Abstract
In this study, we developed lithium–sulfur rechargeable batteries using chemically modified thermoplastic sulfur polymers as cathode active materials, aiming to effectively utilize surplus sulfur resources. The resulting high-sulfur-content resins exhibited self-healing properties, extensibility, and adhesiveness. By leveraging its high solubility in specific organic solvents, we successfully introduced sulfur-based compounds into porous carbon via vacuum impregnation using a solution, rather than conventional thermal impregnation. Charge–discharge measurements of lithium–sulfur (Li-S) secondary batteries assembled with this more uniform composite cathode, compared to those using elemental sulfur, demonstrated an increased discharge capacity in the initial cycles and at higher rates.
1. Introduction
Sulfur is an element abundantly found in volcanic regions and is also produced in large quantities as a byproduct of petroleum refining processes. However, there are no practical products primarily composed of sulfur, and balancing its supply and demand is essential. One of the emerging technologies proposed to address this issue is “inverse vulcanization” (Figure 1).
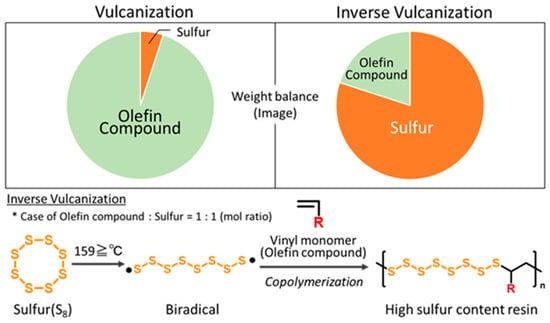
Figure 1.
Images of the weight ratio of olefin compounds to sulfur in vulcanization and inverse vulcanization, and the general reaction scheme of inverse vulcanization.
This method involves heating sulfur with an eight-membered ring structure, which has a melting point of 120–124 °C, to temperatures above 159 °C to induce ring opening. In this process, a polysulfan intermediate with terminal radicals (biradicals) is generated, which then copolymerizes with a small amount of vinyl monomers. As a result, a functional polymer is obtained, in which sulfur domains of random lengths are incorporated between a limited number of C–C bonds. In other words, this method is characterized by its reversed composition ratio compared to the conventional rubber manufacturing process (vulcanization), in which the double bonds of polyisoprene are crosslinked using a small amount of sulfur. Using this inverse vulcanization method, Pyun et al. first reported the development of high-sulfur polymer materials via inverse vulcanization in 2013 [1]. They successfully developed sulfur-containing polymers with easily controllable mechanical and molding properties by heating sulfur (S8) and 1,3-diisopropenylbenzene (DIB) at 185 °C under predetermined mixing ratios. In that study, brownish transparent resins with sulfur contents ranging from 50 to 90 wt% were obtained. Notably, at a sulfur content of 70 wt%, micro-scale patterning was achieved through melting at 185 °C and curing at 200 °C, demonstrating the potential utility of these materials as novel polymers. Following this publication, numerous high-sulfur materials combined with various vinyl compounds have been reported worldwide [2,3,4,5,6,7,8,9,10,11].
Furthermore, these high-sulfur content polymers have also been reported as cathode active materials for application in lithium–sulfur secondary batteries [12,13,14,15]. Pyun et al. were among the first to explore such applications, reporting that high-sulfur content polymers composed of S8 and DIB achieved a capacity of over 800 mAh/g per sulfur weight during 100 charge–discharge cycles [1]. The detailed reasons behind the excellent battery performance were not discussed, but it is speculated that resinifying sulfur improved the bonding and uniformity with the activated carbon particles used as conductive additives [16,17,18,19,20,21,22,23].
We have also been actively conducting research on the synthesis of high-sulfur content resins composed of sulfur and alkenyl compounds using radical polymerization. As a characteristic of these studies, instead of using vinyl compounds that readily undergo radical polymerization, a synthetic strategy is employed to introduce alkene units that are generally less reactive for polymerization (Scheme 1). When radical-polymerizable vinyl monomers, such as styrene-based or acrylic-based monomers, are used, their high polymerizability makes material fabrication easier. However, the bonding between vinyl monomers can potentially disrupt the structural order of the polymer. On the other hand, when using alkenyl compounds with low radical polymerizability, which we focus on, polymerization between these compounds is less likely to occur [24,25,26,27,28,29,30,31,32]. As a result, the number of sulfur atoms between alkenyl units tends to be more evenly distributed, allowing the polymer structure to maintain its orderliness. Based on this concept, we were the first to successfully develop high-sulfur content polymers with crown ethers, which are cyclic oligoethylene oxides, or long-chain alkyl groups as side chains [33,34,35] (Figure 2).
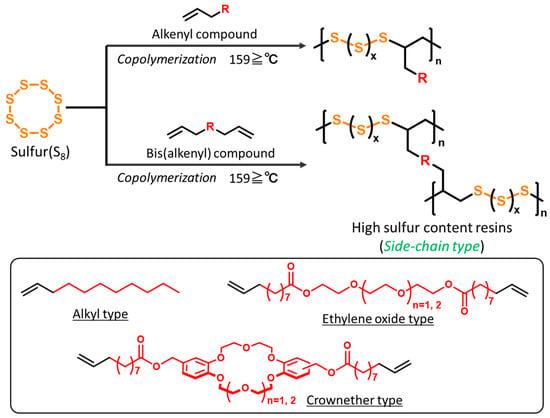
Scheme 1.
Our previous synthetic strategies for high-sulfur-content resins (side-chain type) via inverse vulcanization with various alkenyl compounds.
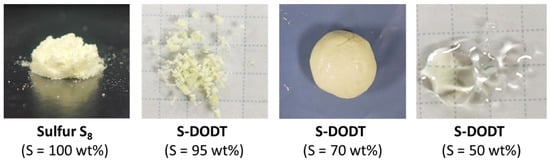
Figure 2.
Photograph of the obtained sulfur resin S-DODTs.
The obtained sulfur polymers exhibited tunable physical states, ranging from rubber-like to liquid or solid, depending on the backbone structure and composition ratio of the alkenyl compounds. Additionally, they showed solubility in specific organic solvents. Furthermore, utilizing these properties made it possible to uniformly introduce sulfur components into porous carbon, demonstrating effectiveness in improving conductivity—one of the challenges associated with sulfur cathodes.
As described above, the high-sulfur-content polymers obtained through inverse vulcanization reported to date have primarily been synthesized from sulfur and compounds containing double bonds, regardless of their polymerizability. However, in recent years, there have been a few reports on the synthesis of high-sulfur-content polymers using compounds other than those containing double bonds [36,37,38,39,40,41]. For example, by reacting sulfur with alkynes, epoxides, halogenated compounds, or thiols, these materials have been explored not only for battery applications but also for use in self-healing and reprocessable materials. Among these methods, when halogenated or thiol compounds are used, the resulting structure significantly differs from conventional polymers with sulfur as a side-chain framework. Instead, the backbone of these compounds is fully incorporated as the main chain between polysulfides. As a result, the crystallinity and solubility of the sulfur polymer undergo significant changes. When these polymers are used as active materials, they are expected to have a positive impact on battery performance.
Herein, we focused on inverse vulcanization involving sulfur and dithiols. Dithiols can generate thiyl radicals at both terminal groups, enabling the formation of linear polymers through radical reactions with sulfur. Specifically, we adopted 3,6-dioxa-1,8-octanedithiol (DODT) as the dithiol component and successfully developed a novel copolymer, S-DODT, with a sulfur content exceeding 50 wt% (Scheme 2). We further investigated its fundamental properties and evaluated its potential as a cathode material for lithium–sulfur secondary batteries.
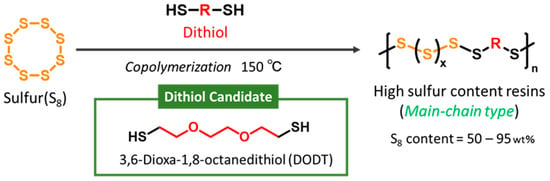
Scheme 2.
Our new synthetic strategy for a high-sulfur-content resin via inverse vulcanization using a dithiol (DODT).
2. Materials and Methods
2.1. Materials and Instruments
1,2-Dimethoxyethane (DME) and 1,3-Dioxolane (DOL, stabilized with BHT) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and used after dehydration with molecular sieves. Other solvents and reagents were used as purchased without further purification. Ketjen Black (KB) ECP600JD was supplied by Lion Specialty Chemicals Co., Ltd. (Tokyo, Japan), and poly(tetrafluoroethylene) (F-104) was supplied by Daikin Industries, Ltd. (Osaka, Japan).
The nuclear magnetic resonance (NMR) measurement was performed using a JMN-LA500 spectrometer (Tokyo, Japan). The glass transition temperature Tg was measured using a highly sensitive differential scanning calorimeter Hitachi High-Tech Science DSC 7020 (Tokyo, Japan). The tensile test was conducted using an A&D Company MCT-1150 (Tokyo, Japan). The Cyclic voltammetry (CV) measurements were performed using a Meiden Hokuto HZ-7000 (Tokyo, Japan). The charge–discharge test was evaluated using a Meiden Hokuto HJ1001SD8 (Tokyo, Japan).
2.2. Synthesis of S–DODT
A mixture of sulfur (S8) and 3,6-Dioxa-1,8-octanedithiol (DODT) in various ratios was heated and stirred at 150–160 °C for 2 h. Subsequently, the reaction mixture was subjected to reduced pressure distillation at 110–120 °C to remove unreacted materials, resulting in the radical copolymer S-DODT of S8 and DODT with a yield of 100%. The sulfur content in the reaction was adjusted to be between 50 and 95 wt%.
1H NMR (CDCl3) δ (ppm from TMS): 4.01-3.65 (m, 8H, -S-CH2-CH2-O-CH2-CH2-O-CH2-CH2-S-), 3.35-2.85 (m, 4H, -S-CH2-CH2-O-CH2-CH2-O-CH2-CH2-S-).
2.3. Preparation of S-DODT@KB Composite
S-DODT (0.417 g, sulfur content: 60 wt%) was dissolved in THF (16.1 g), and the resulting THF solution was mixed with Ketjen Black (KB, 0.211 g). The THF was then removed under reduced pressure conditions, yielding the S-DODT and KB composite, S-DODT@KB.
2.4. Fabrication of S-DODT@KB Electrode
The prepared composite S-DODT@KB (0.590 g) and polytetrafluoroethylene (PTFE, 0.0652 g) were kneaded together and pressed onto a SUS mesh (diameter 1 cm) at room temperature, affording the S-DODT@KB electrode (with a sulfur loading of approximately 4.3 mg/cm2).
2.5. Fabrication of S8@KB Electrode
A mixture of powdered sulfur (S8, 0.500 g) and KB (0.249 g) was heated and stirred at 150 °C for 10 min. The obtained S8@KB composite was kneaded with PTFE at a weight ratio of 9:1 and formed into a sheet. It was then pressed onto a SUS mesh at 120 °C, yielding the S8@KB electrode (with a sulfur loading of approximately 4.4 mg/cm2).
2.6. Fabrication of Li–S Battery and Electrochemical Measurements
The prepared S-DODT@KB or S8@KB electrode was used as the cathode to assemble CR2032 coin cells. The cells consisted of a Celgard #2500 (Asahi Kasei Corp., Tokyo, Japan) separator, a lithium metal foil anode, and an electrolyte composed of 1 M lithium bis(trifluoromethanesulfonyl) imide (LiTFSI) dissolved in a mixed solvent of 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) (volume ratio 1:1). The electrolyte amount was adjusted to an Electrolyte/Sulfur (E/S) ratio of 12. Cyclic voltammetry (CV) was performed using the assembled coin cells, sweeping from 1.0 to 3.0 V at a scan rate of 0.5 mV s−1 under 298 K. Charge–discharge tests were conducted at 298 K under a constant current (rate: 0.1 and 0.5 C) with a cutoff voltage range of 1.6 to 2.7 V. Additionally, when the rate was set to 0.5 C, the electrode composition was adjusted to “active material (S-DODT or S8):KB:PTFE = 3:6:1 (weight ratio)”, and the electrolyte amount was increased to “E/S = 24”.
3. Results and Discussion
The inverse vulcanization of sulfur and DODT proceeded readily under heating and stirring at 150 °C, yielding a yellow oil or resin (Figure 2). When the sulfur content was 50 wt%, the resulting S-DODT (S = 50 wt%) was a viscous liquid, which gradually transformed into a hard resin as the sulfur content increased.
The 1H NMR spectra of S-DODT (S content = 50, 90 wt%) are shown in Figure 3. The proton peak near 1.6 ppm, attributed to the SH group of DODT, disappeared significantly after the reaction with sulfur. Furthermore, the methylene proton peaks (a–c) derived from ethylene oxide near 2.7 ppm and 3.6 ppm shifted downfield, confirming the progress of the reaction. This synthetic mechanism is presumed to proceed via a radical reaction, in which two possible pathways for radical reactions may be involved. The first involves the formation of biradicals through the cleavage of the cyclic sulfur structure, followed by coupling with thiol radicals, similar to Figure 1. The second is a thiol–disulfide exchange reaction, where thiol radicals interact with the disulfide bonds of cyclic sulfur through a radical-mediated rearrangement. Given the reaction environment, both pathways are plausible, suggesting that the reaction progresses through a combination of these two mechanisms.
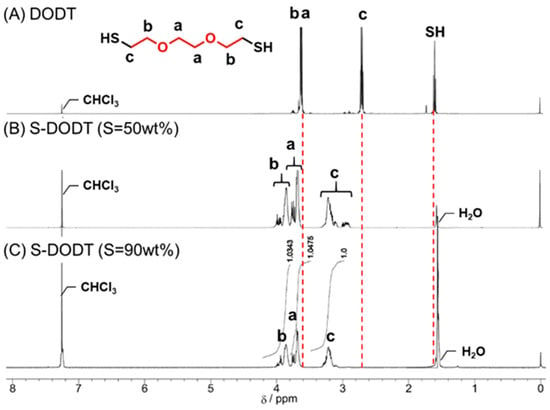
Figure 3.
1H NMR spectra of (A) DODT, (B) S-DODT (S = 50 wt%), and (C) S-DODT (S = 90 wt%) in CDCl3.
Furthermore, as sulfur content increases, the peaks originating from the ethylene oxide units tend to converge. This suggests that as the sulfur ratio increases, the sulfur chain length between the DODT units becomes more uniform.
The differential scanning calorimetry (DSC) chart of S-DODT is shown in Figure 4. Using S-DODT with a sulfur content of 50 wt% or higher, we investigated the melting point (m.p.) and glass transition temperature (Tg). The results showed that as the sulfur content approached 55 wt%, the melting point of sulfur shifted to a lower temperature, and a new glass transition temperature appeared around −50 °C.
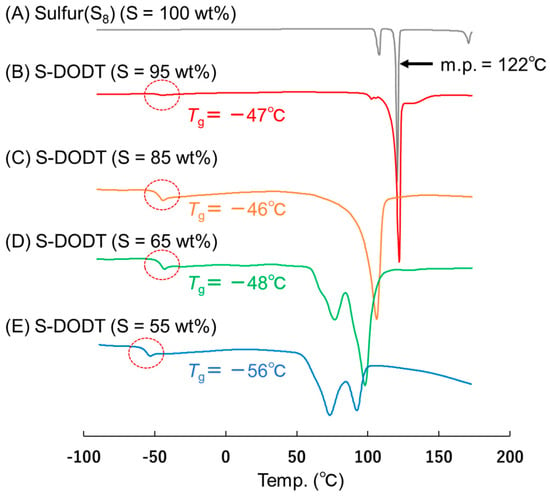
Figure 4.
DSC charts of (A) Sulfur S8, (B) S-DODT (S = 95 wt%), (C) S-DODT (S = 85 wt%), (D) S-DODT (S = 65 wt%) and (E) S-DODT (S = 55 wt%).
The stretchability of S-DODT was also investigated. A rectangular block of S-DODT (S = 70 wt%) was adhered and fixed on both its upper and lower surfaces to the grips of a tensile testing machine. Then, the sample was pulled upward at a constant speed (100 mm/min) to evaluate its elongation properties (Figure 5). The sample exhibited an elongation over 20 times its original height. Additionally, the stress remained within a certain range (2–4 MPa) during elongation, suggesting that continuous stress relaxation occurred within the S-DODT matrix. This phenomenon was suggested to have been caused by the cleavage and recombination of sulfur radicals within the S-DODT matrix. Furthermore, fractured S-DODT could be reattached and molded back to its original state, demonstrating self-healing capabilities. This behavior also supports the presence of reversible sulfur radical bonding.
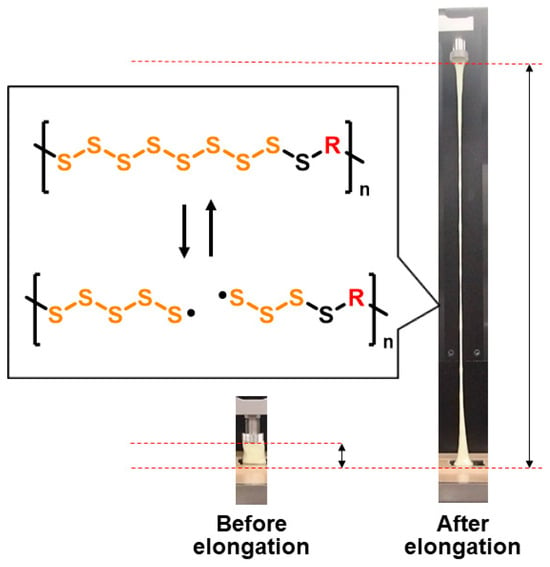
Figure 5.
Photographs of S-DODT (S = 70 wt%) before and after the tensile test (tensile speed: 100 mm/min) and the estimated elongation mechanism.
The obtained S-DODT dissolved in relatively low-polarity solvents such as tetrahydrofuran (THF), but was insoluble in high-polarity solvents like alcohol and water. Additionally, it showed no solubility in typical electrolytes used for Li–S batteries, such as 1M Lithium Bis(trifluoromethanesulfonyl)imide in dioxolane-dimethoxyethane (LiTFSI/DOL-DME) (Figure 6). These results suggest that the introduction of the DODT framework into the nonpolar and highly crystalline sulfur chains imparted partial polarity and amorphous properties to S-DODT.
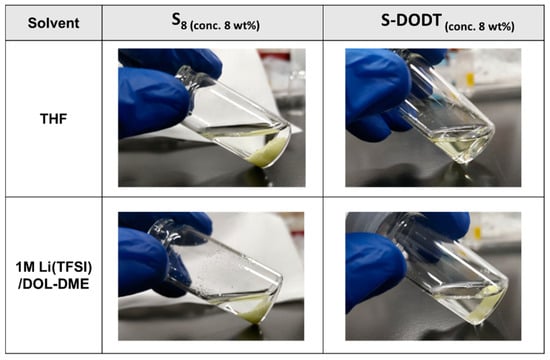
Figure 6.
Solubility test of S₈ and S-DODT (S = 55 wt%) in THF and Li–S battery electrolyte (1M Li(TFSI)/DOL-DME).
Next, a cathode was fabricated using S-DODT as the active material, and its performance was evaluated as a Li–S battery. A key point to emphasize here is that our proposed electrode fabrication method significantly differs from conventional sulfur-based methods, effectively utilizing the unique properties of S-DODT. One of the conventional methods for preparing sulfur electrodes involves mixing the powders of sulfur, carbon (as a conductive additive), and polytetrafluoroethylene (PTFE) (as a binder), followed by molding, a process known as the dry method. In this process, porous carbon is often used, and a sulfur–carbon composite, formed by impregnating molten sulfur into the carbon, is employed as the cathode material. This is expected to suppress the dissolution of polysulfide anions, the discharge products, into the electrolyte.
We also adopted Ketjenblack (KB) as the porous carbon for the aforementioned purpose. Instead of undergoing a thermal melting process, we prepared a sulfur/carbon sheet (S-DODT@KB) by impregnating KB with a THF solution of S-DODT (S = 60, 90 wt%) followed by vacuum drying (Figure 7).
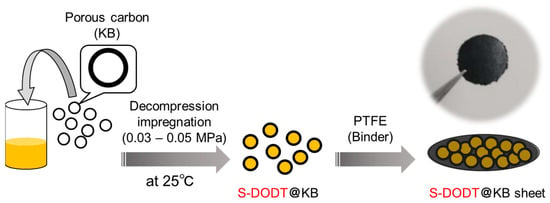
Figure 7.
Overview of the fabrication process of composite sheets for cathode electrodes using S-DODT, porous carbon (KB), and PTFE.
This electrode fabrication method is a novel approach that has not been previously reported. It enables the uniform incorporation of sulfur components into porous carbon in a low-viscosity state without the need for high-temperature heating, adding versatility to the preparation of sulfur cathodes. The obtained composite was kneaded with PTFE powder and used to prepare the S-DODT@KB sheet for the cathode.
The fabricated S-DODT (S = 60, 90)@KB electrodes were employed as a working electrode for cyclic voltammetry (CV) measurements (Figure 8). For comparison, an electrode composed of sulfur (S8@KB) was also prepared and evaluated under similar conditions. The results showed that both the S8@KB and S-DODT@KB electrodes exhibited reduction responses at 1.6 V and 2.1 V, and an oxidation response at 2.6 V, indicating a highly similar reaction mechanism. This suggests that DODT itself is unreactive with lithium ions, while sulfur chains of a certain length react with lithium ions to form lithium sulfide. Additionally, the charge capacity during the reduction reaction of the S-DODT electrodes (S = 60 and 90 wt%) increased by approximately 10–20% compared to that of the S8 electrode, indicating improved reactivity. On the other hand, during the oxidation reaction, the charge capacity was less than half of that observed during the reduction reaction. However, based on the Coulombic efficiency, the suppression effect improved by approximately 10% when using the S-DODT electrodes compared to the S8 electrode.
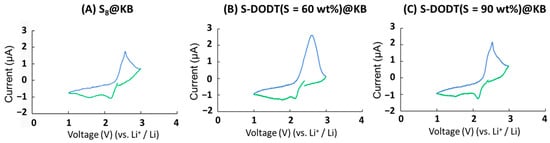
Figure 8.
Cyclic voltammograms (1st cycle) of sulfur cathodes in 1 M LiTFSI-DOL/DME: (A) S8@KB, (B) S-DODT (S = 60 wt%), and (C) S-DODT (S = 90 wt%) at a scan rate of 0.5 mV/s.
As the next step in the electrochemical evaluation, 2032 coin-type Li–S batteries were assembled using S-DODT (S = 60, 90 wt%) or S8@KB as the cathode and lithium metal as the anode, followed by charge–discharge testing. Here, the electrolyte (E)-to-sulfur (S) weight ratio was defined as the E/S ratio using the electrolyte (1.0 M LiTFSI/DOL-DME). Under the conditions of E/S = 12, 0.1 C, and 25 °C, charge–discharge evaluations were conducted. The results showed that within 50 cycles, the cells employing S-DODT exhibited higher capacities compared to those using S8@KB (Figure 9).
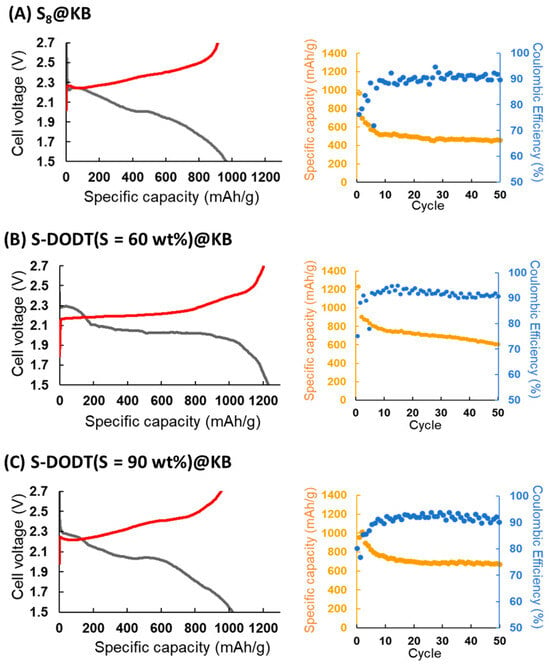
Figure 9.
Charge–discharge curves at initial cycles and changes in–discharge capacity and Coulombic efficiency over cycling (0.1 C, 50 cycles): (A) S8@KB, (B) S-DODT (S = 60 wt%)@KB, and (C) S-DODT (S = 90 wt%)@KB cathodes.
The cathode utilizing S-DODT (S = 60 wt%) achieved even higher capacities compared to S-DODT (S = 90 wt%) and exhibited reduced overpotentials during charge–discharge cycles. The battery using the high-sulfur-content resin S-BMEE (S = 60 wt%) synthesized with bis(2-mercaptoethyl) ether (BMEE), which has one less ethylene oxide unit than DODT, as the active material exhibited a discharge capacity of more than 100 mAh/g lower than that of S-DODT (Figure S1). These results suggested that (i) the high uniformity between S-DODT and carbon within the electrode and (ii) the enhanced reactivity of the sulfur components due to the electrostatic interaction between the ethylene oxide units in DODT (amorphous region) and lithium ions contributed to the improved performance (Figure 10).
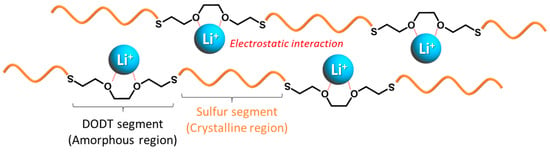
Figure 10.
Illustration of the proposed interaction between S-DODT and lithium ions.
These results were considered to be related to the amount of DODT in the S-DODT. The morphology of S-DODT (S = 60 wt%) was a highly adhesive, flexible, clay-like resin, which was attributed to the incorporation of liquid DODT units throughout the sulfur polymer chains. However, during the discharge reaction, the chemical reaction between S-DODT and lithium ions led to the dissociation of DODT units, some of which dissolved into the electrolyte. This accelerated the degradation of the cathode and significantly deteriorated the long-term cycling performance (Figure S2). In S-DODT (S = 90 wt%) with a lower DODT content, electrode degradation was less likely to occur. Its moderate affinity for lithium ions contributed to stable cycling performance.
Additionally, cells using S-DODT (S = 60 wt%) demonstrated superior performance at higher rates. As a result of charge–discharge tests at 0.5 C, cells with S-DODT exhibited significant improvements in–discharge capacity and Coulombic efficiency during 50 cycles compared to cells with S8 (Figure 11). Furthermore, S-DODT (S = 60 wt%) also showed a reduced voltage gap in the flat regions of both the discharge and charge curves, suggesting that incorporating the DODT units enhances the electrochemical reaction rate. This was attributed to the high uniformity of the sulfur–carbon composite mentioned above and the electrostatic interactions between the DODT units and lithium ions in the polymer containing a highly amorphous segment. On the other hand, improving the long-term capacity retention rate is an important challenge, and efforts toward this improvement will be necessary in the future (Figure S3).
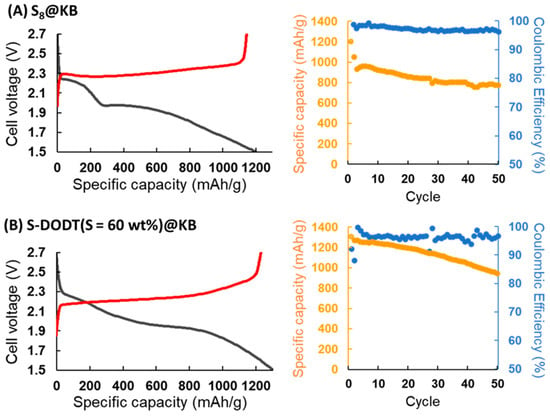
Figure 11.
Charge–discharge curves at initial cycles and changes in discharge capacity and Coulombic efficiency over cycling (0.5 C, 50 cycles): (A) S8@KB and (B) S-DODT (S = 60 wt%)@KB.
4. Conclusions
In this study, we succeeded in synthesizing a high-sulfur-content resin, S-DODT, through the reverse vulcanization of sulfur and the dithiol compound DODT. Additionally, S-DODT exhibited a variety of shapes by controlling the sulfur content, acquiring plasticity, self-healing properties, and solubility in organic solvents. These characteristics are believed to arise from the moderate adjustment of the length of the sulfur chains continuously bonded through the incorporation of DODT units, and from the behavior of the cleavage and recombination of these sulfur chains. Furthermore, by utilizing these properties, it became possible to prepare a highly uniform composite material with the conductive additive, porous carbon KB, leading to the development of a new method for preparing sulfur cathodes. When the obtained S-DODT/KB composite electrode was applied as the cathode in a Li–S battery, the interaction between the ethylene oxide units of DODT and Li ions, together with the high uniformity of the electrode state, led to significant improvements in both capacity and rate performance over 50 cycles. However, the high affinity between S-DODT and the electrolyte means that not only the usual polysulfide anions but also the DODT units are dissolved into the electrolyte, which leads to the degradation of the electrode, and long-term cycle stability has not been achieved. In the future, it will be necessary to investigate the composition and types of electrolytes, as well as to explore the development of cells incorporating gel electrolytes or solid electrolytes, in order to improve long-term stability. We also plan to further investigate the electrochemical reaction mechanisms of S-DODT and its analogs, including the capacity degradation mechanisms and the details of the interaction between DODT and lithium ions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/electrochem6010008/s1, Figure S1: (A) Charge–discharge curves at initial cycles and changes in–discharge capacity and (B) Coulombic efficiency over cycling using S-BMEE@KB cathodes (blue: S-BMEE@KB, black: S-DODT@KB); Figure S2: Charge–discharge curves at initial cycles and changes in–discharge capacity and Coulombic efficiency over cycling (0.1 C, 100 cycles): (A) S8@KB, (B) S-DODT (S = 60 wt%)@KB, and (C) S-DODT (S = 90 wt%)@KB cathodes; Figure S3: Charge–discharge curves at initial cycles and changes in–discharge capacity and Coulombic efficiency over cycling (0.5 C, 100 cycles): (A) S8@KB and (B) S-DODT (S = 60 wt%)@KB.
Author Contributions
Conceptualization, K.O. and K.Y.; synthesis and structural analysis, H.T. and J.T.; writing—original draft preparation, K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Dirlam, P.T.; Simmonds, A.G.; Shallcross, R.C.; Arrington, K.J.; Chung, W.J.; Griebel, J.J.; Hill, L.J.; Glass, R.S.; Char, K.; Pyun, J. Improving the Charge Conductance of Elemental Sulfur via Tandem Inverse Vulcanization and Electropolymerization. ACS Macro Lett. 2015, 4, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Konopka, K.M.; Glass, R.S.; Char, K.; Pyun, J. Chalcogenide hybrid inorganic/organic polymers via inverse vulcanization and dynamic covalent polymerizations. Polym. Chem. 2017, 8, 5167–5173. [Google Scholar] [CrossRef]
- Zhang, Y.; Kleine, T.S.; Carothers, K.J.; Phan, D.D.; Glass, R.S.; MacKay, M.E.; Char, K.; Pyun, J. Functionalized chalcogenide hybrid inorganic/organic polymers (CHIPs) via inverse vulcanization of elemental sulfur and vinylanilines. Polym. Chem. 2018, 9, 2290–2294. [Google Scholar] [CrossRef]
- Park, S.; Lee, D.; Cho, H.; Lim, J.; Char, K. Inverse Vulcanization Polymers with Enhanced Thermal Properties via Divinylbenzene Homopolymerization-Assisted Cross-Linking. ACS Macro Lett. 2019, 8, 1670–1675. [Google Scholar] [CrossRef]
- Berk, H.; Kaya, M.; Cihaner, A. Thermally highly stable polyhedral oligomeric silsesquioxane (POSS)-sulfur based hybrid inorganic/organic polymers: Synthesis, characterization and removal of mercury ion. Polym. Chem. 2022, 13, 5152–5158. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lee, J.M.; Seo, J.H.; Noh, G.Y.; Byun, W.; Kim, S.; Lee, W.; Park, S.; Kim, D.G.; Kim, Y.S. Inverse vulcanization of elemental sulfur catalyzed by trialkyl amines. Green Chem. 2023, 25, 4641–4646. [Google Scholar] [CrossRef]
- Lai, Y.S.; Liu, Y.L. Reaction between 1,3,5-Triisopropylbenzene and Elemental Sulfur Extending the Scope of Reagents in Inverse Vulcanization. Macromol. Rapid Commun. 2023, 44, 2300014. [Google Scholar] [CrossRef]
- Bao, J.; Martin, K.P.; Cho, E.; Kang, K.S.; Glass, R.S.; Coropceanu, V.; Bredas, J.L.; Parker, W.O., Jr.; Njardarson, J.T.; Pyun, J. On the Mechanism of the Inverse Vulcanization of Elemental Sulfur: Structural Characterization of Poly(sulfur-random-(1,3-diisopropenylbenzene)). J. Am. Chem. 2023, 145, 12386–12397. [Google Scholar] [CrossRef]
- Carothers, K.; Lee, K.M.; McConney, M.E.; Stevenson, P.R.; Godman, N.P. Inverse Vulcanization of Vinyl-polycyclic Aromatic Hydrocarbon Monomers and Dynamic Covalent Polymerization with Liquid Crystalline Monomers. ACS Appl. Polym. Mater. 2024, 6, 11118–11126. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.; Tang, Z.; Huang, R.; Xiao, Y.; Chen, J.; Yin, P.; Guo, B.; Zhang, L. Exploring Epoxy-Functionalized Polysulfide as a VOC-Free and Highly Effective Interfacial Modifier for Silica-Filled Rubber Composites. Macromolecules 2024, 57, 470–480. [Google Scholar] [CrossRef]
- Lv, Y.; Shang, M.; Chen, X.; Niu, J. Double-Net Enclosed Sulfur Composite as a New Cathode in Lithium Sulfur Batteries. J. Phys. Chem. C 2019, 123, 17719–17727. [Google Scholar] [CrossRef]
- Parekh, H.M.; Rao, H.; Jokhakar, D.; Parikh, P.V.; Palanisamy, M.; Pol, G.V. Polysulfide shuttle mitigation through a tailored separator for critical temperature energy-dense lithium–sulfur batteries. Sustain. Energy Fuels 2022, 6, 5591–5599. [Google Scholar] [CrossRef]
- Oka, S.S.; Thakur, M.R.; Easley, D.A.; Green, M.J.; Lutkenhaus, L.J. Structural organic battery cathodes comprised of organic redox active polymers, reduced graphene oxide, and aramid nanofibers. Mater. Adv. 2023, 4, 4886–4896. [Google Scholar] [CrossRef]
- Waqas, M.; Niu, Y.; Tang, M.; Pang, Y.; Ali, S.; Donge, Y.; Lv, W.; He, W. A decade of development in cathode-facing surface modified separators for high-performance Li-S batteries. Energy Storage Mater. 2024, 72, 103682. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Cengiz, E.C.; Demir-Cakan, R.; Yagci, Y. Inverse vulcanization of bismaleimide and divinylbenzene by elemental sulfur for Lithium–sulfur batteries. Eur. Polym. J. 2016, 80, 70–77. [Google Scholar] [CrossRef]
- Gomez, I.; Mecerreyes, D.; Blazquez, J.A.; Leonet, O.; Ben Youcef, H.; Li, C.; Gomez-Camer, J.L.; Bundarchuk, O.; Rodriguez-Martinez, L. Inverse vulcanization of sulfur with divinylbenzene: Stable and easy processable cathode material for Lithium–sulfur batteries. J. Power Sources 2016, 329, 72–78. [Google Scholar] [CrossRef]
- Gracia, I.; Benyoucef, H.; Judez, X.; Oteo, U.; Zhang, H.; Li, C.; Rodriguez-Martinez, L.M.; Armand, M. S-containing copolymer as cathode material in poly(ethylene oxide)-based all-solid-state Li–S batteries. J. Power Sources 2018, 390, 148–152. [Google Scholar] [CrossRef]
- Chen, Z.; Droste, J.; Zhai, G.; Zhu, J.; Yang, J.; Hansen, M.R.; Zhuang, X. Sulfur-anchored azulene as a cathode material for Li–S batteries. Chem. Commun. 2019, 55, 9047–9050. [Google Scholar] [CrossRef]
- Yesilot, S.; Kucukkoylu, S.; Demir, E.; Demir-Cakan, R. Phosphazene based star-branched polymeric cathode materials via inverse vulcanization of sulfur for Lithium–sulfur batteries. Polym. Chem. 2020, 11, 124–4132. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Zhou, J.; Fan, X.; Zhang, J.; Jia, Y.; Chen, S.; Meng, X.; Bielawski, C.W.; Geng, J. Covalently grafting sulfur-containing polymers to carbon nanotubes enhances the electrochemical performance of sulfur cathodes. ACS Appl. Polym. Mater. 2022, 4, 939–949. [Google Scholar] [CrossRef]
- Chen, J.M.; Duan, H.; Kong, Y.; Tian, B.; Ning, G.H.; Li, D. Improving Lithium–sulfur Batteries′ Performance via Inverse Vulcanization of Vinylene-Linked Covalent Organic Frameworks. Energy Fuels 2022, 36, 5998–6004. [Google Scholar] [CrossRef]
- Choudhury, S.; Akef, M.; Seifert, A.; Gobel, M.; Gruschwitz, M.; Matsidik, R.; Tegenkamp, C.; Sommer, M. Hybrid Organosulfur Network/MWCNT Composite Cathodes for Li–S Batteries. ACS Appl. Mater. Interfaces 2024, 16, 6301–6314. [Google Scholar] [CrossRef]
- Ko, L.A.; Huang, Y.S.; Lin, Y.A. Bipyridine-containing polysulfide materials for broad-spectrum removal of heavy metals from water. ACS Appl. Polym. Mater. 2021, 3, 3363–3372. [Google Scholar] [CrossRef]
- Eder, M.L.; Call, C.B.; Jenkins, C.L. Jenkins Utilizing Reclaimed Petroleum Waste to Synthesize Water-Soluble Polysulfides for Selective Heavy Metal Binding and Detection. ACS Appl. Polym. Mater. 2022, 4, 1110–1116. [Google Scholar] [CrossRef]
- Scheiger, J.M.; Hoffmann, M.; Falkenstein, P.; Wang, Z.; Rutschmann, M.; Scheiger, V.W.; Grimm, A.; Urbschat, K.; Sengpiel, T.; Matysik, J.; et al. Inverse Vulcanization of Norbornenylsilanes: Soluble Polymers with Controllable Molecular Properties via Siloxane Bonds. Angew. Chem. Int. Ed. Engl. 2022, 61, e202114896. [Google Scholar] [CrossRef]
- Cubero-Cardoso, J.; Cuadri, A.A.; Fermoso, F.G.; Martin-Alfonso, J.E.; Urbano, J. Promising Chalcogenide Hybrid Copolymers for Sustainable Applications as Bio-lubricants and Metal Adsorbents. ACS Appl. Polym. Mater. 2022, 4, 3667–3675. [Google Scholar] [CrossRef]
- Kang, K.S.; Iyer, K.A.; Pyun, J. On the Fundamental Polymer Chemistry of Inverse Vulcanization for Statistical and Segmented Copolymers from Elemental Sulfur. Chem. Eur. J. 2022, 28, e202200115. [Google Scholar] [CrossRef]
- Jia, J.; Yan, P.; Cai, S.D.; Cui, Y.; Xun, X.; Liu, J.; Wang, H.; Dodd, L.; Hu, X.; Lester, D.; et al. Solvated Inverse vulcanisation by photopolymerization. Eur. Polym. J. 2024, 207, 112815. [Google Scholar] [CrossRef]
- Deng, X.; Dop, R.A.; Cai, D.; Neill, D.R.; Hasell, T. Water-Soluble Ionic Liquid-Containing Sulfur Polymers for Mercury Capture, Demulsification, and Antibacterial Activity. Adv. Funct. Mater. 2024, 34, 2311647. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Si, G.; Tan, C. Self-Healing and Recyclable Vulcanized Polyisoprene Based on a Sulfur-Rich Copolymer Cross-Linking Agent Derived from Inverse Vulcanization. ACS Sustain. Chem. Eng. 2024, 12, 2212–2224. [Google Scholar] [CrossRef]
- Shen, H.; Chen, X.; Zheng, B.; Zhang, H. Sorbic Acid-Tung Oil-Sulfur Terpolymer Prepared via Inverse Vulcanization and Its Application as Antibacterial and Toughness Modifier for Polylactide. ACS Appl. Polym. Mater. 2024, 6, 14351–14364. [Google Scholar] [CrossRef]
- Itaoka, K.; Kim, I.T.; Yamabuki, K.; Yoshimoto, N.; Tsutsumi, H. Room temperature rechargeable magnesium batteries with sulfur-containing composite cathodes prepared from elemental sulfur and bis(alkenyl) compound having a cyclic or linear ether unit. J. Power Sources 2015, 297, 323–328. [Google Scholar] [CrossRef]
- Yamabuki, K.; Itaoka, K.; Kim, I.T.; Yoshimoto, N.; Tsutsumi, H. Electrochemically active copolymers prepared from elemental sulfur and bis(alkenyl) compounds having crown ether unit. Polymer 2016, 91, 1–6. [Google Scholar] [CrossRef]
- Yamabuki, K.; Itaoka, K.; Shinchi, T.; Yoshimoto, N.; Ueno, K.; Tsutsumi, H. Soluble sulfur-based copolymers prepared from elemental sulfur and alkenyl alcohol as positive active material for Lithium–sulfur batteries. Polymer 2017, 117, 225–230. [Google Scholar] [CrossRef]
- Dirlam, P.T.; Simmonds, A.G.; Kleine, T.S.; Nguyen, N.A.; Anderson, L.E.; Klever, A.O.; Florian, A.; Costanzo, P.J.; Theato, P.; Mackay, M.E.; et al. Inverse vulcanization of elemental sulfur with 1,4-diphenylbutadiyne for cathode materials in Li–S batteries. RSC Adv. 2015, 5, 24718–24722. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, C.; Wang, Z.; Xia, Z.; Li, R.; Shi, S.; Xu, S.; Yuan, L. Bio-Based Reprocessable and Degradable Epoxy Resins via Inverse Vulcanization. ACS Sustain. Chem. Eng. 2023, 11, 11259–11268. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kitano, D.; Nishimura, R.; Yamagishi, Y.; Horiguchi, A.; Yamaguchi, H. Supramolecular polysulfide polymers cross-linked by metal-ligand interactions. Polym. Chem. 2023, 14, 2577–2580. [Google Scholar] [CrossRef]
- Lee, M.; Oh, Y.; Yu, J.; Jang, S.G.; Yeo, H.; Park, J.J.; You, N.H. Long-wave infrared transparent sulfur polymers enabled by symmetric thiol cross-linker. Nat. Commun. 2023, 14, 2866. [Google Scholar] [CrossRef]
- Ovc-Okene, D.; Gnanavel, A.; Szabo, A.; Szarka, G.; Ivan, B.; Kun, R. Investigation of poly(3,6-dioxa-1,8-octane-dithiol)-based organosulfur polymer as the positive electrode material in rechargeable Li–S battery. J. Electroanal. Chem. 2023, 929, 117113. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Zhu, L.; Gao, L.; Luo, J.; Yang, X.; Fang, Y.; Zhang, Z.; Dong, J. Aziridine-Derived Polysulfide Elastomers for Self-Healable, Strong, and Reusable Adhesives. ACS Appl. Polym. Mater. 2024, 6, 6437–6447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).