3.1. Electrochemical Etching
The search conducted in the Science Citation Index Expanded (SCIE) database yielded a total of 5166 papers on electrochemical etching, with an average of 20.91 citations per document. The majority of these publications were research articles (4937, 95.57%), followed by proceeding papers (560, 10.84%) and review articles (108, 2.09%) (
Table 1). Such a predominance of research articles is typical in bibliometric datasets within the natural sciences and reflects standard publishing practices in the field.
The number of publications on electrochemical etching began to rise significantly in the early 1990s. This growth has continued sharply to the present day, indicating a sustained interest and advancement in the field over the decades (
Figure 2).
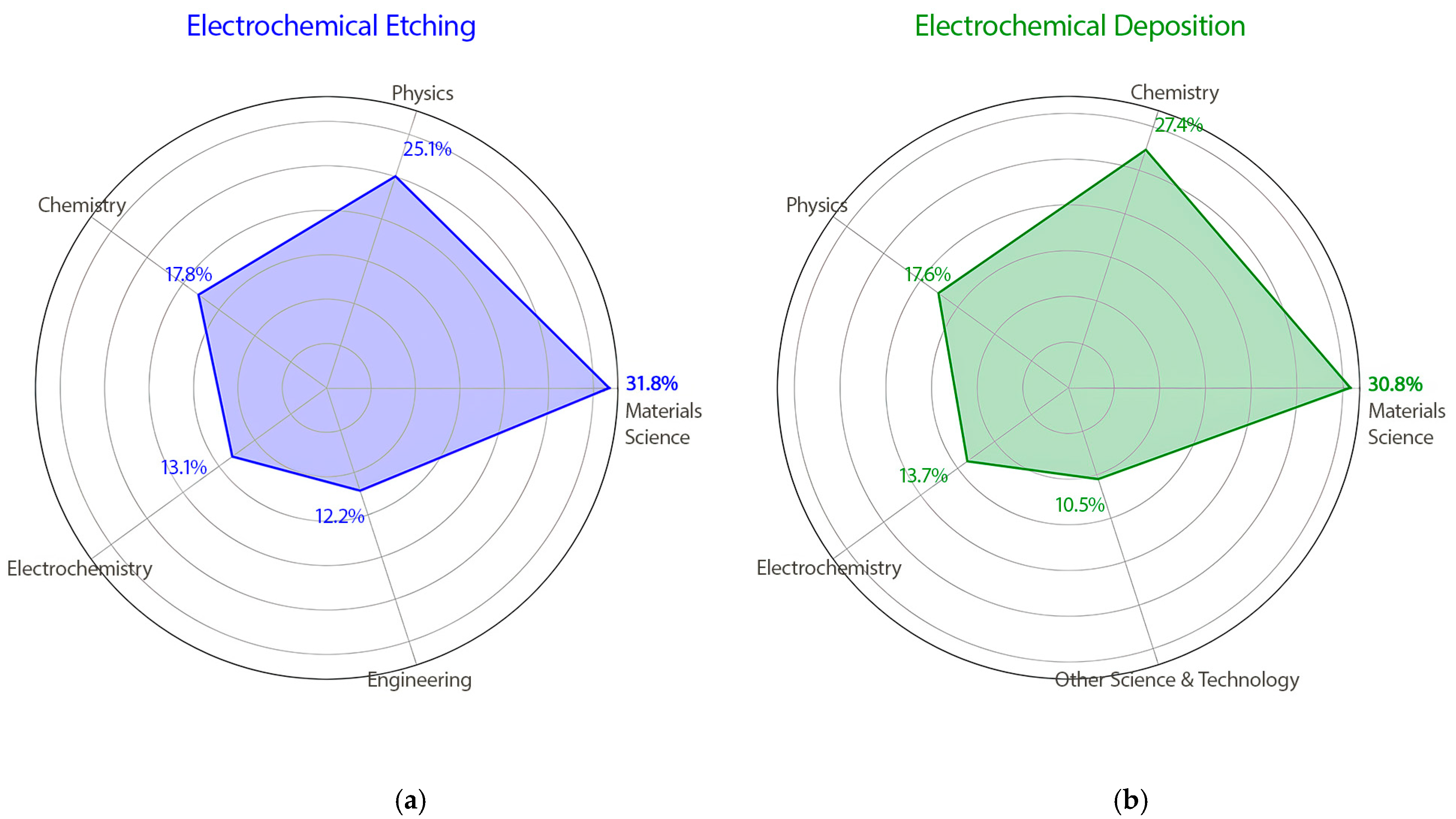
The topic of electrochemical etching spans 55 research areas within SCIE. The largest proportions of publications fall under materials’ science (2215, 42.88%), physics (1745, 33.78%), chemistry (1243, 24.06%), electrochemistry (915, 17.71%), and engineering (847, 16.40%) (
Table 2).
The most prolific journals publishing on electrochemical etching include Journal of the Electrochemical Society (264, 5.11%), Electrochimica Acta (179, 3.47%), Applied Surface Science (104, 2.01%), International Journal of Advanced Manufacturing Technology (96, 1.86%), Applied Physics Letters (90, 1.74%), and Nuclear Tracks and Radiation Measurements (74, 1.43%) (
Table 3).
The leading publishers in this area are Elsevier (1747, 33.82%), Springer Nature (553, 10.71%), Electrochemical Society Inc. (324, 6.27%), American Chemical Society (278, 5.39%), American Institute of Physics (241, 4.67%), Wiley (234, 4.53%), and IOP Publishing Ltd. (228, 4.41%) (
Table 4).
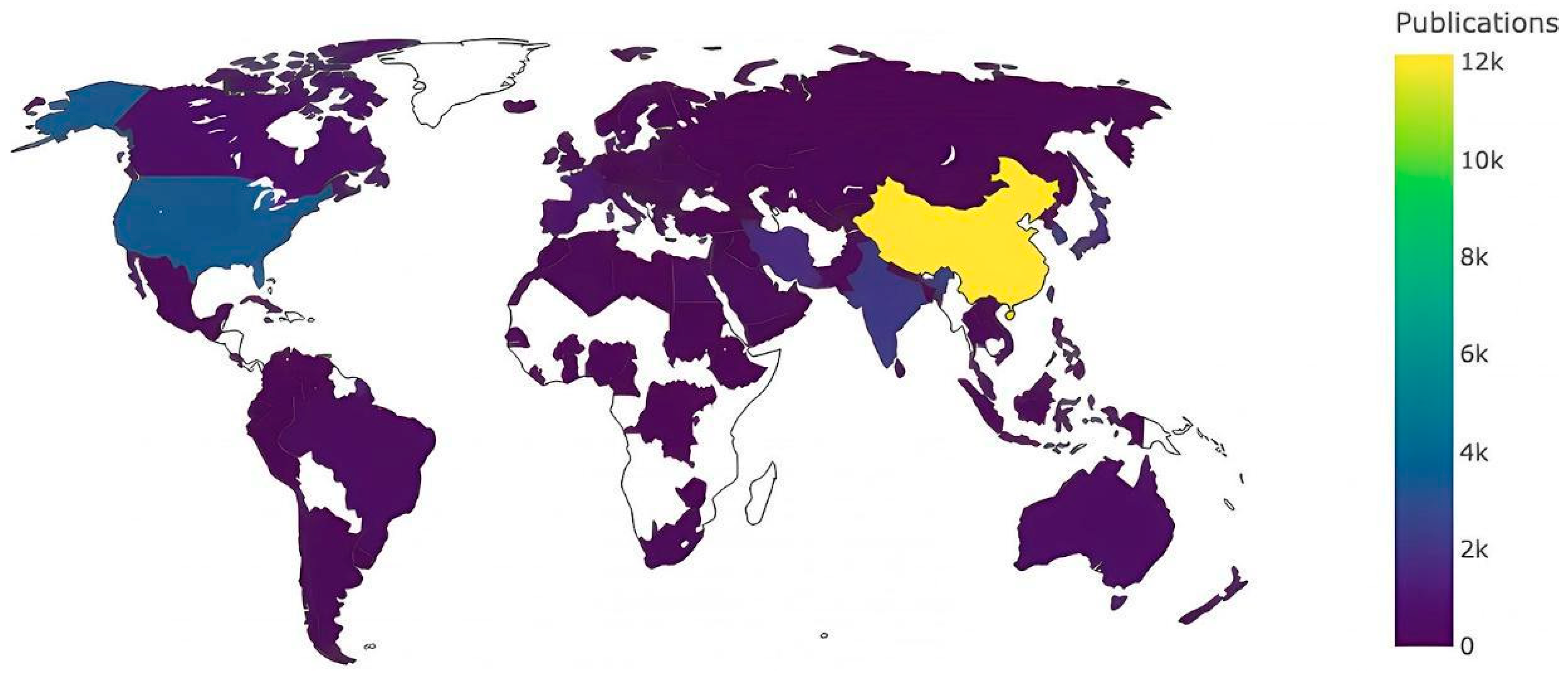
Researchers from China (1208, 23.38%), the USA (585, 11.32%), Germany (403, 7.80%), India (338, 6.54%), Russia (338, 6.54%), and Japan (337, 6.52%) were the most active contributors to this topic (
Figure 3). These nations have established themselves as leaders in electrochemical etching research, fostering significant advancements in the field.
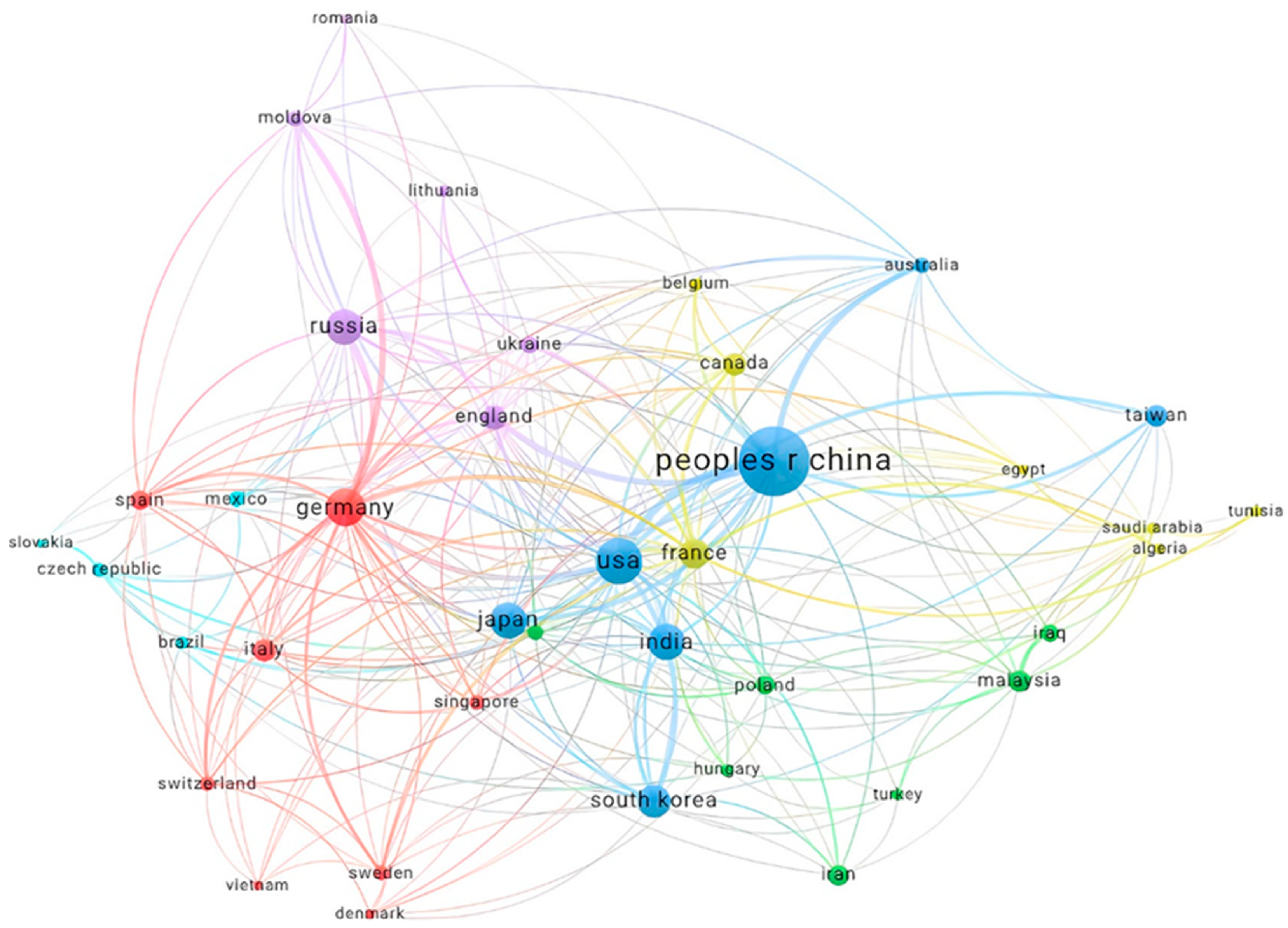
International collaboration plays a critical role in scientific progress, as evidenced by the network of academic partnerships depicted in
Figure 4. This figure illustrates the collaborative relationships between the most productive countries, each contributing at least 24 documents. Nodes represent different countries, and the connecting lines indicate cooperation intensity, with line thickness proportional to collaboration strength.
The diagram shows that China is the most central and interconnected node, indicating its significant role in global research collaboration. The United States also emerges as a major hub, reflecting its active participation in international research projects. Germany, Japan, and South Korea are other prominent countries with substantial collaborative links, showcasing their contributions to scientific advancements through partnerships.
European countries such as the UK, France, and Italy demonstrate strong intra-continental collaborations, underscoring the regional emphasis on joint research initiatives. Interestingly, emerging economies like India, Malaysia, and Brazil also feature in the network, indicating their growing involvement in global research efforts. The presence of smaller countries such as Taiwan and Singapore further emphasises the inclusivity and widespread nature of international scientific collaboration.
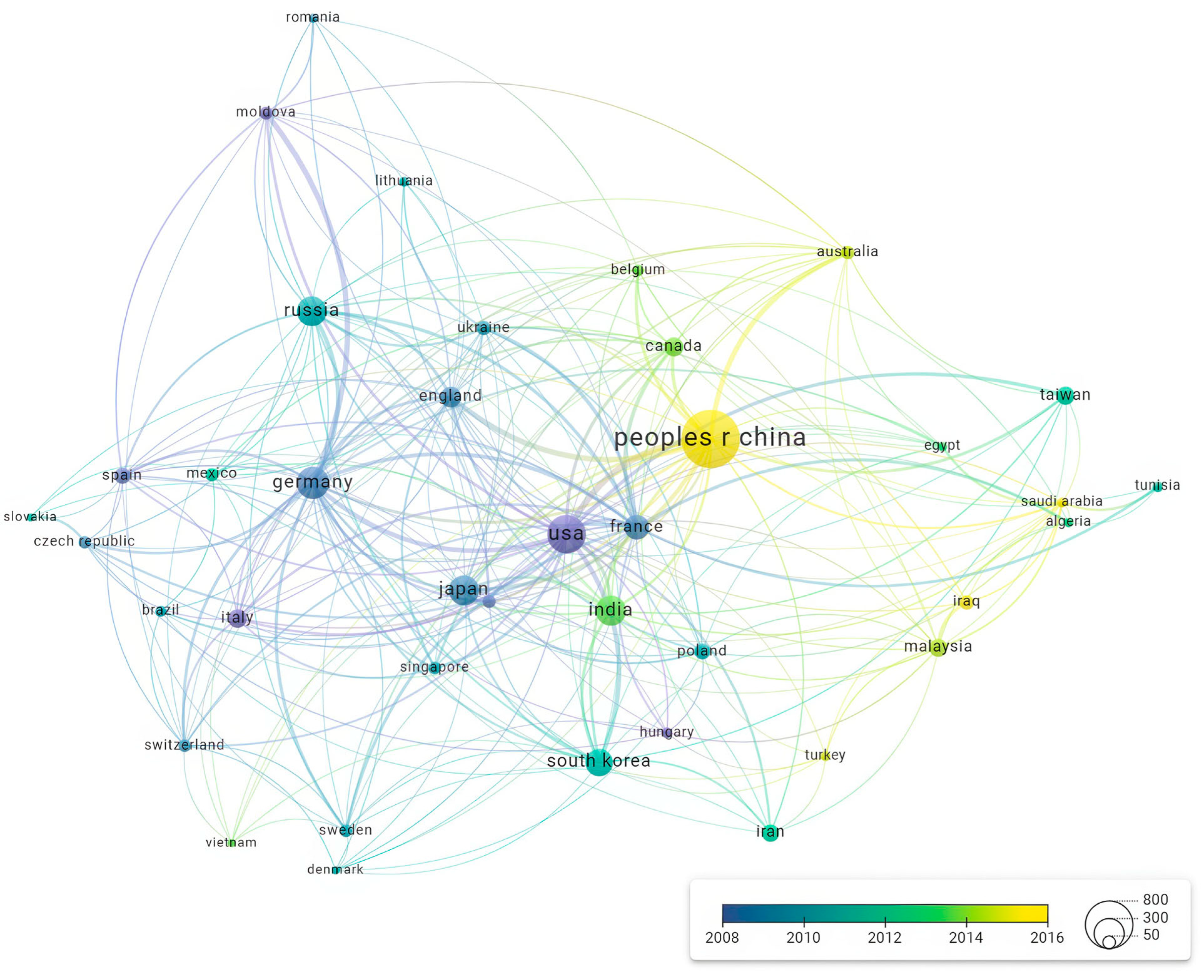
Figure 5 demonstrates the evolution of international collaboration in electrochemical etching research over the years, highlighting the average publication year for each country involved. The colour gradient, ranging from blue to yellow, indicates the average publication year, with blue representing earlier years and yellow representing more recent years.
China stands out as the most prolific contributor, with the highest number of publications (1199 documents), and the average publication year of 2016 indicates a relatively recent surge in research output. The USA also shows a significant number of publications, maintaining a robust presence in the field over time, with an average publication year around 2013. This suggests sustained research activity and collaboration.
Countries like Germany, Japan, and South Korea also feature prominently, with substantial publication records and collaboration networks. Germany’s average publication year is earlier than China’s, reflecting its longer-term engagement in the field. In contrast, Taiwan, Malaysia, and other countries in the Asia-Pacific region show more recent average publication years, highlighting their emerging roles in electrochemical deposition research.
Russia’s position in the network, with its collaborations depicted in purple, indicates earlier average publication years, reflecting a historical focus on this area. However, the recent output from other countries has surpassed it in terms of volume and recency.
The analysis underscores the dynamic nature of international collaboration in electrochemical etching research. While established leaders like China and the USA continue to drive the field, new contributors from the Asia-Pacific region are rapidly increasing their impact, fostering a more diverse and interconnected global research community. This trend highlights the importance of collaborative efforts in advancing the field and suggests a promising future for continued innovation and discovery.
The keywords in scientific papers offer valuable insights into the study’s themes and focal areas. In this analysis, VOSviewer 1.6.20 software was utilised to examine the frequency and co-occurrence of keywords in publications related to electrochemical etching, using SCIE data. A total of 12,621 keywords were identified, of which only 565 appeared 10 or more times.
Figure 6 presents the co-occurrence network map of the most frequent keywords, highlighting connections between terms. Each node represents a keyword, and the connecting lines indicate co-occurrence, with node size proportional to keyword linkage and line thickness reflecting co-occurrence frequency.
For this map, keywords with a minimum of 50 occurrences were selected, resulting in 91 keywords. “Electrochemical etching” emerged as the central keyword, exhibiting 564 co-occurrences. Notably, strong associations were observed with “porous silicon” (677 co-occurrences), “fabrication” (494 co-occurrences), and “photoluminescence” (343 co-occurrences).
The co-occurrence analysis revealed three clusters of closely related keywords, each marked with different colours (
Figure 5). Cluster 1, represented in red, comprises 32 keywords, focusing on electrochemical dissolution and behaviour. The theme of this cluster centres around the processes and mechanisms of electrochemical dissolution and the resulting behaviours of materials. The publications in this cluster mainly reflect research on interactions at the surface level during electrochemical etching, including the dissolution of different metals and alloys and the effects on microstructure and corrosion resistance.
Cluster 2, shown in green, comprises 31 keywords primarily related to silicon and other semiconductors. This cluster emphasises the use of silicon and other semiconductor materials in various applications. The strong association with photoluminescence and optical properties indicates that the publications mainly reflect a focus on the photonic applications of silicon and other semiconductors. The research concerned the structural and functional modifications of these materials, highlighting the use of nanowires, thin films, and semiconductors for enhanced performance in sensors and solar cells.
Cluster 3, depicted in blue, contains 28 keywords, with an emphasis on nanoparticles and oxidation processes. This cluster concentrates on the oxidation and reduction processes involving nanoparticles. The publications in this cluster show attention to surface interactions and dissolution behaviours of metals and nanoparticles during electrochemical processes. The research emphasises the kinetics of these reactions and their implications for material performance, particularly in terms of passivation and oxidation resistance.
In summary, the keyword co-occurrence network analysis provides a comprehensive overview of the major thematic areas within electrochemical etching research. The identified clusters highlight the focus on electrochemical dissolution processes and material behaviours, the applications and modifications of silicon-based materials, and the oxidation and reduction processes involving nanoparticles. These clusters represent distinct but interconnected areas of research, offering a detailed understanding of the various aspects of electrochemical etching and the materials involved.
The analysis of keywords in the publications also shed light on the most frequently studied materials, including porous silicon (677 occurrences), silicon (303), copper (160), titanium (117), aluminium (96), iron (96), nickel (86), gold (68), stainless steel (58), platinum (53), graphene (48), and silver (41). These materials play pivotal roles in electrochemical etching research and are central to ongoing scientific exploration.
The analysis of keyword co-occurrence data over time for electrochemical etching offers insights into the shifting research trends and focal points within this field (
Figure 7). The network map, coloured based on the average publication year, illustrates how different research themes have emerged and evolved from 2010 to 2016. This can be attributed to the year of the appearance of these specific graphs and themes. After 2016, no new dominant themes emerged that had a minimum of 50 occurrences. This may indicate a diversification of research topics, leading to a broader field with a more extensive network that does not exhibit the same dense connections as before.
In the early years, from 2010 to 2012, research primarily focused on fundamental aspects of electrochemical etching. Key topics included “dissolution,” “oxidation,” and “surface” as researchers sought to understand the basic mechanisms and behaviours involved in electrochemical processes. The emphasis was on developing a foundational understanding that could be built upon in subsequent years.
From 2013 to 2014, the focus of research expanded to include more applied aspects, such as “nanoparticles”, “thin films”, and “fabrication”. This period saw significant advancements in the practical applications of electrochemical etching, with an increased emphasis on creating nanostructures and improving fabrication techniques. Researchers explored new materials and methods to enhance the efficiency and effectiveness of electrochemical etching.
In the later years, from 2015 to 2016, the research landscape diversified further, incorporating advanced applications and emerging technologies. Keywords like “photoluminescence”, “optical properties”, and “porous silicon” became more prominent, indicating a growing interest in using electrochemical etching for photonic and optoelectronic applications. The development of “porous silicon” and its integration into various technological applications became a major research focus.
The interconnectedness of different keywords, represented by the strength of the links between them, highlights the collaborative nature of research in this field. Strong connections between keywords such as “electrochemical etching”, “fabrication”, and “porous silicon” demonstrate the multidisciplinary approach adopted by researchers to address complex challenges and innovate within the field.
In summary, the temporal and thematic analysis of electrochemical etching research reveals a dynamic and evolving field. Early research focused on fundamental mechanisms, followed by a shift towards practical applications and advanced technologies. This progression reflects the ongoing efforts of researchers to push the boundaries of what is possible with electrochemical etching and its applications in various industries.
3.2. Electrochemical Deposition
A comprehensive search in the Science Citation Index Expanded (SCIE) database identified a total of 30,759 papers dedicated to electrochemical deposition. The majority of these publications are research articles (29,801, 96.89%), followed by proceeding papers (1451, 4.72%) and review articles (781, 2.54%) (
Table 5). The publication trend, as depicted in
Figure 8, indicates that interest in electrochemical deposition began to surge in the early 2000s and has maintained a robust growth trajectory to the present day.
Electrochemical deposition research spans 63 distinct areas within the SCIE, with the most substantial contributions coming from materials science (15,354, 49.92%), chemistry (13,623, 44.29%), physics (8763, 28.49%), electrochemistry (6813, 22.15%), and other topics in science and technology (5234, 17.02%) (
Table 6).
The most prolific journals publishing on electrochemical deposition include Electrochimica Acta (1558, 5.07%), Journal of the Electrochemical Society (941, 3.06%), Applied Surface Science (752, 2.45%), Journal of Alloys and Compounds (743, 2.42%), Surface Coatings Technology (579, 1.88%), and Journal of Electroanalytical Chemistry (554, 1.80%) (
Table 7). These journals are at the forefront of disseminating significant research findings in this field.
Leading publishers in the domain of electrochemical deposition are Elsevier (12,396, 40.30%), the American Chemical Society (2805, 9.12%), Springer Nature (2696, 8.77%), the Royal Society of Chemistry (2332, 7.58%), Wiley (2029, 6.60%), Electrochemical Society Inc. (1142, 3.71%), and MDPI (897, 2.92%). These publishers are instrumental in curating and advancing the scientific discourse on electrochemical deposition (
Table 8).
Researchers from China (12,168, 39.56%), the USA (3500, 11.38%), India (2118, 6.89%), South Korea (2083, 6.77%), Iran (1701, 5.53%), Japan (1432, 4.66%), and Germany (1406, 4.57%) have been the most active contributors to this research area (
Figure 9). These countries have established themselves as leaders in electrochemical deposition research, demonstrating a strong commitment to advancing the field.
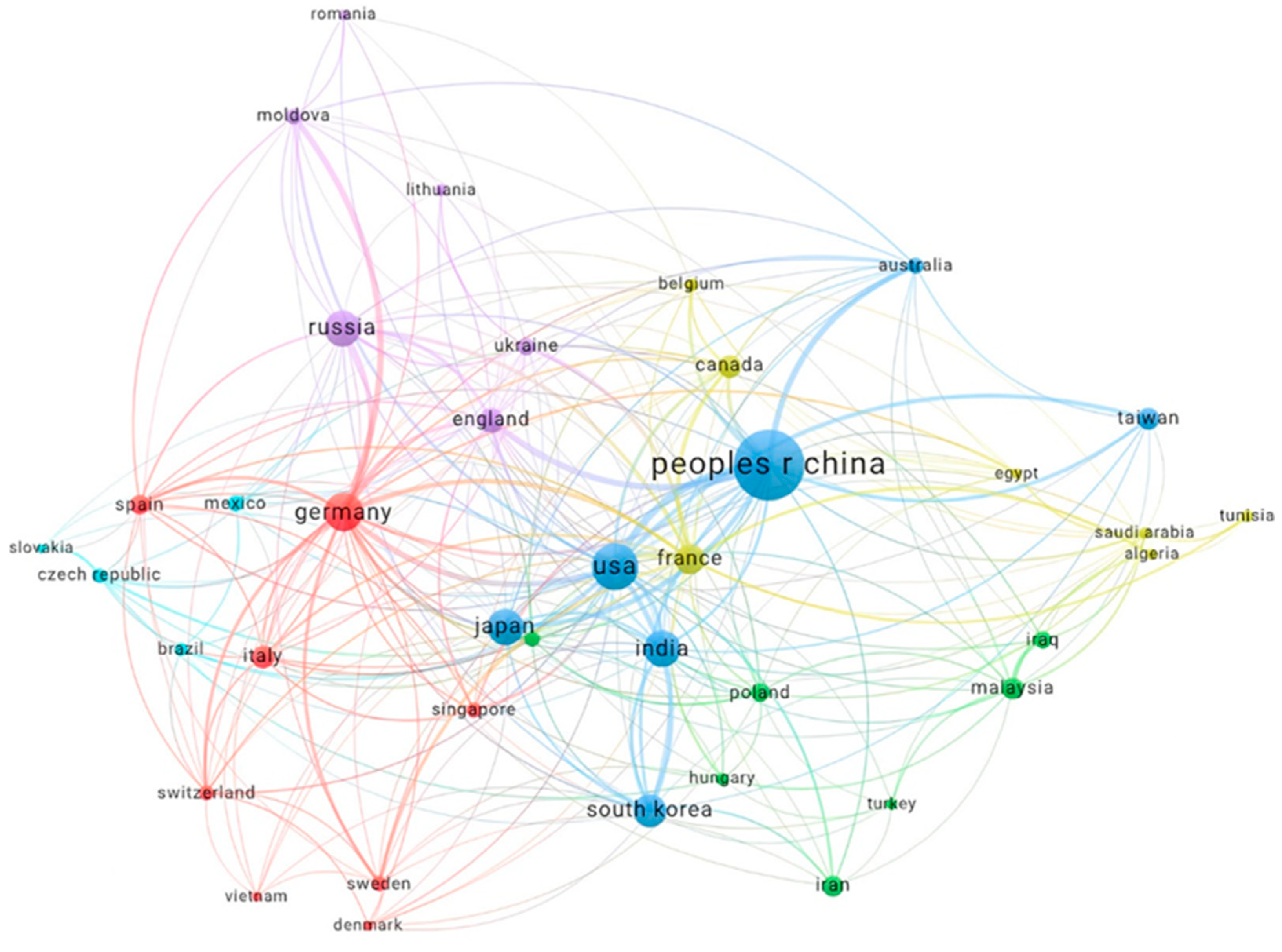
Figure 10 illustrates the international collaboration network among the most prolific countries, each contributing at least 24 documents. China emerges as the most prominent node, reflecting its substantial contribution and central role in global research collaboration. The USA, while also significant, shows fewer connections compared to China, suggesting a more selective collaboration approach.
European countries, particularly Germany, France, and Italy, display dense intra-continental collaboration networks, emphasising the strong regional research partnerships within Europe. Countries like India, South Korea, and Iran are also well-integrated into the global network, showcasing their active participation in international research projects.
The presence of emerging research nations, such as Malaysia, Saudi Arabia, and Egypt, indicates their growing involvement in electrochemical deposition research, contributing to a more diverse and inclusive global research community.
Figure 11 presents an analysis of the international collaboration network in electrochemical deposition research over the years, highlighting the evolution of these collaborations. The nodes are coloured based on the average publication year, with blue indicating earlier years and yellow representing more recent years. This temporal gradient allows us to observe the shifting dynamics of research activity and collaboration intensity.
China stands out as the most prominent node, indicating its significant and recent surge in research output. The concentration of publications around the average year 2016 underscores China’s rapid ascension and dominant role in the field. The USA, while also maintaining a substantial presence, displays a broader range of collaboration over a longer period, with its average publication year around 2013. This suggests a more sustained and consistent research effort.
European countries such as Germany, France, and Italy exhibit dense intra-continental collaboration networks with average publication years indicating a long-term engagement in the field. Germany, with its earlier average publication year, reflects its established history and foundational role, whereas countries like Spain and the UK show a more recent focus, indicating ongoing and evolving research activities.
Emerging research nations, including Malaysia, Saudi Arabia, and Egypt, display more recent average publication years, highlighting their growing involvement and increasing contributions to global research efforts. This trend signifies a broadening of the research community, with new players adding to the diversity and depth of electrochemical deposition research.
In terms of keyword analysis, the VOSviewer software was utilised to evaluate the frequency and co-occurrence of keywords within the publications. This analysis revealed a total of 50,508 keywords, with 3338 occurring 10 times or more.
Figure 12 presents a co-occurrence network map of the most frequently occurring keywords, constructed from keywords with at least 50 occurrences, resulting in a network of 850 keywords.
The term “electrodeposition” is notably central within this research field, exhibiting 12,544 co-occurrences with other keywords, indicating its pivotal role in electrochemical deposition studies. Prominent associations are observed with “nanoparticles” (4995 co-occurrences), “fabrication” (3682 co-occurrences), and “films” (2976 co-occurrences), reflecting key themes in the research.
Keywords with strong interconnections were categorised into six distinct clusters, each depicted in different colours (
Figure 12). Cluster 1 (red) comprises 226 keywords centred on carbon-based materials and their applications, including carbon nanotubes and graphene. This highlights significant research interest in these materials for their unique properties. Cluster 2 (green) includes 188 keywords related primarily to the surface properties and microstructure of materials, indicating a strong emphasis on understanding and manipulating material surfaces and structures at the microscopic level. Cluster 3 (blue) contains 162 keywords associated with the performance and efficiency of various materials and processes, reflecting the importance of optimising performance metrics in electrochemical deposition. Cluster 4 (yellow) comprises 159 keywords connected to thin films and their optical properties, suggesting a research focus on the fabrication and application of thin film materials in various technologies. Cluster 5 (purple) consists of 86 keywords related to the magnetic properties and related applications of materials, indicating an interest in magnetic materials and their potential uses. Cluster 6 (light blue) includes 29 keywords associated with specific electrochemical processes and techniques, reflecting detailed studies on particular methods within the broader field.
The analysis of keywords also highlighted the materials most frequently studied within electrochemical deposition research. These include copper (1247 occurrences), graphene (1435), nickel (1507), carbon (886), gold (767), graphene oxide (616), silver (523), cobalt (523), silicon (346), and titanium (326). These materials are integral to the research and applications in electrochemical deposition, underscoring their significance in ongoing scientific endeavours.
The analysis of keyword co-occurrence data over time using VOSviewer software reveals the evolution of research trends in electrochemical deposition (
Figure 13). The network map, coloured based on the average publication year, provides insights into the shifts in research focus from earlier to more recent studies.
In the early stages of electrochemical deposition research, around the years 2012 to 2014, the focus was predominantly on fundamental aspects and core techniques. Keywords such as “electrodeposition,” “copper,” “nickel,” and “nanoparticles” were central, indicating a primary interest in the basic mechanisms and materials used in electrochemical deposition processes. Studies during this period emphasised understanding the fundamental properties and behaviours of these materials, as well as optimising the deposition techniques for better control and efficiency.
Between 2014 and 2016, there was a noticeable shift towards exploring the applications and performance of deposited materials. Keywords like “performance,” “thin films,” “microstructure,” and “coatings” began to emerge more prominently. This shift reflects a growing interest in applying electrochemical deposition techniques to create advanced materials with specific properties for various applications, such as corrosion resistance, mechanical strength, and electrical conductivity. Research during this period also started to focus on composite materials and the enhancement of material properties through innovative deposition methods.
From 2016 onwards, especially noticeable from 2017 to 2019, the research focus has expanded to include more diverse and application-oriented studies. Keywords such as “nanostructures,” “graphene,” “carbon nanotubes,” “biosensor,” “electrocatalysis,” and “supercapacitor” have become more prevalent. This indicates a strong interest in the functional applications of electrochemically deposited materials in fields such as energy storage, catalysis, and biosensing. The emergence of terms like “facile synthesis,” “efficient,” and “hydrogen evolution reaction” highlights a trend towards developing more efficient and sustainable deposition processes, as well as exploring the potential of these materials in renewable energy technologies.
In summary, the temporal analysis illustrates the dynamic nature of research in electrochemical deposition, with evolving priorities reflecting technological advancements and emerging global challenges. Researchers have progressively moved from foundational studies to application-driven investigations, paving the way for innovative solutions in various high-impact areas.