Review on Electrode Degradation at Fast Charging of Li-Ion and Li Metal Batteries from a Kinetic Perspective
Abstract
1. Introduction
2. Battery Charging Kinetics
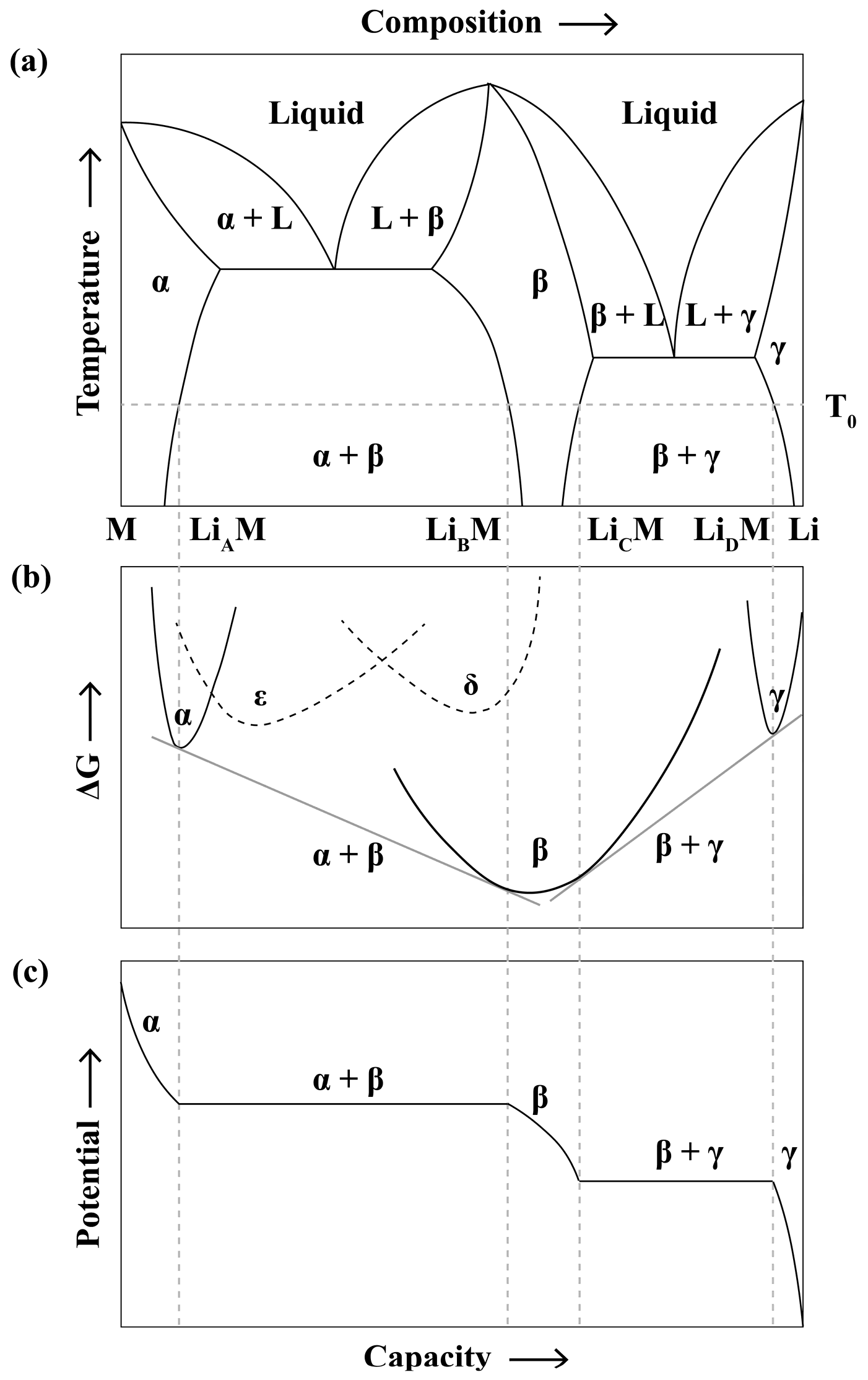
2.1. A Simplified Kinetic Model of Battery Charging and Its Limitations
2.2. Rate-Limiting Steps in Electrode Charging from a Materials Perspective
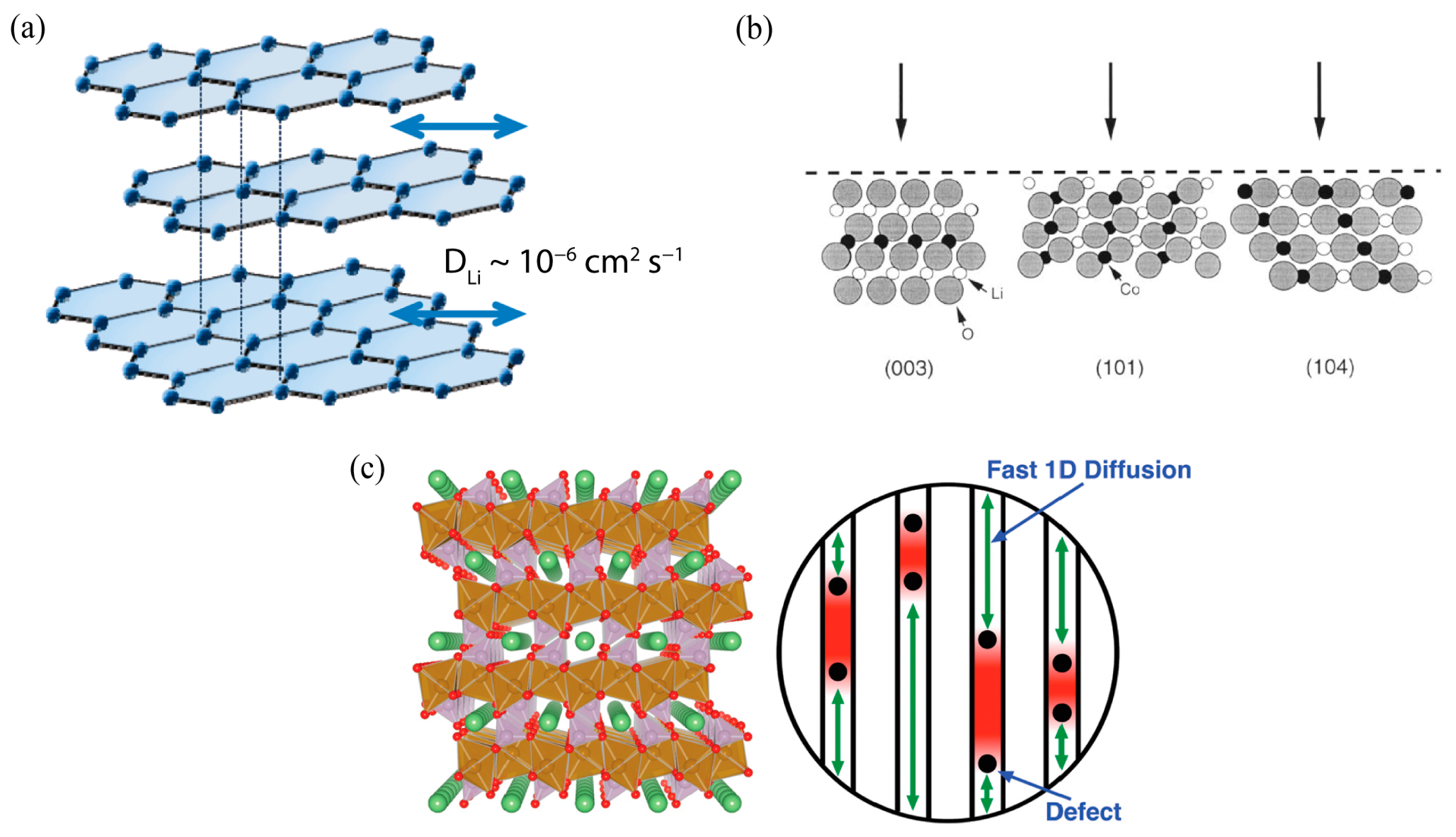
2.2.1. Intrinsic Properties of Electrode Active Materials
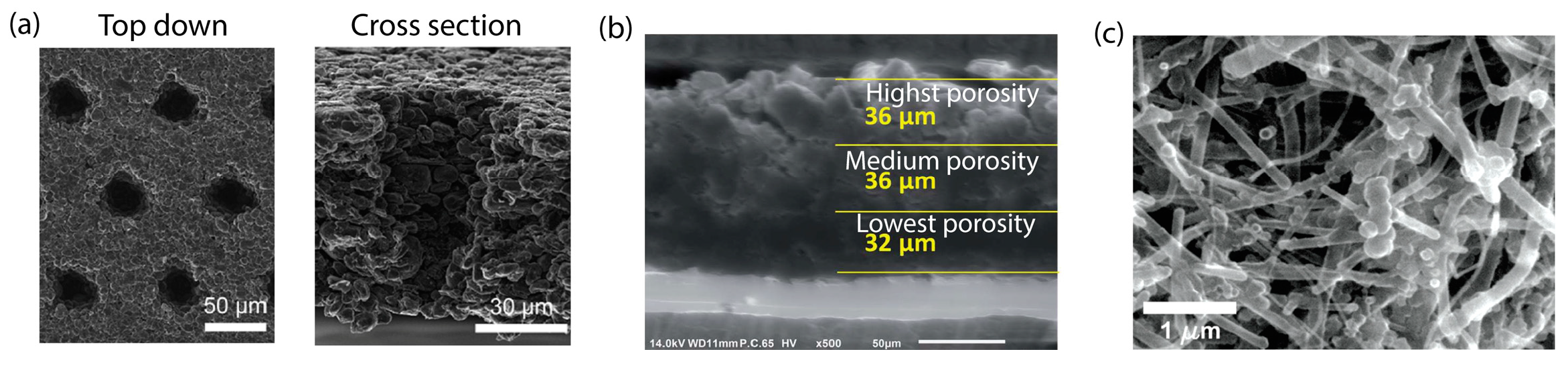
2.2.2. Microstructures of Electrodes
3. Electrode Degradation Mechanisms under Fast Charging
3.1. Effects of Fast Charging
3.1.1. Overpotentials
3.1.2. Spatial Inhomogeneity on the Electrode Level
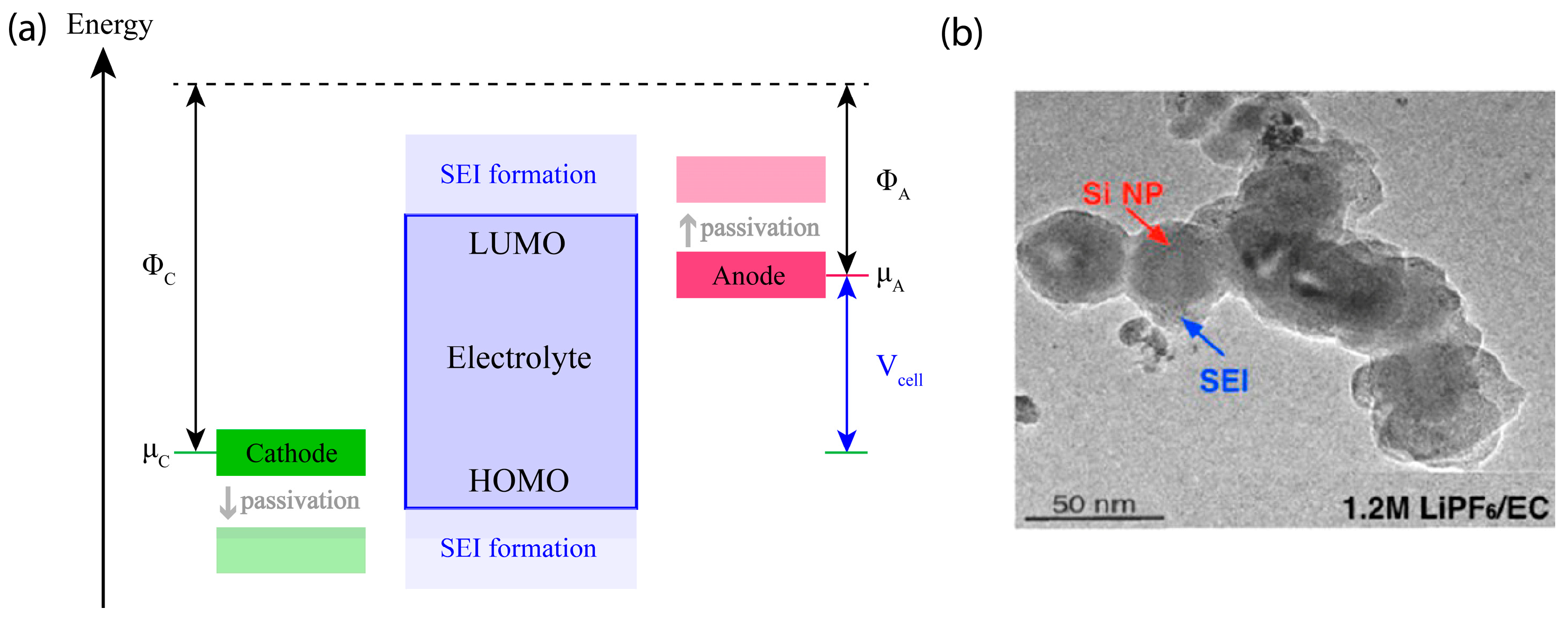
3.2. Anode Degradation at Fast Charging
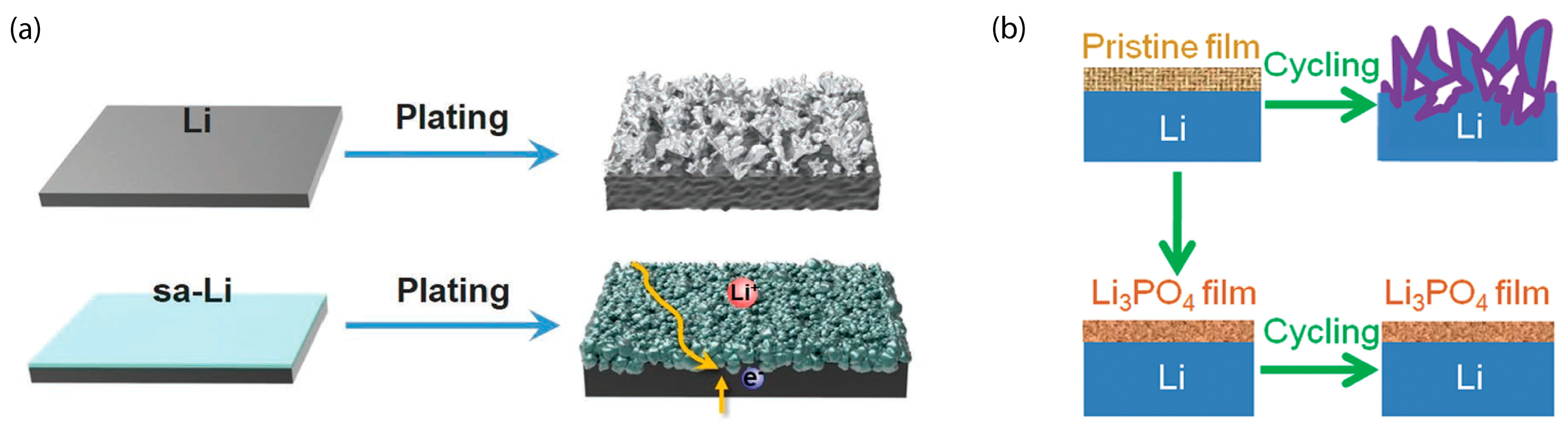
3.2.1. Li Plating in Li-Ion Batteries and Li Metal Batteries
3.2.2. Anode Degradation Modes Aggravated by Fast Charging
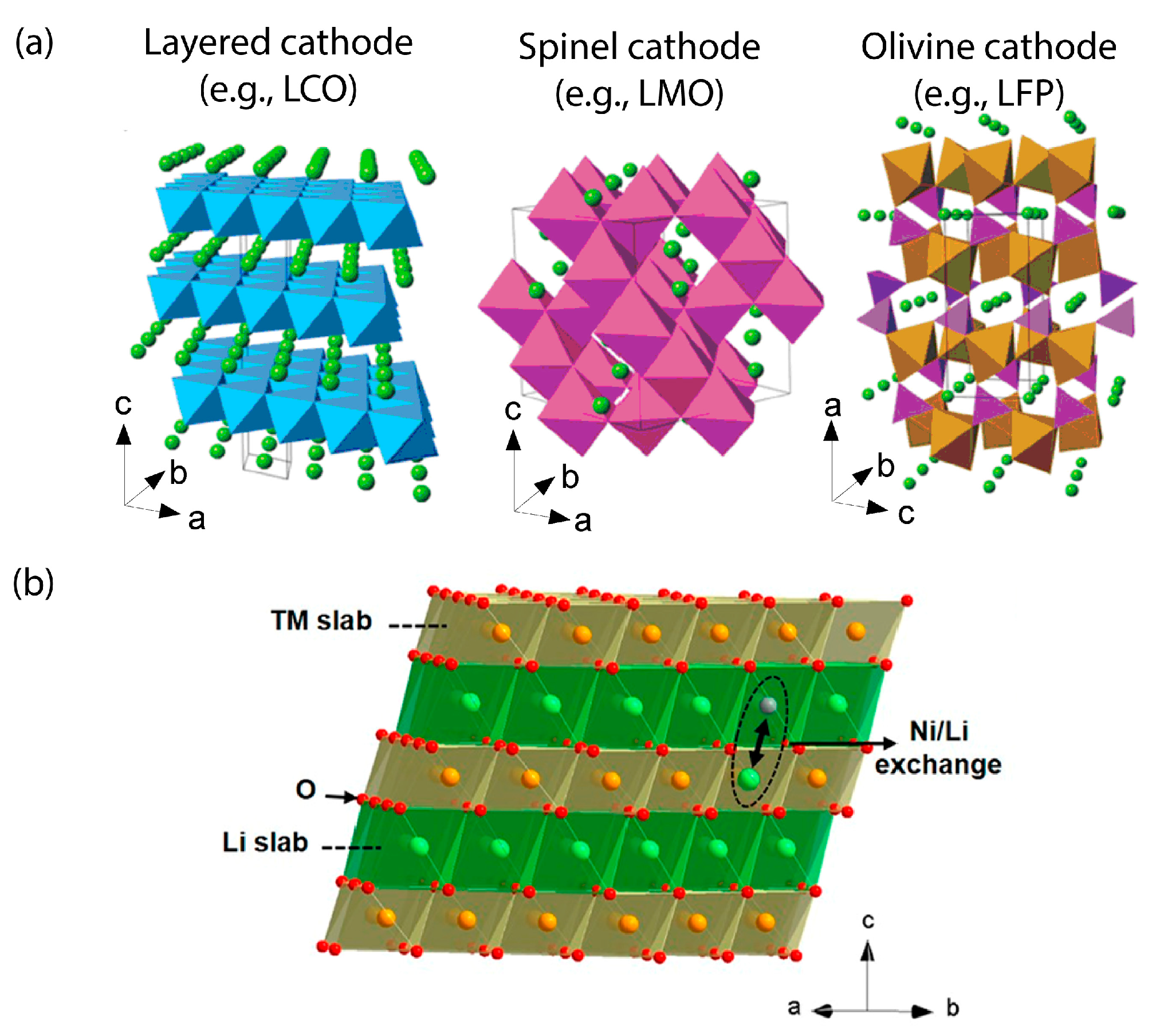
3.3. Cathode Degradation at Fast Charging
3.3.1. Cathode Instability and Decomposition at High Overpotential
3.3.2. Cathode Degradation Modes Aggravated by Fast Charging
4. Solutions to Electrode Degradation for Fast Charging Applications
4.1. Composition Optimization
4.2. Surface Modification
4.3. Microstructure Control
5. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Closing the Gap to Extreme Fast Charge. Available online: https://www.anl.gov/access/research/projects/extreme-fast-charge (accessed on 22 March 2023).
- Supercharger. Available online: https://www.tesla.com/supercharger (accessed on 22 March 2023).
- Introducing V3 Supercharging. Available online: https://www.tesla.com/en_GB/blog/introducing-v3-supercharging (accessed on 22 March 2023).
- Doll, S. Electrify America’s First Megawatt-Level Battery Storage-Backed Charging Station Reduces Stress on the Grid. Available online: https://electrek.co/2022/10/19/electrify-america-megawatt-level-battery-storage-charging-station/ (accessed on 22 March 2023).
- Tian, J.; Li, S.; Liu, X.; Yang, D.; Wang, P.; Chang, G. Lithium-ion battery charging optimization based on electrical, thermal and aging mechanism models. Energy Rep. 2022, 8, 13723–13734. [Google Scholar] [CrossRef]
- Yang, Z.; Charalambous, H.; Lin, Y.; Trask, S.E.; Yu, L.; Wen, J.; Jansen, A.; Tsai, Y.; Wiaderek, K.M.; Ren, Y. Extreme fast charge aging: Correlation between electrode scale and heterogeneous degradation in Ni-rich layered cathodes. J. Power Sources 2022, 521, 230961. [Google Scholar] [CrossRef]
- Tanim, T.R.; Paul, P.P.; Thampy, V.; Cao, C.; Steinrück, H.-G.; Weker, J.N.; Toney, M.F.; Dufek, E.J.; Evans, M.C.; Jansen, A.N. Heterogeneous behavior of lithium plating during extreme fast charging. Cell Rep. Phys. Sci. 2020, 1, 100114. [Google Scholar] [CrossRef]
- Fear, C.; Adhikary, T.; Carter, R.; Mistry, A.N.; Love, C.T.; Mukherjee, P.P. In operando detection of the onset and mapping of lithium plating regimes during fast charging of lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 30438–30448. [Google Scholar] [CrossRef]
- Anseán, D.; Dubarry, M.; Devie, A.; Liaw, B.; García, V.; Viera, J.; González, M. Fast charging technique for high power LiFePO4 batteries: A mechanistic analysis of aging. J. Power Sources 2016, 321, 201–209. [Google Scholar] [CrossRef]
- Al-Saadi, M.; Olmos, J.; Saez-de-Ibarra, A.; Van Mierlo, J.; Berecibar, M. Fast charging impact on the lithium-ion batteries’ lifetime and cost-effective battery sizing in heavy-duty electric vehicles applications. Energies 2022, 15, 1278. [Google Scholar] [CrossRef]
- Li, J.; Dudney, N.J.; Xiao, X.; Cheng, Y.T.; Liang, C.; Verbrugge, M.W. Asymmetric rate behavior of Si anodes for lithium-ion batteries: Ultrafast de-lithiation versus sluggish lithiation at high current densities. Adv. Energy Mater. 2015, 5, 1401627. [Google Scholar] [CrossRef]
- Yang, H.; Wu, H.H.; Ge, M.; Li, L.; Yuan, Y.; Yao, Q.; Chen, J.; Xia, L.; Zheng, J.; Chen, Z. Simultaneously dual modification of Ni-rich layered oxide cathode for high-energy lithium-ion batteries. Adv. Funct. Mater. 2019, 29, 1808825. [Google Scholar] [CrossRef]
- Radin, M.D.; Hy, S.; Sina, M.; Fang, C.; Liu, H.; Vinckeviciute, J.; Zhang, M.; Whittingham, M.S.; Meng, Y.S.; Van der Ven, A. Narrowing the gap between theoretical and practical capacities in Li-ion layered oxide cathode materials. Adv. Energy Mater. 2017, 7, 1602888. [Google Scholar] [CrossRef]
- Hausbrand, R.; Cherkashinin, G.; Ehrenberg, H.; Gröting, M.; Albe, K.; Hess, C.; Jaegermann, W. Fundamental degradation mechanisms of layered oxide Li-ion battery cathode materials: Methodology, insights and novel approaches. Mater. Sci. Eng. B 2015, 192, 3–25. [Google Scholar] [CrossRef]
- Zhang, S.S. Unveiling capacity degradation mechanism of Li-ion battery in fast-charging process. ChemElectroChem 2020, 7, 555–560. [Google Scholar] [CrossRef]
- Xia, S.; Mu, L.; Xu, Z.; Wang, J.; Wei, C.; Liu, L.; Pianetta, P.; Zhao, K.; Yu, X.; Lin, F. Chemomechanical interplay of layered cathode materials undergoing fast charging in lithium batteries. Nano Energy 2018, 53, 753–762. [Google Scholar] [CrossRef]
- Tanim, T.R.; Yang, Z.; Colclasure, A.M.; Chinnam, P.R.; Gasper, P.; Lin, Y.; Yu, L.; Weddle, P.J.; Wen, J.; Dufek, E.J. Extended cycle life implications of fast charging for lithium-ion battery cathode. Energy Storage Mater. 2021, 41, 656–666. [Google Scholar] [CrossRef]
- Son, S.-B.; Robertson, D.; Yang, Z.; Tsai, Y.; Lopykinski, S.; Bloom, I. Fast charge-driven Li plating on anode and structural degradation of cathode. J. Electrochem. Soc. 2020, 167, 140506. [Google Scholar] [CrossRef]
- Colclasure, A.M.; Dunlop, A.R.; Trask, S.E.; Polzin, B.J.; Jansen, A.N.; Smith, K. Requirements for enabling extreme fast charging of high energy density Li-ion cells while avoiding lithium plating. J. Electrochem. Soc. 2019, 166, A1412. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Keyser, M.; Pesaran, A.; Li, Q.; Santhanagopalan, S.; Smith, K.; Wood, E.; Ahmed, S.; Bloom, I.; Dufek, E.; Shirk, M. Enabling fast charging–Battery thermal considerations. J. Power Sources 2017, 367, 228–236. [Google Scholar] [CrossRef]
- Yao, Y.X.; Chen, X.; Yao, N.; Gao, J.H.; Xu, G.; Ding, J.F.; Song, C.L.; Cai, W.L.; Yan, C.; Zhang, Q. Unlocking Charge Transfer Limitations for Extreme Fast Charging of Li-Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202214828. [Google Scholar]
- Ahmed, S.; Bloom, I.; Jansen, A.N.; Tanim, T.; Dufek, E.J.; Pesaran, A.; Burnham, A.; Carlson, R.B.; Dias, F.; Hardy, K. Enabling fast charging–A battery technology gap assessment. J. Power Sources 2017, 367, 250–262. [Google Scholar] [CrossRef]
- Pharr, M.; Zhao, K.; Wang, X.; Suo, Z.; Vlassak, J.J. Kinetics of initial lithiation of crystalline silicon electrodes of lithium-ion batteries. Nano Lett. 2012, 12, 5039–5047. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Thompson, C.V. Kinetic study of the initial lithiation of amorphous silicon thin film anodes. J. Electrochem. Soc. 2018, 165, A650. [Google Scholar] [CrossRef]
- Zlatilova, P.; Balkanov, I.; Geronov, Y. Thin foil lithium-aluminium electrode. The effect of thermal treatment on its electrochemical behaviour in nonaqueous media. J. Power Sources 1988, 24, 71–79. [Google Scholar] [CrossRef]
- Geronov, Y.; Zlatilova, P.; Staikov, G. The secondary lithium—Aluminium electrode at room temperature. II. Kinetics of the electrochemical formation of the lithium—Aluminium alloy. J. Power Sources 1984, 12, 155–165. [Google Scholar] [CrossRef]
- Miao, J.; Wang, B.; Thompson, C.V. Kinetic study of lithiation-induced phase transitions in amorphous germanium thin films. J. Electrochem. Soc. 2020, 167, 090557. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, X.; Zhang, Y.; Zhao, K. Recent advance in understanding the electro-chemo-mechanical behavior of lithium-ion batteries by electron microscopy. Mater. Today Nano 2019, 7, 100040. [Google Scholar] [CrossRef]
- Huo, H.; Janek, J.r. Silicon as Emerging Anode in Solid-State Batteries. ACS Energy Lett. 2022, 7, 4005–4016. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L. Lithium-ion battery fast charging: A review. ETransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Wu, M.; Xu, B.; Ouyang, C. Physics of electron and lithium-ion transport in electrode materials for Li-ion batteries. Chin. Phys. B 2015, 25, 018206. [Google Scholar] [CrossRef]
- Koyama, Y.; Arai, H.; Tanaka, I.; Uchimoto, Y.; Ogumi, Z. Defect chemistry in layered LiMO2 (M = Co, Ni, Mn, and Li1/3Mn2/3) by first-principles calculations. Chem. Mater. 2012, 24, 3886–3894. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, K.; Zhang, H.; Zhu, B. Effect of Ru doping on the properties of LiFePO4/C cathode materials for lithium-ion batteries. ACS Omega 2021, 6, 14122–14129. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, S.U. p-and n-type Doping Effects on the Electrical and Ionic Conductivities of Li4Ti5O12 Anode Materials. J. Phys. Chem. C 2018, 122, 15155–15162. [Google Scholar] [CrossRef]
- Young, D.; Ransil, A.; Amin, R.; Li, Z.; Chiang, Y.M. Electronic conductivity in the Li4/3Ti5/3O4–Li7/3Ti5/3O4 system and variation with state-of-charge as a Li battery anode. Adv. Energy Mater. 2013, 3, 1125–1129. [Google Scholar] [CrossRef]
- Van der Ven, A.; Bhattacharya, J.; Belak, A.A. Understanding Li diffusion in Li-intercalation compounds. Acc. Chem. Res. 2013, 46, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Persson, K.; Sethuraman, V.A.; Hardwick, L.J.; Hinuma, Y.; Meng, Y.S.; Van Der Ven, A.; Srinivasan, V.; Kostecki, R.; Ceder, G. Lithium diffusion in graphitic carbon. J. Phys. Chem. Lett. 2010, 1, 1176–1180. [Google Scholar] [CrossRef]
- Bates, J.; Dudney, N.; Neudecker, B.; Hart, F.; Jun, H.; Hackney, S. Preferred orientation of polycrystalline LiCoO2 films. J. Electrochem. Soc. 2000, 147, 59. [Google Scholar] [CrossRef]
- Electrochemical Behavior and Li Diffusion Study of LiCoO2 Thin Film Electrodes Prepared by PLD. Available online: https://dspace.mit.edu/handle/1721.1/35827 (accessed on 22 March 2023).
- Prosini, P.P.; Lisi, M.; Zane, D.; Pasquali, M. Determination of the chemical diffusion coefficient of lithium in LiFePO4. Solid State Ion. 2002, 148, 45–51. [Google Scholar] [CrossRef]
- Malik, R.; Burch, D.; Bazant, M.; Ceder, G. Particle size dependence of the ionic diffusivity. Nano Lett. 2010, 10, 4123–4127. [Google Scholar] [CrossRef]
- Van der Ven, A.; Ceder, G. Lithium diffusion mechanisms in layered intercalation compounds. J. Power Sources 2001, 97, 529–531. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Q.; Li, J.; Beck, M.J.; Xiao, X.; Cheng, Y.-T. Effects of stress on lithium transport in amorphous silicon electrodes for lithium-ion batteries. Nano Energy 2015, 13, 192–199. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, R.; Gao, W.; Zuo, J.M.; Zhang, X.F.; Misture, S.T.; Chen, Y.; Lockard, J.V.; Zhang, B.; Guo, S. An Ion-Exchange Promoted Phase Transition in a Li-Excess Layered Cathode Material for High-Performance Lithium Ion Batteries. Adv. Energy Mater. 2015, 5, 1401937. [Google Scholar] [CrossRef]
- Nam, K.-W.; Yoon, W.-S.; Yang, X.-Q. Structural changes and thermal stability of charged LiNi1/3Co1/3Mn1/3O2 cathode material for Li-ion batteries studied by time-resolved XRD. J. Power Sources 2009, 189, 515–518. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Kasavajjula, U. Kinetic behavior of LiFeMgPO4 cathode material for Li-ion batteries. J. Power Sources 2006, 162, 1289–1296. [Google Scholar] [CrossRef]
- Barai, A.; Uddin, K.; Dubarry, M.; Somerville, L.; McGordon, A.; Jennings, P.; Bloom, I. A comparison of methodologies for the non-invasive characterisation of commercial Li-ion cells. Prog. Energy Combust. Sci. 2019, 72, 1–31. [Google Scholar] [CrossRef]
- Miao, J.; Wang, B.; Thompson, C.V. First-order amorphous-to-amorphous phase transitions during lithiation of silicon thin films. Phys. Rev. Mater. 2020, 4, 043608. [Google Scholar] [CrossRef]
- Meethong, N.; Huang, H.Y.; Speakman, S.A.; Carter, W.C.; Chiang, Y.M. Strain accommodation during phase transformations in olivine-based cathodes as a materials selection criterion for high-power rechargeable batteries. Adv. Funct. Mater. 2007, 17, 1115–1123. [Google Scholar] [CrossRef]
- Rudraraju, S.; Van der Ven, A.; Garikipati, K. Mechanochemical spinodal decomposition: A phenomenological theory of phase transformations in multi-component, crystalline solids. npj Comput. Mater. 2016, 2, 16012. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J. Spinodal decomposition: A new approach to hierarchically porous inorganic materials for energy storage. Natl. Sci. Rev. 2020, 7, 1635–1637. [Google Scholar] [CrossRef]
- Bai, P.; Cogswell, D.A.; Bazant, M.Z. Suppression of phase separation in LiFePO4 nanoparticles during battery discharge. Nano Lett. 2011, 11, 4890–4896. [Google Scholar] [CrossRef]
- Zheng, T.; Kramer, D.; Mönig, R.; Boles, S.T. Aluminum Foil Anodes for Li-Ion Rechargeable Batteries: The Role of Li Solubility within β-LiAl. ACS Sustain. Chem. Eng. 2022, 10, 3203–3210. [Google Scholar] [CrossRef]
- Yamakawa, S.; Yamasaki, H.; Koyama, T.; Asahi, R. Numerical study of Li diffusion in polycrystalline LiCoO2. J. Power Sources 2013, 223, 199–205. [Google Scholar] [CrossRef]
- Park, M.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A review of conduction phenomena in Li-ion batteries. J. Power Sources 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, L.; Liu, B.; Yu, G. Single-crystalline LiFePO4 nanosheets for high-rate Li-ion batteries. Nano Lett. 2014, 14, 2849–2853. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, B.; Zhao, J.; Zhao, W.; Liang, Z.; Su, Y.; Xie, C.; Zhou, K.; Xiang, Y.; Zhu, J. Electrochemo-mechanical effects on structural integrity of Ni-rich cathodes with different microstructures in all solid-state batteries. Adv. Energy Mater. 2021, 11, 2003583. [Google Scholar] [CrossRef]
- Langdon, J.; Manthiram, A. A perspective on single-crystal layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2021, 37, 143–160. [Google Scholar] [CrossRef]
- Han, Y.; Jung, S.H.; Kwak, H.; Jun, S.; Kwak, H.H.; Lee, J.H.; Hong, S.T.; Jung, Y.S. Single-or poly-crystalline ni-rich layered cathode, sulfide or halide solid electrolyte: Which will be the winners for all-solid-state batteries? Adv. Energy Mater. 2021, 11, 2100126. [Google Scholar] [CrossRef]
- Shi, F.; Song, Z.; Ross, P.N.; Somorjai, G.A.; Ritchie, R.O.; Komvopoulos, K. Failure mechanisms of single-crystal silicon electrodes in lithium-ion batteries. Nat. Commun. 2016, 7, 11886. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Z.; Kuai, C.; Xu, R.; Qin, C.; Zhang, Y.; Rahman, M.M.; Wei, C.; Nordlund, D.; Sun, C.-J. Charge distribution guided by grain crystallographic orientations in polycrystalline battery materials. Nat. Commun. 2020, 11, 83. [Google Scholar] [CrossRef]
- Wang, R.; Chen, X.; Huang, Z.; Yang, J.; Liu, F.; Chu, M.; Liu, T.; Wang, C.; Zhu, W.; Li, S. Twin boundary defect engineering improves lithium-ion diffusion for fast-charging spinel cathode materials. Nat. Commun. 2021, 12, 3085. [Google Scholar] [CrossRef]
- Bläubaum, L.; Röder, F.; Nowak, C.; Chan, H.S.; Kwade, A.; Krewer, U. Impact of Particle Size Distribution on Performance of Lithium-Ion Batteries. ChemElectroChem 2020, 7, 4755–4766. [Google Scholar] [CrossRef]
- Liu, B.; Xu, J. Cracks of silicon nanoparticles in anodes: Mechanics–electrochemical-coupled modeling framework based on the phase-field method. ACS Appl. Energy Mater. 2020, 3, 10931–10939. [Google Scholar] [CrossRef]
- Strauss, F.; Bartsch, T.; de Biasi, L.; Kim, A.-Y.; Janek, J.r.; Hartmann, P.; Brezesinski, T. Impact of cathode material particle size on the capacity of bulk-type all-solid-state batteries. ACS Energy Lett. 2018, 3, 992–996. [Google Scholar] [CrossRef]
- Chen, D.; He, H.; Zhang, D.; Wang, H.; Ni, M. Percolation theory in solid oxide fuel cell composite electrodes with a mixed electronic and ionic conductor. Energies 2013, 6, 1632–1656. [Google Scholar] [CrossRef]
- Pohjalainen, E.; Rauhala, T.; Valkeapää, M.; Kallioinen, J.; Kallio, T. Effect of Li4Ti5O12 particle size on the performance of lithium ion battery electrodes at high C-rates and low temperatures. J. Phys. Chem. C 2015, 119, 2277–2283. [Google Scholar] [CrossRef]
- Ito, Y.; Yamakawa, S.; Hayashi, A.; Tatsumisago, M. Effects of the microstructure of solid-electrolyte-coated LiCoO2 on its discharge properties in all-solid-state lithium batteries. J. Mater. Chem. A 2017, 5, 10658–10668. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Song, Y.; Wu, Z.; Hu, K.; Yue, L.; Zhang, J.; Ming, Y.; Xiang, W.; Wang, G. The structure-activity relationship between precursor fine structure and cathode performance in ultra-high Ni layered oxide. Chem. Eng. Sci. 2022, 260, 117865. [Google Scholar] [CrossRef]
- Wagner, A.C.; Bohn, N.; Geßwein, H.; Neumann, M.; Osenberg, M.; Hilger, A.; Manke, I.; Schmidt, V.; Binder, J.R. Hierarchical Structuring of NMC111-Cathode Materials in Lithium-Ion Batteries: An In-Depth Study on the Influence of Primary and Secondary Particle Sizes on Electrochemical Performance. ACS Appl. Energy Mater. 2020, 3, 12565–12574. [Google Scholar] [CrossRef]
- Taleghani, S.T.; Marcos, B.; Zaghib, K.; Lantagne, G. A study on the effect of porosity and particles size distribution on Li-ion battery performance. J. Electrochem. Soc. 2017, 164, E3179. [Google Scholar] [CrossRef]
- Vishnugopi, B.S.; Verma, A.; Mukherjee, P.P. Fast charging of lithium-ion batteries via electrode engineering. J. Electrochem. Soc. 2020, 167, 090508. [Google Scholar] [CrossRef]
- Elango, R.; Nadeina, A.; Cadiou, F.; De Andrade, V.; Demortière, A.; Morcrette, M.; Seznec, V. Impact of electrode porosity architecture on electrochemical performances of 1 mm-thick LiFePO4 binder-free Li-ion electrodes fabricated by Spark Plasma Sintering. J. Power Sources 2021, 488, 229402. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T. Study of the charging process of a LiCoO2-based Li-ion battery. J. Power Sources 2006, 160, 1349–1354. [Google Scholar] [CrossRef]
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Levartovsky, Y.; Levy, N.R.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D. Fast charging of lithium-ion batteries: A review of materials aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Yao, K.P.; Okasinski, J.S.; Kalaga, K.; Shkrob, I.A.; Abraham, D.P. Quantifying lithium concentration gradients in the graphite electrode of Li-ion cells using operando energy dispersive X-ray diffraction. Energy Environ. Sci. 2019, 12, 656–665. [Google Scholar] [CrossRef]
- Finegan, D.P.; Quinn, A.; Wragg, D.S.; Colclasure, A.M.; Lu, X.; Tan, C.; Heenan, T.M.; Jervis, R.; Brett, D.J.; Das, S. Spatial dynamics of lithiation and lithium plating during high-rate operation of graphite electrodes. Energy Environ. Sci. 2020, 13, 2570–2584. [Google Scholar] [CrossRef]
- Colclasure, A.M.; Tanim, T.R.; Jansen, A.N.; Trask, S.E.; Dunlop, A.R.; Polzin, B.J.; Bloom, I.; Robertson, D.; Flores, L.; Evans, M. Electrode scale and electrolyte transport effects on extreme fast charging of lithium-ion cells. Electrochim. Acta 2020, 337, 135854. [Google Scholar] [CrossRef]
- Mistry, A.; Usseglio-Viretta, F.L.; Colclasure, A.; Smith, K.; Mukherjee, P.P. Fingerprinting redox heterogeneity in electrodes during extreme fast charging. J. Electrochem. Soc. 2020, 167, 090542. [Google Scholar] [CrossRef]
- Lin, X.; Khosravinia, K.; Hu, X.; Li, J.; Lu, W. Lithium plating mechanism, detection, and mitigation in lithium-ion batteries. Prog. Energy Combust. Sci. 2021, 87, 100953. [Google Scholar] [CrossRef]
- Han, X.; Meng, Q.; Wan, X.; Sun, B.; Zhang, Y.; Shen, B.; Gao, J.; Ma, Y.; Zuo, P.; Lou, S. Intercalation pseudocapacitive electrochemistry of Nb-based oxides for fast charging of lithium-ion batteries. Nano Energy 2021, 81, 105635. [Google Scholar] [CrossRef]
- Belharouak, I.; Koenig, G.M.; Tan, T.; Yumoto, H.; Ota, N.; Amine, K. Performance degradation and gassing of Li4Ti5O12/LiMn2O4 lithium-ion cells. J. Electrochem. Soc. 2012, 159, A1165. [Google Scholar] [CrossRef]
- Deng, Q.; Fu, Y.; Zhu, C.; Yu, Y. Niobium-based oxides toward advanced electrochemical energy storage: Recent advances and challenges. Small 2019, 15, 1804884. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Han, Y.; Fraggedakis, D.; Das, S.; Zhou, T.; Yeh, C.-N.; Xu, S.; Chueh, W.C.; Li, J.; Bazant, M.Z. Interplay of lithium intercalation and plating on a single graphite particle. Joule 2021, 5, 393–414. [Google Scholar] [CrossRef]
- Hou, G.; Sun, Q.; Ai, Q.; Ren, X.; Xu, X.; Guo, H.; Guo, S.; Zhang, L.; Feng, J.; Ding, F. Growth direction control of lithium dendrites in a heterogeneous lithiophilic host for ultra-safe lithium metal batteries. J. Power Sources 2019, 416, 141–147. [Google Scholar] [CrossRef]
- He, Y.; Ren, X.; Xu, Y.; Engelhard, M.H.; Li, X.; Xiao, J.; Liu, J.; Zhang, J.-G.; Xu, W.; Wang, C. Origin of lithium whisker formation and growth under stress. Nat. Nanotechnol. 2019, 14, 1042–1047. [Google Scholar] [CrossRef]
- Rangarajan, S.P.; Barsukov, Y.; Mukherjee, P.P. In operando signature and quantification of lithium plating. J. Mater. Chem. A 2019, 7, 20683–20695. [Google Scholar] [CrossRef]
- Ling, C.; Banerjee, D.; Matsui, M. Study of the electrochemical deposition of Mg in the atomic level: Why it prefers the non-dendritic morphology. Electrochim. Acta 2012, 76, 270–274. [Google Scholar] [CrossRef]
- Wasalathanthri, R.N.; Akolkar, R. Perspective—Does the Sand Equation Reliably Predict the Onset of Morphological Evolution in Lithium Electrodeposition? J. Electrochem. Soc. 2022, 169, 092519. [Google Scholar] [CrossRef]
- Shen, L.; Shi, P.; Hao, X.; Zhao, Q.; Ma, J.; He, Y.B.; Kang, F. Progress on lithium dendrite suppression strategies from the interior to exterior by hierarchical structure designs. Small 2020, 16, 2000699. [Google Scholar] [CrossRef]
- Jang, T.; Kang, J.-H.; Kim, S.; Shim, M.; Lee, J.; Song, J.; Kim, W.; Ryu, K.; Byon, H.R. Nanometer-Scale Surface Roughness of a 3-D Cu Substrate Promoting Li Nucleation in Li-Metal Batteries. ACS Appl. Energy Mater. 2021, 4, 2644–2651. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B. Optimizing areal capacities through understanding the limitations of lithium-ion electrodes. J. Electrochem. Soc. 2015, 163, A138. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, J.; Pei, A.; Liu, B.; Wu, Y.; Lin, D.; Li, J.; Wang, H.; Chen, H.; Xu, J. Fast lithium growth and short circuit induced by localized-temperature hotspots in lithium batteries. Nat. Commun. 2019, 10, 2067. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, W.; Hong, L.; Xu, W.; Yang, H.; Wang, F.; Duan, H.; Tang, M.; Jiang, H. Stress-driven lithium dendrite growth mechanism and dendrite mitigation by electroplating on soft substrates. Nat. Energy 2018, 3, 227–235. [Google Scholar] [CrossRef]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J. Lithium ion battery degradation: What you need to know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.J.; Sharafi, A.; Sakamoto, J. Intergranular Li metal propagation through polycrystalline Li6. 25Al0. 25La3Zr2O12 ceramic electrolyte. Electrochim. Acta 2017, 223, 85–91. [Google Scholar] [CrossRef]
- Rangarajan, S.P.; Barsukov, Y.; Mukherjee, P.P. Plating energy as a universal descriptor to classify accelerated cell failure under operational extremes. Cell Rep. Phys. Sci. 2022, 3, 100720. [Google Scholar] [CrossRef]
- Nie, M.; Abraham, D.P.; Chen, Y.; Bose, A.; Lucht, B.L. Silicon solid electrolyte interphase (SEI) of lithium ion battery characterized by microscopy and spectroscopy. J. Phys. Chem. C 2013, 117, 13403–13412. [Google Scholar] [CrossRef]
- Fong, R.; Von Sacken, U.; Dahn, J.R. Studies of lithium intercalation into carbons using nonaqueous electrochemical cells. J. Electrochem. Soc. 1990, 137, 2009. [Google Scholar] [CrossRef]
- Narayanan, S.; Ulissi, U.; Gibson, J.S.; Chart, Y.A.; Weatherup, R.S.; Pasta, M. Effect of current density on the solid electrolyte interphase formation at the lithium∣ Li6PS5Cl interface. Nat. Commun. 2022, 13, 7237. [Google Scholar] [CrossRef]
- Yoon, T.; Milien, M.S.; Parimalam, B.S.; Lucht, B.L. Thermal decomposition of the solid electrolyte interphase (SEI) on silicon electrodes for lithium ion batteries. Chem. Mater. 2017, 29, 3237–3245. [Google Scholar] [CrossRef]
- Ding, J.-F.; Xu, R.; Yan, C.; Li, B.-Q.; Yuan, H.; Huang, J.-Q. A review on the failure and regulation of solid electrolyte interphase in lithium batteries. J. Energy Chem. 2021, 59, 306–319. [Google Scholar] [CrossRef]
- He, Y.; Jiang, L.; Chen, T.; Xu, Y.; Jia, H.; Yi, R.; Xue, D.; Song, M.; Genc, A.; Bouchet-Marquis, C. Progressive growth of the solid–electrolyte interphase towards the Si anode interior causes capacity fading. Nat. Nanotechnol. 2021, 16, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.S.; Son, S.-B.; Kim, J.W.; Kim, S.C.; Choi, Y.S.; Heo, J.Y.; Suh, S.-S.; Kim, Y.-U.; Chu, Y.Y.; Cho, J.S. Electrochemically induced and orientation dependent crack propagation in single crystal silicon. J. Power Sources 2014, 267, 739–743. [Google Scholar] [CrossRef]
- Li, J.; Dozier, A.K.; Li, Y.; Yang, F.; Cheng, Y.-T. Crack pattern formation in thin film lithium-ion battery electrodes. J. Electrochem. Soc. 2011, 158, A689. [Google Scholar] [CrossRef]
- Wang, J.W.; He, Y.; Fan, F.; Liu, X.H.; Xia, S.; Liu, Y.; Harris, C.T.; Li, H.; Huang, J.Y.; Mao, S.X. Two-phase electrochemical lithiation in amorphous silicon. Nano Lett. 2013, 13, 709–715. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Harris, J.T.; Korgel, B.A.; Wang, C.; Nix, W.D.; Cui, Y. In situ TEM of two-phase lithiation of amorphous silicon nanospheres. Nano Lett. 2013, 13, 758–764. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Ryu, I.; Choi, J.W.; Cui, Y.; Nix, W.D. Size-dependent fracture of Si nanowire battery anodes. J. Mech. Phys. Solids 2011, 59, 1717–1730. [Google Scholar] [CrossRef]
- Ma, Z.; Li, T.; Huang, Y.; Liu, J.; Zhou, Y.; Xue, D. Critical silicon-anode size for averting lithiation-induced mechanical failure of lithium-ion batteries. RSC Adv. 2013, 3, 7398–7402. [Google Scholar] [CrossRef]
- Barai, P.; Huang, B.; Dillon, S.J.; Mukherjee, P.P. Mechano-electrochemical interaction gives rise to strain relaxation in Sn electrodes. J. Electrochem. Soc. 2016, 163, A3022. [Google Scholar] [CrossRef]
- Key, B.; Bhattacharyya, R.; Morcrette, M.; Seznec, V.; Tarascon, J.-M.; Grey, C.P. Real-time NMR investigations of structural changes in silicon electrodes for lithium-ion batteries. J. Am. Chem. Soc. 2009, 131, 9239–9249. [Google Scholar] [CrossRef]
- Jung, H.; Allan, P.K.; Hu, Y.-Y.; Borkiewicz, O.J.; Wang, X.-L.; Han, W.-Q.; Du, L.-S.; Pickard, C.J.; Chupas, P.J.; Chapman, K.W. Elucidation of the local and long-range structural changes that occur in germanium anodes in lithium-ion batteries. Chem. Mater. 2015, 27, 1031–1041. [Google Scholar] [CrossRef]
- Key, B.; Morcrette, M.; Tarascon, J.-M.; Grey, C.P. Pair distribution function analysis and solid state NMR studies of silicon electrodes for lithium ion batteries: Understanding the (de) lithiation mechanisms. J. Am. Chem. Soc. 2011, 133, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Trill, J.-H.; Tao, C.; Winter, M.; Passerini, S.; Eckert, H. NMR investigations on the lithiation and delithiation of nanosilicon-based anodes for Li-ion batteries. J. Solid State Electrochem. 2011, 15, 349–356. [Google Scholar] [CrossRef]
- Ogata, K.; Salager, E.; Kerr, C.; Fraser, A.; Ducati, C.; Morris, A.J.; Hofmann, S.; Grey, C.P. Revealing lithium–silicide phase transformations in nano-structured silicon-based lithium ion batteries via in situ NMR spectroscopy. Nat. Commun. 2014, 5, 3217. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, Y.; Peng, C.; Hu, M.Y.; Deng, X.; Lin, M.; Hu, J.Z.; Loh, K.P. Probing lithium germanide phase evolution and structural change in a germanium-in-carbon nanotube energy storage system. J. Am. Chem. Soc. 2015, 137, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiu, J.; Wang, X.; Chen, L.; Cao, G.; Wang, J.; Zhang, H.; He, X. Insights for understanding multiscale degradation of LiFePO4 cathodes. EScience 2022, 2, 125–137. [Google Scholar] [CrossRef]
- Chinnam, P.R.; Colclasure, A.M.; Chen, B.-R.; Tanim, T.R.; Dufek, E.J.; Smith, K.; Evans, M.C.; Dunlop, A.R.; Trask, S.E.; Polzin, B.J. Fast-charging aging considerations: Incorporation and alignment of cell design and material degradation pathways. ACS Appl. Energy Mater. 2021, 4, 9133–9143. [Google Scholar] [CrossRef]
- Daniel, C.; Mohanty, D.; Li, J.; Wood, D.L. Cathode materials review. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2014; pp. 26–43. [Google Scholar]
- Kasnatscheew, J.; Evertz, M.; Kloepsch, R.; Streipert, B.; Wagner, R.; Cekic Laskovic, I.; Winter, M. Learning from Electrochemical Data: Simple Evaluation and Classification of LiMO2-type-based Positive Electrodes for Li-Ion Batteries. Energy Technol. 2017, 5, 1670–1679. [Google Scholar] [CrossRef]
- Li, J.; Lin, C.; Weng, M.; Qiu, Y.; Chen, P.; Yang, K.; Huang, W.; Hong, Y.; Li, J.; Zhang, M. Structural origin of the high-voltage instability of lithium cobalt oxide. Nat. Nanotechnol. 2021, 16, 599–605. [Google Scholar] [CrossRef]
- Wang, H.; Jang, Y.I.; Huang, B.; Sadoway, D.R.; Chiang, Y.M. TEM study of electrochemical cycling-induced damage and disorder in LiCoO2 cathodes for rechargeable lithium batteries. J. Electrochem. Soc. 1999, 146, 473. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Rodkin, A.; Levi, E.; Cohen, Y.; Kim, H.-J.; Schmidt, M. On the capacity fading of LiCoO2 intercalation electrodes:: The effect of cycling, storage, temperature, and surface film forming additives. Electrochim. Acta 2002, 47, 4291–4306. [Google Scholar] [CrossRef]
- Reducing Reliance on Cobalt for Lithium-ion Batteries. Available online: https://www.energy.gov/eere/vehicles/articles/reducing-reliance-cobalt-lithium-ion-batteries (accessed on 22 March 2023).
- Zheng, J.; Ye, Y.; Liu, T.; Xiao, Y.; Wang, C.; Wang, F.; Pan, F. Ni/Li disordering in layered transition metal oxide: Electrochemical impact, origin, and control. Acc. Chem. Res. 2019, 52, 2201–2209. [Google Scholar] [CrossRef]
- Reed, J.; Ceder, G. Role of electronic structure in the susceptibility of metastable transition-metal oxide structures to transformation. Chem. Rev. 2004, 104, 4513–4534. [Google Scholar] [CrossRef]
- Bak, S.-M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.-J.; Kim, K.-B.; Chung, K.Y.; Yang, X.-Q.; Nam, K.-W. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Myung, S.-T.; Maglia, F.; Park, K.-J.; Yoon, C.S.; Lamp, P.; Kim, S.-J.; Sun, Y.-K. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Mijung, N.; Lee, Y.; Cho, J. Water adsorption and storage characteristics of optimized LiCoO2 and LiNi1/3Co1/3Mn1/3O2 composite cathode material for Li-ion cells. J. Electrochem. Soc. 2006, 153, A935. [Google Scholar] [CrossRef]
- Abraham, D.; Twesten, R.; Balasubramanian, M.; Petrov, I.; McBreen, J.; Amine, K. Surface changes on LiNi0.8Co0.2O2 particles during testing of high-power lithium-ion cells. Electrochem. Commun. 2002, 4, 620–625. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, F.-l.; Zhou, X.-a.; Wang, P.; Wang, J.; Ding, H.; Dong, H.; Liang, W.-b.; Zhang, N.-s.; Li, S.-y. Which of the nickel-rich NCM and NCA is structurally superior as a cathode material for lithium-ion batteries? J. Mater. Chem. A 2021, 9, 13540–13551. [Google Scholar] [CrossRef]
- Bloom, I.; Jones, S.A.; Battaglia, V.S.; Henriksen, G.L.; Christophersen, J.P.; Wright, R.B.; Ho, C.D.; Belt, J.R.; Motloch, C.G. Effect of cathode composition on capacity fade, impedance rise and power fade in high-power, lithium-ion cells. J. Power Sources 2003, 124, 538–550. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li [NixCoyMnz]O2 (x= 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative issues of cathode materials for Li-ion batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Alikin, D.; Slautin, B.; Kholkin, A. Revealing Lithiation Kinetics and Battery Degradation Pathway in LiMn2O4-Based Commercial Cathodes via Electrochemical Strain Microscopy. Batteries 2022, 8, 220. [Google Scholar] [CrossRef]
- Luo, F.; Wei, C.; Zhang, C.; Gao, H.; Niu, J.; Ma, W.; Peng, Z.; Bai, Y.; Zhang, Z. Operando X-ray diffraction analysis of the degradation mechanisms of a spinel LiMn2O4 cathode in different voltage windows. J. Energy Chem. 2020, 44, 138–146. [Google Scholar] [CrossRef]
- Sun, S.; Guan, T.; Cheng, X.; Zuo, P.; Gao, Y.; Du, C.; Yin, G. Accelerated aging and degradation mechanism of LiFePO4/graphite batteries cycled at high discharge rates. RSC Adv. 2018, 8, 25695–25703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J. Structure and performance of LiFePO4 cathode materials: A review. J. Power Sources 2011, 196, 2962–2970. [Google Scholar] [CrossRef]
- Kassem, M.; Delacourt, C. Postmortem analysis of calendar-aged graphite/LiFePO4 cells. J. Power Sources 2013, 235, 159–171. [Google Scholar] [CrossRef]
- Woodford, W.H.; Carter, W.C.; Chiang, Y.-M. Strategies to avert electrochemical shock and their demonstration in spinels. J. Electrochem. Soc. 2014, 161, F3005. [Google Scholar] [CrossRef]
- Woodford, W.H.; Chiang, Y.-M.; Carter, W.C. Electrochemical shock in ion-intercalation materials with limited solid-solubility. J. Electrochem. Soc. 2013, 160, A1286. [Google Scholar] [CrossRef]
- Xu, R.; Sun, H.; de Vasconcelos, L.S.; Zhao, K. Mechanical and structural degradation of LiNixMnyCozO2 cathode in Li-ion batteries: An experimental study. J. Electrochem. Soc. 2017, 164, A3333. [Google Scholar] [CrossRef]
- Stenina, I.; Minakova, P.; Kulova, T.; Yaroslavtsev, A. Electrochemical Properties of LiFePO4 Cathodes: The Effect of Carbon Additives. Batteries 2022, 8, 111. [Google Scholar] [CrossRef]
- Ngo, D.T.; Scipioni, R.; Simonsen, S.B.; Jørgensen, P.S.; Jensen, S.H. A TEM study of morphological and structural degradation phenomena in LiFePO4-CB cathodes. Int. J. Energy Res. 2016, 40, 2022–2032. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Yang, Y.; Mu, L.; Wei, C.; Yu, X.; Pianetta, P.; Zhao, K.; Cloetens, P.; Lin, F. Machine-learning-revealed statistics of the particle-carbon/binder detachment in lithium-ion battery cathodes. Nat. Commun. 2020, 11, 2310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. Challenges and strategies for fast charge of Li-ion batteries. ChemElectroChem 2020, 7, 3569–3577. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Z.; Chen, H.; Luo, L.; Zhang, Q.; Chen, J.; Chen, S.; Yang, Y. Bulk boron doping and surface carbon coating enabling fast-charging and stable Si anodes: From thin film to thick Si electrodes. J. Mater. Chem. A 2021, 9, 3628–3636. [Google Scholar] [CrossRef]
- Ali, B.; Muhammad, R.; Anang, D.A.; Cho, M.-k.; Kim, J.-Y.; Nam, K.-W. Ge-doped Li4Ti5-xGexO12 (x= 0.05) as a fast-charging, long-life bi-functional anode material for lithium-and sodium-ion batteries. Ceram. Int. 2020, 46, 16556–16563. [Google Scholar] [CrossRef]
- Kim, N.; Chae, S.; Ma, J.; Ko, M.; Cho, J. Fast-charging high-energy lithium-ion batteries via implantation of amorphous silicon nanolayer in edge-plane activated graphite anodes. Nat. Commun. 2017, 8, 812. [Google Scholar] [CrossRef]
- Liu, X.; Xu, G.-L.; Yin, L.; Hwang, I.; Li, Y.; Lu, L.; Xu, W.; Zhang, X.; Chen, Y.; Ren, Y. Probing the thermal-driven structural and chemical degradation of Ni-rich layered cathodes by Co/Mn exchange. J. Am. Chem. Soc. 2020, 142, 19745–19753. [Google Scholar] [CrossRef]
- Yuge, R.; Tamura, N.; Manako, T.; Nakano, K.; Nakahara, K. High-rate charge/discharge properties of Li-ion battery using carbon-coated composites of graphites, vapor grown carbon fibers, and carbon nanohorns. J. Power Sources 2014, 266, 471–474. [Google Scholar] [CrossRef]
- Gohier, A.; Laïk, B.; Kim, K.H.; Maurice, J.L.; Pereira-Ramos, J.P.; Cojocaru, C.S.; Van, P.T. High-Rate Capability Silicon Decorated Vertically Aligned Carbon Nanotubes for Li-Ion Batteries. Adv. Mater. 2012, 24, 2592–2597. [Google Scholar] [CrossRef]
- Daigle, J.-C.; Asakawa, Y.; Beaupré, M.; Gariépy, V.; Vieillette, R.; Laul, D.; Trudeau, M.; Zaghib, K. Boosting Ultra-Fast Charge Battery Performance: Filling Porous nanoLi4Ti5O12 Particles with 3D Network of N-doped Carbons. Sci. Rep. 2019, 9, 16871. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.; Yu, G. Material and structural design of novel binder systems for high-energy, high-power lithium-ion batteries. Acc. Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gao, P.; Li, P.; Xia, K.; Han, N.; Deng, J.; Li, Y.; Lu, J. Fast-Charging and Ultrahigh-Capacity Lithium Metal Anode Enabled by Surface Alloying. Adv. Energy Mater. 2020, 10, 1902343. [Google Scholar] [CrossRef]
- Li, N.W.; Yin, Y.X.; Yang, C.P.; Guo, Y.G. An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv. Mater. 2016, 28, 1853–1858. [Google Scholar] [CrossRef]
- Ho, V.-C.; An, H.; Hong, M.; Lee, S.; Kim, J.; Park, M.B.; Mun, J. A Low Temperature Self-Assembled ZrO2 Layer as a Surface Modification for High Energy Density Ni-Rich Cathode Materials in a Lithium-Ion Battery. Energy Technol. 2021, 9, 2000800. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, A.-Y.; Liu, G.; Woo, J.-Y.; Kim, H.; Lee, J.K. Li4SiO4-based artificial passivation thin film for improving interfacial stability of Li metal anodes. ACS Appl. Mater. Interfaces 2018, 10, 8692–8701. [Google Scholar] [CrossRef]
- Li, J.; Dudney, N.J.; Nanda, J.; Liang, C. Artificial solid electrolyte interphase to address the electrochemical degradation of silicon electrodes. ACS Appl. Mater. Interfaces 2014, 6, 10083–10088. [Google Scholar] [CrossRef]
- Al-Obeidi, A.; Kramer, D.; Boles, S.T.; Mönig, R.; Thompson, C.V. Mechanical measurements on lithium phosphorous oxynitride coated silicon thin film electrodes for lithium-ion batteries during lithiation and delithiation. Appl. Phys. Lett. 2016, 109, 071902. [Google Scholar] [CrossRef]
- Yan, S.; Chen, X.; Zhou, P.; Wang, P.; Zhou, H.; Zhang, W.; Xia, Y.; Liu, K. Regulating the growth of lithium dendrite by coating an ultra-thin layer of gold on separator for improving the fast-charging ability of graphite anode. J. Energy Chem. 2022, 67, 467–473. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.; Ke, X.; Zhang, Z.; Wu, W.; Lin, G.; Zhou, Z.; Shi, Z. Coupling of triporosity and strong Au–Li interaction to enable dendrite-free lithium plating/stripping for long-life lithium metal anodes. J. Mater. Chem. A 2020, 8, 18094–18105. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, X.; Guan, Y.; Xiang, Y.; Li, M.; Zhang, W.; Zhu, X.; Ming, H.; Lu, L.; Qiu, J. Lithiophilic-lithiophobic gradient interfacial layer for a highly stable lithium metal anode. Nat. Commun. 2018, 9, 3729. [Google Scholar] [CrossRef]
- He, Y.; Zhang, M.; Wang, A.; Zhang, B.; Pham, H.; Hu, Q.; Sheng, L.; Xu, H.; Wang, L.; Park, J. Regulation of Dendrite-Free Li Plating via Lithiophilic Sites on Lithium-Alloy Surface. ACS Appl. Mater. Interfaces 2022, 14, 33952–33959. [Google Scholar] [CrossRef]
- Zhao, B.; Ma, W.; Li, B.; Hu, X.; Lu, S.; Liu, X.; Jiang, Y.; Zhang, J. A fast and low-cost interface modification method to achieve high-performance garnet-based solid-state lithium metal batteries. Nano Energy 2022, 91, 106643. [Google Scholar] [CrossRef]
- Jiang, W.; Dong, L.; Liu, S.; Ai, B.; Zhao, S.; Zhang, W.; Pan, K.; Zhang, L. Improvement of the interface between the lithium anode and a garnet-type solid electrolyte of lithium batteries using an aluminum-nitride layer. Nanomaterials 2022, 12, 2023. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Yuge, R.; Nakahara, K.; Tamura, N.; Miyamoto, S. KOH etched graphite for fast chargeable lithium-ion batteries. J. Power Sources 2015, 284, 258–263. [Google Scholar] [CrossRef]
- Huang, Q.; Ni, S.; Jiao, M.; Zhong, X.; Zhou, G.; Cheng, H.M. Aligned Carbon-Based Electrodes for Fast-Charging Batteries: A Review. Small 2021, 17, 2007676. [Google Scholar] [CrossRef]
- Chen, K.-H.; Namkoong, M.J.; Goel, V.; Yang, C.; Kazemiabnavi, S.; Mortuza, S.; Kazyak, E.; Mazumder, J.; Thornton, K.; Sakamoto, J. Efficient fast-charging of lithium-ion batteries enabled by laser-patterned three-dimensional graphite anode architectures. J. Power Sources 2020, 471, 228475. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, M.; Chen, W.; Liu, Y.; Zhang, L.; Dongfang, N.; Ruan, Y.; Zhang, J.; Wang, P.; Dong, L. Sandwich, vertical-channeled thick electrodes with high rate and cycle performance. Adv. Funct. Mater. 2019, 29, 1809196. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Mijailovic, A.; Wang, G.; Xiong, J.; Mathew, K.; Lu, W.; Sheldon, B.W.; Wu, Q. Gradient porosity electrodes for fast charging lithium-ion batteries. J. Mater. Chem. A 2022, 10, 12114–12124. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, H.; Yang, H.; Yu, Y.; Luo, J.; Li, T.; Li, W.; Zhang, Y.-N.; Kang, Y. Thermodynamic regulation of dendrite-free Li plating on Li3Bi for stable lithium metal batteries. Nano Lett. 2021, 21, 8664–8670. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, H.; Sun, H.; Yu, D.; Wang, Y.; Huang, X.; Chen, L.; Zhang, Z. Controlled Li doping of Si nanowires by electrochemical insertion method. Appl. Phys. Lett. 1999, 75, 2447–2449. [Google Scholar] [CrossRef]
- Park, M.H.; Cho, Y.; Kim, K.; Kim, J.; Liu, M.; Cho, J. Germanium nanotubes prepared by using the kirkendall effect as anodes for high-rate lithium batteries. Angew. Chem. Int. Ed. 2011, 50, 9647–9650. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bao, K.; Lou, Z.; Liang, G.; Zhou, Q. Chemical synthesis of germanium nanoparticles with uniform size as anode materials for lithium ion batteries. Dalton Trans. 2016, 45, 2814–2817. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Hou, Z.; Zhang, T.; Zhu, Y.; Yan, X.; Qian, Y. Honeycomb-like macro-germanium as high-capacity anodes for lithium-ion batteries with good cycling and rate performance. Chem. Mater. 2015, 27, 4156–4164. [Google Scholar] [CrossRef]
- Cui, L.-F.; Yang, Y.; Hsu, C.-M.; Cui, Y. Carbon−silicon core−shell nanowires as high capacity electrode for lithium ion batteries. Nano Lett. 2009, 9, 3370–3374. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Kang, Z.; Liu, W.; Chen, Y. High-density crack-resistant Si-C microparticles for lithium ion batteries. Energy Storage Mater. 2023, 56, 40–49. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Wu, Z.-Y.; Li, J.-T.; Huang, L.; Sun, S.-G. 3D nanostructured multilayer Si/Al film with excellent cycle performance as anode material for lithium-ion battery. J. Alloys Compd. 2016, 657, 559–564. [Google Scholar] [CrossRef]
- Hawley, W.B.; Li, J. Electrode manufacturing for lithium-ion batteries—Analysis of current and next generation processing. J. Energy Storage 2019, 25, 100862. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.; Liou, F.; Park, J. Macro-/micro-controlled 3D lithium-ion batteries via additive manufacturing and electric field processing. Sci. Rep. 2018, 8, 1846. [Google Scholar] [CrossRef]
- Li, L.; Erb, R.M.; Wang, J.; Wang, J.; Chiang, Y.M. Fabrication of Low-Tortuosity Ultrahigh-Area-Capacity Battery Electrodes through Magnetic Alignment of Emulsion-Based Slurries. Adv. Energy Mater. 2019, 9, 1802472. [Google Scholar] [CrossRef]
- Thakur, A.; Dong, X. Additive manufacturing of 3D structural battery composites with coextrusion deposition of continuous carbon fibers. Manuf. Lett. 2020, 26, 42–47. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, Y.; Zhang, Q.; Wan, D.; Huang, C. Directional LiFePO4 cathode structure by freeze tape casting to improve lithium ion diffusion kinetics. J. Power Sources 2021, 506, 230052. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, J. Review on Electrode Degradation at Fast Charging of Li-Ion and Li Metal Batteries from a Kinetic Perspective. Electrochem 2023, 4, 156-180. https://doi.org/10.3390/electrochem4020013
Miao J. Review on Electrode Degradation at Fast Charging of Li-Ion and Li Metal Batteries from a Kinetic Perspective. Electrochem. 2023; 4(2):156-180. https://doi.org/10.3390/electrochem4020013
Chicago/Turabian StyleMiao, Jinghui. 2023. "Review on Electrode Degradation at Fast Charging of Li-Ion and Li Metal Batteries from a Kinetic Perspective" Electrochem 4, no. 2: 156-180. https://doi.org/10.3390/electrochem4020013
APA StyleMiao, J. (2023). Review on Electrode Degradation at Fast Charging of Li-Ion and Li Metal Batteries from a Kinetic Perspective. Electrochem, 4(2), 156-180. https://doi.org/10.3390/electrochem4020013





