, P.M.G.P. A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace 2018, 5, 110. [Google Scholar] [CrossRef]
- Hamerton, I.; Kratz, J. The use of thermosets in modern aerospace applications. In Thermosets; Elsevier: Amsterdam, The Netherlands, 2018; pp. 303–340. [Google Scholar]
- Ahmadi, Z. Epoxy in nanotechnology: A short review. Prog. Org. Coat. 2019, 132, 445–448. [Google Scholar] [CrossRef]
- Kugler, S.; Ossowicz-Rupniewska, P.; Wierzbicka, E.; Łopiński, J. Anhydride-Cured Epoxy Powder Coatings from Natural-Origin Resins, Hardeners, and Fillers. Coatings 2021, 11, 531. [Google Scholar] [CrossRef]
- Ogbonna, V.; Popoola, A.; Popoola, O.; Adeosun, S. A review on the recent advances on improving the properties of epoxy nanocomposites for thermal, mechanical, and tribological applications: Challenges and recommendations. Polym.-Plast. Technol. Mater. 2022, 61, 176–195. [Google Scholar] [CrossRef]
- Nikafshar, S.; McCracken, J.; Dunne, K.; Nejad, M. Improving UV-Stability of epoxy coating using encapsulated halloysite nanotubes with organic UV-Stabilizers and lignin. Prog. Org. Coat. 2020, 158, 105843. [Google Scholar]
- Frigione, M.; Lettieri, M. Recent Advances and Trends of Nanofilled/Nanostructured Epoxies. Materials 2020, 13, 3415. [Google Scholar] [CrossRef]
- Ghahremani, P.; Mostafatabar, A.H.; Bahlakeh, G.; Ramezanzadeh, B. Rational design of a novel multi-functional carbon-based nano-carrier based on multi-walled-CNT-oxide/polydopamine/chitosan for epoxy composite with robust pH-sensitive active anti-corrosion properties. Carbon 2022, 189, 113–141. [Google Scholar] [CrossRef]
- Ji, X.; Seif, A.; Duan, J.; Rashidi, A.; Zhou, Z.; Pourhashem, S.; Mirzaee, M.; Zhai, X.; Zhao, X.; Hou, B. Experimental and DFT studies on corrosion protection performance of epoxy/graphene quantum dots@ TiO2 nanotubes coatings. Constr. Build. Mater. 2022, 322, 126501. [Google Scholar] [CrossRef]
- Haeri, Z.; Ramezanzadeh, B.; Ramezanzadeh, M. Recent progress on the metal-organic frameworks decorated graphene oxide (MOFs-GO) nano-building application for epoxy coating mechanical-thermal/flame-retardant and anti-corrosion features improvement. Prog. Org. Coat. 2022, 163, 106645. [Google Scholar] [CrossRef]
- Auda AbdulAmeer, S.; Thabit, R.S.; Hadi, M.; Taha Ibrahim, I.; Mahmood Saeed, S.; Ali Kadhim, A.; Abbas Sahib, A. Modeling the Kinetics of Degradation of Epoxy Nanocomposites in the Presence of Modified Nanodiamonds with Carboxyl. J. Nanostructures 2022, 12, 975–982. [Google Scholar]
- Morimune-Moriya, S. Polymer/nanocarbon nanocomposites with enhanced properties. Polym. J. 2022, 54, 977–984. [Google Scholar] [CrossRef]
- Kooshksara, M.M.; Mohammadi, S. Investigation of the in-situ solvothermal reduction of multi-layered Graphene oxide in epoxy coating by acetonitrile on improving the hydrophobicity and corrosion resistance. Prog. Org. Coat. 2021, 159, 106432. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Futaba, D.N.; Chen, G.; Chen, C.; Zhou, K.; Zhang, Q.; Li, M.; Ye, Z.; Xu, M. Structural Design and Fabrication of Multifunctional Nanocarbon Materials for Extreme Environmental Applications. Adv. Mater. 2022, 34, 2201046. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C.; Jiang, P. Toward Effective Synergetic Effects from Graphene Nanoplatelets and Carbon Nanotubes on Thermal Conductivity of Ultrahigh Volume Fraction Nanocarbon Epoxy Composites. J. Phys. Chem. C 2012, 116, 23812–23820. [Google Scholar] [CrossRef]
- Jain, N.; Gupta, E.; Kanu, N.J. Plethora of Carbon nanotubes applications in various fields—A state-of-the-art-review. Smart Sci. 2022, 10, 1–24. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, J.; Ming, W.; He, W.; Zhang, G.; Zhang, H.; Cao, Y.; Jiang, Z. Non-traditional processing of carbon nanotubes: A review. Alex. Eng. J. 2022, 61, 597–617. [Google Scholar] [CrossRef]
- Yang, H.; Duan, L.; Zhang, P.; Xu, G.; Cui, J.; Lv, J.; Sun, W.; Li, B.; Wang, D.; Wu, Y. Corrosion resistance of functionalized carbon nanotubes enhanced epoxy coatings on sintered NdFeB magnets. J. Coat. Technol. Res. 2022, 19, 1317–1329. [Google Scholar] [CrossRef]
- Lorwanishpaisarn, N.; Srikhao, N.; Jetsrisuparb, K.; Knijnenburg, J.T.N.; Theerakulpisut, S.; Okhawilai, M.; Kasemsiri, P. Self-healing Ability of Epoxy Vitrimer Nanocomposites Containing Bio-Based Curing Agents and Carbon Nanotubes for Corrosion Protection. J. Polym. Environ. 2022, 30, 472–482. [Google Scholar] [CrossRef]
- Wu, Y.; Weil, T. Recent Developments of Nanodiamond Quantum Sensors for Biological Applications. Adv. Sci. 2022, 9, 2200059. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A.; Tavandashti, N.P. Nanodiamond loaded with corrosion inhibitor as efficient nanocarrier to improve anticorrosion behavior of epoxy coating. J. Ind. Eng. Chem. 2020, 83, 153–163. [Google Scholar] [CrossRef]
- Mohammadkhani, R.; Shojaei, A.; Rahmani, P.; Tavandashti, N.P.; Amouzegar, M. Synthesis and characterization of polyaniline/nanodiamond hybrid nanostructures with various morphologies to enhance the corrosion protection performance of epoxy coating. Diam. Relat. Mater. 2021, 120, 108672. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-Like Two-Dimensional Materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Martinez, A.; Wang, F. Optical modulators with 2D layered materials. Nat. Photon. 2016, 10, 227–238. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: From fabrication to applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Li, F.; Long, L.; Weng, Y. A Review on the Contemporary Development of Composite Materials Comprising Graphene/Graphene Derivatives. Adv. Mater. Sci. Eng. 2020, 2020, 1915641. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, S.; Cui, M.; Qin, S.; Yan, G.; Zhao, H.; Wang, L.; Xue, Q. Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized graphene. Carbon 2017, 114, 356–366. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Zhang, X.; Zhao, Z.; Zhu, Y. A multifunctional super-hydrophobic coating based on PDA modified MoS2 with anti-corrosion and wear resistance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 239–247. [Google Scholar] [CrossRef]
- Yu, B.; Shi, Y.Q.; Yuan, B.H.; Qiu, S.L.; Xing, W.Y.; Hu, W.Z.; Song, L.; Lo, S.M.; Hu, Y. Enhanced thermal and flame retardant properties of flame-retardant-wrapped graphene/epoxy resin nanocomposites. J. Mater. Chem. A 2015, 3, 8034–8044. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Wu, Y.-Y.; Deng, X.; Zheng, Z. The effect of graphene oxide on the mechanical properties, impermeability and corrosion resistance of cement mortar containing mineral admixtures. Constr. Build. Mater. 2021, 288, 123059. [Google Scholar] [CrossRef]
- Prasad, N.E.; Wanhill, R.J. Aerospace Materials and Material Technologies; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Vinokurova, I.; Khlopovskikh, E.; Safonova, L.; Zvyagina, L. Study of the Features of Heat and Mass Transfer in the Interelectrode Space During Electrochemical Processing of Metals for the Aerospace Industry; AIP Publishing LLC: Melville, NY, USA, 2022; p. 050053. [Google Scholar]
- Arrigoni, M. Metallic Materials and Their Applications in Aerospace and Advanced Technologies; MDPI: Basel, Switzerland, 2022; p. 226. [Google Scholar]
- El-Shamy, A.M.; Mouneir, S.M. Medicinal Materials as Eco-friendly Corrosion Inhibitors for Industrial Applications: A Review. J. Bio-Tribo-Corrosion 2023, 9, 1–40. [Google Scholar] [CrossRef]
- Sofi, A.; Jeffrey, J.; Rathor, A.S. Chemical property and characteristics of polymer. In Materials for Lightweight Constructions; CRC Press: Boca Raton, FL, USA, 2023; pp. 61–81. [Google Scholar]
- Lan, J.; Wang, B.; Bo, C.; Gong, B.; Ou, J. Progress on fabrication and application of activated carbon sphere in recent decade. J. Ind. Eng. Chem. 2023, 120, 47–72. [Google Scholar] [CrossRef]
- Kausar, A.; Bocchetta, P. Polymer/Graphene Nanocomposite Membranes: Status and Emerging Prospects. J. Compos. Sci. 2022, 6, 76. [Google Scholar] [CrossRef]
- Stewart, M.G.; Bastidas-Arteaga, E. Corrosion of concrete and steel structures in a changing climate. In Climate Adaptation Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 99–125. [Google Scholar]
- Wong, R. Design, Build and Certification of Composite Airplane Structure. In Design, Manufacturing & Application of Composites; CRC Press: Boca Raton, FL, USA, 2020; pp. 717–725. [Google Scholar]
- Kumar, C.V.; Rajyalakshmi, G.; Kartha, J. Insights on Anti-corrosion Coating of Magnesium Alloy: A Review. J. Bio- Tribo-Corrosion 2023, 9, 1–21. [Google Scholar]
- Wei, J.; Li, B.; Jing, L.; Tian, N.; Zhao, X.; Zhang, J. Efficient protection of Mg alloy enabled by combination of a conventional anti-corrosion coating and a superamphiphobic coating. Chem. Eng. J. 2020, 390, 124562. [Google Scholar] [CrossRef]
- Cui, M.; Wang, B.; Wang, Z. Nature-inspired strategy for anticorrosion. Adv. Eng. Mater. 2019, 21, 1801379. [Google Scholar] [CrossRef]
- Tenjimbayashi, M.; Nishioka, S.; Kobayashi, Y.; Kawase, K.; Li, J.; Abe, J.; Shiratori, S. A Lubricant-Sandwiched Coating with Long-Term Stable Anticorrosion Performance. Langmuir 2018, 34, 1386–1393. [Google Scholar] [CrossRef]
- Al-mashhadani, M.; Ahmed, W.A.; Abdallh, M.; Hussain, Z.; Yousif, E. Eco-friendly green corrosion inhibitors in overview. Res. J. Adv. Sci. 2020, 1, 7–16. [Google Scholar]
- Nematollahzadeh, A.; Seraj, S.; Mirzayi, B. Catecholamine coated maghemite nanoparticles for the environmental remediation: Hexavalent chromium ions removal. Chem. Eng. J. 2015, 277, 21–29. [Google Scholar] [CrossRef]
- Anjum, M.J.; Ali, H.; Khan, W.Q.; Zhao, J.; Yasin, G. Metal/metal oxide nanoparticles as corrosion inhibitors. In Corrosion Protection at the Nanoscale; Elsevier: Amsterdam, The Netherlands, 2020; pp. 181–201. [Google Scholar]
- Wulan, P.P.; Wijardono, S.B. Finding an Optimum Period of Oxidative Heat Treatment on SS 316 Catalyst for Nanocarbon Production from LDPE Plastic Waste. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 552. [Google Scholar] [CrossRef]
- Williams, J.C.; Starke, E.A., Jr. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Pittman, E.; Koumlis, S.; Aung, H.N.; Bellafatto, A.; Lamberson, L. Rate-Dependent Fracture Behavior of Aerospace Epoxies: PR-520 and 3502. J. Aerosp. Eng. 2022, 35, 04021100. [Google Scholar] [CrossRef]
- Ramachandran, K.; Boopalan, V.; Bear, J.C.; Subramani, R. Multi-walled carbon nanotubes (MWCNTs)-reinforced ceramic nanocomposites for aerospace applications: A review. J. Mater. Sci. 2021, 57, 3923–3953. [Google Scholar] [CrossRef]
- Siochi, E.J.; Harrison, J.S. Structural nanocomposites for aerospace applications. MRS Bull. 2015, 40, 829–835. [Google Scholar] [CrossRef]
- Kaiser, A.L.; Acauan, L.; Wardle, B.L. Process-Structure-Property Relations in Dense Aligned Carbon Nanotube/Aerospace-Grade Epoxy Nanocomposites; American Institute of Aeronautics and Astronautics: Lowell, MA, USA, 2022; p. 1095. [Google Scholar]
- Ebrahimzad, P.; Ghasempar, M.; Balali, M. Friction Stir Processing of Aerospace Aluminum Alloy by Addition of Carbon Nano Tube. Trans. Indian Inst. Met. 2017, 70, 2241–2253. [Google Scholar] [CrossRef]
- Pathak, S.; Saha, G.C.; Hadi, M.B.A.; Jain, N.K. Engineered Nanomaterials for Aviation Industry in COVID-19 Context: A Time-Sensitive Review. Coatings 2021, 11, 382. [Google Scholar] [CrossRef]
- Asmatulu, R. Nanocoatings for corrosion protection of aerospace alloys. In Corrosion Protection and Control Using Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2012; pp. 357–374. [Google Scholar]
- Tajuddin, M.H.; Yusof, N.; Abdullah, N.; Abidin, M.N.Z.; Salleh, W.N.W.; Ismail, A.F.; Matsuura, T.; Hairom, N.H.H.; Misdan, N. Incorporation of layered double hydroxide nanofillers in polyamide nanofiltration membrane for high performance of salts rejections. J. Taiwan Inst. Chem. Eng. 2019, 97, 1–11. [Google Scholar] [CrossRef]
- Jakubinek, M.B.; Ashrafi, B.; Zhang, Y.; Martinez-Rubi, Y.; Kingston, C.T.; Johnston, A.; Simard, B. Single-walled carbon nanotube–epoxy composites for structural and conductive aerospace adhesives. Compos. Part B Eng. 2015, 69, 87–93. [Google Scholar] [CrossRef]
- Krishna, A.; Aravinda, L.; Murugan, A.; Kumar, N.S.; Sankar, M.R.; Reddy, K.N.; Balashanmugam, N. A study on wafer scalable, industrially applicable CNT based nanocomposites of Al-CNT, Cu-CNT, Ti-CNT, and Ni-CNT as thermal interface materials synthesised by thin film techniques. Surf. Coat. Technol. 2021, 127926. [Google Scholar] [CrossRef]
- Jyotheender, K.S.; Gupta, A.; Srivastava, C. Grain boundary engineering in Ni-carbon nanotube composite coatings and its effect on the corrosion behaviour of the coatings. Materialia 2020, 9, 100617. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, R.; Dong, X.; Wu, L.; Zhang, X. High Strength Conductive Polyamide 6 Nanocomposites Reinforced by Prebuilt Three-Dimensional Carbon Nanotube Networks. ACS Appl. Mater. Interfaces 2018, 10, 28103–28111. [Google Scholar] [CrossRef]
- Verma, P.; Anoop, S.; Rao, V.S.; Sharma, A.; Rani, R.U. Multiwalled carbon nanotube-poly vinyl alcohol nanocomposite multifunctional coatings on aerospace alloys. Mater. Today Proc. 2018, 5, 21205–21216. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Hu, J.; Lin, T. Formation mechanism and corrosion resistance of the hydrophobic coating on anodized magnesium. Corros. Sci. 2016, 111, 334–343. [Google Scholar] [CrossRef]
- Dassios, K.G.; Galiotis, C. Polymer–nanotube interaction in MWCNT/poly (vinyl alcohol) composite mats. Carbon 2012, 50, 4291–4294. [Google Scholar] [CrossRef]
- Mallakpour, S.; Abdolmaleki, A.; Borandeh, S. l-Phenylalanine amino acid functionalized multi walled carbon nanotube (MWCNT) as a reinforced filler for improving mechanical and morphological properties of poly (vinyl alcohol)/MWCNT composite. Prog. Org. Coat. 2014, 77, 1966–1971. [Google Scholar] [CrossRef]
- Kausar, A. Advances in condensation polymer containing zero-dimensional nanocarbon reinforcement—Fullerene, carbon nano-onion, and nanodiamond. Polym.-Plast. Technol. Mater. 2021, 60, 695–713. [Google Scholar] [CrossRef]
- Kausar, A. Nanocarbon and macrocarbonaceous filler–reinforced epoxy/polyamide: A review. J. Thermoplast. Compos. Mater. 2022, 35, 2620–2640. [Google Scholar] [CrossRef]
- Haleem, Y.A.; Liu, D.; Chen, W.; Wang, C.; Hong, C.; He, Z.; Liu, J.; Song, P.; Yu, S.; Song, L. Surface functionalization and structure characterizations of nanodiamond and its epoxy based nanocomposites. Compos. Part B Eng. 2015, 78, 480–487. [Google Scholar] [CrossRef]
- Bisht, A.; Dasgupta, K.; Lahiri, D. Evaluating the effect of addition of nanodiamond on the synergistic effect of graphene-carbon nanotube hybrid on the mechanical properties of epoxy based composites. Polym. Test. 2020, 81, 106274. [Google Scholar] [CrossRef]
- Farooq, U.; Ali, M.U.; Hussain, S.J.; Ahmad, M.S.; Zafar, A.; Ghafoor, U.; Subhani, T. Improved Ablative Properties of Nanodiamond-Reinforced Carbon Fiber–Epoxy Matrix Composites. Polymers 2021, 13, 2035. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Mohanty, A. Influence of Nanodiamonds on the Mechanical Properties of Glass Fiber-/Carbon Fiber-Reinforced Polymer Nanocomposites. J. Mater. Eng. Perform. 2022, 31, 3847–3858. [Google Scholar] [CrossRef]
- Dhinakaran, V.; Lavanya, M.; Vigneswari, K.; Ravichandran, M.; Vijayakumar, M. Review on exploration of graphene in diverse applications and its future horizon. Mater. Today Proc. 2020, 27, 824–828. [Google Scholar] [CrossRef]
- Monetta, T.; Acquesta, A.; Bellucci, F. Graphene/Epoxy Coating as Multifunctional Material for Aircraft Structures. Aerospace 2015, 2, 423–434. [Google Scholar] [CrossRef]
- Dagdag, O.; Hsissou, R.; Berisha, A.; Erramli, H.; Hamed, O.; Jodeh, S.; El Harfi, A. Polymeric-Based Epoxy Cured with a Polyaminoamide as an Anticorrosive Coating for Aluminum 2024-T3 Surface: Experimental Studies Supported by Computational Modeling. J. Bio-Tribo-Corrosion 2019, 5, 1–13. [Google Scholar] [CrossRef]
- Madhankumar, A.; Nagarajan, S.; Rajendran, N.; Nishimura, T. EIS evaluation of protective performance and surface characterization of epoxy coating with aluminum nanoparticles after wet and dry corrosion test. J. Solid State Electrochem. 2012, 16, 2085–2093. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; Mu, S.; Fu, N.; Liao, Z. Comparative study on Ti/Zr/V and chromate conversion treated aluminum alloys: Anti-corrosion performance and epoxy coating adhesion properties. Appl. Surf. Sci. 2017, 405, 157–168. [Google Scholar] [CrossRef]
- Yuan, C.; Zhao, M.; Sun, D.; Yang, L.; Zhang, L.; Guo, R.; Yao, F.; An, Y. Preparation and properties of few-layer graphene modified waterborne epoxy coatings. J. Appl. Polym. Sci. 2018, 135, 46743. [Google Scholar] [CrossRef]
- Daradmare, S.; Raj, S.; Bhattacharyya, A.R.; Parida, S. Factors affecting barrier performance of composite anti-corrosion coatings prepared by using electrochemically exfoliated few-layer graphene as filler. Compos. Part B Eng. 2018, 155, 1–10. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Aditya, D.M.; Budiman, W.J.; Rahayu, S.; Alvan, F.M.; Karim, G. Fabrication and Evaluation of Graphene-Based Materials through Electrochemical Exfoliation and Expansion Mechanism; AIP Publishing LLC: Melville, NY, USA, 2022; p. 050016. [Google Scholar]
- Raza, M.A.; Westwood, A. Thermal contact resistance of various carbon nanomaterial-based epoxy composites developed for thermal interface applications. J. Mater. Sci. Mater. Electron. 2019, 30, 10630–10638. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Siddaiah, A.; Menezes, P.L. Synergistic wear-corrosion analysis and modelling of nanocomposite coatings. Tribol. Int. 2018, 121, 30–44. [Google Scholar] [CrossRef]
- Chang, K.-C.; Hsu, M.-H.; Lu, H.-I.; Lai, M.-C.; Liu, P.-J.; Hsu, C.-H.; Ji, W.-F.; Chuang, T.-L.; Wei, Y.; Yeh, J.-M.; et al. Room-temperature cured hydrophobic epoxy/graphene composites as corrosion inhibitor for cold-rolled steel. Carbon 2014, 66, 144–153. [Google Scholar] [CrossRef]
- Shaker, M.; Salahinejad, E.; Cao, W.; Meng, X.; Asl, V.Z.; Ge, Q. The effect of graphene orientation on permeability and corrosion initiation under composite coatings. Constr. Build. Mater. 2022, 319, 126080. [Google Scholar] [CrossRef]
- Rafique, I.; Kausar, A.; Muhammad, B. Fabrication and Characterization of High-Performance Diglycidyl Ether of Bisphenol-A/Tetrabromobisphenol-A Blend Reinforced with Multiwalled Carbon Nanotube Composite. Polym.-Plast. Technol. Eng. 2017, 56, 321–333. [Google Scholar] [CrossRef]
- Anwar, Z.; Kausar, A.; Khan, L.A.; Muhammad, B. Modified graphene nanoplatelet and epoxy/block copolymer-based nanocomposite: Physical characteristic and EMI shielding studies. Nanocomposites 2016, 2, 141–151. [Google Scholar] [CrossRef]
- Kausar, A.; Anwar, Z.; Khan, L.A.; Muhammad, B. Functional graphene nanoplatelet reinforced epoxy resin and polystyrene-based block copolymer nanocomposite. Full Nanotub. Carbon Nanostructures 2017, 25, 47–57. [Google Scholar] [CrossRef]
- Kausar, A. Polyimide, polybenzimidazole-in situ-polyaniline nanoparticle and carbon nano-onion-based nanocomposite designed for corrosion protection. Int. J. Polym. Anal. Charact. 2017, 22, 557–567. [Google Scholar] [CrossRef]
- Kausar, A. High performance epoxy/polyester-based nanocomposite coatings for multipurpose applications: A review. J. Plast. Film Sheeting 2020, 36, 391–408. [Google Scholar] [CrossRef]
- Kausar, A. Versatile epoxy/polyaniline and derived nanocomposite: From strategic design to advance application. Mater. Res. Innov. 2021, 25, 321–330. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Sun, H.; Gao, C. Superstructured assembly of nanocarbons: Fullerenes, nanotubes, and graphene. Chem. Rev. 2015, 115, 7046–7117. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Kujur, M.S.; Mallick, A.; Gupta, M. Development of Magnesium Nanocomposites by Powder Metallurgy for Multifunctional Applications: Review. Curr. Nanomater. 2021, 6, 185–206. [Google Scholar] [CrossRef]
- Pavase, T.R.; Lin, H.; Shaikh, Q.-U.-A.; Hussain, S.; Li, Z.; Ahmed, I.; Lv, L.; Sun, L.; Shah, S.B.H.; Kalhoro, M.T. Recent advances of conjugated polymer (CP) nanocomposite-based chemical sensors and their applications in food spoilage detection: A comprehensive review. Sens. Actuators B Chem. 2018, 273, 1113–1138. [Google Scholar] [CrossRef]
- Deng, S.; Djukic, L.; Paton, R.; Ye, L. Thermoplastic–epoxy interactions and their potential applications in joining composite structures—A review. Compos. Part A Appl. Sci. Manuf. 2015, 68, 121–132. [Google Scholar] [CrossRef]
- McMillon-Brown, L. Biomimetic advances in photovoltaics with potential aerospace applications. In Biomimicry for Aerospace; Elsevier: Amsterdam, The Netherlands, 2022; pp. 291–329. [Google Scholar]
- Momoh, A.; Adams, F.; Samuel, O.; Bolade, O.; Olubambi, P. Corrosion Prevention: The Use of Nanomaterials. In Modified Nanomaterials for Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 91–105. [Google Scholar]
- Zhang, J.; Zhu, A. Study on the synthesis of PANI/CNT nanocomposite and its anticorrosion mechanism in waterborne coatings. Prog. Org. Coat. 2021, 159, 106447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).