Characterization of Organic Molecules Grafted to Silica or Bismuth Nanoparticles by NMR
Abstract
:1. Introduction
2. Materials and Methods
3. Results
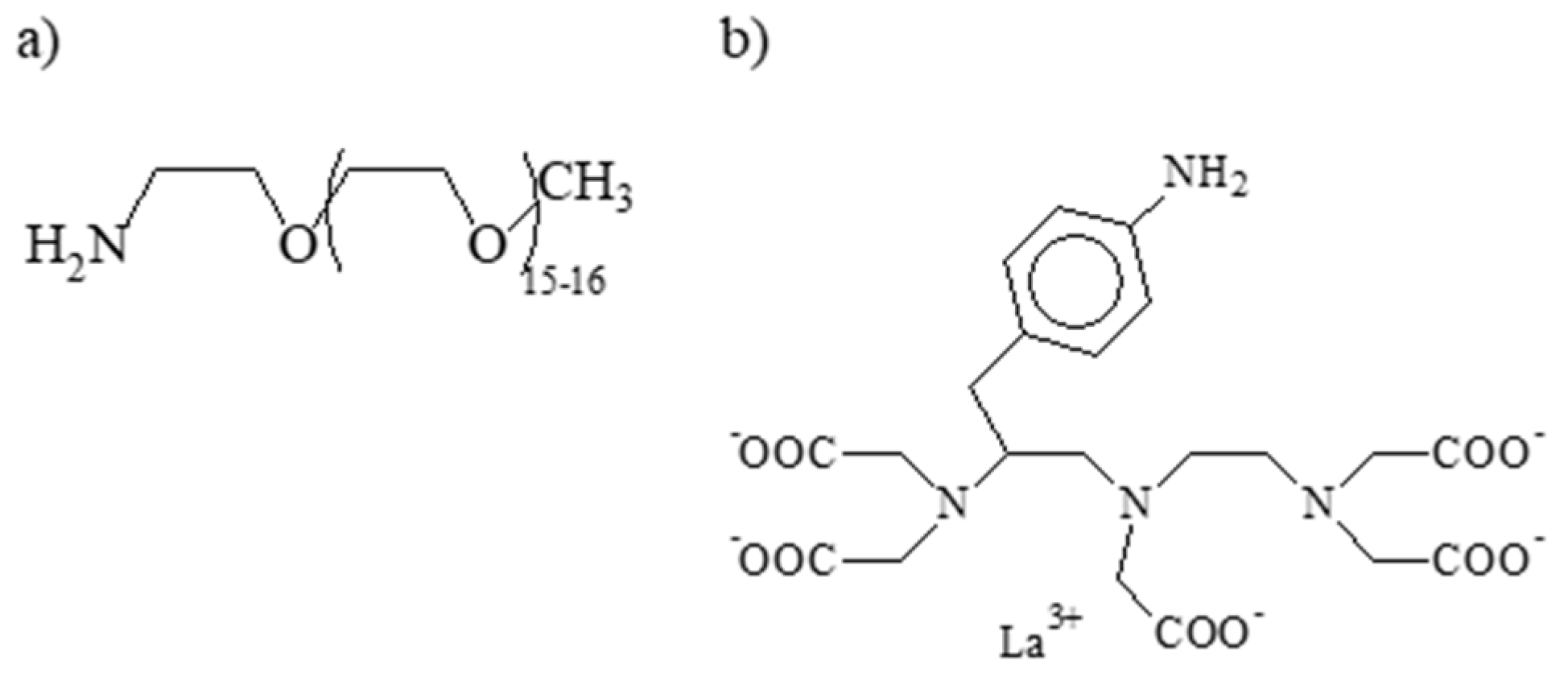
3.1. Silica Nanoparticles
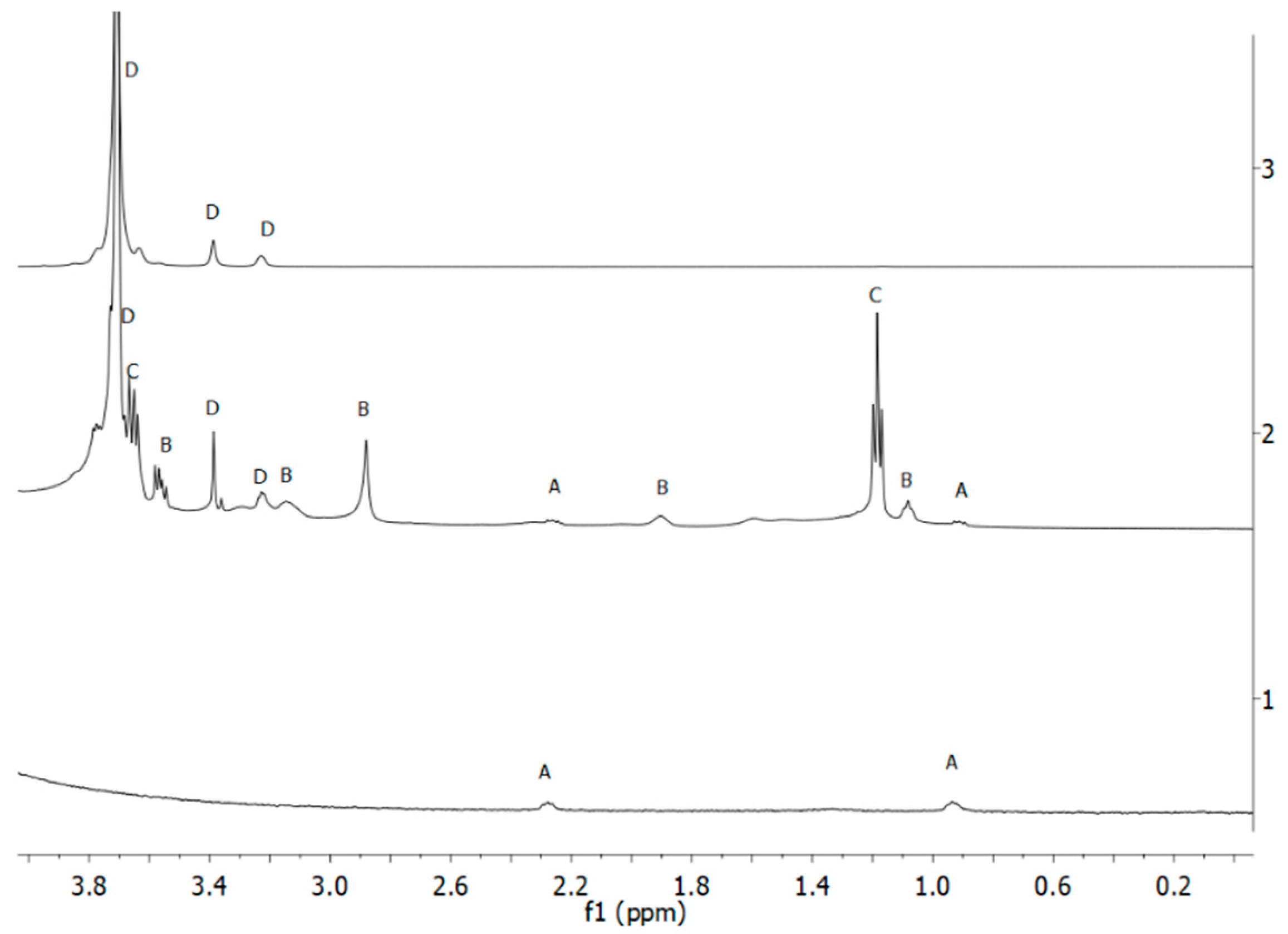
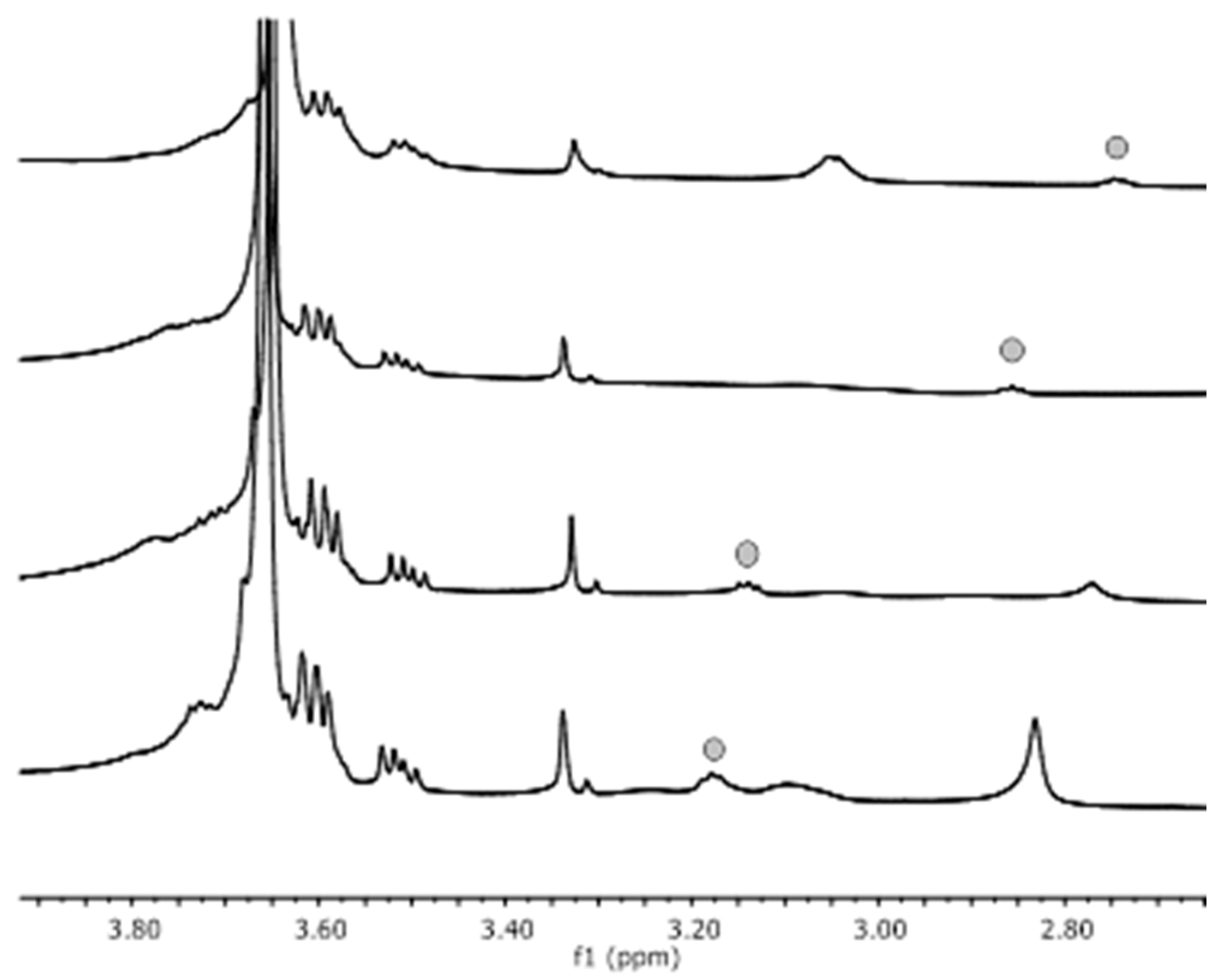
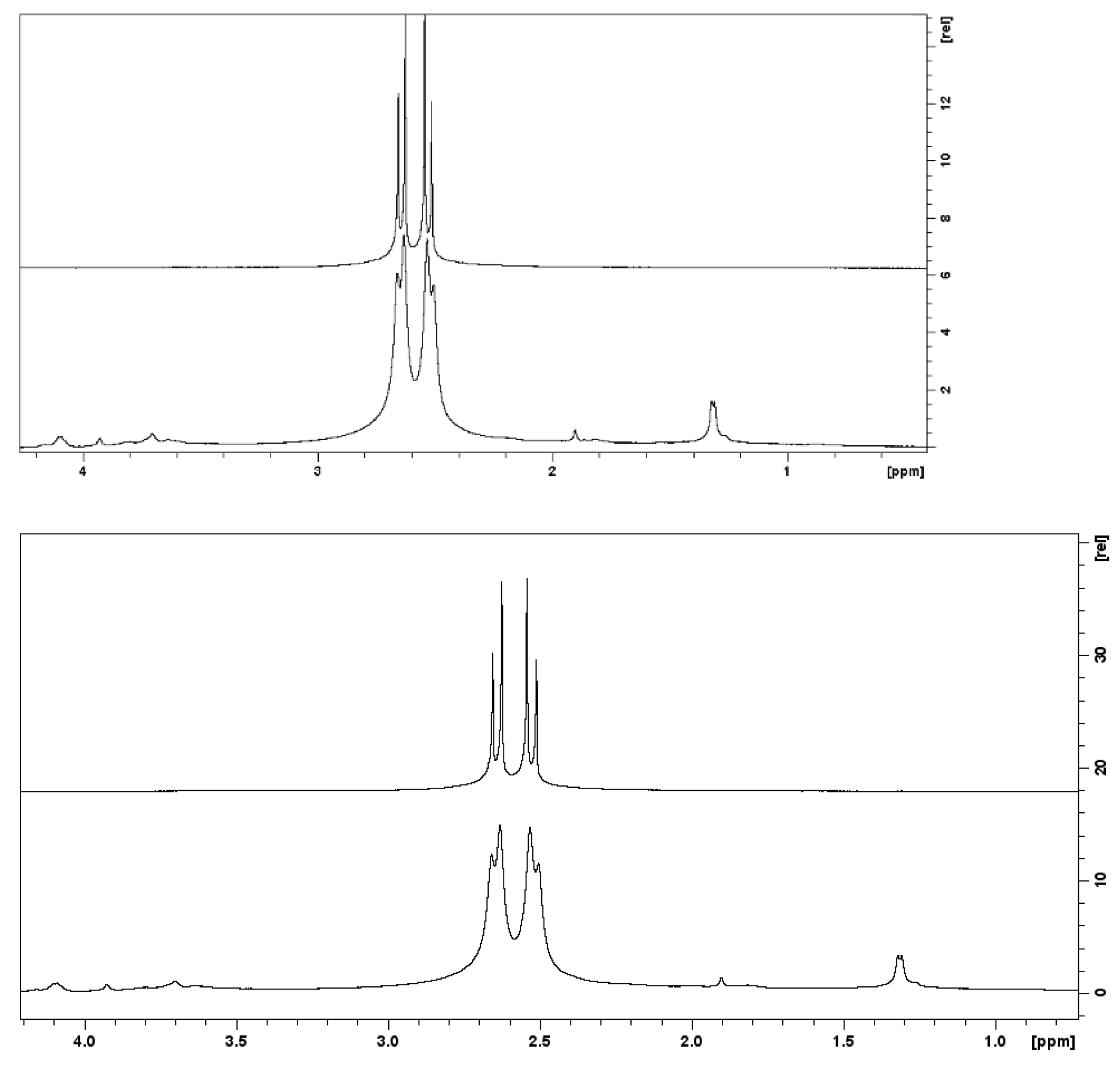
3.1.1. NMR Analysis of the Silica Nanoparticles Grafted with PEG and Synthesized with a Reaction Time of 4 h
3.1.2. NMR Analysis of the Silica Nanoparticles Grafted with PEG and with La-p-NH2-Bn-DTPA Synthesized with a Reaction Time of 24 h
3.2. Bismuth Nanoparticles
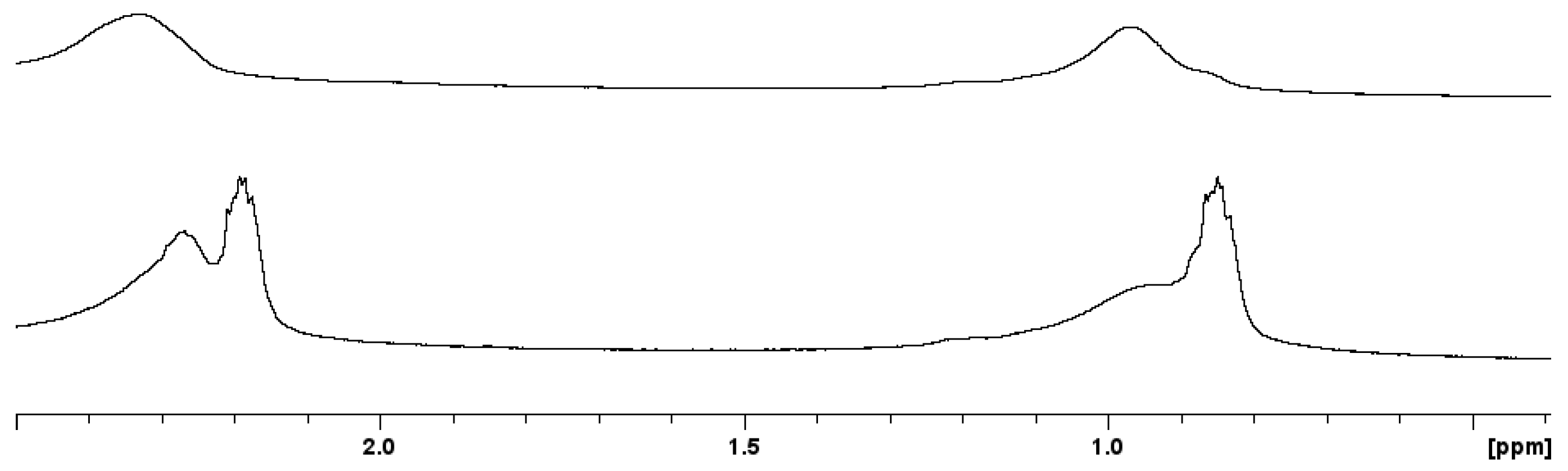
3.2.1. Nanoparticles Stabilized with Citrate
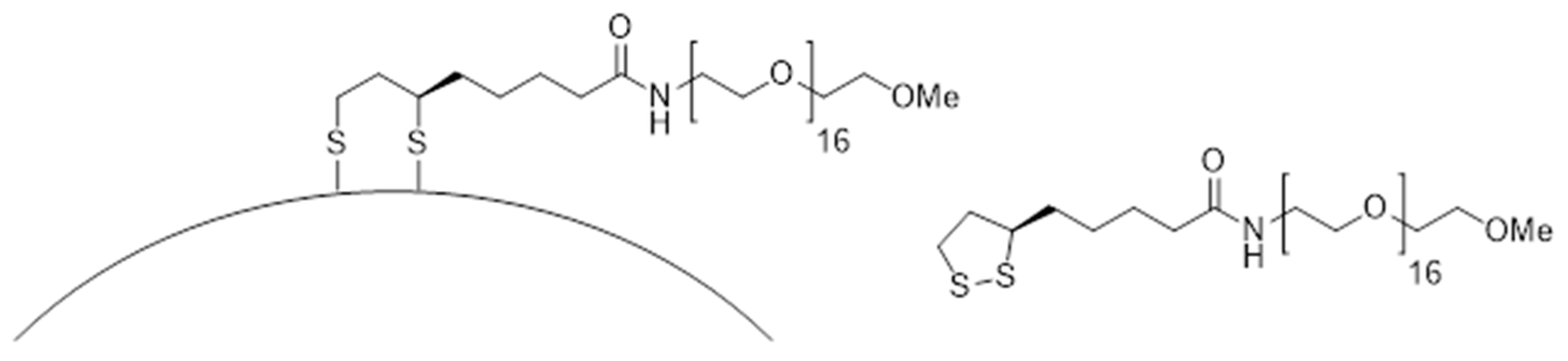
3.2.2. Nanoparticles Stabilized with Lipoic Acid Modified with PEG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Grumezescu, A.M. (Ed.) Inorganic Frameworks as Smart Nanomedicines; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Dumas, C.; Meledandri, C.J. Insights into the Partitioning Behavior of Secondary Surfactants in a Microemulsion-Based Synthesis of Metal Nanoparticles: A DLS and 2D NMR Spectroscopic Investigation. Langmuir 2015, 31, 7193–7203. [Google Scholar] [CrossRef]
- Sarker, M.; Fraser, R.E.; Lumsden, M.D.; Anderson, D.J.; Rainey, J.K. Characterization of Variant Soft Nanoparticle Structure and Morphology in Solution by NMR Spectroscopy. J. Phys. Chem. C 2015, 119, 7461–7471. [Google Scholar] [CrossRef]
- Henoumont, C.; Laurent, S.; Muller, R.N.; Vander Elst, L. HR-MAS NMR spectroscopy: An innovative tool for the characterization of iron oxide nanoparticles tracers for molecular imaging. Anal. Chem. 2015, 87, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Van Zijl, P.C.M.; Sehgal, A.A. Proton chemical exchange saturation transfer (CEST) MRS and MRI. eMagRes 2016, 5, 1307–1332. [Google Scholar]
- Ferrauto, G.; Delli Castelli, D.; Di Gregorio, E.; Terreno, E.; Aime, S. LipoCEST and cellCEST imaging agents: Opportunities and challenges. WIREs Nanomed. Nanobiotechnol. 2016, 8, 602–618. [Google Scholar] [CrossRef]
- McMahon, M.T.; Gilad, A.A. Cellular and Molecular Imaging Using Chemical Exchange Saturation Transfer. Top. Magn. Reson. Imaging 2016, 25, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, C.F.G.C.; Laurent, S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Henoumont, C.; Stanicki, D.; Boutry, S.; Lipani, E.; Belaid, S.; Muller, R.N.; Vander Elst, L. MRI Contrast Agents, from molecules to particles. In SpringerBriefs in Applied Sciences and Technology; Springer Nature: Singapore, 2017. [Google Scholar]
- Laurent, S.; Henoumont, C.; Vander Elst, L.; Muller, R.N. Synthesis and Physicochemical Characterisation of Gd-DTPA Derivatives as Contrast Agents for MRI. Eur. J. Inorg. Chem. 2012, 2012, 1889–1915. [Google Scholar] [CrossRef]
- Bonnet, C.S.; Toth, E. Molecular magnetic resonance imaging probes based on Ln3+ complexes. Adv. Inorg. Chem. 2016, 68, 43–96. [Google Scholar]
- Aime, S.; Botta, M.; Terreno, E. Gd(III)-based contrast agents for MRI. Adv. Inorg. Chem. 2005, 57, 173–237. [Google Scholar]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008, 108, 2064–2110, Erratum in Chem. Rev. 2010, 110, 2574. [Google Scholar] [CrossRef]
- Mathieu, P.; Coppel, Y.; Respaud, M.; Thi Quyen, N.; Boutry, S.; Laurent, S.; Stanicki, D.; Henoumont, C.; Novio, F.; Lorenzo, J.; et al. Silica Coated Iron/Iron Oxide Nanoparticles as a Nano-Platform for T2 Weighted Magnetic Resonance Imaging. Molecules 2019, 24, 4629. [Google Scholar] [CrossRef] [Green Version]
- Washner, J.; Gale, E.M.; Rodriguez-Rodriguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Devreux, M.; Henoumont, C.; Dioury, F.; Stanicki, D.; Boutry, S.; Larbanoix, L.; Ferroud, C.; Muller, R.N.; Laurent, S. Bimodal Probe for Magnetic Resonance Imaging and Photoacoustic Imaging Based on a PCTA-Derived Gadolinium(III) Complex and ZW800–1. Eur. J. Inorg. Chem. 2019, 29, 3354–3365. [Google Scholar] [CrossRef]
- Botta, M.; Tei, L. Relaxivity Enhancement in Macromolecular and Nanosized GdIII-Based MRI Contrast Agents. Eur. J. Inorg. Chem. 2012, 12, 1945–1960. [Google Scholar] [CrossRef]
- Mertz, D.; Affolter-Zbaraszczuk, C.; Barthès, J.; Cui, J.; Caruso, F.; Baumert, T.F.; Voegel, J.-C.; Ogier, J.; Meyer, F. Templated assembly of albumin-based nanoparticles for simultaneous gene silencing and magnetic resonance imaging. Nanoscale 2014, 6, 11676–11680. [Google Scholar] [CrossRef]
- Chen, W.; Cormode, D.P.; Vengrenyuk, Y.; Herranz, B.; Feig, J.E.; Klink, A.; Mulder, W.J.M.; Fisher, E.A.; Fayad, Z.A. Collagen-specific peptide conjugated HDL nanoparticles as MRI contrast agent to evaluate compositional changes in atherosclerotic plaque regression. JACC Cardiovasc. Imaging 2013, 6, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.E.; Shkumatov, A.; Withers, S.G.; Yang, B.; Glockner, J.F.; Misra, S.; Roy, E.J.; Wong, C.-H.; Zimmerman, S.C.; Kong, H. A Polymeric Fastener Can Easily Functionalize Liposome Surfaces with Gadolinium for Enhanced Magnetic Resonance Imaging. ACS Nano 2013, 7, 9599–9610. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, M.J.; De Campo, L.; Hirabayashi, M.; Bean, P.A.; Waddington, L.J.; Scoble, J.A.; Coia, G.; Drummond, C.J. Gadolinium-DTPA amphiphile nanoassemblies: Agents for magnetic resonance imaging and neutron capture therapy. Biomater. Sci. 2014, 2, 924–935. [Google Scholar] [CrossRef] [Green Version]
- Ndiaye, M.; Malytskyi, V.; Vangijzegem, T.; Sauvage, F.; Wels, M.; Cadiou, C.; Moreau, J.; Henoumont, C.; Muller, R.N.; Harakat, D.; et al. Comparison of MRI Properties between Multimeric DOTAGA and DO3A Gadolinium-Dendron Conjugates. Inorg. Chem. 2019, 58, 12798–12808. [Google Scholar] [CrossRef] [PubMed]
- Granato, L.; Longo, D.; Boutry, S.; Vander Elst, L.; Henoumont, C.; Aime, S.; Muller, R.N.; Laurent, S. Synthesis and Relaxometric Characterization of New Poly[N,N-bis(3-aminopropyl)glycine] (PAPGly) Dendrons Gd-Based Contrast Agents and Their in Vivo Study by Using the Dynamic Contrast-Enhanced MRI Technique at Low Field (1 T). Chem. Biodivers. 2019, 16, e1900322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Duong, H.T.T.; Laurent, S.; Macmillan, A.; Whan, R.M.; Vander Elst, L.; Muller, R.N.; Hu, J.; Lowe, A.; Boyer, C.; et al. Nanoparticles based on star polymers as theranostic vectors: Endosomal-triggered drug release combined with MRI sensitivity. Adv. Healthc. Mater. 2015, 4, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-K.; Singh, A.; Heo, J.; Kim, D.; Lee, K.E.; Jeon, H.; Koh, J.; Kwon, I.-C.; Kim, S. Gadolinium-coordinated elastic nanogels for in vivo tumor targeting and imaging. Biomaterials 2013, 34, 6846–6852. [Google Scholar] [CrossRef] [PubMed]
- Abdukayum, A.; Yang, C.-X.; Zhao, Q.; Chen, J.-T.; Dong, L.-X.; Yan, X.-P. Gadolinium Complexes Functionalized Persistent Luminescent Nanoparticles as a Multimodal Probe for Near-Infrared Luminescence and Magnetic Resonance Imaging in Vivo. Anal. Chem. 2014, 86, 4096–4101. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Yao, X.; Zhu, Y.; Shen, J.; Yang, X.; Li, C. Multifunctional gadolinium-labeled silica-coated core/shell quantum dots for magnetic resonance and fluorescence imaging of cancer cells. RSC Adv. 2014, 4, 20641–20648. [Google Scholar] [CrossRef]
- Lipani, E.; Laurent, S.; Surin, M.; Vander Elst, L.; Leclère, P.; Muller, R.N. High-Relaxivity and Luminescent Silica Nanoparticles as Multimodal Agents for Molecular Imaging. Langmuir 2013, 29, 3419–3427. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hossain, M.; Wang, C.; Qiao, Y.; An, J.J.; Ma, L.; Su, M. Targeted nanoparticles for enhanced X-ray radiation killing of multidrug-resistant bacteria. Nanoscale 2013, 5, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Su, M. Nanoparticle location and material dependent dose enhancement in X-ray radiation therapy. J. Phys. Chem. C Nanomater. Interfaces 2012, 116, 23047–23052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Xu, S.; Hu, W.; Xun, X.; Zheng, L.; Su, M. Tumor targeted, stealthy and degradable bismuth nanoparticles for enhanced X-ray radiation therapy of breast cancer. Biomaterials 2018, 154, 24–33. [Google Scholar] [CrossRef]
- Jiao, L.; Li, Q.Q.; Deng, J.; Okosi, N.; Xia, J.; Su, M. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Nanoscale 2018, 10, 6751–6757. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Naha, P.C.; Benavides-montes, V.; Litt, H.I.; Goforth, A.M.; Cormode, D.P. Synthesis, X-ray Opacity, and Biological Compatibility of Ultra-High Payload Elemental Bismuth Nanoparticle X-ray Contrast Agents. Chem. Mater. 2014, 26, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Unold, J.; Shuboni-mulligan, D.D.; Blanco-fernandez, B.; Shapiro, E.M. Surface engineering of bismuth nanocrystals to counter dissolution. Nanoscale 2016, 8, 13217–13222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swy, E.R.; Schwartz-Duval, A.S.; Shuboni, D.D.; Latourette, M.T.; Mallet, C.L.; Parys, M.; Cormode, D.P.; Shapiro, E.M. Dual-modality, fluorescent, PLGA encapsulated bismuth nanoparticles for molecular and cellular fluorescence imaging and computed tomography. Nanoscale 2014, 6, 13104–13112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.; Zhang, X.; Zhang, C.; Jiang, Y.; Fu, Y.Y.; Yu, C.; Sun, S.K.; Yan, X.P. Facile Synthesis of Uniform-Sized Bismuth Nanoparticles for CT Visualization of Gastrointestinal Tract in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 12720–12726. [Google Scholar] [CrossRef]
- Gomez, C.; Hallot, G.; Pastor, A.; Laurent, S.; Brun, E.; Sicard-Roselli, C.; Port, M. Metallic bismuth nanoparticles: Towards a robust, productive and ultrasound assisted synthesis from batch to flow-continuous chemistry. Ultrason. Sonochem. 2019, 56, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Branca, M.; Corp, K.; Ciuculescu-Pradines, D.; Coppel, Y.; Lecante, P.; Amiens, C. Insights into the chemistry of bismuth nanoparticles. New J. Chem. 2017, 41, 5960–5966. [Google Scholar] [CrossRef]
- Hallot, G.; Cagan, V.; Laurent, S.; Gomez, C.; Port, M. A greener chemistry process using microwaves in continuous flow to synthesize metallic bismuth nanoparticles. ACS Sustain. Chem. Eng. 2021, 9, 9177–9187. [Google Scholar] [CrossRef]
- Johnson Jr, C.S. Diffusion ordered nuclear magnetic resonance spectroscopy: Principles and applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. [Google Scholar] [CrossRef]
- Augé, S.; Amblard-Blondel, B.; Delsuc, M.-A. Investigation of the diffusion measurement using PFG and test of the robustness against experimented conditions and parameters. J. Chim. Phys. 1999, 96, 1559–1565. [Google Scholar] [CrossRef]
- Aureli, F.; D’Amato, M.; De Berardis, B.; Raggi, A.; Chiara Turco, A.; Cubadda, F. Investigating agglomeration and dissolution of silica nanoparticles in aqueous suspensions by dynamic reaction cell inductively coupled plasma-mass spectrometry in time resolved mode. J. Anal. At. Spectrom. 2012, 27, 1540–1548. [Google Scholar] [CrossRef]
- Fontecave, T.; Sanchez, C.; Azaïs, T.; Boissière, C. Chemical Modification As a Versatile Tool for Tuning Stability of Silica Based Mesoporous Carriers in Biologically Relevant Conditions. Chem. Mater. 2012, 24, 4326–4336. [Google Scholar] [CrossRef]
- Mahon, E.; Hristov, D.R.; Dawson, K.A. Stabilising fluorescent silica nanoparticles against dissolution effects for biological studies. Chem. Commun. 2012, 48, 7970–7972. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, M.; Jiang, S.; Förster, S. Two Growth Mechanisms of Thiol-Capped Gold Nanoparticles Controlled by Ligand Chemistry. Langmuir 2019, 35, 12130–12138. [Google Scholar] [CrossRef] [PubMed]
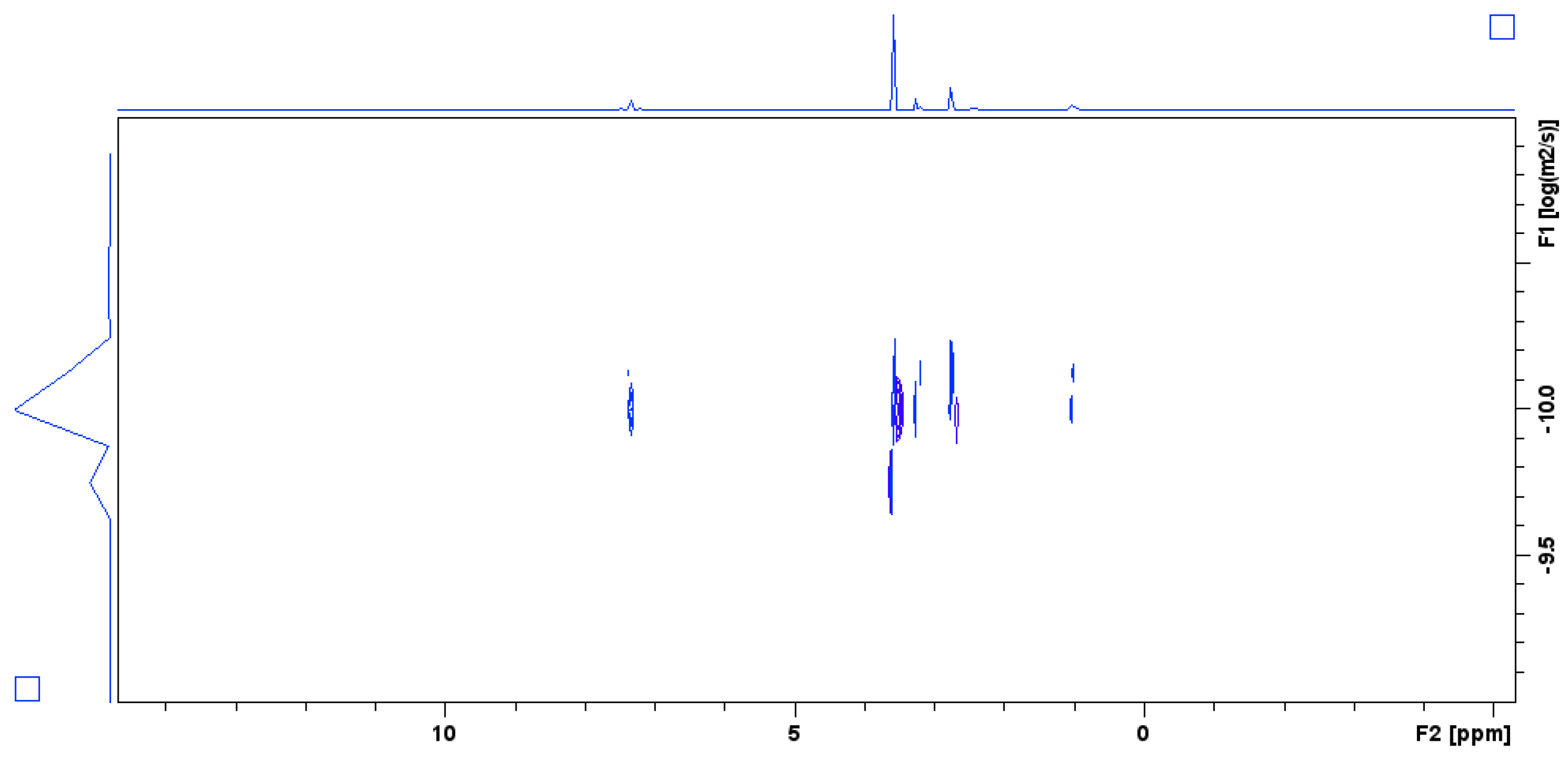
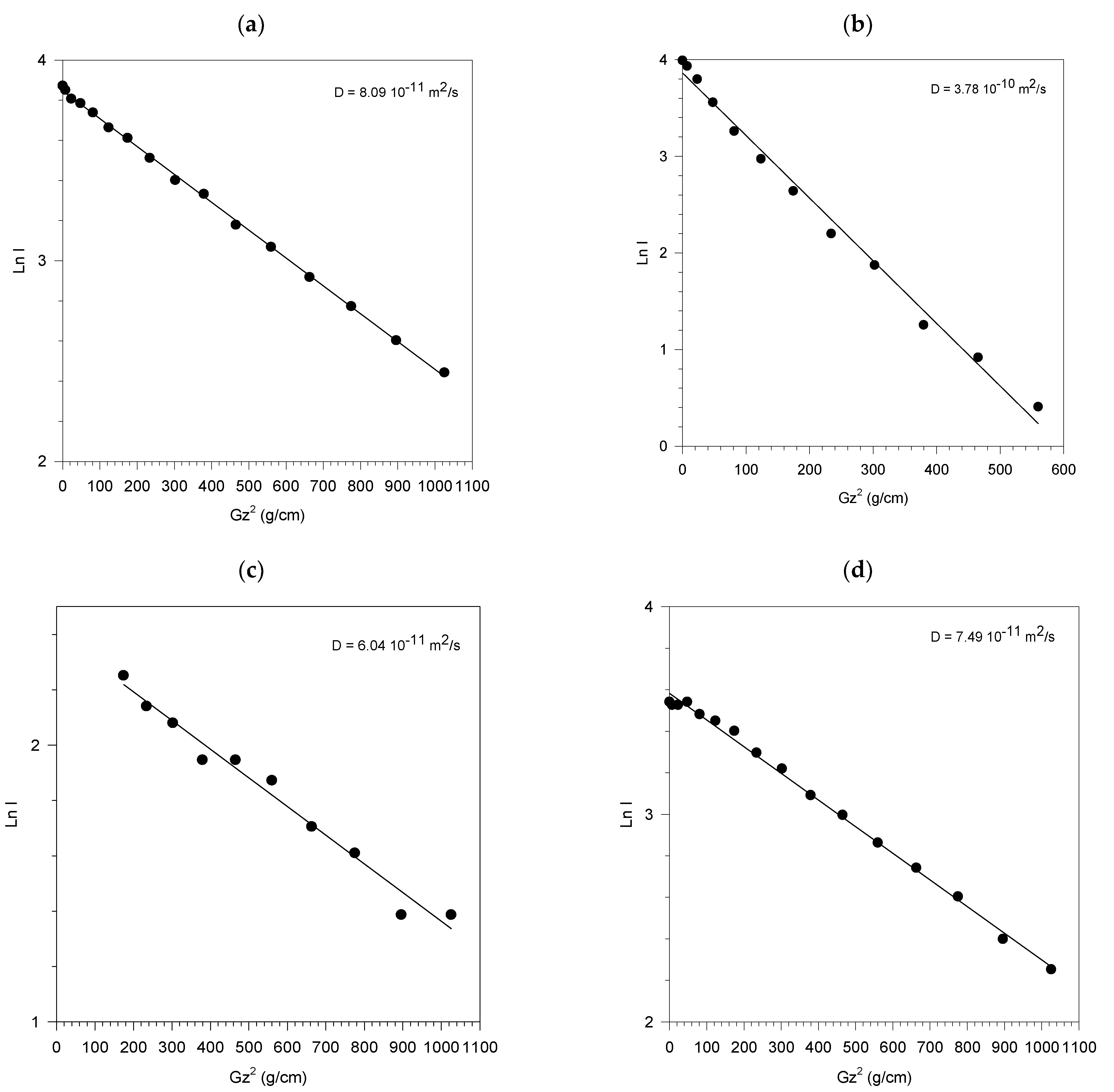


| ∆ = 250 ms | ∆ = 600 ms | ||
|---|---|---|---|
| SiO2-PEG | D (×10−10 m2/s) | SiO2-PEG | D (×10−10 m2/s) |
| pH = 6 | 2.49 | pH = 7 | D1 = 3.1 (I1 = 0.98) D2 = 0.16 (I2 = 0.02) |
| pH = 9 | 2.32 | ||
| pH = 10.5 | 2.64 | ||
| pH = 12 | 2.71 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henoumont, C.; Hallot, G.; Lipani, E.; Gomez, C.; Muller, R.N.; Vander Elst, L.; Port, M.; Laurent, S. Characterization of Organic Molecules Grafted to Silica or Bismuth Nanoparticles by NMR. Appl. Nano 2021, 2, 330-343. https://doi.org/10.3390/applnano2040024
Henoumont C, Hallot G, Lipani E, Gomez C, Muller RN, Vander Elst L, Port M, Laurent S. Characterization of Organic Molecules Grafted to Silica or Bismuth Nanoparticles by NMR. Applied Nano. 2021; 2(4):330-343. https://doi.org/10.3390/applnano2040024
Chicago/Turabian StyleHenoumont, Céline, Gauthier Hallot, Estelle Lipani, Catherine Gomez, Robert N. Muller, Luce Vander Elst, Marc Port, and Sophie Laurent. 2021. "Characterization of Organic Molecules Grafted to Silica or Bismuth Nanoparticles by NMR" Applied Nano 2, no. 4: 330-343. https://doi.org/10.3390/applnano2040024
APA StyleHenoumont, C., Hallot, G., Lipani, E., Gomez, C., Muller, R. N., Vander Elst, L., Port, M., & Laurent, S. (2021). Characterization of Organic Molecules Grafted to Silica or Bismuth Nanoparticles by NMR. Applied Nano, 2(4), 330-343. https://doi.org/10.3390/applnano2040024







