Synthesis, Characterization and Catalytic Activity of UiO-66-NH2 in the Esterification of Levulinic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
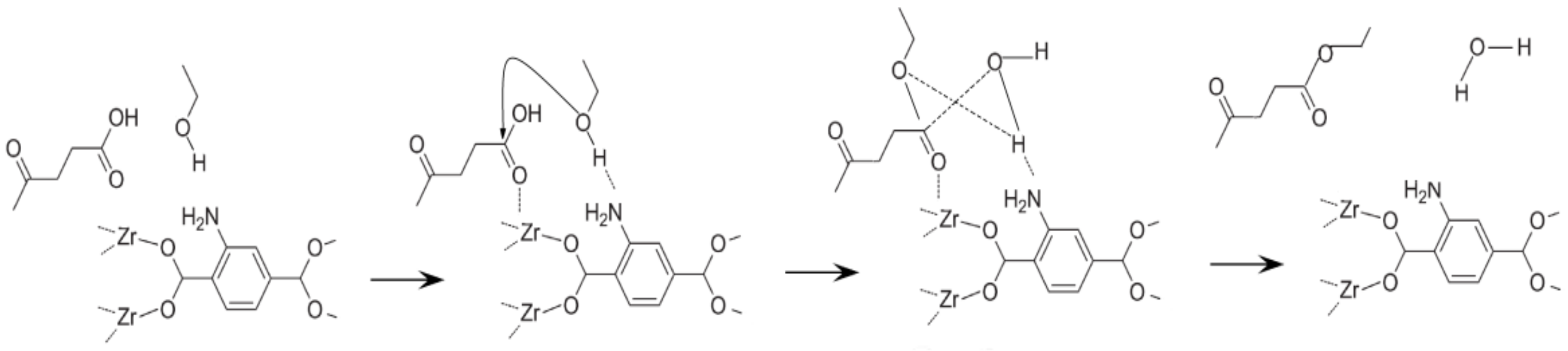
2.2. Synthesis of MOF UiO-66-NH2
2.3. Characterization of MOF UiO-66-NH2
2.4. Catalytic Esterification Reaction
3. Results
3.1. Physicochemical Characterization
3.1.1. X-ray Diffraction
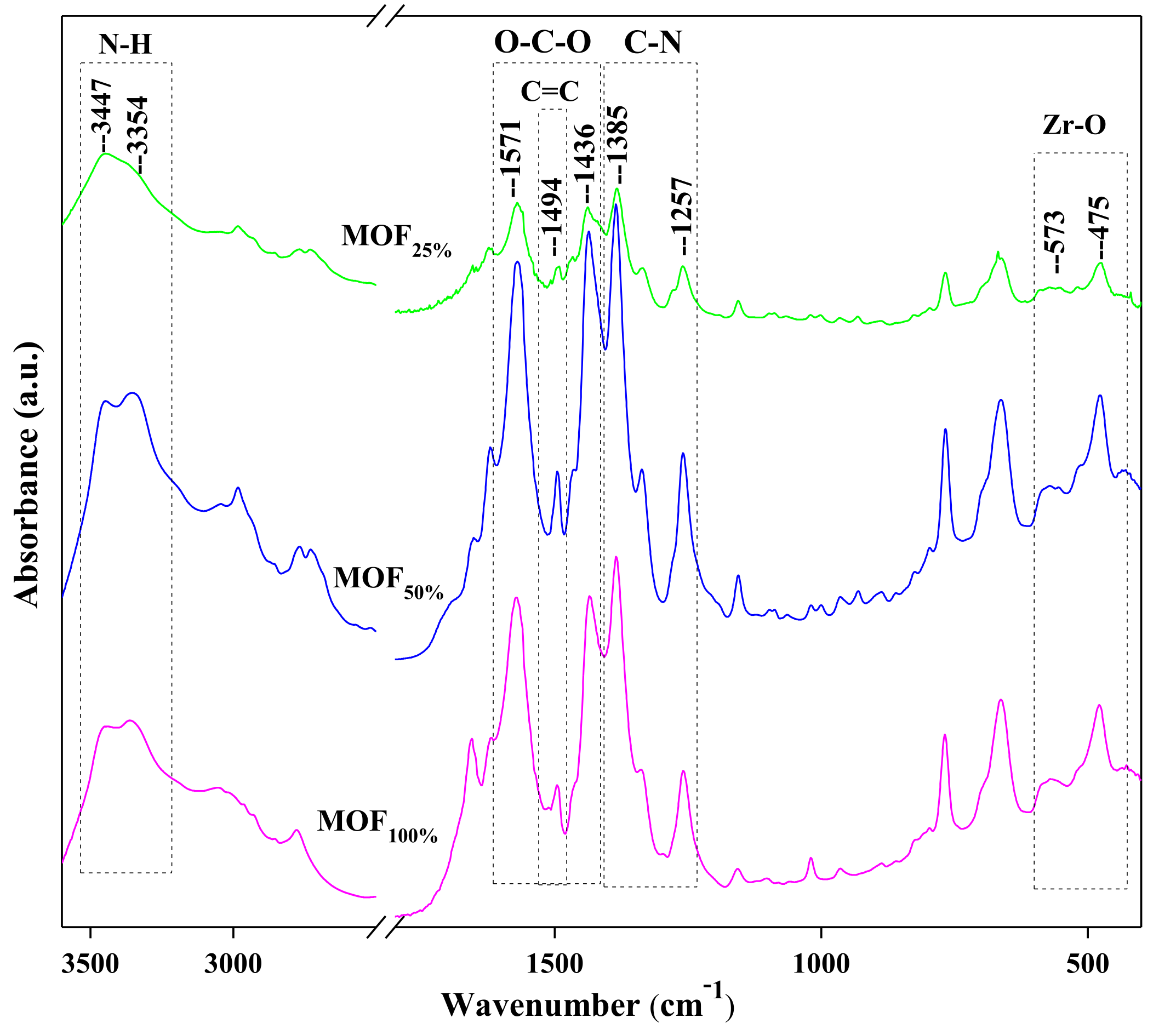
3.1.2. FTIR Spectroscopy
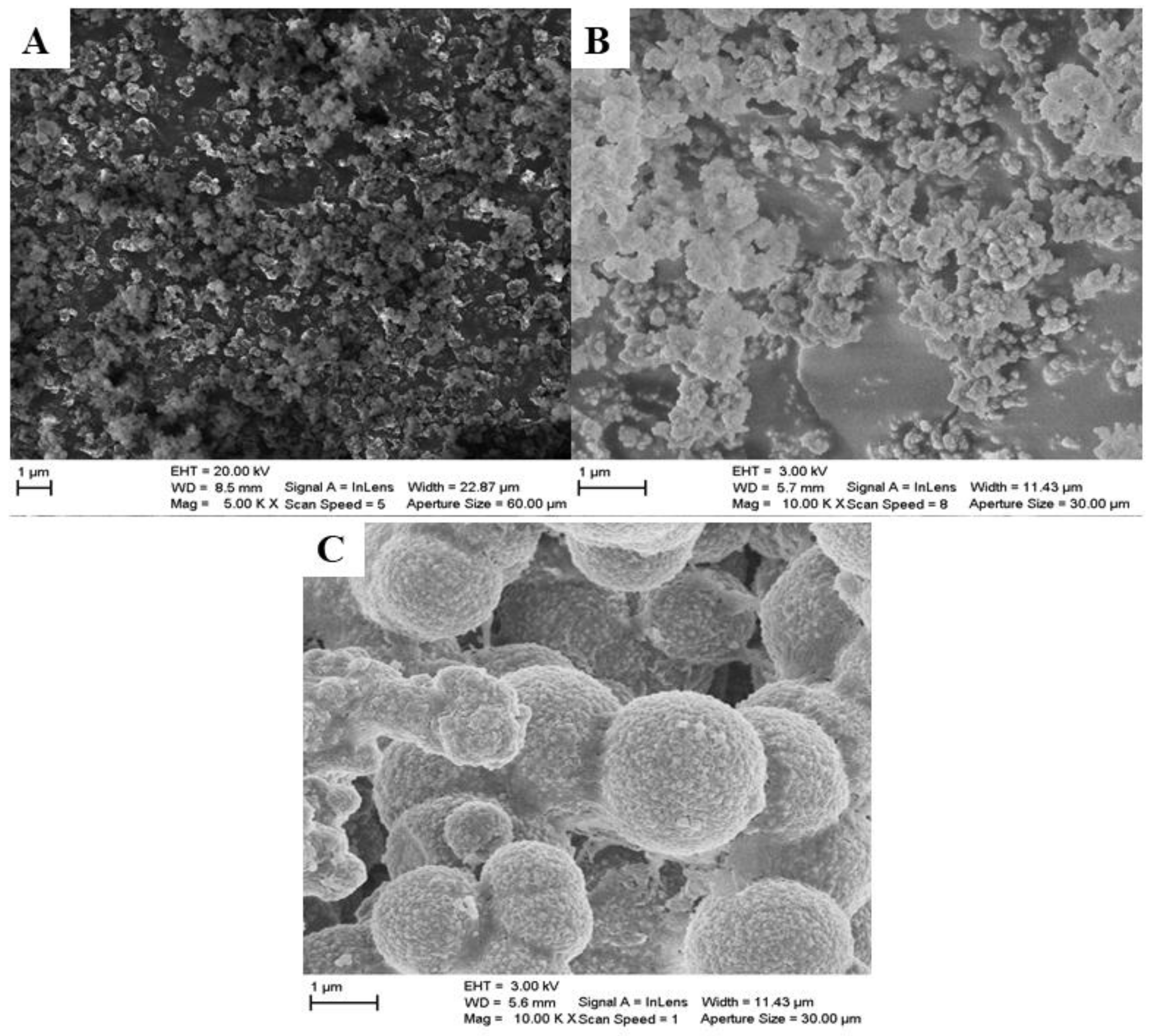
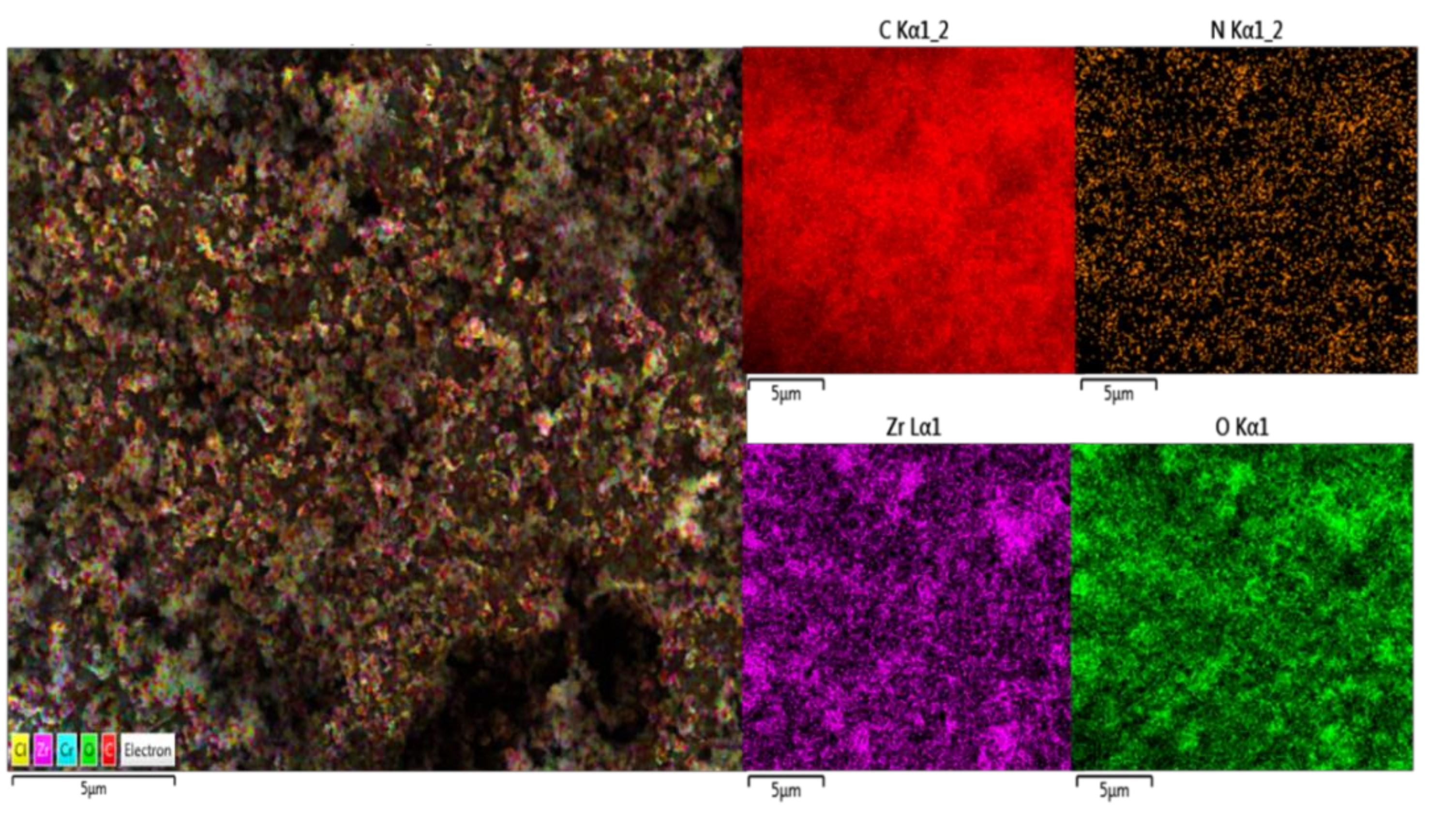
3.1.3. SEM-EDS Analysis
3.1.4. Microwave Plasma Atomic Emission Spectrometry (MP-AES) and Surface Area by BET
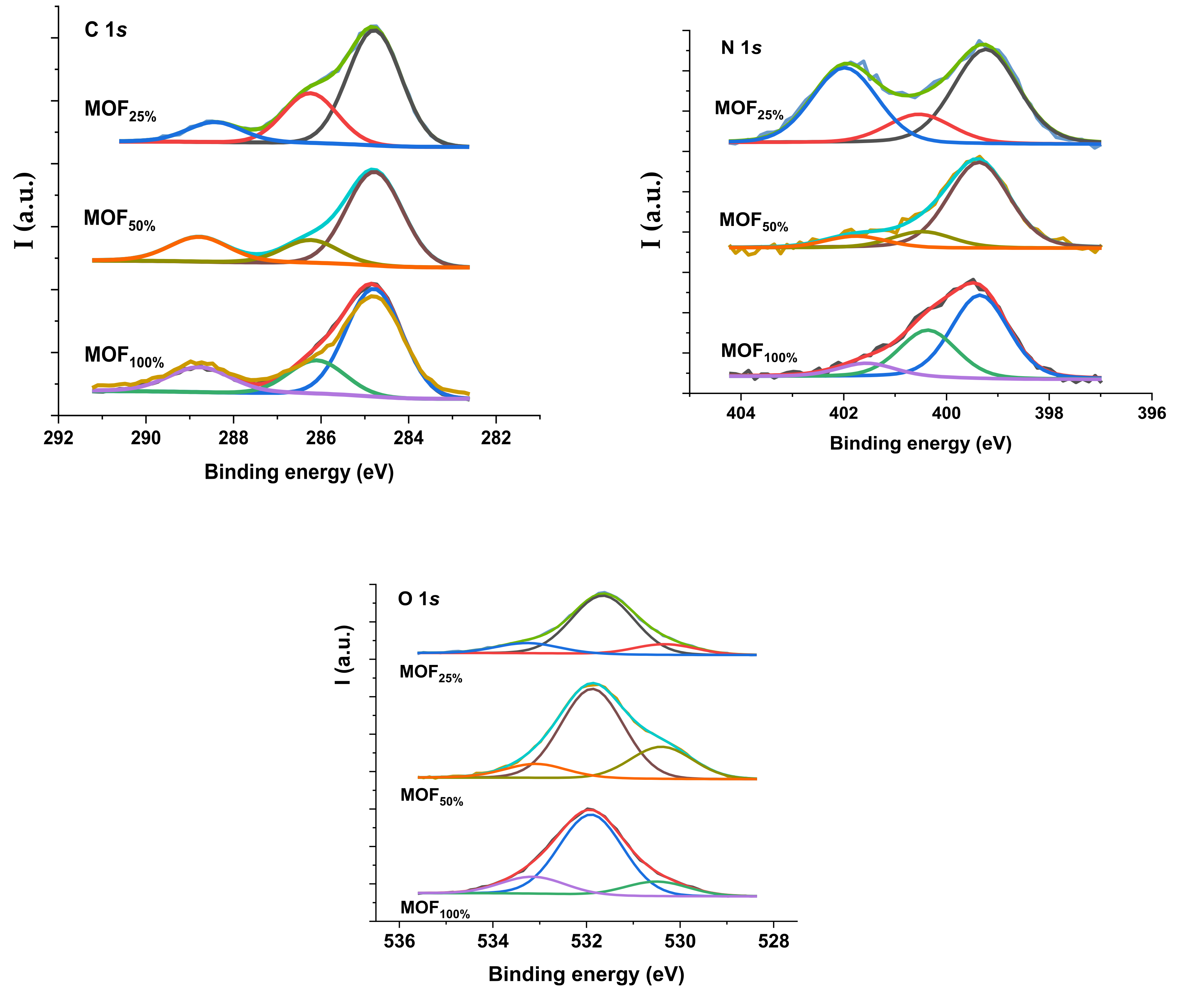
3.1.5. XPS-Spectroscopy
3.1.6. Acid Analysis by FTIR-CO
3.2. Catalytic Activity: Esterification for Ethyl Levulinate Production
3.3. Estimation of Kinetic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Factors governing dissolution process of lignocellulosic biomass in ionic liquid: Current status, overview and challenges. Bioresour. Technol. 2015, 178, 2–18. [Google Scholar] [CrossRef]
- Mikola, M.; Ahola, J.; Tanskanen, J. Production of levulinic acid from glucose in sulfolane/water mixtures. Chem. Eng. Res. Des. 2019, 148, 291–297. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Sarmah, A.K.; Sen, R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour. Technol. 2018, 247, 1144–1154. [Google Scholar] [CrossRef]
- Comba, M.B.; Tsai, Y.H.; Sarotti, A.M.; Mangione, M.I.; Suárez, A.G.; Spanevello, R.A. Levoglucosenone and its new applications: Valorization of cellulose residues. Eur. J. Org. Chem. 2018, 5, 590–604. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Chang, C.; Zhu, X.; Liu, X.; Han, J.; Ge, Q. Enhancing tungsten oxide/SBA-15 catalysts for hydrolysis of cellobiose through doping ZrO2. Appl. Catal. A Gen. 2016, 523, 182–192. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Shen, S.; Wang, C.; Han, Y.; Cai, B.; Li, H. Influence of reaction conditions on heterogeneous hydrolysis of cellulose over phenolic residue-derived solid acid. Fuel 2014, 134, 573–578. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lin, K.Y.A. Microwave-enhanced catalytic transfer hydrogenation of levulinic acid to γ-valerolactone using zirconium-based metal organic frameworks: A comparative study with conventional heating processes. J. Taiwan Inst. Chem. Eng. 2019, 96, 321–328. [Google Scholar] [CrossRef]
- Ogino, I.; Suzuki, Y.; Mukai, S.R. Esterification of levulinic acid with ethanol catalyzed by sulfonated carbon catalysts: Promotional effects of additional functional groups. Catal. Today 2018, 314, 62–69. [Google Scholar] [CrossRef]
- Xu, G.; Chang, C.; Zhu, W.; Li, B.; Ma, X.J.; Du, F.G. A comparative study on direct production of ethyl levulinate from glucose in ethanol media catalysed by different acid catalysts. Chem. Pap. 2013, 67, 1355–1363. [Google Scholar] [CrossRef]
- Bart, H.J.; Reidetschlager, J.; Dchatka, K.; Lehmann, A. Kinetics of esterification of levulinic acid with n-butanol by homogeneous catalysts. Ind. Eng. Chem. Res. 1994, 33, 21–25. [Google Scholar] [CrossRef]
- Omoruyi, U.; Page, S.; Hallett, J.; Miller, P.W. Homogeneous Catalyzed Reactions of Levulinic Acid: To γ-Valerolactone and Beyond. ChemSusChem 2016, 9, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, H.; Fábos, V.; Tuba, R.; Bodor, A.; Mika, L.T.; Horváth, I.T. Integration of Homogeneous and Heterogeneous Catalytic Processes for a Multi-step Conversion of Biomass: From Sucrose to Levulinic Acid, γ-Valerolactone, 1,4-Pentanediol, 2-Methyl-tetrahydrofuran, and Alkanes. Top. Catal. 2008, 48, 49–54. [Google Scholar] [CrossRef]
- Fernandes, D.R.; Rocha, A.S.; Maia, E.F.; Mota, C.J.A.; da Silva, V.T. Levulinic acid esterification with ethanol to ethyl levulinate production over solid acid catalysts. Appl. Catal. A Gen. 2012, 425, 199–204. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Nemoto, K.; Fujitani, T. Esterification of levulinic acid with ethanol over sulfated Si-dopedZrO2solid acid catalyst: Study of the structure–activity relationships. Appl. Catal. A Gen. 2014, 476, 186–196. [Google Scholar] [CrossRef]
- Popova, M.; Shestakova, P.; Lazarova, H.; Dimitrov, M.; Kovacheva, D.; Szegedi, A.; Mali, G.; Dasireddy, V.; Likozar, B.; Wilde, N.; et al. Efficient solid acid catalysts based on sulfated tin oxides for liquid phase esterification of levulinic acid with ethanol. Appl. Catal. A Gen. 2018, 560, 119–131. [Google Scholar] [CrossRef]
- Huang, Y.B.; Yang, T.; Cai, B.; Chang, X.; Pan, H. Highly efficient metal salt catalyst for the esterification of biomass derived levulinic acid under microwave irradiation. RSC Adv. 2016, 6, 2106–2111. [Google Scholar] [CrossRef]
- Melero, J.A.; Morales, G.; Iglesias, J.; Paniagua, M.; Hernández, B.; Penedo, S. Efficient conversion of levulinic acid into alkyl levulinates catalyzed by sulfonic mesostructured silicas. Appl. Catal. A Gen. 2013, 466, 116–122. [Google Scholar] [CrossRef]
- Antunes, M.M.; Lima, S.; Neves, P.; Magalhaes, A.L.; Fazio, E.; Neri, F.; Pereira, M.T.; Silva, A.F.; Silva, C.M.; Rocha, S.M.; et al. Integrated reduction and acid-catalysed conversion of furfural in alcohol medium using Zr,Al-containing ordered micro/mesoporous silicates. Appl. Catal. B 2016, 182, 485–503. [Google Scholar] [CrossRef] [Green Version]
- Pachamuthu, M.P.; Srinivasan, V.V.; Karvembu, R.; Luque, R. Preparation of mesoporous stannosilicates SnTUD-1 and catalytic activity in levulinic acid esterification. Microporous Mesoporous Mat. 2019, 287, 159–166. [Google Scholar] [CrossRef]
- Enumula, S.S.; Chada, V.R.B.G.R.R.; Burri, D.R.; Kamaraju, S.R.R. Clean synthesis of alkyl levulinates from levulinic acid over one pot synthesized WO3-SBA-16 catalyst. J. Mol. Catal. A Chem. 2017, 426, 30–38. [Google Scholar] [CrossRef]
- Chermahini, A.N.; Nazeri, M. Esterification of the levulinic acid with n-butyl and isobutyl alcohols over aluminum-containing MCM-41. Fuel Process. Technol. 2017, 167, 442–450. [Google Scholar] [CrossRef]
- Song, D.; An, S.; Sun, Y.; Guo, Y. Efficient conversion of levulinic acid or furfuryl alcohol into alkyl levulinates catalyzed by heteropoly acid and ZrO2 bifunctionalized organosilica nanotubes. J. Catal. 2016, 333, 184–199. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Tabassum, M.; Coutts, S.; Ttitirici, M.M. Esterification of levulinic acid into ethyl levulinate catalysed by sulfonated hydrothermal carbons. Chin. J. Catal. 2014, 35, 929–936. [Google Scholar] [CrossRef]
- Tejero, M.A.; Ramírez, E.; Fité, C.; Tejero, J.; Cunill, F. Esterification of levulinic acid with butanol over ion exchange resins. Appl. Catal. A Gen. 2016, 517, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Qiu, M.; Qi, X. Selective conversion of biomass-derived levulinic acid to ethyl levulinate catalyzed by metal organic framework (MOF)-supported polyoxometalates. Appl. Catal. A Gen. 2019, 572, 168–175. [Google Scholar] [CrossRef]
- Llorente, P.L. Síntesis y Aplicación Catalítica de Materiales MOF en Reacciones de Química Fina. Ph.D. Thesis, Universidad Rey Juan Carlos, Móstoles, Spain, 2017. [Google Scholar]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. J. Am. Chem. Soc. 2012, 112, 933–969. [Google Scholar]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials: A Review. J. Am. Chem. Soc. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Yujia, S.J.; Hong-Cai, Z. Recent progress in the synthesis of metal–organic frameworks. Sci. Technol. Adv. Mater. 2015, 16, 54202. [Google Scholar]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mat. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Abid, H.R.; Shang, J.; Ang, H.M.J.; Wang, S. Amino-functionalized Zr-MOF nanoparticles for adsorption of CO2 and CH4. Int. J. Smart Nano Mater. 2013, 4, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Zhang, H.; Song, F.; Huang, T.; Ji, J.; Zhong, Q.; Chu, W.; Xu, Q. UiO-66-NH2/GO Composite: Synthesis, Characterization and CO2 Adsorption Performance. Materials 2018, 11, 589. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Corma, A.; Xamena, F.X.L.i. Conversion of levulinic acid into chemicals: Synthesis of biomass derived levulinate esters over Zr-containing MOFs. Chem. Eng. Sci. 2015, 124, 52–60. [Google Scholar] [CrossRef]
- Negus, M.P.; Mansfield, A.C.; Leadbeater, N.E. The preparation of ethyl levulinate facilitated by flow processing: The catalyzed and uncatalyzed esterification of levulinic acid. J. Flow Chem. 2015, 5, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, A.F.; Ramos, R.; Moreira, M.M.; Soares, O.S.G.P.; Ribeiro, L.S.; Pereira, M.F.R.; Delerue-Matos, C.; Freire, C. Production of ethyl levulinate fuel bioadditive from 5-hydroxymethylfurfural over sulfonic acid functionalized biochar catalysts. Fuel 2021, 303, 121227. [Google Scholar] [CrossRef]
- Tukacs, J.M.; Sylvester, Á.; Kmecz, I.; Jones, R.V.; Óvári, M.; Mika, L.T. Continuous flow hydrogenation of methyl and ethyl levulinate: An alternative route to g-valerolactone production. R. Soc. Open Sci. 2019, 6, 182233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, L.; Iglesias, C.; Faroldi, B.; Zamaro, M.U.y.J. Efficient solvothermal synthesis of highly porous UiO-66 nanocrystals in dimethylformamide-free media. J. Mater. Sci. 2018, 53, 1862–1873. [Google Scholar] [CrossRef]
- Caratelli, C.; Hajek, J.; Cirujano, F.G.; Waroquier, M.; Xamenab, F.X.L.i.; van Speybroeck, V. Nature of active sites on UiO-66 and beneficial influence of water in the catalysis of Fischer esterification. J. Catal. 2017, 352, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Cirujano, F.G.; Corma, A.; Xamena, F.X.L.i. Zirconium-containing metal organic frameworks as solid acid catalysts for the esterification of free fatty acids: Synthesis of biodiesel and other compounds of interest. Catal. Today 2014, 274, 213–220. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Zaharudin, N.H.; Amin, N.A.S. Esterification of renewable levulinic acid to levulinate esters using amberlyst-15 as a solid acid catalyst. J. Teknol. 2017, 79, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Desidery, L.; Yusubov, M.S.; Zhuiykov, S.; Verpoort, F. Fully-sulfonated hydrated UiO66 as efficient catalyst for ethyl levulinate production by esterification. Catal. Commun. 2018, 117, 33–37. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Liu, Y.T.J.; Chen, S.Y. Adsorption of fluoride to UiO-66-NH2 in water: Stability, kinetic, isotherm and thermodynamic studies. J. Colloid Interface Sci. 2016, 461, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Wan, L.; Caro, J. Microwave-assisted synthesis of well-shaped UiO-66-NH2 with high CO2 adsorption capacity. Mater. Res. Bull. 2018, 98, 308–313. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Batten, M.P.; Polyzos, A.; Carey, K.C.; Mardel, J.I.; Lim, K.S.; Hill, M.R. Versatile, High Quality and Scalable Continuous Flow Production of Metal-Organic Frameworks. Sci. Rep. 2014, 4, 5443. [Google Scholar] [CrossRef]
- Wang, J.; Xia, T.; Zhang, X.; Zhang, Q.; Cui, Y.; Yang, Y.; Qian, G. A turn-on fluorescent probe for Cd 2+ detection in aqueous environments based on an imine functionalized nanoscale metal–organic framework. RSC Adv. 2017, 7, 54892–54897. [Google Scholar] [CrossRef] [Green Version]
- Arrozi, U.S.F.; Wijaya, H.W.; Patah, A.; Permana, Y. Efficient acetalization of benzaldehydes using UiO-66 and UiO-67: Substrates accessibility or Lewis acidity of zirconium. Appl. Catal. A Gen. 2015, 506, 77–84. [Google Scholar] [CrossRef]
- Lu, A.X.; McEntee, M.; Browe, M.A.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W. MOFabric: Electrospun Nanofiber Mats from PVDF/UiO-66-NH2 for Chemical Protection and Decontamination. Appl. Mater. Interfaces 2017, 9, 13632–13636. [Google Scholar] [CrossRef]
- Luu, C.L.; van Nguyen, T.T.; Nguyen, T.; Hoang, T.C. Synthesis, characterization and adsorption ability of UiO-66-NH2. Adv. Nat. Sci.-Nanosci. 2015, 6, 25004. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Liu, M.; Li, K.; Guo, X. Facile synthesis of morphology and size-controlled zirconium metal–organic framework UiO-66: The role of hydrofluoric acid in crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Hou, J.; Luan, Y.; Tang, J.; Wensley, A.M.; Yanga, M.; Lu, Y. Synthesis of UiO-66-NH2 derived heterogeneous copper (II) catalyst and study of its application in the selective aerobic oxidation of alcohols. J. Mol. Catal. A Chem. 2015, 407, 53–59. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, G.; Wang, L.; Li, J.; An, Q.; Ji, S. Pervaporation dehydration of acetic acid using NH2 -UiO-66/PEI mixed matrix membranes. Sep. Purif. Technol. 2017, 186, 20–27. [Google Scholar] [CrossRef]
- Tang, J.; Dong, W.; Wang, G.; Yao, Y.; Cai, L.; Liu, Y.; Tan, L. Efficient molybdenum (vi) modified Zr-MOF catalysts for epoxidation of olefins. RSC Adv. 2014, 4, 42977–42982. [Google Scholar] [CrossRef]
- Venturi, D.M.; Campana, F.; Marmottini, F.; Costantino, F.; Vaccaro, L. Extensive Screening of Green Solvents for Safe and Sustainable UiO-66 Synthesis. ACS Sustain. Chem. Eng. 2020, 8, 17154–17164. [Google Scholar] [CrossRef]
- Ploskonka, A.M.; Marzen, S.E.; DeCoste, J.B. Facile Synthesis and Direct Activation of Zirconium Based Metal–Organic Frameworks from Acetone. Ind. Eng. Chem. Res. 2017, 56, 1478–1484. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.; Reinoso, F.R.; Rouquerol, J.; Sing, K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Groen, J.C.; Peffer, L.A.; Pérez-Ramírez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Review. Microporous Mesoporous Mat. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Gomes, I.S.; de Carvalho, D.C.; Oliveira, A.C.; Rodríguez-Castellón, E.; Lang, R. On the reasons for deactivation of titanate nanotubes with metals catalysts in the acetalization of glycerol with acetone. Chem. Eng. 2018, 334, 1927–1942. [Google Scholar] [CrossRef]
- Travlou, N.A.; Ginnakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S- and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 04–111. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Dai, P.; Yan, L.; Cao, L.; Gu, X.; Zhao, X. Missing-node directed synthesis of hierarchical pores on a zirconium metal–organic framework with tunable porosity and enhanced surface acidity via a microdroplet flow reaction. J. Mater. Chem. A 2017, 5, 22372–22379. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Chakarova, K.; Strauss, I.; Mihaylov, M.; Drenchev, N.; Hadjiivanov, K. Evolution of acid and basic sites in UiO-66 and UiO-66-NH2 metal-organic frameworks: FTIR study by probe molecules. Microporous Mesoporous Mat. 2019, 281, 110–122. [Google Scholar] [CrossRef]
- DeStefano, M.R.; Islamoglu, T.; Garibay, S.J.; Hupp, J.T.; Farha, O.K. Room temperature synthesis of uio-66 and the thermal modulation of densities of defect sites. Chem. Mat. 2017, 29, 1357–1361. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Sonar, S.K.; Niphadkar, P.S.; Joshi, P.N.; Deshpande, S.S.; Patil, V.S.; Bokade, V.V. Catalytic upgrading of renewable levulinic acid to ethyl levulinate biodiesel using dodecatungstophosphoric acid supported on desilicated H-ZSM-5 as catalyst. Appl. Catal. A Gen. 2013, 460, 90–98. [Google Scholar] [CrossRef]
- Zubir, M.I.; Chin, S.Y. Kinetics of Modified Zirconia-catalyzed Heterogeneous Esterification Reaction for Biodiesel Production. Appl. Sci. 2010, 10, 2584–2589. [Google Scholar] [CrossRef] [Green Version]
- Jrad, A.; Tarboush, B.J.A.; Hmadeh, M.; Ahmad, M. Tuning acidity in zirconium-based metal organic frameworks catalysts for enhanced production of butyl butyrate. Appl. Catal. A Gen. 2019, 570, 31–41. [Google Scholar] [CrossRef]
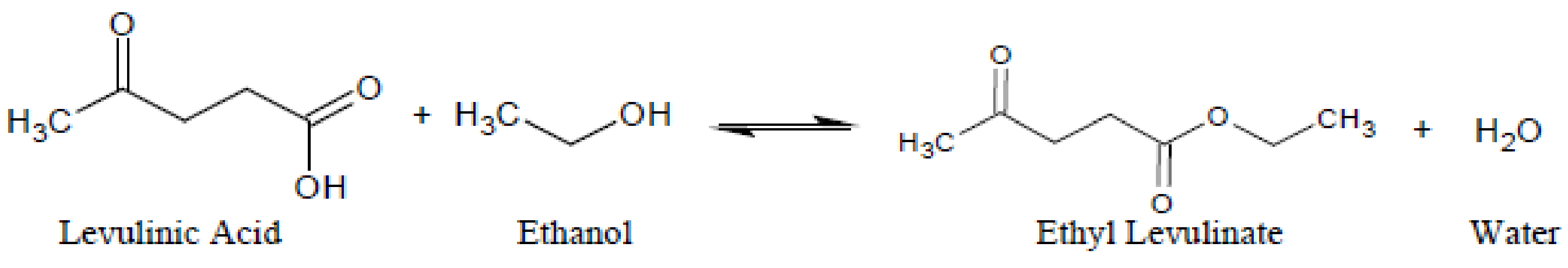

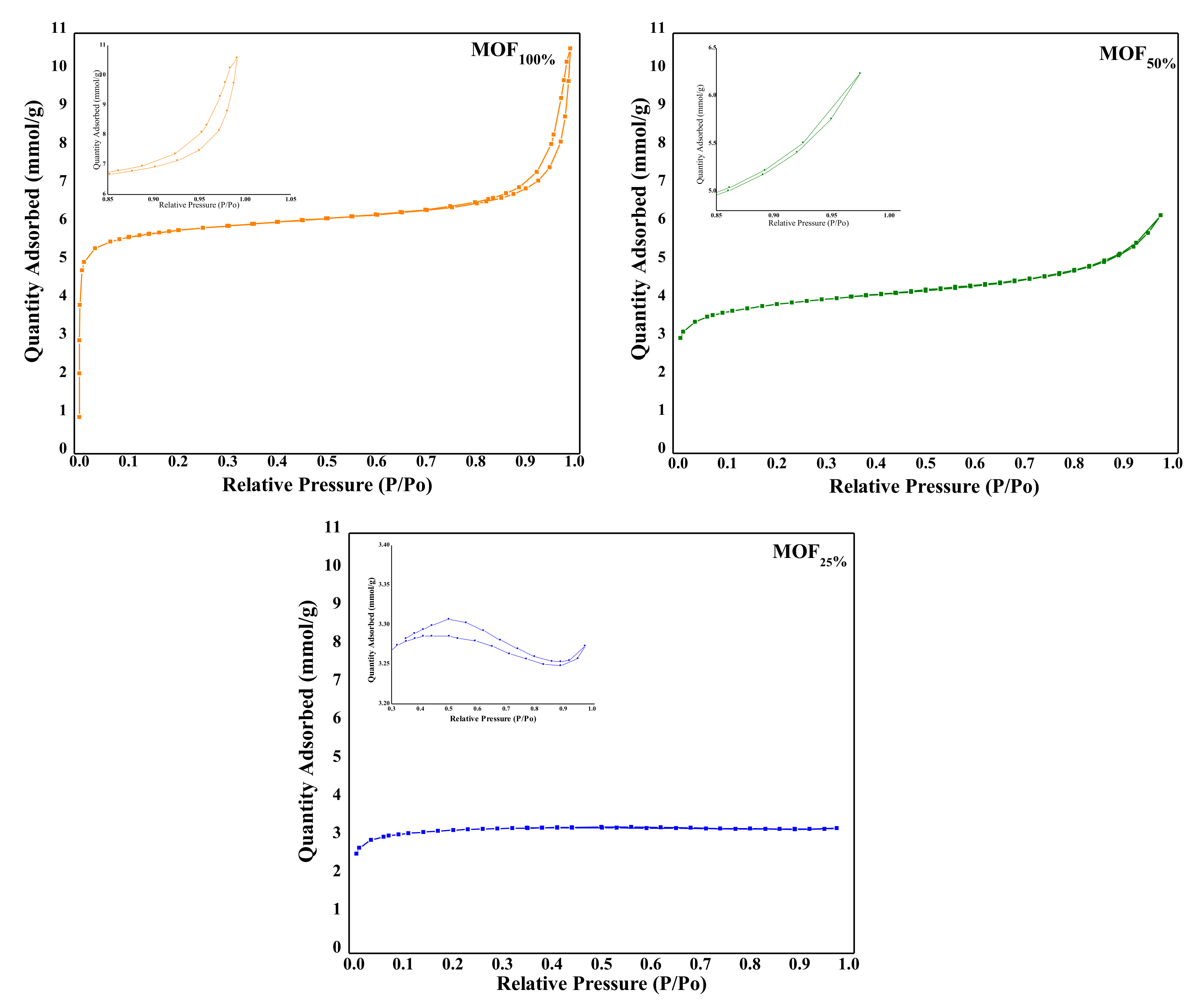
| Catalyst | Zr (wt%) Nominal | Zr (wt%) Experimental | BET (m2 g−1) | Pore Size (nm) | Micropore Area (m2/g) | Mesopore Area (m2/g) |
|---|---|---|---|---|---|---|
| MOF100% | 31 | 20.5 ± 0.4 | 474 ± 12 | 3.60 | 168 ± 4 | 219 ± 5 |
| MOF50% | 31 | 21.0 ± 0.4 | 424 ± 22 | 2.34 | 179 ± 4 | 156 ± 4 |
| MOF25% | 31 | 16.8 ± 0.3 | 407 ± 24 | 1.15 | 216 ± 5 | 151 ± 4 |
| Sample | C% | N% | O% | Zr% |
|---|---|---|---|---|
| MOF100% | 60.54 | 4.62 | 29.87 | 4.97 |
| MOF50% | 51.95 | 3.85 | 36.33 | 7.87 |
| MOF25% | 67.11 | 7.61 | 22.36 | 2.92 |
| Sample | C 1s | N 1s | O 1s | Zr 3d5/2 |
|---|---|---|---|---|
| MOF100% | 284.8 (62) | 399.3 (57) | 530.5 (13) | 182.9 |
| 282.1 (20) | 400.4 (33) | 531 (15) | ||
| 288.8 (18) | 401.6 (10) | 533.2 (15) | ||
| MOF50% | 284.8 (64) | 399.4 (76) | 530.4 (24) | 182.9 |
| 286.2 (16) | 400.5 (14) | 531.9 (65) | ||
| 288.8 (20) | 401.8 (10) | 533.1 (11) | ||
| MOF25% | 284.8 (61) | 399.2 (48) | 530.3 (13) | 182.9 |
| 286.2 (27) | 400.5 (15) | 531.6 (74) | ||
| 288.4 (12) | 402.0 (15) | 533.3 (13) |
| Sample | % Area (50 °C) Weak Sites | % Area (100 °C) Medium Sites | % Area Total Sites | |||
|---|---|---|---|---|---|---|
| Lewis | Brønsted | Lewis | Brønsted | Lewis | Brønsted | |
| MOF100% | 55.38 | 44.62 | 50.23 | 49.77 | 52.81 | 47.19 |
| MOF50% | 69.32 | 30.68 | 50.29 | 49.71 | 59.81 | 40.20 |
| MOF25% | 48.35 | 51.65 | 45.86 | 54.14 | 47.11 | 52.90 |
| Catalyst | Conversion % | Selectivity EL % | TON |
|---|---|---|---|
| MOF100% | 12.85 | 40.75 | 3 |
| MOF50% | 20.07 | 46.66 | 5.21 |
| MOF25% | 18.37 | 24.65 | 3.14 |
| Catalyst | Conversion % | Selectivity EL % | TON |
|---|---|---|---|
| MOF100% | 96.16 | 100 | 54.25 |
| MOF50% | 96.09 | 100 | 53.01 |
| MOF25% | 96.20 | 100 | 66.18 |
| MOF25% Reuse | 97.66 | 88.96 | 59.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo Fuchineco, D.A.; Heredia, A.C.; Mendoza, S.M.; Rodríguez-Castellón, E.; Crivello, M.E. Synthesis, Characterization and Catalytic Activity of UiO-66-NH2 in the Esterification of Levulinic Acid. Appl. Nano 2021, 2, 344-358. https://doi.org/10.3390/applnano2040025
Bravo Fuchineco DA, Heredia AC, Mendoza SM, Rodríguez-Castellón E, Crivello ME. Synthesis, Characterization and Catalytic Activity of UiO-66-NH2 in the Esterification of Levulinic Acid. Applied Nano. 2021; 2(4):344-358. https://doi.org/10.3390/applnano2040025
Chicago/Turabian StyleBravo Fuchineco, Daiana A., Angélica C. Heredia, Sandra M. Mendoza, Enrique Rodríguez-Castellón, and Mónica E. Crivello. 2021. "Synthesis, Characterization and Catalytic Activity of UiO-66-NH2 in the Esterification of Levulinic Acid" Applied Nano 2, no. 4: 344-358. https://doi.org/10.3390/applnano2040025
APA StyleBravo Fuchineco, D. A., Heredia, A. C., Mendoza, S. M., Rodríguez-Castellón, E., & Crivello, M. E. (2021). Synthesis, Characterization and Catalytic Activity of UiO-66-NH2 in the Esterification of Levulinic Acid. Applied Nano, 2(4), 344-358. https://doi.org/10.3390/applnano2040025







