Optical tweezers, also more colloquially known as optical traps, are an exceptionally helpful tool for noninvasive and precise manipulation of microscopic items ranging in size from attached proteins to optical beads to Eukaryotic multicellular cells [
1]. In addition to this remarkable plasticity in size manipulation, optical tweezers can manipulate a host of different objects. Cellular components as small as proteins and DNA can be manipulated by attaching optically trapped glass beads, while objects as large as cell aggregates can be manipulated without beads. Optical tweezers were initially pioneered by Arthur Ashkin, whose seminal research into single-beam gradient traps laid the groundwork for advances over the past 30 years. Ashkin’s work outlying the single-beam gradient trap demonstrated how converging lenses could facilitate the study of microscopic biology species [
2]. Optical tweezers work by utilizing a set of focused lasers to hold an object in place, as shown in
Figure 1. This manipulation is carried out by opposing forces applied to the object on different sides, thus holding the object static in space [
1]. Two opposing forces implicated in this manipulation include scattering/absorbing and gradient forces. The scattering/absorbing force points in the direction of the main beam and tend to destabilize the trap in excess of the gradient force. The gradient force is proportional to the intensity of the laser and points toward the trap’s focal point. In order for the trap to remain stabilized, the sum of the gradient force must be in excess of the scattering/absorbing force [
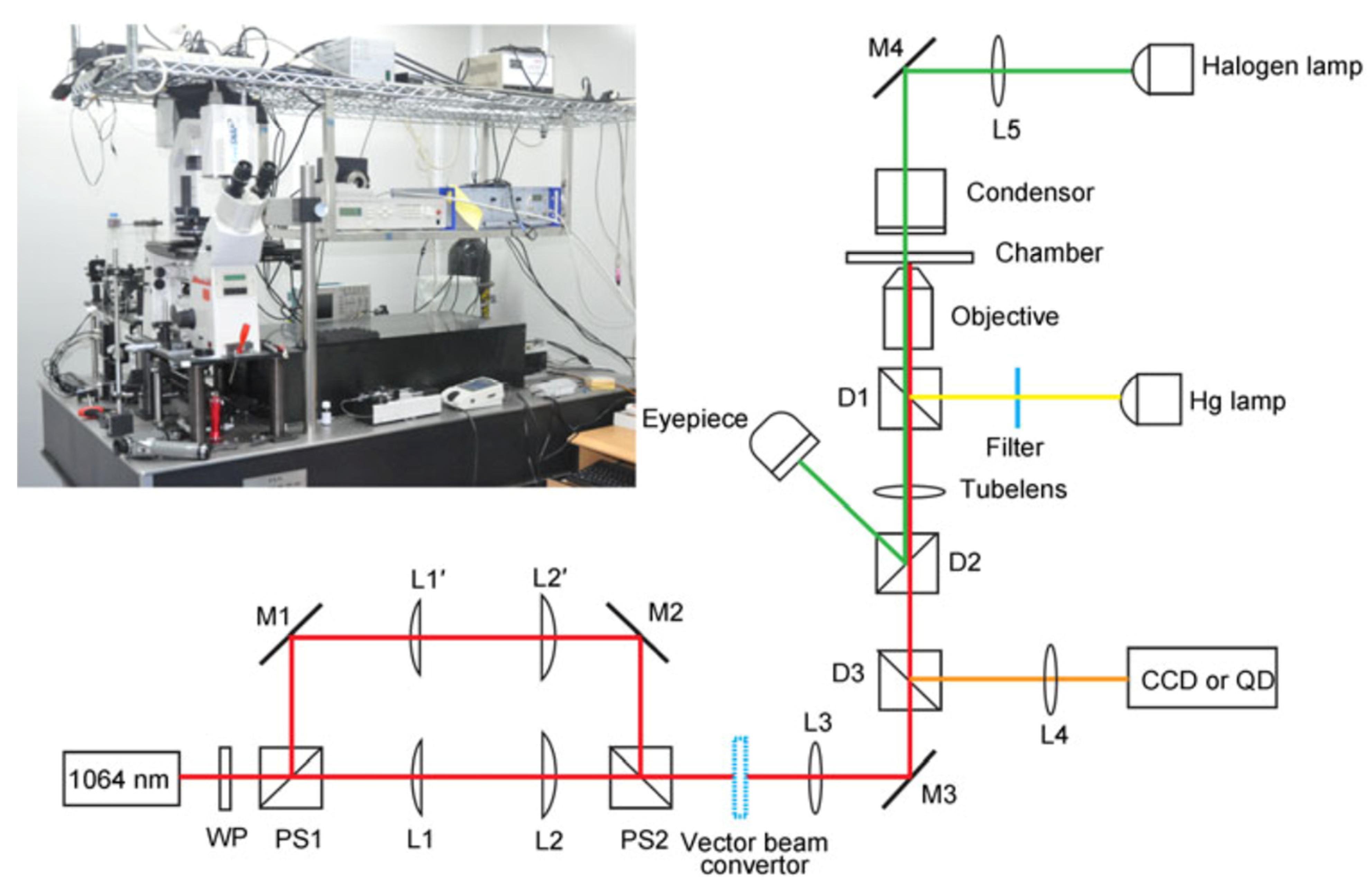
1]. As such, the varied functions of the optical tweezer model are applicable to a host of subset fields in nanotechnology. In the larger scope, the baseline model for optical tweezers is made by an infrared laser shone onto a series of mirrors which reflect the laser into a series of focusing lenses. The laser then reflects off another mirror into a microscopic objective, which then travels to a condenser. The area between the microscopic objective and condenser serves as the site in which the sample is manipulated. From the condenser, the laser passes through another reflective mirror and off another lens before finally reaching the Quadrant Photo Diode [QPD]. The QPD is utilized to measure the displacements of the laser and transmit them into force measurements. A reflective mirror is placed before the microscope objective to reflect the infrared light into another laser and then onto a camera. The camera is then able to record the movement of the sample in real-time [
3]. Originally the manipulation of biology species was significantly hindered by the damaging effect of the lasers. However, a transition to infrared lasers facilitated longer handling times of living organisms and thus the broader use of optical tweezers within the realm of biophysics [
4]. The damaging effect of high-intensity lasers along a specimen proves another consideration even when using infrared lasers. The process of opticution, or damage resulting from laser adsorption, and studies examining the phenomenon classify an optimal wavelength of 790–1064 nanometers when handling living organisms [
5]. Within the realm of membrane interaction studies, optical tweezers can be used to assess the strength of the membrane, fluidity, and protein concentration among the extracellular surface. The majority of studies and protocols utilizing optical tweezers while studying membranes use a membrane tether connected to an optically trapped bead [
3]. The importance of this tether is primarily associated with determining the equilibrium constants of the cell membrane. These constants can provide insights into the elasticity modulus of the membrane [
3]. The combination of variations in tether dynamics and the basal optical trap depicts the various assays which can be utilized in membrane studies. Arthur Ashkin’s work with light and latex beads has resulted in the emergence of optical tweezers as a prominent discipline within the greater clade of nanotechnology [
6]. Following a discussion on the advancement of membrane interaction studies due to the versatility of optical tweezers, a discussion of specific assays utilizing optical tweezers will be explored.
1.1. Holistic Membrane Interaction
The most pervasive causal factor for the myriad of assays derived from the basal optical tweezer state includes membrane interaction studies. These studies encompass a broad study base in which two cells can be placed near each other to evaluate their effects on their membranes, or a single membrane of a cell can be studied to determine its force measurements. In addition, external stimuli, such as polar molecules, can be applied to determine their effects on certain membrane protein channels. Such studies also have implications in the secondary messengers and other biochemical pathways. Membrane studies host the potential to explore many different optical trapping assays, primarily stemming from the cellular membrane’s complex and intricate makeup. Pulling assays can be introduced by applying a tether to an aspect of the cell membrane. If two tethers are applied to opposite ends of the cell, stretching assays can be applied to test the elasticity of the membrane or the cell as a whole. In addition, dielectric particles can be utilized to introduce indentation assays, whereby a part of the cell membrane is pushed down, which serves as another mode of measuring cell membrane elasticity. This section aims to illustrate the variety of assays that result from membrane interaction studies and their implications in further creating novel assays centered around the theory of optical tweezers.
Before the advent of optical tweezers within membrane interaction studies, membrane excitation was cited as a precursor and laid the groundwork for full cell body manipulations. Originally, Ashkin utilized radiation pressure from lasers to excite micro-sized particles. While exploring the effect of radiation pressure on particle movement and trapping, Ashkin would posit the hypothesis that the acceleration and trapping of a particle depend on the optical transitions [
7]. By building off his hypothesis for later studies, Ashkin utilized membrane excitation studies to excite microparticles and determine their effect on the function of macroscopic particles [
4]. As such, future studies, such as those conducted by Bar-Ziv et al., utilized optical lasers to excite the lipid bilayer leading to changes in function. Through initiating dynamic instabilities, the lipid bilayer performed functions ranging from exocytosis to breaks within the membrane [
8].
One of the major difficulties with using optical tweezers includes adequately and safely manipulating the specimen of interest while curbing the effects of the high output lasers. Ashkin utilized a novel optical CW atom laser in 2004, which utilized evaporative cooling to condense the atoms within the trap. The novel laser, thus, facilitated the study of membrane interaction by conserving the specimen of interest [
9]. Such studies include examining the effects of forces applied to the membrane and their cumulative effect on function. A study conducted by Sorre et al., examined the effect of force measurements on the function of lipid sorting. By using optical tweezers to measure the force necessary to act upon the membrane, the study’s results illustrated that the behavior of lipids within the cell membrane is augmented by the presence of lipid-clustering proteins and the presence of a high concentration of lipids in a particular section of the membrane [
10]. In similar regard, changes in membrane tension can lead to changes in cell motility [
11].
When studying the membrane interaction with optical tweezers, a critical cellular structure is the extracellular matrix. The extracellular matrix is an external skeleton of protein components that aid the cell in adhesion, transportation, communication, and protection. In order to further study the function of the extracellular matrix, studies performed by Sheetz et al. utilized ligand-binding receptors to pinpoint certain areas of attachment to assess the strength of the matrix and quantify its plasticity in force measurements [
12]. This protocol is also implicated in measuring molecular bond strength. Subsequent measurements include evaluating the ligand and molecular bond [
12]. This study also highlights many critical optical trapping assays in measuring the mechanical properties of cells. One of these assays includes nanoindentation, whereby a dielectric particle is utilized in an optical motility assay to indent part of a cell membrane [
12]. Another assay that further evaluates the elasticity of a cell or its subcellular components includes the application of a stretching assay [
12]. By applying a low-intensity optical laser to a cell held in an optical trap, force-displacement measurements can be taken to the elasticity of that particular part of the cell. Within the context of this review article, stretching assays can be applied to membranes to evaluate their elasticity. Such stretching assays can also be applied to different unicellular organisms such as bacteria which display a cell wall of peptidoglycan or lipopolysaccharides. Another critical component of optical trapping assays includes a tether extension. Tether extensions provide a host of different applications to optical traps, primarily in the realm of membrane interaction studies. Tethers can be spontaneously induced by the cell being studied or artificially [
12]. Tethers were utilized by attaching optically trapped beads to them in order to pinpoint a more precise effect on the cell membrane of interest. More specifically, tethers can be attached to specific receptors or aspects of the extracellular matrix and can subsequently be used to apply a pulling force on the membrane. A study examining the effect of plasma membrane tension on cell motility, utilized an optically trapped bead attached to a membrane tether to guide the elasticity of the cell membrane. The results of the study displayed that plasma membrane tension is inversely related to cell motility and thus a threshold for plasma membrane tension determines whether a cell can display motility [
13]. In particular, plasma membrane tension acts to coordinate and direct the movement of the cell by regulating polymerization in the direction of interest [
14]. Organelle-level interaction includes utilizing membrane tethers to study to the coalescence of vesicles within the Golgi apparatus. By pulling an artificial vesicle through a micropipette, the pulling force acting upon the tethers was observed and measured to calculate the rate of coalescence [
15].
When examining a microbiological perspective for optical tweezers, the single-cell bacterium was optically trapped and moved toward each other to determine the molecular mechanisms behind bacteria membranes [
16]. The bacteria utilized, Bacillus subtilis is a gram-positive bacterium with an exceptionally large cell wall consisting of peptidoglycan. The thick extracellular peptidoglycan sugar works to compensate for the lack of extracellular components present in higher-order cells such as Eukaryotes. An optical trap was utilized to hold a single bacterium in place, while another moving optical trap held a bacterium, which was brought in proximity to the static organism. The subsequent displacements of the lasers culminating in the optical traps were then translated into force measurements [
16]. Quorum sensing, a process by which two bacteria communicate, was concluded [
16]. This was because force measurements resulting from the displacement of the optical lasers depicted the two bacterial organisms were signaling that they were too close to each other. This particular setup of the optical tweezers is specifically referred to as Holographic optical tweezers. As opposed to the basal optical trap setup, Holographic optical tweezers utilize a spatial light modulator to manipulate and control two optical traps simultaneously. In addition, the laser power for this assay was dialed down to 3–5 milliwatts to avoid damaging the bacteria model organisms. In order to avoid overlapping the two optical lasers, the traps were not brought in less than 1 micrometer away from each other. The totality of the assay encompassed slowly moving one of the optical traps toward the static condition in 200 to 500-nanometer steps before moving in the opposite direction. The total process took 20 min to complete, while measurements were taken every 20 s [
16]. This study illustrates that the utility of optical tweezers extends beyond ligand-receptor interactions and can quantify the effects of signaling molecules on a cohort of organisms.
When examining the effects of malaria on Erythrocytes, optical tweezers were utilized to explore the effects of Merozoite and Erythrocyte complex. Malarial merozoites are implicated in invading red blood cells and rapidly proliferating throughout the body. In this study, Malarial merozoite was optically trapped and moved into proximity of an optically trapped red blood cell [
17]. When placed in proximity of the erythrocyte, the merozoite began to undergo membrane deformations which were characteristic of its invasive potential. In addition, to examining moving trap assays and the effects of proximity on cell membrane interactions, the effects of the merozoite on erythrocyte membrane composition were examined after the invasive target had attached to the red blood cell. By utilizing optical tweezers to study attached membranes, force measurements were derived from examinations of the merozoite binding and erythrocyte deformation [
17]. The study utilizes optical tweezers further by studying the effects of a known inhibitor of merozoite invasion into a red blood cell. The known inhibitor utilized was Heparin which acts by blocking the merozoite from attaching to the red blood cell. The result of the applied inhibitor displayed a statistically significant decrease in detachment force as compared to the wild-type, which leads to the implication of Heparin in curbing merozoite attachment and detachment [
17]. The totality of this study illustrates the versatility of optical tweezers in a host of different assays. Optical tweezers have similarly been applied to induce deformations in erythrocytes. Induced deformation studies demonstrate how optical tweezers can be utilized to investigate the viscoelastic properties of erythrocytes [
18]. Subsequent variations of the optical trapping and motility assays enabled the examination of the effects of the merozoite from the point of proximity, the point of preinvasive deformations of the merozoite, the subsequent deformations of the red blood cell from merozoite invasion, and an in-depth understanding of the mechanism in which the merozoite inhibitor Heparin works to curb red blood cell deformation.
Another critical cell component that is an important aspect of membrane interactions includes the mechanism by which they fuse. SNARE proteins or Soluble N-ethylmaleimide-sensitive factor attachment protein receptors act to tether cell membranes together and, through a zipper-like motion, force both the membranes to fuse by drawing them into proximity [
19]. As such, vesicle fusion through the SNARE complex poses an exceptional field of study. However, recent efforts to model the energetics of the SNARE complex have been extremely difficult to quantify. Thus, optical tweezers were utilized to calculate force-related measurements surrounding vesicle fusion through the SNARE complex. Coiled-coil SNARE complex was tethered to two optically trapped beads. One side of the coiled-coil complex was attached to a biotin tether receptor, while the other was attached to an anchor DNA. The anchor DNA was attached to the manipulated bead [
19]. From this point, extension force of the coiled-coil could be measured when a mechanical force is applied to the DNA-bound bead [
19]. This study illustrates the utility of a simple optical pulling assay in determining finer biochemical measurements associated with membrane-to-membrane interactions.
1.2. Pulling Assays
As depicted in the study which investigated SNARE complex mechanics, pulling assays play a critical role in membrane studies. In particular, membrane studies centered around attaching a moving optical bead to a part of the membrane or cytoskeleton provide a sufficient anchor for testing membrane elasticity. Since membrane elasticity is tied to cellular function, pulling assays can aid in further uncovering the mechanics behind certain biochemical functions. One study examined the relationship between cell elasticity and the regulation of function on macrophages and the various cells that make up the immune system [
20]. A single standard optical tweezer setup was utilized with the addition of a moving optical bead that was bound to the cell of the interest’s membrane. A glass slip was coated with 5 × 10
4 of the cells of interest. Uncoated polystyrene beads were subsequently placed on the slips and manipulated with optical tweezers. The optical tweezers captured each bead and held it close to the cell of interest for 5 s to allow for a proper membrane tether to form. Van der Waal forces are likely implicated in the formation of these tethers [
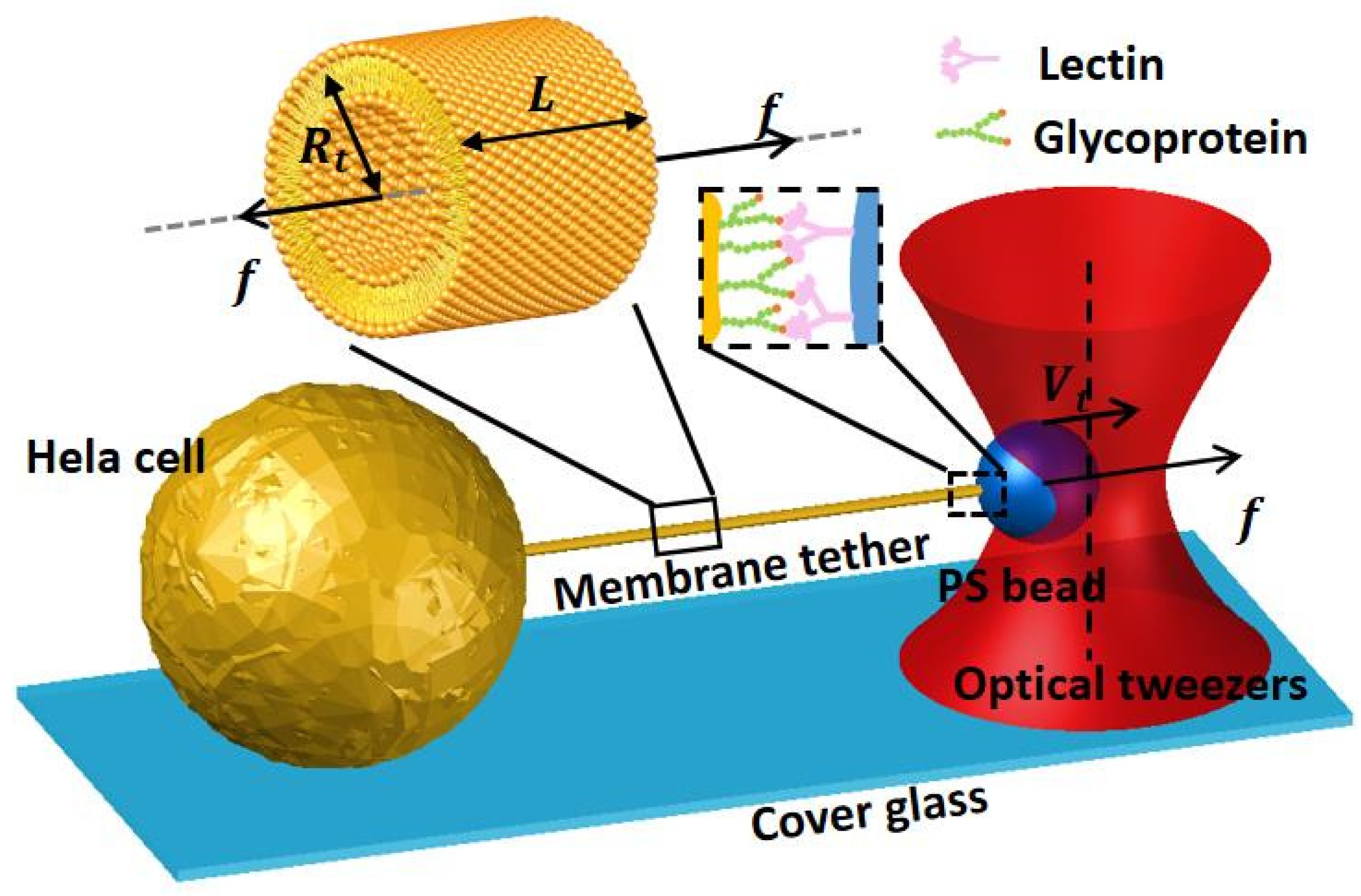
20]. Following the tether formation between the cell and bead, conventional pulling assays utilize the optically trapped bead to calculate force measurements surrounding membrane pulling. Another study examined the effects of a pulling assay on a HeLa cell, as shown in
Figure 2. A HeLa cell is an immortal cancer cell utilized in scientific studies. This particular study sought to examine the finer details of cell membrane stretching [
21]. A lectin-coated bead that formed a tether with the HeLa cell enabled them to measure the forces surrounding membrane bending [
21].
A significant area of study regarding pulling assays includes membrane tension studies. When cells undergo exocytosis, part of their membrane bubbles out and forms an independent vesicle. Many studies examine the effects of tension during cell exocytosis. As with other pulling assays, coated beads are attached to a tether that connects to a particular section of the cell membrane. Subsequently, optical tweezers are utilized to pull tension across the point of exocytosis [
22]. In studies examining plasma membrane morphology changes to exocytosis, a higher resolution capture is required. As such, simulation emission depletion microscopy [STED], can be utilized along with the optical trap to capture a higher resolution [
22]. A clearer visualization of membrane interaction can also be conducted through fluorescence applications followed by electroporation, or the process of deliberately increasing the permeability of the cell membrane [
23]. When requiring less precise methods of pulling assays, magnetic force transducers stand as an effective replacement for optical tweezers. While utilizing magnetic force transducers, a paramagnetic bead is tethered to the phospholipid bilayer of the cell membrane, and tension is applied by a mounted electromagnet [
24]. However, the standard procedure is best demonstrated in the studies performed by Li et al., in which optical tweezers were used to measure the membrane viscosity of outer hair cells by pulling tethers attached to the cell membrane at different rates, resulting in more precise measurements [
25].
1.3. Indentation Assays
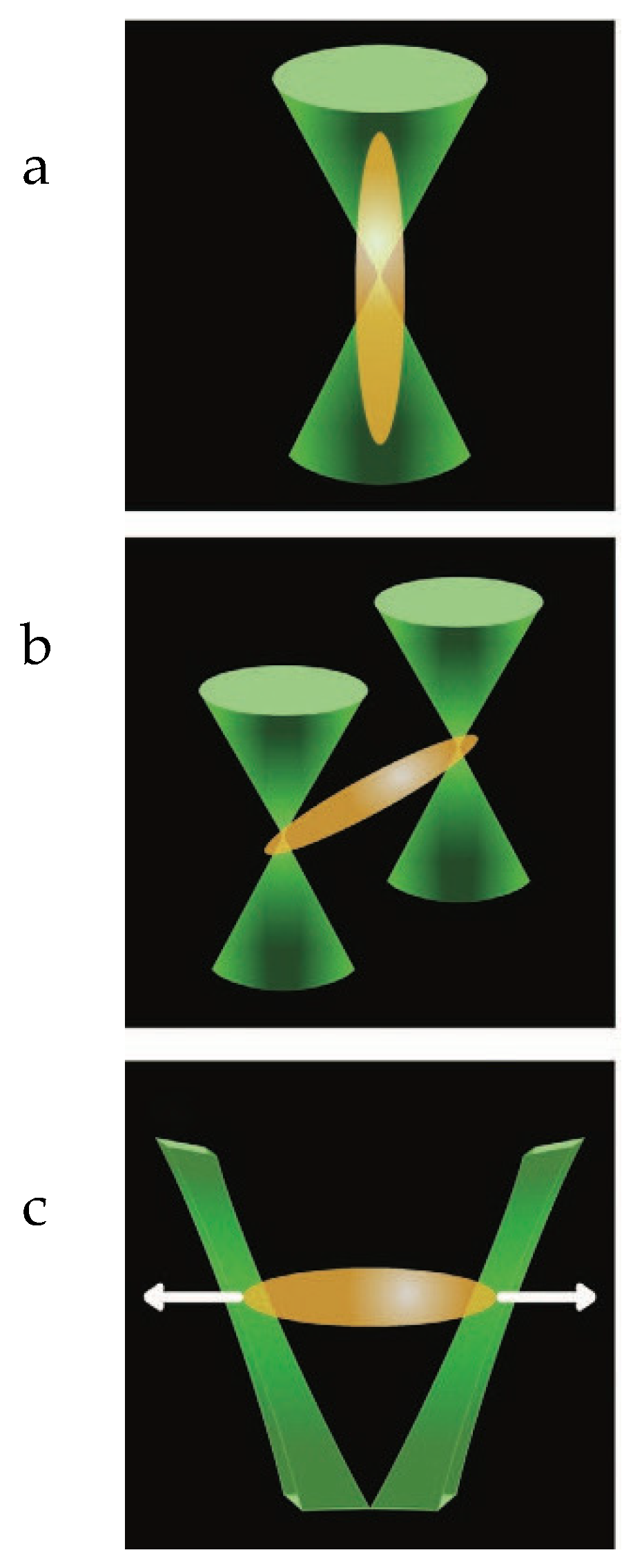
In similar regard to pulling assays, indentation assays also utilize an optically trapped bead to impose a force on the cell membrane. However, as opposed to pulling assays, indentation assays are utilized to measure the forces related to pushing down or indenting the cell membrane. In a study examining the effectiveness of cell indentation studies, an optical laser is conventionally set up with similar focal lenses and a Nikon camera. However, instead of utilizing a movable bead and a single optical trap, the effectiveness of indentation assays in optically trapped a bead was examined when utilizing optical tweezers to manipulate the cell [
26]. Specifically, the optically trapped bead is placed over the cell, and no contact is ensured to be present. The cell is then moved towards the bead. As the cell is moved closer, the bead begins to displace from its trapped condition. The displacement the bead travels can then be measured and quantified [
26]. As shown in
Figure 3a. In this study, the bead’s displacement was measured to calculate the elasticity modulus of the cell membrane. In a similar study performed by the same head author, the indentation assay was used to determine and compare the elasticity moduli of different cancer cell lines. The virulence of each cell line was assessed by examining the stiffness of the cell membrane when indented with an optically trapped bead. In addition, the previous indentation protocol was expanded upon by placing the cells on different coverslips with varying substrate flexibilities. This was done to mimic the epithelial tissue in which the cancer cells incubate and proliferate [
27]. The significance of this study illustrates the clinical application of indentation assays in cell identification and pathology. The use of indentation studies also facilitates other avenues of measurement beyond bead displacement. Another potential measurement of indentation displacement includes the placement and control of micron-sized beads on the surface of vesicles, which stand-in for the roles of vesicles and the cell membrane respectively [
28]. Further research into the elasticity modulus of cancer cell lines determined force measurements from vertical indentation by measuring the lateral motions in which the cell traveled [
29]. A composite evaluation of both lateral displacements of the cell and displacement of the optically trapped bead can provide an ample sampling of measurements within indentation assays. A different study that examined the stiffness values of keratinocytes depicted a variation of the indentation protocol by optically manipulating a bead into the cell [
30]. As shown in
Figure 3b. As opposed to the protocol followed by Yousafzai et al., this study laterally indented a moving bead into a cell instead of vertically moving the cell into a bead. The bead was moved laterally into the cell to avoid any unnecessary interactions from cytoskeletal elements and effectively make contact with the most consistent conformation of the lipid bilayer [
30]. Another important characteristic of membrane plasticity includes BAR domain. By examining the presence of endolymph when accompanying the indentation of a membrane, the potential curvature can be predicted [
31]. Indentation assays exhibit exceptional plasticity within their protocol to adapt to various cell and membrane types.