Enhanced Performance of a Microbial Fuel Cell Using Double Oxidant-Treated Carbon Felts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Electrodes
2.3. Microbial Fuel Cell Setup
2.4. Calculation and Analysis
3. Results
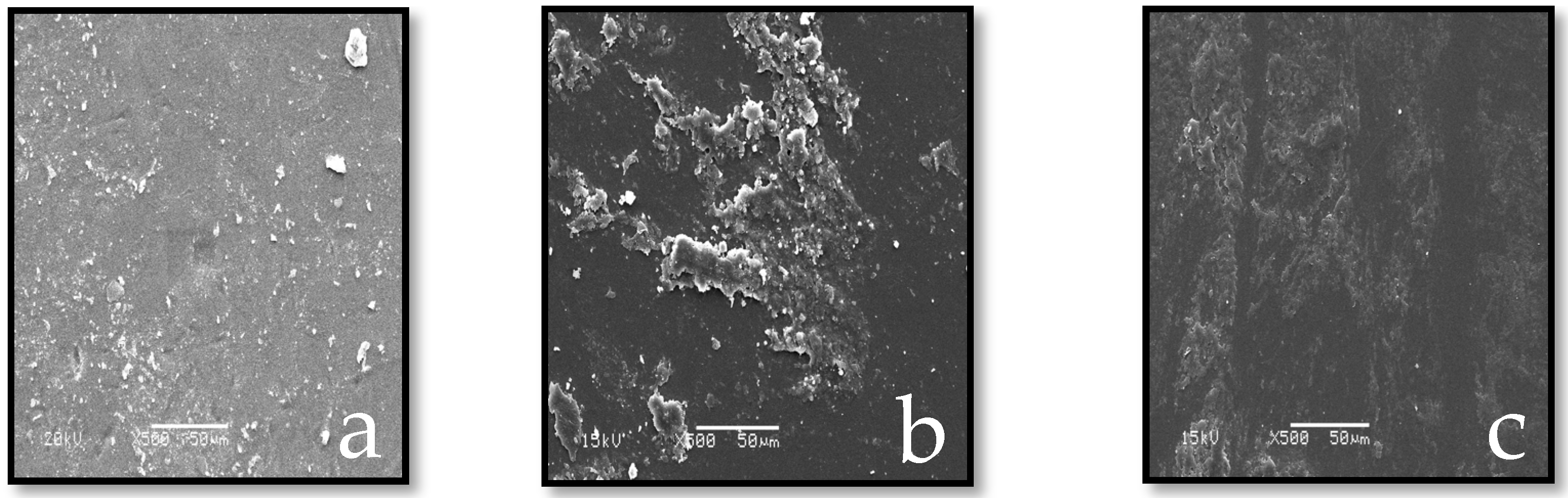
3.1. Electrode Characterization
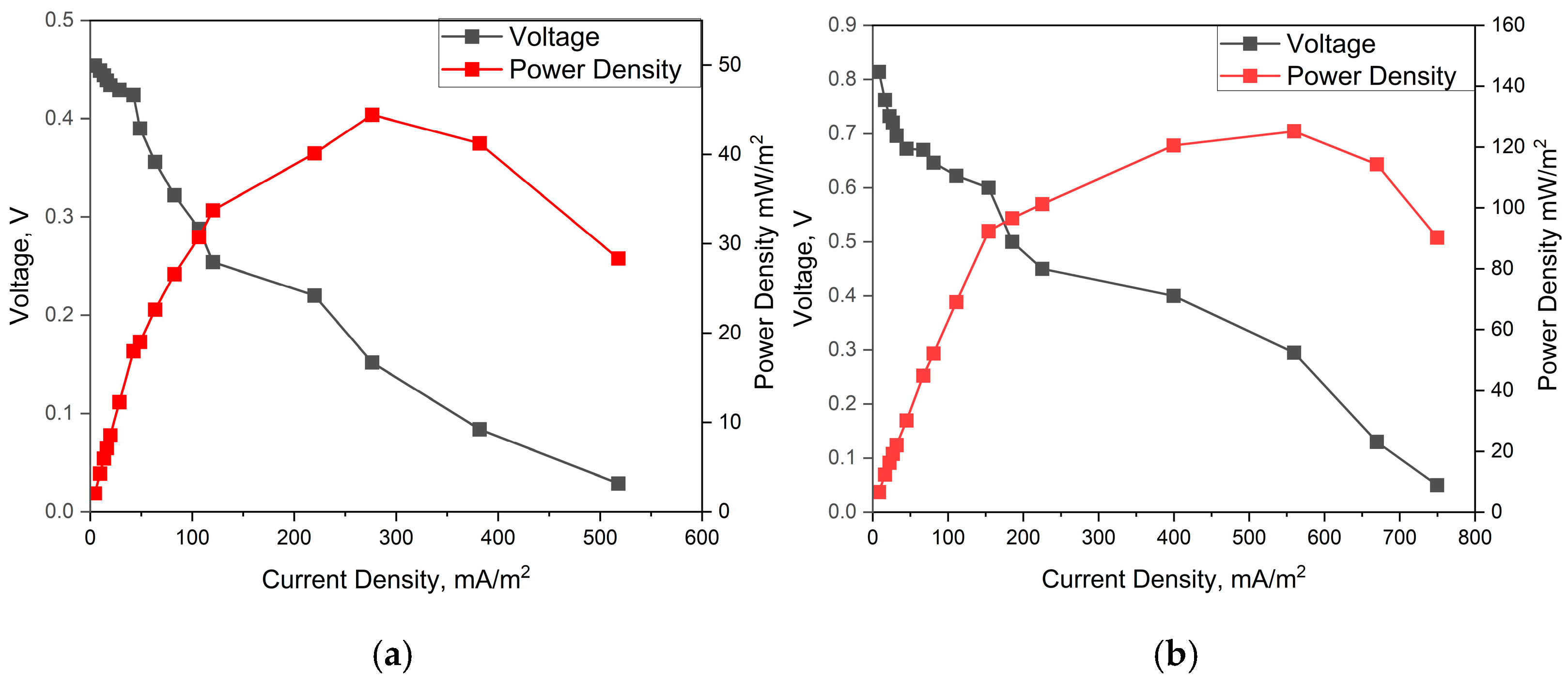
3.2. Voltage and Power Generation
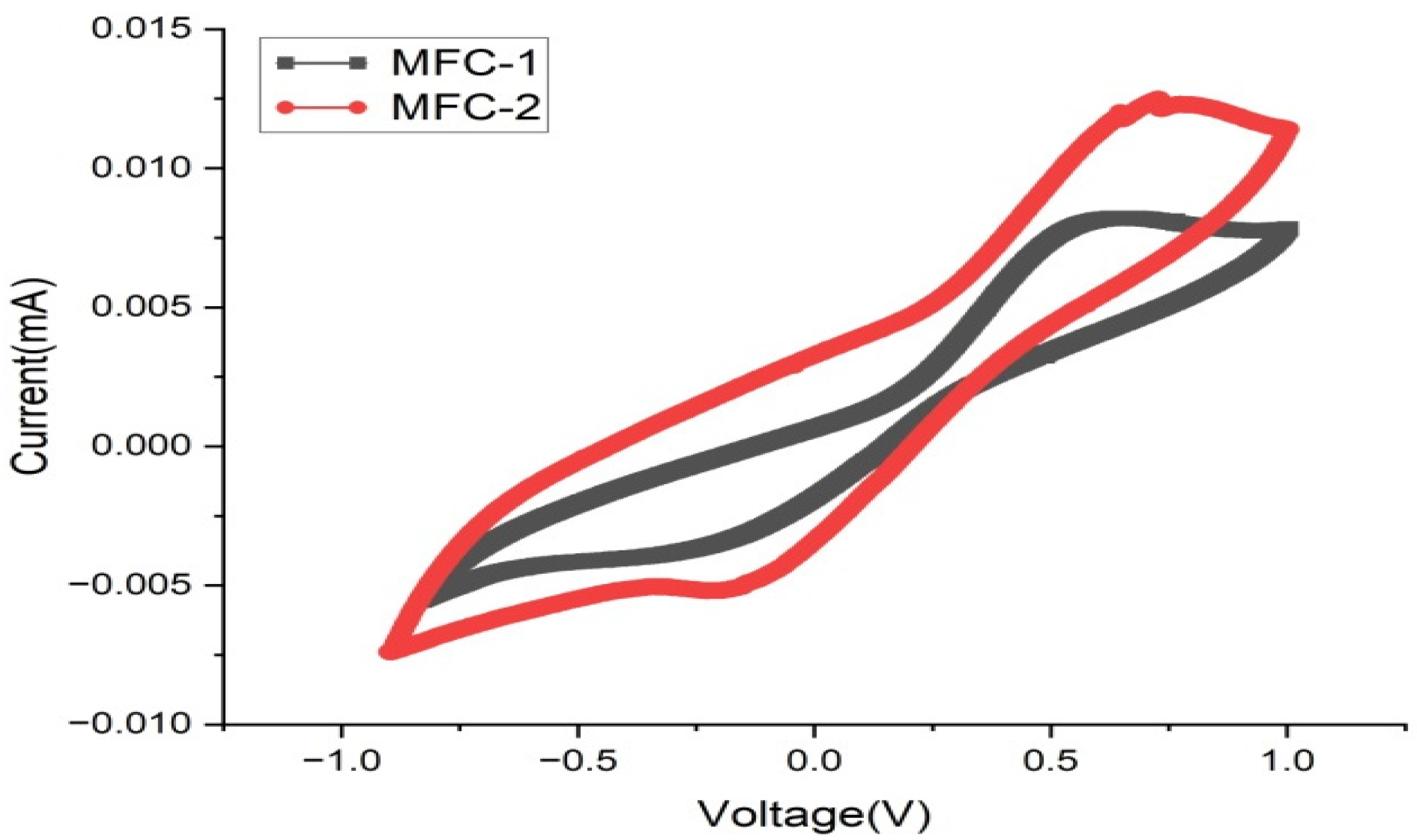
3.3. Cyclic Voltammetry (CV)
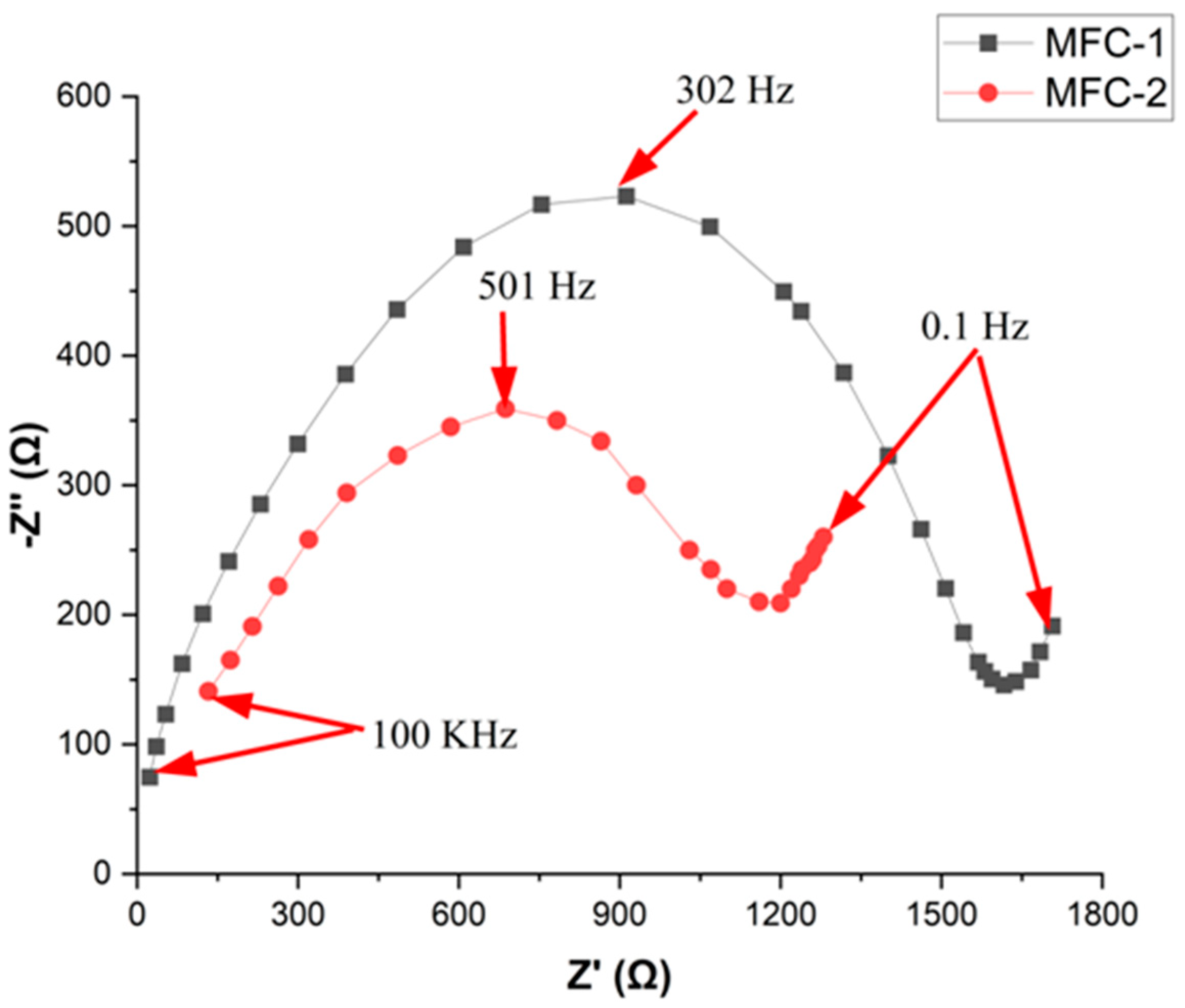
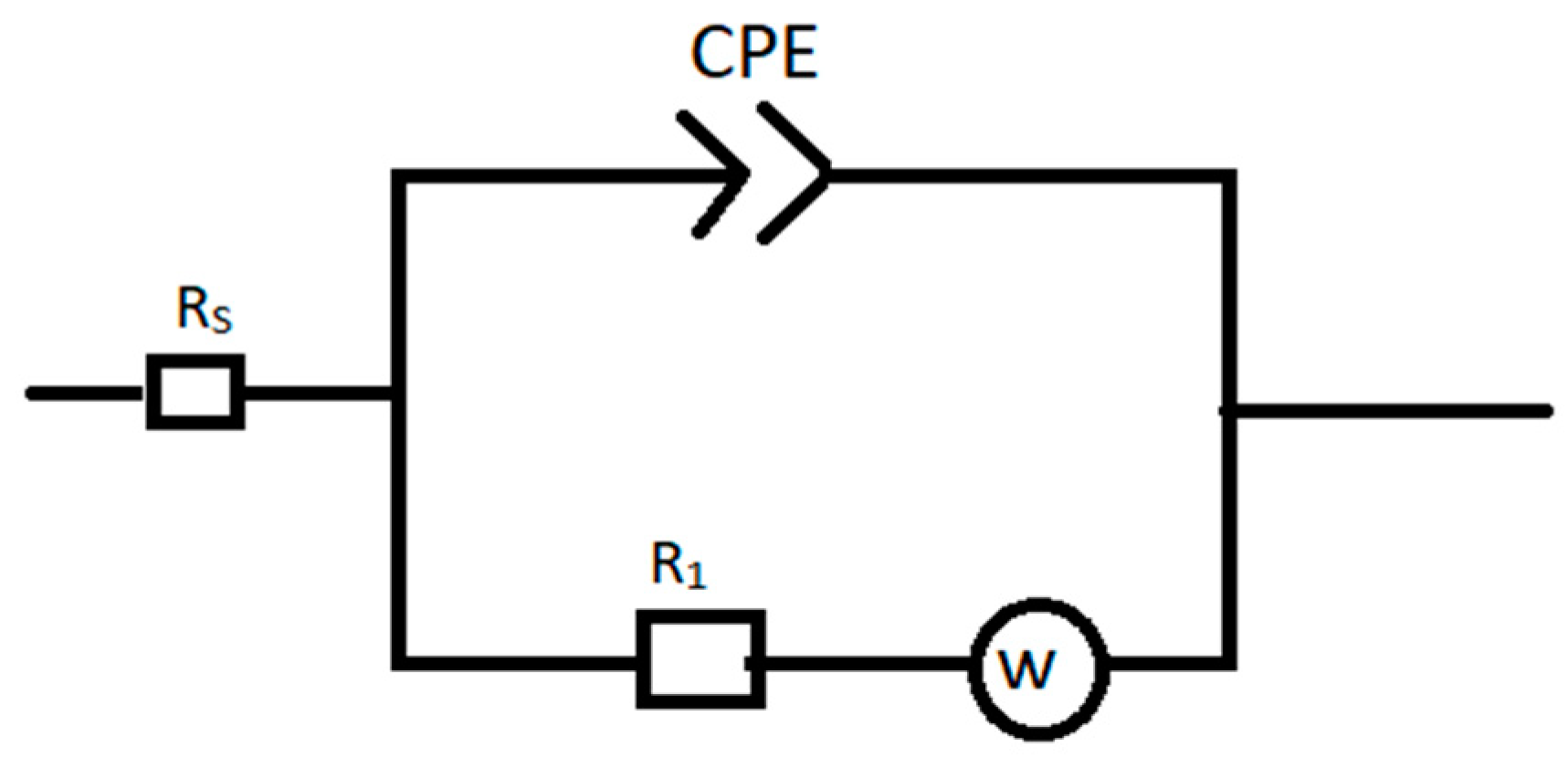
3.4. Electrochemical Impedance Spectroscopy
3.5. Membrane Fouling
3.6. Limitations and Future Scope
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asaithambi, P.; Yesuf, M.B.; Govindarajan, R.; Niju, S.; Periyasamy, S.; Rabba, Z.A.; Pandiyarajan, T.; Kadier, A.; Mani, D.; Alemayehu, E. Combined ozone, photo, and electrocoagulation technologies-An innovative technique for treatment of distillery industrial wastewater. Environ. Eng. Res. 2024, 29, 230042. [Google Scholar] [CrossRef]
- Ratna, S.; Rastogi, S.; Kumar, R. Current trends for distillery wastewater management and its emerging applications for sustainable environment. J. Environ. Manag. 2021, 290, 112544. [Google Scholar] [CrossRef]
- Selvamurugan, M.; Doraisamy, P.; Maheswari, M.; Valliappan, K. An integrated approach to achieve a circular bioeconomy in the treatment and disposal of distillery spentwash. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Das, I.; Ghangrekar, M.M.; Pant, D. Moving towards practical applications of microbial fuel cells for sanitation and resource recovery. J. Water Process Eng. 2020, 38, 101566. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Carmona-Martínez, A.A.; Chendake, A.D.; Pandit, S.; Pant, D. Modeling and optimization strategies towards performance enhancement of microbial fuel cells. Bioresour. Technol. 2021, 320, 124256. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Chen, X.; Yuan, X.; Li, N.; He, W.; Feng, Y. Enhanced electricity generation and extracellular electron transfer by polydopamine–reduced graphene oxide (PDA–rGO) modification for high-performance anode in microbial fuel cell. Chem. Eng. J. 2020, 387, 123408. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rodríguez-Couto, S. Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): An overview. Biochem. Eng. J. 2020, 164, 107779. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Li, T.; Dong, Z.; Wang, Y. Modification of carbon felt anodes using double-oxidant HNO3/H2O2 for application in microbial fuel cells. RSC Adv. 2018, 8, 2059–2064. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Khan, M.R.; Azam, M.; AlAmmari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2021, 13, 135. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zeng, Y.K.; Zhu, X.B.; Wei, L.; Zhao, T.S. A high-performance dual-scale porous electrode for vanadium redox flow batteries. J. Power Sources 2016, 325, 329–336. [Google Scholar] [CrossRef]
- Sutrisnoh, N.A.A.; Lim, G.J.H.; Chan, K.K.; Raju, K.; Teh, V.; Lim, J.J.N.; Fam, D.W.H.; Srinivasan, M. Toward High-Capacity Carbon Fiber Cathodes for Structural Batteries using Electrophoretic Deposition: Effects of Oxidative Surface Treatment on Carbon Fibers. Adv. Eng. Mater. 2023, 25, 2300694. [Google Scholar] [CrossRef]
- Altın, N.; Uyar, B. Impact of anode surface modifications on microbial fuel cell performance and algal biomass production. Environ. Technol. 2024, 1–12. [Google Scholar] [CrossRef]
- Ozer, T. Electrochemical activation and characterization of carbon cloth. Eur. Chem. Biotechnol. J. 2025, 3, 11–20. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Tan, W.; Ye, J.; Liu, C.; Feng, Q.; Xu, L. Effect of hydrofluoric acid-modified Co3O4/Y-type molecular sieves on MFC performance. J. Water Process Eng. 2024, 59, 104946. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Sayed, E.T.; Cho, H.; Park, M.; Obaid, M.; Kim, H.-Y.; Barakat, N.A.M. Effective strategies for anode surface modification for power harvesting and industrial wastewater treatment using microbial fuel cells. J. Environ. Manag. 2018, 206, 228–235. [Google Scholar] [CrossRef]
- Huang, W.; Chen, J.; Hu, Y.; Chen, J.; Sun, J.; Zhang, L. Enhanced simultaneous decolorization of azo dye and electricity generation in microbial fuel cell (MFC) with redox mediator modified anode. Int. J. Hydrogen Energy 2017, 42, 2349–2359. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Sathishkumar, Y.; Lee, Y.S.; Kim, A.R.; Yoo, D.J.; Kumar, G.G. The Influence of Chitosan Substrate and Its Nanometric Form Toward the Green Power Generation in Sediment Microbial Fuel Cell. J. Nanosci. Nanotechnol. 2017, 17, 558–563. [Google Scholar] [CrossRef]
- Qiang, Y.; Ouyang, D.; You, L.; Liu, D.; Zhao, X. Liquid flow fuel cell with an electrodeposition-modified nickel foam anode for efficient oxidation of 5-hydroxymethylfurfural to produce 2, 5-furandicarboxylic acid with co-generation of electricity. Chem. Eng. J. 2023, 469, 143832. [Google Scholar] [CrossRef]
- Santos, J.S.; Tarek, M.; Sikora, M.S.; Praserthdam, S.; Praserthdam, P. Anodized TiO2 nanotubes arrays as microbial fuel cell (MFC) electrodes for wastewater treatment: An overview. J. Power Sources 2023, 564, 232872. [Google Scholar] [CrossRef]
- Wu, J.; Liu, R.; Dong, P.; Li, N.; He, W.; Feng, Y.; Liu, J. Enhanced electricity generation and storage by nitrogen-doped hierarchically porous carbon modification of the capacitive bioanode in microbial fuel cells. Sci. Total Environ. 2023, 858, 159688. [Google Scholar] [CrossRef]
- Zahran, M. Iron- and carbon-based nanocomposites as anode modifiers in microbial fuel cells for wastewater treatment and power generation applications. J. Water Process Eng. 2024, 64, 105679. [Google Scholar] [CrossRef]
- Mahmoud, R.H.; Gomaa, O.M.; Hassan, R.Y.J.R.a. Bio-electrochemical frameworks governing microbial fuel cell performance: Technical bottlenecks and proposed solutions. RCS Adv. 2022, 12, 5749–5764. [Google Scholar]
- Zhong, D.; Liu, Y.; Liao, X.; Zhong, N.; Xu, Y. Facile preparation of binder-free NiO/MnO2-carbon felt anode to enhance electricity generation and dye wastewater degradation performances of microbial fuel cell. Int. J. Hydrogen Energy 2018, 43, 23014–23026. [Google Scholar] [CrossRef]
- Najihah Mohamad Daud, N.; Nasir Mohamad Ibrahim, M.; Ali Yaqoob, A.; Suriaty Yaakop, A.; Hazwan Hussin, M.; Shen, C.Y.; AlObaid, A.A. Optimizing microbial fuel cells performance: An innovative approach integrating anode materials, dual-pollutant treatment, and long-term operation. Fuel 2024, 364, 131160. [Google Scholar] [CrossRef]
- Tian, E.; Liu, Y.; Yin, F.; Lu, S.; Zheng, L.; Wang, X.; Wang, Z.; Liu, H. Facilitating proton transport by endowing forward osmosis membrane with proton conductive sites in osmotic microbial fuel cell. Chem. Eng. J. 2023, 451, 138767. [Google Scholar] [CrossRef]
- Guo, W.; Chen, Y.; Wang, J.; Cui, L.; Yan, Y. Enhanced electroactive bacteria enrichment and facilitated extracellular electron transfer in microbial fuel cells via polydopamine coated graphene aerogel anode. Bioelectrochemistry 2024, 160, 108769. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, B.; Qiu, Y.; Liu, K.; Fang, Z.; Qi, J.; Ma, Z.; Sun, T.; Liu, S. Enhanced microorganism attachment and flavin excretion in microbial fuel cells via an N,S-codoped carbon microflower anode. J. Colloid Interface Sci. 2023, 648, 327–337. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Yaakop, A.S.; Ahmad, A. Application of microbial fuel cells energized by oil palm trunk sap (OPTS) to remove the toxic metal from synthetic wastewater with generation of electricity. Appl. Nanosci. 2021, 11, 1949–1961. [Google Scholar] [CrossRef]
- Arkatkar, A.; Mungray, A.K.; Sharma, P. Bioelectrochemical behaviour of a sequentially added biocatalytic coculture in a microbial fuel cell. J. Basic Microbiol. 2020, 60, 562–573. [Google Scholar] [CrossRef]
- Dolatabadi, M.; Ahmadzadeh, S. A rapid and efficient removal approach for degradation of metformin in pharmaceutical wastewater using electro-Fenton process; optimization by response surface methodology. Water Sci. Technol. 2019, 80, 685–694. [Google Scholar] [CrossRef]
- Jin, X.; Guo, F.; Liu, Z.; Liu, Y.; Liu, H. Enhancing the Electricity Generation and Nitrate Removal of Microbial Fuel Cells with a Novel Denitrifying Exoelectrogenic Strain EB-1. Front. Microbiol. 2018, 9, 2633. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Al-Zaqri, N.; Alamzeb, M.; Hussain, F.; Oh, S.-E.; Umar, K. Bioenergy Generation and Phenol Degradation through Microbial Fuel Cells Energized by Domestic Organic Waste. Molecules 2023, 28, 4349. [Google Scholar] [CrossRef]
- Tahir, K.; Miran, W.; Jang, J.; Maile, N.; Shahzad, A.; Moztahida, M.; Ghani, A.A.; Kim, B.; Lee, D.S. MnCo2O4 coated carbon felt anode for enhanced microbial fuel cell performance. Chemosphere 2021, 265, 129098. [Google Scholar] [CrossRef]
- Ahmad, A.; Alshammari, M.B.; Ibrahim, M.N. Impact of Self-Fabricated Graphene–Metal Oxide Composite Anodes on Metal Degradation and Energy Generation via a Microbial Fuel Cell. Processes 2023, 11, 163. [Google Scholar] [CrossRef]
- Paudel, D.R.; Kaphle, G.C.; Poudel, B.R.; Kc, M.; Singh, M.; Ojha, G.P. Enhanced Hydrogen Evolution Reaction of a Zn+2-Stabilized Tungstate Electrocatalyst. Electrochem 2025, 6, 3. [Google Scholar] [CrossRef]
- Hu, M.; Li, X.; Xiong, J.; Zeng, L.; Huang, Y.; Wu, Y.; Cao, G.; Li, W. Nano-Fe3C@PGC as a novel low-cost anode electrocatalyst for superior performance microbial fuel cells. Biosens. Bioelectron. 2019, 142, 111594. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Yaakop, A.S.; Rafatullah, M. Utilization of biomass-derived electrodes: A journey toward the high performance of microbial fuel cells. Appl. Water Sci. 2022, 12, 99. [Google Scholar] [CrossRef]
- Sonawane, A.V.; Rikame, S.; Sonawane, S.H.; Gaikwad, M.; Bhanvase, B.; Sonawane, S.S.; Mungray, A.K.; Gaikwad, R. A review of microbial fuel cell and its diversification in the development of green energy technology. Chemosphere 2024, 350, 141127. [Google Scholar] [CrossRef]
- Bazina, N.; Ahmed, T.G.; Almdaaf, M.; Jibia, S.; Sarker, M. Power generation from wastewater using microbial fuel cells: A review. J. Biotechnol. 2023, 374, 17–30. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, G.-P.; Luo, H.-W.; Li, W.-W.; Wang, L.-F.; Yu, H.-Q. Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res. 2012, 46, 1817–1824. [Google Scholar] [CrossRef]
- Rikame, S.S.; Mungray, A.A.; Mungray, A.K. Electricity generation from acidogenic food waste leachate using dual chamber mediator less microbial fuel cell. Int. Biodeterior. Biodegrad. 2012, 75, 131–137. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Organic Fouling and Chemical Cleaning of Nanofiltration Membranes: Measurements and Mechanisms. Environ. Sci. Technol. 2004, 38, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-J.; Chae, K.-J.; Ajayi, F.F.; Kim, K.-Y.; Yu, H.-W.; Kim, C.-w.; Kim, I.S. Effects of biofouling on ion transport through cation exchange membranes and microbial fuel cell performance. Bioresour. Technol. 2011, 102, 298–303. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandya, B.; Chaudhari, L.; Vaghela, N.R. Enhanced Performance of a Microbial Fuel Cell Using Double Oxidant-Treated Carbon Felts. Electrochem 2025, 6, 12. https://doi.org/10.3390/electrochem6020012
Pandya B, Chaudhari L, Vaghela NR. Enhanced Performance of a Microbial Fuel Cell Using Double Oxidant-Treated Carbon Felts. Electrochem. 2025; 6(2):12. https://doi.org/10.3390/electrochem6020012
Chicago/Turabian StylePandya, Bhavi, Latesh Chaudhari, and Naresh R. Vaghela. 2025. "Enhanced Performance of a Microbial Fuel Cell Using Double Oxidant-Treated Carbon Felts" Electrochem 6, no. 2: 12. https://doi.org/10.3390/electrochem6020012
APA StylePandya, B., Chaudhari, L., & Vaghela, N. R. (2025). Enhanced Performance of a Microbial Fuel Cell Using Double Oxidant-Treated Carbon Felts. Electrochem, 6(2), 12. https://doi.org/10.3390/electrochem6020012





