Reconstruction of Electrochemical Impedance Spectroscopy from Time-Domain Pulses of a 3.7 kWh Lithium-Ion Battery Module
Abstract
:1. Introduction
2. Materials and Methods
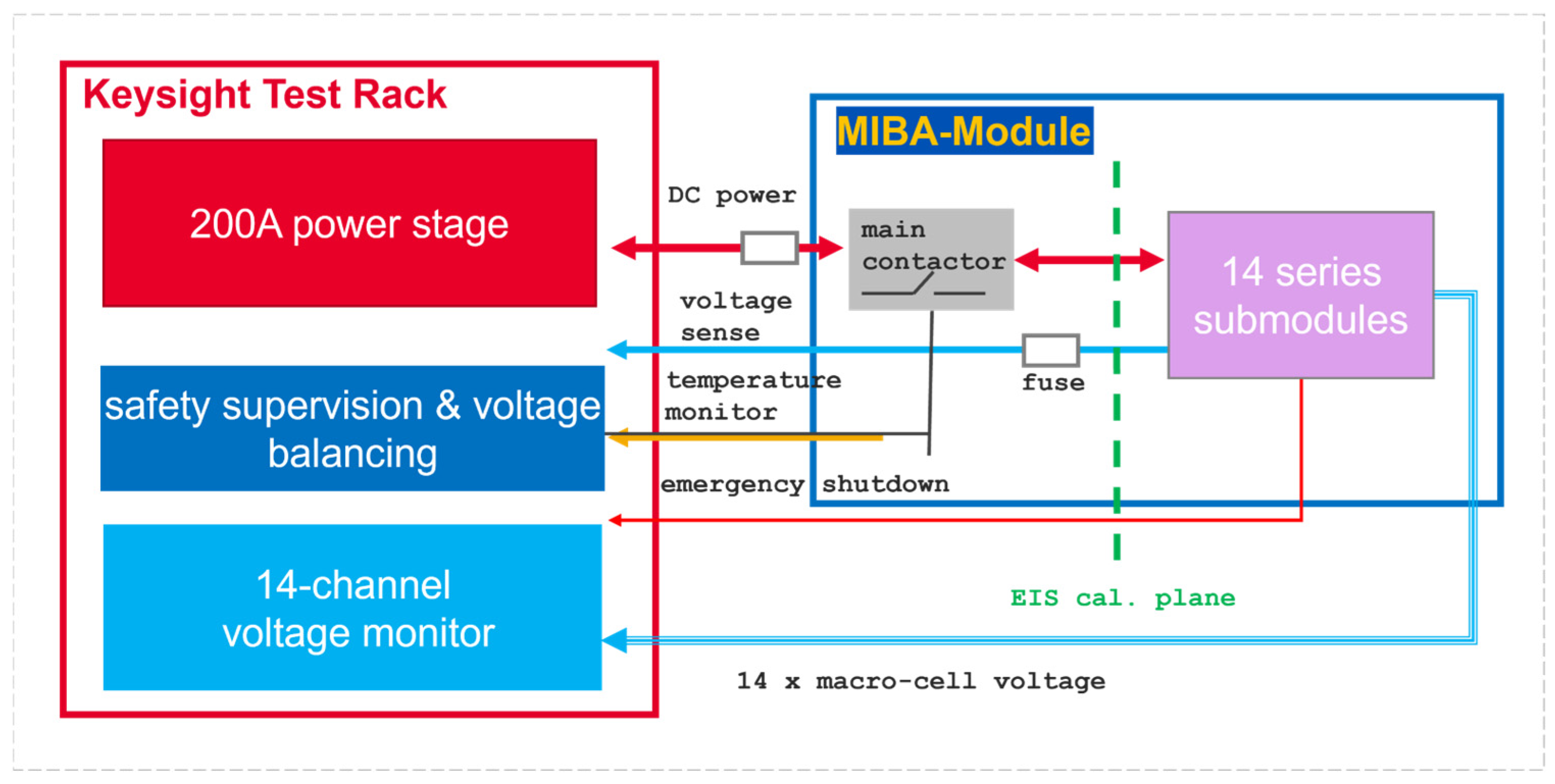
2.1. Battery Test Hardware
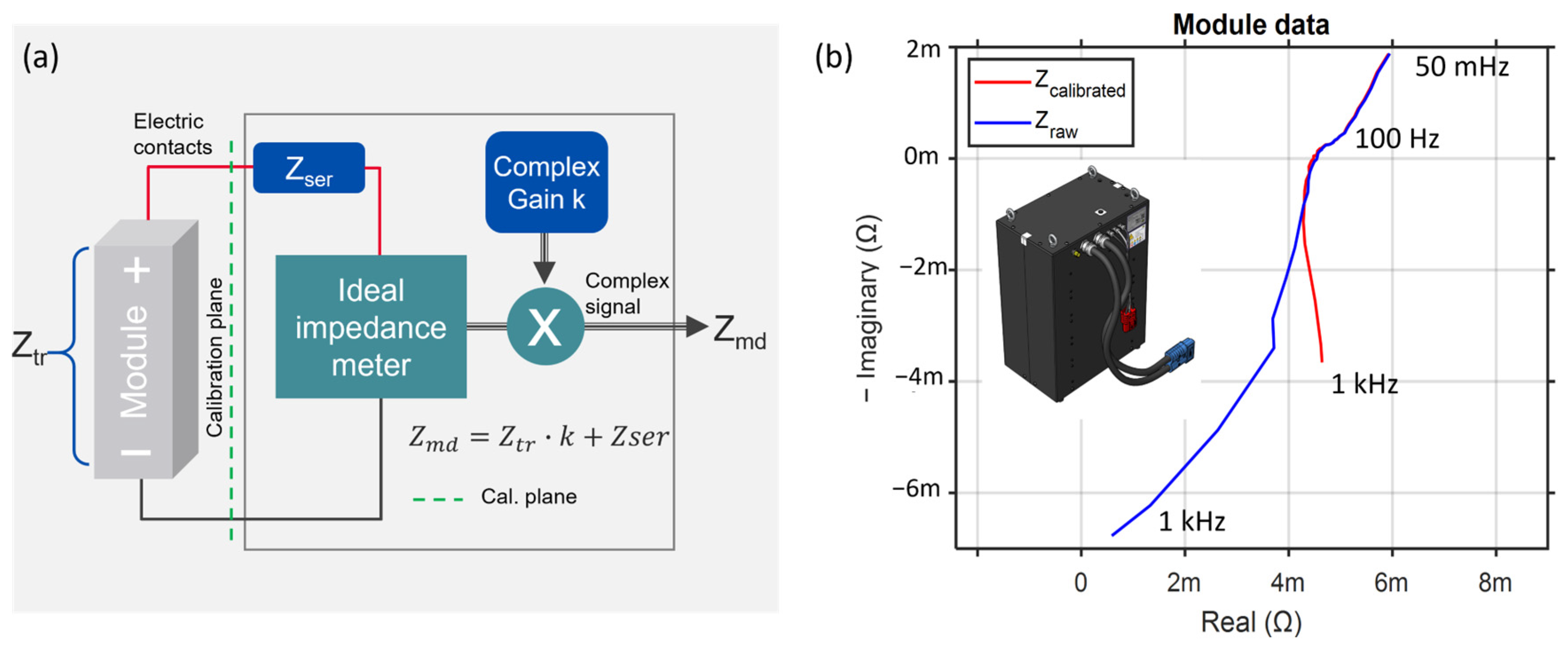
2.2. Impedance Spectroscopy Calibration
2.3. Time-Domain Pulse Analysis
- Discrete solution of an RC filter
- b.
- Calculation of the time-domain distribution of the relaxation time (DRT)
- c.
- Converting the time-domain results to the frequency domain
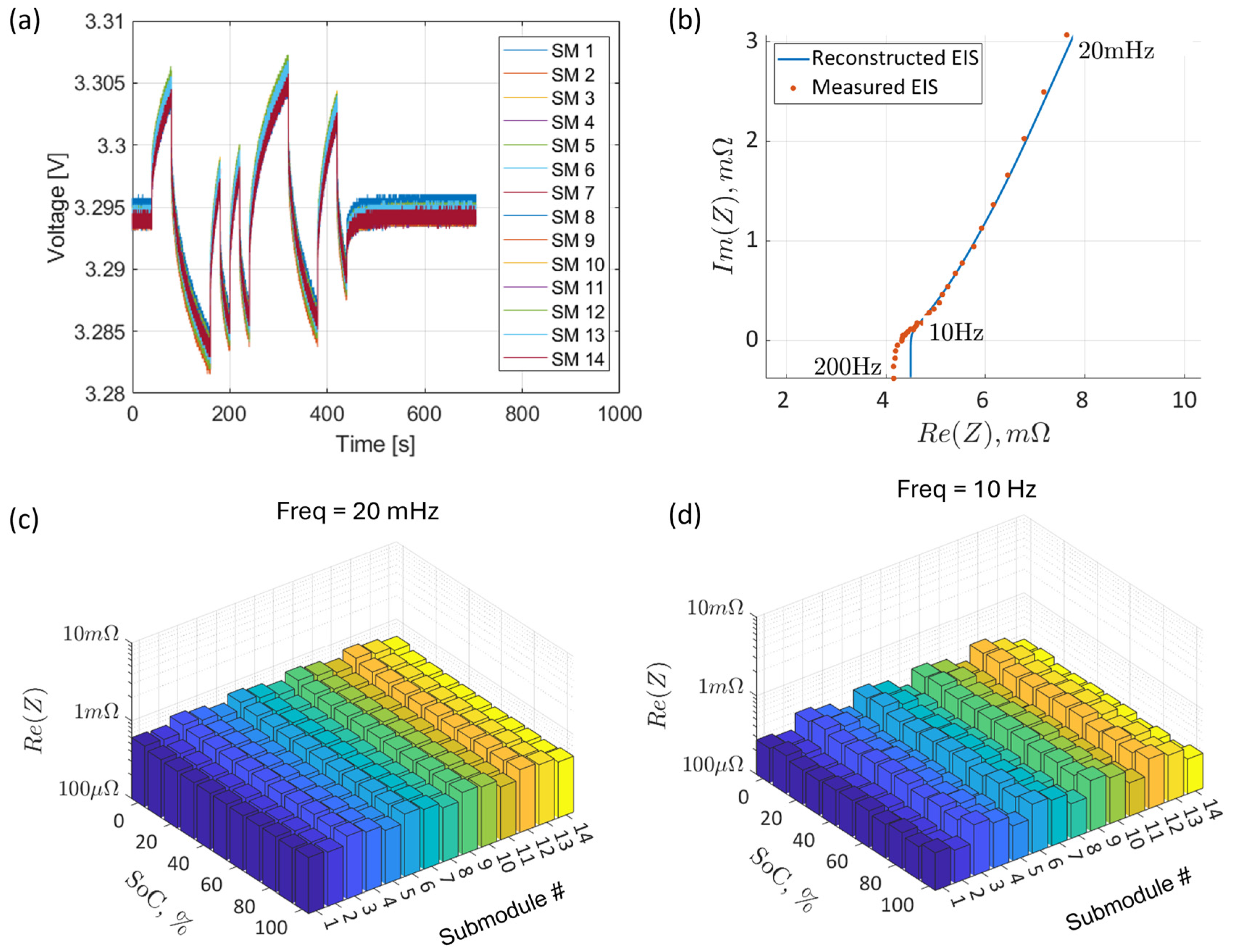
2.4. Battery Module, Submodule, and Cells
3. Results and Discussion
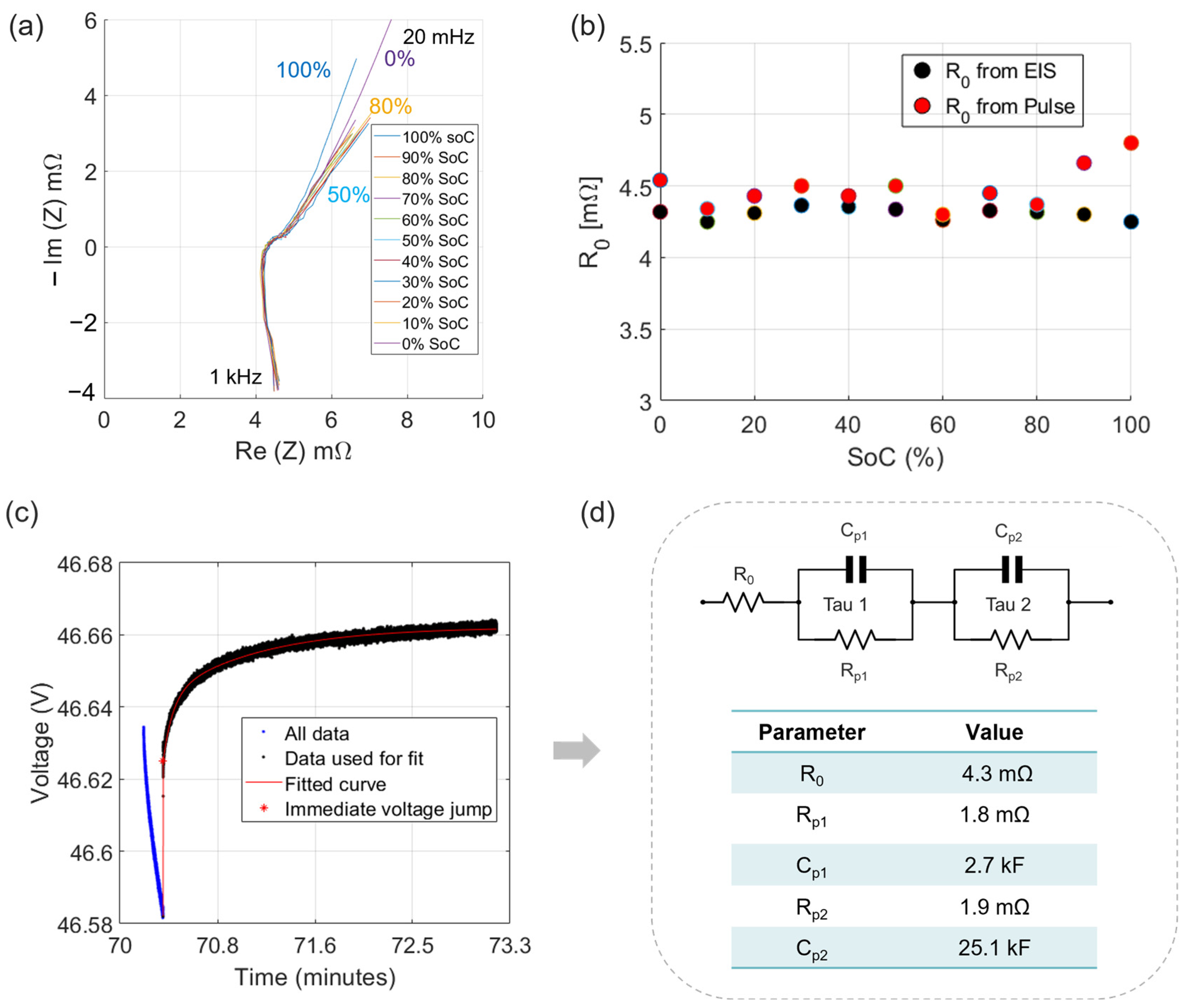
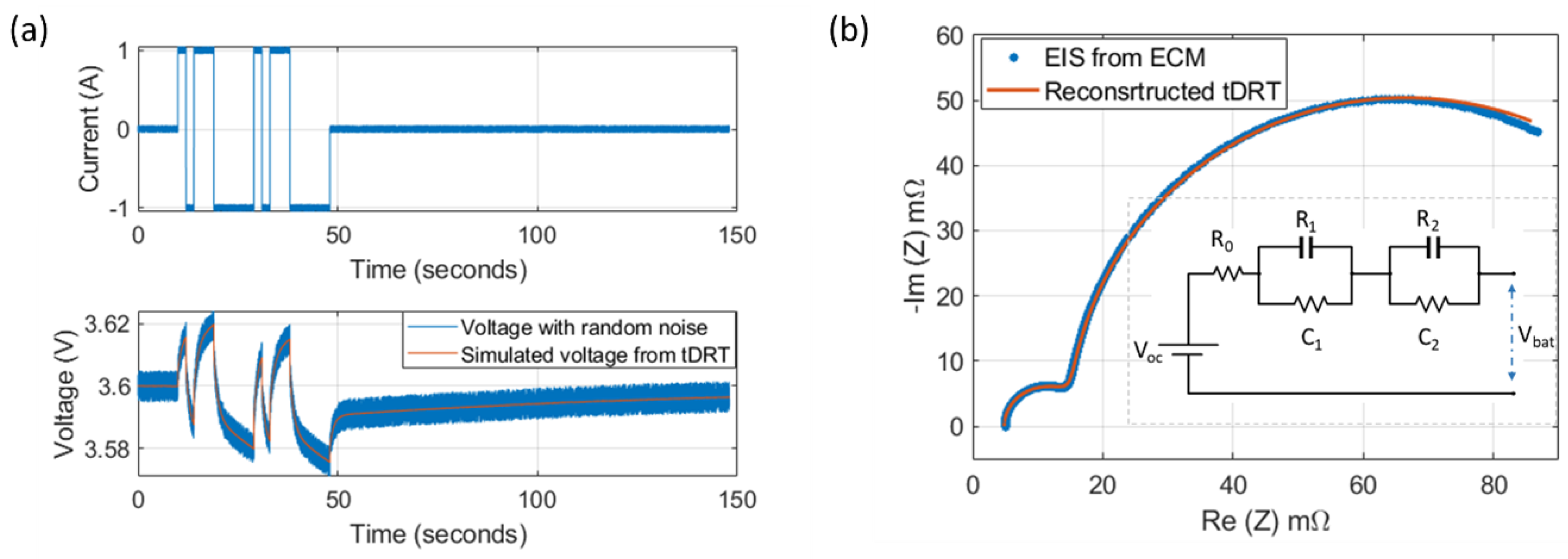
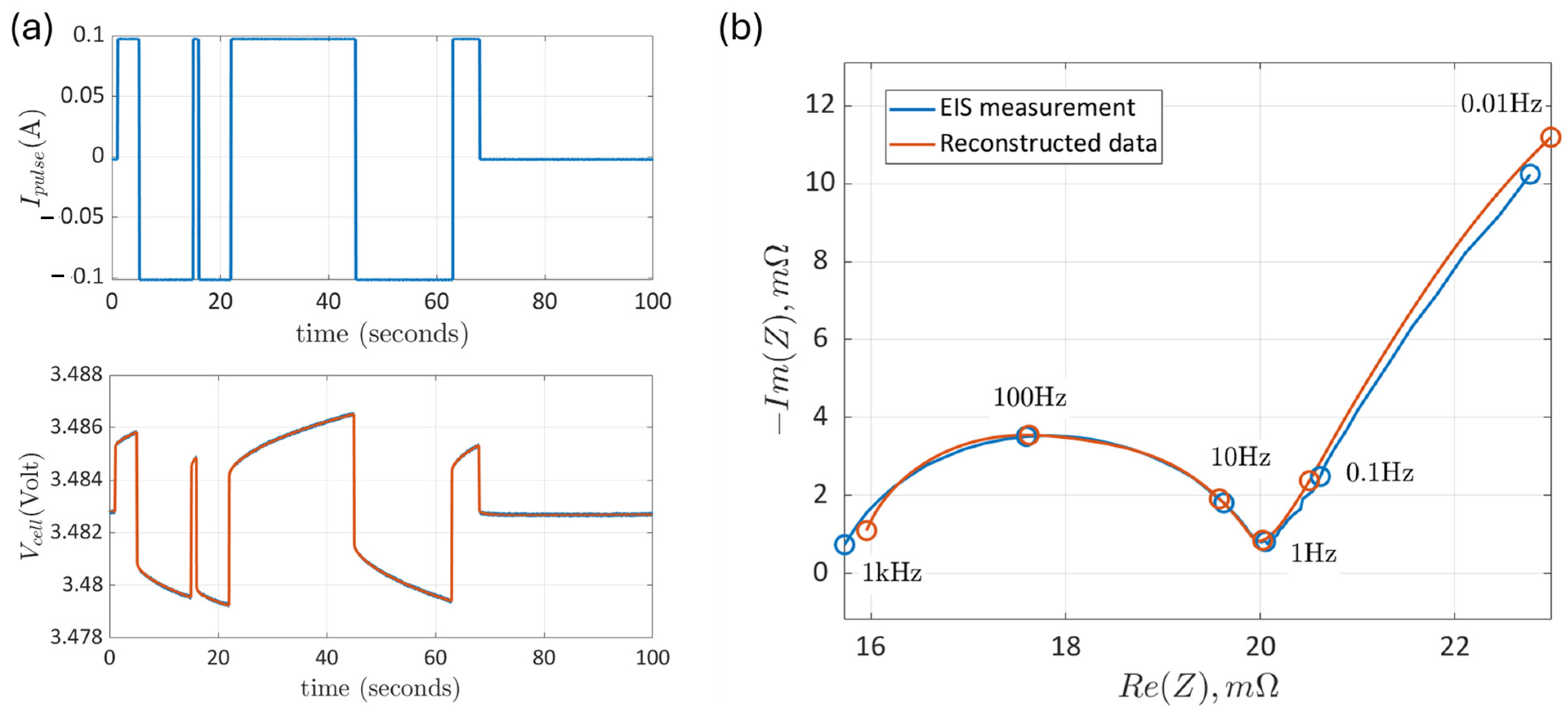
3.1. Calibrated EIS and Pulse Tests
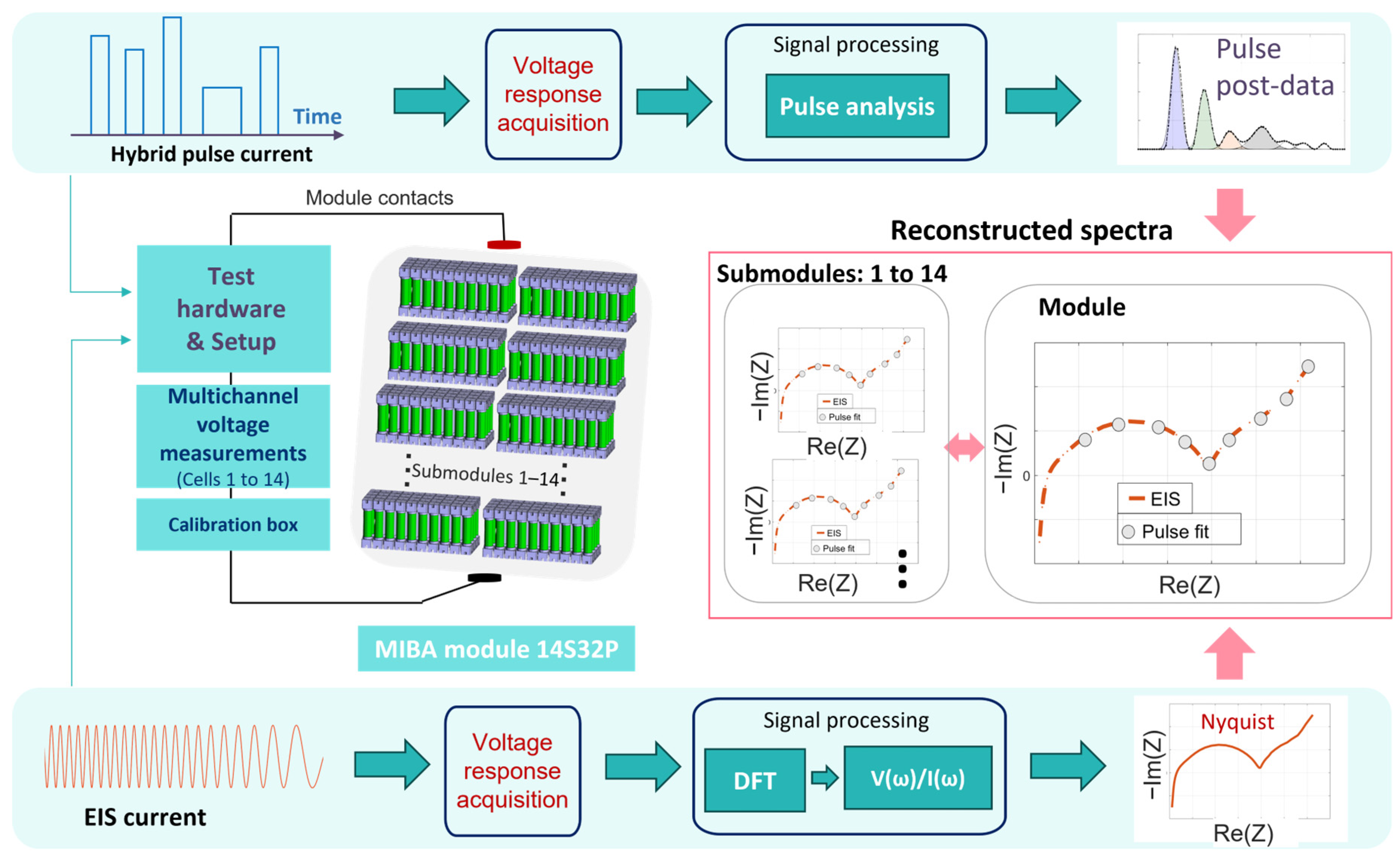
3.2. Reconstruction of the Impedance Data from the Time-Domain Pulse
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Yadav, S.; Salman, M.; Chavan, S.; Kim, S.C. Review of thermal coupled battery models and parameter identification for lithium-ion battery heat generation in EV battery thermal management system. Int. J. Heat Mass Transf. 2024, 218, 124748. [Google Scholar] [CrossRef]
- Tashakor, N.; Naseri, F.; Fang, J.; Zadeh, A.H.; Goetz, S. Module Voltage and Resistance Estimation of Battery-Integrated Cascaded Converters Through Output Sensors Only for EV Applications. IEEE Trans. Transp. Electrif. 2024, 10, 7984–7995. [Google Scholar] [CrossRef]
- Dixon, J.; Bell, K. Electric vehicles: Battery capacity, charger power, access to charging and the impacts on distribution networks. eTransportation 2020, 4, 100059. [Google Scholar] [CrossRef]
- McGovern, M.E.; Bruder, D.D.; Huemiller, E.D.; Rinker, T.J.; Bracey, J.T.; Sekol, R.C.; Abell, J.A. A review of research needs in nondestructive evaluation for quality verification in electric vehicle lithium-ion battery cell manufacturing. J. Power Sources 2023, 561, 232742. [Google Scholar] [CrossRef]
- Galeotti, M.; Cinà, L.; Giammanco, C.; Cordiner, S.; Di Carlo, A. Performance analysis and SOH (state of health) evaluation of lithium polymer batteries through electrochemical impedance spectroscopy. Energy 2015, 89, 678–686. [Google Scholar] [CrossRef]
- R-Smith, N.A.-Z.; Leitner, M.; Alic, I.; Toth, D.; Kasper, M.; Romio, M.; Surace, Y.; Jahn, M.; Kienberger, F.; Ebner, A.; et al. Assessment of lithium ion battery ageing by combined impedance spectroscopy, functional microscopy and finite element modelling. J. Power Sources 2021, 512, 230459. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Zhang, C.; Zhang, W.; Ma, Z.; Jiang, Y. Lithium-ion battery aging mechanisms and life model under different charging stresses. J. Power Sources 2017, 356, 103–114. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Li, D.; Wang, K. State-of-health estimation of lithium-ion batteries based on electrochemical impedance spectroscopy: A review. Prot. Control. Mod. Power Syst. 2023, 8, 1–17. [Google Scholar] [CrossRef]
- Kasper, M.; Leike, A.; Thielmann, J.; Winkler, C.; R-Smith, N.A.-Z.; Kienberger, F. Electrochemical impedance spectroscopy error analysis and round robin on dummy cells and lithium-ion-batteries. J. Power Sources 2022, 536, 231407. [Google Scholar] [CrossRef]
- Böttiger, M.; Paulitschke, M.; Bocklisch, T. Systematic experimental pulse test investigation for parameter identification of an equivalent based lithium-ion battery model. Energy Procedia 2017, 135, 337–346. [Google Scholar] [CrossRef]
- Białoń, T.; Niestrój, R.; Skarka, W.; Korski, W. HPPC Test Methodology Using LFP Battery Cell Identification Tests as an Example. Energies 2023, 16, 6239. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, S.-Y.; Chen, Y.; Zhang, H.; Park, S.-J. An SMPS-Based Lithium-Ion Battery Test System for Internal Resistance Measurement. IEEE Trans. Transp. Electrif. 2023, 9, 934–944. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Li, D.; Cui, X.; Wang, L.; Li, L.; Wang, K. Electrochemical Impedance Spectroscopy: A New Chapter in the Fast and Accurate Estimation of the State of Health for Lithium-Ion Batteries. Energies 2023, 16, 1599. [Google Scholar] [CrossRef]
- Iurilli, P.; Brivio, C.; Wood, V. On the use of electrochemical impedance spectroscopy to characterize and model the aging phenomena of lithiumion batteries: A critical review. J. Power Sources 2021, 505, 229860. [Google Scholar] [CrossRef]
- Chan, H.S.; Dickinson, E.J.; Heins, T.P.; Park, J.; Gaberšček, M.; Lee, Y.Y.; Heinrich, M.; Ruiz, V.; Napolitano, E.; Kauranen, P.; et al. Comparison of methodologies to estimate state-of-health of commercial Li-ion cells from electrochemical frequency response data. J. Power Sources 2022, 542, 231814. [Google Scholar] [CrossRef]
- Kalogiannis, T.; Hosen, S.; Sokkeh, M.A.; Goutam, S.; Jaguemont, J.; Jin, L.; Qiao, G.; Berecibar, M.; Van Mierlo, J. Comparative Study on Parameter Identification Methods for Dual-Polarization Lithium-Ion Equivalent Circuit Model. Energies 2019, 12, 4031. [Google Scholar] [CrossRef]
- Moradpour, A.; Kasper, M.; Hoffmann, J.; Kienberger, F. Measurement Uncertainty in Battery Electrochemical Impedance Spectroscopy. IEEE Trans. Instrum. Meas. 2022, 71, 1006209. [Google Scholar] [CrossRef]
- Meddings, N.; Heinrich, M.; Overney, F.; Lee, J.-S.; Ruiz, V.; Napolitano, E.; Seitz, S.; Hinds, G.; Raccichini, R.; Gaberšček, M.; et al. Application of electrochemical impedance spectroscopy to commercial Li-ion cells: A review. J. Power Sources 2020, 480, 228742. [Google Scholar] [CrossRef]
- Moradpour, A.; Kasper, M.; Kienberger, F. Quantitative Cell Classification Based on Calibrated Impedance Spectroscopy and Metrological Uncertainty. Batter. Supercaps 2023, 6, e202200524. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Acharya, A.B.; Sui, X.; Meng, J.; Teodorescu, R.; Stroe, D.-I. A Review of Pulsed Current Technique for Lithium-ion Batteries. Energies 2020, 13, 2458. [Google Scholar] [CrossRef]
- Barai, A.; Uddin, K.; Widanage, W.D.; McGordon, A.; Jennings, P. A study of the influence of measurement timescale on internal resistance characterisation methodologies for lithium-ion cells. Sci. Rep. 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, D.; Schaltz, E.; Stroe, D.-I.; Gismero, A.; Yang, B. Degradation mechanism analysis and State-of-Health estimation for lithium-ion batteries based on distribution of relaxation times. J. Energy Storage 2022, 55, 105386. [Google Scholar] [CrossRef]
- Goldammer, E.; Kowal, J. Determination of the Distribution of Relaxation Times by Means of Pulse Evaluation for Offline and Online Diagnosis of Lithium-Ion Batteries. Batteries 2021, 7, 36. [Google Scholar] [CrossRef]
- Plank, C.; Rüther, T.; Jahn, L.; Schamel, M.; Schmidt, J.P.; Ciucci, F.; Danzer, M.A. A review on the distribution of relaxation times analysis: A powerful tool for process identification of electrochemical systems. J. Power Sources 2024, 594, 233845. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Q.-A.; Li, W.; Wang, J.; Bai, Y.; Zhao, Y.; Li, X.; Zhang, J. Insight into the origin of pseudo peaks decoded by the distribution of relaxation times/ differential capacity method for electrochemical impedance spectroscopy. J. Electroanal. Chem. 2022, 910, 116176. [Google Scholar] [CrossRef]
- Oppenheim, A.V.; Schafer, R.W.; Buck, J.R. Discrete-Time Signal Processing, 2nd ed.; Prentice-Hall Signal Processing Series; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Tan, L.; Jiang, J. Chapter 6—Digital Signal Processing Systems, Basic Filtering Types, and Digital Filter Realizations. In Digital Signal Processing, 2nd ed.; Tan, L., Jiang, J., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 161–215. [Google Scholar] [CrossRef]
- Parks, T.W.; Burrus, C.S. Digital Filter Design; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Schmidt, J.P.; Ivers-Tiffée, E. Pulse-fitting—A novel method for the evaluation of pulse measurements, demonstrated for the low frequency behavior of lithium-ion cells. J. Power Sources 2016, 315, 316–323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasper, M.; Moertelmaier, M.; Popp, H.; Kienberger, F.; Al-Zubaidi R-Smith, N. Reconstruction of Electrochemical Impedance Spectroscopy from Time-Domain Pulses of a 3.7 kWh Lithium-Ion Battery Module. Electrochem 2025, 6, 17. https://doi.org/10.3390/electrochem6020017
Kasper M, Moertelmaier M, Popp H, Kienberger F, Al-Zubaidi R-Smith N. Reconstruction of Electrochemical Impedance Spectroscopy from Time-Domain Pulses of a 3.7 kWh Lithium-Ion Battery Module. Electrochem. 2025; 6(2):17. https://doi.org/10.3390/electrochem6020017
Chicago/Turabian StyleKasper, Manuel, Manuel Moertelmaier, Hartmut Popp, Ferry Kienberger, and Nawfal Al-Zubaidi R-Smith. 2025. "Reconstruction of Electrochemical Impedance Spectroscopy from Time-Domain Pulses of a 3.7 kWh Lithium-Ion Battery Module" Electrochem 6, no. 2: 17. https://doi.org/10.3390/electrochem6020017
APA StyleKasper, M., Moertelmaier, M., Popp, H., Kienberger, F., & Al-Zubaidi R-Smith, N. (2025). Reconstruction of Electrochemical Impedance Spectroscopy from Time-Domain Pulses of a 3.7 kWh Lithium-Ion Battery Module. Electrochem, 6(2), 17. https://doi.org/10.3390/electrochem6020017






