Trends and Opportunities in Enzyme Biosensors Coupled to Metal-Organic Frameworks (MOFs): An Advanced Bibliometric Analysis
Abstract
1. Introduction
- RQ1: How has the scientific literature related to MOF been developed?
- RQ2: What is the relevance of biosensors created from MOFs coupled to enzymes related to other topics?
- RQ3: In which field of applications did the MOFs generate more interest from the researchers of the collected database?
- RQ4: What are the overall results on search trends (keywords and search areas) related to MOFs in emerging research fields?
2. Methodology
2.1. Data Source
2.2. Data Analysis
3. Results and Discussion
3.1. Bibliometric Analysis
3.1.1. Publication Result: Overall Results
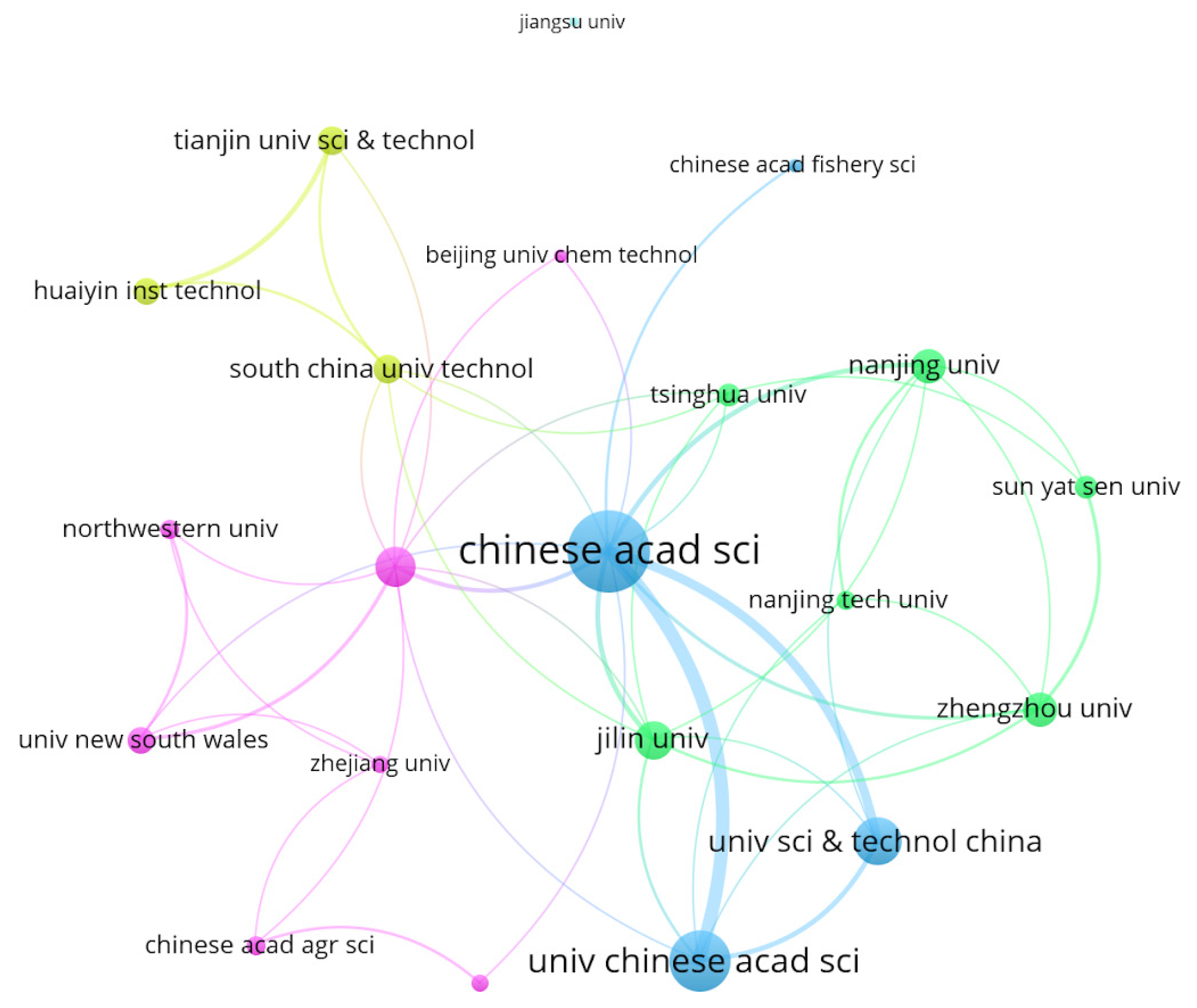
3.1.2. The Distribution by Country and Institution
3.1.3. The Most Cited Articles
3.1.4. The Research Areas
3.2. Biosensors Design
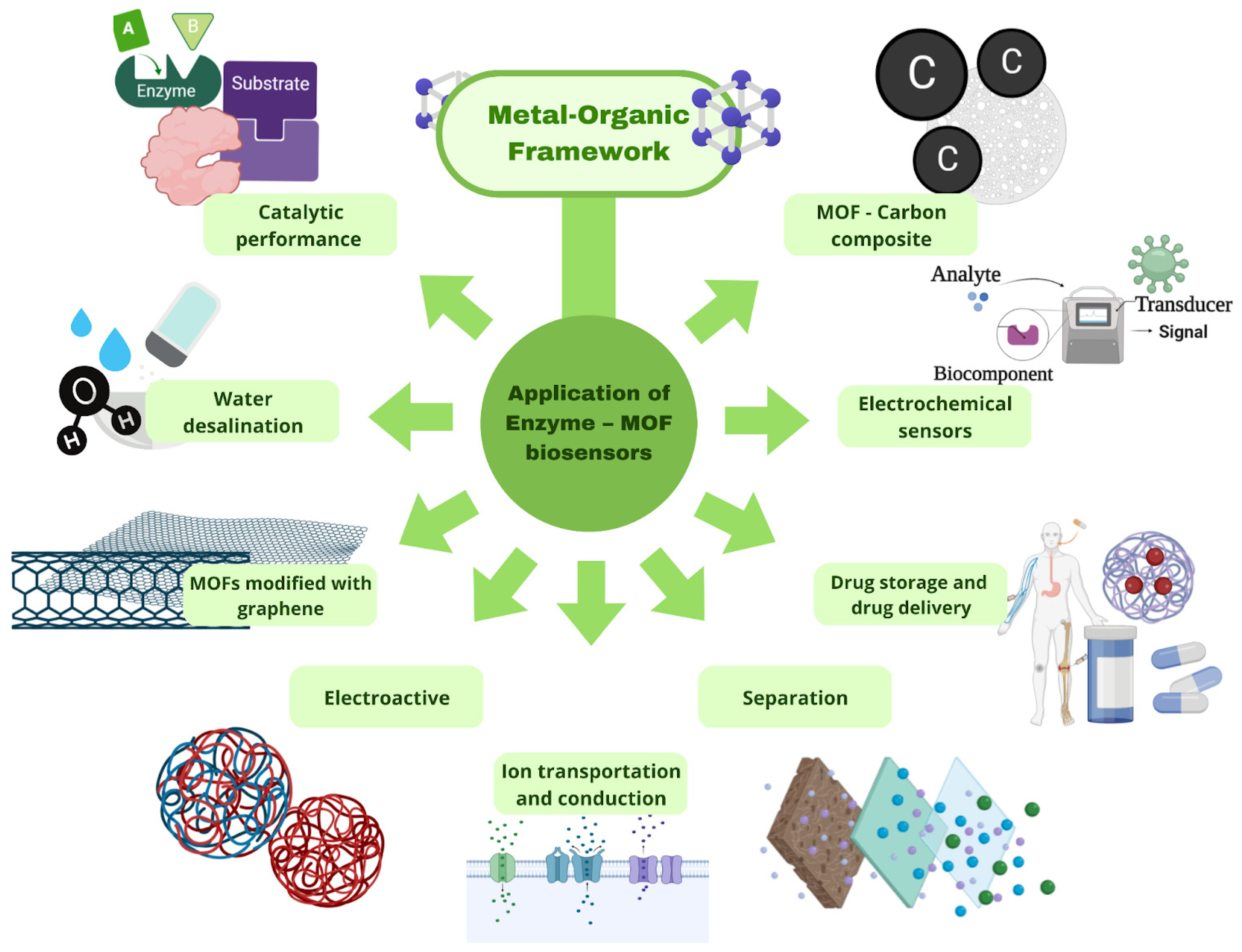
3.2.1. MOF-Based Chemosensors and Biosensors
3.2.2. MOFs for Catalytic Performance
3.2.3. Nonenzymatic Electrochemical Detection
3.2.4. Enzyme–MOF Composites
3.2.5. Point-of-Care Sensors
4. Trendy Research Topics
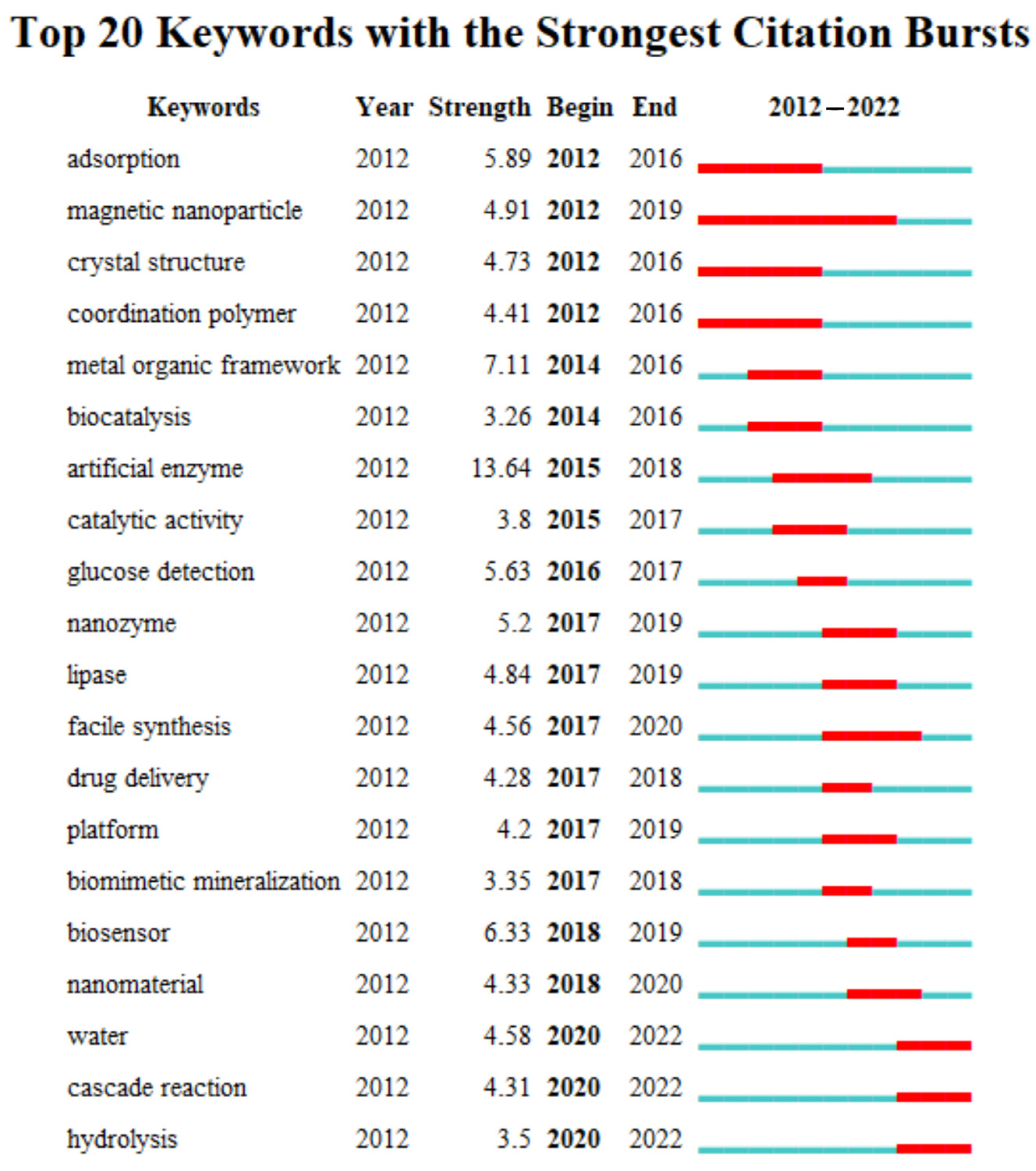
4.1. Quantitative Analysis of Frequent Keywords
4.2. Research Areas
4.2.1. Research Fields
4.2.2. Emerging Trends
4.2.3. Two Key Insights
5. Conclusions
- Through the analysis conducted, it was found that biosensors were a highly researched topic during the period analyzed, indicating a growing interest in this emerging field of scientific literature. The promising applications of MOFs coupled with various materials, especially enzymes, suggest the potential for future developments.
- The non-enzymatic use of MOFs is more prominent due to its long history of use in various fields of research. This combination has been explored more extensively than the enzyme-MOF combination, which is a relatively recent development. As a result, areas that have received more research attention in the past have not included the enzyme–MOF combination, resulting in a lack of studies in this area of science.
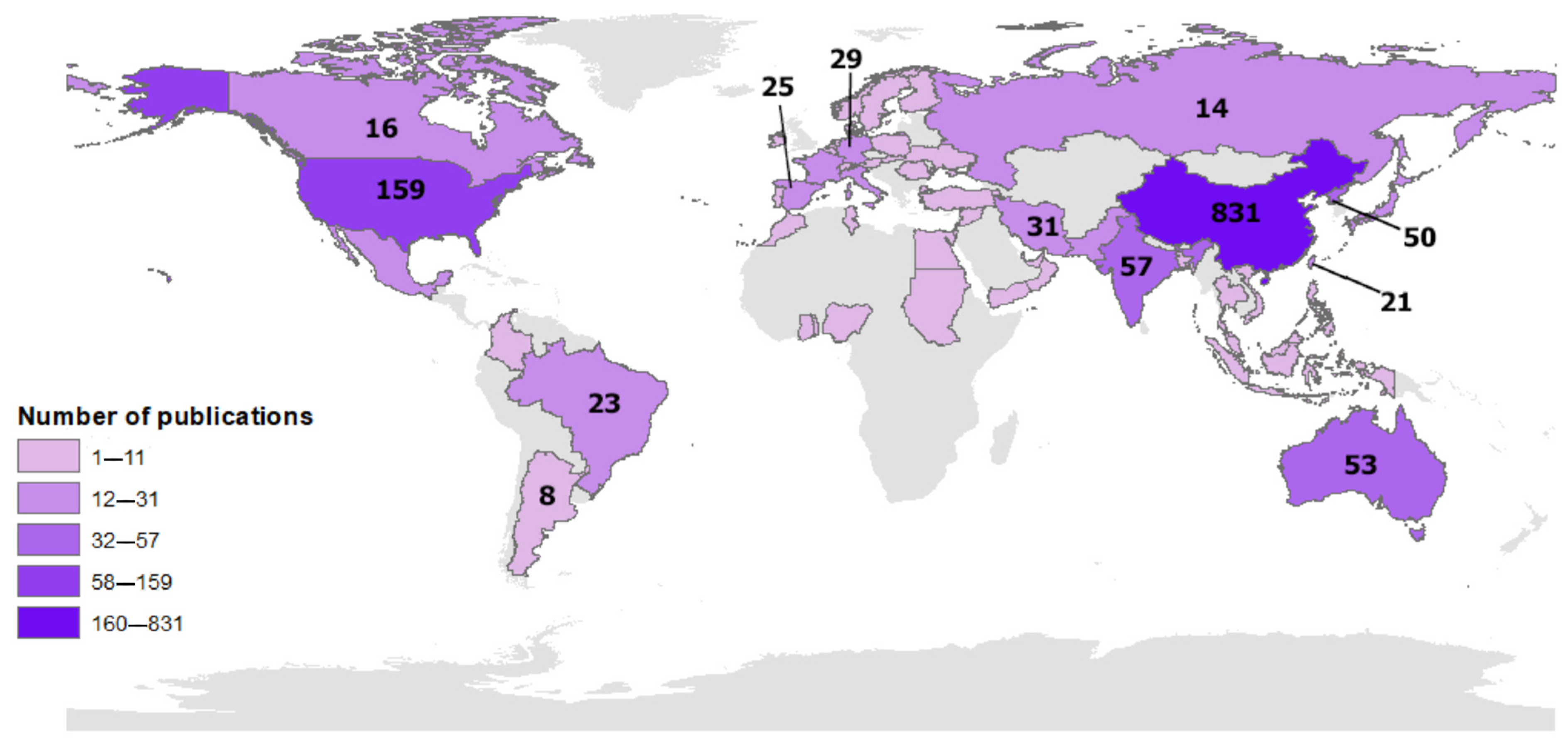
- The analysis of publishing countries revealed that China (831 articles), followed by the United States (159 articles) and India (57 articles), had the most significant contributions in this research area. This is a common trend in several research fields as these countries have a significant engagement in building scientific knowledge. Furthermore, this information indicates the relative importance of this research area to China compared to the United States, as evident from the quantitative data of the documents produced.
- Based on the analysis of the topics addressed in each article and the post-processing of the Citespace data, it can be concluded that the field of medicine is the most prominent research area in the application of MOFs. This is mainly due to the large number of articles, identified by relevance levels, that have focused on the detection and removal of harmful compounds that affect human health. Energy applications of MOFs are also considered important, but to a lesser extent compared to medicine.
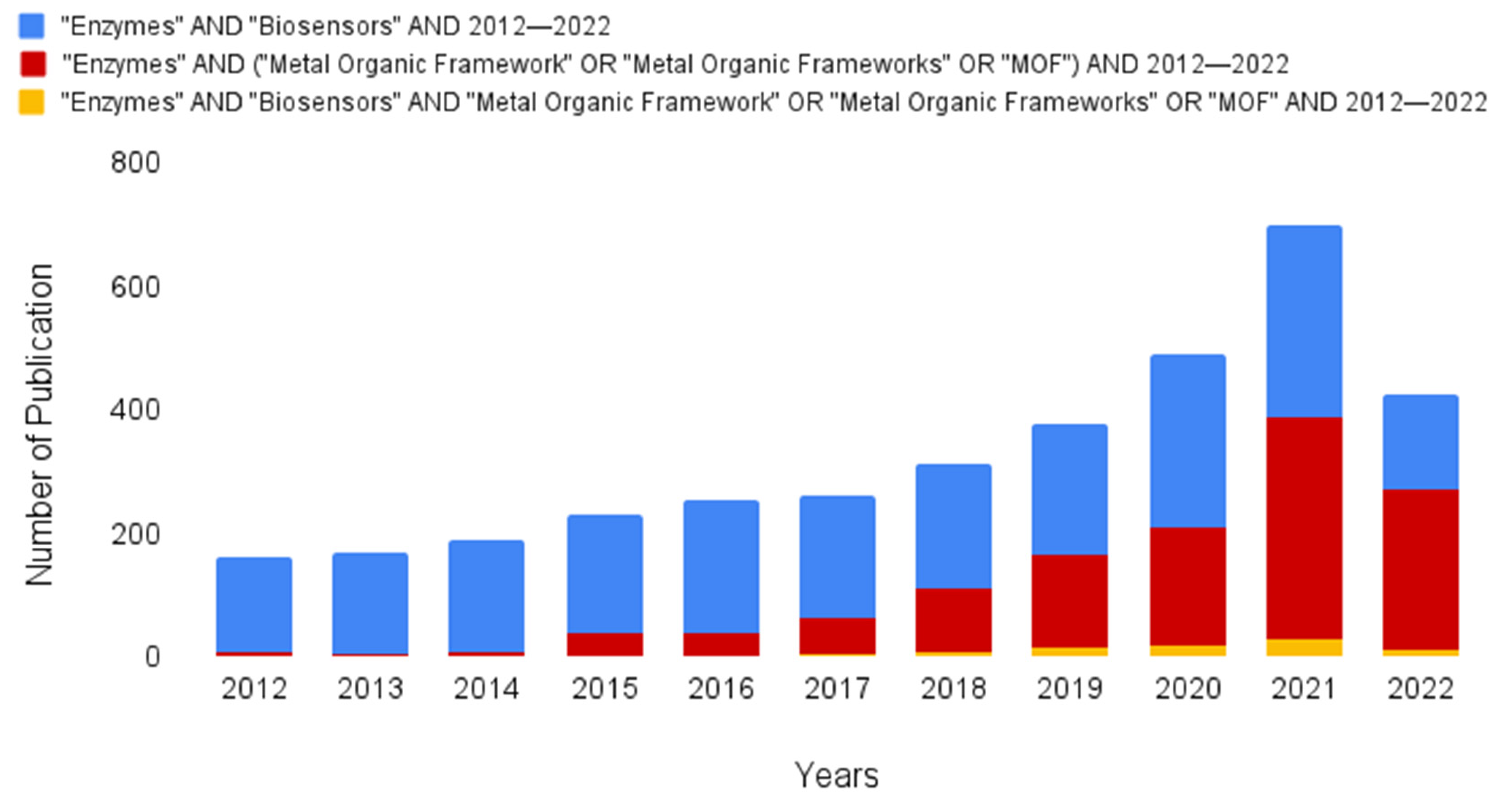
- An important aspect to note in the methodology is the comparison of the number of articles found in the two largest databases. A search using the keywords “Enzymes” and “Biosensors” returned 2264 articles. However, by adding the keywords “Metal-Organic Framework”, “Metal-Organic Frameworks”, or “MOF”, the number of articles increased to 1220. Therefore, searches related to enzymes and MOFs represented about 53% of the total database related to enzymes and biosensors. However, when all of these keywords were combined, the number decreased to 87, indicating that there were significantly more studies on other applications of MOFs coupled with enzymes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ouyang, B.; Ouyang, P.; Shi, M.; Maimaiti, T.; Li, Q.; Lan, S.; Luo, J.; Wu, X.; Yang, S.-T. Low toxicity of metal-organic framework MOF-199 to bacteria Escherichia coli and Staphylococcus aureus. J. Hazard. Mater. Adv. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Souza, J.E.D.S.; de Oliveira, G.P.; Alexandre, J.Y.N.H.; Neto, J.G.L.; Sales, M.B.; Junior, P.G.D.S.; de Oliveira, A.L.B.; de Souza, M.C.M.; dos Santos, J.C.S. A Comprehensive Review on the Use of Metal–Organic Frameworks (MOFs) Coupled with Enzymes as Biosensors. Electrochem 2022, 3, 89–113. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, R.; Ma, G.; Deng, L.; Liu, H.; Ding, Y.; Jiang, J. Preparation of a cobalt metal-organic framework (Co-MOF) and its application as a polypropylene flame retardant by compounding with melamine polyphosphate. Polym. Test. 2022, 116, 107765. [Google Scholar] [CrossRef]
- Naghdi, S.; Shahrestani, M.M.; Zendehbad, M.; Djahaniani, H.; Kazemian, H.; Eder, D. Recent advances in application of metal-organic frameworks (MOFs) as adsorbent and catalyst in removal of persistent organic pollutants (POPs). J. Hazard. Mater. 2023, 442, 130127. [Google Scholar] [CrossRef] [PubMed]
- López-R, M.; Barrios, Y.; Perez, L.D.; Soto, C.; Sierra, C. Metal-Organic Framework (MOFs) tethered to cotton fibers display antimicrobial activity against relevant nosocomial bacteria. Inorg. Chim. Acta 2022, 537, 120955. [Google Scholar] [CrossRef]
- Cui, L.; Hu, J.; Li, C.-C.; Wang, C.-M.; Zhang, C.-Y. An electrochemical biosensor based on the enhanced quasi-reversible redox signal of prussian blue generated by self-sacrificial label of iron metal-organic framework. Biosens. Bioelectron. 2018, 122, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.M.; Alexandre, J.Y.N.H.; Souza, J.E.S.; Neto, J.G.L.; Júnior, P.G.D.S.; Rocha, M.V.P.; dos Santos, J.C.S. The Chemistry and Applications of Metal–Organic Frameworks (MOFs) as Industrial Enzyme Immobilization Systems. Molecules 2022, 27, 4529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lou, Y.; Guo, C.; Jia, Q.; Song, Y.; Tian, J.-Y.; Zhang, S.; Wang, M.; He, L.; Du, M. Metal–organic frameworks (MOFs) based chemosensors/biosensors for analysis of food contaminants. Trends Food Sci. Technol. 2021, 118, 569–588. [Google Scholar] [CrossRef]
- Yadav, A.K.; Verma, D.; Dalal, N.; Kumar, A.; Solanki, P.R. Molecularly imprinted polymer-based nanodiagnostics for clinically pertinent bacteria and virus detection for future pandemics. Biosens. Bioelectron. X 2022, 12, 100257. [Google Scholar] [CrossRef]
- Cavalcante, F.; Falcão, I.d.A.; Souza, J.D.S.; Rocha, T.; de Sousa, I.; Cavalcante, A.; de Oliveira, A.; de Sousa, M.; dos Santos, J. Designing of Nanomaterials-Based Enzymatic Biosensors: Synthesis, Properties, and Applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Lv, M.; Zhou, W.; Tavakoli, H.; Bautista, C.; Xia, J.; Wang, Z.; Li, X. Aptamer-functionalized metal-organic frameworks (MOFs) for biosensing. Biosens. Bioelectron. 2020, 176, 112947. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, Q.; Lu, K.; Huang, J.; Zhang, Y.; Hou, Y.; Qiao, H.; Li, D.; Wei, Q. Encapsulating enzyme into metal-organic framework during in-situ growth on cellulose acetate nanofibers as self-powered glucose biosensor. Biosens. Bioelectron. 2020, 171, 112690. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Vaidya, L.B.; Nadar, S.S.; Rathod, V.K. Metal-Organic Frameworks (MOFs) for Enzyme Immobilization. In Metal-Organic Frameworks for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 491–523. [Google Scholar]
- Bonazza, H.L.; Manzo, R.M.; dos Santos, J.C.S.; Mammarella, E.J. Operational and Thermal Stability Analysis of Thermomyces lanuginosus Lipase Covalently Immobilized onto Modified Chitosan Supports. Appl. Biochem. Biotechnol. 2017, 184, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Valério, R.B.R.; Cavalcante, A.L.G.; Mota, G.F.; de Sousa, I.G.; da Silva Souza, J.E.; Cavalcante, F.T.T.; de Aguiar Falcão, I.R.; da Silva Moreira, K. Understanding the Biocatalytic Potential of Lipase from Rhizopus chinensis. Biointerface Res. Appl. Chem. 2021, 12, 4230–4260. [Google Scholar] [CrossRef]
- Monteiro, R.; Dos Santos, J.; Alcántara, A.; Fernandez-Lafuente, R. Enzyme-Coated Micro-Crystals: An Almost Forgotten but Very Simple and Elegant Immobilization Strategy. Catalysts 2020, 10, 891. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Immobilization of Lipases on Hetero-functional Octyl–Glyoxyl Agarose Supports. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2016; pp. 73–85. [Google Scholar]
- Bilal, M.; Rashid, E.U.; Zdarta, J.; dos Santos, J.C.; Fernandes, P.C.; Cheng, H.; Jesionowski, T. Engineering magnetic nanobiocatalytic systems with multipurpose functionalities for biocatalysis, biotechnology and bioprocess applications. Sustain. Chem. Pharm. 2022, 30, 100866. [Google Scholar] [CrossRef]
- Cavalcante, A.L.G.; Cavalcante, C.G.; Colares, R.P.; Ferreira, D.A.; da Silva, F.F.M.; de Sousa, E.Y.A.; Souza, J.E.D.S.; Monteiro, R.R.D.C.; de Oliveira, A.L.B.; dos Santos, J.C.S.; et al. Preparation, Characterization, and Enantioselectivity of Polyacrylate Microcapsules Entrapping Ananas comosus Extract. Rev. Virtual Química 2021, 13, 1319–1329. [Google Scholar] [CrossRef]
- Alexandre, J.Y.N.H.; Cavalcante, F.T.T.; Freitas, L.M.; Castro, A.P.; Borges, P.T.; Junior, P.G.d.S.; Filho, M.N.R.; Lopes, A.A.S.; da Fonseca, A.M.; Lomonaco, D.; et al. A Theoretical and Experimental Study for Enzymatic Biodiesel Production from Babassu Oil (Orbignya sp.) Using Eversa Lipase. Catalysts 2022, 12, 1322. [Google Scholar] [CrossRef]
- Júnior, J.B.; Nascimento, J.G.A.D.; Silva, M.P.F.; Brandão, E.D.A.L.; Bizerra, V.D.C.; dos Santos, K.M.; Serpa, J.D.F.; dos Santos, J.C.S.; da Fonseca, A.M.; de Oliveira, D.L.V.; et al. Performance of Eversa Transform 2.0 Lipase in Ester Production Using Babassu Oil (Orbignya sp.) and Tucuman Oil (Astrocaryum vulgar): A Comparative Study between Liquid and Immobilized Forms in Fe3O4 Nanoparticles. Catalysts 2023, 13, 571. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; dos Santos, J.C.; Ortiz, C.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.; Fernandez-Lafuente, R. Lecitase ultra: A phospholipase with great potential in biocatalysis. Mol. Catal. 2019, 473, 110405. [Google Scholar] [CrossRef]
- Lima, P.J.M.; da Silva, R.M.; Neto, C.A.C.G.; e Silva, N.C.G.; Souza, J.E.D.S.; Nunes, Y.L.; dos Santos, J.C.S. An overview on the conversion of glycerol to value-added industrial products via chemical and biochemical routes. Biotechnol. Appl. Biochem. 2021, 69, 2794–2818. [Google Scholar] [CrossRef] [PubMed]
- Moreira, K.S.; Júnior, L.S.M.; Monteiro, R.R.C.; De Oliveira, A.L.B.; Valle, C.P.; Freire, T.M.; Fechine, P.B.A.; De Souza, M.C.M.; Fernandez-Lorente, G.; Guisan, J.M.; et al. Optimization of the Production of Enzymatic Biodiesel from Residual Babassu Oil (Orbignya sp.) via RSM. Catalysts 2020, 10, 414. [Google Scholar] [CrossRef]
- Mota, G.F.; de Sousa, I.G.; de Oliveira, A.L.B.; Cavalcante, A.L.G.; Moreira, K.D.S.; Cavalcante, F.T.T.; Souza, J.E.D.S.; Falcão, R.D.A.; Rocha, T.G.; Valério, R.B.R.; et al. Biodiesel production from microalgae using lipase-based catalysts: Current challenges and prospects. Algal Res. 2022, 62, 102616. [Google Scholar] [CrossRef]
- Souza, J.E.S.; Monteiro, R.R.C.; Rocha, T.G.; Moreira, K.S.; Cavalcante, F.T.T.; Braz, A.K.D.S.; de Souza, M.C.M.; dos Santos, J.C.S. Sonohydrolysis using an enzymatic cocktail in the preparation of free fatty acid. 3 Biotech 2020, 10, 254. [Google Scholar] [CrossRef]
- Pinheiro, M.P.; Monteiro, R.R.; Silva, F.F.; Lemos, T.L.; Fernandez-Lafuente, R.; Gonçalves, L.R.; dos Santos, J.C. Modulation of Lecitase properties via immobilization on differently activated Immobead-350: Stabilization and inversion of enantiospecificity. Process. Biochem. 2019, 87, 128–137. [Google Scholar] [CrossRef]
- Rocha, T.G.; Gomes, P.H.D.L.; de Souza, M.C.M.; Monteiro, R.R.C.; dos Santos, J.C.S. Lipase Cocktail for Optimized Biodiesel Production of Free Fatty Acids from Residual Chicken Oil. Catal. Lett. 2020, 151, 1155–1166. [Google Scholar] [CrossRef]
- Synthesis, Biological Activity, and In silico Study of Bioesters Derived from Bixin by the CALB Enzyme. Biointerface Res. Appl. Chem. 2021, 12, 5901–5917. [CrossRef]
- Cavalcante, F.T.T.; da Fonseca, A.M.; Alexandre, J.Y.N.H.; dos Santos, J.C. A stepwise docking and molecular dynamics approach for enzymatic biolubricant production using Lipase Eversa® Transform as a biocatalyst. Ind. Crops Prod. 2022, 187, 115450. [Google Scholar] [CrossRef]
- de Sousa, I.G.; Mota, G.F.; Cavalcante, A.L.G.; Rocha, T.G.; Sousa, P.D.S.; Alexandre, J.Y.N.H.; Souza, J.E.D.S.; Neto, F.S.; Cavalcante, F.T.T.; Lopes, A.A.S.; et al. Renewable processes of synthesis of biolubricants catalyzed by lipases. J. Environ. Chem. Eng. 2023, 11, 109006. [Google Scholar] [CrossRef]
- Bezerra, F.D.A.; Lima, G.D.C.; Carvalho, A.C.L.D.M.; Vega, K.B.; Oliveira, M.C.F.; de Lemos, T.L.G.; dos Santos, J.C.S.; Gonçalves, L.R.B.; Rios, N.S.; Fernandez-Lafuente, R.; et al. Chemoenzymatic synthesis of both enantiomers of propafenone hydrochloride through lipase-catalyzed process. Mol. Catal. 2022, 529, 112540. [Google Scholar] [CrossRef]
- Moreira, K.D.S.; de Oliveira, A.L.B.; Júnior, L.S.D.M.; de Sousa, I.G.; Cavalcante, A.L.G.; Neto, F.S.; Valério, R.B.R.; Chaves, A.V.; Fonseca, T.D.S.; Cruz, D.M.V.; et al. Taguchi design-assisted co-immobilization of lipase A and B from Candida antarctica onto chitosan: Characterization, kinetic resolution application, and docking studies. Chem. Eng. Res. Des. 2021, 177, 223–244. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Cavalcante, A.L.G.; de Sousa, I.G.; Neto, F.S.; dos Santos, J.C.S. Current Status and Future Perspectives of Supports and Protocols for Enzyme Immobilization. Catalysts 2021, 11, 1222. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Neto, D.M.A.; Fechine, P.B.A.; Lopes, A.A.S.; Gonçalves, L.R.B.; dos Santos, J.C.S.; de Souza, M.C.M.; Fernandez-Lafuente, R. Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation. Int. J. Mol. Sci. 2019, 20, 5807. [Google Scholar] [CrossRef] [PubMed]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Reactivation of lipases by the unfolding and refolding of covalently immobilized biocatalysts. RSC Adv. 2015, 5, 55588–55594. [Google Scholar] [CrossRef]
- de Oliveira, A.L.B.; Cavalcante, F.T.T.; Moreira, K.S.; Monteiro, R.R.C.; Rocha, T.G.; Souza, J.E.S.; da Fonseca, A.M.; Lopes, A.A.S.; Guimarães, A.P.; de Lima, R.K.C.; et al. Lipases Immobilized onto Nanomaterials as Biocatalysts in Biodiesel Production: Scientific Context, Challenges, and Opportunities. Rev. Virtual Química 2021, 13, 875–891. [Google Scholar] [CrossRef]
- da Fonseca, A.M.; dos Santos, J.C.S.; de Souza, M.C.M.; de Oliveira, M.M.; Colares, R.P.; de Lemos, T.L.G.; Braz-Filho, R. The use of new hydrogel microcapsules in coconut juice as biocatalyst system for the reaction of quinine. Ind. Crops Prod. 2019, 145, 111890. [Google Scholar] [CrossRef]
- Monteiro, R.R.; de Oliveira, A.L.B.; de Menezes, F.L.; de Souza, M.C.M.; Fechine, P.B.; dos Santos, J.C. Improvement of enzymatic activity and stability of lipase A from Candida antartica onto halloysite nanotubes with Taguchi method for optimized immobilization. Appl. Clay Sci. 2022, 228, 106634. [Google Scholar] [CrossRef]
- Cavalcante, A.L.G.; Chaves, A.V.; Fechine, P.B.A.; Alexandre, J.Y.N.H.; Freire, T.M.; Davi, D.M.B.; Neto, F.S.; de Sousa, I.G.; Moreira, K.d.S.; de Oliveira, A.L.B.; et al. Chemical modification of clay nanocomposites for the improvement of the catalytic properties of Lipase A from Candida antarctica. Process. Biochem. 2022, 120, 1–14. [Google Scholar] [CrossRef]
- de Sousa, I.G.; Chaves, A.V.; de Oliveira, A.L.B.; Moreira, K.d.S.; Junior, P.G.d.S.; Neto, F.S.; de Carvalho, S.C.F.; Valério, R.B.R.; Lima, G.V.; Lopes, A.A.S.; et al. A novel hybrid biocatalyst from immobilized Eversa® Transform 2.0 lipase and its application in biolubricant synthesis. Biocatal. Biotransform. 2022, 28, 1–22. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Xu, Q. Porous metal–organic frameworks as platforms for functional applications. Chem. Commun. 2011, 47, 3351–3370. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.; Vaidya, L.; Rathod, V.K. Enzyme embedded metal organic framework (enzyme–MOF): De novo approaches for immobilization. Int. J. Biol. Macromol. 2020, 149, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.F.; da Silva, A.F.; da Silva, F.L.; dos Santos, K.M.; de Oliveira, M.P.; Nobre, M.M.; Catumba, B.D.; Sales, M.B.; Silva, A.R.; Braz, A.K.S.; et al. A scientometric analysis of research progress and trends in the design of laccase biocatalysts for the decolorization of synthetic dyes. Process. Biochem. 2023, 126, 272–291. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Fakhar, M.; Keighobadi, M.; Hezarjaribi, H.Z.; Montazeri, M.; Banimostafavi, E.S.; Sayyadi, S.; Hamadani, M.M.G.; Sharifpour, A.; Tabaripour, R.; Asadi, S.; et al. Two decades of echinococcosis/hydatidosis research: Bibliometric analysis based on the web of science core collection databases (2000–2019). Food Waterborne Parasitol. 2021, 25, e00137. [Google Scholar] [CrossRef]
- Sales, M.B.; Borges, P.T.; Filho, M.N.R.; da Silva, L.R.M.; Castro, A.P.; Lopes, A.A.S.; de Lima, R.K.C.; Rios, M.A.D.S.; dos Santos, J.C.S. Sustainable Feedstocks and Challenges in Biodiesel Production: An Advanced Bibliometric Analysis. Bioengineering 2022, 9, 539. [Google Scholar] [CrossRef]
- Catumba, B.D.; Sales, M.B.; Borges, P.T.; Filho, M.N.R.; Lopes, A.A.S.; Rios, M.A.D.S.; Desai, A.S.; Bilal, M.; dos Santos, J.C.S. Sustainability and challenges in hydrogen production: An advanced bibliometric analysis. Int. J. Hydrogen Energy 2023, 48, 7975–7992. [Google Scholar] [CrossRef]
- Sweileh, W.M.; Al-Jabi, S.W.; AbuTaha, A.S.; Zyoud, S.H.; Anayah, F.M.A.; Sawalha, A.F. Bibliometric analysis of worldwide scientific literature in mobile-health: 2006–2016. BMC Med. Inform. Decis. Mak. 2017, 17, 72. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Drout, R.J.; Robison, L.; Farha, O.K. Catalytic applications of enzymes encapsulated in metal–organic frameworks. Coord. Chem. Rev. 2018, 381, 151–160. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, M.; Lu, L.; Lou, X.; Dong, M.; Zhu, L. Metal-organic framework/enzyme coated optical fibers as waveguide-based biosensors. Sens. Actuators B Chem. 2019, 288, 12–19. [Google Scholar] [CrossRef]
- Liang, J.; Liang, K. Nano-bio-interface engineering of metal-organic frameworks. Nano Today 2021, 40, 101256. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2018, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Gu, Z.-Y.; Li, J.-R.; Jiang, H.-L.; Wei, Z.; Zhou, H. Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal-Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, M.; Zhang, X.; Liu, Q.; Lin, D.; Xu, C.; Xie, F.; Sun, X. Co-MOF nanosheet array: A high-performance electrochemical sensor for non-enzymatic glucose detection. Sens. Actuators B Chem. 2018, 278, 126–132. [Google Scholar] [CrossRef]
- Zhou, R.; Zhuang, X.; Wu, Q.; Jin, M.; Zheng, C.; Jiang, Y.; Lou, Y.; Zheng, L. Cu-MOF@Pt 3D nanocomposites prepared by one-step wrapping method with peroxidase-like activity for colorimetric detection of glucose. Colloids Surf. B Biointerfaces 2022, 216, 112601. [Google Scholar] [CrossRef]
- Hassanzadeh, J.; Al Lawati, H.A.; Bagheri, N. On paper synthesis of multifunctional CeO2 nanoparticles@Fe-MOF composite as a multi-enzyme cascade platform for multiplex colorimetric detection of glucose, fructose, sucrose, and maltose. Biosens. Bioelectron. 2022, 207, 114184. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.-J.; Zhang, Q.; Zhang, J.-Y.; Zhang, N.; Fang, Y.-Z.; Yan, J.; Ke, Q. The multifunctional BODIPY@Eu-MOF nanosheets as bioimaging platform: A ratiometric fluorescencent sensor for highly efficient detection of F-, H2O2 and glucose. Sens. Actuators B Chem. 2021, 354, 131140. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, F.; Yin, X.-B. A ratiometric fluorescence platform based on boric-acid-functional Eu-MOF for sensitive detection of H2O2 and glucose. Biosens. Bioelectron. 2019, 135, 208–215. [Google Scholar] [CrossRef]
- Dong, S.; Niu, H.; Sun, L.; Zhang, S.; Wu, D.; Yang, Z.; Xiang, M. Highly dense Ni-MOF nanoflake arrays supported on conductive graphene/carbon fiber substrate as flexible microelectrode for electrochemical sensing of glucose. J. Electroanal. Chem. 2022, 911, 116219. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.M.; Mason, J.A.; Kong, X.; Bloch, E.D.; Gygi, D.; Dani, A.; Crocellà, V.; Giordanino, F.; Odoh, S.O.; Drisdell, W.S.; et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 2015, 519, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Shieh, F.-K.; Wang, S.-C.; Yen, C.-I.; Wu, C.-C.; Dutta, S.; Chou, L.-Y.; Morabito, J.V.; Hu, P.; Hsu, M.-H.; Wu, K.C.-W.; et al. Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by a de Novo Approach: Size-Selective Sheltering of Catalase in Metal–Organic Framework Microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Liu, C.; Wang, Z.; Kang, L.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. Nanozyme Decorated Metal–Organic Frameworks for Enhanced Photodynamic Therapy. ACS Nano 2018, 12, 651–661. [Google Scholar] [CrossRef]
- Feng, D.; Liu, T.-F.; Su, J.; Bosch, M.; Wei, Z.; Wan, W.; Yuan, D.; Chen, Y.-P.; Wang, X.; Wang, K.; et al. Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat. Commun. 2015, 6, 5979. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Wei, H. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond. Inorg. Chem. Front. 2015, 3, 41–60. [Google Scholar] [CrossRef]
- Mirsadoughi, E.; Pebdeni, A.B.; Hosseini, M. Sensitive colorimetric aptasensor based on peroxidase-like activity of ZrPr-MOF to detect Salmonella Typhimurium in water and milk. Food Control. 2023, 146, 109500. [Google Scholar] [CrossRef]
- Yang, H.; Sun, Z.; Qin, X.; Wu, H.; Zhang, H.; Liu, G. Ultrasmall Au nanoparticles modified 2D metalloporphyrinic metal-organic framework nanosheets with high peroxidase-like activity for colorimetric detection of organophosphorus pesticides. Food Chem. 2022, 376, 131906. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zeng, M.; Zhao, Y.; Zuo, X.; Meng, F.; Lv, F.; Lu, Y. Zr(IV)-based metal-organic framework nanocomposites with enhanced peroxidase-like activity as a colorimetric sensing platform for sensitive detection of hydrogen peroxide and phenol. Environ. Res. 2021, 203, 111818. [Google Scholar] [CrossRef]
- Ran, F.; Xu, Y.; Ma, M.; Liu, X.; Zhang, H. Flower-like ZIF-8 enhance the peroxidase-like activity of nanoenzymes at neutral pH for detection of heparin and protamine. Talanta 2022, 250, 123702. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Xu, W.; Wu, Y.; Jiao, L.; Chen, Y.; Huang, J.; Tang, Y.; Gu, W.; Zhu, C. Histidine-engineered metal-organic frameworks with enhanced peroxidase-like activity for sensitive detection of metallothioneins. Sens. Actuators B Chem. 2022, 366, 131927. [Google Scholar] [CrossRef]
- Xie, F.; Ma, X.; Liu, W.; Wang, Y.; Dong, H.; Mi, T.; Jiang, X.; Sha, J. An unprecedented molybdenum oxide based helical MOF with peroxidase-like activity synthesized by surfactant-thermal method. Inorg. Chem. Commun. 2018, 97, 93–97. [Google Scholar] [CrossRef]
- Li, P.; Feng, Y.; Cheng, D.; Wei, J. Self-template synthesis of mesoporous vanadium oxide nanospheres with intrinsic peroxidase-like activity and high antibacterial performance. J. Colloid Interface Sci. 2022, 625, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Liu, M.; Wu, X.; Cai, Z.; Zhang, X.; Xi, Z.; Wang, Y.; Dai, C.; Kang, X.; Liu, Z.; et al. A novel ternary Ag-Cu2O/Ti3C2 heterostructure with high peroxidase-like activity for on-site colorimetric detection of galactose. Sens. Actuators B Chem. 2022, 369, 132343. [Google Scholar] [CrossRef]
- Kaur, N. Gauri Anthraquinone appended chemosensors for fluorescence monitoring of anions and/or metal ions. Inorg. Chim. Acta 2022, 536, 120917. [Google Scholar] [CrossRef]
- Huo, R.; Wang, C.; Xu, F.; Xing, Y.-H.; Wang, Y.-F.; Bai, F.-Y. Multistimuli-responsive pyrene-based lanthanide (III)-MOF construction and applied as dual-function fluorescent chemosensors for trace water and vitamins molecules. Mater. Today Chem. 2023, 27, 101292. [Google Scholar] [CrossRef]
- Ye, W.; Yang, W. Exploring metal-organic frameworks in electrochemistry by a bibliometric analysis. J. Ind. Eng. Chem. 2022, 109, 68–78. [Google Scholar] [CrossRef]
- Dashtian, K.; Shahbazi, S.; Tayebi, M.; Masoumi, Z. A review on metal-organic frameworks photoelectrochemistry: A headlight for future applications. Coord. Chem. Rev. 2021, 445, 214097. [Google Scholar] [CrossRef]
- Amini, S.; Ebrahimzadeh, H.; Seidi, S.; Jalilian, N. Application of electrospun polyacrylonitrile/Zn-MOF-74@GO nanocomposite as the sorbent for online micro solid-phase extraction of chlorobenzenes in water, soil, and food samples prior to liquid chromatography analysis. Food Chem. 2021, 363, 130330. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; He, T.; Wang, G.-M. Zirconium-based metal-organic frameworks for fluorescent sensing. Coord. Chem. Rev. 2023, 476, 214930. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Diao, Y.; He, Q.; Lu, C.; Singh, A.; Kumar, A.; Liu, J.; Lan, Q. Recent advances in the electrochemical applications of Ni-based metal organic frameworks (Ni-MOFs) and their derivatives. Chemosphere 2022, 307, 135729. [Google Scholar] [CrossRef]
- Yu, H.; Tan, X.; Zhang, L.; Yang, H.; Zhu, P.; Yan, Z.; Gao, C.; Yu, J. Metal-organic framework-enabled surface state passivation integrating with single-nuclease-propelled multistage amplification for ultrasensitive lab-on-paper photoelectrochemical biosensing. Chem. Eng. J. 2022, 450, 137955. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Zhinzhilo, V.A.; Bryantseva, J.D.; Uflyand, I.E.; Kharisov, B.I. ZrIV metal–organic framework based on terephthalic acid and 1,10-phenanthroline as an adsorbent for solid phase extraction of tetracycline antibiotics. Mendeleev Commun. 2022, 32, 661–663. [Google Scholar] [CrossRef]
- Kotova, A.A.; Thiebaut, D.; Vial, J.; Tissot, A.; Serre, C. Metal-organic frameworks as stationary phases for chromatography and solid phase extraction: A review. Coord. Chem. Rev. 2022, 455, 214364. [Google Scholar] [CrossRef]
- Qin, P.; Chen, D.; Li, D.; Li, M.; Mu, M.; Gao, Y.; Zhu, S.; Lu, M. Synthesis of spindle-like amino-modified Zn/Fe bimetallic metal-organic frameworks as sorbents for dispersive solid-phase extraction and preconcentration of phytohormoes in vegetable samples. Food Chem. 2023, 409, 135272. [Google Scholar] [CrossRef]
- Abbasalizadeh, A.; Sorouraddin, S.M.; Farajzadeh, M.A.; Nemati, M.; Mogaddam, M.R.A. Dispersive solid phase extraction of several pesticides from fruit juices using a hydrophobic metal organic framework prior to HPLC-MS/MS determination. J. Food Compos. Anal. 2022, 114, 104788. [Google Scholar] [CrossRef]
- Yeganeh, M.; Farzadkia, M.; Jafari, A.J.; Sobhi, H.R.; Esrafili, A.; Gholami, M. Application of a magnetic solid-phase extraction method using a novel magnetic metal organic framework nanocomposite for extraction of malathion and diazinon pesticides from environmental water samples. Microchem. J. 2022, 183, 108082. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Wang, X.; Wang, H.; Zhao, J.; Zhou, Z.; Du, X.; Lu, X. In situ anchor of multi-walled carbon nanotubes into iron-based metal-organic frameworks for enhanced adsorption of polycyclic aromatic hydrocarbons by magnetic solid-phase extraction. J. Chromatogr. A 2022, 1681, 463459. [Google Scholar] [CrossRef]
- Zhang, S.; Rong, F.; Guo, C.; Duan, F.; He, L.; Wang, M.; Zhang, Z.; Kang, M.; Du, M. Metal–organic frameworks (MOFs) based electrochemical biosensors for early cancer diagnosis in vitro. Coord. Chem. Rev. 2021, 439, 213948. [Google Scholar] [CrossRef]
- Nangare, S.N.; Sangale, P.M.; Patil, A.G.; Boddu, S.H.; Deshmukh, P.K.; Jadhav, N.R.; Tade, R.S.; Patil, D.R.; Pandey, A.; Mutalik, S.; et al. Surface architectured metal organic frameworks-based biosensor for ultrasensitive detection of uric acid: Recent advancement and future perspectives. Microchem. J. 2021, 169, 106567. [Google Scholar] [CrossRef]
- Hou, J.; Wan, J.; Yan, Z.; Wang, Y.; Ma, Y.; Xie, Y.; Chen, H.; Xue, Y. A novel polydopamine-modified metal organic frameworks catalyst with enhanced catalytic performance for efficient degradation of sulfamethoxazole in wastewater. Chemosphere 2022, 297, 134100. [Google Scholar] [CrossRef]
- Feng, H.; Liu, X.; Li, Y.; Ma, X.; Yan, Q.; Zhao, F. Novel powder catalysts of ferrocene-based metal-organic framework and their catalytic performance for thermal decomposition of ammonium perchlorate. Powder Technol. 2021, 397, 117035. [Google Scholar] [CrossRef]
- Liang, X.; Ji, S.; Chen, Y.; Wang, D. Synthetic strategies for MOF-based single-atom catalysts for photo- and electro-catalytic CO2 reduction. iScience 2022, 25, 104177. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Huang, Z. Structure and function tailored metal-organic frameworks for heterogeneous catalysis. Chem. Catal. 2022, 2, 3304–3319. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Bryliakov, K.P. Asymmetric catalysis using metal-organic frameworks. Coord. Chem. Rev. 2021, 437, 213845. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Q. Metal-Organic Framework Composites for Catalysis. Matter 2019, 1, 57–89. [Google Scholar] [CrossRef]
- Jiao, L.; Jiang, H.-L. Metal-organic frameworks for catalysis: Fundamentals and future prospects. Chin. J. Catal. 2023, 45, 1–5. [Google Scholar] [CrossRef]
- Panahi, Z.; Custer, L.; Halpern, J.M. Recent advances in non-enzymatic electrochemical detection of hydrophobic metabolites in biofluids. Sens. Actuators Rep. 2021, 3, 100051. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Hou, M.; Li, X.; Wu, X.; Ge, J. Immobilization on Metal–Organic Framework Engenders High Sensitivity for Enzymatic Electrochemical Detection. ACS Appl. Mater. Interfaces 2017, 9, 13831–13836. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhao, Y.; Ge, J. Impact of the size effect on enzymatic electrochemical detection based on metal-organic frameworks. Anal. Chim. Acta 2021, 1149, 238191. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, D.; Song, N.; Wang, C.; Lu, X. Promoting non-enzymatic electrochemical sensing performance toward glucose by the integration of conducting polypyrrole with metal-organic framework. Compos. Commun. 2022, 30, 101074. [Google Scholar] [CrossRef]
- Mahmood, A.; Guo, W.; Tabassum, H.; Zou, R. Metal-Organic Framework-Based Nanomaterials for Electrocatalysis. Adv. Energy Mater. 2016, 6, 1600423. [Google Scholar] [CrossRef]
- Lopa, N.S.; Rahman, M.; Ahmed, F.; Sutradhar, S.C.; Ryu, T.; Kim, W. A base-stable metal-organic framework for sensitive and non-enzymatic electrochemical detection of hydrogen peroxide. Electrochim. Acta 2018, 274, 49–56. [Google Scholar] [CrossRef]
- Du, Q.; Liao, Y.; Shi, N.; Sun, S.; Liao, X.; Yin, G.; Huang, Z.; Pu, X.; Wang, J. Facile synthesis of bimetallic metal–organic frameworks on nickel foam for a high performance non-enzymatic glucose sensor. J. Electroanal. Chem. 2022, 904, 115887. [Google Scholar] [CrossRef]
- Daud, A.; Lim, H.; Ibrahim, I.; Endot, N.; Gowthaman, N.; Jiang, Z.; Cordova, K.E. An effective metal-organic framework-based electrochemical non-enzymatic glucose sensor. J. Electroanal. Chem. 2022, 921, 116676. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Ma, J.; He, W.; Zhang, B.; Zhai, X.; Fu, Y. Hierarchical hollow CuO/Cu2O and Cu2O/Cu/C derived from metal–organic framework for non-enzymatic oxidation toward glucose. J. Mol. Liq. 2023, 375, 121317. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, K.; Duan, J.; Zheng, S.; Jiang, J. The nickel phosphate rods derived from Ni-MOF with enhanced electrochemical activity for non-enzymatic glucose sensing. Talanta 2022, 247, 123587. [Google Scholar] [CrossRef]
- Kachouei, M.A.; Shahrokhian, S.; Ezzati, M. Bimetallic CoZn-MOFs easily derived from CoZn-LDHs, as a suitable platform in fabrication of a non-enzymatic electrochemical sensor for detecting glucose in human fluids. Sens. Actuators B Chem. 2021, 344, 130254. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.; Lee, H.-N.; Park, Y.M.; Bae, Y.-S.; Kim, H.-J. Electrochemically derived CuO nanorod from copper-based metal-organic framework for non-enzymatic detection of glucose. Appl. Surf. Sci. 2019, 479, 720–726. [Google Scholar] [CrossRef]
- Kim, S.E.; Muthurasu, A. Metal-organic framework–assisted bimetallic Ni@Cu microsphere for enzyme-free electrochemical sensing of glucose. J. Electroanal. Chem. 2020, 873, 114356. [Google Scholar] [CrossRef]
- Vignesh, A.; Vajeeston, P.; Pannipara, M.; Al-Sehemi, A.G.; Xia, Y.; Kumar, G.G. Bimetallic metal-organic framework derived 3D hierarchical NiO/Co3O4/C hollow microspheres on biodegradable garbage bag for sensitive, selective, and flexible enzyme-free electrochemical glucose detection. Chem. Eng. J. 2021, 430, 133157. [Google Scholar] [CrossRef]
- Gokila, N.; Muthumalai, K.; Haldorai, Y.; Kumar, R.T.R. Electrochemical Non-enzymatic sensor based on Co-H2ABDC Metal Organic Framework for detection of glyphosate. Chem. Phys. Lett. 2022, 795, 139481. [Google Scholar] [CrossRef]
- Janjani, P.; Bhardwaj, U.; Gupta, R.; Kushwaha, H.S. Bimetallic Mn/Fe MOF modified screen-printed electrodes for non-enzymatic electrochemical sensing of organophosphate. Anal. Chim. Acta 2022, 1202, 339676. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; He, Y.; Gao, J.; Li, W.; Cheng, L.; Sun, F.; Xia, P.; Wang, Q. A generic and non-enzymatic electrochemical biosensor integrated molecular beacon-like catalyzed hairpin assembly circuit with MOF@Au@G-triplex/hemin nanozyme for ultrasensitive detection of miR-721. Biosens. Bioelectron. 2022, 203, 114051. [Google Scholar] [CrossRef]
- Golsheikh, A.M.; Yeap, G.-Y.; Yam, F.K.; Lim, H.S. Facile fabrication and enhanced properties of copper-based metal organic framework incorporated with graphene for non-enzymatic detection of hydrogen peroxide. Synth. Met. 2019, 260, 116272. [Google Scholar] [CrossRef]
- Xia, L.; Luan, X.; Qu, F.; Lu, L. Co-MOF/titanium nanosheet array: An excellent electrocatalyst for non-enzymatic detection of H2O2 released from living cells. J. Electroanal. Chem. 2020, 878, 114553. [Google Scholar] [CrossRef]
- Abrori, S.A.; Septiani, N.L.W.; Nugraha; Anshori, I.; Suyatman; Suendo, V.; Yuliarto, B. Metal-Organic-Framework FeBDC-Derived Fe3O4 for Non-Enzymatic Electrochemical Detection of Glucose. Sensors 2020, 20, 4891. [Google Scholar] [CrossRef]
- Song, S.; Ma, X.; Li, W.; Zhang, B.; Shao, B.; Chang, X.; Liu, X. Novel stylophora coral-like furan-based Ni/Co bimetallic metal organic framework for high-performance capacitive storage and non-enzymatic glucose electrochemical sensing. J. Alloys Compd. 2023, 931, 167413. [Google Scholar] [CrossRef]
- Du, Y.; Jia, X.; Zhong, L.; Jiao, Y.; Zhang, Z.; Wang, Z.; Feng, Y.; Bilal, M.; Cui, J.; Jia, S. Metal-organic frameworks with different dimensionalities: An ideal host platform for enzyme@MOF composites. Coord. Chem. Rev. 2021, 454, 214327. [Google Scholar] [CrossRef]
- Feng, C.-Y.; Wang, K.-H.; Li, S.; Liu, D.-S.; Yang, Z. Use of tyrosinase-inorganic salt hybrid nanoflowers and tyrosinase-MOF hybrid composites for elimination of phenolic pollutants from industrial wastewaters. Chemosphere 2023, 317, 137933. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, Z.; Ji, X.; Xue, Y.; Ren, B.; Zhao, H.; Huang, Y. Novel enzyme-metal-organic framework composite for efficient cadaverine production. Biochem. Eng. J. 2021, 176, 108222. [Google Scholar] [CrossRef]
- Feng, Y.; Cao, X.; Zhang, L.; Li, J.; Cui, S.; Bai, Y.; Chen, K.; Ge, J. Defect engineering of enzyme-embedded metal–organic frameworks for smart cargo release. Chem. Eng. J. 2022, 439, 135736. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, S. Controllable immobilization of enzymes in metal-organic frameworks for biocatalysis. Chem Catal. 2021, 1, 20–22. [Google Scholar] [CrossRef]
- Wu, H.; Li, T.; Bao, Y.; Zhang, X.; Wang, C.; Wei, C.; Xu, Z.; Tong, W.; Chen, D.; Huang, X. MOF-enzyme hybrid nanosystem decorated 3D hollow fiber membranes for in-situ blood separation and biosensing array. Biosens. Bioelectron. 2021, 190, 113413. [Google Scholar] [CrossRef]
- Wang, X.; Lan, P.C.; Ma, S. Metal–Organic Frameworks for Enzyme Immobilization: Beyond Host Matrix Materials. ACS Central Sci. 2020, 6, 1497–1506. [Google Scholar] [CrossRef]
- Xu, W.; Jiao, L.; Yan, H.; Wu, Y.; Chen, L.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Glucose Oxidase-Integrated Metal–Organic Framework Hybrids as Biomimetic Cascade Nanozymes for Ultrasensitive Glucose Biosensing. ACS Appl. Mater. Interfaces 2019, 11, 22096–22101. [Google Scholar] [CrossRef]
- Fu, X.; Ding, B.; D’Alessandro, D. Fabrication strategies for metal-organic framework electrochemical biosensors and their applications. Coord. Chem. Rev. 2023, 475, 214814. [Google Scholar] [CrossRef]
- Jangi, S.R.H.; Akhond, M. High throughput urease immobilization onto a new metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) for water treatment and safe blood cleaning. Process. Biochem. 2021, 105, 79–90. [Google Scholar] [CrossRef]
- Sohrabi, H.; Ghasemzadeh, S.; Ghoreishi, Z.; Majidi, M.R.; Yoon, Y.; Dizge, N.; Khataee, A. Metal-organic frameworks (MOF)-based sensors for detection of toxic gases: A review of current status and future prospects. Mater. Chem. Phys. 2023, 299, 127512. [Google Scholar] [CrossRef]
- Pang, S.; Wu, Y.; Zhang, X.; Li, B.; Ouyang, J.; Ding, M. Immobilization of laccase via adsorption onto bimodal mesoporous Zr-MOF. Process. Biochem. 2015, 51, 229–239. [Google Scholar] [CrossRef]
- Yuan, X.; Ou, J.; Zhang, P.; Xu, W.; Jiang, B.; Tang, K. PEG-modified lipase immobilized onto NH2-MIL-53 MOF for efficient resolution of 4-fluoromandelic acid enantiomers. Int. J. Biol. Macromol. 2020, 165, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Ulu, A. Metal–organic frameworks (MOFs): A novel support platform for ASNase immobilization. J. Mater. Sci. 2020, 55, 6130–6144. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Dong, F.; Li, Y.; Guo, Y.; Liu, X.; Xu, J.; Wu, X.; Zheng, Y. Ultrasensitive immunoassay for detection of zearalenone in agro-products using enzyme and antibody co-embedded zeolitic imidazolate framework as labels. J. Hazard. Mater. 2021, 412, 125276. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Fan, X.; Yu, S.; Li, X.; Wang, S.; Lu, L. Metal-organic frameworks (MOFs): A novel platform for laccase immobilization and application. J. Environ. Chem. Eng. 2022, 10, 125276. [Google Scholar] [CrossRef]
- Farahani, A.; Azimi, S.; Azimi, M. Developing an integrated POC spectrophotometric device for discrimination and determination of opioids based on gold nanoparticles. Microchem. J. 2022, 182, 107930. [Google Scholar] [CrossRef]
- Kaci, K.; del Caño, R.; Luna, M.; Milán-Rois, P.; Castellanos, M.; Abreu, M.; Cantón, R.; Galán, J.C.; Somoza, Á.; Miranda, R.; et al. Paving the way to point of care (POC) devices for SARS-CoV-2 detection. Talanta 2022, 247, 123542. [Google Scholar] [CrossRef]
- Bueno, L.; de Araujo, W.R.; Paixão, T.R.L.C. Point of Care (POC) Medical Biosensors for Cancer Detection. In Medical Biosensors for Point of Care (POC) Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 183–201. [Google Scholar]
- Zhang, W.; Bu, S.; Zhang, J.; Ma, L.; Liu, X.; Wang, X.; Li, Z.; Hao, Z.; Li, Z.; Wan, J. Point-of-care detection of pathogenic bacteria based on pregnancy test strips and metal–organic frameworks. Microchem. J. 2022, 175, 107142. [Google Scholar] [CrossRef]
- Gowri, A.; Kumar, N.A.; Anand, B.S. Recent advances in nanomaterials based biosensors for point of care (PoC) diagnosis of Covid-19—A minireview. TrAC Trends Anal. Chem. 2021, 137, 116205. [Google Scholar] [CrossRef]
- Chinnapaiyan, S.; Rajaji, U.; Chen, S.-M.; Liu, T.-Y.; Filho, J.I.D.O.; Chang, Y.-S. Fabrication of thulium metal–organic frameworks based smartphone sensor towards arsenical feed additive drug detection: Applicable in food safety analysis. Electrochim. Acta 2021, 401, 139487. [Google Scholar] [CrossRef]
- Nehra, M.; Kanika; Dilbaghi, N.; Kumar, R.; Kumar, S. Trends in point-of-care optical biosensors for antibiotics detection in aqueous media. Mater. Lett. 2021, 308, 131235. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, B. A point-of-care diagnostics logic detector based on glucose oxidase immobilized lanthanide functionalized metal–organic frameworks. Nanoscale 2019, 11, 22946–22953. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, J.; Zhang, M.; Shi, G. Lanthanide metal-organic framework as a paper strip sensor for visual detection of sulfonamide with smartphone-based point-of-care platform. Talanta 2021, 237, 122920. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Ma, Y.; Wang, X.; Zhang, J.; Bai, B.; Yu, L.; Guo, C.; Zhang, F.; Qin, S. A novel metal–organic frameworks composite-based label-free point-of-care quartz crystal microbalance aptasensing platform for tetracycline detection. Food Chem. 2022, 392, 133302. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Zhang, Y.; Xu, J. A bibliometric review on stability and reinforcement of special soil subgrade based on CiteSpace. J. Traffic Transp. Eng. 2022, 9, 223–243. [Google Scholar] [CrossRef]
- Wilks, D. Cluster Analysis. In International Geophysics; Elsevier: Amsterdam, The Netherlands, 2011; pp. 603–616. [Google Scholar] [CrossRef]
- Qureshi, A.M.I.; Sofi, M.U.; Dar, N.; Khan, M.; Mahdi, S.; Dar, Z.A.; Bangroo, S.; El-Serehy, H.A.; Hefft, D.I.; Popescu, S.M. Insilco identification and characterization of superoxide dismutase gene family in Brassica rapa. Saudi J. Biol. Sci. 2021, 28, 5526–5537. [Google Scholar] [CrossRef]
- Wang, T.-W.; Bu, S.; Wang, K.; Zhang, L.; Yi, Z.-X.; Zhu, S.-G.; Zhang, J.-G. Synthesis of energetic coordination polymers based on 4-nitropyrazole by solid-melt crystallization in non-ionization condition. Def. Technol. 2022, in press. [Google Scholar] [CrossRef]
- Liu, W.-L.; Wu, C.-Y.; Chen, C.-Y.; Singco, B.; Lin, C.-H.; Huang, H.-Y. Fast Multipoint Immobilized MOF Bioreactor. Chem. A Eur. J. 2014, 20, 8923–8928. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; Qu, X. Nano-Gold as Artificial Enzymes: Hidden Talents. Adv. Mater. 2014, 26, 4200–4217. [Google Scholar] [CrossRef]
- Hatano, M.; Ishihara, K. Conformationally flexible chiral supramolecular catalysts for enantioselective Diels–Alder reactions with anomalous endo/exo selectivities. Chem. Commun. 2012, 48, 4273–4283. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Mrozinski, J.; Bharadwaj, P.K. Solvent-Induced Carboxylate Shift and Movement of an Anthryl Side-Group in Single-Crystal to Single-Crystal Structural Dynamics in a Gadolinium Coordination Polymer. Cryst. Growth Des. 2014, 14, 3623–3633. [Google Scholar] [CrossRef]
- Yuan, H.; Rossetto, D.; Mellert, H.; Dang, W.; Srinivasan, M.; Johnson, J.; Hodawadekar, S.; Ding, E.C.; Speicher, K.; Abshiru, N.; et al. MYST protein acetyltransferase activity requires active site lysine autoacetylation. EMBO J. 2011, 31, 58–70. [Google Scholar] [CrossRef]
- Yang, C.; Mi, J.; Feng, Y.; Ngo, L.; Gao, T.; Yan, L.; Zheng, Y.G. Labeling Lysine Acetyltransferase Substrates with Engineered Enzymes and Functionalized Cofactor Surrogates. J. Am. Chem. Soc. 2013, 135, 7791–7794. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.J.; Bloch, E.D.; Mason, J.A.; Queen, W.; Hudson, M.R.; Planas, N.; Borycz, J.; Dzubak, A.; Verma, P.; Lee, K.; et al. Oxidation of ethane to ethanol by N2O in a metal–organic framework with coordinatively unsaturated iron(II) sites. Nat. Chem. 2014, 6, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Yin, H.; Xing, C.; Xu, C. A Sensitive DNAzyme-Based Chiral Sensor for Lead Detection. Materials 2013, 6, 5038–5046. [Google Scholar] [CrossRef]
- Zhou, Z.; Hartmann, M. Recent Progress in Biocatalysis with Enzymes Immobilized on Mesoporous Hosts. Top. Catal. 2012, 55, 1081–1100. [Google Scholar] [CrossRef]
- Ai, L.; Li, L.; Zhang, C.; Fu, J.; Jiang, J. MIL-53(Fe): A Metal-Organic Framework with Intrinsic Peroxidase-Like Catalytic Activity for Colorimetric Biosensing. Chem. A Eur. J. 2013, 19, 15105–15108. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J. Multicopper complexes and coordination polymers for mild oxidative functionalization of alkanes. Coord. Chem. Rev. 2012, 256, 2741–2759. [Google Scholar] [CrossRef]
- Mohan, B.; Kumar, S.; Xi, H.; Ma, S.; Tao, Z.; Xing, T.; You, H.; Zhang, Y.; Ren, P. Fabricated Metal-Organic Frameworks (MOFs) as luminescent and electrochemical biosensors for cancer biomarkers detection. Biosens. Bioelectron. 2021, 197, 113738. [Google Scholar] [CrossRef]
- Rabiee, N.; Fatahi, Y.; Ahmadi, S.; Abbariki, N.; Ojaghi, A.; Rabiee, M.; Radmanesh, F.; Dinarvand, R.; Bagherzadeh, M.; Mostafavi, E.; et al. Bioactive hybrid metal-organic framework (MOF)-based nanosensors for optical detection of recombinant SARS-CoV-2 spike antigen. Sci. Total. Environ. 2022, 825, 153902. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Sun, K.; Liu, L. Carbon-dots-referenced metal-organic frameworks for chemical sensing of tumor/mood biomarker 5-hydroxyindoleacetic acid in human urine: Covalent grafting blue-emitting carbon dots onto red-emitting MOF. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122244. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.; Singh, G.; Pombeiro, A.J.; Solovev, A.A.; Sharma, P.K.; Chen, Q. Metal-organic frameworks (MOFs) for milk safety and contaminants monitoring. TrAC Trends Anal. Chem. 2023, 159, 116921. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Jiao, T.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based sensors for the detection of food contaminants. TrAC Trends Anal. Chem. 2022, 154, 116642. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Eshaghi, M.M.; Ostovar, S.; Shamsabadipour, A.; Safakhah, S.; Mousavi, M.S.; Rahdar, A.; Pandey, S. UiO-66 metal-organic framework nanoparticles as gifted MOFs to the biomedical application: A comprehensive review. J. Drug Deliv. Sci. Technol. 2022, 76, 103758. [Google Scholar] [CrossRef]
- Rezaee, T.; Fazel-Zarandi, R.; Karimi, A.; Ensafi, A.A. Metal-organic frameworks for pharmaceutical and biomedical applications. J. Pharm. Biomed. Anal. 2022, 221, 115026. [Google Scholar] [CrossRef]
- Aza, P.; Molpeceres, G.; Vind, J.; Camarero, S. Multicopper oxidases with laccase-ferroxidase activity: Classification and study of ferroxidase activity determinants in a member from Heterobasidion annosum s. l. Comput. Struct. Biotechnol. J. 2023, 21, 1041–1053. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Tao, C.; Ding, R. Research hotspots and trends of cardiopulmonary exercise test: Visualization analysis based on citespace. Med. Nov. Technol. Devices 2022, 16, 100191. [Google Scholar] [CrossRef]
- Goud, B.S.; Shin, G.; Vattikuti, S.P.; Mameda, N.; Kim, H.; Koyyada, G.; Kim, J.H. Enzyme-integrated biomimetic cobalt metal-organic framework nanozyme for one-step cascade glucose biosensing via tandem catalysis. Biochem. Eng. J. 2022, 188, 108669. [Google Scholar] [CrossRef]
- Ouyang, Y.; O’Hagan, M.P.; Willner, I. Functional catalytic nanoparticles (nanozymes) for sensing. Biosens. Bioelectron. 2022, 218, 114768. [Google Scholar] [CrossRef]
- Zhong, N.; Gao, R.; Shen, Y.; Kou, X.; Wu, J.; Huang, S.; Chen, G.; Ouyang, G. Enzymes-Encapsulated Defective Metal–Organic Framework Hydrogel Coupling with a Smartphone for a Portable Glucose Biosensor. Anal. Chem. 2022, 94, 14385–14393. [Google Scholar] [CrossRef]
- Adeel, M.; Asif, K.; Rahman, M.; Daniele, S.; Canzonieri, V.; Rizzolio, F. Glucose Detection Devices and Methods Based on Metal–Organic Frameworks and Related Materials. Adv. Funct. Mater. 2021, 31, 2106023. [Google Scholar] [CrossRef]
- Singh, R.; Musameh, M.; Gao, Y.; Ozcelik, B.; Mulet, X.; Doherty, C.M. Stable MOF@enzyme composites for electrochemical biosensing devices. J. Mater. Chem. C 2021, 9, 7677–7688. [Google Scholar] [CrossRef]
- Cheng, X.; Zheng, Z.; Zhou, X.; Kuang, Q. Metal–Organic Framework as a Compartmentalized Integrated Nanozyme Reactor to Enable High-Performance Cascade Reactions for Glucose Detection. ACS Sustain. Chem. Eng. 2020, 8, 17783–17790. [Google Scholar] [CrossRef]
- Koua, X.; Tonga, L.; Shena, Y.; Zhub, W.; Yina, L.; Huangb, S.; Zhua, F.; Chena, G.; Ouyanga, G. Smartphone-assisted robust enzymes@MOFs-based paper biosensor for point-of-care detection. Biosens. Bioelectron. 2020, 156, 112095. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, X.-Z.; Gu, H.-W.; Yi, H.-C.; Sun, W.-Y. A dual-response biosensor for electrochemical and glucometer detection of DNA methyltransferase activity based on functionalized metal-organic framework amplification. Biosens. Bioelectron. 2019, 134, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, L.; Dai, H.; Li, Z.; Fu, Y.; Li, Y. Biomineralization-mimetic preparation of robust metal-organic frameworks biocomposites film with high enzyme load for electrochemical biosensing. J. Electroanal. Chem. 2018, 823, 40–46. [Google Scholar] [CrossRef]
- Liu, X.; Qi, W.; Wang, Y.; Su, R.; He, Z. A facile strategy for enzyme immobilization with highly stable hierarchically porous metal–organic frameworks. Nanoscale 2017, 9, 17561–17570. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, C.; Zhang, Y.; He, F.; Liu, M.; Li, X. Preparation of graphene nano-sheet bonded PDA/MOF microcapsules with immobilized glucose oxidase as a mimetic multi-enzyme system for electrochemical sensing of glucose. J. Mater. Chem. B 2016, 4, 3695–3702. [Google Scholar] [CrossRef]
- Patra, S.; Crespo, T.H.; Permyakova, A.; Sicard, C.; Serre, C.; Chaussé, A.; Steunou, N.; Legrand, L. Design of metal organic framework–enzyme based bioelectrodes as a novel and highly sensitive biosensing platform. J. Mater. Chem. B 2015, 3, 8983–8992. [Google Scholar] [CrossRef]
- Ilacas, G.C.; Basa, A.; Nelms, K.J.; Sosa, J.D.; Liu, Y.; Gomez, F.A. Paper-based microfluidic devices for glucose assays employing a metal-organic framework (MOF). Anal. Chim. Acta 2019, 1055, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, W.; Nguyen, W.; Chen, W.; Wang, J.; Chen, M. Advances of metal organic frameworks in analytical applications. Mater. Today Adv. 2022, 15, 100273. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Osazuwa, O.U.; Elimian, E.A.; Eshiemogie, S.O.; Oyefolu, P.K.; Kusuma, H.S. A comprehensive review of recent advances in the synthesis and application of metal-organic frameworks (MOFs) for the adsorptive sequestration of pollutants from wastewater. Sep. Purif. Technol. 2023, 311, 123246. [Google Scholar] [CrossRef]
- Yan, C.; Jin, J.; Wang, J.; Zhang, F.; Tian, Y.; Liu, C.; Zhang, F.; Cao, L.; Zhou, Y.; Han, Q. Metal–organic frameworks (MOFs) for the efficient removal of contaminants from water: Underlying mechanisms, recent advances, challenges, and future prospects. Coord. Chem. Rev. 2022, 468, 214595. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Adegboyega, S.A.; Giwa, A.-R.A. Remediation potentials of composite metal-organic frameworks (MOFs) for dyes as water contaminants: A comprehensive review of recent literatures. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100568. [Google Scholar] [CrossRef]
- Shamim, M.A.; Zia, H.; Zeeshan, M.; Khan, M.Y.; Shahid, M. Metal organic frameworks (MOFs) as a cutting-edge tool for the selective detection and rapid removal of heavy metal ions from water: Recent progress. J. Environ. Chem. Eng. 2021, 10, 106991. [Google Scholar] [CrossRef]
- Biswal, L.; Chatterjee, S. Metal Organic Frameworks (MOFs) in Aiding Water Purification from Emerging and Ionic Contam-inants. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 651–668. [Google Scholar]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Shivam; Megha, R.; Lakhani, V.; Vala, S.; Dharaskar, S.; Paluvai, N.R.; Sinha, M.K.; Jampa, S.S. Removal of heavy metals and dyes from its aqueous solution utilizing metal organic Frameworks (MOFs): Review. Mater. Today Proc. 2023, 77, 188–200. [Google Scholar] [CrossRef]
- Poonia, K.; Patial, S.; Raizada, P.; Ahamad, T.; Khan, A.A.P.; Van Le, Q.; Nguyen, V.-H.; Hussain, C.M.; Singh, P. Recent advances in Metal Organic Framework (MOF)-based hierarchical composites for water treatment by adsorptional photocatalysis: A review. Environ. Res. 2023, 222, 115349. [Google Scholar] [CrossRef]
- Gouda, S.P.; Dhakshinamoorthy, A.; Rokhum, S.L. Metal-organic framework as a heterogeneous catalyst for biodiesel production: A review. Chem. Eng. J. Adv. 2022, 12, 100415. [Google Scholar] [CrossRef]


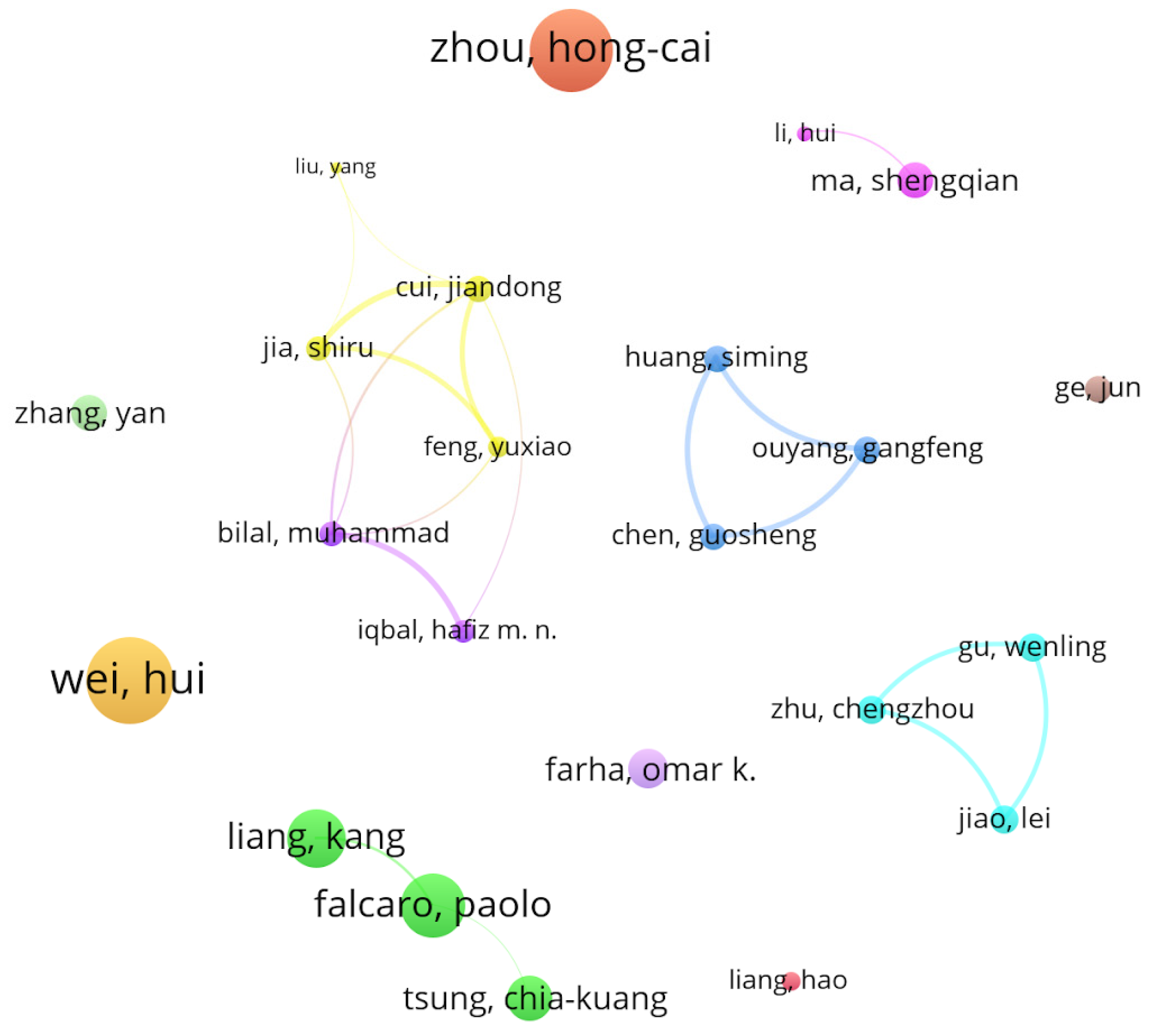
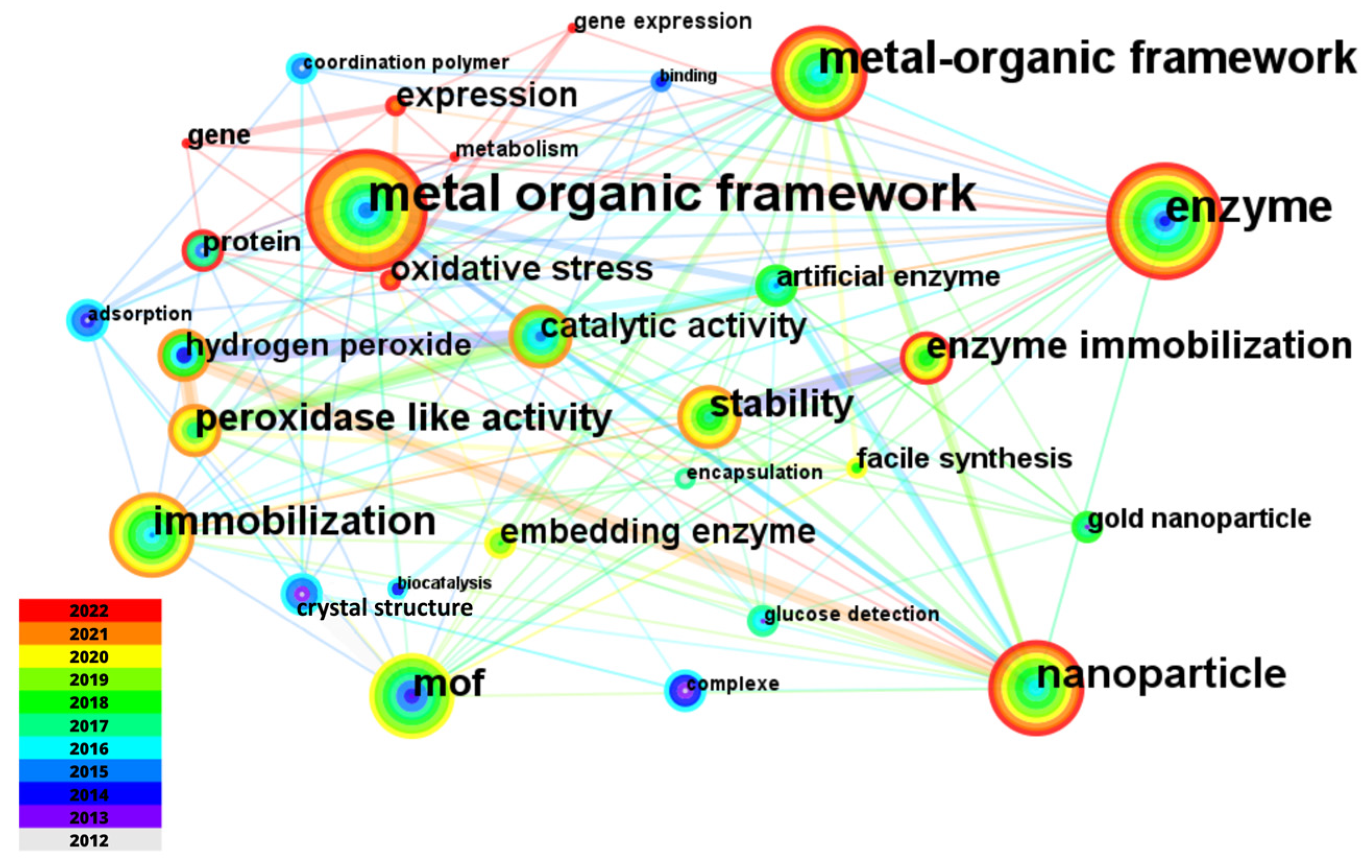
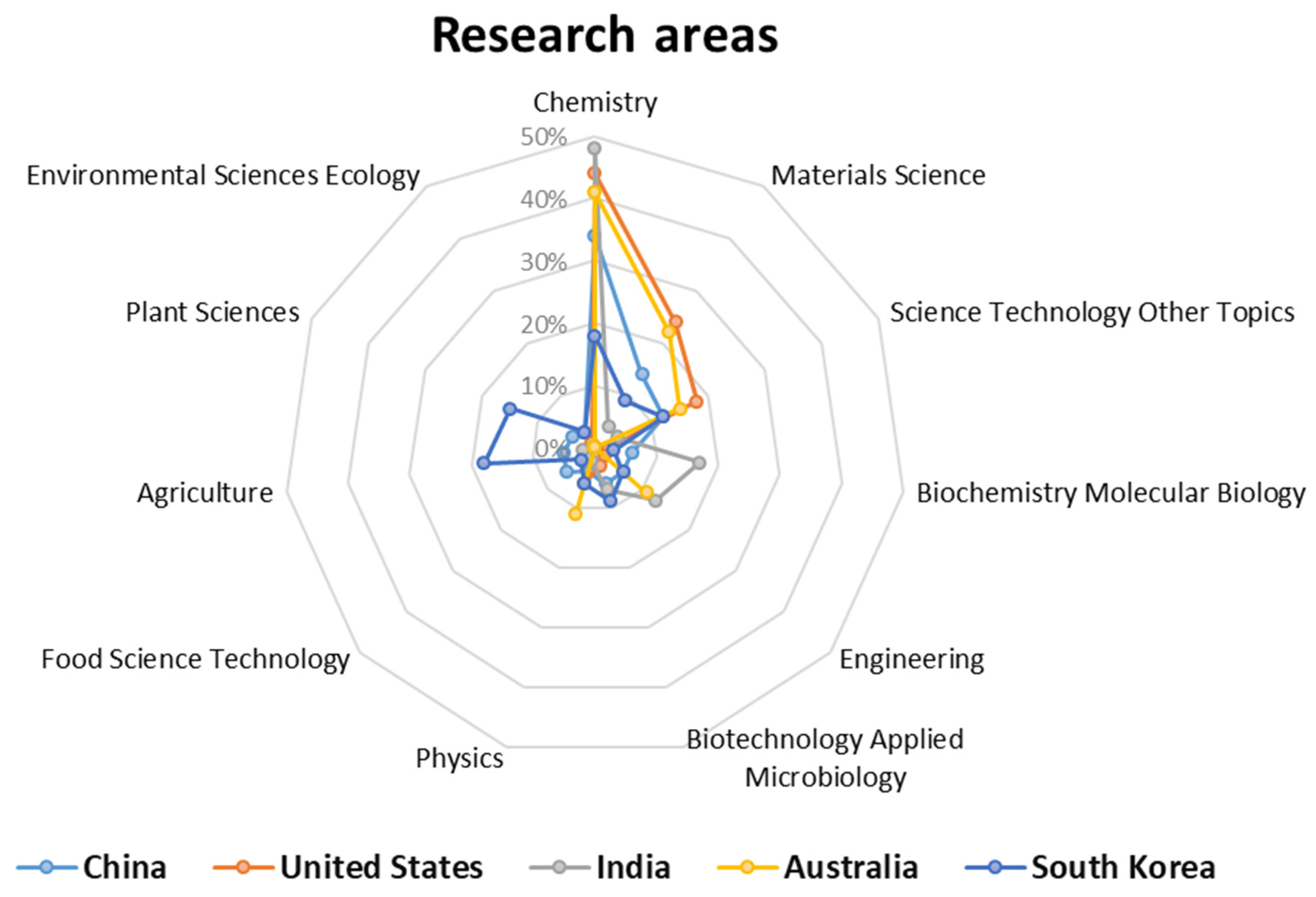

| Rank | Journal | C | IF | NP | NC | AC | P |
|---|---|---|---|---|---|---|---|
| 1 | Chemical Society Reviews | ENG | 60.615 | 14 | 3564 | 254.571 | 1.14% |
| 2 | Angewandte Chemie-International Edition | GER | 16.823 | 30 | 3231 | 107.700 | 2.45% |
| 3 | Journal of the American Chemical Society | USA | 16.383 | 33 | 3209 | 97.242 | 2.70% |
| 4 | ACS Applied Materials & Interfaces | USA | 10.383 | 54 | 1787 | 33.092 | 4.42% |
| 5 | Advanced Materials | GER | 32.086 | 12 | 1519 | 126.583 | 0.98% |
| 6 | Coordination Chemistry Reviews | NL | 24.833 | 22 | 1476 | 67.090 | 1.80% |
| 7 | Nature Communications | ENG | 17.694 | 11 | 1436 | 130.545 | 0.90% |
| 8 | Chemical Communications | ENG | 6.065 | 18 | 969 | 53.833 | 1.45% |
| 9 | RSC Advances | ENG | 4.036 | 14 | 807 | 57.642 | 1.14% |
| 10 | Analytical Chemistry | USA | 8.008 | 16 | 755 | 47.187 | 1.31% |
| Rank | Country | NP | NC | AC | TLS | P |
|---|---|---|---|---|---|---|
| 1 | People’s Republic of China | 831 | 25,877 | 31.139 | 4205 | 51.87% |
| 2 | USA | 159 | 12,665 | 79.654 | 2833 | 9.92% |
| 3 | India | 57 | 1304 | 22.877 | 573 | 3.55% |
| 4 | Australia | 53 | 3206 | 60.490 | 1483 | 3.30% |
| 5 | South Korea | 50 | 1440 | 28.800 | 321 | 3.12% |
| 6 | Iran | 31 | 650 | 20.967 | 280 | 1.93% |
| 7 | Germany | 29 | 1143 | 39.413 | 242 | 1.81% |
| 8 | Spain | 25 | 635 | 25.400 | 247 | 1.56% |
| 9 | Brazil | 23 | 507 | 22.043 | 188 | 1.43% |
| 10 | Taiwan | 21 | 1891 | 90.047 | 708 | 1.31% |
| Rank | Papers | Authors | Year Published | Citation |
|---|---|---|---|---|
| 1 | Nanomaterials with Enzyme-Like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II) [55] | Wu, Jiangjiexing; Wang, Xiaoyu; Wang, Quan; Lou, Zhangping; Li, Sirong; Zhu, Yunyao; Qin, Li; Wei, Hui | 2019 | 1651 |
| 2 | Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal-Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts [56] | Feng, Dawei; Gu, Zhi-Yuan; Li, Jian-Rong; Jiang, Hai-Long; Wei, Zhangwen; Zhou, Hong-Cai | 2012 | 1187 |
| 3 | Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications [63] | Huang, Yanyan; Ren, Jinsong; Qu, Xiaogang | 2019 | 992 |
| 4 | Cooperative Insertion of CO2 in Diamine-Appended Metal-Organic Frameworks [64] | McDonald, Thomas M.; Mason, Jarad A.; Kong, Xueqian; Bloch, Eric D.; Gygi, David; Dani, Alessandro; Crocella, Valentina; Giordanino, Filippo; Odoh, Samuel O.; Drisdell, Walter S.; Vlaisavljevich, Bess; Dzubak, Allison L.; Poloni, Roberta; Schnell, Sondre K.; Planas, Nora; Lee, Kyuho; Pascal, Tod; Wan, Liwen F.; Prendergast, David; Neaton, Jeffrey B.; Smit, Berend; Kortright, Jeffrey B.; Gagliardi, Laura; Bordiga, Silvia; Reimer, Jeffrey A.; Long, Jeffrey R. | 2015 | 778 |
| 5 | Biomimetic Mineralization of Metal-Organic Frameworks as Protective Coatings for Biomacromolecules [65] | Liang, Kang; Ricco, Raffaele; Doherty, Cara M.; Styles, Mark J.; Bell, Stephen; Kirby, Nigel; Mudie, Stephen; Haylock, David; Hill, Anita J.; Doonan, Christian J.; Falcaro, Paolo | 2015 | 754 |
| 6 | Enzyme–MOF (Metal-Organic Framework) Composites [66] | Lian, Xizhen; Fang, Yu; Joseph, Elizabeth; Wang, Qi; Li, Jialuo; Banerjee, Sayan; Lollar, Christina; Wang, Xuan; Zhou, Hong-Cai | 2017 | 703 |
| 7 | Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by a de Novo Approach: Size-Selective Sheltering of Catalase in Metal-Organic Framework Microcrystals [67] | Shieh, Fa-Kuen; Wang, Shao-Chun; Yen, Chia-I; Wu, Chang-Cheng; Dutta, Saikat; Chou, Lien-Yan; Morabito, Joseph V.; Hu, Pan; Hsu, Ming-Hua; Wu, Kevin C. -W.; Tsung, Chia-Kuang | 2015 | 550 |
| 8 | Nanozyme Decorated Metal-Organic Frameworks for Enhanced Photodynamic Therapy [68] | Zhang, Yan; Wang, Faming; Liu, Chaoqun; Wang, Zhenzhen; Kang, LiHua; Huang, Yanyan; Dong, Kai; Ren, Jinsong; Qu, Xiaogang | 2018 | 446 |
| 9 | Stable Metal-Organic Frameworks Containing Single-Molecule Traps for Enzyme Encapsulation [69] | Feng, Dawei; Liu, Tian-Fu; Su, Jie; Bosch, Mathieu; Wei, Zhangwen; Wan, Wei; Yuan, Daqiang; Chen, Ying-Pin; Wang, Xuan; Wang, Kecheng; Lian, Xizhen; Gu, Zhi-Yuan; Park, Jihye; Zou, Xiaodong; Zhou, Hong-Cai | 2015 | 410 |
| 10 | Nanozymes in Bionanotechnology: from Sensing to Therapeutics and Beyond [70] | Wang, Xiaoyu; Hu, Yihui; Wei, Hui | 2016 | 408 |
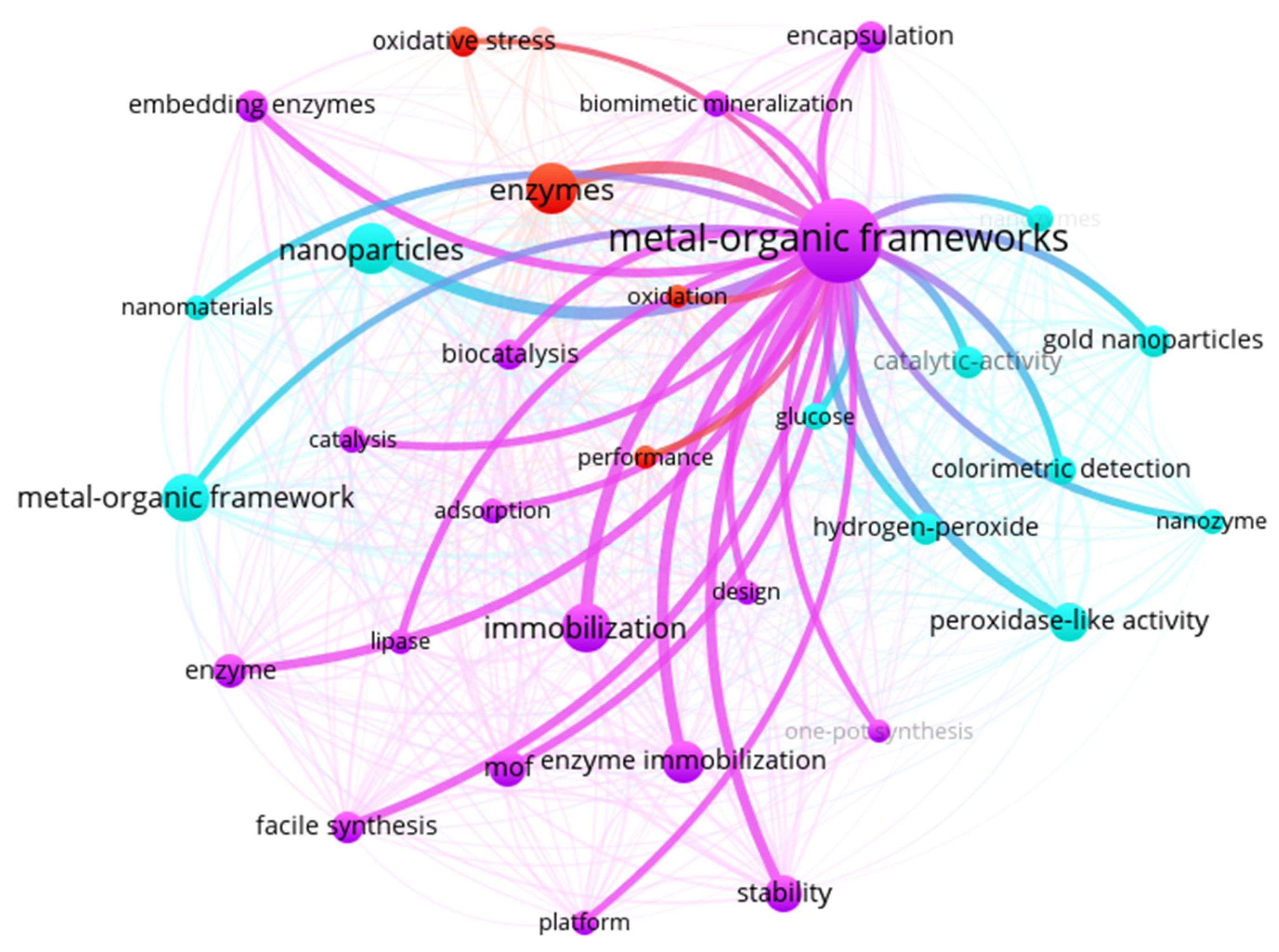
| Rank | Keyword | Frequency | TLS | Rank | Keyword | Frequency | TLS |
|---|---|---|---|---|---|---|---|
| 1 | Metal-Organic Frameworks | 430 | 1066 | 13 | Catalytic-activity | 77 | 297 |
| 2 | Nanoparticles | 185 | 553 | 14 | Hydrogen-peroxide | 77 | 252 |
| 3 | Enzymes | 178 | 431 | 15 | Encapsulation | 74 | 289 |
| 4 | Immobilization | 172 | 585 | 16 | Facile synthesis | 73 | 285 |
| 5 | Metal-Organic Framework | 155 | 392 | 17 | Biocatalysis | 68 | 260 |
| 6 | Enzyme immobilization | 129 | 417 | 18 | Oxidative stress | 66 | 48 |
| 7 | Peroxidase-like activity | 110 | 412 | 19 | Colorimetric detection | 65 | 270 |
| 8 | Stability | 104 | 377 | 20 | Expression | 63 | 38 |
| 9 | MOF | 103 | 337 | 21 | Biomimetic mineralization | 56 | 220 |
| 10 | Enzyme | 87 | 282 | 22 | Glucose | 55 | 194 |
| 11 | Embedding enzymes | 79 | 244 | 23 | Catalysis | 54 | 163 |
| 12 | Gold nanoparticles | 78 | 245 | 24 | Nanozymes | 54 | 191 |
| CID | Label | NS | Mean | Top Five Terms | Representative Papers |
|---|---|---|---|---|---|
| #0 | metal-organic framework | 32 | 2014 | metal-organic framework; ascorbic acid; tandem catalysis; sensitive detection; oxidase-like activity | [153,154] |
| #1 | exo selectivities | 19 | 2012 | exo selectivities; enantioselective diels-alder reaction; flexible chiral supramolecular catalyst; anthryl side-group; single-crystal structural dynamics | [155,156] |
| #2 | metal-organic framework | 18 | 2012 | metal-organic framework; myst protein acetyltransferase activity; active site lysine autoacetylation; cofactor surrogate; labeling lysine acetyltransferase substrate | [157,158] |
| #3 | signaling pathway | 17 | 2014 | signaling pathway; metal-organic framework; antioxidant capacity; large yellow croaker; lipid metabolism | [159,160] |
| #4 | metal-organic framework | 17 | 2012 | metal-organic framework; intrinsic peroxidase-like catalytic activity; pH-responsive drug delivery; coordination polymer; prepared using metal-organic framework template | [161,162] |
| #5 | multicopper complex | 16 | 2012 | multicopper complex; mild oxidative functionalization; coordination polymer; alkane; cooperative insertion | [64,163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, M.B.; Neto, J.G.L.; De Sousa Braz, A.K.; De Sousa Junior, P.G.; Melo, R.L.F.; Valério, R.B.R.; Serpa, J.d.F.; Da Silva Lima, A.M.; De Lima, R.K.C.; Guimarães, A.P.; et al. Trends and Opportunities in Enzyme Biosensors Coupled to Metal-Organic Frameworks (MOFs): An Advanced Bibliometric Analysis. Electrochem 2023, 4, 181-211. https://doi.org/10.3390/electrochem4020014
Sales MB, Neto JGL, De Sousa Braz AK, De Sousa Junior PG, Melo RLF, Valério RBR, Serpa JdF, Da Silva Lima AM, De Lima RKC, Guimarães AP, et al. Trends and Opportunities in Enzyme Biosensors Coupled to Metal-Organic Frameworks (MOFs): An Advanced Bibliometric Analysis. Electrochem. 2023; 4(2):181-211. https://doi.org/10.3390/electrochem4020014
Chicago/Turabian StyleSales, Misael Bessa, José Gadelha Lima Neto, Ana Kátia De Sousa Braz, Paulo Gonçalves De Sousa Junior, Rafael Leandro Fernandes Melo, Roberta Bussons Rodrigues Valério, Juliana de França Serpa, Ana Michele Da Silva Lima, Rita Karolinny Chaves De Lima, Artemis Pessoa Guimarães, and et al. 2023. "Trends and Opportunities in Enzyme Biosensors Coupled to Metal-Organic Frameworks (MOFs): An Advanced Bibliometric Analysis" Electrochem 4, no. 2: 181-211. https://doi.org/10.3390/electrochem4020014
APA StyleSales, M. B., Neto, J. G. L., De Sousa Braz, A. K., De Sousa Junior, P. G., Melo, R. L. F., Valério, R. B. R., Serpa, J. d. F., Da Silva Lima, A. M., De Lima, R. K. C., Guimarães, A. P., de Souza, M. C. M., Lopes, A. A. S., Rios, M. A. d. S., Serafim, L. F., & dos Santos, J. C. S. (2023). Trends and Opportunities in Enzyme Biosensors Coupled to Metal-Organic Frameworks (MOFs): An Advanced Bibliometric Analysis. Electrochem, 4(2), 181-211. https://doi.org/10.3390/electrochem4020014









