Comparative Analysis of the Corrosion Behavior of Plain and Nanoporous Copper
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Electrode Preparation and Cell Setup
2.3. Stripping Analysis
2.4. Establishing Calibration Curve for Stripping Analysis
2.5. Preparation of NP Cu
2.6. Polarization Test
2.7. Characterization Techniques
3. Results and Discussion
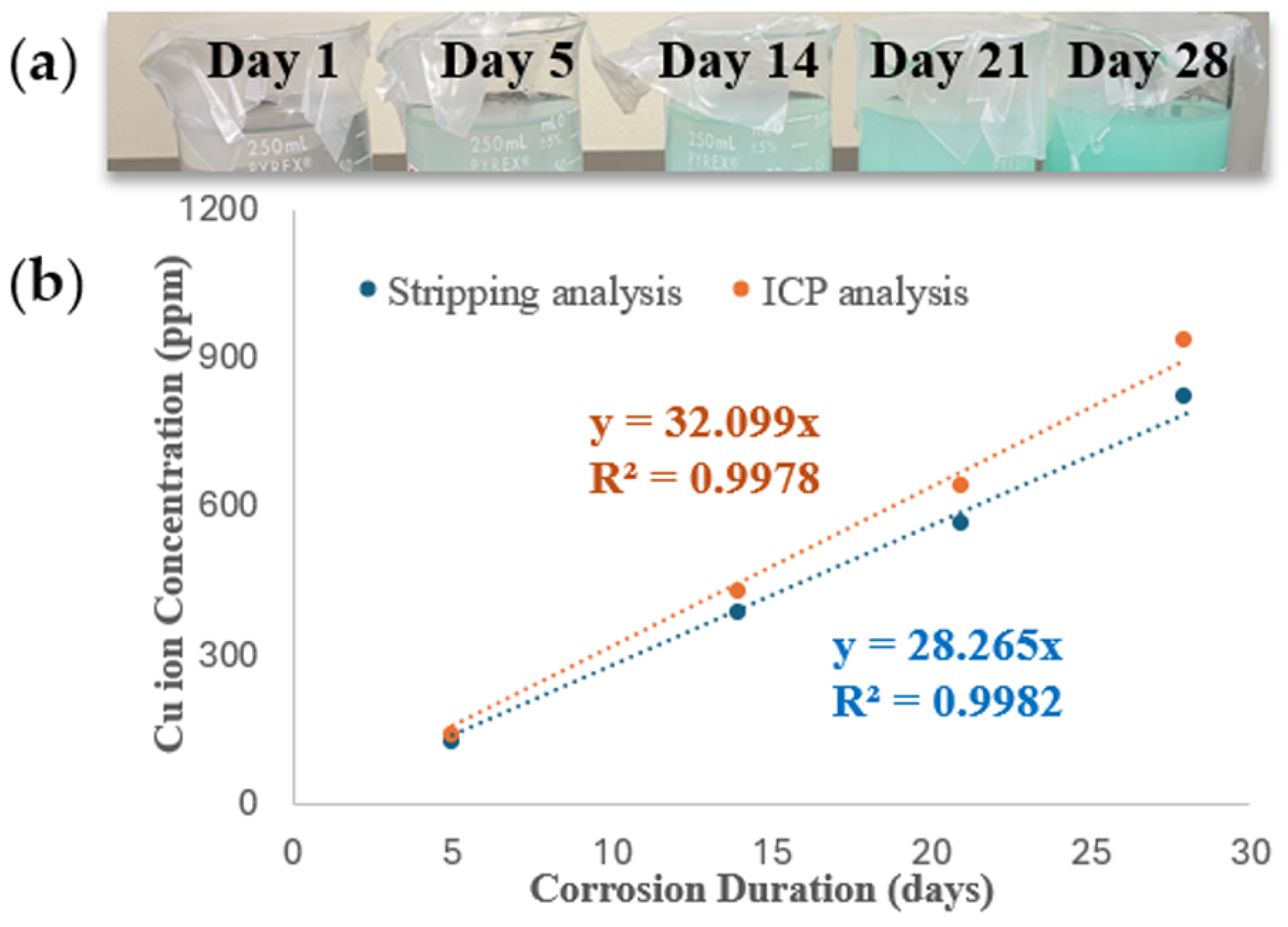
3.1. Quantitative Analysis of Plain Cu Corrosion Behavior Using Stripping Analysis and ICP-OES
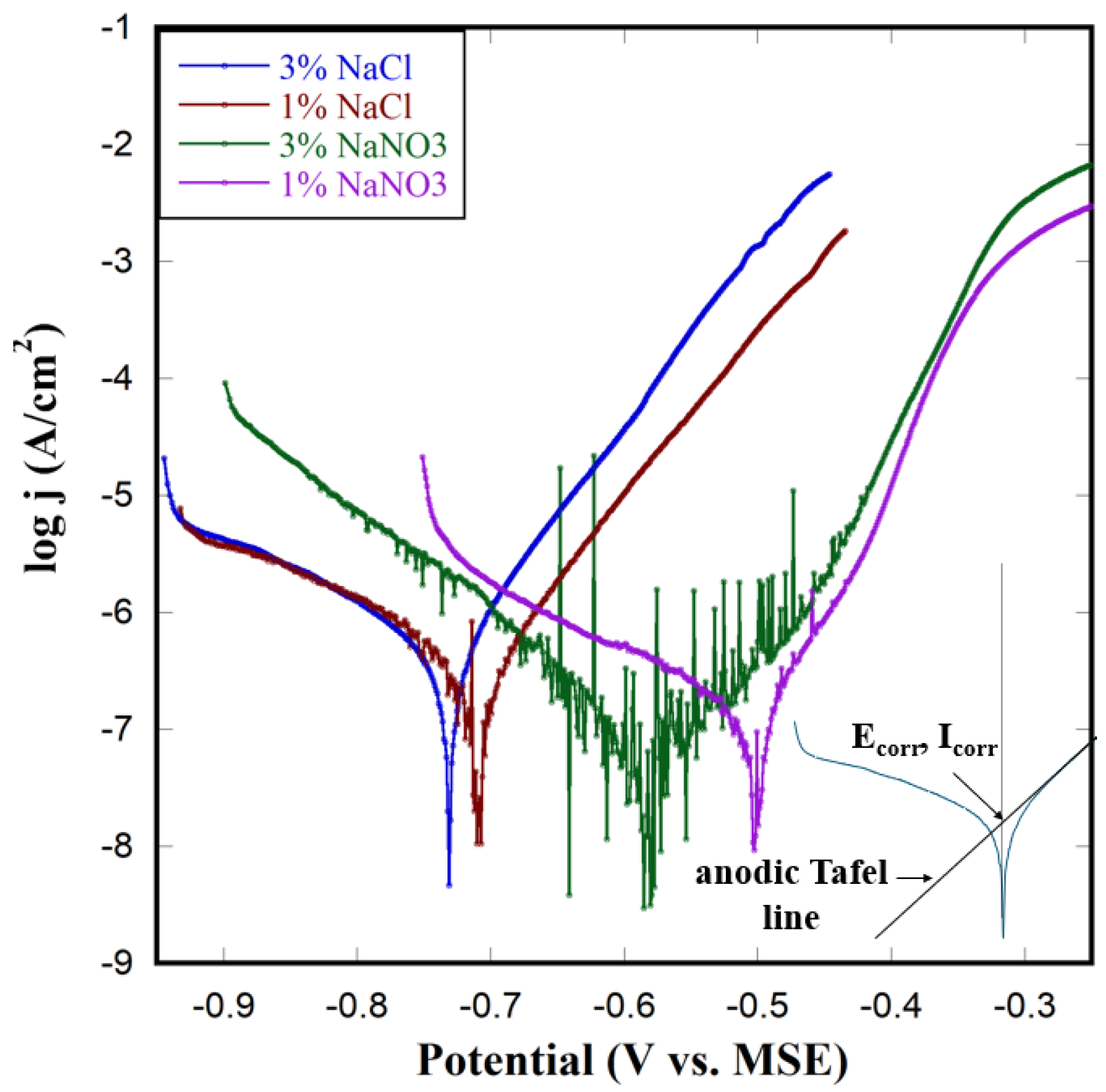
3.2. Polarization Tests on Plain Cu Within N2 Environment
- 1st pathway
3.3. Polarization Test on Plain Cu Within Air Environment
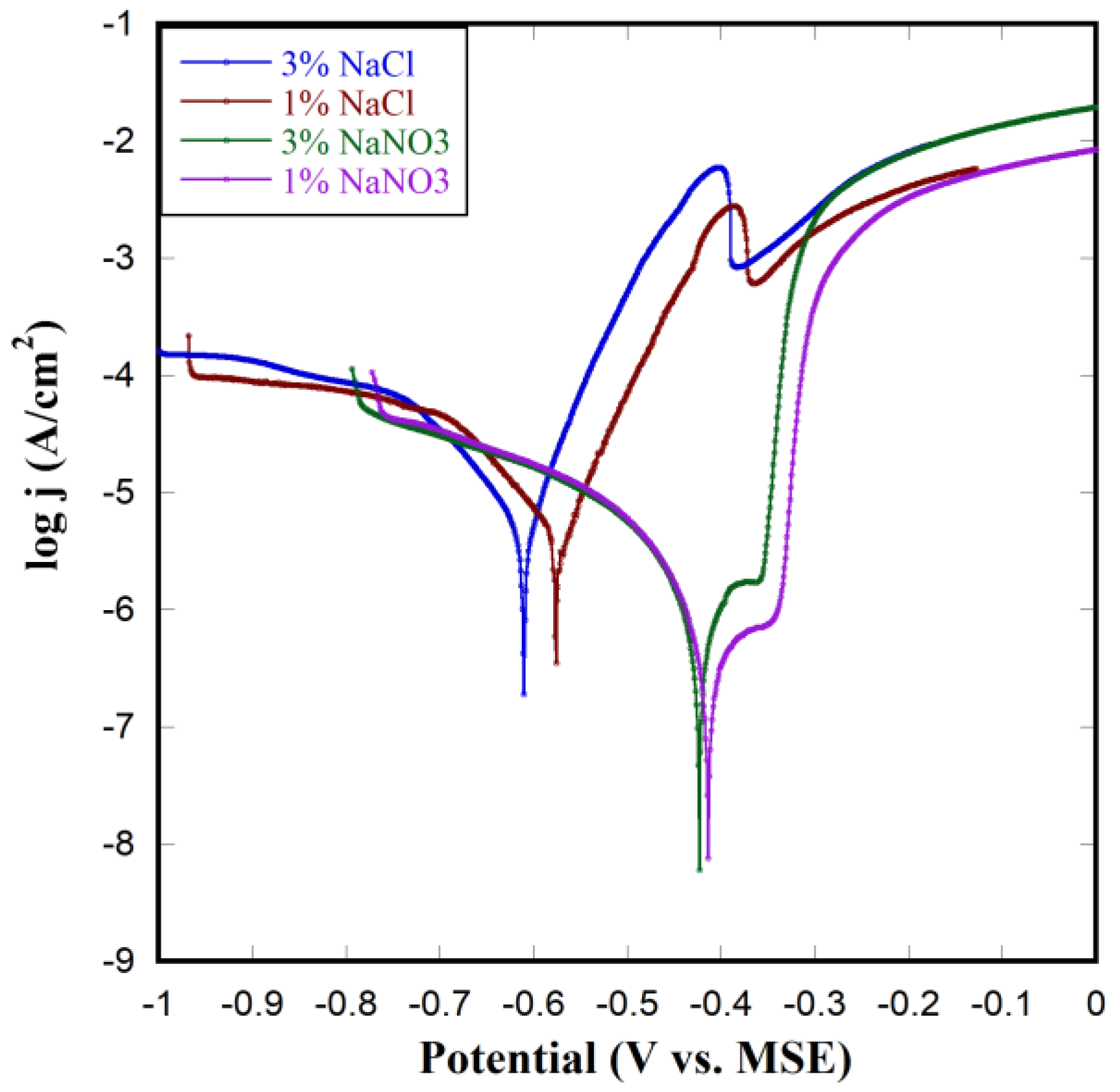
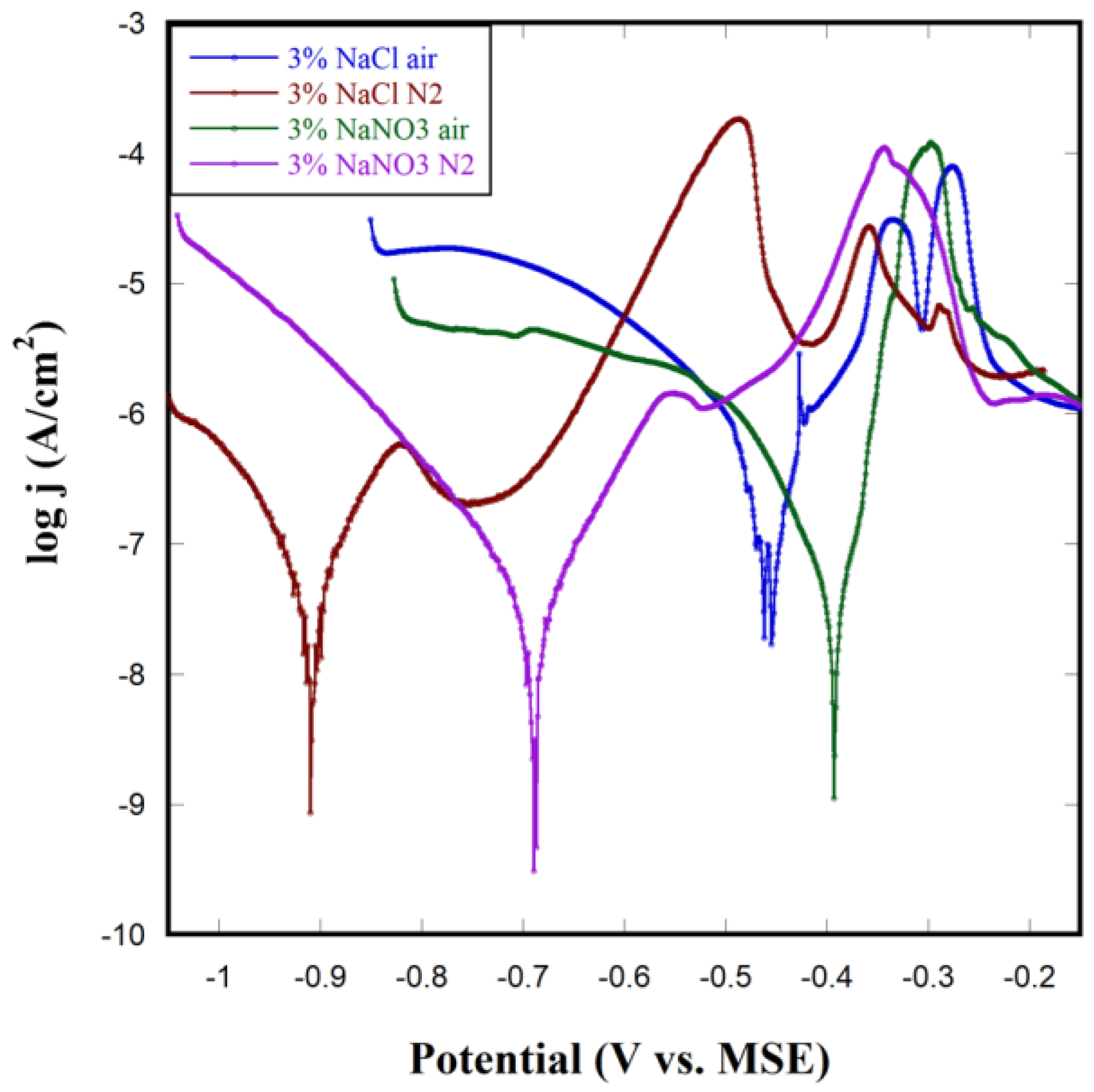
3.4. Corrosion of NP Cu in Different Solutions
3.5. Polarization Test on NP Cu
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wlasny, I.; Dabrowski, P.; Rogala, M.; Pasternak, I.; Strupinski, W.; Baranowski, J.; Klusek, Z. Impact of electrolyte intercalation on the corrosion of graphene-coated copper. Corros. Sci. 2015, 92, 69–75. [Google Scholar] [CrossRef]
- Çakmakcı, İ.; Duran, B.; Duran, M.; Bereket, G. Experimental and theoretical studies on protective properties of poly (pyrrole-co-N-methyl pyrrole) coatings on copper in chloride media. Corros. Sci. 2013, 69, 252–261. [Google Scholar] [CrossRef]
- Lanzani, G.; Kangas, T.; Laasonen, K. Copper passivation by metal doping. J. Alloys Compd. 2009, 482, 33–42. [Google Scholar] [CrossRef]
- Rao, B.A.; Iqbal, M.Y.; Sreedhar, B. Electrochemical and surface analytical studies of the self-assembled monolayer of 5-methoxy-2-(octadecylthio) benzimidazole in corrosion protection of copper. Electrochim. Acta 2010, 55, 620–631. [Google Scholar]
- Khaled, K. Studies of the corrosion inhibition of copper in sodium chloride solutions using chemical and electrochemical measurements. Mater. Chem. Phys. 2011, 125, 427–433. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, D. Study of corrosion behavior of copper in 3.5 wt.% NaCl solution containing extracellular polymeric substances of an aerotolerant sulphate-reducing bacteria. Corros. Sci. 2018, 136, 275–284. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Gong, Y.; Gao, F.; Luo, Z.; Zhang, S.; Li, H. Water soluble corrosion inhibitors for copper in 3.5 wt% sodium chloride solution. Corros. Sci. 2017, 123, 339–350. [Google Scholar] [CrossRef]
- Polarz, S.; Smarsly, B. Nanoporous materials. J. Nanosci. Nanotechnol. 2002, 2, 581–612. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef]
- Snyder, J.; Fujita, T.; Chen, M.; Erlebacher, J. Oxygen reduction in nanoporous metal–ionic liquid composite electrocatalysts. Nat. Mater. 2010, 9, 904–907. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M.; Erlebacher, J. Metallic mesoporous nanocomposites for electrocatalysis. J. Am. Chem. Soc. 2004, 126, 6876–6877. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.; McCue, I.; Livi, K.; Erlebacher, J. Structure/processing/properties relationships in nanoporous nanoparticles as applied to catalysis of the cathodic oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 8633–8645. [Google Scholar] [CrossRef] [PubMed]
- Biener, J.; Wittstock, A.; Zepeda-Ruiz, L.; Biener, M.; Zielasek, V.; Kramer, D.; Viswanath, R.; Weissmüller, J.; Bäumer, M.; Hamza, A. Surface-chemistry-driven actuation in nanoporous gold. Nat. Mater. 2009, 8, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Weissmüller, J. Bulk nanoporous metal for actuation. Adv. Eng. Mater. 2010, 12, 714–723. [Google Scholar] [CrossRef]
- Biener, J.; Nyce, G.W.; Hodge, A.M.; Biener, M.M.; Hamza, A.V.; Maier, S.A. Nanoporous plasmonic metamaterials. Adv. Mater. 2008, 20, 1211–1217. [Google Scholar] [CrossRef]
- Spitzberg, J.D.; Zrehen, A.; van Kooten, X.F.; Meller, A. Plasmonic-nanopore biosensors for superior single-molecule detection. Adv. Mater. 2019, 31, 1900422. [Google Scholar] [CrossRef]
- An, C.; Wang, Z.; Xi, W.; Wang, K.; Liu, X.; Ding, Y. Nanoporous Cu@Cu2O hybrid arrays enable photo-assisted supercapacitor with enhanced capacities. J. Mater. Chem. A 2019, 7, 15691–15697. [Google Scholar] [CrossRef]
- Kondrat, S.; Perez, C.; Presser, V.; Gogotsi, Y.; Kornyshev, A. Effect of pore size and its dispersity on the energy storage in nanoporous supercapacitors. Energy Environ. Sci. 2012, 5, 6474–6479. [Google Scholar] [CrossRef]
- Liu, Z.; Du, J.; Qiu, C.; Huang, L.; Ma, H.; Shen, D.; Ding, Y. Electrochemical sensor for detection of p-nitrophenol based on nanoporous gold. Electrochem. Commun. 2009, 11, 1365–1368. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, J.; Liu, J.; Tang, H.; Cao, C.; Zhao, D.; Ding, Y. Molecularly imprinted polymer decorated nanoporous gold for highly selective and sensitive electrochemical sensors. Sci. Rep. 2015, 5, 7699. [Google Scholar] [CrossRef]
- Feng, Y.; Siow, K.-S.; Teo, W.-K.; Tan, K.-L.; Hsieh, A.-K. Corrosion mechanisms and products of copper in aqueous solutions at various pH values. Corrosion 1997, 53, 389–398. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, X.; Sun, H.; Li, Y.; Zhang, K.; Wu, Y. Corrosion behavior of copper at elevated temperature. Int. J. Electrochem. Sci. 2012, 7, 7902–7914. [Google Scholar] [CrossRef]
- Abbott, W. The corrosion of copper and porous gold in flowing mixed gas environments. IEEE Trans. Compon. Hybrids Manuf. Technol. 1990, 13, 40–45. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Larios-Lopez, L.; Liu, C.; Garcia-Gutierrez, D.; Camacho-Bragado, A.; Yacaman, M.J. Corrosion at the nanoscale: The case of silver nanowires and nanoparticles. Chem. Mater. 2005, 17, 6042–6052. [Google Scholar] [CrossRef]
- Scendo, M. Inhibition of copper corrosion in sodium nitrate solutions with nontoxic inhibitors. Corros. Sci. 2008, 50, 1584–1592. [Google Scholar] [CrossRef]
- Drogowska, M.; Brossard, L.; Ménard, H. Influence of anions on the passivity behavior of copper in alkaline solutions. Surf. Coat. Technol. 1988, 34, 383–400. [Google Scholar] [CrossRef]
- Munoz, A.I.; Espallargas, N.; Mischler, S. Tribocorrosion; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Jones, D.A. Principles and prevention. Corrosion 1996, 2, 168. [Google Scholar]
- Lei, Z.; Borgesen, P.; Dimitrov, N. Electrodeposition Complexity and the Root Cause of Interfacial Voiding in Solder Joints with Plated Nickel. ACS Appl. Electron. Mater. 2024, 6, 457–464. [Google Scholar] [CrossRef]
- Herrero, E.; Clavilier, J.; Feliu, J.M.; Aldaz, A. Influence of the geometry of the hanging meniscus contact on the hydrogen oxidation reaction on a Pt (111) electrode in sulphuric acid. J. Electroanal. Chem. 1996, 410, 125–127. [Google Scholar] [CrossRef]
- Lei, Z.; Mull, K.; Dimitrov, N. New Electrochemical Approach for Synthesis of Nanoporous Silver. J. Electrochem. Soc. 2024, 171, 092502. [Google Scholar] [CrossRef]
- C110-GRADE COPPER. Available online: https://optimuspc.com/pages/materials (accessed on 4 October 2024).
- Shao, W.; Pattanaik, G.; Zangari, G. Influence of chloride anions on the mechanism of copper electrodeposition from acidic sulfate electrolytes. J. Electrochem. Soc. 2007, 154, D201. [Google Scholar] [CrossRef]
- Castillo, E.; Dimitrov, N. Electrodeposition of Zn-rich CuxZn(1−x) films with controlled composition and morphology. J. Electrochem. Soc. 2021, 168, 062513. [Google Scholar] [CrossRef]
- Castillo, E.; Zhang, J.; Dimitrov, N. All-electrochemical synthesis of tunable fine-structured nanoporous copper films. Mrs Bull. 2022, 47, 913–925. [Google Scholar] [CrossRef]
- Papavinasam, S. Electrochemical polarization techniques for corrosion monitoring. In Techniques for Corrosion Monitoring; Elsevier: Amsterdam, The Netherlands, 2021; pp. 45–77. [Google Scholar]
- G102-89; Astm, G. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2004.
- Corrosion of Metals. Available online: https://xapps.xyleminc.com/Crest.Grindex/help/grindex/contents/Metals.htm (accessed on 23 September 2024).
- Kear, G.; Barker, B.; Walsh, F. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Milić, S.M.; Petrović, M.B. Films formed on copper surface in chloride media in the presence of azoles. Corros. Sci. 2009, 51, 1228–1237. [Google Scholar] [CrossRef]
- Dima, G.; De Vooys, A.; Koper, M. Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions. J. Electroanal. Chem. 2003, 554, 15–23. [Google Scholar] [CrossRef]
- Nishimura, K.; Machida, K.; Enyo, M. On-line mass spectroscopy applied to electroreduction of nitrite and nitrate ions at porous Pt electrode in sulfuric acid solutions. Electrochim. Acta 1991, 36, 877–880. [Google Scholar] [CrossRef]
- Xie, Y.; Dimitrov, N. Highly Active and Durable CuxAu(1–x) Ultrathin-Film Catalysts for Nitrate Electroreduction Synthesized by Surface-Limited Redox Replacement. ACS Omega 2018, 3, 17676–17686. [Google Scholar] [CrossRef]
- Salgado, J.A.M.; Chavarín, J.U.; Cruz, D.M. Observation of copper corrosion oxide products reduction in metallic samples by means of digital image correlation. Int. J. Electrochem. Sci. 2012, 7, 1107–1117. [Google Scholar] [CrossRef]
- Altun, H.; Sen, S. Studies on the influence of chloride ion concentration and pH on the corrosion and electrochemical behaviour of AZ63 magnesium alloy. Mater. Des. 2004, 25, 637–643. [Google Scholar] [CrossRef]
- Kearns, J.R.; Kearns, J.R.; Little, B.J. Microbiologically Influenced Corrosion Testing; ASTM International: West Conshohocken, PA, USA, 1994; Volume 1232. [Google Scholar]
- Wang, J.; Wen, W.; Xie, F.; Wu, B.; Yang, Y.; Cheng, J.; Zhang, S.; Zhang, X. Effects of oxygen concentration on the corrosion behavior of high entropy alloy AlCoCrFeNi in simulated deep sea. Heliyon 2024, 10, e32793. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Adan, N. Effect of oxygen concentration on corrosion rate of carbon steel in seawater. Am. J. Eng. Res. 2014, 3, 64–67. [Google Scholar]
- Metal Corrosion: Understanding the Causes, Effects, and Prevention. Available online: https://fakhriengineering.com/metal-corrosion-understanding-the-causes-effects-and-prevention/#:~:text=Oxygen%3A%20The%20presence%20of%20oxygen%20in%20the%20environment%2C,commonly%20known%20as%20oxidation%2C%20weakening%20the%20metal%E2%80%99s%20structure (accessed on 23 September 2024).
- Kamundi, M.; Bromberg, L.; Fey, E.; Mitchell, C.; Fayette, M.; Dimitrov, N. Impact of structure and composition on the dealloying of AuxAg(1–x) alloys on the nanoscale. J. Phys. Chem. C 2012, 116, 14123–14133. [Google Scholar] [CrossRef]
- Xia, J.; Ambrozik, S.; Crane, C.C.; Chen, J.; Dimitrov, N. Impact of structure and composition on the dealloying of CuxAu(1–x) bulk and nanoscale alloys. J. Phys. Chem. C 2016, 120, 2299–2308. [Google Scholar] [CrossRef]
- Al-Khateeb, M.; Barker, R.; Neville, A.; Thompson, H. The effect of surface roughness on diffusion and chemical reaction controlled limiting currents on a Rotating Cylinder Electrode in deaerated solutions with and without CO2. Corrosion 2018, 74, 971–983. [Google Scholar] [CrossRef]
- Erlebacher, J.; Baris, J.; Zhou, C.; Hou, S.; Wang, C.; Anber, E.; Lech, S.; Taheri, M. Dealloyed Materials for Corrosion Protection. In Proceedings of the Electrochemical Society Meeting Abstracts 244, Gothenburg, Sweden, 8–10 October 2023; p. 1277. [Google Scholar]
- Corcoran, S.; Sieradzki, K. Chaos during the growth of an artificial pit. J. Electrochem. Soc. 1992, 139, 1568. [Google Scholar] [CrossRef]
- Cao, Q.; Shao, Z.; Hensley, D.K.; Lavrik, N.V.; Venton, B.J. Influence of geometry on thin layer and diffusion processes at carbon electrodes. Langmuir 2021, 37, 2667–2676. [Google Scholar] [CrossRef]


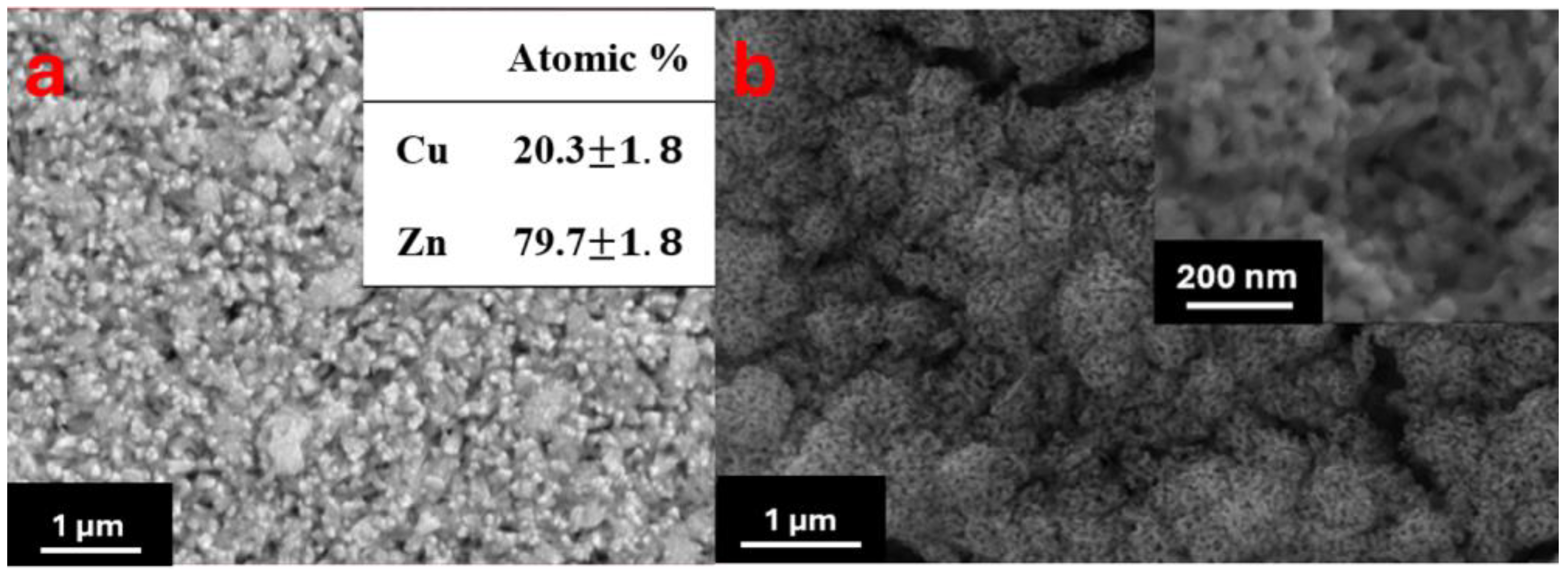
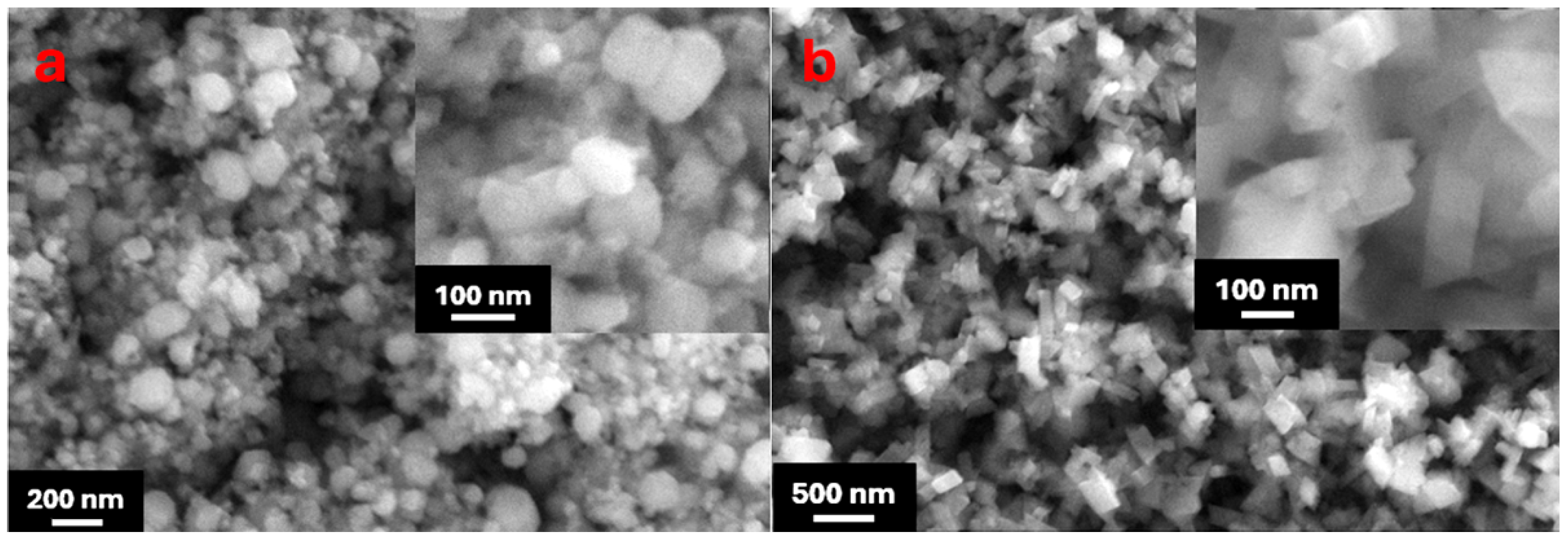
| Solutions | Corrosion Potential (V vs. MSE) | Corrosion Current Density (A/cm2) | Corrosion Rate (×10−3 mpy) |
|---|---|---|---|
| 3% NaCl | −0.73 | 4.17 × 10−7 | 381 |
| 1% NaCl | −0.71 | 2.88 × 10−7 | 263 |
| 3% NaNO3 | −0.57 | 2.51 × 10−7 | 229 |
| 1% NaNO3 | −0.50 | 1.51 × 10−7 | 138 |
| Solutions | Corrosion Potential (V vs. MSE) | Corrosion Current Density (A/cm2) | Corrosion Rate (×10−3 mpy) |
|---|---|---|---|
| 3% NaCl | −0.62 | 4.26 × 10−6 | 3890 |
| 1% NaCl | −0.57 | 3.98 × 10−6 | 3630 |
| 3% NaNO3 | −0.42 | 3.72 × 10−7 | 340 |
| 1% NaNO3 | −0.41 | 2.24 × 10−7 | 204 |
| NP Cu in Different Conditions | Corrosion Potential (V vs. MSE) | Corrosion Current Density (A/cm2) | Corrosion Rate (×10−3 mpy) |
|---|---|---|---|
| 3% NaCl N2 | −0.9 | 6.46 × 10−8 | 59.0 |
| 3% NaCl air | −0.46 | 3.72 × 10−7 | 340 |
| 3% NaNO3 N2 | −0.69 | 2.63 × 10−8 | 24.0 |
| 3% NaNO3 air | −0.39 | 5.25 × 10−8 | 47.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Z.; Mull, K.; Dimitrov, N. Comparative Analysis of the Corrosion Behavior of Plain and Nanoporous Copper. Electrochem 2025, 6, 1. https://doi.org/10.3390/electrochem6010001
Lei Z, Mull K, Dimitrov N. Comparative Analysis of the Corrosion Behavior of Plain and Nanoporous Copper. Electrochem. 2025; 6(1):1. https://doi.org/10.3390/electrochem6010001
Chicago/Turabian StyleLei, Zhen, Ksenya Mull, and Nikolay Dimitrov. 2025. "Comparative Analysis of the Corrosion Behavior of Plain and Nanoporous Copper" Electrochem 6, no. 1: 1. https://doi.org/10.3390/electrochem6010001
APA StyleLei, Z., Mull, K., & Dimitrov, N. (2025). Comparative Analysis of the Corrosion Behavior of Plain and Nanoporous Copper. Electrochem, 6(1), 1. https://doi.org/10.3390/electrochem6010001







