Abstract
In a plant microbial fuel cell (P-MFC), the plant provides the fuel in the form of exudates secreted by the roots, which are oxidised by electroactive bacteria. The immature plant is hampered by low energy yields. Several factors may explain this situation, including the low open-circuit voltage of the plant cell. This is a function of the development of the biofilm formed by the electroactive bacteria on the surface of the anode, in relation to the availability of the exudates produced by the roots. In order to exploit the fertilising role of biochars, a plant cell was developed from C. citratus and grown in a medium to which 5% by mass of coconut shell biochar had been added. Its effect was studied as well as the distance between the electrodes. The potential of Cymbopogon citratus was also evaluated. Three samples without biochar, with inter-electrode distances of 2, 5 and 7 cm, respectively, identified as SCS2, SCS5 and SCS7, and three with the addition of 5 % biochar, with the same inter-electrode distance values, identified as S2, S5 and S7, were prepared. Open-circuit voltage (OCV) measurements were taken at 6 a.m., 1 p.m. and 8 p.m. The results showed that all the samples had high open-circuit voltage values at 1 p.m. Samples containing 5% biochar had open-circuit voltages increased by 16 %, 8.94% and 5.78%, respectively, for inter-electrode distances of 2, 5 and 7 cm compared with those containing no biochar. Furthermore, the highest open-circuit voltage values were obtained for all samples with C. citratus at an inter-electrode distance of 5 cm. The maximum power output of the PMFC with C. citratus in this study was 75.8 mW/m2, which is much higher than the power output of PMFCs in recent studies.
1. Introduction
As the world’s population grows and becomes more affluent, so does the demand for energy [1,2]. Fossil fuels are unevenly distributed around the world, are becoming scarce, and are increasingly difficult to extract [3,4]. Other sustainable alternative energy sources (wind, geothermal, photovoltaic, hydroelectric) are geographically dependent and deform the landscape [3]. The conversion of solar energy by plants into chemical and then electrical energy is a promising and environmentally friendly biotechnology, thanks to the rhizodeposition process that produces exudates (sugars, organic acids, etc.), secretions (polymeric carbohydrates, enzymes), lysates (dead cell material) and gases (ethylene, CO2) [5,6] by the process of rhizodeposition [5]. The plant microbial fuel cell stores solar energy as chemical energy and converts it into electricity by degrading the organic matter released in the rhizosphere by electroactive bacteria [7,8,9]. Photosynthesis is the process by which a plant uses light energy to convert carbon dioxide and water into chemical energy that can be used for plant growth. The production of bioelectricity from living plants is a new and exiting concept in the field of microbial fuel cells (MFC) known as plant microbial fuel cells (PMFCs) [7]. In addition, the use of plants in PMFCs reduces CO2 emissions by converting them into biomass through photosynthesis. This process is one of the methods used to produce alternative energy without releasing compounds that are harmful to the environment. The PMFC concept was first proposed by Strik et al. (2008), who used a two-chamber MFC where the roots of a plant are coupled in the anode chamber to use the carbohydrate reserves of the rhizome as a substrate for microorganisms. They designed a PMFC with a Glyceria maxima plant using graphite felt as electrodes and a precautionary exchange membrane. The maximum voltage they achieved was 256 mW with a power of 67 mW/m2, showing the potential of the PMFC as a starting point for future researchers. During PMFC operation, anodes are inserted into the rhizosphere. Recent studies have attempted several strategies to improve the electricity production of PMFC, such as the use of different plant species [10], growth medium [10] cell configurations, inter-electrode distance and electrode materials. The rhizosphere provides a favourable environment for the proliferation of bacteria, which is beneficial for plant growth [11]. Several root exudates and microbial metabolites have been reported to mediate electricity transfer. Plant type is one of the factors influencing PMFC, as the amount of electricity produced by each plant is different. Certain types of microorganisms are only found in certain plant roots or in certain environments. In general, the types of plant commonly used in PMFC to produce more electricity are those that live in water because of their electrolytic capacity. PMFC technology has the potential to become an alternative source of bioenergy in the future, which is green, clean, renewable, sustainable and much cheaper than any other form of bioenergy [12]. Despite its advantages, the PMFC system is limited by its low bioelectricity production capacity and relatively low cost. Helder and Marjolein have developed a new plant-growth medium: Hoagland medium with added phosphate buffer [13]. In their study, the influence of the Hoagland solution on electro-active bacteria was not studied. Wilgince Appolon et al. used cattle urine to increase plant growth and electricity production [14]. The use of bovine urine for electricity generation would be problematic once the system is scaled up. P. Chiranjeevi et al. varied the distance between the anode and the root in the rhizosphere from 0, 2 cm, 4 cm, 8 cm and 16 cm [15]. Kazuka Takanezawa et al. conducted a study that varied the depth of the anode by 5 cm and 2 cm. They experienced solid performance at a depth of 5 cm; at a depth of 2 cm the anode is not sufficiently anaerobic [16].
The aim of this study was to evaluate the influence of coconut shell biochar and the optimal anode-to-cathode distance on voltage generation in the C. citratus-based PMFC. The analysis includes open-circuit voltage measurements, which take into account electrochemical reactions at the electrodes and voltage and current measurement with a 1000 Ω load. C. citratus was used as the fuel source. It has never been used before in P-MFC technologies. This is the first time that this species has been used to generate electricity using PMFC. C. citratus is an important plant because of its advantages. The long-term power production of C. citratus was investigated as a function of the inter-electrode distance and the addition of coconut shell biochar. In addition, power ratings were calculated to determine the potential power generated by the C. citratus plant compared to other plants used in the PMFC microbial fuel cell.
2. Materials and Methods
2.1. Materials
Cymbopogon citratus (a member of the grass family Poaceae), known worldwide as citronella, was used in this study. Its characteristic lemon scent is derived from the prefix ‘lemon’, which is mainly due to the presence of a cyclic monoterpene called ‘citral’ [17]. It is a fast-growing, bushy, aromatic perennial herb that can reach a height of at least 1 metre, with numerous stems with stiff leaves arising from short rhizomatous roots. Its green, linear leaf blades are tapered at both ends, characteristic of grasses. It is a C4 monocot (plants with a high photosynthetic rate) [18]. Although very well adapted to temperate climates, C. citratus can grow in a wide range of environmental conditions. It is an economically important plant with a reasonable life span of about five years. It is widely used in Ayurvedic medicine. It is believed to have a variety of pharmacological activities, including anti-amoebic, antibacterial, antidiarrhoeal, antifilarial, antifungal and anti-inflammatory properties [17]. Its biomass is used to produce essential oils of commercial value and in food technology and traditional medicine. The characteristics of this plant listed above favoured its selection and use in our experiments. The graphite rod of the Leclanché battery was used as an anode. This rod can conduct electricity to collect the electrons released by the exo-electrogenic bacteria [19]. The same type of material is used for the cathode. These graphite electrodes are extracted from the UM1.R20 SIZE D batteries manufactured by Sunwatt (Hangzhou, China). The characteristics of the stem can be found at http: www.sunwatt.com. Power is better when the same type of material is used for the electrodes [20]. In our experiment, oxygen was used as the electron acceptor. Part of the cathode was in contact with the ambient air to make use of atmospheric oxygen [21]. The cathode exposed to air generated an exceptional power density due to the abundant availability of oxygen and its high reducing power. Current production depends directly on the availability of pure oxygen on the cathode, which could be produced by the photosynthetic biofilm; atmospheric oxygen could also be used. Copper wire was used as an electron collector. The wires were then connected to the metal component of the graphite electrode, utilising a pair of bending pliers.
2.2. Methodology
The setup for PMFC entailed six prepared pots in all cases. All the pots were identical in volume and geometry. The pots used were crafted from clay, a material that possesses the capacity to retain water and maintain an optimal level of moisture, thereby facilitating effective cation (proton) migration. In the event of excess water, the pots possess inherent porosity that enables the water to be expelled, thereby ensuring that the plants, which are not of an aquatic nature, are able to thrive. Each pot had a volume of 2.5 L. Each pot had an upper diameter of 18 cm, a lower diameter of 10 cm and a height of 12 cm. Pots S2, S5 and S7 represent pots without biochar with an inter-electrode distance of 2 cm, 5 cm and 7 cm, respectively. Pots SCS2, SCS5 and SCS7 represent pots with biochar with inter-electrode distances of 2 cm, 5 cm and 7 cm, respectively. Poultry manure was utilised for its elevated nitrogen content, which is a pivotal element for plant growth. The manure was amassed from CERSA (Regional Centre of Excellence for Poultry Science) at the University of Lome in Togo. Table 1 summarises the composition of each pot and Figure 1 shows a diagram of the experimental set-up.

Table 1.
Showing the different pots and their compositions.
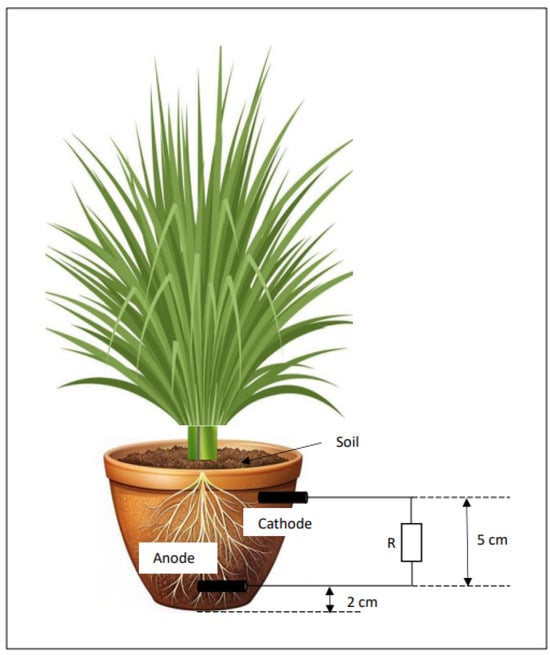
Figure 1.
Showing the different pots and their compositions.
The soil used was collected in the vicinity of Solar Energy Laboratory and the samples were sieved using a 1.5 mm sieve. The mass of soil for each soil was weighed using an analytical balance. Before placing the anodes, each pot was filled to a height of 2 cm. The anodes were placed horizontally in the soil close to the rhizosphere/root zone of the plant, i.e., covered by the fibrous roots. Since the depth of an anode in the soil greatly affects electricity production, the anode was placed 2 cm from the bottom of the pot as shown in Figure 1. The length of all the plants was 30 cm. The mass of C. citratus stems in each pot was 50 g. The cathodes were half-sunk into the pots using a hand-held drill to leave part of the cathodes open to the air. The cathode was pressed in halfway so that its surface area reactive with air was smaller than the surface area of the anode, to avoid a concentration overvoltage that limits the life of the technology. These electrodes (0.7 cm diameter; 5.7 cm height) were used as anodes and cathodes for the first time in PMFC research and the circuit was completed using an external 1000 ohm resistor. Three replicates were used in this experiment. The performance of the PMFCs was evaluated for 18 days (June 2022) at room temperature. The experimental setup was exposed to direct sunlight and supplied with tap water twice a day (6 a.m.; 5 p.m.). The amount of water for each pot was 0.33 L. The water supply was gradually increased three to four times each day as the plants grew. The OCV (open-circuit voltage) and the on-load voltage (U: measured at 1000 ohm) were measured using an AstroAl multimeter https://www.amazon.com.be/AstroAI-Multim%C3%A8tre-%C3%89lectrique-Automatique-R%C3%A9sistance/dp/B0842HTN8C (accessed on 3 January 2024). Plant growth is evidenced by the increase in voltage over time. Electrochemical reactions and electron flow were assessed by measuring open-circuit voltage and on-load voltage. All the PMFCs were placed in the laboratory under natural light and humidity conditions. Measurements were taken at (6 a.m., 1 p.m. and 8 p.m.). The power density (P) in mW/m2 was calculated by correlating the overall voltage generated, the current and the electrode area (A) according to the equation:
where
P is the power in mW/m2
V is the voltage in V
A is surface in m2
I is current in mA
For power normalisation, we used a surface power density instead of the volumetric power density or the planted surface area. In fact, out of 102 publications consulted, 81 used a surface power density and 21 used a volumetric power density, with the anode surface area being the most important. 58 studies out of 102 used the anode surface area, as in the case of our study. The anode surface area was calculated by taking into account the lateral surface area of the electrode used, which has a cylindrical shape.
3. Results and Discussion
This section is divided into subheadings. It should provide a concise and accurate description of the experimental results, their interpretation and the experimental conclusions that can be drawn.
3.1. Effect of Photosynthesis on Bioelectricity Production
The electroactive microorganisms growing at the anode oxidise the organic carbon (present in root exudates) and release electrons that can be harvested in the form of electricity. The potential developed between two unconnected electrodes (anode and cathode) of the system was measured as the OCV. The conversion of solar energy by plants into chemical and then electrical energy represents a promising and environmentally friendly biotechnology, thanks to the form of exudates (sugars, organic acids, etc.), secretions (polymeric carbohydrates, enzymes), lysates (dead cell material) and gases (ethylene, CO2) [4] through the rhizodeposition process [5]. These substances include low molecular weight compounds (organic acids, monosaccharides, etc.) which are degraded by microorganisms, whereas high molecular weight compounds, dead cells and root litter, are degraded over a relatively long period of time. In the anodic chamber, microorganisms, acting as catalysts for the oxidation process, convert organic substances (e.g., glucose) according to the following reaction:
The released electrons pass through the anode into the external circuit and move towards the cathode, while the hydrogen ions pass through the ion-selective membrane (in this case the sol) and enter the cathode space. In the cathode chamber with the participation of incoming electrons and hydrogen ions, the reduction of molecular oxygen in the air and the formation of water molecules can take place under aerobic conditions [15]:
Under anaerobic conditions, it is possible to reduce hydrogen ions to diatomic H2 gas during the cathodic reaction, discharging hydrogen ions at the cathode and adsorbing hydrogen atoms at the electrode:
The bioelectrogenic activity in terms of the OCV was evaluated after the integration of all the electrodes in the system. Measurements were taken at 6 a.m. (sunrise), 1 p.m. (sun at its zenith) and at 8 p.m. (night). The PMFC operation showed an effective response to direct sunlight (Figure 2). Oxillation of the OCV voltage was obtained. This oxillation could depend on solar radiation (Figure 2). An increase in electrochemical activity was observed, especially at 1 p.m.
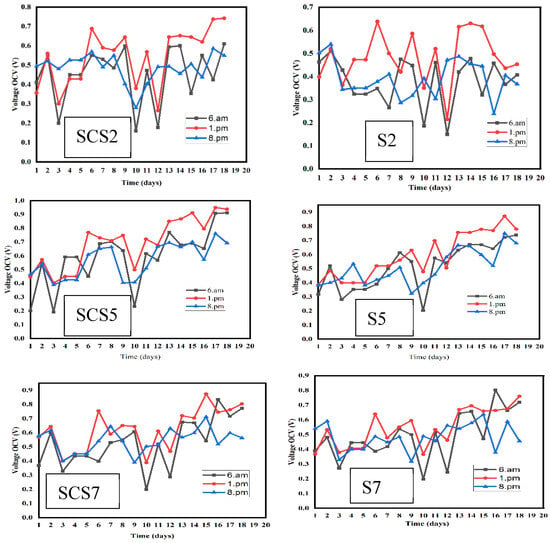
Figure 2.
Change in OCV voltage for each pot as a function of time.
Table 1 shows that the maximum value for each electrochemical parameter was obtained at 1 p.m. for each pot. These graphs show that photosynthetic activity has a direct influence on compound (substrate) synthesis. The maximum OCV for each pot was obtained at 1 p.m. (Figure 2) due to high photosynthetic activity. In fact, by capturing the sun’s rays, the CO2 is fixed by the leaves and green stems in the form of carbohydrates, around 70% of which migrate to the roots, which release 60% of the 70% into the rhizosphere [22]. The maximum OCVs recorded at 1 p.m. were as follows SCS2 (742 mV), SCS5 (950 mV), SCS7 (804 mV), S2 (638 mV), S5 (872 mV) and S7 (760 mV). The photosynthesis process allows the synthesis of a large number of exudates or compounds in the rhizosphere [23]. These observations have shown that photosynthetic activity directly influences energy production through the rhizodeposition phenomenon. Moqsud et al. [24] reported that the paddy cell, in which plant photosynthesis is combined with microbial conversion of organic matter into energy, proved that solar radiation affects the paddy system. It was noted that voltage generation was high when solar radiation was high. In the following subheadings, we will consider the 1 p.m. measurements for all samples.
3.2. Effect of Inter-Electrode Distance by Electrochemical Response
The distance between the electrodes (anode and cathode) varied from 2 cm to 7 cm. The current density and voltage values observed for each distance applied are shown in Table 2 One of the determining factors for good performance of PMFCs with the tubular design is the inter-electrode distance. To get an idea of the distance between the anode and cathode in the Cymbopogon citratus-based PMFC and to determine it for the rest of our work, we prepared samples with inter-electrode distance values of 2 cm, 5 cm and 7 cm. Figure 3 and Figure 4 show the evolution of the open-circuit voltage of the cells realised.

Table 2.
Showing maximum values for electrochemical parameters as a function of time.
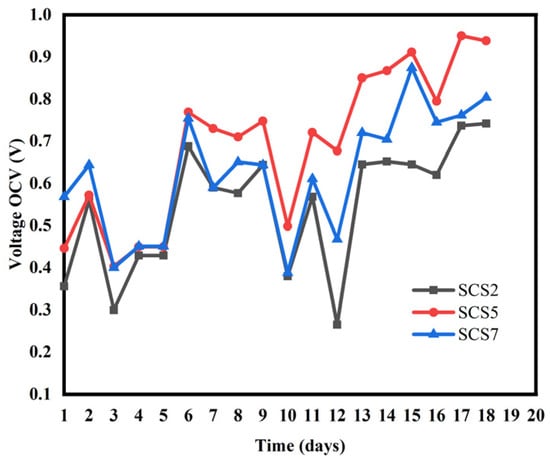
Figure 3.
Evolution of the OCV voltage of pots with biochar as a function of the inter-electrode distance.

Figure 4.
Evolution of the OCV voltage of pots without biochar as a function of the inter-electrode distance.
Latency times were almost 5 days for all PMFCs. Between day 1 and day 2, there was a slight increase (Figure 3 and Figure 4). This is due to the degradation of the substrate present in the soil by the bacteria without any input from the plant. This phenomenon corresponds to the formation of the biofilm. Measurements on days 4 and 5 were virtually constant. This finding would indicate that the plant provides a continuous supply of organic matter to the soil throughout its life [25]. These fluctuations are thought to be due to the complex nature of the substrate synthesised by the plant, electrochemical reactions, the interaction between the soil and the batteries, the nature of the plant and the microorganisms, etc. In fact, the plant’s roots release into the rhizosphere root exudates (sugars, organic acids, etc.), polymeric secretions rich in carbohydrates and enzymes, lysates (dead cell material) and gases (ethylene and carbon dioxide) [26].
Observation of Figure 3 and Figure 4 shows that the optimum distance is 5 cm. It can be seen that the SCS2 pot has a maximum OCV: SCS2 (742 mV), lower than that of SCS5 and SCS7 which are, respectively, SCS5 (950 mV), [SCS7 (804 mV) (Figure 3). The same observation is made in Figure 4, where sample S2 has a lower OCV: S2 (638 mV) than S5 (872 mV) and S7 (760 mV). This result, obtained for an inter-electrode distance of 2 cm, is thought to be due to a phenomenon known as oxygen crossover, in which oxygen is introduced into the anode instead of being concentrated in the cathode, thereby reducing its performance. This phenomenon also occurred in the work of Kristopher Ray et al. [27]. A shorter distance between the electrodes favours diffusion of oxygen through the anode because the anode is not completely in an anaerobic environment, as pointed out by Takanezawa in 2010 [16]. Our analysis shows that the SCS5 sample has a higher OCV: SCS5 (950 mV) than SCS2 (742 mV) and SCS7 (804 mV) (Figure 3). The same applies to Figure 3 where S5 has a higher OCV: S5 (872 mV) than S2 (638 mV)] and S7 (760 mV). This high value obtained for a distance of 5 cm between the electrodes would obviously be due to a thicker layer of soil (a layer compared with that of a distance of 2 cm) which limits the diffusion of oxygen at the surface of the anode on the one hand. On the other hand, internal resistance increases with the thickness of the soil layer. The distance of 5 cm therefore represents an optimum for better performance of plant microbial fuel cells based on Cymbopogon citratus. Finally, in Figure 3 and Figure 4, we see that the OCV voltages of SCS7 and S7 are higher than those of SCS2 and S2, respectively. The 7 cm distance maintains a much thicker soil layer than the 2 cm distance. The 7 cm distance makes the anode zone completely anaerobic, which favours higher microbial activity, but when the PMFC has a long distance, the protons generated at the anode (base of the PMFC reactor) have to travel a longer distance to reach the cathode surface [28]. To reach the cathode, the protons have to face an obstacle in the form of a gravitational force that hinders the process of moving the anode part. Consequently, in the PMFC with a large distance, there is a greater possibility that the inability of the proton to reach the cathode will undergo a reduction process with oxygen. This results in a high internal resistance. As a result, oxygen diffusion through the anode would have a more pronounced effect than internal resistance. Table 2 also shows that the maximum values for all the electrochemical parameters were measured for pots with an inter-electrode distance of 5 cm. These results are in agreement with those of Takanezawa and Deng et al. (2010) [10] where a distance of 5 cm was found for rice plants for electricity generation from plants when he worked in the rice field. Our results disagree with those of Gregory Bataillon (2021) [29] for whom a distance of less than 7.5 disadvantages the technology because of oxygen diffusion. Secondly, the results obtained complement those of Kamaran Raman (2012) and M. Ghangrekar and V.B. Shinde (2006). In their study, M. Ghangrekar and V.B. Shinde [1] varied the distance between 20 cm, 24 cm and 28 cm. They concluded that battery performance increased as the distance between the electrodes decreased.
3.3. Electrogenic Activity of PMFCs by Electrochemical Response as a Function of Biochar
To compare the voltage and power obtained from PMFCs with or without biochar, we considered PMFCs that differed only by the presence of biochar. Figure 5, Figure 6 and Figure 7 illustrate the variation of the open-circuit voltage for PMFC with biochar and without biochar. Even if the open-circuit voltage does not measure the flow of the electric current produced by the plant, nevertheless it measures the activity of the anodic and cathodic reagents on the surface of the electrodes. All the pots present the same appearance for the open-circuit voltage. The curves initially present a low voltage value. This low voltage value is linked to the accommodation of the plant to the new environment [30]. This low value could be due to the process of the formation of the electroactive biofilm at the anode and the production of the enzymes necessary for the transfer of electrons [31]. These results indicate that living compost and soil as a matrix substrate already contain exo-electrogenic microorganisms and that the addition of biochar would have made for a competitive situation on the electrode surface. This is because the plant has not yet begun to release root exudates, which are responsible for initiating metabolic activity. From the fourth day onwards, the plant begins to release rhizopods into the rhizosphere, where the continuous availability of substrate is possible for the production of sustainable electricity. Pots SCS2, SCS5 and SCS7 showed higher values of open-circuit voltage than pots S2, S5 and S7, respectively (Figure 5, Figure 6 and Figure 7).
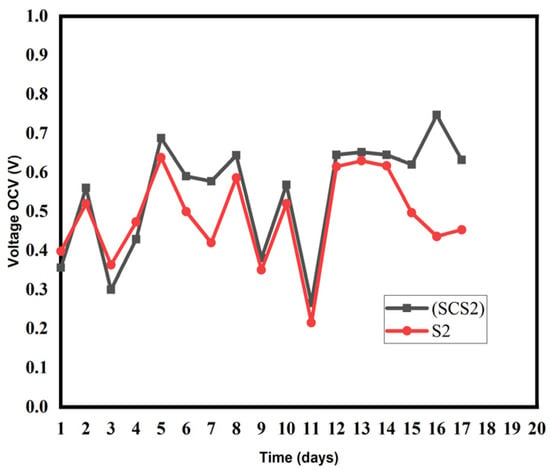
Figure 5.
Evolution of the open-circuit voltage of samples SCS2 and S2.
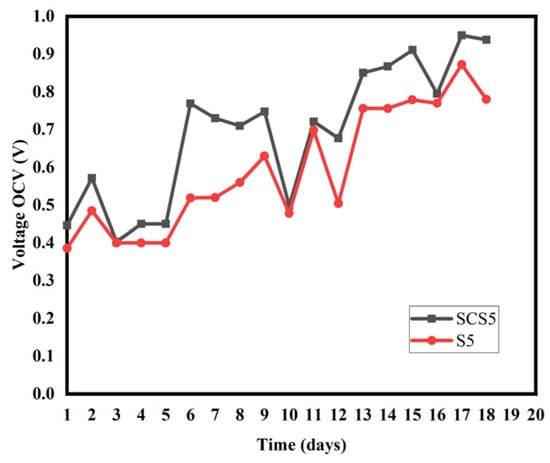
Figure 6.
Evolution of the open-circuit voltage of samples SCS5 and S5.
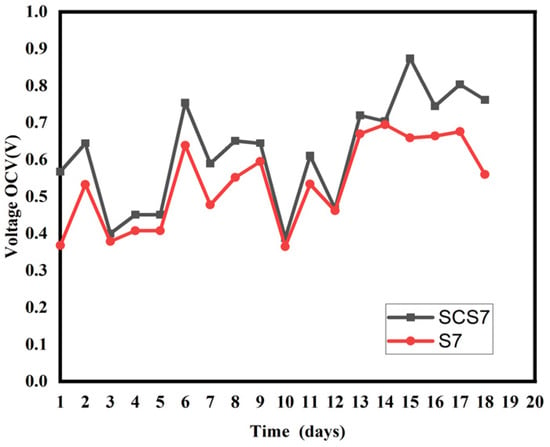
Figure 7.
Evolution of the open-circuit voltage of samples SCS7 and S7.
For Figure 5, Figure 6 and Figure 7, the maximum OCV voltages in the biochar-differentiated pots are as follows: [SCS2 (742 mV), S2 (638 mV)]; [SCS5 (950 mV), S5 (872 mV)], [SCS7 (804 mV, S7 (760 mV)]. This increase in performance is thought to be due to the presence of biochar. The addition of biochar would have reduced the resistance of the soil, increasing the bioelectric production of the PMFC and maintaining good performance even in low humidity conditions. Porosity allows electroactive bacteria to proliferate [32]. The high values observed in biochar-amended pots are due to the proliferation of electro-active bacteria and the water retention of the biochar. The retention of water in the biochar allows the moist environment to be maintained, which has a dual benefit. The wet environment provides an anaerobic environment for the anode and also allows a good flow of ions. The retention of water would allow good contact between the cathode and the substrate, which would facilitate good PMFC performance. In addition, under drought conditions, PMFC with added biochar could show better drought resistance due to their ability to improve soil moisture characteristics and improve soil conductivity. This study highlights the essential role of biochar in optimising the performance of PMFC. It improves our understanding of the optimisation of PMFC and could offer a new method of energy production for the future, as well as presenting a new soil-monitoring strategy.
3.4. Comparison of Voltage Generation and Power Density with Some Articles Reported in the Literature
The highest voltage generation and power density were obtained for PMFC with biochar, reaching values of 968 mV and 75.8 mW/m2 for SCS5, respectively, the highest values for pots with biochar, while PMFC without biochar showed a voltage of 872 mV and a power density of 47.56 mW/cm2 for S5, which were the highest values for PMFC without biochar (Table 3).

Table 3.
Voltage generation and power density of different PMFCs with C. citratus.
The maximum voltage obtained in this work was found to be higher than others reported in PMFC studies. Carmalin and Sreeja found 323 mV, 485 mV and 505 mV, respectively, for Brassica juncea, Trigonella foenum-graecum and Cana Stuttgart [11]. JC Gomora et al. found a maximum OCV of 630 mV when using Agapanthus africanus with compost for PMFC [33]. This difference in the open-circuit voltage value shows the importance of the microbial community at the rhizosphere level. The utility of photosynthetic plants has been well studied using Glyceria maxima as a model and has achieved a maximum power production of 67 mW/m2. An average power density of 50 mW/m2 was achieved by Spartina anglica for 33 days among the different PMFCs studied by Timmers et al. [34]. Moqsud et al. [24] used 1% and 3 % compost on rice-based PMFCs. The maximum power density obtained in the study was 23 mW/m2. In the present study, we observed a much higher power density than that obtained in a recent study [8]. A maximum power density of 15.73 mW/m2 was obtained for a Canna indica-MFC system [35], which was about five times lower than that obtained in this study. The high power density obtained in our study can be attributed to the type of plant and the addition of biochar to the soil, resulting in root deposits that are degradable by microorganisms and therefore responsible for electron donation [36].
In agreement with the data obtained in this work, Cymbopogon citratus has the capacity to generate a large amount of voltage in a PMFC. However, in order to improve electrochemical performance, further studies are needed to evaluate the effect of certain variables on voltage generation in order to gain a deeper understanding of the production of green electricity from this plant.
4. Conclusions
In conclusion, the efficiency of C. citratus in PMFC was studied with the addition of coconut shell biochar and the distance between the two electrodes to generate bioelectricity. The incorporation of biochar into the soil showed a better open-circuit voltage compared to pots without biochar. The addition of biochar promotes the growth and proliferation of bacteria. The interelectrode distance is a determining factor for good performance; an optimum distance of 5 cm was found to limit the diffusion of oxygen through the anode as well as provide a high value of internal resistance. All the cells showed a higher electrogenic activity at 1 p.m., which can be directly attributed to photosynthetic activity. The PMFC technology based on C. citratus with the addition of biochar in the rhizosphere and a distance of 5 cm between the electrodes gives a better power of 75.8 mW/m2. In order to increase energy production, PMFC can be designed and studied by developing the electrodes using an ecological method and finding the optimum percentage for the addition of this biochar.
Author Contributions
Conceptualization, E.Z., D.M.K. and P.K.; methodology, E.Z. and E.M.; solware, E.Z.; validation, E.Z., D.M.K. and B.M.A.; formal analysis, E.Z.; investigation, E.Z.; resources, data curation, E.M.; writing-original draft preparation, E.Z.; writing-review and editing, E.Z.; visualization, E.M.; supervision, D.M.K.; project administration, E.Z.; funding acquisition, E.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by World Bank, grant number Credit 6512-TG through a PhD scholarship grant for E.Z.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
This work was supported by Regional Center of Excellence for Electricity Management (CERME)-TOGO Funding: IDA Credit 6512-TG (World Bank). The authors would like to thank all the staff at LES (Solar Energy Laboratory) for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ghangrekar, M.; Shinde, V. Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour. Technol. 2007, 98, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J. World Population Growth and the Role of Annual Energy Use per Capita. Technol. Forecast. Soc. Change 1998, 59, 55–87. [Google Scholar] [CrossRef]
- Bombelli, P.; Iyer, D.M.R.; Covshoff, S.; McCormick, A.J.; Yunus, K.; Hibberd, J.M.; Fisher, A.C.; Howe, C.J. Comparison of power output by rice (Oryza sativa) and an associated weed (Echinochloa glabrescens) in vascular plant bio-photovoltaic (VP-BPV) systems. Appl. Microbiol. Biotechnol. 2013, 97, 429–438. [Google Scholar] [CrossRef]
- Zingbe, E.; Kongnine, D.M.; Agbomahena, B.M.; Kpelou, P.; Mouzou, E. Elaboration and Characterization of Electrodes from Robinia pseudoacacia and Azadirachta indica Charcoal Powder with Coconut Bio-Pitch as a Binder. Materials 2024, 17, 5156. [Google Scholar] [CrossRef]
- Rudrappa, T.; Biedrzycki, M.L.; Bais, H.P. Causes and consequences of plant-associated biofilms. FEMS Microbiol. Ecol. 2008, 64, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L.; Chuah, J.H.; Chow, C.-O.; Ng, P.K. Plant microbial fuel cells: A comprehensive review of influential factors, innovative configurations, diverse applications, persistent challenges, and promising prospects. Int. J. Green Energy 2024, 22, 599–648. [Google Scholar] [CrossRef]
- Türker, O.C.; Yakar, A. A hybrid constructed wetland combined with microbial fuel cell for boron (B) removal and bioelectric production. Ecol. Eng. 2017, 102, 411–421. [Google Scholar] [CrossRef]
- Sonu, K.; Sogani, M.; Syed, Z.; Rajvanshi, J.; Pandey, S.C. Performance evaluation of Epipremnum aureum plant-based microbial fuel cell using composite anode made up of carbonized corncob and carbon rod. Biomass Convers. Biorefin. 2024, 14, 5149–5156. [Google Scholar] [CrossRef]
- Bataillou, G.; Ondel, O.; Haddour, N. 900-Days long term study of plant microbial fuel cells and complete application for powering wireless sensors. J. Power Sources 2024, 593, 233965. [Google Scholar] [CrossRef]
- Sophia, A.C.; Sreeja, S. Green energy generation from plant microbial fuel cells (PMFC) using compost and a novel clay separator. Sustain. Energy Technol. Assess. 2017, 21, 59–66. [Google Scholar] [CrossRef]
- Gravel, V.; Dorais, M.; Ménard, C. Organic potted plants amended with biochar: Its effect on growth and Pythium colonization. Can. J. Plant Sci. 2013, 93, 1217–1227. [Google Scholar] [CrossRef]
- Sonu, K.; Sogani, M.; Syed, Z.; Dongre, A.; Sharma, G. Improved decolorization of dye wastewater and enhanced power output in the electrically stacked microbial fuel cells with H2O2 modified corncob anodes. Environ. Prog. Sustain. Energy 2021, 40, ep13638. [Google Scholar] [CrossRef]
- Helder, M. Design Criteria for the Plant-Microbial Fuel Cell: Electricity Generation with Living Plants: From Lab Tot Application; Wageningen University and Research: Wageningen, The Netherlands, 2012. [Google Scholar]
- Apollon, W.; Luna-Maldonado, A.I.; Kamaraj, S.K.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Aranda-Ruíz, J. Progress and recent trends in photosynthetic assisted microbial fuel cells: A review. Biomass Bioenergy 2021, 148, 106028. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Mohanakrishna, G.; Mohan, S.V. Rhizosphere mediated electrogenesis with the function of anode placement for harnessing bioenergy through CO2 sequestration. Bioresour. Technol. 2012, 124, 364–370. [Google Scholar] [CrossRef]
- Takanezawa, K.; Nishio, K.; Kato, S.; Hashimoto, K.; Watanabe, K. Factors affecting electric output from rice-paddy microbial fuel cells. Biosci. Biotechnol. Biochem. 2010, 74, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Fatima, K. Review on Pharmacological Activity of Amygdalin. Arch. Cancer Res. 2017, 5, 10–12. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C.; Dahal, K.; Alber, N.A.; Chadee, A. Photosynthesis, respiration and growth: A carbon and energy balancing act for alternative oxidase. Mitochondrion 2020, 52, 197–211. [Google Scholar] [CrossRef]
- Thress, K.S.; Paweletz, C.P.; Felip, E.; Cho, B.C.; Stetson, D.; Dougherty, B.; Lai, Z.; Markovets, A.; Vivancos, A.; Kuang, Y.; et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non–small cell lung cancer harboring EGFR T790M. Nat. Med. 2015, 21, 560–562. [Google Scholar] [CrossRef]
- Hong, S.W.; Chang, I.S.; Choi, Y.S.; Chung, T.H. Experimental evaluation of influential factors for electricity harvesting from sediment using microbial fuel cell. Bioresour. Technol. 2009, 100, 3029–3035. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef]
- Mehdinia, A.; Dejaloud, M.; Jabbari, A. Nanostructured polyaniline-coated anode for improving microbial fuel cell power output. Chem. Pap. 2013, 67, 1096–1102. [Google Scholar] [CrossRef]
- Pham, T.H.; Aelterman, P.; Verstraete, W. Bioanode performance in bioelectrochemical systems: Recent improvements and prospects. Trends Biotechnol. 2009, 27, 168–178. [Google Scholar] [CrossRef]
- Moqsud, M.A.; Yoshitake, J.; Bushra, Q.S.; Hyodo, M.; Omine, K.; Strik, D. Compost in plant microbial fuel cell for bioelectricity generation. Waste Manag. 2015, 36, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Strik, D.P.; Timmers, R.A.; Helder, M.; Steinbusch, K.J.; Hamelers, H.V.; Buisman, C.J. Microbial solar cells: Applying photosynthetic and electrochemically active organisms. Trends Biotechnol. 2011, 29, 41–49. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Yeruva, D.K.; Kumar, A.K.; Mohan, S.V.; Varjani, S. Chapter 3.8—Plant-Microbial Fuel Cell Technology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Pamintuan, K.R.S.; Reyes, C.S.A.; Lat, D.K.O. Compartmentalization and polarization studies of a Plant-Microbial Fuel Cell assembly with Cynodon dactylon. E3S Web Conf. 2020, 181, 1–5. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, S.K. Effect of Hydrothermal Carbonization Reaction Parameters on the properties of hydrochar and pellets. Environ. Prog. Sustain. Energy 2014, 33, 676–680. [Google Scholar] [CrossRef]
- Bataillou, G.; Haddour, N.; Vollaire, C. Bioelectricity production of PMFC using Lobelia Queen Cardinalis in individual and shared soil configurations. E3S Web Conf. 2022, 334, 08001. [Google Scholar] [CrossRef]
- Azri, Y.M.; Tou, I.; Sadi, M.; Bouzidi, Y. Production d’électricité verte via une plante vivante ‘Watsonia sp.’dans la pile à combustible microbienne. J. Renew. Energ. 2015, 18, 63–70. [Google Scholar] [CrossRef]
- Jayawardhane, J.; Cochrane, D.W.; Vyas, P.; Bykova, N.V.; Vanlerberghe, G.C.; Igamberdiev, A.U. Roles for Plant Mitochondrial Alternative Oxidase Under Normoxia, Hypoxia, and Reoxygenation Conditions. Front. Plant Sci. 2020, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, S.; Sophia, S.J.; Pandian, K. High surface graphene nanoflakes as sensitive sensing platform for simultaneous electrochemical detection of metronidazole and chloramphenicol. Mater. Sci. Eng. C 2018, 90, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.; Cunalata, G. Infusiones de Moringa oleifera (moringa) combinada con Cymbopogon citratus (hierba luisa) y Lippia alba (mastranto). Unemi 2020, 13, 118. [Google Scholar]
- Timmers, R.A.; Rothballer, M.; Strik, D.P.B.T.B.; Engel, M.; Schulz, S.; Schloter, M.; Hartmann, A.; Hamelers, B.; Buisman, C. Microbial community structure elucidates performance of Glyceria maxima plant microbial fuel cell. Appl. Microbiol. Biotechnol. 2012, 94, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Dash, P.; Mohanty, A.; Abbassi, R.; Mishra, B.K. Performance assessment of innovative constructed wetland-microbial fuel cell for electricity production and dye removal. Ecol. Eng. 2012, 47, 126–131. [Google Scholar] [CrossRef]
- Deng, H.; Chen, Z.; Zhao, F. Energy from Plants and Microorganisms: Progress in Plant–Microbial Fuel Cells. ChemSusChem 2012, 5, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


